R.R.O. 1990, Reg. 551: GENERAL, Drug and Pharmacies Regulation Act, R.S.O. 1990, c. H.4
Drug and Pharmacies Regulation Act
Loi sur la réglementation des médicaments et des pharmacies
R.R.O. 1990, REGULATION 551
GENERAL
Historical version for the period April 23, 1999 to June 3, 2008.
Last amendment: O. Reg. 179/99.
This Regulation is made in English only.
Definitions
1. (1) In this Regulation,
“external application” means application to the outer surface of the body;
“internal use” means local or systemic absorption upon introduction into the body by the parenteral route or through a body orifice;
“safe medication”, for the purpose of the Schedules, means medication in a dose or doses within the usual therapeutic limits of dosage for a drug named in the following publications:
1. Pharmacopoeia Internationalis, 2nd ed. 1967
2. The Canadian Formulary, 7th ed. 1949
3. The British Pharmacopoeia, 1973 and addendum 1975
4. The British Pharmaceutical Codex, 1973 and supplement 1976
5. The European Pharmacopoeia,
Volume I 1969
Volume II 1971
Volume III 1975 and
Supplement 1977
6. The Food and Drugs Act (Canada) and the regulations thereunder
7. The Pharmacopoeia of the United States of America, XIX ed. and 3rd Supplement 1977
8. Martindale, The Extra Pharmacopoeia, 27th ed. 1977
9. The National Formulary, 14th ed. and 3rd Supplement 1977
10. AMA Drug Evaluations, 3rd ed.
11. Pharmacopée Française, VIII ed. 1965 and supplement 1968
12. Pediatric Dosage Handbook (American Pharmaceutical Association), 1973;
“Schedule G preparation” means a drug that contains one drug referred to in Schedule G and one or more active medicinal ingredients not referred to in Schedule G in a recognized therapeutic dose or a drug that contains as the only medicinal ingredient phenobarbital or any of its salts in an amount not exceeding 32.4 milligrams per unit dosage;
“Schedule N preparation” means a drug that,
(a) contains one drug referred to in Schedule N and two or more active medicinal ingredients not referred to in Schedule N in a recognized therapeutic dose, and
(b) is not intended for parenteral administration;
“sell” includes offer to sell, dispense, distribute, give away and supply. R.R.O. 1990, Reg. 551, s. 1; O. Reg. 784/94, s. 2.
(2) A reference in this Regulation to Schedule A, B, C, D or E is a reference to such Schedule referred to in Ontario Regulation 297/96. O. Reg. 179/99, s. 1.
2. - 40. Revoked: O. Reg. 784/94, s. 3.
41. A member, in the practice of a pharmacist, shall use only a vocational designation set out in paragraph 1, 2, 3, 4 or 5 of subsection 125 (2) of the Act but may use academic degrees in association with the member’s name. R.R.O. 1990, Reg. 551, s. 41.
42. Revoked: O. Reg. 120/97, s. 1.
43. The owner of a pharmacy shall at the request of the Council complete and file with the Registrar within thirty days after the request a return in Form 1. R.R.O. 1990, Reg. 551, s. 43.
44. The Council may at any time require an examination and audit to be made by a public accountant designated by it for the purpose of ascertaining and reporting to the Council whether the information furnished by the owner of a pharmacy in a return in Form 1 is correct and the owner shall provide to the public accountant all the evidence, vouchers, records, books and papers that may be required by the public accountant for the purpose of the examination and audit and the public accountant shall report to the Council in the manner required by the Council. R.R.O. 1990, Reg. 551, s. 44.
45. Revoked: O. Reg. 784/94, s. 3.
46. (1) The practice of pharmacy shall not be carried on by a member where there is a conflict of interest.
(2) It is a conflict of interest for a member to,
(a) knowingly operate or be associated as owner, manager, employee or corporate director, in the operation of a pharmacy that is supplying drugs to a nursing home owned or operated by the same person that owns or operates the pharmacy unless the drugs are supplied to not more than twenty persons who are not more than 40 per cent of the residents of the nursing home;
(b) knowingly be involved in the operation of a pharmacy where the owner or a partner, shareholder or director of the owner of the pharmacy owns, controls or has a beneficial interest in 25 per cent or more of the ownership of an entity that manufactures drugs;
(c) participate in an arrangement by reason of which the interest of the member or any person associated with the member in the operation of a pharmacy influences, or is likely to influence adversely, the discharge of the member’s professional obligation as a pharmacist. R.R.O. 1990, Reg. 551, s. 46.
