R.R.O. 1990, Reg. 645: GENERAL, Immunization of School Pupils Act, R.S.O. 1990, c. I.1
Immunization of School Pupils Act
Loi sur l’immunisation des élèves
R.R.O. 1990, REGULATION 645
GENERAL
Historical version for the period September 18, 2013 to June 30, 2014.
Last amendment: O. Reg. 260/13.
This Regulation is made in English only.
Record of Immunization
1. A record of immunization maintained by a medical officer of health with respect to a pupil shall contain the following information, except for any items of information that are not reasonably possible for the medical officer of health to obtain:
1. The pupil’s full name, address and telephone number.
2. If applicable, every alternate name of the pupil.
3. The pupil’s sex.
4. The pupil’s date of birth.
5. The pupil’s country of birth.
6. The name of the school attended by the pupil and the identifying number, if any, assigned by the Ministry of Education to the school.
7. The pupil’s school grade or class.
8. The pupil’s health number assigned by the General Manager under the Health Insurance Act.
9. The full name, address and telephone number of every parent of the pupil.
10. The preferred language or languages of the pupil’s parents.
11. A record of all the pupil’s immunizations against designated diseases showing,
i. the type of vaccine given,
ii. the date of administration of the vaccine, and
iii. any reactions to the vaccine.
12. Any statement of medical exemption that pertains to the pupil, showing the effective time period on the statement.
13. Any statement of conscience or religious belief that pertains to the pupil. O. Reg. 260/13, s. 1.
Reports from schools
1.1 (1) For the purposes of assisting in the accurate maintenance of records of immunization, every person who operates a school shall submit reports containing records for each pupil in the school to the medical officer of health for the health unit in which the school is located. O. Reg. 260/13, s. 1.
(2) Subject to subsection (3), a report under subsection (1) shall be made,
(a) upon the request of the medical officer of health;
(b) within the time frame stipulated by the medical officer of health; and
(c) employing a secure mechanism of information transfer stipulated by the medical officer of health. O. Reg. 260/13, s. 1.
(3) A medical officer of health may only stipulate time frames and secure mechanisms of information transfer that are reasonable under the circumstances. O. Reg. 260/13, s. 1.
(4) If a person who operates a school has collected and maintains any of the following information about a pupil, the pupil’s record in a report under subsection (1) shall contain that information:
1. The pupil’s full name, address and telephone number.
2. If applicable, every alternate name of the pupil.
3. The pupil’s sex.
4. The pupil’s date of birth.
5. The pupil’s country of birth.
6. The name of the school attended by the pupil and the identifying number, if any, assigned by the Ministry of Education to the school.
7. The pupil’s school grade or class.
8. The pupil’s Ontario education number, if one has been assigned to the pupil.
9. The full name, address and telephone number of every parent of the pupil.
10. The preferred language or languages of the pupil’s parents. O. Reg. 260/13, s. 1.
(5) A medical officer of health shall comply with the following with respect to collecting, retaining, using and disclosing a pupil’s Ontario education number under this section:
1. The medical officer of health shall only collect the Ontario education number for the purpose of matching the personal information in the report submitted by a person who operates a school with the personal health information contained in a record of immunization.
2. The medical officer of health shall not use the Ontario education number for any purpose other than for the purpose of matching the personal information contained in the report submitted by a person who operates a school with the personal health information contained in a record of immunization.
3. The medical officer of health shall only retain the Ontario education number in a record that is not the record of immunization.
4. The medical officer of health shall take steps that are reasonable in the circumstances to ensure that the record in which the Ontario education number is retained is only used for the purpose of matching the personal information in the report submitted by a person who operates a school with the personal health information contained in a record of immunization.
5. The medical officer of health shall not disclose the Ontario education number. O. Reg. 260/13, s. 1.
(6) In this section,
“Ontario education number” means the number assigned to the pupil by the Minister of Education under the Education Act. O. Reg. 260/13, s. 1.
2. A statement of medical exemption shall be in Form 1. R.R.O. 1990, Reg. 645, s. 2.
Note: On July 1, 2014, section 2 is revoked and the following substituted: (See: O. Reg. 260/13, ss. 2, 4 (2))
Forms
2. For the purposes of the Act, the forms for the statement of medical exemption, the statement of conscience or religious belief and the notice of transfer from a school are the forms with those names, dated August, 2013, that are available through the website of the Government of Ontario Central Forms Repository. O. Reg. 260/13, s. 2.
