R.R.O. 1990, Reg. 569: REPORTS, Health Protection and Promotion Act, R.S.O. 1990, c. H.7
Health Protection and Promotion Act
R.R.O. 1990, REGULATION 569
Amended to O. Reg. 84/95
REPORTS
Historical version for the period April 3, 1995 to January 11, 2005.
Disclaimer: This consolidation is not an official copy of the law because some portion of it may not be fully legible in HTML or Word format.
This is the English version of a bilingual regulation.
1. (1) A report required under section 25, 26 or 27 of the Act shall, with respect to the person to whom the report relates, contain the following information:
1. Name and address in full.
2. Date of birth in full.
3. Sex.
4. Date of onset of symptoms. R.R.O. 1990, Reg. 569, s. 1 (1).
(2) A person who makes a report under section 25 or 26 of the Act and gives the information set out in subsection (1) shall, upon the request of the medical officer of health, give to the medical officer of health such additional information respecting the reportable disease or communicable disease, as the case may be, as the medical officer of health considers necessary. R.R.O. 1990, Reg. 569, s. 1 (2).
(3) Despite subsection (1), a report under section 25 or 26 of the Act with respect to tuberculosis shall be made in Form 1 or Form 2, as the case may be, and with respect to leprosy shall be made in Form 3. R.R.O. 1990, Reg. 569, s. 1 (3).
2. A report required under section 28 of the Act shall, with respect to the pupil to whom the report relates, contain the following information:
1. Name and address in full.
2. Date of birth in full.
3. Sex.
4. Name and address in full of the school that the pupil attends. R.R.O. 1990, Reg. 569, s. 2.
3. A report made under subsection 29 (1) of the Act shall, with respect to the person to whom the finding was made, be made within twenty-four hours of the making of the finding and shall contain the following information:
1. Name and address in full.
2. Date of birth in full.
3. Sex.
4. Date when the specimen was taken that yielded the positive finding.
5. Name and address in full of the physician or dentist attending the person. R.R.O. 1990, Reg. 569, s. 3.
4. A report made under section 30 of the Act shall, with respect to the deceased, contain the following information:
1. Name and address in full.
2. Date of birth in full.
3. Date of death in full.
4. Name and address in full of the physician who attended the deceased. R.R.O. 1990, Reg. 569, s. 4.
5. A report under section 25 or 26 of the Act shall contain the following information in addition to the information required under subsection 1 (1):
1. Syphilis:
i. The date of diagnosis.
ii. The name and address of the physician attending the person.
iii. The name of the hospital and the date of admission if the person is admitted to a hospital.
iv. Duration and stage of infection.
v. Drugs and dosage used for previous treatment, if any, of the infection.
vi. If previous treatment given, the place, date and physician responsible for the administration of the treatment.
vii. Current treatment, if any, of the infection, setting out the drugs and dosage used.
viii. If current treatment is being given, the place, date and physician responsible for the administration of treatment.
ix. Laboratory findings including serological tests, microscopic examination, cerebrospinal fluid examination.
x. The person responsible for tracing contacts of the person.
2. Gonorrhoea due to penicillinase producing strain of Neisseria gonorrhoeae:
i. The date of diagnosis.
ii. The name and address of the physician attending the person.
iii. The name of the hospital and the date of admission if the person is admitted to a hospital.
iv. Place where infection is believed to have been acquired.
v. Initial treatment, if any, of the infection setting out drugs and dosage used.
vi. If initial treatment given, give place, date and physician responsible for administration of treatment.
vii. Final effective treatment setting out drugs and dosage used.
viii. If effective treatment has been given, place, date and physician responsible for administration of treatment.
ix. The agency responsible for tracing contacts of the person.
x. The number of contacts of the person who have been traced.
xi. The number of contacts of the person found to be infected with penicillinase producing strain of Neisseria gonorrhoeae.