47. - 51. Revoked: O. Reg. 784/94, s. 3.
52. An application for a certificate of accreditation of a pharmacy shall be in Form 3. R.R.O. 1990, Reg. 551, s. 52.
53. A certificate of accreditation of a pharmacy shall be in Form 4 and shall be displayed in the pharmacy. R.R.O. 1990, Reg. 551, s. 53.
54. Every certificate of accreditation expires with the 9th day of March in each year. R.R.O. 1990, Reg. 551, s. 54.
55. An application for renewal of a certificate of accreditation of a pharmacy shall be in Form 5. R.R.O. 1990, Reg. 551, s. 55.
56. (1) An oral prescription in respect of a drug referred to in Schedule E, F or G or in respect of a Schedule N preparation may be given only to a person referred to in subsection 149 (1) of the Act. R.R.O. 1990, Reg. 551, s. 56 (1).
(2) An oral prescription referred to in subsection (1) shall be reduced to writing forthwith by the person receiving the prescription from the prescriber. R.R.O. 1990, Reg. 551, s. 56 (2).
(3) A prescription may be given only in writing in respect of a drug, other than a Schedule N preparation, referred to in Schedule N. R.R.O. 1990, Reg. 551, s. 56 (3).
57. A prescription for a drug referred to in Schedule N shall not be refilled. R.R.O. 1990, Reg. 551, s. 57.
58. A person shall refill a prescription for a drug referred to in Schedule E or F only where a prescriber so directs and specifies the number of times it may be refilled. R.R.O. 1990, Reg. 551, s. 58.
59. Every person who receives an oral direction to refill a prescription for a drug referred to in Schedule E or F, subsequent to the time the prescription is issued, shall forthwith record on the original prescription,
(a) the date the refill direction is received;
(b) the number of times specified that it may be refilled; and
(c) the name and address of the prescriber issuing the direction if the prescriber of the refill is not the prescriber of the original prescription,
or record in a record of prescriptions kept under the name of each patient,
(d) the name and quantity of drug prescribed and where applicable the strength of the drug;
(e) the date the refill direction is received;
(f) the number of times specified that the prescription may be refilled; and
(g) the name and address of the prescriber issuing the direction if the prescriber of the refill is not the prescriber of the original prescription,
and the person shall sign the prescription or the record of prescriptions, as the case may be. R.R.O. 1990, Reg. 551, s. 59.
60. A person shall only refill a prescription for a drug referred to in Schedule G where the prescriber, at the time the prescription is issued,
(a) directs in writing that the prescription be refilled; and
(b) specifies the number of times it may be refilled and the dates for or intervals between refilling it. R.R.O. 1990, Reg. 551, s. 60.
61. A prescription, except for a drug referred to in Schedule N, may only be refilled where the person refilling the prescription records,
(a) on the original prescription therefor,
(i) the date of the refill,
(ii) the quantity of the drug dispensed, and
(iii) his or her signature; or
(b) in a record of prescriptions kept under the name of each patient,
(i) the date of the refill,
(ii) the identification number that is on the prescription therefor,
(iii) the name, strength where applicable, and quantity of the drug dispensed,
(iv) the identity of the manufacturer of the drug dispensed,
(v) the name of the prescriber,
(vi) the price charged, and
(vii) the signature of the person refilling the prescription. R.R.O. 1990, Reg. 551, s. 61.
62. (1) A pharmacist may transfer a prescription to another pharmacist for the purpose of refilling the prescription, except with respect to a prescription for a drug referred to in Schedule G or N, where,
(a) the prescriber has authorized the prescription to be refilled a specific number of times and there are authorized refills remaining;
(b) the pharmacist transferring the prescription gives a copy of the prescription either,
(i) in writing to the person named in the prescription, his or her agent or a pharmacist acting on behalf of such person or agent, or
(ii) orally to a pharmacist acting on behalf of the person named in the prescription;
(c) the transferred prescription is marked “transferred copy”;
(d) the transferred copy contains,
(i) the name and address of the person for whom the drug is prescribed,
(ii) the name and quantity of the drug prescribed and where applicable the strength of the drug,
(iii) the quantity of the drug dispensed if different from the quantity prescribed,
(iv) the directions for use as prescribed,
(v) the name and address of the prescriber,
(vi) the identity of the manufacturer of the drug dispensed,
(vii) the identification number of the prescription,
(viii) the name and address of the pharmacy transferring the prescription,
(ix) the date the prescription was issued by the prescriber,
(x) the number of refills authorized originally,
(xi) the number of authorized refills remaining,
(xii) the date of the last refill, and
(xiii) the name of the pharmacist transferring the prescription; and
(e) the pharmacist transferring the prescription records on the original prescription or in a record of prescriptions kept under the name of each patient that the prescription has been transferred, the date of the transfer and his or her signature. R.R.O. 1990, Reg. 551, s. 62 (1).
