This page is no longer current and is provided for archival and research purposes.
Ontario Toxics Reduction Program - Reference Tool for Assessing Safer Chemical Alternatives
This document is intended to increase awareness of green chemistry and assist industries and businesses in evaluating which chemicals are safer alternatives.
This document has been prepared from a review and analysis performed in 2011 for the Ministry of the Environment by Senes Consultants.
Important Notices
There may be websites linked to and from this document that are operated or created for organizations outside of the Government of Ontario. Those organizations are solely responsible for the operation and information (including the right to display such information) found on their respective websites. These linked websites may or may not be available in French. The linking to or from this document does not imply on the part of the Government of Ontario any endorsement or guarantee of any of the organizations or information (including the right to display such information) found on their respective websites.
No Liability
The Ontario Ministry of the Environment assumes no responsibility for errors or omissions in any of the information contained on any of the websites. Ministry of the Environment (MOE) makes no representation or warranty of any kind whatsoever with respect to the web sites. More specifically disclaims any express or implied warranties related to the use of the websites and all contents including without limitation warranties of non-infringement or fitness for any particular purpose.
In no event shall the Ministry of the Environment, the Province of Ontario and their respective officers, employees, servants or agents be liable for any failure to keep the contents of the websites up to date, for errors or omissions contained on the website, or for any damages (including without limitation, damages for loss of profits, business interruption, loss of information, or direct, indirect, incidental, special, consequential or punitive damages) arising out of or related to the use or inability to use this document (including all contents), whether under contract, in tort or under any other basis of liability.
Preamble
This document was developed under the Ontario Toxics Reduction Program and is intended to increase awareness of green chemistry and to assist industries and businesses in evaluating chemicals as safer alternatives. The scope of the document is intentionally broad to assist a range of industries and sectors in understanding green chemistry principles and considerations for the assessment of safer alternatives. This document is not intended to be prescriptive, and is not meant to reflect endorsement, guidance or mandatory action in the Province on Ontario. Instead, it serves as a starting point for those enterprises/individuals looking to consider conducting an alternatives assessment and provides references and a general approach to help move towards safer chemical alternatives.
The framework outlined in this reference tool for the assessment of safer chemical alternatives is based on a thorough review of safer chemical assessment programs and approaches, assessment tools and frameworks, and is also a matter of professional judgement. The background information on alternatives assessment provided in this tool is meant to help companies and also facilitate conversations with contractors, who may be enlisted to complete an alternatives assessment on their behalf. It should be noted that an alternatives assessment should have the involvement of professionals with a scientific background with an understanding of the toxicity of chemicals as an alternatives assessment is not a simple task.
The MOE recognizes that there are challenges that may be encountered in the course of assessing and implementing safer chemical alternatives that may include restrictions due to global formulations, data gaps, interpretation of data (especially environmental and human toxicity), capital investments required, customer approval and satisfaction, evaluation costs, supply assessments, research and development, and facility down-time due to a change in chemical. Furthermore, the MOE recognizes that alternatives assessment is not an absolute process as the end result will not be a chemical or process that is ‘greener’ or ‘safer’ in its entirety; rather, alternatives assessment is a comparative methodology with the end result being a greener/safer chemical or process.
For additional information and any addenda or revisions to this guide, please visit the MOE website.
Public Information Centre (PIC)
Ontario Ministry of the Environment
135 St. Clair Avenue West
Toronto, Ontario
M4V 1M2
Tel: 416-325-4000 Toll-free: 1-800-565-4923 Toll-free TTY: 1-800-515-2759 Fax: 416-325-3195
Cette publication hautement spécialisée n’est disponible qu’en anglais en vertu du reglement 441/97, qui en exempte I’application de la Loi sur les services en français. Pour obtenir de I’aide en français, veuillez communiquer avec Ie ministere de I’Environnement au 416 314-7938 (Fax: 416 314-7949)
Acronyms
- AA
- Alternatives Assessment
- ACGIH
- American Conference of Governmental Industrial Hygienists
- ACS
- American Chemical Society
- AIM
- Analog Identification Methodology
- ASTM
- American Society for Testing and Materials
- ATSDR
- Agency for Toxic Substances and Disease Registry
- BAF
- Bioaccumulation Factor
- BCF
- Bioconcentration Factor
- Cal/EPA
- California Environmental Protection Agency
- CARB
- California Air Resources Board
- CARS
- Chemical Assessment and Ranking System
- CAS® RN
- Chemical Abstracts Service Registry Number
- CCOHS
- Canadian Centre for Occupational Health and Safety
- CDD
- Chlorinated Dibenzo-p-dioxin
- CDF
- Chlorinated Dibenzofuran
- CEPA
- Canadian Environmental Protection Act
- C
- Ceiling Limit
- CGCEN
- Canadian Green Chemistry and Engineering Network
- CMP
- Chemicals Management Plan
- CPA
- Clean Production Action
- CTSA
- Cleaner Technologies Substitutes Assessment
- DEHP
- Di(2-ethylhexyl) phthalate
- DfE
- Design for the Environment
- DSL
- Domestic Substances List
- EC/Dx
- Effect/Dose Concentration (that affects x per cent or organisms)
- ECICS
- European Customs Inventory of Chemical Substances
- Eco-SSLs
- Ecological Soil Screening Levels
- ECOSAR
- Ecological Structure Activity Relationships
- ECOTOX
- Ecotoxicology Database
- EFDB
- Environmental Fate Database
- EPCRA
- Emergency Planning & Community Right-to-Know Act
- EPI
- Estimation Programs Interface
- GATT
- General Agreement on Tariffs and Trade
- GCC
- GreenCentre Canada
- GCES
- Green Chemistry Expert System
- GCI
- Green Chemistry Institute (of the ACS)
- GCN
- Green Chemistry Network
- GHS
- Globally Harmonized System
- GLGCN
- Great Lakes Green Chemistry Network
- GWP
- Global Warming Potential
- HMIS
- Hazardous Materials Identification System
- HSDB
- Hazardous Substance Data Bank
- IARC
- International Agency for Research on Cancer
- IC/Dx
- Inhibitory Concentration/Dose (that affects x per cent of organisms)
- IDLH
- Immediately Dangerous to Life or Health
- IRIS
- Integrated Risk Information System
- IRR
- Internal Rate of Return
- ISO
- International Organization for Standardization
- LCA
- Life Cycle Analysis
- LC/Dx
- Lethal Concentration/Dose (that kills x per cent of organisms)
- LCI
- Life Cycle Inventory
- LCIA
- Life Cycle Impact Assessment
- LO(A)EL/C
- Lowest Observed (Adverse) Effect Level/Concentration
- LSS
- Lab Safety Supply
- Koc
- Organic carbon-normalized sorption coefficients
- Kow
- Octanol-Water Partition Coefficient
- MATC
- Maximum Acceptable Toxicant Concentration
- MBDC
- McDonough Braungart Design Chemistry
- mg/kg
- Milligrams per kilogram
- mg/kg-d
- Milligrams per kilogram per day
- mg/L
- Milligrams per litre
- mg/m3
- Milligrams per cubic metre
- MIR
- Maximum Incremental Reactivity
- MMC
- McMaster-Carr
- MOE
- Ontario Ministry of the Environment
- MSDS
- Material Safety Data Sheets
- NAFTA
- North American Free Trade Agreement
- NATO/CCMS
- North Atlantic Treaty Organization/Committee on the Challenges of Modern Society
- NDSL
- Non-Domestic Substances List
- NESHAP
- National Emissions Standard for Hazardous Air Pollutants
- NFPA
- National Fire Protection Association
- NIOSH
- National Institute for Occupational Safety and Health
- NJDEP
- New Jersey Department of Environmental Protection
- NO(A)EC/L
- No Observed (Adverse) Effect Concentration/Level
- NOHSC
- National Occupational Health & Safety Commission
- NOx
- Nitrogen Oxides
- NPCA
- National Paint & Coating Association
- NPRI
- National Pollutant Release Inventory
- NPV
- Net Present Value
- NTP
- National Toxicology Program
- O3
- Ozone
- OCETA
- Ontario Centre for Environmental Technological Advancement
- ODP
- Ozone Depleting Potential
- OECD
- Organisation for Economic Cooperation and Development
- OELs
- Occupational Exposure Limits
- OPPT
- Office of Pollution Prevention and Toxics
- OSHA
- Occupational Safety and Health Administration
- P2OASys
- Pollution Prevention Options Analysis System
- PAH
- Polycyclic Aromatic Hydrocarbon
- PBT
- Persistent, Bioaccumulative and Toxic
- PCB
- Polychlorinated Biphenyl
- PCE
- Perchloroethylene (Tetrachloroethylene)
- PEL
- Permissible Exposure Limit
- PentaBDE
- Pentabromodiphenyl Ether
- PHYSPROP
- Physical Properties Database
- pKa
- Acid-base dissociation constant
- PPE
- Personal Protective Equipment
- PSL
- Priority Substances List
- QSAR
- Quantitative Structure Activity Relationship
- REACH
- Registration, Evaluation, Authorization and Restriction of Chemical Substances
- REL
- Recommended Exposure Limit
- RfD
- Reference Dose
- RfC
- Reference Concentration
- RoC
- Report on Carcinogens
- RSEI
- Risk-Screening Environmental Indicators
- SCRAM
- Scoring and Ranking Assessment Model
- SETAC
- Society of Environmental Toxicology and Chemistry
- SF
- Sustainable Futures Initiative
- SIN
- Substitute It Now
- SMART
- Synthetic Methodology Assessment for Reduction Techniques
- SNAc
- Significant New Activity (requirements)
- SRC
- Syracuse Research Corporation
- STEL
- Short-term Exposure Limit
- T1/2
- Half-life
- TEF
- Toxicity Equivalency Factor
- TEQ
- Toxic Equivalent
- TLV
- Threshold Limit Value
- TRA
- Toxics Reduction Act
- TRI
- Toxics Release Inventory
- TRV
- Toxicological Reference Value
- TSCA
- Toxic Substances Control Act
- TURI
- Toxics Use Reduction Institute
- TWA
- Time-Weighted Average
- U.S. EPA
- United States Environmental Protection Agency
- VE
- Virtual Elimination
- VOC
- Volatile Organic Compound
- WHO
- World Health Organization
- WSIB
- Workplace Safety and Insurance Board
- ZWA
- Zero Waste Alliance
1.0 Introduction
1.1 Project Background
The Ontario Ministry of the Environment (MOE) has introduced the Toxics Reduction Program with the purpose of preventing pollution and protecting human health and the environment through the reduction in the use and creation of substances which may pose a threat to human health and the environment, and informing Ontarians about these substances. The Toxics Reduction Program includes promotion of green chemistry and engineering, and support to industry and other stakeholders. Towards this end, the MOE has completed a review of programs and approaches for assessing safer chemical alternatives, and has developed this reference tool to provide support and guidance for stakeholders to identify and consider safer chemical alternatives.
The MOE conducted a jurisdictional review of safer alternative practices (Jurisdictional Review of Safer Chemical Alternatives) and, based on this review, developed a reference tool for chemical substitution. This tool is intended to provide information and increase awareness of green chemistry and assessment of safer chemical alternatives, and to inform individuals who are in decision-making roles or negotiating positions with their suppliers.
This report is the second part of the safer chemical alternatives initiative and provides references and guidance to assist the Ontario government, industry and other stakeholders in assessing chemicals that may be used as safer alternatives. The information contained herein was obtained from a review of existing programs, networks, and assessment tools (programs) related to the promotion, evaluation and implementation of green chemistry and safer chemical alternatives. The complete review of these programs is provided in the 2012 document entitled Jurisdictional Review of Safer Chemical Alternatives.
This document is not intended as a prescriptive tool, and is purposely as general as possible so that it may be applied broadly across multiple industries and sectors. It is aimed at drop-in chemical substitution, although other aspects of safer alternatives and green chemistry in general can encompass production processes, materials, products, economic systems and functions, as well as product redesign to eliminate the need for a current activity or function of a chemical substance. In addition, it should be noted that an evaluation of safer chemical alternatives may not find a better alternative at present but that does not preclude an examination of a safer alternative using the guidance provided in this tool.
The framework outlined in this reference tool for the assessment of safer chemical alternatives is based on a thorough review of other assessment tools, frameworks and programs, and is also a matter of professional judgement. It is recognized that each industry and manufacturer may have to adapt the tool to their specific needs and, as such, is intended to provide a general approach that can be followed to help industry move towards a greener economy.
As a small or medium size company, possibly with limited resources to devote to an alternatives assessment, this reference tool is provided as an educational overview for the procedure and considerations for completing an alternatives assessment. Some components discussed in this document may be outside the scope and resources available to small or medium-sized companies. However, a broad understanding of alternatives assessment will enhance discussions with, for example, chemical suppliers and possibly create opportunities for improvements within the guiding principles of green chemistry. Additionally, an awareness of the green chemistry principles and considerations for safer alternatives might lead to other process or formulation improvements.
As a large company, some components of alternative assessment may already exist within the organization. This reference tool is not intended to override existing functions or procedures; however, it may provide guidance for the enhancement of existing procedures or help with the expansion of green chemistry programs within the organization.
As a facility that is subject to the Toxics Reduction Act, 2009 (TRA), the facility is required to develop a plan to reduce their use and creation of a prescribed toxic substance. As part of this, the facility must identify and assess at least one option from each of the seven categories listed in Ontario Regulation 455/09, or explain why an option could not be identified for any set category. One of those seven categories is materials or feedstock substitution (i.e., a safer chemical alternative). An identified option cannot contravene another law nor have a greater net negative impact on human health and the environment. The TRA and Regulation provide a facility with the flexibility to determine an appropriate approach for undertaking the identification and analysis of options.
As part of the assessment of the option, technical feasibility and economic feasibility must be considered. A useful starting point for a facility may be to identify the specific technical and economic factors that should be considered as part of this. And while there are a number of factors that can be considered by a facility, it must include the anticipated savings for each option, if any, and the anticipated payback period as part of the analysis of economic feasibility. For more information, please refer to the MOE website for additional information or guidance.
If you are a company that receives parts and materials from other suppliers, you may not have the control to modify the parts and materials; however, discussions with suppliers could be initiated to encourage the supplier to implement improvements. This reference tool is also not necessarily relevant for a company or facility that does not produce chemicals; again, discussions could be initiated with suppliers to encourage the implementation of improvements. In addition, the selection of an alternative chemical may be done through a sector based approach so that all members can benefit from the change in a common chemical.
1.2 Green Chemistry & Safer Chemical Alternatives
Traditionally, environmental protection has focused on controlling risk by controlling or reducing exposure. Green chemistry, on the other hand, looks to reduce risks at their source by stimulating innovation in safer and cleaner forms of production, products and activities. In other words, green chemistry looks at developing manufacturing processes that do not use or lead to the generation of harmful substances, rather than finding end-of-pipe solutions to dispose of toxics and reduce emissions (GLGCN 2010, Rossi et al. 2006).
Green chemistry is the design of chemical products and processes that reduce or eliminate the use or generation of hazardous substances (U.S. EPA 2010a). This is usually accomplished through the reduction of waste, use of non-toxic components, and improvement in process efficiency. Green chemistry typically encompasses the life cycle of a product, including by-products of the manufacturing process and use. The 12 principles of green chemistry developed by Anastas and Warner (1998) are as follows:
- It is better to prevent waste than to treat or clean up waste after it is formed.
- Synthetic methods should be designed to maximize the incorporation of all materials used in the process into the final product.
- Wherever practicable, synthetic methodologies should be designed to use and generate substances that possess little or no toxicity to human health and the environment.
- Chemical products should be designed to preserve efficacy of function while reducing toxicity.
- The use of auxiliary substances (e.g., solvents, separation agents, etc.) should be made unnecessary wherever possible and, innocuous when used.
- Energy requirements should be recognized for their environmental and economic impacts and should be minimized. Synthetic methods should be conducted at ambient temperature and pressure.
- A raw material or feedstock should be renewable rather than depleting wherever technically and economically practicable.
- Unnecessary derivization (block group, protection/deprotection, temporary modification of physical/chemical processes) should be avoided whenever possible.
- Catalytic reagents (as selective as possible) are superior to stoichiometric reagents.
- Chemical products should be designed so that at the end of their function they do not persist in the environment and break down into innocuous degradation products.
- Analytical methodologies need to be further developed to allow for real-time, in-process monitoring and control prior to the formation of hazardous substances.
- Substances and the form of a substance used in a chemical process should be chosen so as to minimize the potential for chemical accidents, including releases, explosions, and fires.
It should be noted that these 12 principles are not to be used as assessment tools, and merely serve to provide suggestions of how the concepts of green chemistry may be implemented into an alternatives assessment process. Therefore, following any one principle does not necessarily imply that a chemical or process is ‘green’ but would serve to move towards a ‘greener’ process or ‘safer’ chemical.
