
R.R.O. 1990, Reg. 645: GENERAL
under Immunization of School Pupils Act, R.S.O. 1990, c. I.1
Skip to content| current | March 29, 2018 – (e-Laws currency date) |
| September 1, 2017 – March 28, 2018 | |
| August 3, 2017 – August 31, 2017 | |
| July 1, 2014 – August 2, 2017 | |
| September 18, 2013 – June 30, 2014 | |
| December 19, 2003 – September 17, 2013 |
Immunization of School Pupils Act
Loi sur l’immunisation des élèves
R.R.O. 1990, REGULATION 645
GENERAL
Historical version for the period December 19, 2003 to September 17, 2013.
Last amendment: O. Reg. 443/03.
This Regulation is made in English only.
1. A record of immunization maintained by a medical officer of health with respect to a pupil shall contain,
(a) the name of the pupil in full;
(b) the date of birth of the pupil;
(c) the sex of the pupil;
(d) the name of the school attended by the pupil;
(d.1) the pupil’s health number, as defined in the Health Cards and Numbers Control Act, 1991;
(e) a record of all the pupil’s immunization against designated diseases showing,
(i) the type of vaccine given,
(ii) the date of administration of the vaccine, and
(iii) any reactions to the vaccine;
(f) any statement of medical exemption that pertains to the pupil showing the effective time period on the statement; and
(g) any statement of religious belief that pertains to the pupil. R.R.O. 1990, Reg. 645, s. 1; O. Reg. 299/96, s. 1.
2. A statement of medical exemption shall be in Form 1. R.R.O. 1990, Reg. 645, s. 2.
3. A statement of conscience or religious belief shall be in Form 2. R.R.O. 1990, Reg. 645, s. 3.
4. A notice of transfer of pupil referred to in section 14 of the Act shall be in Form 3. R.R.O. 1990, Reg. 645, s. 4.
5. The following program of immunization in respect of designated diseases is prescribed:
SCHEDULE
Item |
Disease |
Type of Vaccine to be Used |
Minimum Number of Doses Accepted |
Recommended Schedule of Primary Immunization |
Interval Between Booster Doses |
1. |
Diphtheria |
TOXOID |
3 |
Two injections, 1 to 2 months apart with a further dose one year later. Children immunized in infancy require three doses 1 to 2 months apart, a further dose one year later and a booster dose at age 4-6. |
10 years |
2. |
Tetanus |
TOXOID |
3 |
Two injections, 1 to 2 months apart with a further dose one year later. Children immunized in infancy require three doses 1 to 2 months apart, a further dose one year later and a booster dose at age 4-6. |
10 years |
3. |
Poliomyelitis |
Inactivated Polio vaccine (IPV) or |
3 |
Two injections, 1 to 2 months apart with a further dose one year later. Children immunized in infancy require three doses 1 to 2 months apart, a further dose one year later and a booster dose at age 4-6. |
NONE required |
Live Oral Polio vaccine (OPV) |
3 |
Two doses 1 to 2 months apart with a further dose 2 to 12 months later. Children immunized in infancy require a booster dose at age 4-6. |
NONE required | ||
4. |
Measles |
Live attenuated virus vaccine |
2 |
One dose after the first birthday with a further dose more than one month later and preferably at age 4-6. |
NONE required |
5. |
Mumps |
Live attenuated virus vaccine |
1 after one year of age |
One dose after the first birthday. |
NONE required |
6. |
Rubella |
Live attenuated virus vaccine |
1 after one year of age |
One dose after the first birthday. |
NONE required |
R.R.O. 1990, Reg. 645, s. 5; O. Reg. 299/96, s. 2; O. Reg. 443/03, s. 1.
FORM 1
STATEMENT OF MEDICAL EXEMPTION
Immunization of School Pupils Act
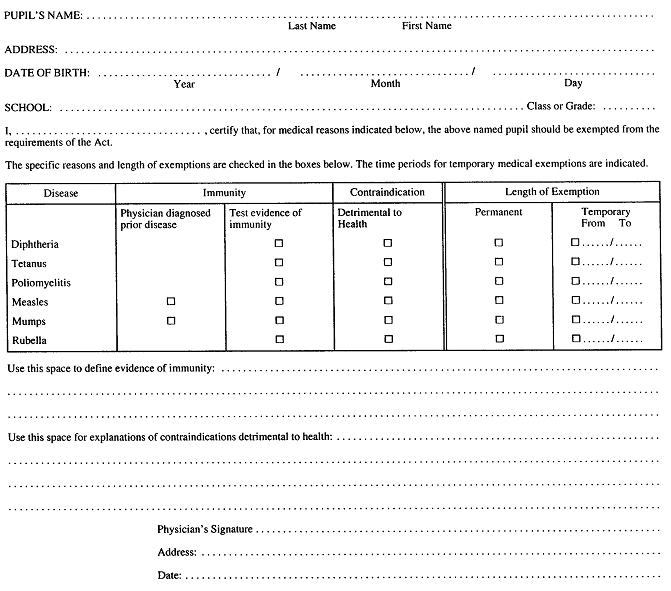
R.R.O. 1990, Reg. 645, Form 1.
FORM 2
STATEMENT OF CONSCIENCE OR RELIGIOUS BELIEF
Immunization of School Pupils Act


R.R.O. 1990, Reg. 645, Form 2.
FORM 3
NOTICE OF TRANSFER FROM A SCHOOL
Immunization of School Pupils Act

R.R.O. 1990, Reg. 645, Form 3.