(2) A prescription that has been transferred from a pharmacist shall not be refilled in the transferring pharmacy and shall not be transferred further. R.R.O. 1990, Reg. 551, s. 62 (2).
(3) A pharmacist to whom a prescription has been transferred shall not dispense a drug pursuant thereto until he or she has obtained from the pharmacist transferring the prescription the information set out in clause (1) (d) and, where the prescription has been transferred orally, reduced the prescription to writing indicating therein the information specified in clause (1) (d). R.R.O. 1990, Reg. 551, s. 62 (3).
63. Every manager of a pharmacy shall keep or cause to be kept a record of every purchase of a drug referred to in Schedule G or N by entering or causing to be entered in a register or other record maintained for that purpose forthwith upon such purchase,
(a) the date of the purchase;
(b) the name, strength where applicable, and quantity of the drug; and
(c) the name and address of the person from whom the drug was purchased or received. R.R.O. 1990, Reg. 551, s. 63.
64. Every manager of a pharmacy shall keep or cause to be kept a record of every sale of a drug referred to in Schedule G, other than a Schedule G preparation, or in Schedule N, other than a Schedule N preparation, by entering or causing to be entered in a register maintained for that purpose forthwith upon such sale,
(a) the date of the sale;
(b) the name, strength where applicable, and quantity of the drug;
(c) the name and address of the purchaser or person named in the prescription,
and, where applicable,
(d) the name and address of the prescriber; and
(e) the identification number on the prescription. R.R.O. 1990, Reg. 551, s. 64.
65. Every manager of a pharmacy shall keep or cause to be kept a record of every sale of a Schedule G preparation or a Schedule N preparation other than by prescription, by entering or causing to be entered in a register or other record maintained for that purpose forthwith upon such sale,
(a) the date of the sale;
(b) the name, strength where applicable, and quantity of the drug; and
(c) the name and address of the purchaser. R.R.O. 1990, Reg. 551, s. 65.
66. The prescriptions and other records required by this Regulation shall be retained for not less than six years and shall be open to inspection by an inspector appointed under a by-law of the Council and an inspector may make copies of or take extracts from the prescriptions and other records. R.R.O. 1990, Reg. 551, s. 66.
67. (1) A record of every sale of a drug referred to in Part I of Schedule D shall be entered in a book kept by the seller for that purpose. R.R.O. 1990, Reg. 551, s. 67 (1).
(2) The record of a sale referred to in subsection (1) shall include,
(a) the date of the sale;
(b) the name and address of the purchaser;
(c) the name of the drug sold;
(d) the quantity of the drug sold; and
(e) the purpose for which it is required as stated by the purchaser. R.R.O. 1990, Reg. 551, s. 67 (2).
(3) After the record referred to in subsection (1) has been completed, the seller shall cause the purchaser to sign the record and the seller shall sign it. R.R.O. 1990, Reg. 551, s. 67 (3).
(4) The seller of a drug referred to in Part I of Schedule D shall not deliver it to the purchaser until a record of the sale has been completed in accordance with this section. R.R.O. 1990, Reg. 551, s. 67 (4).
68. Revoked: O. Reg. 179/99, s. 2.
69. A container in which a substance referred to in Part II of Schedule B is sold at retail shall include on the label, legibly and conspicuously displayed on the outer surface of the container, the name of the substance and a caution or warning that the substance should be kept out of the reach of children, but this section does not apply where the substance is referred to in the Hazardous Products Act (Canada). R.R.O. 1990, Reg. 551, s. 69.
70. A container in which a substance referred to in Part III of Schedule B is sold at retail shall include on the label, legibly and conspicuously displayed on the outer surface of the container, the name of the substance and a caution or warning that the substance should be used only with adequate ventilation, but this section does not apply where the substance is referred to in the Hazardous Products Act (Canada). R.R.O. 1990, Reg. 551, s. 70.