There are many benefits to the use of green chemistry outside of the direct benefits of reducing the use and creation of toxic chemicals. Green chemistry can reduce costs through a reduction in capital investment, insurance costs, required inputs, energy, disposal and treatment of hazardous waste, and improved process efficiency. Many countries have approval processes that favour green chemicals through reduced permitting time (e.g., the U.S. EPA Sustainable Futures Initiative [SF]) and special labelling programs (e.g., the U.S. EPA Design for the Environment [DfE]). The manufacture and use of green chemicals minimizes the potential for future liabilities, either through waste management, chemical accidents, or workplace exposures. Green chemistry can also present business opportunities for suppliers and manufacturers and marketing advantages for products and companies. Additionally, investors are becoming increasingly wary of toxic risks, not just as health risks but also as a business risk.
The selection of safer chemical alternatives is an important but complex component of green chemistry. Substituting with alternatives can be more efficient at reducing multiple risks in the long term, as alternative assessment examines a broader range of factors and options than traditional problem-based approaches. Alternatives can encompass production processes, chemicals, materials, products, economic systems and functions, as well as product redesign to eliminate the need for a current activity or function of a product (Rossi et al. 2006). This reference tool, however, is limited to the assessment of chemicals that may be used as drop-in safer chemical alternatives.
The selection of a safer chemical alternative includes consideration of possible chemical substitutes and an assessment of a full range of criteria, such as persistence, toxicity, degradation, cost, performance, etc. It should be noted that alternatives assessment is not an absolute process as the end result will not be a chemical or process that is ‘green’ in its entirety; rather, alternatives assessment is a comparative methodology with the end result being a greener chemical or process. For example, an alternative chemical may be safer (i.e., less persistent or toxic) in one aspect, but it may introduce other hazards that the original chemical did not present. The information gathered for the assessment is geared towards promoting informed decisions, minimizing the potential for unintended consequences such as switching to a poorly understood and potentially more hazardous alternative (Lavoie et al. 2010). As such, the selection of an alternative is not a black and white decision and requires prioritization of criteria.
Currently, Canada has very few regulations and programs geared towards large-scale adoption of green chemistry and the evaluation of safer chemical alternatives, with the exception of the International Organization for Standardization (ISO) 14040 standards. Instead, technologies to control emissions are often used in an effort to manage hazards and risks. There is therefore a need to provide industry with guidance and tools on how to voluntarily implement the principles of green chemistry and the potential selection of safer chemical alternatives (Thorpe 2005). Alternatives assessment is a process that has been implemented by a number of organizations, and various regulations, frameworks assessment tools and manuals have been developed around this subject internationally; however, a standard method for alternatives assessment has not been established to date.
1.3 Existing Programs
The MOE conducted a review of existing programs, networks and assessment tools for the evaluation of safer chemical alternatives (Jurisdictional Review of Safer Chemical Alternatives). From this review, several key conclusions were drawn with respect to what features are necessary in the development and implementation of a reference tool aimed at promoting green chemistry. The most challenging aspect of a green chemistry initiative is the establishment of a defined metric system for the assessment of chemicals and processes so that a preferred alternative can be selected. The criteria that form the basis of this metric system must be specific to industry use, but should also have some consistency between multiple industries.
Many of the programs that were reviewed focused on the assessment of safer alternatives based on human and environmental health. For example, the comparison of chemicals based on some definition of persistence, bioaccumulative potential and toxicity was common to almost every program reviewed. While most of the programs neglected to evaluate other crucial aspects such as economic and technical feasibility and social impacts, there were some assessment tools and frameworks that did recognize the importance of economics and technical requirements. For example, the Lowell Center for Sustainable Production has published an Alternative Assessment Framework (Rossi et al. 2006) that comparatively assesses chemicals, materials or products based on their human health and environmental effects, social justice, economic feasibility and technical performance. Although the U.S. EPA DfE Safer Product Labeling Program only considers the hazards of a product, the DfE has also developed the Cleaner Technologies Substitutes Assessment (CTSA) Methodology which evaluates the comparative human health and environmental risks, competitiveness (e.g., performance, cost) and resource conservation of traditional and alternative chemicals, manufacturing methods and technologies (DfE 1996). This methodology has been updated recently and renamed to the Alternatives Assessment (AA) Methodology (Lavoie et al. 2010). McDonough Braungart Design Chemistry (MBDC) has created their own Cradle to Cradle® Certification program which recognizes companies that choose chemicals, materials or processes for health and perpetual recyclability and social responsibility (MBDC 2010).
All of the programs reviewed in detail in the Jurisdictional Review of Safer Chemical Alternatives have aspects that are relevant to green chemistry and safer alternatives; however, the Lowell Center Alternatives Assessment Framework (Rossi et al. 2006) seems to be one of the most comprehensive, and therefore serves as the basis for this reference tool. Information and concepts from other programs, such as the U.S. EPA DfE CTSA Methodology (DfE 1996) and AA Methodology (Lavoie et al. 2010), the Alternatives Assessment Process Guidance provided by the Toxics Use Reduction Institute (TURI 2006a) and MBDC’s Cradle to Cradle® program (MBDC 2010) have also been incorporated into the development of this reference tool.
2.0 Evaluating Safer Chemical Alternatives
Purpose: This section provides an overview of the evaluation of safer chemical alternatives.
If you are a small/medium company… It is important to be aware of all components of the evaluation, although not all components may be relevant to your company.
If you are a large company… This framework provides the major components of an evaluation of safer chemical alternatives.
Chemicals, materials and products are terms which cannot be entirely separated from one another. In industry, chemicals (raw materials, by products, end-products) can be sold directly as products, but they can also be used to make materials, which can in turn be sold as is or used in the fabrication of other products which are then sold to consumers. All of these terms play a role in green chemistry, and safer chemical alternatives can be implemented at multiple stages in the production process. The framework presented herein pertains only to the evaluation of safer chemical alternatives; however, a similar framework can also be applied to the evaluation of materials and products. The framework is outlined in Figure 2.1, and the components are discussed in the following sections. It should be noted that the framework provided in this tool is intended to be as general as possible and not be prescriptive so that it can be applied by companies and industries of varying sizes. As seen from the framework (Figure 2.1), there are several stages to the evaluation of a chemical alternative and it is not expected that every aspect of the assessment framework will necessarily be applicable to all manufacturers and companies. For example, some small companies may only be able to accomplish the first part of the assessment (the preliminary assessment) whereas large industries may be able to carry out a complete detailed assessment including a life cycle analysis. The information provided in this reference tool may be explicit in some instances; however, in many cases, the information that is presented serves only to provide suggestions for factors to consider in the assessment. Examples have been provided whenever possible.
Additionally, the guidance provided in this reference tool is intended to be applicable to all industries in Ontario, and not just for those industries subject to reporting under the Toxics Reduction Act, 2009 (TRA, Ontario Regulation 455/09). Evaluation steps within this reference tool that overlap with TRA reporting requirements are highlighted as appropriate. Reporting requirements under the TRA can be found on the Service Ontario e-laws home page.
Figure 2.1 Framework for Assessing Safer Chemical Alternatives
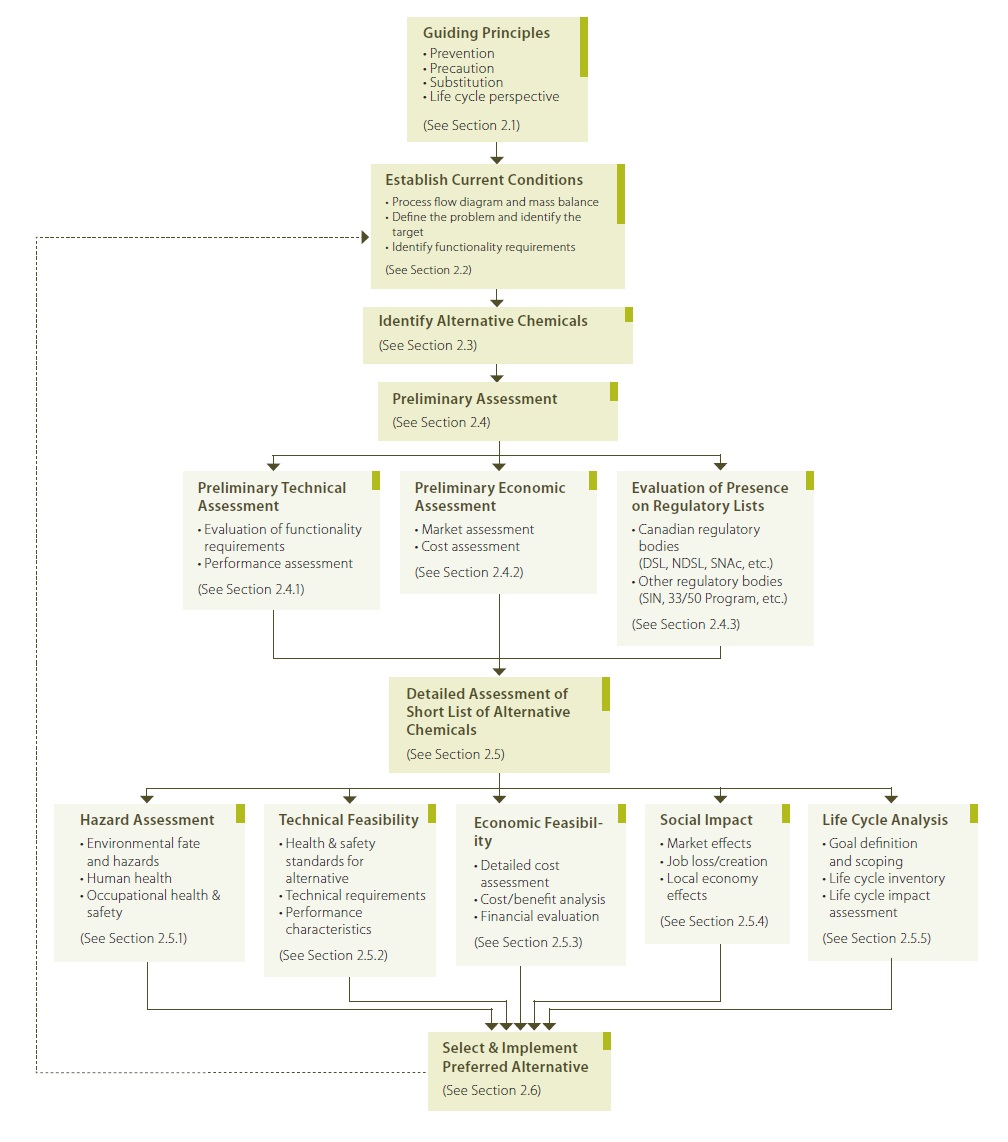
Note: Adapted from Rossi et al. (2006)
2.1 Guiding Principles
It is recognized that there are no regulations in Ontario related to alternatives assessment; thus, any alternatives assessment in Ontario will be voluntary. This reference tool has been developed with this in mind and is based on a set of guiding principles to help direct the decision-making process. Although implementation of safer chemicals is voluntary in Ontario, it should be highlighted that there is a worldwide movement in this direction and therefore companies and industries in Ontario that consider alternatives assessments would be better placed to implement programs related to green chemistry and alternatives assessment in the future.
In developing this reference tool, a number of guiding principles were considered including prevention, precaution, substitution and life cycle perspective. In addition, an alternatives assessment can be complex and resource intensive, especially when a large number of alternative chemicals are available. As such, preliminary technical and economic analyses should be conducted in an effort to pare down a potentially long list of alternative chemicals by removing those that may, for example, be prohibitively expensive or which may not offer the required functionality. This step may be the only step completed by smaller companies with limited resources. Those alternative chemicals which appear feasible would then be carried forward to a more detailed assessment. A major part of the detailed assessment examines the potential hazards of the alternative chemicals (Section 2.5.1) since an alternative should be selected to reduce, avoid or eliminate the use of hazardous chemicals in order to result in a reduction of risk to the health of workers, the general public and the environment. In addition, an alternative chemical must undergo detailed technical and economic feasibility analyses (Sections 2.5.2 and 2.5.3), a social impact analysis (Section 2.5.4), and show that overall it is an improvement over the current chemical over the entire life cycle (Section 2.5.5). A qualitative or quantitative life cycle analysis can provide a broad consideration of the environmental, economic and social aspects across the life cycle of the product.
2.2 Establish Current Conditions
Purpose: This section provides information onestablishing current use and function conditions for the alternatives assessment.
If you are a small/medium company… This may be the only component of your evaluation.
If you are a large company… This is an important component of your evaluation.
Before an alternatives assessment can be conducted, the process should be examined to identify whether a safer chemical alternative may be required or beneficial. Therefore, a good starting point is the development of a process flow diagram and mass balance, which can help to identify the problem and target chemical, as well as any functionality requirements of that chemical.
2.2.1 Process Flow Diagram
A process flow diagram is useful in identifying the target chemical for the alternatives assessment, and also provides information for the other steps in the assessment such as the economic feasibility analysis (Section 2.5.3).
A process flow diagram can have varying levels of detail. In its simplest form, it can show the flows into and out of a production process (i.e., raw material inputs, products, and non-product output streams), while more detailed diagrams may include piping information, operating conditions, etc. It may be useful1 to prepare a more detailed flow diagram by including a mass balance (i.e., labelling all process streams with flow rates of all chemicals) (NJDEP 2002). At the simplest level the assessor can determine the total periodic (e.g., annual) mass or volume inputs and outputs of a chemical (i.e., as a raw material, and as waste) at the facility.
Process flow diagrams should be prepared for every process in each stage of the manufacturing cycle2. This helps to ensure that the assessor fully understands the process(es), and serves to highlight processes or process streams which are problematic and which may benefit from an alternative assessment (NJDEP 2002). Even if a problematic process or process stream has already been identified, the process flow diagram still represents a useful tool in the assessment. By fully understanding the process and identifying all operating conditions and chemicals used, opportunities for improvement will be easier to recognize.
Although process flow diagrams can add value to the assessment, and the more detail the better, it is recognized that a detailed flow diagram of every production process may not be feasible for all companies due to resource constraints3. In this case, the process flow diagram can focus on the production process of interest, showing only the flows into and out of that process.
If desired, emissions can be estimated at a higher level of detail. Under the National Pollutant Release Inventory (NPRI) reporting, facilities are required to estimate releases, disposals and transfers of NPRI substances. If the current chemical is an NPRI substance, then the emitted mass of the current chemical should be easy to determine from NPRI reporting. The same methodology can be applied on a more simplistic level to the alternatives or current chemicals which are not NPRI substances. Environment Canada has an online resource for General NPRI Tools, including equations and conversion factors, example calculations and a listing of software which may be useful when estimating releases.
More information on the basic concepts of process flow diagrams can be found in many engineering textbooks (e.g., Himmelblau 1990, Luyben and Wenzel 1988). The Synthetic Methodology Assessment for Reduction Techniques (SMART) module of the U.S. EPA’s Green Chemistry Expert System (GCES) may be of use when developing the mass balance. Information on the batches per year, reaction yield, reagents and products is entered into the module and the module calculates the amounts of wastes generated. It also categorizes the wastes according to EPA’s level of concern. The results are summarized in a report which outlines the overall level of concern for the process, identifies the waste sources of most concern, and also suggests other modules within the GCES which may help in identifying possible alternatives. The GCES is available for free via download from the U.S. EPA.
More information on chemical manufacturing process and product formulation can be found in the Hazardous Substance Data Bank (HSDB), the Kirk-Othmer Encyclopedia of Chemical Technology, or Ullmann’s Encyclopedia of Industrial Chemistry (information obtained from DfE 1996).
2.2.2 Define the Problem and Identify the Target
From the process flow diagram that was developed in the previous step, the potential benefits of conducting an alternatives assessment should become clear. An assessment may be advantageous if any of the following apply:
- A chemical in one or more processes is going to be subject to new or stricter regulations;
- A chemical in one or more processes requires separate, costly disposal options (e.g., hazardous waste);
- There is high potential for environmental or human exposure to a hazardous chemical used or produced in the process;
- A chemical in one or more processes is on an existing list of chemicals of concern (e.g., priority list, see Section 2.4.3);
- A particular process requires a hazardous substance for cleaning; or
- A chemical in one or more processes is a non-renewable feedstock.
This list is by no means exhaustive. There may be any number of other reasons to conduct an alternatives assessment, and this list is intended merely to serve as a starting point to help assessors determine chemicals in their processes for which an alternatives assessment may be warranted. An alternatives assessment may also be warranted if concerns have been initiated by industry or consumer requests and by policy or regulatory drivers (Lavoie et al. 2010).
