R.R.O. 1990, Reg. 569: REPORTS, Health Protection and Promotion Act, R.S.O. 1990, c. H.7
Health Protection and Promotion Act
R.R.O. 1990, REGULATION 569
Amended to O. Reg. 1/05
REPORTS
Historical version for the period January 12, 2005 to March 23, 2005.
This is the English version of a bilingual regulation.
1. (1) A report required under section 25, 26 or 27 of the Act shall, with respect to the person to whom the report relates, contain the following information:
1. Name and address in full.
2. Date of birth in full.
3. Sex.
4. Date of onset of symptoms. R.R.O. 1990, Reg. 569, s. 1 (1).
(2) A person who makes a report under section 25 or 26 or subsection 27 (1) or (2) of the Act and gives the information set out in subsection (1) shall, upon the request of the medical officer of health, give to the medical officer of health such additional information respecting the reportable disease or communicable disease, as the case may be, as the medical officer of health considers necessary. R.R.O. 1990, Reg. 569, s. 1 (2); O. Reg. 1/05, s. 1 (1).
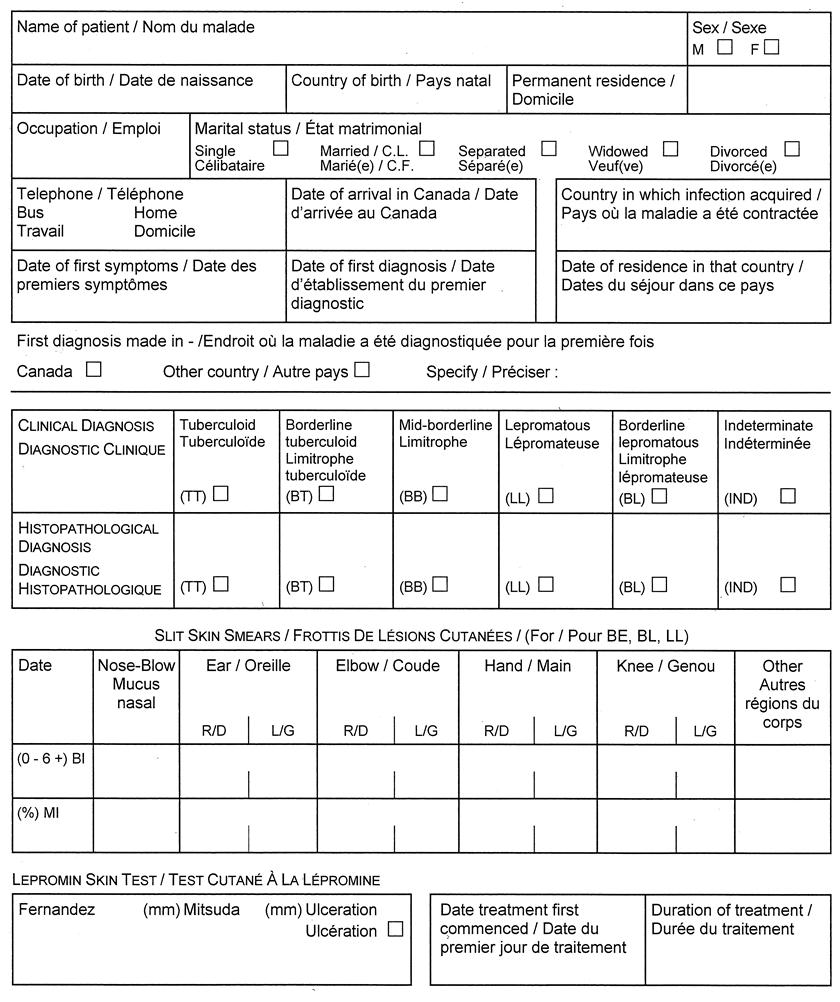
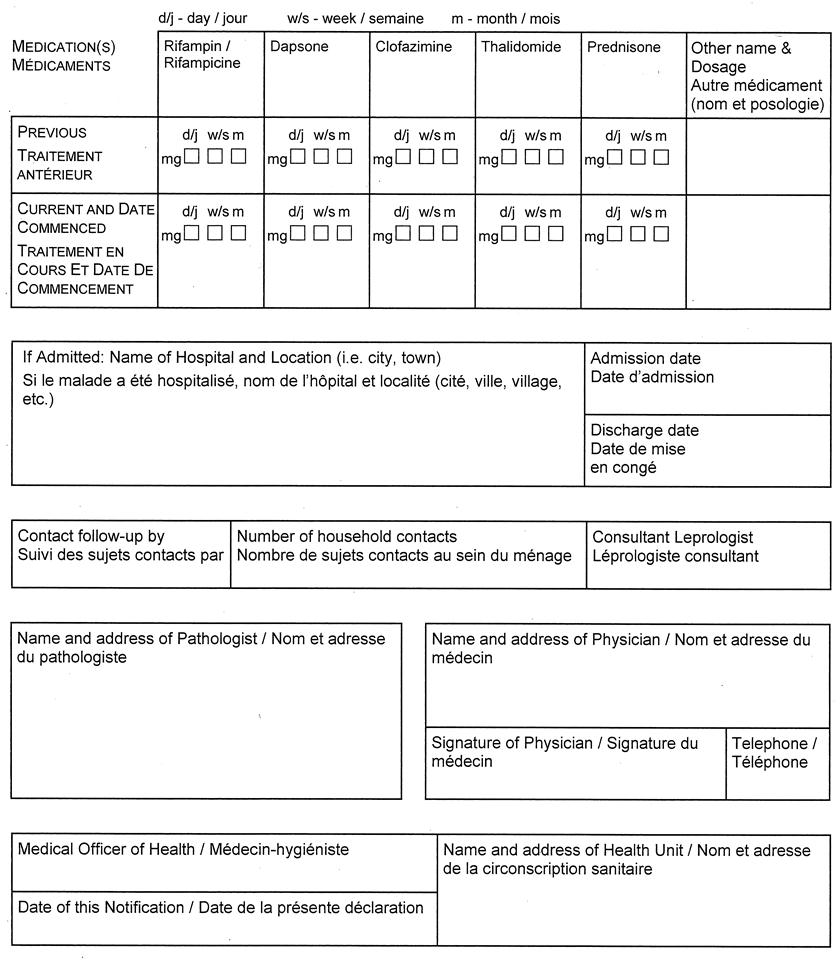
(3) Despite subsection (1), a report under section 25 or 26 of the Act with respect to leprosy shall be made in Form 3. O. Reg. 1/05, s. 1 (2).