71. (1) A container in which a drug specified in this section is dispensed pursuant to a prescription and in a form intended for systemic or internal use shall bear the following words legibly and conspicuously displayed on the outer surface of the container:
“WARNING: Do not exceed the dose prescribed by your physician. If difficulty in breathing persists, contact your physician immediately.”
R.R.O. 1990, Reg. 551, s. 71 (1).
(2) The following drugs are specified for the purposes of subsection (1):
1. Ephedrine and its salts.
2. Epinephrine and its salts.
3. Ethylnorepinephine and its salts.
4. Fenoterol and its salts.
5. Ipratropium and its salts.
6. Isoetharine and its salts.
7. Isoproterenol (Isoprenaline) and its salts.
8. Metaproterenol (Orciprenaline) and its salts.
9. Salbutamol (Albuterol) and its salts.
10. Terbutaline and its salts. R.R.O. 1990, Reg. 551, s. 71 (2).
72. Every pharmacy shall be so constructed that,
(a) it contains a prescription laboratory in which drugs are stored and prescriptions compounded or dispensed, located in a well defined area having a floor area adequate for the efficient operation of the pharmacy but of not less than 9.3 square metres;
(b) it is free from every condition that may,
(i) be dangerous to health,
(ii) injuriously affect its efficient operation, or
(iii) injuriously affect the drugs prepared, compounded, dispensed or stored therein;
(c) a separate room, compartment, locker or cupboard is provided for keeping the wearing apparel of employees;
(d) floors and floor coverings may be readily cleaned in rooms where,
(i) drugs are prepared, compounded, dispensed or stored,
(ii) equipment is washed, or
(iii) washing fixtures and toilet fixtures are located;
(e) the walls and ceilings of rooms and passageways may be readily cleaned and the painting or decorating maintained in good condition;
(f) all rooms and passageways are well lighted and ventilated; and
(g) suitable areas are provided for the storage and controlled sale of drugs by the pharmacist. R.R.O. 1990, Reg. 551, s. 72.
73. (1) Every pharmacy shall be provided with,
(a) a supply of hot and cold water adequate for the efficient operation of the pharmacy;
(b) facilities for washing utensils used in the preparation, service or storage of drugs;
(c) separate hand-washing facilities available for employees and located in a convenient location in the pharmacy;
(d) a system for filing prescriptions;
(e) a typewriter in good working condition;
(f) a prescription counter adequate for the efficient operation of the prescription laboratory with not less than 1.12 square metres of free working space;
(g) a refrigerator for the exclusive storage of drugs requiring refrigeration;
(h) sufficient containers for storing refuse in a sanitary manner; and
(i) the compounding and dispensing equipment set out in the following Table:
TABLE
Item |
Equipment |
Minimum Number Required |
Specifications |
1. |
Prescription Balance |
1 |
Class “A” with sensibility reciprocal of 10 mg., and with lid which allows draft-free weighing to be made when the lid is closed. |
2. |
Weights, Metric |
1 set |
From 10 mg. to 50 mg. where not an integral part of the prescription balance. |
3. |
Graduates, Metric |
3 |
One each of 10 ml., 25 ml. and 100 ml. |
4. |
Mortars and Pestles |
Glass or earthenware, | |
1 |
60 ml. or 120 ml. | ||
1 |
240 ml. | ||
1 |
480 ml. | ||
5. |
Spatulas |
3 |
Stainless steel, one each of small, medium and large: |
1 |
Non-metal. | ||
6. |
Funnels |
2 |
Glass or plastic, one each of small (approximately 7.62 cm. diameter) and large (approximately 15.24 cm. diameter). |
7. |
Stirring Rods |
2 |
Glass or plastic. |
8. |
Ointment Slab, Pill Tile |
1 |
|
or Parchment Paper |
1 book |
||
9. |
Prescription Numbering Device |
1 |
(j) a quantity of the following consumable material sufficient for the efficient operation of the pharmacy:
1. Bottles with caps.
2. Tablet vials (glass or plastic with caps).
3. Labels.
4. Filter papers.
5. Weighing papers.
6. Ointment jars with caps.
7. Distilled or de-ionized water.
8. Dropper bottles.
9. Child-resistant packages;
(k) a library including as a minimum, the following texts, pharmacopoeias, periodicals and other books:
1. A current edition of,
i. A Compendium of Pharmaceutical Specialties.
ii. A Drug Interaction Publication.
iii. A Pharmacology or Therapeutics Text.
iv. Parts I and VI of the Health Disciplines Act and the regulations and amendments.
v. The Narcotic Control Act (Canada), the regulations thereunder and amendments.
vi. The Food and Drugs Act (Canada), the regulations thereunder and amendments, pertinent to the sale of drugs, devices and vitamins.