3. A statement of conscience or religious belief shall be in Form 2. R.R.O. 1990, Reg. 645, s. 3.
Note: On July 1, 2014, section 3 is revoked and the following substituted: (See: O. Reg. 260/13, ss. 2, 4 (2))
Program of immunization
3. The program of immunization in respect of designated diseases set out in the Tables to this section is prescribed.
table 1
Program of immunization
|
Item |
Disease |
Minimum Number of Valid Doses Required |
Required Minimum Intervals Between Primary Series Doses |
Required Booster Doses |
|
1. |
Diphtheria |
If age at first dose is under 7 years: |
||
|
4 or 5 doses required (depending on intervals as described) |
First valid dose administered no earlier than 6 weeks (42 days) of age. Second valid dose administered at a minimum interval of 4 weeks (28 days) following previous dose. Third valid dose administered at a minimum interval of 4 weeks (28 days) following previous dose. Fourth valid dose administered at a minimum interval of 24 weeks following previous dose. Fifth valid dose administered at a minimum interval of 24 weeks following previous dose, and no earlier than 4 years of age. (Note: Fifth valid dose is not required if fourth valid dose is administered at 4 years of age or older.) |
10 years following last dose given |
||
|
If age at first dose is 7 years or older: |
||||
|
3 doses required |
Second valid dose administered at a minimum interval of 4 weeks (28 days) following previous dose. Third valid dose administered at a minimum interval of 24 weeks following previous dose. |
10 years following last dose given |
||
|
2. |
Tetanus |
If age at first dose is under 7 years: |
||
|
4 or 5 doses required (depending on intervals as described) |
First valid dose administered no earlier than 6 weeks (42 days) of age. Second valid dose administered at a minimum interval of 4 weeks (28 days) following previous dose. Third valid dose administered at a minimum interval of 4 weeks (28 days) following previous dose. Fourth valid dose administered at a minimum interval of 24 weeks following previous dose. Fifth valid dose administered at a minimum interval of 24 weeks following previous dose, and no earlier than 4 years of age. (Note: Fifth valid dose is not required if fourth valid dose is administered at 4 years of age or older.) |
10 years following last dose given |
||
|
If age at first dose is 7 years or older: |
||||
|
3 doses required |
Second valid dose administered at a minimum interval of 4 weeks (28 days) following previous dose. Third valid dose administered at a minimum interval of 24 weeks following previous dose. |
10 years following last dose given |
||
|
3. |
Pertussis |
If age at first dose is under 7 years: |
||
|
4 or 5 doses required (depending on intervals as described) |
First valid dose administered no earlier than 6 weeks (42 days) of age. Second valid dose administered at a minimum interval of 4 weeks (28 days) following previous dose. Third valid dose administered at a minimum interval of 4 weeks (28 days) following previous dose. Fourth valid dose administered at a minimum interval of 24 weeks following previous dose. Fifth valid dose administered at a minimum interval of 24 weeks following previous dose, and no earlier than 4 years of age. (Note: Fifth valid dose is not required if fourth valid dose is administered at 4 years of age or older.) |
10 years following last dose given |
||
|
If age at first dose is 7 years or older: |
||||
|
3 doses required |
Second valid dose administered at a minimum interval of 4 weeks (28 days) following previous dose. Third valid dose administered at a minimum interval of 24 weeks following previous dose. |
10 years following last dose given |
||
|
4. |
Poliomyelitis |
If age at first dose is under 7 years: |
||
|
3 or 4 doses required (depending on intervals as described) |
First valid dose administered no earlier than 6 weeks (42 days) of age. Second valid dose administered at a minimum interval of 4 weeks (28 days) following previous dose. Third valid dose administered at a minimum interval of 24 weeks following previous dose. Fourth valid dose administered at a minimum interval of 24 weeks following previous dose, and no earlier than 4 years of age. (Note: Fourth valid dose is not required if third valid dose is administered at 4 years of age or older.) |
None required |
||
|
If age at first dose is 7 years or older: |
||||
|
3 doses required |
Second valid dose administered at a minimum interval of 4 weeks (28 days) following previous dose. Third valid dose administered at a minimum interval of 24 weeks following previous dose. |