Once the chemical to be evaluated is identified, the key issue(s) with this chemical needs to be defined. For example, is it being evaluated because it is persistent or bioaccumulative? Are purchasing and/or disposal costs significantly higher for this chemical than other chemicals in the process? This information will help in the evaluation of the alternative(s) since the selection of a preferred alternative will address the identified issue(s).
2.2.3 Identify Functionality Requirements
The assessor may also need to determine whether there are any functionality requirements that the chemical, and hence any alternative, must fulfill. This is generally determined by the function and use of the chemical. A good starting point for identifying functionality requirements is to describe the role of the chemical in the process and define all operating conditions (e.g., temperature, pressure, etc.). Are there ignition sources directly near the process area so that highly volatile chemicals must be avoided? Does the end product require that all raw materials be water soluble? Some examples of chemical properties that may influence the functionality of a chemical include:
- Ability to withstand extreme reaction conditions — is the process temperature- or pressure-sensitive? For example, polymerization reactions generally occur at temperatures of approximately 50°C to 60°C and atmospheric pressure to prevent evaporation of organic solvents, while ammonia synthesis occurs at a higher temperature of between 300°C and 550°C at a pressure of approximately 150 to 250 atmospheres.
- Low volatility — does the process require the raw materials to be non-volatile at the reaction temperature? A higher vapour pressure of a liquid at a given temperature signifies a higher volatility, meaning that the liquid will readily evaporate. For example, the vapour pressures of propane and water at 20°C are 16,500 mm Hg and 17.5 mm Hg, respectively, indicating that propane is more volatile and will therefore evaporate at a lower temperature than water.
- Physical state — does the process require any or all of the raw materials to be in solid, liquid or gaseous form?
- Viscosity — does the process require the raw materials to be of low viscosity (‘thickness’)? For example, castor oil is a thicker fluid with a viscosity of approximately 0.985 Pa·s (Pascal second) at 25°C, while water is a thinner fluid with a viscosity of approximately 0.0009 Pa·s at the same temperature.
Once the functionality requirements are identified, the physical and chemical properties that an alternative must possess should become clear. This information will then be used for both the preliminary (Section 2.4.1) and the detailed (Section 2.5.2) technical evaluations of the identified alternative(s).
2.3 Identify Alternatives
Purpose: This section provides information on identifying possible chemical alternatives for the alternatives assessment.
If you are a small/medium company… This is a key component of your evaluation.
If you are a large company… This is a key component of your evaluation.
Once the chemical and its associated process are identified and described, the next step in the assessment is the identification of any alternatives that exist for the chemical, paying particular attention to those that are designed for that particular use.
The framework presented in this reference tool focuses on the replacement of a chemical with a safer alternative (i.e., a drop-in replacement); however, it should be noted that the term ‘alternative’ does not only refer to a drop-in replacement, but it may also refer to a process or product change that would eliminate the function or need for the chemical in the first place as per Principle 5 of the 12 Principles of Green Chemistry (Section 1.2). For example, TURI conducted an alternatives assessment study of five chemicals in which it identified UV sterilization as a safer process alternative to the use of para-formaldehyde as a dry sterilant (TURI 2006b).
Alternatives can be identified from other sources, or from brainstorming based on a detailed understanding of the function of the chemical that has been developed from the previous steps. Alternatives can include those that are already available, as well as those that may be on the horizon. It is highly recommended that if a chemical or process is common across an industry sector, that the individual industries in the sector may combine their resources in the identification and assessment of safer alternative chemicals.
Sources that may have a list of possible alternatives include the following:
- U.S. EPA Green Chemical Expert System (GCES) — available for free download.
- Green Synthetic Reactions Module — a searchable database that provides details on alternative techniques that have been used successfully to design green reactions.
- Designing Safer Chemicals Module — through examples or through information based on chemical class (e.g., polyesters), chemical characteristics (e.g., persistence) or chemical use (e.g., surfactants), this module provides guidance on how safer chemical substances can be designed. It should be noted that not all of the features were fully operational at the time this tool was prepared.
- Green Solvents/Reactions Conditions — provides information on greener solvent systems and is also a searchable database that can provide suggestions for replacement solvents based on physiochemical properties.
- U.S. EPA Sustainable Futures Initiative (SF) — Analog Identification Methodology (AIM).
- Although the intended purpose of AIM is to estimate potential hazards (toxicity) of chemicals that have not yet been experimentally tested, it can also be used to identify chemical analogs (chemicals of similar structure) which may behave similarly but with different hazardous properties.
- Green Chemical Alternatives Purchasing Wizard
- A publicly available tool aimed at reducing hazardous waste by replacing hazardous chemicals with greener substitutes. Greener chemicals can be identified by searching by the chemical or process that needs replacing or by known alternative chemicals or processes.
- CleanGredients® — accessible through subscription
- An online database aimed at promoting the flow of information between chemical suppliers and chemical product formulators, by providing technical information on the chemicals used in various cleaning products to enable identification of chemicals with potential environmental and human health benefits.
- Toxics Use Reduction Institute (TURI) — Five Chemicals Alternatives Assessment Study (TURI 2006b)
- A thorough assessment of possible alternatives for five chemicals (lead, formaldehyde, perchloroethylene (PCE), hexavalent chromium, di(2-ethylhexyl) phthalate (DEHP)) for a total of 16 different use categories including manufacturing, consumer products, and other applications.
This list is not exhaustive and serves only as a starting point for a thorough search for alternative chemicals. Identified alternatives are carried forward in the next steps of the evaluation.
2.4 Preliminary Assessment
Purpose: This section provides information on a preliminary assessment for the alternatives assessment by identifying any alternative chemicals that may be a potential concern and therefore, not ideal as a safer chemical alternative.
If you are a small/medium company… This may be the final step; however, further analysis is recommended if possible in your assessment.
If you are a large company… This step has the potential to remove unfavourable alternatives from your evaluation and reduce the scope of your evaluation.
As discussed previously, alternatives assessment can be a time and labour intensive exercise. While a larger industry may have the resources to do a full assessment of all alternatives, it is recognized that smaller companies may not. As such, this preliminary assessment can serve to conserve resources by paring down the list of alternative chemicals identified in Section 2.3, eliminating chemicals that are not economically or technically feasible or that may be of potential concern based on their presence on one or more priority lists. This may also be the final step of an assessment for a smaller company without the resources to complete a full assessment.
2.4.1 Preliminary Technical Assessment
As discussed in Section 2.2.3, there may be several functionality requirements that an alternative chemical must meet in order to be a suitable replacement for the current chemical. The preliminary technical assessment evaluates the alternative chemicals on the basis of their fulfillment of these requirements. Functionality requirements for alternative chemicals may include properties such as density, water solubility, colour, boiling point/melting point, odour, and vapour pressure. Information on these properties can generally be found on a chemical’s Material Safety Data Sheet (MSDS). As an example, if an end product requires that the raw materials are water soluble and an alternative chemical is not water soluble, then the chemical is not technically feasible for that end product and therefore it would not be logical to carry this alternative chemical through the detailed assessment.
Other considerations can include performance characteristics such as durability or longevity of the end product, maintenance requirements, energy consumption, etc. Table 2.1 provides an example of some physical properties that may be important in the technical assessment of alternatives to the use of pentabromodiphenyl ether (PentaBDE) as a flame retardant. Physical properties for both the current chemical (PentaBDE) as well as three identified alternative chemicals are provided in the table (based on information obtained from the U.S. EPA (2005)). As seen from this table, data can be found for some of the physical properties from literature sources; however, some data are not available and can be estimated from structural relationships as described in this reference tool (Section 2.5.1). This example also demonstrates how an alternatives assessment can benefit from consulting scientific experts.
Of note, facilities subject to the Toxics Reduction Act, 2009 (TRA) are required to consider technical feasibility to reduce their use and creation of prescribed toxic substances.4
2.4.2 Preliminary Economic Assessment
Many factors must be considered in an alternative assessment; however, one of the most important factors to consider is that the alternative should yield a profitable end product. As will be discussed in Section 2.5.3, there are many components to an economic analysis including a detailed cost assessment, cost/benefit analysis and financial evaluation.
A market assessment can be considered as part of this preliminary economic assessment and compiles price information for chemicals for use in the cost assessment and identifies trends in the manufacturing and use of the chemicals for consideration in the future supply and demand of the chemicals (DfE 1996). Availability of the alternative chemical should also be considered. Will it be relatively easy to obtain the alternative chemical, and is it available locally or only from suppliers that are great distances away? Are there multiple suppliers so that if one supplier shuts down there are other options for obtaining the chemical?
Some consideration of the international market is also important, such as taxes or tariffs on chemicals imported from foreign suppliers and any international trade regulations or agreements (e.g., General Agreement on Tariffs and Trade [GATT] and the North American Free Trade Agreement [NAFTA]). Additionally, it should be determined if any of the alternatives fall under a chemical or category that might affect the ability of an industry to market the product (either positively or negatively). For example, one of the alternative chemicals may be a restricted substance under a jurisdiction, thereby precluding its sale in that jurisdiction.
In terms of the preliminary cost assessment, at the most basic level, this can compare the direct and indirect costs of the current chemical and its alternatives. In general, the raw material costs play a large role in determining the profitability of the product. If no major process change is expected as a result of implementation of an alternative chemical, then to be profitable, the raw material cost for the alternative chemical should be similar to that for the current chemical. Therefore, in the preliminary assessment, a search can be conducted of various chemical suppliers to determine approximate raw material costs for the alternative chemicals. Of note, facilities subject to the Toxics Reduction Act, 2009 (TRA) are required to consider economic feasibility to reduce their use and creation of a prescribed toxic substance.5
As an example, in the TURI Five Chemicals Alternative Assessment Study (TURI 2006b) one of the main considerations for the alternative selection for DEHP in resilient floor covering was plasticizer cost. The flooring market is so competitive that even a small change in product cost could make the product unattractive to customers. Therefore, for the DEHP alternatives in resilient floor covering it was concluded that the plasticizer cost must not be more than 10 per cent greater than DEHP on a processed per pound basis. From Table 2.2, it can be seen that only two of the six alternative chemicals meet the 10 per cent cost requirement and therefore the other four alternative chemicals would be dropped from a more detailed assessment.
| Factor | Current: PentaBDE | Alternative Chemicals: Triphenyl Phosphate | Alternative Chemicals: Tribromopentyl Alcohol | Alternative Chemicals: Tris(1,3-dichloro-2-propyl) Phosphate |
|---|---|---|---|---|
| Physical Form | Solid | Solid | Solid | Liquid |
| Melting Point (°C) | -5 (E) | 50.5 | 62-67 | -58 |
| Boiling Point (°C) | 436 (E) | 389 (E) | 760 (E) | > 200 |
| Vapour Pressure (mm Hg) | 3.1 x 10-8 (E) | 6.3 x 10-6 | 6.2 x 10-5 (E) | < 10-6 (E) |
| Water Solubility (g/L) | 9.0 x 10-4 (E) | 1.9 x 10-3 | 2 | 0.042 |
| Log Kow | 6.84 (E) | 4.59 | 2.6 | 2.4 |
Note: Data for alternative chemicals as cited in U.S. EPA (2005); data for PentaBDE estimated using U.S. EPA PBT Profiler (2006a); E indicates data are estimated.
| Plasticizer | Raw Material ($/lb) | Adjustment Factor | Adjusted Cost (Processed $/lb) | Less than 10 per cent higher than DEHP? |
|---|---|---|---|---|
| DEHP (current) | 0.70 | 1 | 0.70 | - |
| Di(2-ethylhexyl) adipate | 0.74 | 0.94 | 0.70 | Yes |
| Di(2-ethylhexyl) phosphate | 2-3 | 1 | 2-3 | No |
| Di(isononyl) phthalate | 0.73 | 1.06 | 0.77 | Yes |
| Di(isononyl) phthalate | 0.77 | 1.1 | 0.85 | No |
| Trioctyl trimellitate | 0.95 | 1.17 | 1.11 | No |
| Trioctyl trimellitate | 2.10 | 1 | 2.10 | No |
Note: Data obtained from TURI (2006b).
2.4.3 Evaluation of Presence on Regulatory Lists
There are a number of chemicals that are present on one or more regulatory lists as a result of high use, production rates, emissions rates, etc. within the jurisdiction which publishes the list, or as a result of hazardous properties or high potential for exposure. The chemicals that have been identified by the organizations and programs discussed below may be considered among the more hazardous and/or high use/release substances and may currently be subject to some legislative control, or may be subject to it in the future.
If an alternative chemical is on one or more of these lists it does not mean that it necessarily should be dropped from further assessment since it may, for example, still be less toxic than the current chemical used in the process. Furthermore, the alternative chemical may be used in smaller quantities such that the exposure is lower (see Section 2.5.5.3) and thus may be considered as an alternative.
The following paragraphs discuss some of the regulatory lists that should be consulted.
Part 5 of the Canadian Environmental Protection Act, 1999 (CEPA 1999) focuses on understanding and reducing the risks posed by new and existing substances. As part of the Act, various substance inventories and lists have been established, including the following:
- Domestic Substances List (DSL) — The DSL is an inventory of approximately 23,000substances that, between January 1, 1984 and December 31, 1986, were manufactured in, imported into or used in Canada on a commercial scale (in a quantity of or exceeding 100 kg per calendar year). All substances not on this list, with a few exceptions, are considered to be ‘new’ and are subject to notification which includes an assessment of toxicity. The list is amended regularly to include additional substances that have been deemed eligible following their assessment under the new substances notification and assessment regime. A searchable database is available online.
- Non-Domestic Substances List (NDSL) — The NDSL is an inventory of more than 58,000 substances based on the U.S. EPA’s Toxic Substances Control Act (TSCA) Chemical Substances Inventory for 1985. The list is updated annually to add or delete substances incorporated into, or removed from, the TSCA inventory. Substances not on the DSL but listed on the NDSL are subject to lesser information requirements. A searchable database is available online.
- Priority Substances List (PSL) — This is a list of substances that need to be assessed on a priority basis to determine whether they are ‘toxic’ (as defined under Section 64 of CEPA 1999) and pose a risk to the health of Canadians or to the environment. A total of 44 substances are included in the first list (PSL1), while 25 substances are included in the second list (PSL2). A substance must be assessed within five years of addition to the list. The PSL substances are listed at the PSL website.
- Toxic Substances List (Schedule 1 of CEPA 1999) — Substances are recommended for addition to this list if they are found to be ‘toxic’ or capable of becoming ‘toxic’ (as defined under Section 64 of CEPA 1999) after having undergone a PSL assessment, a screening assessment, or the review of a decision by another jurisdiction. This list is available online at the Toxic Substances List webpage.
- Virtual Elimination (VE) List — This is a newer list developed as part of the Government of Canada’s Chemical Management Plan (CMP) and includes substances that are designated for virtual elimination. The compilation of this list is mandated under Section 65 of CEPA 1999. The level of quantification is set for each substance on this list and the quantity or concentration of the substance that may be released into the environment either alone or in combination with any other substances from any source or type of source is prescribed. Hexachlorobutadiene and perfluoroctane sulfonate (and its salts) are the only substances currently on this list.
- Non-Statutory List — This is a list of substances that have been found to meet at least one of the criteria for ‘toxic’ as set out in Section 64 of CEPA 1999 but that have not been added to Schedule 1 of the Act (i.e., the Toxic Substances List). These substances have effective risk management measures in place under other provincial and territorial programs or federal acts; however, these substances may be upgraded to Schedule 1 if the Government of Canada finds that those existing preventive and/or control actions are not effective. This list is available online at the Non-Statutory List webpage.
Any chemical on these lists can be determined by using the Search Engine for Chemicals and Polymers.
As mentioned above, the Government of Canada has recently introduced the Chemical Management Plan (CMP), which includes a number of new, proactive measures to make sure that chemical substances are managed properly in an effort to improve the degree of protection against hazardous chemicals. As part of the plan, the Government of Canada has begun issuing Significant New Activity (SNAc) requirements under subsection 81(3) of CEPA 1999 for approximately 300 highly hazardous substances that have been or currently are used in Canada (i.e., are on the DSL). Data must be submitted to Environment Canada and Health Canada for review before re-introduction or increased use of these substances in quantities of greater than 100 kg per year. Substances on the DSL that are subject to SNAc requirements can be determined with the Search Engine for Chemicals and Polymers. Alternatively, a listing of the 145 substances that are currently on the SNAc list can be found online.
As part of the MOE’s Toxics Reduction Act, 2009 (TRA) and the Toxics Reduction Program to reduce the use and creation of toxic substances, a total of approximately 350 substances on NPRI and acetone (Ontario Regulation 127/01) are prescribed. From this list, various review processes have resulted in the prioritization of 47 substances and substance groups (Phase 1) while the remainder form part of Phase 2 implementation. A complete listing of Toxic Substances and Substances of Concern is available electronically.