2. A report required under section 28 of the Act shall, with respect to the pupil to whom the report relates, contain the following information:
1. Name and address in full.
2. Date of birth in full.
3. Sex.
4. Name and address in full of the school that the pupil attends. R.R.O. 1990, Reg. 569, s. 2.
3. A report made under subsection 29 (1) of the Act shall, with respect to the person to whom the finding was made, be made within twenty-four hours of the making of the finding and shall contain the following information:
1. Name and address in full.
2. Date of birth in full.
3. Sex.
4. Date when the specimen was taken that yielded the positive finding.
5. Name and address in full of the physician or dentist attending the person. R.R.O. 1990, Reg. 569, s. 3.
4. A report made under section 30 of the Act shall, with respect to the deceased, contain the following information:
1. Name and address in full.
2. Date of birth in full.
3. Date of death in full.
4. Name and address in full of the physician who attended the deceased. R.R.O. 1990, Reg. 569, s. 4.
5. A report under section 25 or 26 of the Act shall contain the following information in addition to the information required under subsection 1 (1):
1. Syphilis:
i. The date of diagnosis.
ii. The name and address of the physician attending the person.
iii. The name of the hospital and the date of admission if the person is admitted to a hospital or the name of the hospital and the date of each visit if the person is seen as an out-patient of the hospital.
iv. Duration, stage and site of infection.
v. Drugs and dosage used for previous treatment, if any, of the infection.
vi. If previous treatment given, the place, date and physician responsible for the administration of the treatment.
vii. Current treatment, if any, of the infection, setting out the drugs and dosage used.
viii. If current treatment is being given, the place, date and physician responsible for the administration of treatment.
ix. Laboratory findings and investigative tests including, without being limited to, serological tests, microscopic examination and cerebrospinal fluid examinations, together with the results of the tests.
x. The person responsible for tracing contacts of the person.
xi. Place where infection is believed to have been acquired.
xii. The number of contacts of the person who have been traced.
2. Chancroid, Chlamydia trachomatis infections, Gonorrhoea:
i. The date of diagnosis.
ii. The name and address of the physician attending the person.
iii. The name of the hospital, the date of admission and the date of discharge if the person is admitted to hospital.
iv. Place where infection is believed to have been acquired.
v. The person responsible for tracing the contacts of the person.
vi. The number of contacts who have been traced.
vii. The agent of disease.
viii. Medical condition of the person including signs and symptoms of the infection.
ix. The case classification of the person.
x. Laboratory findings and investigative tests including, without being limited to, culture and antimicrobial sensitivity, serological tests, microscopic examination and cerebrospinal fluid examination, together with the results of the tests.
xi. The source of infection including history of exposures.
xii. Risk factors for the disease.
xiii. The travel history of the person, including:
A. Date and place of entry into country where disease acquired.
B. Date of departure from country where disease acquired.
C. Travel within country where disease acquired by date, place and length of stay.
xiv. Initial treatment, if any, of the infection, including, without being limited to, the drugs and dosage used.
xv. If initial treatment has been given, the place, date and physician responsible for administration of treatment.
xvi. Final effective treatment including, without being limited to, the drugs and dosage used.
xvii. If effective treatment has been given, the place, date and physician responsible for administration of treatment.
xviii. The date of death and relation of the infection to the cause of death, if the person is deceased.
3. Acquired Immune Deficiency Syndrome (AIDS):
i. The date of diagnosis.
ii. The name and telephone number of the physician attending the person.
iii. The name of the hospital if the person is admitted to a hospital or is an outpatient.
iv. Medical conditions of the person including laboratory findings and date of onset of symptoms that are indicative of Acquired Immune Deficiency Syndrome.
v. Other medical conditions of the person that may have caused immuno-suppression (exclusion criteria).
vi. Country of birth, date of arrival in Canada, race and residence of the person at onset of illness.
vii. Current status of person infected (alive or dead) (if dead give date of death).
viii. Information preceding the diagnosis of Acquired Immune Deficiency Syndrome with respect to,
A. sexual relations of the person with a male partner,
B. sexual relations of the person with a female partner,
C. use by the person of needles for self-injection of drugs not prescribed by a physician, or
D. receipt by the person of blood or blood products (give dates).
ix. Information, preceding the diagnosis of Acquired Immune Deficiency Syndrome, with respect to heterosexual relations of the person with another person who is,
A. an intravenous abuser,
B. a bisexual man,
C. a person with hemophilia or a coagulation disorder,
D. a blood transfusion recipient with Acquired Immune Deficiency Syndrome or documented Human Immune Virus infection,
E. a person with Acquired Immune Deficiency Syndrome or documented Human Immune Virus infection,
F. a person who was born or resided in a country where heterosexual transmission of Acquired Immune Deficiency Syndrome predominates (specify country).