None required |
||
|
5. |
Measles |
2 doses required |
First valid dose administered no earlier than 1 year of age, and at a minimum interval of 4 or 6 weeks following any previous dose of a live vaccine, depending on the live vaccine (see Table 2). Second valid dose administered at a minimum interval of 4 or 6 weeks following any previous dose of a live vaccine, depending on the live vaccine (see Table 2). |
None required |
|
6. |
Mumps |
2 doses required |
First valid dose administered no earlier than 1 year of age, and at a minimum interval of 4 or 6 weeks following any previous dose of a live vaccine, depending on the live vaccine (see Table 2). Second valid dose administered at a minimum interval of 4 or 6 weeks following any previous dose of a live vaccine, depending on the live vaccine (see Table 2). |
None required |
|
7. |
Rubella |
1 dose required |
First valid dose administered no earlier than 1 year of age, and at a minimum interval of 4 or 6 weeks following any previous dose of a live vaccine, depending on the live vaccine (see Table 2). |
None required |
|
8. |
Meningococcal Disease |
If born on or after September 1, 2004 and age is under 12 years: |
||
|
1 dose of meningococcal conjugate-C vaccine required |
One valid dose administered no earlier than 1 year of age and at a minimum interval of 4 weeks (28 days) following any previous dose (if given). |
None required |
||
|
If born on or after January 1, 1997 and age is 12 years or older (Grade 7 or above): |
||||
|
1 dose of meningococcal conjugate-ACYW-135 vaccine required |
One valid dose administered at a minimum interval of 4 weeks (28 days) following any previous dose (if given). |
None required |
||
|
9. |
Varicella |
If born on or after January 1, 2010: |
||
|
2 doses required |
First valid dose administered no earlier than 1 year of age, and at a minimum interval of 4 or 6 weeks following any previous dose of a live vaccine, depending on the live vaccine (see Table 2). Second valid dose administered at a minimum interval of 4 or 6 weeks following any previous dose of a live vaccine, depending on the live vaccine (see Table 2). |
None required |
||
Table 2
Minimum intervals between live vaccines
|
Live Vaccines (given in any order) |
Minimum interval between doses |
|
MMR and MMR |
4 weeks (28 days) |
|
MMR and Varicella |
|
|
MMR and MMRV |
6 weeks (42 days) |
|
MMRV and MMRV |
|
|
MMRV and Varicella |
|
|
Varicella and Varicella |
|
|
Other live vaccines and MMR, MMRV, or Varicella |
4 weeks (28 days) |
MMR = combined measles, mumps, rubella vaccine
MMRV = combined measles, mumps, rubella, varicella vaccine
O. Reg. 260/13, s. 2.
4. A notice of transfer of pupil referred to in section 14 of the Act shall be in Form 3. R.R.O. 1990, Reg. 645, s. 4.
Note: On July 1, 2014, section 4 is revoked. (See: O. Reg. 260/13, ss. 2, 4 (2))
5. The following program of immunization in respect of designated diseases is prescribed:
Schedule
|
Item |
Disease |
Type of Vaccine to be Used |
Minimum Number of Doses Accepted |
Recommended Schedule of Primary Immunization |
Interval Between Booster Doses |
|
1. |
Diphtheria |
TOXOID |
3 |
Two injections, 1 to 2 months apart with a further dose one year later. Children immunized in infancy require three doses 1 to 2 months apart, a further dose one year later and a booster dose at age 4-6. |
10 years |
|
2. |
Tetanus |
TOXOID |
3 |
Two injections, 1 to 2 months apart with a further dose one year later. Children immunized in infancy require three doses 1 to 2 months apart, a further dose one year later and a booster dose at age 4-6. |
10 years |
|
3. |
Poliomyelitis |
Inactivated Polio vaccine (IPV) or |
3 |
Two injections, 1 to 2 months apart with a further dose one year later. Children immunized in infancy require three doses 1 to 2 months apart, a further dose one year later and a booster dose at age 4-6. |
NONE required |
|
|
|
Live Oral Polio vaccine (OPV) |
3 |
Two doses 1 to 2 months apart with a further dose 2 to 12 months later. Children immunized in infancy require a booster dose at age 4-6. |
NONE required |
|
4. |
Measles |
Live attenuated virus vaccine |
2 |
One dose after the first birthday with a further dose more than one month later and preferably at age 4‑6. |
NONE required |
|
5. |
Mumps |
Live attenuated virus vaccine |
1 after one year of age |
One dose after the first birthday. |
NONE required |
|
6. |
Rubella |
Live attenuated virus vaccine |
1 after one year of age |
One dose after the first birthday. |
NONE required |
R.R.O. 1990, Reg. 645, s. 5; O. Reg. 299/96, s. 2; O. Reg. 443/03, s. 1.