In other jurisdictions, chemicals on regulatory lists have generally been identified as chemicals that are persistent, bioaccumulative or toxic (i.e., PBTs). For example, the Substitute It Now! (SIN) list is a listing of 356 chemicals that have been identified as substances of very high concern based on criteria established by the European Union REACH program (Registration, Evaluation, Authorization and Restriction of Chemical Substances). The SIN list can be accessed from the website of the International Chemical Secretariat. The Norwegian Pollution Control Authority has also developed criteria for health and environmental hazards and, based on these criteria, have produced three lists: (1) the List of Dangerous Substances; (2) the Priority List; and (3) the Observation List (online listing available). TURI (2006b) has completed a detailed alternatives assessment study on five chemicals that have been identified as hazardous or toxic. These include lead, formaldehyde, perchloroethylene (tertrachloroethylene [PCE]), hexavalent chromium, and di(2-ethylhexyl)phthalate (DEHP). In 1988, the U.S. EPA organized a voluntary pollution prevention program, the 33/50 program, which targeted 17 chemicals for which a 33 per cent reduction in releases and transfers of these chemicals to the environment should be achieved by 1992, and 50 per cent by 1995, measured against a 1988 baseline. These chemicals included (U.S. EPA 1999):
- benzene
- carbon tetrachloride
- chloroform
- dichloromethane
- methyl ethyl ketone
- methyl isobutyl ketone
- tetrachloroethylene
- toluene
- 1,1,1-trichloroethane
- trichloroethylene
- xylenes
- cadmium and cadmium compounds
- chromium and chromium compounds
- cyanide compounds
- lead and lead compounds
- mercury and mercury compounds
- nickel and nickel compounds
In summary, the selection of an alternative chemical from one of these lists serves only to caution the assessor that there may be issues or implications associated with the use of the substance such as stricter or more cumbersome reporting requirements. For example, if an alternative chemical is identified that is not on the DSL, then the alternative chemical would be considered ‘new’ to Canada and would be subject to the New Substances Notification Regulations (NSNR), more information available online.
Alternatively, if an alternative chemical is on the DSL, the assessor should check to see if the alternative is considered ‘toxic’ or is capable of becoming ‘toxic’ (i.e., is listed on Schedule 1 under CEPA 1999 [List of Toxic Substances]. It should be noted that the lists presented here are not exhaustive and high priority substances may also be identified elsewhere.
2.5 Detailed Assessment of Short List of Alternative Chemicals
Purpose: This section provides information on a detailed assessment for alternative chemicals.
If you are a small/medium company… This may be outside the scope of your assessment; an awareness of the detailed assessment would be useful for future chemical considerations, purchasing or for discussions with chemical suppliers, for example.
If you are a large company… This is the main component of your evaluation; all aspects should be considered, although the depth and level of detail will depend on your individual requirements and resources.
As discussed in the previous section, only those alternative chemicals that are determined to be economically and technically feasible based on preliminary analyses are carried forward into this more detailed assessment. As indicated previously, some small and medium size enterprises may not be able to progress to this step of the alternatives process and may instead end the assessment after the preliminary assessment (Section 2.4). In addition, the preliminary assessment may not identify any viable or reasonable chemical alternatives that are currently available. In such a case, steps could be taken with chemical suppliers or formulators to develop alternatives for the future or process changes could also be considered. Process changes are not discussed in this reference tool. The alternatives assessment is a continuous process and industries should always be examining their chemicals and processes.
The following sections outline the information that is useful for comparing the current chemical to potential alternative chemicals on the basis of their environmental fate and hazards, technical feasibility, economic feasibility, social impacts and life cycle analysis to aid in the selection of a preferred safer chemical alternative in Section 2.6. The information provided in the following sections allows for a broad evaluation of the alternative chemicals, providing a company or institution with the ability to select an alternative that is consistent with their environmental, technical economic and social priorities. It should be noted that the evaluation of environmental fate and hazards (including toxicity) forms part of a chemical risk assessment process that could potentially be used in an alternatives assessment. Caution needs to be applied when considering a risk assessment process for the evaluation of chemical alternatives as it is a highly technical process and involves implicit judgement of what is a ‘safe’ level of risk. The scoring matrices provided in this tool are a simpler way of evaluating some of the fundamental steps in a risk assessment process.
2.5.1 Hazard Assessment
Purpose: This section provides information on particular components of a hazard assessment.
If you are a small/medium company… Information provided on a hazard assessment can be used for discussions with chemical suppliers to encourage the development of chemicals/products with less hazardous properties or ingredients.
If you are a large company… The evaluation of chemical hazards outlined in the hazard assessment should be completed by or include input from a scientist or others trained in the evaluation of chemical hazards and toxicity.
The hazard assessment involves the collection of data on environmental fate and hazards, human health hazards, and occupational health and safety for the current chemical used in the process and all identified alternative chemicals.
As per the 12 Principles of Green Chemistry (Section 1.2), a safer chemical alternative should possess little to no toxicity to human health and the environment, and should break down into innocuous degradation products. As mentioned previously, priority lists are often developed based on the persistence, bioaccumulative potential and toxicity of chemicals (PBTs); as such, these attributes are part of the Hazard Assessment. The criteria for PBTs adopted by the various jurisdictions and programs that were evaluated in the Jurisdictional Review of Safer Chemical Alternatives are summarized in Appendix A.
These criteria for PBTs have been examined and values that are considered to be best suited for application in the Ontario context have been selected for the evaluation of PBT in this reference tool. The selection is based on a review of all values, professional judgement and in keeping with current regulations and guidelines for Canada and Ontario. For example, the DSL provides ‘cut-off’ values for persistence (i.e., half life) and bioaccumulation (i.e., bioconcentration factor (BCF), bioaccumulation factor (BAF), log octanol-water partition coefficient (log Kow). If these values are exceeded then that substance is classified as persistent and/or bioaccumulative. It should be noted, however, that other jurisdictions and programs such as the DfE’s Safer Products Labeling Program have set more stringent cut-off values. For example, the cut-off value for the half-life of a chemical in water is six months for the DSL but only 60 days for other jurisdictions; as such, values from other jurisdictions have also been provided as reference in order to assess a potential chemical alternative.
Well-defined metrics need to be developed in order to aid in the evaluation and interpretation of the hazards of a chemical. Various ranking methodologies have been developed by the programs that were reviewed in the Jurisdictional Review of Safer Chemical Alternatives. Examples include, but are not limited to, the following:
- Clean Production Action Green Screen for Safer Chemicals (Rossi and Heine 2007)
- In the Green Screen, a chemical is evaluated by comparison to a series of four benchmarks, with each benchmark defining a progressively safer chemical. Similar to other programs, the criteria used in this program are persistence, bioaccumulation and toxicity. The levels of concern are defined by threshold values that are quantitative, qualitative, or based on expert references and aim to harmonize existing hazard classification and labelling systems.
- TURI Five Chemicals Alternative Assessment Study (TURI 2006b)
- Alternatives that passed screening based on health and environmental effects and PBT were prioritized for further study by assessment of chemical- and application-specific criteria in the categories of technical performance, health and environmental effects, economic considerations, and importance to stakeholders. Each alternative was evaluated against the reference (current) chemical by assigning a ‘+’, ‘−’ or ‘=’ to each criterion (better than, worse than or equal to the reference). No conclusions were drawn as to which alternative was preferred.
- TURI Alternatives Assessment Process Guidance (TURI 2006a)
- This document defines a consistent process for setting priorities for study and evaluating the alternatives for the five chemicals evaluated by TURI (2006b). Metrics are provided to evaluate the feasibility of each alternative based on technical, financial, environmental hazards and human health and safety criteria. This document also includes suggestions for specific types of resources for certain phases of the study.
- The Scoring and Ranking Assessment Model (SCRAM) (Snyder et al. 2000a, b, c and d)
- SCRAM was developed as a risk-based screening tool to provide relative rankings of 140 chemicals based on persistence, bioaccumulation and toxicity. Additionally, the model provides a score based on the uncertainty of the information available for each of these categories.
- The MOE categorization procedure of Phase I and Phase II substances (MOE 2009a)
- This document provides a ranking procedure for identifying toxic substances and substances of concern. Hazard scoring information was obtained from RSEI (Risk-Screening Environmental Indicators) and SCRAM (the Scoring and Ranking Assessment Model). Scores from RSEI and SCRAM were converted to rankings. The relevance of the chemical to MOE program areas, and also other programs and agencies, was a consideration.
- The U.S. EPA Design for the Environment (DfE) Cleaner Technologies Substitutes Assessment (CTSA) Methodology (DfE 1996)
- The CTSA methodology provides a way of evaluating the comparative human health and environmental risks, competitiveness (e.g., performance, cost) and resource conservation of traditional and alternative chemicals manufacturing methods and technologies using a systematic methodology. Considerations in the methodology include: environmental fate, human health, environmental hazards, exposure assessment and workplace risk, performance assessment, and energy impacts, among other things.
- TURI Pollution Prevention Options Analysis System (P2OASys) (TURI, unknown year)
- The P2OASys scores alternative chemicals according to an established metric system. Users also input a score for the certainty of the data to reflect the confidence in the value, which is then accounted for in the final scoring. The score for each criterion is computed and a final score for a chemical or process is calculated to incorporate the certainty of the data. Considerations include: human effects, hazards (physical, aquatic, atmospheric, disposal, chemical, product), persistence and bioaccumulation, exposure potential, and energy and resource use.
- MBDC Cradle to Cradle® design framework (MBDC 2010)
- The materials and manufacturing practices of a product are assessed in five categories: Material Health, Material Reutilization, Renewable Energy Use, Water Stewardship, and Social Responsibility. Material health includes the evaluation of every chemical or material for its health and safety for humans and the environment against 19 criteria. Criteria include carcinogenicity, toxicity, persistence and bioaccumulation, among other things. A Material Reutilization Score is calculated for a material based on the recyclable, reusable, and compostable content. Energy use and water use are also considered, along with labour practices.
- The Australian Government Criteria for Classifying Hazardous Substances (NOHSC 2004)
- This document provides guidance on the classification of carcinogenicity, mutagenicity and reproductive toxicology. Criteria are also provided for health effects and determining whether a substance is hazardous based on its ecotoxicological and physicochemical properties.
Based on a review of the above-mentioned metrics, a ranking system has been developed for the hazard assessment in this reference tool and is provided in Table 2.3 through to Table 2.7. The outcome of this step is a matrix of shaded cells (i.e., high (dark green), moderate (medium green) or low (light green) concern)) for both the current chemical and the various alternative chemicals. In most cases, the comparison is relative to set criteria that have been determined to be appropriate for the given categories; however, for a few of the categories in the occupational exposure evaluation, the comparison is relative to the current chemical used in the process. In general, if no value for a parameter is available in the literature or it cannot be estimated based on empirical or other relationships, then the chemical should receive a moderate risk score for that parameter (i.e., medium green). If a range of values are reported, then the value used in the matrix should be the most conservative of the individual values. The factors to consider in this part of the assessment are discussed below. It must be noted that ‘low concern’ (i.e., light green) does not imply that the chemical is ‘safe’.
Interpretation of the matrix is a complex step when determining whether a chemical is a reasonable alternative since a number of the factors are inter-related and often data may not be available for multiple categories. It may at first seem logical to drop a chemical from further assessment based solely on the fact that there are numerous dark green cells in the matrix; however, this is not appropriate. For example, a chemical may have a BCF of greater than 1,000 (i.e., a dark green score (high concern)), but a half life (T1/2) in water of only two days (i.e., a light green score (low concern)) and therefore would not be as much of a concern as the shaded matrix would lead one to believe. Adding complication to interpretation is the fact that there is often a lack of toxicological information available. Although estimation tools and analogs can sometimes be used, caution must be used in applying these techniques and may require the input of a scientist with a background in toxicology. Interpretation of the hazards for selecting a preferred alternative may require the use of ranking and weighting factors such as those developed for the TURI P2OASys tool. This is discussed further in Section 2.6.
2.5.1.1 Environmental Fate and Hazards
As discussed above, persistence, bioaccumulation and toxicity are the major factors to be considered in the assessment of environmental fate and hazards. Additionally, there should also be some consideration of the toxicity of the degradation products. Although it is difficult to provide well-defined metrics for degradation products, the general hazards of any potential degradation products can be evaluated from a search of peer-reviewed literature. If there are documented degradation products which are of concern, then the chemical should be scored as being of high concern (i.e., dark green). The factors and criteria that are deemed appropriate for the Ontario context are presented in Table 2.3.
This step in the Hazard Assessment is a data intensive step. Material Safety Data Sheets (MSDS’s) will have some of the information that is pertinent to this screening step but there are also other sources of data. For example, Chapter 5 of the CTSA Methodology developed by the DfE (1996) provides an exhaustive listing of potential sources of environmental fate and toxicity data, and references for estimating environmental fate parameters. Some of the most comprehensive sources for these data include, but are not limited to, the following:
- Hazardous Systems Data Bank (HSDB)
- Provides information on chemical manufacturing processes and product formulations.
- Syracuse Research Board Environmental Fate Data Base (EFDB) (SRC 1994, as cited in DfE 1996)
- Provides information on environmental fate data, microbial toxicity and biodegradation, and physical/chemical properties.
- The U.S. EPA ECOTOXicology Database (ECOTOX) (U.S. EPA 2000)
- Provides toxicity data for ecological species.
- Ecological Soil Screening Levels (Eco-SSLs) documents (U.S. EPA various years)
- Provides soil screening levels for terrestrial plants and animals.
- Handbook of Environmental Degradation Rates (Howard et al. 1991, as cited in DfE 1996) (not available online)
- Provides environmental degradation rates for select chemicals.
If data on environmental fate and/or toxicity are not available for the chemical or identified alternatives, values can often be estimated using various analytical models and quantitative structure-activity relationship (QSAR) models. There are several sources which provide guidance on estimating values for these parameters. Again, the CTSA Methodology (DfE 1996) provides an extensive list or sources, including the following:
- BioByte Inc. Bio-Loom program
- Provides log octanol-water partition coefficient (log Kow) and acid-base dissociation constant (pKa) values, as well as hydrophobic and molecular refractivity parameters.
- Handbook of Chemical Property Estimation Methods (Lyman et al. 1990)
- Provides methods for estimating chemical properties such as density, vapour pressure, solubility, etc.
- U.S. EPA PBT Profiler (U.S. EPA 2006a)
- Predicts physical/chemical properties, bioconcentration factors, environmental fate, carcinogenicity, toxicity to aquatic organisms, worker and general population exposure, and other information for chemicals lacking experimental data.
- The Estimation Programs Interface (EPI) Suite developed jointly by the U.S. EPA’s Office of Pollution Prevention and Toxics (OPPT) and Syracuse Research Corporation (SRC)
- Provides estimations of chemical parameters, such as log octanol-water partition coefficients (log Kow), gas-phase reaction rates, air-water partition coefficients (Henry’s Law constants), melting points, boiling points, vapour pressures, aerobic and anaerobic biodegradability, biodegradation half-life, organic carbon-normalized sorption coefficients for soil and sediment (Koc), water solubility, fish bioconcentration factors, aqueous hydrolysis rate constants, rates of volatilization from rivers and lakes, partitioning of a chemical among air, soil, sediment, and water, and toxicity to fish, aquatic invertebrates and algae.
- U.S. EPA Ecological Structure Activity Relationships (ECOSAR) Class program (U.S. EPA 2009a)
- Estimates the aquatic toxicity of industrial chemicals.