x. Information preceding the diagnosis of Acquired Immune Deficiency Syndrome, as to whether the person has worked or is working in a health care or clinical laboratory setting (give occupation and setting).
xi. Information, preceding the diagnosis of Acquired Immune Deficiency Syndrome, as to whether there are no identifiable risk factors or any other exposures that could have been the source of the infection.
xii. Information, in the case of a child who is one year of age or older but less than sixteen years of age, as to whether the child was infected as a result of perinatal transmission.
4. Lassa Fever, Hemorrhagic fevers including Ebola virus disease, Marburg virus disease and Hemorrhagic fevers from other viral causes and Plague:
i. The date of diagnosis.
ii. The name and address of the physician attending the person.
iii. The name of the hospital and the date of admission if the person is admitted to a hospital.
iv. Travel history outside Canada.
A. Date and place of entry into country where disease acquired.
B. Date of departure from country where disease acquired.
C. Date and time of entry into Canada and carrier and flight number if applicable.
D. Travel within country where disease acquired by date, place and length of stay.
E. Any other places visited en route to Canada.
v. List places and method of travel within Canada in the week prior to and since onset of illness.
vi. Exposure to any of the following. (Give date and time).
A. Rodents or monkeys.
B. Persons with a similar illness.
C. Virus in a laboratory.
vii. Clinical history.
A. Date of onset of illness.
B. Symptoms and signs of the illness.
C. History of malaria or malaria prophylaxis.
viii. Laboratory specimens.
A. List all specimens collected by type and date.
B. Name of laboratory where specimens may be located.
ix. State if ambulance was used and date of use.
5. Chickenpox (Varicella), Diphtheria, Haemophilus influenzae b disease, invasive, Measles, Meningitis, acute, Meningococcal disease, invasive, Mumps, Pertussis (Whooping Cough), Pneumococcal disease, invasive, Poliomyelitis, acute, Rubella, Rubella, congenital syndrome, Tetanus:
i. The date of the diagnosis.
ii. The agent of disease.
iii. The name and address of the physician attending the person.
iv. Medical condition and status of the person including signs, symptoms and site, if any, of the infection.
v. The clinical history of the person, including:
A. The name of the hospital, date of admission and the date of discharge from the hospital if the person is admitted to hospital or the name of the hospital if the person is seen as an out-patient of the hospital.
B. The date and duration of isolation, if isolated.
C. Vaccination history.
vi. The case classification of the person.
vii. Laboratory findings and investigative tests including, without being limited, to culture and antimicrobial sensitivity, serological tests, microscopic examination and cerebrospinal fluid examination, together with the results of the tests.
viii. Association with outbreak and outbreak number, if applicable.
ix. Current treatment, if any, of the infection, setting out the drugs and dosage used and the date treatment commenced and ended.
x. Completion of the course of treatment including the major mode of therapy and the treatment compliance.
xi. Place where infection is believed to have been acquired.
xii. The source of infection including history of exposures and potential for community transmission.
xiii. Risk factors for the disease.
xiv. The immigration status and origin of the person, including:
A. Country of birth.
B. Country of last residence.
C. Date of arrival in Canada.
D. Immigration status at time of arrival in Canada.
xv. The travel history of the person, including:
A. Date and place of entry into country where disease acquired.
B. Date of departure from country where disease acquired.
C. Date and time of entry into Canada and carrier and flight number, if applicable.
D. Travel within country where disease acquired by date, place and length of stay.
E. Any other places visited en route to and from Canada.
xvi. List places and method of travel within Canada prior to and since the onset of illness.
xvii. The employment details of the person including job title and place of employment.
xviii. The name and address of the school the person attends, if applicable, including the classroom.
xix. Health unit responsible for identifying contacts.
xx. Names of health units with contacts.
xxi. Number of contacts identified.
xxii. Number of contacts traced.
xxiii. Number of contacts tested and treated, if applicable.
xxiv. Results of testing of contacts, if applicable.
xxv. Outcome:
A. If the person is deceased, date and cause of death.
B. Complications.
C. Absconded — lost to follow-up before treatment completion.
D. Other.
6. Tuberculosis:
i. The date of the diagnosis.
ii. The agent of disease.
iii. The name and address of the physician attending the person.
iv. Medical condition and status of the person including signs, symptoms and site, if any, of the infection.
v. The clinical history of the person, including:
A. The name of the hospital, date of admission and the date of discharge from the hospital if the person is admitted to hospital or the name of the hospital if the person is seen as an out-patient of the hospital.