2. A current edition or edition immediately preceding the current edition of,
i. A Pharmaceutics Text.
ii. A Dispensatory.
iii. A Medical Dictionary; and
(l) a telephone that is listed in the local telephone directory. R.R.O. 1990, Reg. 551, s. 73 (1).
(2) Only a potable water supply shall be used in any room where drugs are prepared, compounded, dispensed or stored. R.R.O. 1990, Reg. 551, s. 73 (2).
(3) All drugs stored in a pharmacy shall be stored on or in shelves, drawers or fixtures provided for that purpose. R.R.O. 1990, Reg. 551, s. 73 (3).
(4) Every pharmacy shall maintain,
(a) furniture, equipment and appliances used in the interior of the pharmacy so that thorough cleaning of all areas is possible;
(b) in a clean and sanitary condition,
(i) all furniture, equipment and appliances, and
(ii) all rooms in the pharmacy, whether used for the storage, compounding or dispensing of drugs or not; and
(c) the painting and decorating of the interior and exterior of the pharmacy in good condition. R.R.O. 1990, Reg. 551, s. 73 (4).
(5) Every room where drugs are prepared, compounded, dispensed or stored in a pharmacy shall be kept free from materials and equipment not regularly used in the room. R.R.O. 1990, Reg. 551, s. 73 (5).
(6) Refrigerators for the storage of drugs in a pharmacy shall,
(a) be maintained at a temperature between 1.3o Celsius and 10o Celsius;
(b) be kept clean and in a sanitary condition; and
(c) be located in an area not accessible to the public. R.R.O. 1990, Reg. 551, s. 73 (6).
(7) All refuse and waste materials in a pharmacy,
(a) shall be removed from the premises at least twice weekly and more often if necessary to maintain a sanitary condition; and
(b) contained in filled containers shall be removed from any room in which drugs are prepared, compounded, dispensed or stored. R.R.O. 1990, Reg. 551, s. 73 (7).
74. (1) Every pharmacist, at the time of payment of his or her annual fee and at any other time within seven days after a request by the Registrar, shall file with the Registrar a signed statement setting out,
(a) the pharmacist’s residential address; and
(b) the location of the place of practice of the pharmacist. R.R.O. 1990, Reg. 551, s. 74 (1).
(2) Every pharmacist shall notify the Registrar in writing of any change in the information required by subsection (1) within seven days of the change. R.R.O. 1990, Reg. 551, s. 74 (2).
75. (1) Every owner of a pharmacy at the time of payment of the fee for renewal of a certificate of accreditation of the pharmacy, or at any other time within seven days after a request by the Registrar, shall file with the Registrar a signed statement setting out,
(a) the full name of the owner of the pharmacy and, where the owner is a corporation, the full name and residential addresses of the directors of the corporation;
(b) the address of the owner of the pharmacy;
(c) the name by which the pharmacy is known to the public;
(d) the location of the pharmacy;
(e) the full name of the manager of the pharmacy;
(f) the residential address of the manager of the pharmacy; and
(g) the names of the pharmacists, interns and registered pharmacy students employed in the pharmacy. R.R.O. 1990, Reg. 551, s. 75 (1).
(2) The owner of a pharmacy shall notify the Registrar in writing of any change in the information required by subsection (1) within seven days of the change. R.R.O. 1990, Reg. 551, s. 75 (2).
76. (1) Every person who proposes to open a new pharmacy, acquire an existing pharmacy or relocate an existing pharmacy shall, within the time prescribed by subsection (2), file with the Registrar a signed statement setting out,
(a) the full name of the owner of the pharmacy;
(b) the address of the owner of the pharmacy;
(c) the name by which the pharmacy will be known to the public;
(d) the location of the pharmacy; and
(e) the proposed date of opening, acquiring or relocating the pharmacy. R.R.O. 1990, Reg. 551, s. 76 (1).
(2) A person who proposes to open a new pharmacy or relocate an existing pharmacy shall file the information required by subsection (1) at least thirty days before opening or relocating the pharmacy and, where the person proposes to operate an existing pharmacy, the person shall file the information before commencing to operate the pharmacy. R.R.O. 1990, Reg. 551, s. 76 (2).