3. Acquired Immune Deficiency Syndrome (AIDS):
i. The date of diagnosis.
ii. The name and telephone number of the physician attending the person.
iii. The name of the hospital if the person is admitted to a hospital or is an outpatient.
iv. Medical conditions of the person including laboratory findings and date of onset of symptoms that are indicative of Acquired Immune Deficiency Syndrome.
v. Other medical conditions of the person that may have caused immuno-suppression (exclusion criteria).
vi. Country of birth, date of arrival in Canada, race and residence of the person at onset of illness.
vii. Current status of person infected (alive or dead) (if dead give date of death).
viii. Information preceding the diagnosis of Acquired Immune Deficiency Syndrome with respect to,
A. sexual relations of the person with a male partner,
B. sexual relations of the person with a female partner,
C. use by the person of needles for self-injection of drugs not prescribed by a physician, or
D. receipt by the person of blood or blood products (give dates).
ix. Information, preceding the diagnosis of Acquired Immune Deficiency Syndrome, with respect to heterosexual relations of the person with another person who is,
A. an intravenous abuser,
B. a bisexual man,
C. a person with hemophilia or a coagulation disorder,
D. a blood transfusion recipient with Acquired Immune Deficiency Syndrome or documented Human Immune Virus infection,
E. a person with Acquired Immune Deficiency Syndrome or documented Human Immune Virus infection,
F. a person who was born or resided in a country where heterosexual transmission of Acquired Immune Deficiency Syndrome predominates (specify country).
x. Information preceding the diagnosis of Acquired Immune Deficiency Syndrome, as to whether the person has worked or is working in a health care or clinical laboratory setting (give occupation and setting).
xi. Information, preceding the diagnosis of Acquired Immune Deficiency Syndrome, as to whether there are no identifiable risk factors or any other exposures that could have been the source of the infection.
xii. Information, in the case of a child who is one year of age or older but less than sixteen years of age, as to whether the child was infected as a result of perinatal transmission.
4. Lassa Fever, Marburg virus disease, Ebola virus disease and Plague:
i. The date of diagnosis.
ii. The name and address of the physician attending the person.
iii. The name of the hospital and the date of admission if the person is admitted to a hospital.
iv. Travel history outside Canada.
A. Date and place of entry into country where disease acquired.
B. Date of departure from country where disease acquired.
C. Date and time of entry into Canada and carrier and flight number if applicable.
D. Travel within country where disease acquired by date, place and length of stay.
E. Any other places visited en route to Canada.
v. List places and method of travel within Canada in the week prior to and since onset of illness.
vi. Exposure to any of the following. (Give date and time).
A. Rodents or monkeys.
B. Persons with a similar illness.
C. Virus in a laboratory.
vii. Clinical history.
A. Date of onset of illness.
B. Symptoms and signs of the illness.
C. History of malaria or malaria prophylaxis.
viii. Laboratory specimens.
A. List all specimens collected by type and date.
B. Name of laboratory where specimens may be located.
ix. State if ambulance was used and date of use. R.R.O. 1990, Reg. 569, s. 5.
5.1 (1) In this section,
“AIDS” means Acquired Immune Deficiency Syndrome; (“sida”)
“HIV” means Human Immunodeficiency Virus. (“VIH”) O. Reg. 749/91, s. 1.
(2) A physician who provides professional services to a patient in a clinic set out in the Schedule and who is required to report under section 26 of the Act following a test to determine if the patient is infected with an agent of AIDS is exempt from reporting the patient’s name and address if, before the test was ordered, the patient received counselling about preventing the transmission of HIV infection. O. Reg. 749/91, s. 1; O. Reg. 84/95, s. 1 (1).
(3) The operator of a laboratory is exempt from reporting, under section 29 of the Act, the name and address of a person who has tested positive for an agent of AIDS if the test is in relation to professional services provided at a clinic set out in the Schedule. O. Reg. 749/91, s. 1; O. Reg. 84/95, s. 1 (2).
(4) Revoked: O. Reg. 84/95, s. 1 (3).
6. (1) Where a medical officer of health receives a report made under section 25, 26, 27 or 28, subsection 29 (2) or section 30 of the Act, he or she shall forward a copy of the report to the Public Health Branch of the Ministry. R.R.O. 1990, Reg. 569, s. 6 (1).
(2) Where a copy of a report referred to in subsection (1) concerns a person who has,
(a) amebiasis;
(b) chickenpox;
(c) epidemic diarrhoea;
(d) genital chlamydia trachomatis infections;
(e) genital herpes;
(f) gonorrhoea, other than gonorrhoea due to penicillinase producing strain of Neisseria gonorrhoeae;
(g) giardiasis;
(h) influenza;
(i) measles;
(j) mumps;
(k) pertussis; or
(l) rubella,
the copy shall be forwarded with the name of the person deleted. R.R.O. 1990, Reg. 569, s. 6 (2).