B. The date and duration of isolation, if isolated.
C. Vaccination history.
D. Reactivation of old disease and years of previous treatment setting out the drugs and dosages used and the dates treatment commenced and ended.
vi. The case classification of the person.
vii. Laboratory findings and investigative tests including, without being limited to, culture and antimicrobial sensitivity, serological tests, X-ray examination, microscopic examination and cerebrospinal fluid examination, together with the results of the tests.
viii. Current treatment, if any, of the infection, setting out the drugs and dosage used and the date treatment commenced and ended.
ix. Completion of the course of treatment including the major mode of therapy (Directly Observed Therapy — daily or intermittent or Daily, self-administered) and the treatment compliance estimate (80%, 50-79%, less than 50% or unknown).
x. Place where infection is believed to have been acquired.
xi. The source of infection including history of exposures.
xii. Risk factors for the disease.
xiii. The immigration status and origin of the person, including:
A. Country of birth.
B. Country of last residence.
C. Immigration Medical Surveillance serial number or Inland Processing Number.
D. Date of arrival in Canada.
E. Reported for medical surveillance (has made contact with health unit or equivalent agency in other jurisdiction.)
F. Has had medical assessment in Canada for immigration surveillance.
G. Immigration status at time of arrival in Canada.
H. Country of birth of parents if person is under 20 years of age and Canadian born non-Aboriginal.
xiv. The registered Indian status of the person.
xv. The travel history of the person, including:
A. Date and place of entry into country where disease acquired.
B. Date of departure from country where disease acquired.
C. Date and time of entry into Canada and carrier and flight number, if applicable.
D. Travel within country where disease acquired by date, place and length of stay.
E. Any other places visited en route to and from Canada.
xvi. List places and method of travel within Canada prior to and since the onset of illness.
xvii. The employment details of the person including job title and place of employment.
xviii. The name and address of the school the person attends, if applicable, including the classroom.
xix. Health unit responsible for identifying contacts.
xx. Names of health units with contacts.
xxi. Number of contacts identified.
xxii. Number of contacts traced.
xxiii. Number of contacts tested and number of contacts treated.
xxiv. Results of testing of contacts.
xxv. Outcome:
A. If the person is deceased, date of death and cause of death.
B. Complications.
C. Absconded — lost to follow-up before treatment completion.
D. Other.
7. Cytomegalovirus infection, congenital, Group B Streptococcal Disease, neonatal, Herpes, neonatal, Ophthalmia Neonatorum:
i. The date of the diagnosis.
ii. The name and address of the physician attending the person.
iii. The name of the hospital, the date of admission and the date of discharge if the person is admitted to hospital.
iv. The contacts who have been traced.
v. Medical condition of the person including signs and symptoms of the infection.
vi. The case classification of the person.
vii. Laboratory findings and other investigative test results including, without being limited to, culture and antimicrobial sensitivity, serological tests, microscopic examination and cerebrospinal fluid examination, together with the results of the tests.
viii. Initial treatment, if any, of the infection including, without being limited to, the drugs and dosage used.
ix. Final effective treatment including, without being limited to, the drugs and dosage used.
x. Risk factors for the disease.
xi. The date of death and relation of the infection to the cause of death, if deceased.
8. Malaria, Yellow Fever:
i. The date of the diagnosis.
ii. The name and address of the physician attending the person.
iii. The name of the hospital, the date of admission and the date of discharge if the person is admitted to hospital.
iv. Place where infection is believed to have been acquired.
v. The agent of disease and sub-type.
vi. Medical condition of the person including signs and symptoms of the infection.
vii. The case classification of the person.
viii. Association with outbreak and outbreak number, if applicable.
ix. Laboratory findings and investigative tests including, without being limited to, culture and antimicrobial sensitivity, serological tests, microscopic examination and cerebrospinal fluid examination, together with the results of the tests.
x. The source of infection including history of exposures.
xi. Risk factors for the disease.
xii. The travel history of the person, including:
A. Date and place of entry into country where disease acquired.
B. Date of departure from country where disease acquired.
C. Travel within country where disease acquired by date, place and length of stay.
xiii. History of malaria and malaria prophylaxis or history of yellow fever vaccination.
xiv. Initial treatment, if any, of the infection including, without being limited to, the drugs and dosage used.
xv. If initial treatment given, the place, date and physician responsible for administration of treatment.
xvi. Final effective treatment including, without being limited to, the drugs and dosage used.
xvii. If effective treatment has been given, place, date and physician responsible for administration of treatment.
xviii. The date of death and relation of the infection to the cause of death, if deceased.
9. Group A Streptococcal disease, invasive:
i. The date of the diagnosis.
ii. The agent of disease.
iii. The name and address of the physician attending the person.
iv. Medical condition and status of the person including clinical severity, signs, symptoms and site, if any, of the infection.
v. The clinical history of the person, including:
A. The name of the hospital, date of admission and the date of discharge from the hospital if the person is admitted to hospital.
B. The date and duration of isolation, if isolated.
vi. The case classification of the person.
vii. Laboratory findings and investigative tests including, without being limited to, culture and antimicrobial sensitivity, serological tests, microscopic examination and cerebrospinal fluid examination, together with the results of the tests.
viii. Association with outbreak and outbreak number, if applicable.
ix. Current treatment, if any, of the infection, setting out the drugs and dosage used and the date treatment commenced and ended.
x. Antibiotic resistance, if applicable.
xi. Place where infection is believed to have been acquired.
xii. The source of infection including history of exposures.
xiii. Risk factors for the disease.
xiv. The employment details of the person including job title and place of employment.
xv. The name and address of the school the person attends, if applicable, including the classroom.
xvi. Health unit responsible for identifying contacts.
xvii. Names of health units with contacts.
xviii. Number of contacts identified.
xix. Number of contacts traced.
xx. Number of contacts tested and treated, if applicable.
xxi. Results of testing contacts, if applicable.
xxii. The date of death and relation of the infection to the cause of death, if the person is deceased.