Note: On July 1, 2014, section 5 is revoked. (See: O. Reg. 260/13, ss. 2, 4 (2))
Form 1
STATEMENT OF MEDICAL EXEMPTION
Immunization of School Pupils Act
Insert regs\graphics\1990\645\645001au.tif
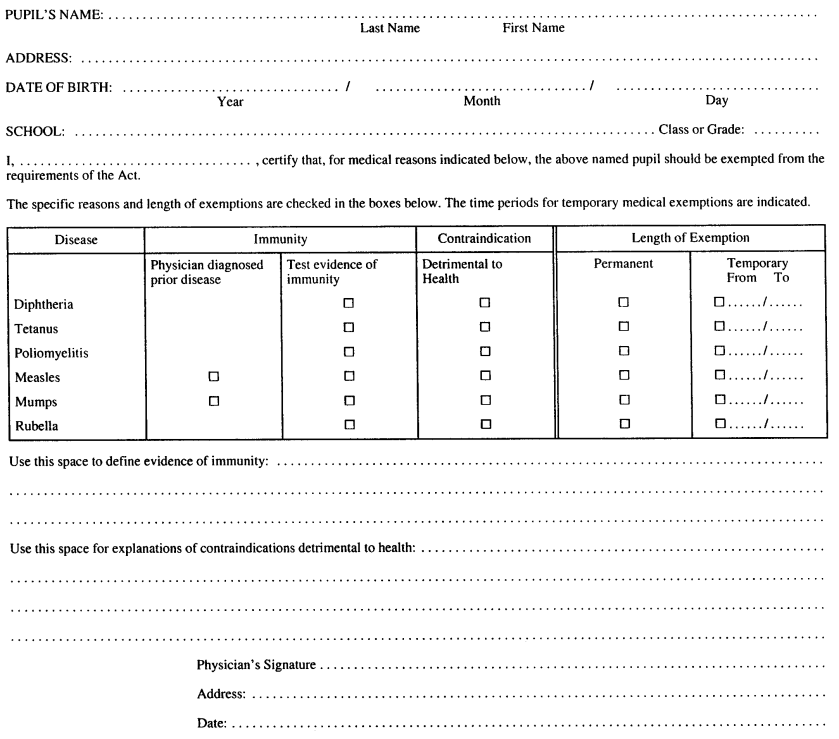
R.R.O. 1990, Reg. 645, Form 1.
Note: On July 1, 2014, Form 1 is revoked. (See: O. Reg. 260/13, ss. 3, 4 (2))
Form 2
STATEMENT OF CONSCIENCE OR RELIGIOUS BELIEF
Immunization of School Pupils Act
Insert regs\graphics\1990\645\645002au.tif
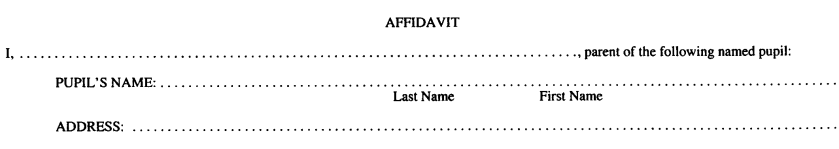
Insert regs\graphics\1990\645\645002bu.tif

R.R.O. 1990, Reg. 645, Form 2.
Note: On July 1, 2014, Form 2 is revoked. (See: O. Reg. 260/13, ss. 3, 4 (2))
Form 3
NOTICE OF TRANSFER FROM A SCHOOL
Immunization of School Pupils Act
Insert regs\graphics\1990\645\645003au.tif
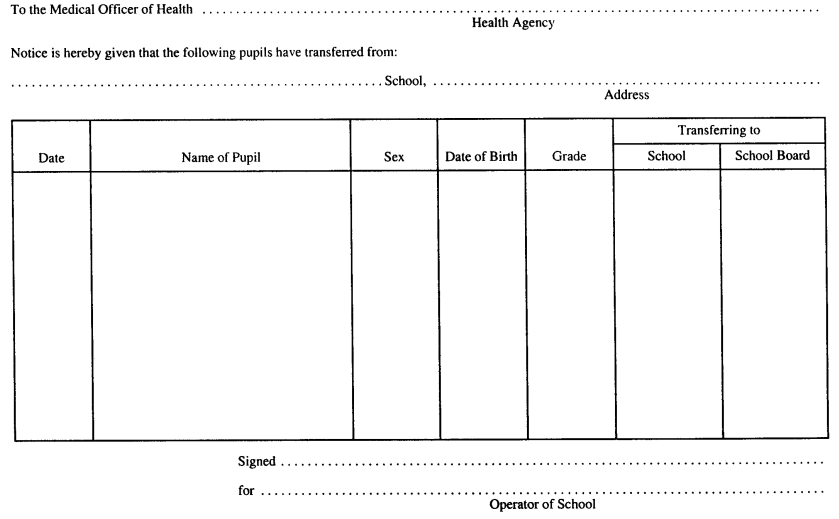
R.R.O. 1990, Reg. 645, Form 3.
Note: On July 1, 2014, Form 3 is revoked. (See: O. Reg. 260/13, ss. 3, 4 (2))