- U.S. EPA Sustainable Futures Initiative [SF] — Analog Identification Methodology (AIM)
- AIM is used to help predict the potential hazards of untested chemicals by comparing the untested chemical to analogs (chemicals with similar structures) for which experimental (measured) data are available in a specific set of publicly available databases. AIM is to be used for neutral organic compounds, and not metals, inorganic or organic salts.
| Factor | Measure/Metric | Concern Level/Evaluation(1) | Source of Data(2) |
|---|---|---|---|
| Persistence (Half-life, T1/2) | T1/2 in water |
|
HSDB, U.S. EPA PBT Profiler |
| Persistence (Half-life, T1/2) | T1/2 in soil/sediment | HSDB, U.S. EPA PBT Profiler | |
| Bioaccumulation | BCF or BAF |
|
HSDB, U.S. EPA PBT Profiler |
| Bioaccumulation | log Kow |
|
DSL HSDB, U.S. EPA PBT Profiler |
| Aquatic Toxicity (fish, invertebrates, algae) Acute | LC50, EC50 or IC50 |
|
HSDB, U.S. EPA PBT Profiler, U.S. EPA ECOTOX |
| Aquatic Toxicity (fish, invertebrates, algae) Chronic | NOEC, LOEC or MATC |
|
HSDB, U.S. EPA PBT Profiler, U.S. EPA ECOTOX |
| Wildlife Toxicity (including mammals, amphibians, reptiles, birds) Acute | ED50 or LD50 |
|
HSDB, U.S. EPA PBT Profiler, U.S. EPA Eco-SSL |
| Wildlife Toxicity (including mammals, amphibians, reptiles, birds) Chronic | Chronic NOAEL or LOAEL |
|
HSDB, U.S. EPA PBT Profiler, U.S. EPA Eco-SSL |
| Degradation Products (HC) | Degradation products of concern |
|
Peer-reviewed literature |
(1) HC - high concern, MC - moderate concern, LC - low concern
(2) Suggested sources; other sources are discussed in main text
(3) DSL criteria; however, other values should also be considered
- T1/2
- Half life
- BCF
- Bioconcentration Factor
- BAF
- Bioaccumulation Factor
- log Kow
- log octanol-water partition coefficient
- EC/D50
- Effect Concentration/Dose affecting 50 per cent of test organisms
- LC/D50
- Lethal Concentration/Dose killing 50 per cent of test organisms
- IC50
- Concentration providing 50 per cent inhibition to a reaction
- NOEC
- No Observed Effect Concentration
- NOAEL
- No Observed Adverse Effect Level
- LOEC
- Lowest Observed Effect Concentration
- LOAEL
- Lowest Observed Adverse Effect Level
- MATC
- Maximum Acceptable Toxicant Concentration
It should be noted that when evaluating toxicity values caution needs to be taken to make sure that the appropriate basis is used for comparison purposes. For example, it may not be straight forward to directly compare LC50s or NOAELs for a substance due to differences in studies such as the length of the study, the species tested, data gaps associated with the studies, etc. It is therefore recommended that a scientist with a background in toxicology should be involved in the assessment of safer chemical alternatives.
Table 2.4 provides an example of an environmental fate and hazards matrix for PentaBDE (a flame retardant) and three alternative chemicals (based on information obtained from the U.S. EPA (2005)). This table again illustrates the fact that a combination of measured data and estimated values using the various estimation tools discussed above are needed to complete a fate and hazards matrix, and that even still there are often remaining data gaps which lead to uncertainty in the assessment.
| Factor | Measure/Metric(1) | Current: PentaBDE | Alternative Chemicals: Triphenyl Phosphate | Alternative Chemicals: Tribromopentyl Alcohol | Alternative Chemicals: Tris (1,3-dichloro-2-propyl) Phosphate |
|---|---|---|---|---|---|
| Persistence | T1/2 in water(days) | 180 (E) (HC) | 38 (E) (MC) | 38 (E) (MC) | 180 (E) (HC) |
| Persistence | T1/2 in soil/ sediment (days) | 360 (soil) 1600 (sediment (E) (HC) |
75 (soil) 340 (sediment) (E) (HC) |
75 (soil) 340 (sediment) (E) (HC) |
360 (soil) 1600 (sediment) (E) (HC) |
| Bioaccumulation | BCF or BAF | 15,000(E) (HC) | 132-1743 (HC) | 10.8(E)(LC) | 3-11 (LC) |
| Bioaccumulation | log Kow | 6.84 (E) (HC) | 4.59 (MC) | 2.6 (LC) | 2.4 (LC) |
| Aquatic Toxicity - Acute | LC50, EC50 or IC50 (mg/L) | ND (MC) | 0.87 (fish) 1.1 (daphnid) 2.0 (algae) (HC) |
32 (fish) 64 (daphnid) 28 (algae) (MC) |
1.9 (fish) 3.8 (daphnid, E) 12 (algae) (MC) |
| Aquatic Toxicity - Chronic | NOEC, LOEC or MATC (mg/L) | 0.00069 mg/L (E) (HC) | 0.14 (fish) 0.1 (daphnid, E) 0.14-0.5 (algae, E) (MC) |
3.2 (fish, E) 6.4 (daphnid, E) 7 (algae, E) MC |
0.2 (fish, E) 0.4 (daphnid, E) 5 (algae) (MC) |
| Wildlife Toxicity - Acute | ED50 or LD50 (mg/kg-d) | ND (MC) | > 5,000 (oral) > 8,000 (dermal) (LC) |
1630 to > 2000 (oral, dermal) > 714 mg/m3 (inhalation) (MC) |
2250-6800 (oral) > 4640 (dermal) (MC) |
| Wildlife Toxicity - Chronic | NOAEL or LOAEL (mg/kg-d) | 1.77 (NOAEL for liver enzyme induction [Carlson 1980]) (HC) | 161 – 711 (NOAEL range for various effects) (MC) | 141 (NOAEL for a closely related compound (MC) | 0.25-200 (NOAEL range for various effects) (HC) |
| Degra-dation Products | ND (MC) | Diphenyl phosphate, phenol (MC) | Expected to be less persistent than parent compound (MC) | Expected to be less persistent than parent compound (MC) |
(1) HC - high concern, MC - moderate concern, LC - low concern
Data for PentaBDE and persistence data for alternative chemicals estimated using PBT Profiler (U.S. EPA 2006a); all other data obtained from U.S. EPA (2005)
- ND
- No data
- T1/2
- Half life
- BCF
- Bioconcentration Factor
- BAF
- Bioaccumulation Factor
- log Kow
- log octanol-water partition coefficient
- EC/D50
- Effect Concentration/Dose affecting 50 per cent of test organisms
- LC/D50
- Lethal Concentration/Dose killing 50 per cent of test organisms
- IC50
- Concentration providing 50 per cent inhibition to a reaction
- NOEC
- No Observed Effect Concentration
- NOAEL
- No Observed Adverse Effect Level
- LOEC
- Lowest Observed Effect Concentration
- LOAEL
- Lowest Observed Adverse Effect Level
- MATC
- Maximum Acceptable Toxicant Concentration
2.5.1.2 Human Health
In addition to environmental fate and hazards, potential effects on human health need to be evaluated. Human health effects evaluated in this step are related to the protection of the general public and sensitive sub-populations. Section 2.5.1.3 discusses evaluation criteria for the protection of worker health and safety. Human health effects are scored by examining acute and chronic toxicological reference values for both carcinogenic and non-carcinogenic effects as presented in Table 2.5.
Acute effects are evaluated using lethal doses (oral or dermal exposure) or concentrations (inhalation exposure) causing lethality in 50 per cent of the test population. Chronic effects are evaluated for non-cancer and cancer endpoints. For the former, the concern level of a chemical is measured using NOAEL (oral or dermal exposure) or NOAEC (inhalation) values. Other non-cancer effects can be evaluated based on whether they are considered to affect development or are endocrine disruptors.
| Factor | Measure/Metric | Concern Level/Evaluation(1) | Source of Data(2) |
|---|---|---|---|
| Human Toxicity - Acute | LD50 (oral, dermal) | ≤ 200mg/kg (HC) > 200 to ≤ 2,000 mg/kg (MC) > 2,000 mg/kg (LC) |
HSDB, U.S. EPA IRIS, MSDS |
| Human Toxicity - Acute | LC50 (inhalation) | ≤ 400 mg/m3 (HC) < 400 to ≤ 4,000 mg/m3 (MC) > 4,000 mg/m3 (LC) |
HSDB, U.S. EPA IRIS, MSDS |
| Human Toxicity - Chronic | RfD (oral, dermal) | ≤ 0.001 mg/kg-d (HC) > 0.001 to ≤ 4,000 mg/m3 (MC) > 4,000 mg/m3 (LC) |
HSDB, U.S. EPA IRIS, Health Canada, MSDS |
| Human Toxicity - Chronic | RfC (inhalation) | ≤ 1 × 10-6 mg/m3 (HC) > 1 × 10-6 to ≤ 1 × 10-3 mg/m3 (MC) > 1 × 10-3 mg/m3 (LC) |
HSDB, U.S. EPA IRIS, Health Canada, MSDS |
| Human Toxicity - Chronic | Carcinogenicity | EPA: A, B1, B2; IARC: 1, 2A (HC) EPA: C, D; IARC: 2B, 3 (MC) EPA: E; IARC: 4 (LC) |
U.S. EPA IRIS, IARC, NTP |
| Human Toxicity - Chronic | Mutagenicity | Positive evidence (HC) No information (no reported studies) (MC) No evidence (LC) |
Peer-reviewed literature, MSDS |
| Human Toxicity - Chronic | Endocrine Disruption | Positive evidence (HC) No information (no reported studies) (MC) No evidence from reported studies (LC) |
Peer-reviewed literature |
| Human Toxicity | Reproductive or Developmental Effects | Positive evidence )HC) No information (no reported studies) (MC) No evidence from reported studies (LC) |
Peer-reviewed literature, MSDS |
(1) HC - high concern, MC - moderate concern, LC - low concern
(2) Suggested sources; other sources are discussed in main text
- LC/D50
- Lethal Concentration/Dose killing 50 per cent of test organisms
- RfD
- Reference Dose (oral) (mg/kg-d)
- RfC
- Reference Concentration (mg/m3)
In terms of carcinogenic effects, the International Agency for Research on Cancer (IARC) has assessed a vast number of substances in their IARC monographs and classified them as one of the following:
- Group 1 - Carcinogenic to humans
- Group 2 – Probably carcinogenic to humans
- Group 2B – Possibly Carcinogenic to humans
- Group 3 – Not classifiable as to its carcinogenicity to humans
- Group 4 – Probably not carcinogenic to humans
The IARC classifications were used by the MOE in the prioritization of substances under the TRA and are considered appropriate for use in the screening process. Listings of classifications by alphabetical order, Chemical Abstracts Service Registry Number (CAS® RN) or Group are available from IARC (IARC 2010).
In prioritizing chemicals under the TRA, the MOE also relied upon substances identified as carcinogens in the Report on Carcinogens (RoC) published by the National Toxicology Program (NTP). These reports are published biennially and are available from the NTP website.
Additionally, in 1986 the U.S. EPA developed a similar system of classification, the results of which are provided on a chemical by chemical basis in the U.S. EPA Integrated Risk Information System (IRIS) (U.S. EPA 2010b) and can also be used in the screening process. The classification system is as follows:
- Group A – Human carcinogen
- Group B1 – Probable human carcinogen (limited evidence from epidemiologic studies)
- Group B2 – Probable human carcinogen (sufficient evidence from animal studies, inadequate evidence from epidemiologic studies)
- Group C – Possible human carcinogen
- Group D – Not classifiable as to human carcinogenicity
- Group E – Evidence of non-carcinogenicity for humans
This step is also very data intensive. Some of the more common sources for these data include, but are not limited to, the following:
- Health Canada Toxicological Reference Values (TRVs) (HC 2004) (Health Canada)
- A listing of carcinogenic slope factors and unit risks and non-carcinogenic tolerable daily intakes and concentrations developed by Health Canada.
- The U.S. EPA Integrated Risk Information System (IRIS) (U.S. EPA 2010b)
- A database containing qualitative and quantitative information on human health effects that may result from exposure to more than 540 chemicals. Oral and inhalation TRVs are derived based on a review of internal and external human and animal studies.
- MOE Toxicological Reference Values (MOE 2009b)
- A summary of Ministry of the Environment recommended oral and inhalation TRVs for chronic and/or sub-chronic exposure based on a review of values from other health and environmental agencies.
- The California Environmental Protection Agency Toxicity Criteria Database (Cal/EPA 2003)
- A searchable database providing California Public Health goals, cancer potency information, oral and inhalation TRVs, and details on derivation of these values.
- The Agency for Toxic Substances and Disease Registry Toxicological Profiles (ATSDR various years)
- Detailed, public-reviewed documents providing qualitative and quantitative information for 308 priority toxics. Detailed derivations of the oral and inhalation TRVs are provided within each document.
- Hazardous Systems Data Bank (HSDB)
- Provides information on chemical manufacturing processes and product formulations.
- Material Safety Data Sheets (MSDS)
- Documents summarizing important information regarding the physical, chemical and toxicological properties of a particular substance.
A more comprehensive listing is provided in the CTSA Methodology (DfE 1996); however, the above-listed references are among the more comprehensive and are reputable regulatory agencies and sources.
An example of a completed human health hazard evaluation matrix is provided in Table 2.6 for the flame retardant example (based on information obtained from the U.S. EPA (2005)). From this table, it can be seen that there are significant data gaps for human health toxicological data, which adds uncertainty to the assessment and illustrates one of the challenges of completing an alternatives assessment. It must be reiterated that the use of analogs to fill in data gaps and interpretation of the toxicological data may require input from a scientist with a background in toxicology.
2.5.1.3 Occupational Health and Safety
The evaluation metrics provided in the previous section are applicable to the general public and sensitive subpopulations. However, different threshold values are available for workers, and numerous agencies have derived occupational exposure limits. In Ontario, regulated under the Occupational Health and Safety Act (R.R.O. 1990, Regulation 833 and O.Reg.490/09; available from the Service Ontario e-laws home page, the Ontario Ministry of Labour provides the Occupational Exposure Limits (OELs) as the maximum airborne concentration of a biological or chemical agent to which a worker may be exposed in a work day or work week (time-weighted average, or TWA), in a 15-minute period (short term exposure, or STEL) or at any time (ceiling limit, or C). These values have been used to develop the metrics for occupational exposure, as shown in Table 2.7. A table with a complete list of OELs is available on the Ontario Ministry of Labour’s Website (‘Occupational Exposure Limits for Ontario workplaces’). For mixtures of airborne chemical agents that exert an additive health effect, if analytical results of individual airborne agents are available, the formula found in Regulation 833 should be used to determine respective exposure limits for agents.
If OELs are not available for a chemical, then other agencies should be checked. Examples include the Recommended Exposure Limits (RELs) from the National Institute for Occupational Health and Safety (NIOSH), the Permissible Exposure Limits (PELs) from the U.S. Occupational Safety & Health Administration (OSHA) and the American Conference of Governmental Industrial Hygienists (ACGIH). Values from NIOSH and OSHA can be found by searching the NIOSH Pocket Guide to Chemical Hazards. Additional references can be found on the Canadian Centre for Occupational Health and Safety site.
In addition to OELs, there are other chemical hazards that should be considered in terms of occupational health and safety. Alternative chemicals should be selected to protect workers from hazards such as fire and explosion, and from highly corrosive chemicals. These goals are in sync with Principle 12 of the 12 Principles of Green Chemistry (Section 1.2). As such, the reactivity, flammability and corrosivity of the alternative chemicals need to be assessed. The National Fire Protection Association (NFPA) has developed a standard (NFPA 704) to describe the relative hazards of many common chemicals and is intended primarily for fire fighters and emergency responders. Similarly, National Paint & Coatings Association (NPCA) has developed the Hazardous Materials Identification System (HMIS®) that attempts to convey full health warning information for the same categories to all employees, not just emergency responders. Both systems provide relative hazard rankings (generally on a scale from 0 to 4 with 4 being a high hazard) for the categories of health, flammability, reactivity, and special hazards (such as an oxidizing agent). The hazard rankings for flammability and reactivity, which can be found on a chemical’s MSDS, can be used to evaluate the relative safety of alternative chemicals.