10. Influenza:
i. The date of diagnosis.
ii. The agent of disease, including subtype.
iii. The name and address of the physician attending the person.
iv. Medical condition and status of the person including signs, symptoms and site, if any, of the infection.
v. The clinical history of the person, including:
A. The name of the hospital, date of admission and the date of discharge from the hospital if the person is admitted to hospital or the name of the hospital if the person is seen as an out-patient of the hospital.
B. The date and duration of isolation, if isolated.
C. Vaccination history.
vi. The case classification of the person.
vii. Laboratory findings including, without being limited to, antigen detection, culture and viral strain identification, genetic typing and serological tests.
viii. Association with outbreak and outbreak number, if applicable.
ix. Current treatment, if any, of the infection, setting out the drugs and dosage used and the date treatment commenced and ended.
x. Place where infection is believed to have been acquired.
xi. The source of infection including history of exposures.
xii. Risk factors for the disease.
xiii. The travel history of the person, if applicable, including:
A. Date and place of entry into country where disease acquired.
B. Date of departure from country where disease acquired.
C. Date and time of entry into Canada and carrier and flight number, if applicable.
D. Travel within country where disease acquired by date, place and length of stay.
E. Any other places visited en route to Canada.
xiv. List places and method of travel within Canada prior to and since the onset of illness, if applicable.
xv. The employment details of the person including job title and place of employment.
xvi. The name and address of the school the person attends, if applicable, including the classroom.
xvii. Health unit responsible for identifying contacts.
xviii. Names of health units with contacts.
xix. Number of contacts identified.
xx. Number of contacts traced.
xxi. Number of contacts tested and treated, if applicable.
xxii. The date of death and relation of the infection to the cause of death, if the person is deceased.
11. Severe Acute Respiratory Syndrome (SARS):
i. The date of the diagnosis.
ii. The agent of disease.
iii. The name and address of the physician attending the person.
iv. Medical condition and status of the person including signs, symptoms and site, if any, of the infection.
v. The clinical history of the person, including:
A. The name of the hospital, date of admission and the date of discharge from the hospital if the person is admitted to hospital, or transferred to another hospital or the name of the hospital if the person is seen as an out-patient of the hospital.
B. The date and duration of isolation, if isolated.
C. The date and duration of quarantine, if quarantined.
D. Vaccination history.
vi. The case classification of the person.
vii. The date of any change of case classification and details of the change.
viii. Laboratory findings and investigative tests including, without being limited to, culture and antimicrobial sensitivity, serological tests, microscopic examination, X-ray examination and cerebrospinal fluid examination, together with the results of the tests.
ix. Association with outbreak and outbreak number, if applicable.
x. Current treatment, if any, of the infection, setting out the drugs and dosage used and the date treatment commenced and ended.
xi. Place where infection is believed to have been acquired.
xii. The source of infection including history of exposures, and potential for community transmission.
xiii. Risk factors for the disease.
xiv. The travel history of the person, including:
A. Date and place of entry into country where disease acquired.
B. Date of departure from country where disease acquired.
C. Date and time of entry into Canada and carrier and flight number, if applicable.
D. Travel within country where disease acquired by date, place and length of stay.
E. Any other places visited en route to Canada.
xv. List places and method of travel within Canada prior to and since the onset of illness.
xvi. The employment details of the person including job title and place of employment.
xvii. The name and address of the school the person attends, if applicable, including the classroom.
xviii. Health unit responsible for identifying contacts.
xix. Names of health units with contacts.
xx. Number of contacts identified.
xxi. Number of contacts traced.
xxii. Number of contacts quarantined.
xxiii. Number of contacts tested and treated, if applicable.
xxiv. Results of testing contacts, if applicable.
xxv. The date of death and relation of the infection to the cause of death, if deceased.
12. Respiratory infection outbreaks in institutions:
i. The name and address of the institution and the contact person.
ii. The agent of disease, if known.
iii. The onset date and clinical details of symptoms in first and last cases.
iv. A description of the outbreak and an outbreak definition including, without being limited to a description of symptoms and laboratory findings.
v. The date the outbreak was declared and the outbreak number.
vi. The date the outbreak was declared over.
vii. The total number of cases in residents and all persons who carry on activities in the facility including employees, nurses, students, medical house staff, physicians, contract workers and volunteers (“staff”).
viii. The total number of residents and staff vaccinated prior to and during the outbreak and the total number of cases in residents and staff that were vaccinated prior to and during the outbreak.
ix. The total number of cases in residents and staff of admissions to hospital, X-ray confirmation of pneumonia, and deaths during the outbreak period.
x. Measures taken to monitor the facility for signs and symptoms consistent with the outbreak in persons who are residents or staff of the institution including the line list which shall include the name and location of residents and staff within the institution exhibiting signs and symptoms consistent with the description of the outbreak including clinical details and when the symptoms commenced and ended.
xi. Number of residents and staff in the entire institution and in areas of the institution affected by the outbreak.
xii. The name of the hospital, the date of admission and the date of discharge of any person who is a resident or staff member or any person who is admitted to hospital with signs and symptoms consistent with the definition of the outbreak.
xiii. Medical condition and status of persons exhibiting signs and symptoms consistent with the definition of the outbreak.
xiv. The name of any resident or staff member of the institution who dies during the outbreak period whether the cause of death is the respiratory infection or any other cause and including the time and date of death, the location of the death and cause of death.
xv. The details of any notification made to any other institution regarding the declaration of an outbreak in the institution for the purposes of preventing the spread of infection.