Schedule
1. The District of Algoma Health Unit, Sexual Health Clinic, 99 Foster Drive, Sault Ste. Marie.
2. Anishnawbe Health Toronto, 225 Queen Street East, Toronto.
3. Barrie STD Clinic, 370 Dunlop Street, Barrie.
4. Bay Centre for Birth Control, Regional Women’s Health Centre, 790 Bay Street, Toronto.
5. Birth Control & STD Information Centre, 2828 Bathurst Street, North York.
6. Brampton-Caledon STD Clinic, 180B Sandalwood Parkway East, Brampton.
7. Centre médico-social communautaire, 22 College Street, Toronto.
8. Centretown Community Health Centre, 340 MacLaren Street, Ottawa.
9. Community Health Department, HIV Clinic, 99 Regina Street South, Waterloo.
10. Elgin-St. Thomas Health Unit, AIDS Division, 99 Edward Street, St. Thomas.
11. Hassle Free Clinic, 556 Church Street, Toronto.
12. HIV Care Program Clinic, Metropolitan General Hospital, 2240 Kildare Road, Windsor.
13. InterCommunity Health Centre, 659 Dundas Street East, London.
14. Kingston, Frontenac and Lennox and Addington Health Unit, STD Clinic, 221 Portmouth Avenue, Kingston.
15. Mississauga East STD Clinic, 3038 Hurontario Street, Mississauga.
16. Mississauga West STD Clinic, 2227 South Millway, Mississauga.
17. Peterborough County-City Health Unit, Sexual Health Clinic, 10 Hospital Drive, Peterborough.
18. Regional Niagara Health Services Department, Falls Clinic, 5710 Kitchener Street, Niagara Falls.
19. Rexdale Community Health Centre, 2267 Islington Avenue, Rexdale.
20. Sandwich Community Health Centre, 749 Felix Avenue, Windsor.
21. Sandy Hill Community Health Centre, 24 Selkirk Avenue, Vanier.
22. SITE, 480A Somerset Street West, Ottawa.
23. Somerset West Community Health Centre, 755 Somerset Street West, Ottawa.
24. STD Clinic, 237 Barton Street East, Hamilton.
25. STD Clinic, 250 Besserer Street, Ottawa.
26. Sudbury and District Health Unit, STD Clinic, 1300 Paris Crescent, Sudbury.
27. Thunder Bay District Health Unit, STD Clinic, 999 Balmoral Street, Thunder Bay.
28. Wellington-Dufferin-Guelph Health Unit, Sexual Health Clinic, 125 Delhi Street, Guelph.
29. West Central Community Health Centres, Alexandra Park Health Centre, 64 Augusta Avenue, Toronto.
30. West Central Community Health Centres, Niagara Neighborhood Health Centre, 674 Queen Street West, Toronto.
31. West Central Community Health Centres, SHOUT Clinic, 467 Jarvis Street, Toronto.
32. Windsor-Essex County Health Unit, HIV Clinic, 1005 Ouellette Avenue, Windsor.
33. City of York Health Unit, Sexual Health Clinic, 662 Jane Street, York.
34. City of York Health Unit, Sexual Health Clinic, 504 Oakwood Avenue, York.
O. Reg. 84/95, s. 2.
Form 1
NOTIFICATION OF NEW ACTIVE OR REACTIVE TUBERCULOSIS CASE
Health Protection and Promotion Act
Insert regs\graphics\1990\569\569001au.tif
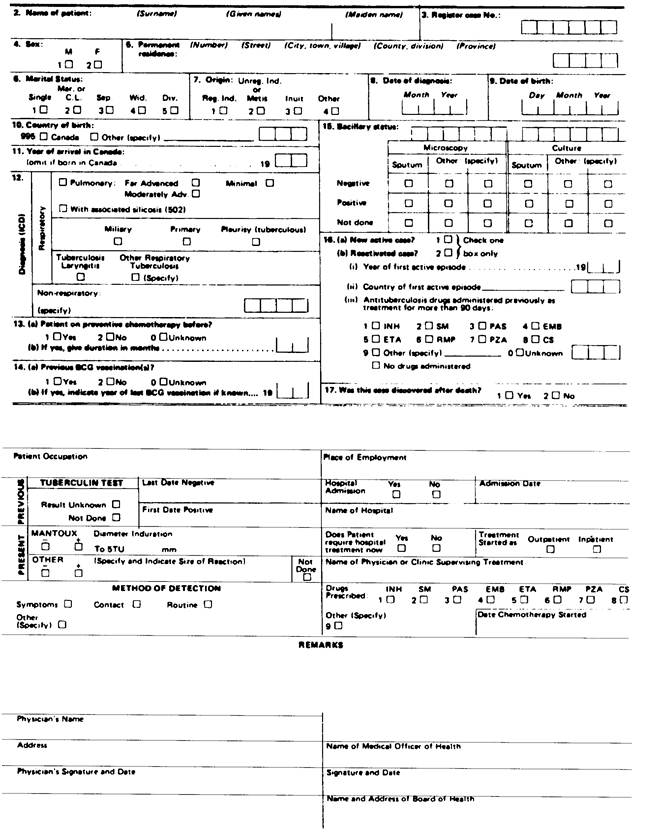
R.R.O. 1990, Reg. 569, Form 1.