In the absence of an NFPA or HMIS® hazard ranking for a chemical, the vapour pressure can be used to assess the flammability of an alternative chemical relative to the current chemical. For example, a lower vapour pressure is associated with a chemical of lower flammability. The corrosivity of a chemical can be evaluated by consideration of a chemical’s pH; a chemical with a very low pH (i.e., very acidic) or very high pH (i.e., very basic, or caustic) is generally more corrosive than one with a more neutral pH.
| Factor | Measure/Metric(1) | Current: PentaBDE | Alternative Chemicals: Triphenyl Phosphate | Alternative Chemicals: Tribromopentyl Alcohol | Alternative Chemicals: Tris (1,3-dichloro-2-propyl) Phosphate |
|---|---|---|---|---|---|
| Human Toxicity - Acute | LD50 (oral, dermal) | ND (MC) | ND (MC) | ND (MC) | ND (MC) |
| Human Toxicity - Acute | LC50 (inhalation) | ND (MC) | ND (MC) | ND (MC) | ND (MC) |
| Human Toxicity - Chronic | RfD (mg/kg-d) | 0.002 (MC) | 1.61-7.11(2) (LC) | 1.41 (2) (LC) | 0.0025-2 (2) (MC) |
| Human Toxicity - Chronic | RfC (mg/m3) | ND (MC) | ND (MC) | ND (MC) | ND (MC) |
| Human Toxicity - Chronic | Carcinogenicity | EPA Group D (Not classifiable) (MC) | ND (MC) | ND (MC) | ND (MC) |
| Human Toxicity - Chronic | Mutagenicity | ND (MC) | No evidence (LC) | Positive and negative evidence (MC) | Positive and negative evidence (MC) |
| Human Toxicity - Chronic | Endocrine Disruption | ND (MC) | ND (MC) | ND (MC) | ND (MC) |
| Human Toxicity - Chronic | Reproductive or Developmental Effects | ND (MC) | Little to no evidence (MC) | Positive evidence (but only by analogy to closely related compound) (MC) | Positive evidence (HC) |
(1) HC - high concern, MC - moderate concern, LC - low concern
(2) RfDs estimated from chronic wildlife toxicity NOAELs by dividing by a factor of 100
Data for PentaBDE obtained from the U.S. EPA (2010a); data for alternative chemicals obtained from the U.S. EPA (2005)
- ND
- No data
- LC/D50
- Lethal Concentration/Dose killing 50 per cent of test organisms
- RfD
- Reference dose (oral)
- RfC
- Reference concentration (inhalation)
| Factor | Measure/Metric | Concern Level/Evaluation(1) | Source of Data(2) |
|---|---|---|---|
| Occupational Health and Safety - Occupational Exposure Limits | TWA, STEL, C | Lowest of values < that of current chemical (MC) Lowest of values > that of current chemical (LC) |
Ontario OELs |
| Occupational Health and Safety - Other Physical Hazards | Corrosivity (pH scale) | pH < 2, pH > 12 (MC) ≥ 7 pH ≤ 12 (LC) |
MSDS |
| Occupational Health and Safety - Other Physical Hazards | Reactivity | Reactivity of 4 (HC) Reactivity of 2 or 3 (MC) Reactivity of 0 or 1 (LC) |
NFPA, HMIS, MSDS |
| Occupational Health and Safety - Other Physical Hazards | Flammability | Flammability of 4 (HC) Flammability of 2 or 3 (MC) Flammability of 0 or 1 (LC) |
NFPA, MSDS |
| Occupational Health and Safety - Other Physical Hazards | Vapour Pressure (at ambient conditions) | > that of current chemical (MC) < that of current chemical (LC) |
MSDS |
(1) HC - high concern, MC - moderate concern, LC - low concern
(2) Suggested sources; other sources are discussed in main text
- OEL
- Occupational Exposure Limit (Ontario)
- NFPA
- National Fire Protection Association
- TWA
- time-weighted average
- STEL
- Short-term exposure limit
- C
- Ceiling limit
Vapour pressure and pH data are often listed on MSDS sheets, and can also be found in the following sources:
- SRC Physical Properties Database (PHYSPROP) — basic data (full access requires subscription).
- Basic database provides information on physical and chemical properties of over 25,000 compounds while full access will also provide full reference citations and structure depictions.
- Aldrich Handbook of Fine Chemicals (Aldrich 2010)
- A catalogue often used as a reference book (available for free) providing information on structures, physical and chemical data, and literature references.
- Perry’s Chemical Engineers’ Handbook (Green and Perry 2008)
- A handbook providing extensive chemical engineering knowledge containing, among other things, information on physical properties of chemicals. The electronic copy of the current (8th) edition includes interactive tables of physical properties for over 2,300 organic and inorganic substances.
2.5.2 Technical Feasibility
Purpose: This section provides information on completing a technical feasibility analysis as part of the alternatives assessment.
If you are a small/medium company… This section may be for informational purposes only, depending on the resources and decisions made by your company in pursuing a safer chemical alternative.
If you are a large company… This is an important aspect of the alternatives assessment to ensure that performance requirements are achieved by the alternative chemical(s).
The technical feasibility of an alternative chemical is arguably the most important aspect of its evaluation — an alternative is not viable if it does not achieve performance requirements. However, the technical evaluation can be limited by the availability of technical data for the alternative chemicals. A preliminary evaluation of technical feasibility is important at the preliminary stage and was discussed in Section 2.4.1 which considered the functionality requirements and some performance characteristics; however, there are other factors to consider when doing the more detailed analysis. Table 2.8 provides a summary of some considerations for a technical feasibility evaluation and these elements are discussed further in the following paragraphs. The applicable criteria for the technical feasibility evaluation should be defined prior to the evaluation of the alternative chemicals.
As discussed as part of the Hazard Assessment (Section 2.5.1.3), health and safety standards for chemicals exist to ensure worker safety, either during chemical handling, storage, use, or transportation. Although these standards have already been discussed, they are considered here again in the technical assessment since they impact not only safety but also the facility operations. Consideration of these requirements should include facility limitations, handling and storage precautions, and safety as a result of, for example, flammability, reactivity, etc. Any special precautions or actions that are necessary for the use of a chemical could affect the feasibility or cost associated with the alternative chemical.
Additionally, there should be a consideration of the functionality requirements and performance characteristics that were identified in Section 2.2.3. Functionality requirements are related to the chemical function and include properties such as density, water solubility, colour, boiling point/melting point, odour, and vapour pressure. This evaluation was previously done as part of the Preliminary Assessment (Section 2.4.1) and this information can further be used at this stage of the more detailed analysis. Performance characteristics are properties such as maintenance requirements, durability, longevity, resistance to high temperatures, energy consumption, process change requirements, and product quality. These too were considered briefly in the Preliminary Assessment and are further considered at this stage of the assessment.
The assessor(s) would need to consider a number of these factors in determining the technical feasibility of the alternative chemical.
| Parameter | Example | Measure/Metric | Source of Data |
|---|---|---|---|
| Health & Safety Standards for Alternative |
|
Are there any standards which a chemical must meet for workplace health and safety? | MSDS, industry associations |
| Functionality Requirements |
|
Does alternative meet all requirements for its intended function? | MSDS |
| Performance Characteristics |
|
Will changing to a new chemical require significant equipment and process adjustments? Is this possible or feasible? Will customers continue to be satisfied with the quality of the product? | Industry; chemical and equipment suppliers |
2.5.3 Economic Feasibility
Purpose: This section provides information on completing an economic feasibility assessment as part of the alternatives assessment.
If you are a small/medium company… This section may be for informational purposes only, depending on the resources and decisions made by your company in pursuing a safer chemical alternative.
If you are a large company… This is an important aspect of the alternatives assessment to ensure that a chemical alternative is economically viable.
As discussed in the Preliminary Assessment (Section 2.4), an alternative should be economically feasible (sustainable) to be practical, and this can be evaluated by conducting a market assessment, cost assessment, cost/benefit analysis, and financial evaluation for each alternative. At the most basic level, economic feasibility can compare the direct and indirect costs of the current chemical and its alternatives. The preliminary assessment (Section 2.4.1) considered a basic market and cost assessment. A more complete assessment of economic feasibility includes the following:
- Detailed Cost Assessment
- Cost/Benefit Analysis
- Financial Evaluation
These are discussed in more detail in the following section. Information from the process flow diagram (Section 2.2.1) such as the annual raw material requirements, are needed in this evaluation.
2.5.3.1 Detailed Cost Assessment
The cost assessment quantifies the direct and indirect costs associated with the use of the current chemical and the identified alternatives. Some key considerations include:
- Direct costs
- Capital costs
- Transition costs
- One-time costs for conducting laboratory- or pilot-scale experiments, research and development, plant down time, etc.
- Operations and maintenance costs
- Includes energy costs, resource costs, disposal/treatment costs.
- Chemical costs
- Includes consideration of the quantity of chemical (current chemical vs. alternatives) required, as well as the shelf life/replacement rate of the chemicals.
- Includes costs of additional reagents or other chemicals for required formulation.
- Key end of life costs
- Includes costs of re-cycling, disposal of finished product.
- Includes costs of disposal of chemical.
- Indirect costs
- Insurance costs, taxes and fees, costs of meeting regulatory requirements, safety and employee training costs, non-compliance liability.
- Changes in worker productivity, liability claims due to accidents or releases, sales due to negative or positive publicity.
The cost assessment should also consider future price changes. Are there potential future cost reductions, such as economies of scale due to higher volume production, or cost increases due to increased demand and limited production? Table 2.9 provides some additional guidance and key considerations for the cost assessment.
Some examples of software that may be useful for completing cost assessments include:
- U.S. EPA DfE CTSA Methodology (DfE 1996) recommends Tellus Institute’s P2/Finance Pollution Prevention Financial Analysis and Cost Evaluation System
- Analysis tool for the financial evaluation of pollution prevention (and other) projects. The tool is available free of charge for government agencies (through contacting the P2 Information Clearinghouse, but charges apply for all other organizations.
- EstPro Conceptual Cost Estimating Software, available from Chempute Software
- Software for producing quick, accurate cost estimates for new plants, major equipment acquisitions or upgrades, large maintenance projects, etc.
- Cleopatra Enterprise
- Software tool for estimating costs for the full life cycle of a project. The tool is modular so that it can be tailored to a company’s specific needs.
- SEER for Manufacturing
- Project estimation and management solution to assess up-front project feasibility and optimize project costs and schedules.
- Costimator, available from MTI Systems
- Cost estimating software to model the capabilities of a manufacturing facility, with data obtained from various manufacturing organizations worldwide that can be used as is or edited to fit the user’s needs.
2.5.3.2 Cost/Benefit Analysis
The cost/benefit analysis is completed to quantify the costs of a chemical use versus the benefits received through the chemical use, using information from the detailed cost assessment. Some components of a cost/benefit analysis are difficult to quantify. However, a cost/benefit analysis can include consideration of the capital costs and investments required for a technology change (e.g., equipment costs, facility costs) and benefits from the technology change (e.g., financial incentives/tax breaks, increased market potential). Regulatory impact analyses are cost/benefit analysis forecasts for industry-wide impacts for regulatory scenarios based on levels of pollution allowed and these can also be used for the cost/benefit assessment. Table 2.9 provides some additional guidance and key considerations for the cost/benefit assessment.
2.5.3.3 Financial Evaluation
The financial evaluation investigates the long-term financial implications of using the current and alternative chemicals. Long-term financial indicators can be used to evaluate the time value of money and cash flows associated with the current and alternative chemicals. Possible indicators are net present value (NPV), payback period and internal rate of return (IRR). Table 2.9 provides some additional guidance and key considerations for the financial evaluation.
| Component | Parameter | Measure/Metric | Source of Data |
|---|---|---|---|
| Detailed Cost Assessment | Purchase price of alternative chemical | Retail Price | Price catalogues or websites (e.g., of manufacturers, distributors, retailers) |
| Detailed Cost Assessment | Purchase price of current chemical | Retail price | Price catalogues or websites (e.g., of manufacturers, distributors, retailers) |
| Detailed Cost Assessment | Relative cost of alternative to current chemical | Alternative : Current cost | Calculated |
| Detailed Cost Assessment | Manufacturing costs | Energy consumption, maintenance costs | Industry trade associations |
| Detailed Cost Assessment | Replacement rate | Product life, shelf life | Product data sheets |
| Detailed Cost Assessment | Disposal costs | Fees | e.g., Clean Harbours |
| Detailed Cost Assessment | Training costs | Employee training costs | Industry trade associations, government organizations, CCOHS, WSIB |
| Detailed Cost Assessment | Transition costs | One-time costs such as those incurred from laboratory scale experiments, pilot scale experiments, manufacturing scale trials, etc. | Green Centre Canada might be able to provide some insight or possible resources |
| Detailed Cost Assessment | Regulatory compliance costs | Costs associated with reporting and other compliance issues | Government organizations |
| Detailed Cost Assessment | Safety costs | PPE, control devices, special storage measures | MSDS, supply websites (e.g., Lab Safety Supply [LSS], McMaster-Carr [MMC]) |
| Detailed Cost Assessment | Insurance costs | Insurance costs associated with chemical use | Industry trade associations |
| Detailed Cost Assessment | Liability costs | Costs associated with accidents or releases | Industry trade associations |
| Detailed Cost Assessment | Taxes, fees | Taxes and other fees associated with chemical use | Industry trade associations |
| Cost/Benefit Analysis | Capital and operating costs of technology change | Additional equipment costs or material costs | Industry experts, trade associations |
| Cost/Benefit Analysis | Benefits of technology change | Financial incentives (e.g., research and development tax breaks), increased market potential | Government organizations, industry experts |
| Cost/Benefit Analysis | Regulatory impact analysis | Forecasts for industry-wide impacts for various regulatory scenarios | |
| Financial Evaluation | Return on investment | Internal rate of return, payback period, NPV | Based on financial requirements |
Note: Challenge for economic assessments is that prices are not static and to capture all true costs of a chemical is difficult, especially for high hazard chemicals
- NPRI
- National Pollutant Release Inventory
- ECICS
- European Customs Inventory of Chemical Substances
- TRI
- Toxics Release Inventory
- WSIB
- Workplace Safety and Insurance Board
- ATSDR
- Agency for Toxic Substances and Disease Registry
- PPE
- personal protective equipment
- MSDS
- Material Safety Data Sheets
- CCOHS
- Canadian Centre for Occupational Health and Safety
- LSS
- Lab Safety Supply
- MMC
- McMaster
2.5.4 Social Impact
Purpose: This section provides information on completing an assessment of social impacts as part of the alternatives assessment.
If you are a small/medium company… This section may be for informational purposes only, depending on the resources and decisions made by your company in pursuing a safer chemical alternative.
If you are a large company… This aspect of the alternatives assessment considers socio-economic impacts on the local economy/jobs.
Social impacts from the substitution of a current chemical with an alternative chemical consider socio-economic impacts on the market/jobs/local economy. Some factors to evaluate are:
- How does the alternative affect the market/jobs/local economy?
- Does the alternative replace a locally sourced material with a foreign material, leading to job loss locally?
- Does the alternative create jobs locally?
- Are there effects from pollution on health and recreation?
- Does the alternative affect worker productivity, job satisfaction or education?
2.5.5 Life Cycle Analysis
Purpose: This section provides information on completing a life cycle analysis as part of the alternatives assessment.
If you are a small/medium company… This section may be for informational purposes only, depending on the resources available.
If you are a large company… This is an important aspect of the alternatives assessment to prevent the inadvertent transfer of environmental impacts from one media or life stage to another; the depth and detail of the life cycle analysis will depend on the resources available, but some consideration of life cycle impacts should be completed.
A life cycle analysis (LCA) should be considered as part of the evaluation and comparison of safer chemical alternatives, as it prevents the inadvertent transfer of environmental impacts from one medium to another or one life stage to another. LCA provides a systematic method for identifying and evaluating the environmental burdens of a product at all stages in its life cycle (resource extraction, production of materials, product parts, and the product itself, use of the product, disposal of the product) and provides a bigger picture comparison of chemicals. It is important to consider the results of an LCA in the selection of alternatives since it is a broad consideration of environmental, social, and/or economic issues across the life cycle of a chemical, as shown in Figure 2.2. Additionally, the 12 Principles of Green Chemistry support the consideration of life cycle impacts of a chemical or process (Section 1.2).
A comprehensive LCA can be complex and at times resource and time intensive, and therefore may be outside the scope of an alternative assessment, particularly for some facilities. It is important to consider the availability of data, time requirements, and financial resources against the anticipated benefits of the LCA before conducting a detailed LCA. Therefore, some general guidance and considerations for conducting an LCA are provided, along with references for further information.
Figure 2.2 Overview of Life Cycle Analysis Process
Note: From U.S. EPA (1993, cited in U.S. EPA 2006b)
The International Organization for Standardization (ISO) has developed two ISO Standards related to Life Cycle Analysis: ISO 14040:2006 Life Cycle Assessment, Principles and Framework and ISO 14044:2006 Life Cycle Assessment, Requirements and Guidelines. ISO 14040:2006 defines the principles and framework for LCA:
- Goal definition and scoping (Section 2.5.5.1)
- Life cycle inventory (Section 2.5.5.2)
- Life cycle impact assessment (Section 2.5.5.3)
- Interpretation of results (Section 2.5.5.4)
ISO 14040:2006 also reports on the limitations of LCA, the relationship between the phases and conditions for the use of value choices and optional elements. ISO14044:2006 outlines the specific requirements for an LCA and provides guidelines. The framework for LCA is discussed in more detail in the following sections.
2.5.5.1 Goal Definition and Scoping
The boundaries of the LCA and data requirements are defined, typically by the project partners, in this initial step. Assumptions and limitations of the assessment should also be identified and outlined during this step. The breadth and depth of the analysis needs to be compatible with the goals of the analysis and sufficient to address the data requirements of the scope. The boundary of an LCA can be cradle-to-gate, cradle-to-grave, or cradle-to-cradle, for example, and the boundary should be selected based on available resources and specified. A lack of transparency is a detriment to an LCA; therefore, assumptions should be clearly stated and visible. The outcome of the goal definition and scoping phase is a statement of the boundaries, methodologies and data categories covered by the LCA. The LCA will be more manageable to complete with a small scope and tighter boundaries; however, it will not capture all components and has a greater risk of overlooking potential impacts. The accounting of the complex and interconnected industrial systems is often impossible or difficult to achieve.