xvi. Laboratory findings and investigative tests including, without being limited to, antigen detection, culture and antimicrobial sensitivity, serological tests, microscopic examination, cerebrospinal fluid examination and X-ray examination, together with the results of the tests.
xvii. Current treatment, if any, of the persons exhibiting signs and symptoms consistent with the outbreak, setting out the drugs and dosage used and the date treatment commenced.
xviii. Infection control measures utilized to minimize the impact of the outbreak on the residents and staff and to prevent the spread of the infection including, but not limited to, influenza immunization, exclusion of non-immunized persons from the facility, the use of antiviral medications, isolation of ill persons, increased environmental sanitation and restriction of visitors.
xix. Place where infection is believed to have been acquired.
xx. The source of infection including history of exposures.
xxi. Risk factors for the disease.
xxii. Health units responsible for identifying contacts.
xxiii. Names of health units with contacts.
xxiv. Number of contacts identified.
xxv. Number of contacts traced.
xxvi. Number of contacts tested and treated, if applicable.
xxvii. Results of testing contacts, if applicable.
xxviii. Verification of staff immunization policies.
13. Encephalitis, including primary, viral, post-infectious, vaccine-related, subacute sclerosing panencephalitis, and unspecified:
i. The date of the diagnosis.
ii. The agent of disease.
iii. The name and address of the physician attending the person.
iv. Medical condition and the current status of the person including signs and symptoms.
v. The name of the hospital and the date of admission and the date of discharge if the person is admitted to hospital or the name and date of visits if the person is seen as an out-patient of the hospital.
vi. The case classification of the person.
vii. The outcome of the disease.
viii. Laboratory findings and investigative tests including, without being limited to, culture and antimicrobial sensitivity, serological tests, microscopic examination and cerebrospinal fluid examination, together with the results of the tests.
ix. Association with outbreak and outbreak number, if applicable.
x. Current treatment, if any, of the infection, setting out the drugs and dosage used and the date treatment commenced and ended.
xi. Place where infection is believed to have been acquired.
xii. The source of infection including history of exposures.
xiii. Risk factors for the disease.
xiv. The travel history of the person, including:
A. Date and place of entry into country where disease acquired.
B. Date of departure from country where disease acquired.
C. Date and time of entry into Canada and carrier and flight number, if applicable.
D. Travel within country where disease acquired by date, place and length of stay.
E. Any other places visited en route to Canada.
xv. List places and method of travel within Canada prior to and since the onset of illness.
xvi. The employment details of the person including job title and place of employment.
xvii. The name and address of the school the person attends, if applicable, including classroom.
xviii. The date of death and relation of the infection to the cause of death, if deceased.
14. Hepatitis B, Hepatitis C, Hepatitis D (Delta hepatitis):
i. The date of the diagnosis.
ii. The agent of disease.
iii. The name and address of the physician attending the person.
iv. The name of the hospital, the date of admission and the date of discharge if the person is admitted to hospital or the name of the hospital if the person is seen as an out-patient of the hospital.
v. History of immunization and post exposure prophylaxis as appropriate.
vi. The case classification of the person.
vii. Laboratory findings and investigative tests including, without being limited to, serological tests, microscopic examination and cerebrospinal fluid examination, together with the results of the tests.
viii. Association with outbreak and outbreak number, if applicable.
ix. The source of infection including history of exposures.
x. Risk factors for the disease.
xi. Place where infection is believed to have been acquired.
xii. The travel history of the person, including:
A. Date and place of entry into country where disease acquired.
B. Date of departure from country where disease acquired.
C. Travel within country where disease acquired by date, place and length of stay.
xiii. The employment details of the person including job title and place of employment, if applicable.
xiv. The name and address of the school the person attends, if applicable, including classroom.
xv. The person responsible for tracing the contacts of the person (hepatitis B and Delta only).
xvi. The contacts who have been traced (hepatitis B and Delta only).
xvii. The date of death and relation of the infection to the cause of death, if deceased.
15. Transmissible Spongiform Encephalopathy, including Creutzfeldt-Jakob Disease, all types, Gerstmann-Sträussler-Scheinker Syndrome, Fatal Familial Insomnia and Kuru:
i. The date of the diagnosis.
ii. The name and address of the physicians attending the person.
iii. The name of the hospital and the date of admission if the person is admitted to a hospital or is seen as an out-patient of the hospital.
iv. Laboratory findings and investigative tests including, without being limited to, 14-3-3 protein test, cerebrospinal fluid examination, microscopic examination, electroencephalogram, magnetic resonance imaging, computerized axial tomography and biopsy, together with the results of the tests.
v. History and physical examination findings of the person.
vi. Dates of organ, blood or blood product donated or received.
vii. Name of institution where performed, and dates, with respect to invasive procedures to person including, without being limited to, lumbar puncture, surgery and endoscopy.
viii. Countries of residence and duration of residence or travel.
ix. Genetic history of transmissible spongiform encephalopathy.
x. Date of death, if the person is deceased.
xi. Autopsy findings.