(3) Every person who proposes to open a new pharmacy, acquire an existing pharmacy or relocate an existing pharmacy shall, on or before the day the person commences to operate the pharmacy, notify the Registrar of the name of the manager of the pharmacy. R.R.O. 1990, Reg. 551, s. 76 (3).
77. Every person who permanently closes a pharmacy shall, within seven days of closing the pharmacy, notify the Registrar of the closing and within thirty days of the closing shall file with the Registrar a signed statement setting out,
(a) the full name of the owner of the pharmacy;
(b) the name by which the pharmacy was known to the public;
(c) the location of the pharmacy;
(d) the name of the manager of the pharmacy;
(e) the date of closing;
(f) the disposition of the drugs in stock in the pharmacy at the time of closing;
(g) the disposition of the prescription files, drug registers and other records required to be kept under this Regulation; and
(h) the date on which all signs and symbols relating to the practice of pharmacy either within or outside the premises were removed. R.R.O. 1990, Reg. 551, s. 77.
78. The parts of a pharmacy in which prescriptions are compounded and dispensed for the public or drugs are stored or sold by retail shall be so constructed that they may be locked and made not accessible to the public in the absence of a pharmacist. R.R.O. 1990, Reg. 551, s. 78.
79. Revoked: O. Reg. 179/99, s. 3.
80. Revoked: O. Reg. 298/96, s. 1.
SCHEDULE A Revoked: O. Reg. 179/99, s. 4.
SCHEDULE B Revoked: O. Reg. 179/99, s. 4.
SCHEDULE C Revoked: O. Reg. 179/99, s. 4.
SCHEDULE D Revoked: O. Reg. 179/99, s. 4.
SCHEDULE E Revoked: O. Reg. 179/99, s. 4.
SCHEDULE F Revoked: O. Reg. 179/99, s. 4.
SCHEDULE G Revoked: O. Reg. 179/99, s. 4.
SCHEDULE N Revoked: O. Reg. 179/99, s. 4.
FORM 1
RETURN BY OWNER OF A PHARMACY
Drug and Pharmacies Regulation Act



R.R.O. 1990, Reg. 551, Form 1; O. Reg. 784/94, s. 5.
FORM 2 Revoked: O. Reg. 784/94, s. 6.
FORM 3
APPLICATION FOR CERTIFICATE OF ACCREDITATION OF A PHARMACY
Drug and Pharmacies Regulation Act
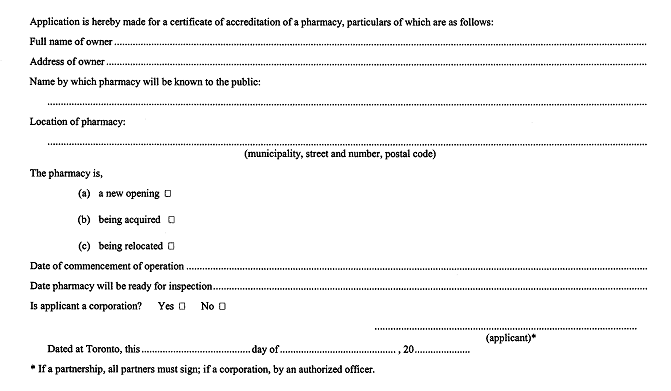
R.R.O. 1990, Reg. 551, Form 3; O. Reg. 784/94, s. 7.
FORM 4
CERTIFICATE OF ACCREDITATION OF A PHARMACY — ONTARIO COLLEGE OF PHARMACISTS
Drug and Pharmacies Regulation Act

R.R.O. 1990, Reg. 551, Form 4; O. Reg. 784/94, s. 8; O. Reg. 120/97, s. 2.
FORM 5
APPLICATION FOR RENEWAL OF CERTIFICATE OF ACCREDITATION OF A PHARMACY
Drug and Pharmacies Regulation Act
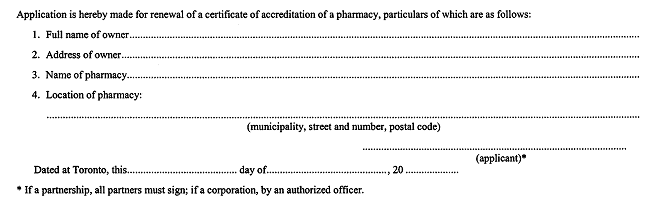
R.R.O. 1990, Reg. 551, Form 5; O. Reg. 784/94, s. 9.