Some considerations for this phase of the LCA include:
- What are the issues, questions, and decisions to be made?
- What levels of data, data quality, and model accuracy are required?
- Who is the anticipated audience?
- What is the anticipated use of the study?
- Which specific categories and issues should be included and excluded?
- What valuation approach or weighting methods will be used?
2.5.5.2 Life Cycle Inventory
The life cycle inventory (LCI) component of the LCA quantifies the relevant emissions, materials, and energy flows (inputs and outputs) for the LCA within the scope and boundaries defined in the previous step. These include processes and products that fall within the system boundaries and considerations include:
- Resource use
- Water (especially freshwater)
- Energy
- Land
- Other non-renewable
- System inputs
- Are they recycled, reused, and/or renewable?
- Quantity
- Transportation energy
- Potential safety risks with process
- Physical hazards such as heat, noise generation, vibration, ergonomic hazards
- Chemical waste and use
- Hazardous and non-hazardous?
- Upstream effects, consumer hazard, disposal hazard
- Catalysts and reagents
- System outputs
- Emissions
- Waste
- Releases to air, water and land
- Air: greenhouse gas, ozone depletion, acid rain formation
- Land: landfill, reportable quantities, incineration, recycling
- End-product
- Reusable, recyclable, closed-loop recyclable, compostable
- Useful life
The U.S. EPA (1993, 1995) recommends the following steps for completing an LCI:
- Develop a flow diagram of the processes being evaluated
- Develop a data collection plan
- Collect data
- Evaluate and report results
The inventory analysis provides a list of quantities of pollutants released to the environment, as well as quantities of energy and materials used. This data can be organized by environmental media, life cycle stage, process, etc.6
2.5.5.3 Life Cycle Impact Assessment
The life cycle impact assessment (LCIA) uses the data collected in the LCI to determine the impacts on the environment and humans for the complete life cycle of the chemical product (as defined in the goal definition and scoping, e.g., cradle-to-cradle). The LCIA should establish a link between the chemical and its potential impacts. The LCIA is intended to provide relative comparisons of the potential to cause human or environmental damage by way of a systematic procedure for classifying and characterizing environmental effects. The results are not an absolute quantification of risk and impacts but rather a comparison of the potential risks of one chemical to another. The U.S. EPA (2006b) provides an example:
What are the impacts of 9,000 tons of carbon dioxide or 5,000 tons of methane emissions released in the atmosphere? Which is worse? What are their potential impacts on smog? On global warming?
As discussed previously, evaluation of the hazards of a chemical are important when conducting an alternatives assessment (Section 2.5.1); however, there may be other factors that are relevant to the LCIA such as the Global Warming Potential (GWP), Ozone Depleting Potential (ODP), etc. of a chemical. For example, the GWP is the ratio of the warming caused by a greenhouse gas to the warming caused by a similar mass of carbon dioxide; if a chemical has a GWP is greater than 1 then it has the potential for a greater impact on global warming than does carbon dioxide. A list of GWPs can be found on the U.S. EPA website (U.S. EPA 2009b) as well as Chapter 2 of Climate Change 2007: The Physical Science Basis (Forster et al. 2007).
Additionally, the assessor should consider not only the hazard but also the risk presented by an alternative chemical by evaluating the potential exposure to the chemical using information on the amount of chemical used, produced or released over its life cycle (i.e., the mass of the chemical). Information from the process flow diagram (Section 2.2.1) may be useful here. For example, when comparing two pesticides, pesticide A may be significantly less toxic than pesticide B and therefore be less hazardous; however, it may also be significantly less effective such that 100 times more of pesticide A is required to do the same job as pesticide B and thus represents a greater risk to human health and the environment. Additionally, within a process chemical A may be more toxic than chemical B, but chemical A may be used within a closed loop system (unlike chemical B) and, as a result, the potential for exposure to chemical A is less than that to chemical B. As such, the Toxic Equivalent (TEQ) (i.e., risk) associated with the alternative chemical needs to be determined as part of the LCIA. The TEQ can be expressed in a simplified format by the following equation:
TEQ = Mass × Toxic Potential
In the above equation, mass represents some measure of release of the chemical, for example from emissions from a manufacturing facility to the surrounding environment, while the toxic potential is a measure of how toxic a chemical is relative to a reference chemical. For example, for many volatile organic compounds, benzene is used as the reference chemical. It must be noted that this approach is not standard and is limited as a tool to assess exposure potential. It only allows for comparison within the same group of chemicals and not between different groups. However, it can be used with caution to provide some relative estimate of the potential for exposure to chemicals within the same group.
| PAH | TEF |
|---|---|
| Acenaphthene | 0.001 |
| Acenaphthylene | 0.01 |
| Benzo(a)anthracene | 0.1 |
| Benzo(a)pyrene | 1 |
| Benzo(b)fluoranthene | 0.1 |
| Benzo(g,h,i)perylene | 0.01 |
| Benzo(k)fluoranthene | 0.1 |
| Chrysene | 0.01 |
| Dibenzo(a,h)anthracene | 1 |
| Fluoranthene | 0.01 |
| Indeno(1,2,3-c,d)pyrene | 0.1 |
| Pyrene | 0.001 |
Note: TEFs obtained from Kalberlah et al. (1995), as cited in MOE (2009b)
Toxic potentials are well documented in literature for some classes of compounds, and minimally or not at all for others. Toxic potentials are available for the class of compounds referred to as polycyclic aromatic hydrocarbons (PAHs), which includes benzo(a)pyrene, benzo(a,h)anthracene, benzo(k)fluoranthene, etc. Rather than establishing unique toxicological reference values for each PAH, toxicity equivalency factors (TEFs) (provided in Table 2.10) are used which are based on the observed toxicity of a given PAH relative to that of benzo(a)pyrene (i.e., based on a statistically derived relationship between observed cancer incidences from a PAH and benzo(a)pyrene).
Under NPRI reporting, TEQs for dioxins and furans must be reported using the TEFs (values provided in Table 2.11) from the North Atlantic Treaty Organization/Committee on the Challenges of Modern Society (NATO/CCMS 1998). As such, if the current chemical in the alternatives assessment is a dioxin or furan, then under NPRI reporting the facility should already have a TEQ calculated. The World Health Organization (WHO) also provides TEFs, and the most current values for human and mammalian exposure to dioxin-like polychlorinated biphenyls (PCBs) are also provided in Table 2.11 (van den Berg et al. 2006).
In the absence of TEFs for chemicals such as volatile organic compounds (VOCs) and metals, the TEQs can be assessed relative to one another using aquatic, mammalian or human toxicity values. For example, if the current chemical is lead, then the NOAEL for lead is the reference value and lead is assigned a TEF of 1. To estimate the TEF for alternative chemical A, the NOAEL for alternative chemical A can be divided by the NOAEL for lead. The result will be a TEF > 1 if alternative chemical A is more toxic than lead, and < 1 if it is less toxic. The TEQs can then be calculated relative to one another using the known and estimated mass emissions previously calculated. TEFs must be calculated using the same benchmark (e.g., all NOAEL values for humans for non-carcinogenic effects), and for consistency TEFs toxicity values from the same source (e.g., all from IRIS (U.S. EPA 2010b), Health Canada (2004), etc.) should be used whenever possible.
| Congener | TEF |
|---|---|
| 2,3,7,8-TCDD | 1 |
| 1,2,3,7,8-PeCDD | 0.5 |
| 1,2,3,4,7,8-HxCDD | 0.1 |
| 1,2,3,6,7,8-HxCDD | 0.1 |
| 1,2,3,7,8,9-HxCDD | 0.1 |
| 1,2,3,4,6,7,8- HpCDD | 0.01 |
| OCDD | 0.001 |
a TEFs obtained from NATO/CCMS (1998)
Note : T – tetra, P – penta, Hx – hexa, Hp –hepta, O – octa
| Congener | TEF |
|---|---|
| 2,3,7,8-TCDF | 0.1 |
| 1,2,3,7,8-PeCDF | 0.5 |
| 2,3,4,7,8-PeCDF | 0.05 |
| 1,2,3,4,7,8-HxCDF | 0.1 |
| 1,2,3,6,7,8-HxCDF | 0.1 |
| 1,2,3,7,8,9-HxCDF | 0.1 |
| 2,3,4,6,7,8-HpCDF | 0.1 |
| 1,2,3,4,6,7,8-HpCDF | 0.01 |
| 1,2,3,4,7,8,9-HpCDF | 0.01 |
| OCDF | 0.001 |
a TEFs obtained from NATO/CCMS (1998)
Note : T – tetra, P – penta, Hx – hexa, Hp –hepta, O – octa
| Congener | TEF |
|---|---|
| PCB 77 | 0.0001 |
| PCB 81 | 0.0003 |
| PCB 126 | 0.1 |
| PCB 169 | 0.03 |
b TEFs obtained from van den Berg et al. (2006)
| Congener | TEF |
|---|---|
| 105 | 0.00003 |
| 114 | 0.00003 |
| 118 | 0.00003 |
| 123 | 0.00003 |
| 156 | 0.00003 |
| 157 | 0.00003 |
| 167 | 0.00003 |
| 189 | 0.00003 |
b TEFs obtained from van den Berg et al. (2006)
A simpler methodology is to evaluate the relative risk of an alternative chemical by multiplying the mass emitted by the environmental risk potential, rather than by the toxic potential. For example, the contribution to global warming (the risk) of a chemical can be evaluated as the mass emitted times the GWP. Likewise, the contribution to acidification (acid rain) can be evaluated as the mass emitted times the chemical’s acidification potential. VOCs differ significantly in their impacts on ozone (O3) and, as such, reactivity scales such as the Maximum Incremental Reactivity (MIR) Scale developed by William P.L. Carter for the California Air Resources Board (CARB 2010) can be used in place of TEFs. The MIR Scale is a measure of the relative impacts of the different types of VOCs on O3 formation (in units of g O3 per g VOC) and is derived from the scenarios where the NOx inputs are adjusted to yield highest incremental reactivities (changes in O3) caused by small VOC additions, divided by the amount of VOC added. The complete listing of MIR values can be found in Table B-1 of the up to date reactivity scales report (CARB 2010).
As an example of an LCIA, the U.S. EPA (2006b) recommends the following steps and provides the example presented in Table 2.12:
- Selection and definition of impact categories — identify relevant environmental impact categories, such as global warming, acidification, etc. (see Table 2.12)
- Classification — assign LCI results to impact categories
- Characterization — model the LCI results within each impact category using science-based conversion factors (e.g., GWP)
- Normalization — express potential impacts in ways that can be compared (e.g., TEQs)
- Grouping — sort or rank the indicators
- Weighting — emphasize the most important potential impacts
- Evaluating and Reporting — gain a better understanding of the reliability of the results.
| Impact Category | Scale | Examples of LCI Data (i.e., classification) | Common Possible Characterization Factor | Description of Characterization Factor |
|---|---|---|---|---|
| Global Warming | Global | Carbon Dioxide (CO2) Nitrogen Dioxide (NO2) Methane (CH4) Chlorofluorocarbons (CFCs) Hydrochlorofluorocarbons (HCFCs) Methyl Bromide (CH3Br) |
Global Warming Potential | Converts LCI data to carbon dioxide (CO2) equivalents Note: global warming potentials can be 50, 100, or 500 year potentials. |
| Stratospheric Ozone Depletion | Global | Chlorofluorocarbons (CFCs) Hydrochlorofluorocarbons (HCFCs) Halons Methyl Bromide (CH3Br) |
Ozone Depleting Potential | Converts LCI data to trichlorofluoromethane (CFC-11) equivalents. |
| Acidification | Regional Local |
Sulfur Oxides (SOx) Nitrogen Oxides (NOx) Hydrochloric Acid (HCL) Hydroflouric Acid (HF) Ammonia (NH4) |
Acidification Potential | Converts LCI data to hydrogen (H+) ion equivalents. |
| Eutrophication | Local | Phosphate (PO4) Nitrogen Oxide (NO) Nitrogen Dioxide (NO2) Nitrates Ammonia (NH4) |
Eutrophication Potential | Converts LCI data to phosphate (PO4) equivalents. |
| Photochemical Smog | Local | Non-methane hydrocarbon (NMHC) | Photochemical Oxident Creation Potential | Converts LCI data to ethane (C2H6) equivalents. |
| Terrestrial Toxicity | Local | Toxic chemicals with a reported lethal concentration to rodents | LC50 | Converts LC50 data to equivalents; uses multi-media modeling, exposure pathways. |
| Aquatic Toxicity | Local | Toxic chemicals with a reported lethal concentration to fish | LC50 | Converts LC50 data to equivalents; uses multi-media modeling, exposure pathways. |
| Human Health | Global Regional Local |
Total releases to air, water, and soil. | LC50 | Converts LC50 data to equivalents; uses multi-media modeling, exposure pathways. |
| Resource Depletion | Global Regional Local |
Quantity of minerals used Quantity of fossil fuels used |
Resource Depletion Potential | Converts LCI data to a ratio of quantity of resource used versus quantity of resource left in reserve. |
| Land Use | Global Regional Local |
Quantity disposed of in a landfill or other land modifications | Land Availability | Converts mass of solid waste into volume using an estimated density. |
| Water Use | Regional Local |
Water used or consumed | Water Shortage Potential | Converts LCI data to a ratio of quantity of water used versus quantity of resource left in reserve. |
Note: From U.S. EPA (2006b) Note that for acidification, ISO uses SO2 equivalents
2.5.5.4 Interpretation of Results
This phase of the LCA summarizes and analyzes the results of the LCI and LCIA, with a discussion of limitations and uncertainties, especially within the context of the defined scope. The preferred chemical can be identified with a clear understanding of the uncertainty and assumptions used in the analysis. This information can be used with the other considerations in the evaluation (i.e., technical feasibility, economic feasibility, and social impact) to select the preferred alternative. The U.S. EPA (2006b) provides the following steps for completing the interpretation of results:
- Identification of significant issues based on the LCI and LCIA
- Evaluation to consider completeness, sensitivity, and consistency checks
- Conclusions, recommendations, and reporting
2.5.5.5 Resources
The components and description of LCA provided above are a general overview and outline of a complicated process. The following resources provide further guidance, more detailed information and available software for the completion of LCA7:
- ISO 2006a. Life Cycle Assessment, Principles and Framework. ISO 14040
- Describes the principles and framework for LCA; it does not, however, describe the LCA technique in detail nor does it specify methodologies for the individual phases of the LCA.
- ISO 2006b. Life Cycle Assessment, Requirements and Guidelines. ISO 14044
- Specifies requirements and provides guidelines for LCA, including the individual phases (LCIA and LCI).
- Ecobilan, Life Cycle Analysis Software, TEAM: Tool for Environmental Analysis and Management.
- Enables the user to describe any industrial system and to calculate the associated life cycle inventories and potential environmental impacts according to the ISO 14040 series of standards.
- Handbook on Life Cycle Assessment – Operational Guide to the ISO Standards (Guinée (ed.) 2002)7
- A step by step guide with operational guidelines for conducting an LCA study based on the ISO Standards for LCA. The different ISO elements and requirements are made operational to the 'best available practice' for each step.
- Todd and Curran 1999. Streamlined Life cycle Assessment: A Final Report from the SETAC (Society of Environmental Toxicology and Chemistry) North America Streamlined LCA Workgroup
- Provides recommendations for applying the goal-and-scope definition process to design and streamline an LCA study, describing how to decide what is and what is not to be included in a study while still being consistent with the original study goals and anticipated uses.
- PE International, GaBi 4 Software.
- A software tool for modelling products and systems from a life cycle perspective, packaged with the life cycle database of the user’s choice to provide the most relevant life cycle data and social parameters. Allows users to build models for any product, balance emissions and material and energy inputs and outputs, aggregate results, generate charts, and create interactive reports.
- PRé Consultants, SimaPro 7.2 LCA Software
- A tool to collect, analyze and monitor the environmental performance of products and services by modelling complex life cycles in a systematic and transparent way, following the ISO 14040 series recommendations.
- State of California 2009. Life Cycle Cost Assessment (LCCA) Model
- The Life Cycle Cost Assessment (LCCA) Model) model is a key tool in determining the cost effectiveness of implementing energy conservation measures in state facilities, which can have a higher first cost than standard measures.
- Sustainable Minds LCA Software
- A comprehensive and standardized web-based system that allows you to estimate, evaluate, compare and track the life cycle environmental and human health performance of products in the earliest stages of design.