16. Amebiasis, Anthrax, Botulism, Brucellosis, Campylobacter enteritis, Cholera, Cryptosporidiosis, Cyclosporiasis, Food poisoning — all causes, Gastroenteritis, institutional outbreaks, Giardiasis, Hantavirus Pulmonary Syndrome, Hepatitis A viral infections, Legionellosis, Listeriosis, Lyme Disease, Paratyphoid Fever, Psittacosis/Ornithosis, Q Fever, Rabies, Salmonellosis, Shigellosis, Trichinosis, Tularemia, Typhoid Fever, West Nile Virus Illness, Verotoxin-producing E. coli infection indicator conditions, including Haemolytic Uraemic Syndrome (HUS), Yersiniosis:
i. The date of the diagnosis.
ii. The agent of disease.
iii. The name and address of the physician attending the person.
iv. Medical condition of the person including signs and symptoms of the infection.
v. The name of the hospital and the date of admission and the date of discharge if the person is admitted to hospital or the name of the hospital if the person is seen as an out-patient of the hospital.
vi. The case classification of the person.
vii. Laboratory findings and investigative tests including, without being limited to, culture and antimicrobial sensitivity, serological tests, microscopic examination and cerebrospinal fluid examination, together with the results of the tests.
viii. Association with outbreak and outbreak number, if applicable.
ix. Current treatment, if any, of the infection, setting out the drugs and dosage used and the dates treatment commenced and ended.
x. Place, including geographic location, where infection is believed to have been acquired.
xi. The source of infection including history of exposures and potential for community spread.
xii. Risk factors for the disease.
xiii. The travel history of the person, including:
A. Date and place of entry into country or countries where disease is believed to have been acquired.
B. Date of departure from country or countries where disease is believed to have been acquired.
C. Date and time of entry into Canada and carrier and flight number, if applicable.
D. Travel within country or countries where disease is believed to have been acquired by date, place and length of stay.
E. Any other places visited en route to and from Canada.
xiv. The immigration status and origin of the person, including:
A. Country of birth.
B. Country of last residence.
C. Date of arrival to Canada.
xv. List places and method of travel within Canada for the period of time equal to at least two incubation periods of the disease prior to and since the onset of illness.
xvi. The employment details of the person including job title and place of employment.
xvii. The name and address of the school the person attends, if applicable, including classroom.
xviii. The outcome of the disease.
xix. The date of death and relation of the infection to the cause of death, if the person is deceased.
17. Smallpox:
i. The date of the diagnosis.
ii. The agent of disease.
iii. The name and address of the physician attending the person.
iv. Medical condition and status of the person including signs and symptoms of the infection.
v. The clinical history of the person, including:
A. The name of the hospital, date of admission and the date of discharge from the hospital if the person is admitted to hospital, or transferred to another hospital or the name of the hospital if the person is seen as an out-patient of the hospital.
B. The date and duration of isolation, if isolated.
C. The date and duration of quarantine, if quarantined.
D. Vaccination history.
vi. The case classification of the person.
vii. The date of any change of case classification and details of the change.
viii. Laboratory findings and investigative tests including, without being limited to culture and antimicrobial sensitivity, serological tests, microscopic examination and cerebrospinal fluid examination, together with the results of the tests.
ix. Association with outbreak and outbreak number, if applicable.
x. Current treatment, if any, of the infection, setting out the drugs and dosage used and the date treatment commenced and ended.
xi. Place, including geographical location, where infection is believed to have been acquired.
xii. The source of infection including history of exposures and potential for community transmission.
xiii. Risk factors for the disease.
xiv. The travel history of the person, including:
A. Date and place of entry into country where disease acquired.
B. Date of departure from country where disease acquired.
C. Date and time of entry into Canada and carrier and flight number, if applicable.
D. Travel within country where disease acquired by date, place and length of stay.
E. Any other places visited en route to Canada.
xv. List places and method of travel within Canada prior to and since the onset of illness.
xvi. The employment details of the person including job title and place of employment.
xvii. The name and address of the school the person attends, if applicable, including the classroom.
xviii. Health unit responsible for identifying contacts.
xix. Names of health units with contacts.
xx. Number of contacts identified.
xxi. Number of contacts traced.
xxii. Number of contacts quarantined.
xxiii. Number of contacts tested and treated, if applicable.
xxiv. Results of testing contacts, if applicable.
xxv. The date of death and relation of the infection to the cause of death, if deceased. R.R.O. 1990, Reg. 569, s. 5; O. Reg. 1/05, s. 2.
5.1 (1) In this section,
“AIDS” means Acquired Immune Deficiency Syndrome; (“sida”)
“HIV” means Human Immunodeficiency Virus. (“VIH”) O. Reg. 749/91, s. 1.
(2) A physician who provides professional services to a patient in a clinic set out in the Schedule and who is required to report under section 26 of the Act following a test to determine if the patient is infected with an agent of AIDS is exempt from reporting the patient’s name and address if, before the test was ordered, the patient received counselling about preventing the transmission of HIV infection. O. Reg. 749/91, s. 1; O. Reg. 84/95, s. 1 (1).
(3) The operator of a laboratory is exempt from reporting, under section 29 of the Act, the name and address of a person who has tested positive for an agent of AIDS if the test is in relation to professional services provided at a clinic set out in the Schedule. O. Reg. 749/91, s. 1; O. Reg. 84/95, s. 1 (2).
(4) Revoked: O. Reg. 84/95, s. 1 (3).
6. (1) Where a medical officer of health receives a report made under section 25, 26, 27 or 28, subsection 29 (2) or section 30 of the Act, he or she shall immediately forward a copy of the report to the Public Health Division of the Ministry in a secure manner. O. Reg. 1/05, s. 3.
(2) Where a copy of a report referred to in subsection (1) concerns a person who has,
(a) amebiasis;
(b) chickenpox;
(c) epidemic diarrhoea;
(d) genital chlamydia trachomatis infections;
(e) genital herpes;
(f) gonorrhoea, other than gonorrhoea due to penicillinase producing strain of Neisseria gonorrhoeae;
(g) giardiasis;
(h) influenza;
(i) measles;
(j) mumps;
(k) pertussis; or
(l) rubella,
the copy shall be forwarded with the name of the person deleted. R.R.O. 1990, Reg. 569, s. 6 (2).