Form 3
NOTIFICATION OF NEW ACTIVE — LEPROSY (HANSEN’S DISEASE)
Health Protection and Promotion Act
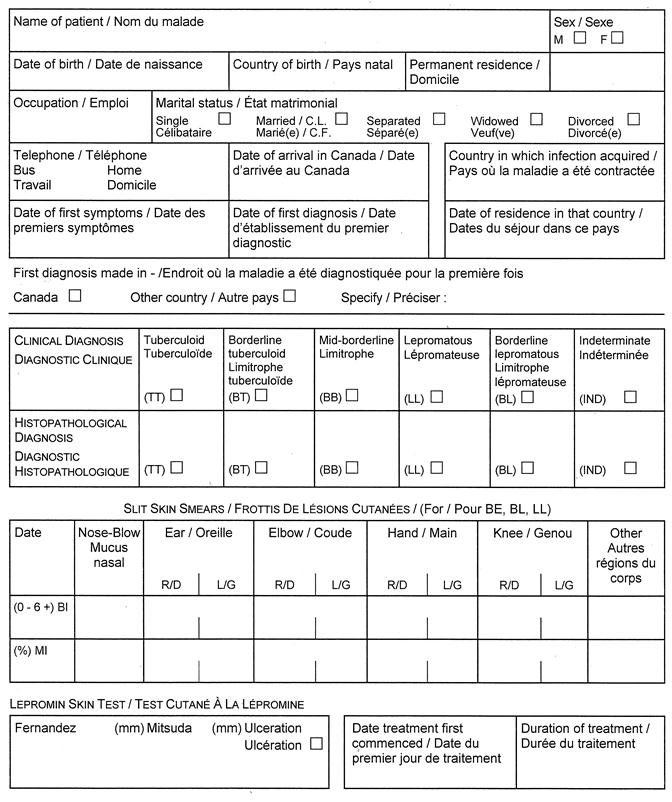
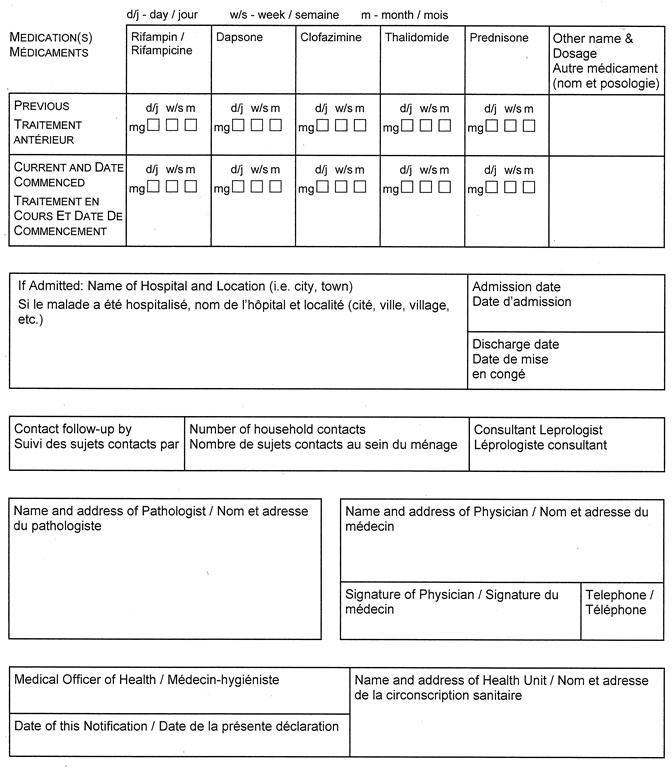
O. Reg. 606/91, s. 2.