- U.S. EPA 2006a. Life Cycle Assessment: Principles and Practice.
- An introductory overview of LCA, describing the general uses and four basic stages of conducting an analysis.
2.6 Select and Implement Preferred Alternative
Purpose: This section provides guidance for the selection of a preferred chemical alternative.
If you are a small/medium company… This section may not be applicable, but should be considered.
If you are a large company… This is the final step in the alternatives assessment and selection criteria will vary based on individual goals and considerations.
There are many criteria which need to be considered when conducting an alternatives assessment. Not only must the evaluation consider the human and environmental toxicology of the chemical or process, but it must also consider the technical feasibility, financial feasibility and social impact of the alternative and impacts over the life cycle of the alternative. The selected alternative chemical should be less hazardous than the current chemical to humans and the environment, and should also pose less risk (i.e., hazard combined with exposure potential). However, the alternative must be technically and financially feasible while still producing a product that is acceptable to consumers.
A clear and documented decision process with specified decision parameters allows for discussion and interpretation by other parties. Decisions will have a framework behind them, with transparency and consistency that an informal decision making process lacks. A formal process removes the arbitrary appearance of the decision and also allows for constructive conversations with other parties. However, it should be noted that the selection of an alternative chemical is a subjective process which involves trade-offs among multiple decision criteria that depend on the preferences of the decision makers.
2.6.1 Selecting the Preferred Alternative
The selection of a preferred alternative is complex and as discussed previously should only be evaluated by experts with knowledge in the area. This will minimize the potential for unintended consequences, such as switching to a poorly understood and potentially more hazardous chemical (Lavoie et al. 2010). It is not likely that one alternative chemical will be superior for all attributes and considerations; therefore, prioritization of the various attributes will be necessary for the selection of a preferred alternative. Prioritization can be qualitative or quantitative and is subject to the procedures and goals of the organization conducting the alternatives assessment. The goal of prioritization is to consider the attributes of the alternatives within the context of the specific features that are the most important for the selection of the preferred alternative and, conversely, to place less importance on factors that are not as relevant for the selection.
For a qualitative selection, some possible guidelines are suggested which are in sync with the 12 Principles of Green Chemistry (Section 1.2):
- Avoid alternatives that are PBTs or which generate PBTs over their life cycle.
- Favour alternatives that eliminate undesirable chemicals/components from a process/formulation.
- Favour alternatives that reduce resource consumption and use renewable feedstocks.
It is important to reiterate that, as discussed in Section 1.2, an alternative chemical is not necessarily ‘safer’ simply because it follows one or more of the 12 Principles of Green Chemistry.
Specific guidelines for a qualitative selection can be developed for an organization based on their principles and goals. Discussions among decision makers and stakeholders could be important at this stage of the assessment.
A quantitative evaluation typically involves the development of ranking metrics and weighting factors. Weighting factors will allow for the prioritization of the various components of the alternatives assessment and also provide an opportunity for stakeholder involvement in the decision making process. Rossi et al. (2006) offer some suggestions for a quantitative evaluation:
- Create summary tables/decision matrices from the evaluation modules to support the selection process (i.e., for each alternative under consideration, summarize toxicity data, persistence, bioaccumulation, hazard information (e.g., Section 2.5.1).
- Consider assigning each performance attribute a ranking and a weight depending on the importance to the organization and calculate a score for each alternative using the ranking and weight criteria; applying a consistent ranking and weighting system allows for a relative comparison between the alternatives under consideration.
The TURI Pollution Prevention Options Analysis System (P2OASys) tool provides an example of the application of ranking and weighting of the evaluated categories. Figure 2.3 provides a portion of the P2OASys Standardized Hazard Score Database, showing a summary of the scoring methodology for some of the criteria from P2OASys that are common to the criteria considered in this reference tool (the complete Database is provided in Appendix A). The user inputs a value for each criterion, and that criterion is then assigned a score of 2, 4, 6, 8 or 10. The user also inputs a certainty value (from 0 to 100) to provide an estimate of the reliability of the input data. The products of the individual scores and certainties are computed, and the criteria with the two greatest values of the product are selected. The corresponding individual scores are averaged, as are the certainties, to provide overall scores for each general category (i.e., acute health effects). Each general category is then assigned a user-defined weighting value (default is 10), and the weighted final score for each chemical is computed by the following equation:
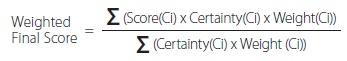
| Category | Units | Score 2.00 | Score 4.00 | Score 6.00 | Score 8.00 | Score 10.00 |
|---|---|---|---|---|---|---|
| Inhalation LC50 | ppm | 10,000 | 1,000 | 150 | 15 | < 15 |
| PEL/TLV | ppm | 200 | 100 | 25 | 5. | < 5 |
| PEL/TLV (dusts/particles) | mg/m3 | 10 | 5 | 1 | 0.1 | < 0.1 |
| Oral LD50 | mg/kg | 5,000 | 500 | 50 | 5 | < 5 |
| Dermal LD50 | mg/kg | 5,000 | 500 | 50 | 5 | < 5 |
TURI, year not reported, available online (P2OASys Tool to Compare Materials).
| Category | Units | Score 2.00 | Score 4.00 | Score 6.00 | Score 8.00 | Score 10.00 |
|---|---|---|---|---|---|---|
| Reference Dose RfD | mg/kg/day | 0.1 | 0.05 | 0.01 | 0.001 | < 0.001 |
| Carcinogen | IARC/EPA Class | 4,E | 3,D | 2B,C | 2A,B | 1,A |
| Mutagen | L/M/H | L | L/M | M | M/H | H |
| Reproductive effects | L/M/H | L | L/M | M | M/H | H |
| Neurotoxicity | L/M/H | L | L/M | M | M/H | H |
| Developmental effects | L/M/H | L | L/M | M | M/H | H |
Note: L = low, M = medium, H = high (level of concern)
TURI, year not reported, available online (P2OASys Tool to Compare Materials).
| Category | Units | Score 2.00 | Score 4.00 | Score 6.00 | Score 8.00 | Score 10.00 |
|---|---|---|---|---|---|---|
| Water Quality Criteria | mg/L | > 10 | 6-8 | 4-6 | 1-4 | < 1 |
| Aquatic LC50 | mg/L | 1,000 | 50 | 1 | 0.1 | < 0.1 |
| Fish NOAEC | mg/L | 0.2 | 0.02 | 0.002 | 0.0002 | < 0.0002 |
| Plant EC50 | mg/L | 100 | 10 | 1 | 0.1 | < 0.1 |
TURI, year not reported, available online (P2OASys Tool to Compare Materials).
| Category | Units | Score 2.00 | Score 4.00 | Score 6.00 | Score 8.00 | Score 10.00 |
|---|---|---|---|---|---|---|
| Persistence | L/M/H | L | L/M | M | M/H | H |
| Bioconcentration | log Kow | 1 | 2 | 4 | 6 | > 6 |
| Bioconcentration factor (BCF) | kg/L | 10 | 100 | 200 | 1,000 | > 1,000 |
Note: L = low, M = medium, H = high (level of concern)
TURI, year not reported, available online (P2OASys Tool to Compare Materials).
| Category | Units | Score 2.00 | Score 4.00 | Score 6.00 | Score 8.00 | Score 10.00 |
|---|---|---|---|---|---|---|
| Greenhouse gas | Y/N | No metrics provided | No metrics provided | No metrics provided | No metrics provided | No metrics provided |
| Ozone depletory | ODP units | No metrics provided | No metrics provided | No metrics provided | No metrics provided | No metrics provided |
| Acid rain formation | Y/N | No metrics provided | No metrics provided | No metrics provided | No metrics provided | No metrics provided |
TURI, year not reported, available online (P2OASys Tool to Compare Materials).
| Category | Units | Score 2.00 | Score 4.00 | Score 6.00 | Score 8.00 | Score 10.00 |
|---|---|---|---|---|---|---|
| Vapour pressure | mm Hg | 0.1 | 1 | 10 | 100 | > 100 |
| Flammability | 0,1,2,3,4 | 0 | 1 | 2 | 3 | 4 |
| Flash point | °C | 100 | 75 | 25 | 10 | < 10 |
| Reactivity | 0,1,2,3,4 | 0 | 1 | 2 | 3 | 4 |
| pH | pH units | 7 | 6-7, 7-8 | 5-6, 8-9 | 3-5, 9-11 | 1-3, 11-14 |
| Corrosivity | L/M/H | L | L/M | M | M/H | H |
Note: L = low, M = medium, H = high (level of concern)
TURI, year not reported, available online (P2OASys Tool to Compare Materials).
| Category | Units | Score 2.00 | Score 4.00 | Score 6.00 | Score 8.00 | Score 10.00 |
|---|---|---|---|---|---|---|
| Upstream effects | L/M/H | L | L/M | M | M/H | H |
| Consumer hazard | L/M/H | L | L/M | M | M/H | H |
| Disposal hazard | L/M/H | L | L/M | M | M/H | H |
Note: L = low, M = medium, H = high (level of concern)
TURI, year not reported, available online (P2OASys Tool to Compare Materials).
| Category | Units | Score 2.00 | Score 4.00 | Score 6.00 | Score 8.00 | Score 10.00 | |
|---|---|---|---|---|---|---|---|
| Exposure potential | Disposal hazard | L/M/H | L | L/M | M | M/H | H |
Note: L = low, M = medium, H = high (level of concern)
TURI, year not reported, available online (P2OASys Tool to Compare Materials).
This is illustrated in Figure 2.4 using trichloroethylene as the current chemical and acetone as the alternative (example obtained from TURI). The assessor must use professional judgement or obtain expert input to evaluate the information collected in the previous sections in order to assign weighting factors to each category.
| Category | Current Process (Trichloroethylene): Score | Current Process (Trichloroethylene): Certainty | Alternative 1 (Acetone): Score | Alternative 1 (Acetone): Certainty | Value: Weight |
|---|---|---|---|---|---|
| Acute human effects | 10 | 100 | 9 | 100 | 10 |
| Chronic human effects | 7 | 100 | 5 | 100 | 10 |
| Physical hazards | - | - | - | - | 10 |
| Aquatic hazard | 7 | 100 | 2 | 100 | 10 |
| Persistence/bioaccumulation | 9 | 100 | 7 | 100 | 10 |
| Atmospheric hazard | - | - | - | - | 10 |
| Disposal hazard | 6 | 100 | 2 | 100 | 10 |
| Chemical hazard | 9 | 100 | 10 | 100 | 10 |
| Energy/resource use | - | - | - | - | 10 |
| Product hazard | 5 | 100 | 2 | 100 | 10 |
| Exposure potential | 8 | 100 | 4 | 100 | 10 |
| Final | 61 | - | 41 | - | 110 |
| Weighted Final | 7.63 | 100.00 | 5.13 | 100.00 | - |
TURI, year not reported, available online (P2OASys Tool to Compare Materials).
As discussed in the Hazard Assessment (Section 2.5.1), toxicity data may not be available for all categories. A lack of data does not preclude a chemical from further assessment, but it does increase the uncertainty in the assessment and caution is therefore critical when interpreting the data and applying weighting factors. If resources are available, laboratory testing can be conducted to obtain the missing information (see Section 3.0). It must also be noted that ranking and comparison of toxicity values is not straightforward as there are many factors which can influence a value. For example, an LC50 value may be available for one of the alternative chemicals while only a NOAEL is available for another alternative chemical, both of which represent different effects on a population of organisms and therefore cannot be compared directly. Additionally, comparison of toxicity values of the same endpoint effect may be confounded by the fact that these values are generally derived experimentally and experimental conditions and results can vary greatly from one test to another (e.g., length of study, organism studied, etc.). In short, interpretation of the hazard assessment data is complex and caution must be exercised by the assessor who should be an expert in the field.
Some additional considerations from DfE (1996) include the availability of funds for capital investments, if required, as well as the outlook for the overall market of the product being developed. Additionally, an alternative may appear less attractive due to higher costs in one or more areas as a result of emerging technologies; these costs may be subject to changing economic conditions as the technologies develop and this should be kept in mind when evaluating the alternative chemicals. Again, discussion among decision makers and stakeholders is critical in the development of weighting factors specific to the organization to aid in the selection process.
2.6.2 Implementation and Continuous Monitoring
Once an alternative chemical is selected, it is ideal that the alternative chemical is actually implemented. However, before implementation, the process flow diagram and mass balance of the process should be reconstructed with the selected alternative to ensure that the process will be functional. If everything is in order, then sourcing of any new equipment or facility upgrades can commence. Once the alternative is in place and the process is operational, the process should be monitored carefully to ensure that it is operating as expected. It must be reiterated that this reference tool has been developed for a ‘drop-in’ alternative chemical; however, alternatives assessments can be conducted for process changes as well. The field of green chemistry is a rapidly evolving field; as such, alternatives assessment should not be viewed as a finite process. Periodic revisiting of the alternatives assessment to update it with information on, for example, new and emerging alternatives, new data on environmental fate and human health effects, etc., may be beneficial.
2.6.3 Resources
In addition to the TURI P2OASys tool, the following is a summary list of the programs that have been discussed in more detail previously in the reference tool and which provide examples of the selection of preferred alternatives:
- DfE CTSA Methodology (1996) (now replaced by the Alternatives Assessment (AA) Methodology (Lavoie et al. 2010))
- Neither the CTSA nor the updated AA processes recommend alternative chemicals; however, they do provide the information for informed business decisions that account for risk, performance, and cost concerns, potentially reducing their regulatory burden or potential liability costs or avoiding regulation altogether. Considerations for the selection of an alternative are provided.
- SCRAM (Snyder et al. 2000a, b, c and d, U.S. EPA 2002)
- SCRAM provides a score based on the uncertainty of the information available for a chemical. The final composite scores can be used to make the ultimate decision on the selection of an alternative chemical.
- Chemical and Ranking Assessment System (CARS) (ZWA 2003)
- The CARS database is a decision support tool for assessing chemicals. The user of the database identifies relative rankings of the various assessment properties and the database provides a ranking of the chemicals of interest to aid in the selection of an alternative chemical.
- Clean Production Action (CPA) Green Screen for Safer Chemicals (Rossi and Heine 2007)
- In the Green Screen, a chemical is evaluated by comparison to a series of four benchmarks, with each benchmark defining a progressively safer chemical. Similar to other programs, the criteria used in this program are persistence, bioaccumulation and toxicity. The levels of concern are defined by threshold values that are quantitative, qualitative, or based on expert references and aim to harmonize existing hazard classification and labelling systems.
- TURI Five Chemicals Alternative Assessment Study (TURI 2006b)
- Alternatives that passed screening based on health and environmental effects and PBT were prioritized for further study by assessment of chemical- and application-specific criteria in the categories of technical performance, health and environmental effects, economic considerations, and importance to stakeholders. Each alternative was evaluated against the reference (current) chemical by assigning a ‘+’, ‘−’ or ‘=’ to each criterion (better than, worse than or equal to the reference). No conclusions were drawn as to which alternative was preferred.
- TURI Alternatives Assessment Process Guidance (TURI 2006a)
- This document defines a consistent process for setting priorities for study and evaluating the alternatives for the five chemicals evaluated by TURI (2006b). Metrics are provided to evaluate the feasibility of each alternative based on technical, financial, environmental hazards and human health and safety criteria. This document also includes suggestions for specific types of resources for certain phases of the study.
- The MOE categorization procedure of Phase I and Phase II substances (MOE 2009a)
- This document provides a ranking procedure for identifying toxic substances and substances of concern. Hazard scoring information was obtained from RSEI (Risk-Screening Environmental Indicators) and SCRAM (the Scoring and Ranking Assessment Model). Scores from RSEI and SCRAM were converted to rankings. The relevance of the chemical to MOE program areas, and also other programs and agencies, was a consideration.
1 Under the Toxics Reduction Act, 2009 and O. Reg. 455/09 (toxic substance accounting), regulated facilities must track and quantify prescribed substances on a process level.
2 Under the Toxics Reduction Act, 2009 and O. Reg. 455/09 regulated facilities must prepare a process flow diagram for prescribed substances.
3 Under the Toxics Reduction Act, 2009 and O. Reg. 455/09 regulated facilities must prepare a process flow diagram for prescribed substances.
4 Under the Toxics Reduction Act, 2009 and O. Reg. 455/09 regulated facilities must identify technically feasible reduction options.
5 Under the Toxics Reduction Act, 2009 and O. Reg. 455/09 regulated facilities must undertake an economic feasibility analysis for all technically feasible options.
6 Under the Toxics Reduction Act, 2009 and O. Reg. 455/09 regulated facilities must consider LCI for prescribed substances.
7 The following programs and software are examples of the available tools and resources only and do not represent endorsement by the Ontario Ministry of the Environment.