7. (1) A report required under subsection 38 (3) of the Act shall, with respect to the person to whom the report relates, contain the following information:
1. Name, address and telephone number in full.
2. Date of birth in full.
3. Sex.
4. The name, address and telephone number of the parent or guardian of the person experiencing the adverse event if the person is a minor.
5. The name and address of the physician attending the person.
6. The name of the hospital, the date of admission, and date of discharge, if the person was admitted to hospital as a result of the reportable event.
7. Signs and symptoms relating to the reportable event and, if known, the time and date of onset of each symptom relating to the reportable event.
8. The treatment prescribed, setting out drugs and dosage used, if the person sought medical care as a result of the reportable event.
9. The name of the hospital, the date seen, and the name of the physicians attending the person, if the person was seen as an out-patient of a hospital as a result of the reportable event.
10. Laboratory findings including the dates and results of testing and other investigative procedures relating to the reportable event.
11. The outcome of the reportable event on the date of the report; specifically, whether the person who had experienced the reportable event had fully recovered, had a residual health effect or died.
12. The name and manufacturer of each vaccine related to the reportable event, the date and time on which the vaccine was administered, and the dose, site of administration, route, lot number and expiry date of the vaccine related to the reportable event, and any prior dates on which the same vaccine had been administered.
13. The name and manufacturer of each vaccine received prior to and associated with the reportable event. O. Reg. 1/05, s. 4.
(2) A medical officer of health who receives a report under subsection 38 (3) of the Act shall report to the Ministry in respect of the adverse event report. O. Reg. 1/05, s. 4.
8. Any report made under the Act that is referred to in this Regulation shall be forwarded to the Ministry using the integrated Public Health Information System (iPHIS), or any other method specified by the Ministry. O. Reg. 1/05, s. 4.
Schedule
1. The District of Algoma Health Unit, Sexual Health Clinic, 99 Foster Drive, Sault Ste. Marie.
2. Anishnawbe Health Toronto, 225 Queen Street East, Toronto.
3. Barrie STD Clinic, 370 Dunlop Street, Barrie.
4. Bay Centre for Birth Control, Regional Women’s Health Centre, 790 Bay Street, Toronto.
5. Birth Control & STD Information Centre, 2828 Bathurst Street, North York.
6. Brampton-Caledon STD Clinic, 180B Sandalwood Parkway East, Brampton.
7. Centre médico-social communautaire, 22 College Street, Toronto.
8. Centretown Community Health Centre, 340 MacLaren Street, Ottawa.
9. Community Health Department, HIV Clinic, 99 Regina Street South, Waterloo.
10. Elgin-St. Thomas Health Unit, AIDS Division, 99 Edward Street, St. Thomas.
11. Hassle Free Clinic, 556 Church Street, Toronto.
12. HIV Care Program Clinic, Metropolitan General Hospital, 2240 Kildare Road, Windsor.
13. InterCommunity Health Centre, 659 Dundas Street East, London.
14. Kingston, Frontenac and Lennox and Addington Health Unit, STD Clinic, 221 Portmouth Avenue, Kingston.
15. Mississauga East STD Clinic, 3038 Hurontario Street, Mississauga.
16. Mississauga West STD Clinic, 2227 South Millway, Mississauga.
17. Peterborough County-City Health Unit, Sexual Health Clinic, 10 Hospital Drive, Peterborough.
18. Regional Niagara Health Services Department, Falls Clinic, 5710 Kitchener Street, Niagara Falls.
19. Rexdale Community Health Centre, 2267 Islington Avenue, Rexdale.
20. Sandwich Community Health Centre, 749 Felix Avenue, Windsor.
21. Sandy Hill Community Health Centre, 24 Selkirk Avenue, Vanier.
22. SITE, 480A Somerset Street West, Ottawa.
23. Somerset West Community Health Centre, 755 Somerset Street West, Ottawa.
24. STD Clinic, 237 Barton Street East, Hamilton.
25. STD Clinic, 250 Besserer Street, Ottawa.
26. Sudbury and District Health Unit, STD Clinic, 1300 Paris Crescent, Sudbury.
27. Thunder Bay District Health Unit, STD Clinic, 999 Balmoral Street, Thunder Bay.
28. Wellington-Dufferin-Guelph Health Unit, Sexual Health Clinic, 125 Delhi Street, Guelph.
29. West Central Community Health Centres, Alexandra Park Health Centre, 64 Augusta Avenue, Toronto.
30. West Central Community Health Centres, Niagara Neighborhood Health Centre, 674 Queen Street West, Toronto.
31. West Central Community Health Centres, SHOUT Clinic, 467 Jarvis Street, Toronto.
32. Windsor-Essex County Health Unit, HIV Clinic, 1005 Ouellette Avenue, Windsor.
33. City of York Health Unit, Sexual Health Clinic, 662 Jane Street, York.
34. City of York Health Unit, Sexual Health Clinic, 504 Oakwood Avenue, York.
O. Reg. 84/95, s. 2.
Forms 1, 2 Revoked: O. Reg. 1/05, s. 5.
Form 3
NOTIFICATION OF NEW ACTIVE — LEPROSY (HANSEN’S DISEASE)
Health Protection and Promotion Act
O. Reg. 606/91, s. 2.