1,4-Dichlorobenzene, 3,3-Dichlorobenzidine, Hexachlorobenzene, and Pentachlorophenol
This document identifies Best Management Practices (BMPs) to eliminate or reduce four substances in wastewater effluents of industrial sectors: 1,4-dichlorobenzene, 3,3-dichlorobenzidine, hexachloro-benzene (HCB), and pentachlorophenol (PCP). The two primary audiences for this document are municipal and industrial facility representatives that potentially use or produce these substances.
March 6, 2007
Prepared for:
Ministry of the Environment
Land and Water Policy Branch
135 St. Clair Avenue West, 6th Floor
Toronto, Ontario
M4V 1P5
PIBS 6212e
This Best Management Practices (BMPs) document has been prepared by a consultant team headed by XCG Consultants Ltd. to describe various BMP activities available through literature review and through application of the consultant’s knowledge and professional opinion of potential BMP approaches to achieve pollution prevention and a reduction of specific contaminants that may be present in effluent discharges from selected industrial, institutional and commercial (IC&I) facilities.
The document also outlines preliminary opinions of estimated costs for treatment processes that may be required to reduce and/or remove specific contaminants as a function of effluent reference discharge criteria. The document provides qualitative and quantitative estimates of potential reductions achievable through treatment measures for specific pollutants and is intended to be a 'guidance' document only. Site-specific analysis of each facility is required to identify the most effective pollution prevention and treatment measures.
The document does not reflect the official position or the views of the Government of Ontario or the Ministry of the Environment.
Executive Summary
This Best Management Practices (BMPs) document is one in a series of documents to identify BMPs to eliminate or reduce specific harmful pollutants potentially found in wastewater effluents of six industrial sectors in Ontario. This document differs from others in the series because it does not focus on a specific industrial sector. This and the sector-specific documents in this series provide qualitative and quantitative estimates of the potential reductions achievable through pollution prevention and treatment measures for specific pollutants of concern. This BMP document is a guide only; site-specific analysis of each facility is required to identify the most effective pollution prevention and treatment measures.
This document identifies BMPs to eliminate or reduce four substances in wastewater effluents of industrial sectors: 1,4-dichlorobenzene, 3,3-dichlorobenzidine, hexachloro-benzene (HCB), and pentachlorophenol (PCP). The two primary audiences for this document are municipal representatives and industrial facility representatives that potentially use or produce these substances.
Benefits of implementing BMPs, specifically pollution prevention measures, include but are not limited to, the following:
- Increased cost-effectiveness and lower long-term costs;
- Increased customer satisfaction;
- Social benefits, such as good community relations;
- Reductions in energy, water and materials used; and
- Reduced risk and liability.
The common use of 1,4-dichlorobenzene is as a space deodorant for restrooms and waste containers, and as a fumigant for control of moths, moulds, and mildews. Use of 1,4-dichlorobenzene in the production of polyphenylene sulfide (PPS) resin has increased steadily over recent years. 1,4-Dichlorobenzene is also used as an intermediate in the production of other chemicals and may be used for manufacture of dyes and pharmaceuticals. 1,4-Dichlorobenzene is a United States Environmental Protection Agency (USEPA) registered pesticide. The substance is highly volatile, and most references in the literature are made with respect to air emissions. In Canada, 1,4-dichlorobenzene is classified as a Tier II substance targeted for reduction in the Canada-Ontario Agreement (COA) respecting the Great Lakes Basin Ecosystem.
The main use of 3,3-dichlorobenzidine is in the production of pigments for the printing ink, textile, paper, paint, rubber, leather, plastic, and related industries. Although 3,3-dichlorobenzidine is not currently produced, imported, or used in Canada, the dihydrochloride salt of 3,3-dichlorobenzidine is currently used in Canada, primarily for manufacture of yellow and orange "azo" pigments. The 3,3-dichlorobenzidine-based pigments are mainly used for printing inks; almost all yellow printing inks are coloured with this type of pigment. In Canada, 3,3-dichlorobenzidine is classified as a Tier II substance targeted for reduction in the COA respecting the Great Lakes Basin Ecosystem.
Hexachlorobenzene (HCB) was used as a fungicide (pesticide) from the 1940s to the late 1970s. In Canada, it has not been used commercially since 1972. Although HCB itself is no longer used directly as a pesticide, it is currently formed as a by-product in the production of chemical solvents, chlorine, and chlorine-containing compounds, such as ferric/ferrous chloride, and is a component of several currently used pesticides. HCB is also found in certain pigments, which are used for paints, inks, plastics, and coatings. In Canada, HCB is classified as a Tier I substance targeted for virtual elimination in the COA respecting the Great Lakes Basin Ecosystem.
Pentachlorophenol (PCP) is used as a pesticide, and in oil-based paints, glue preservatives (e.g., in leather and toilet paper), and as an intermediate in pharmaceutical synthesis and colouring substances in the chemical manufacturing industry. PCP compounds are also used in the textiles industry as a fungicide on heavy-duty textiles and fibres subject to attack by fungi and bacteria during storage and use (e.g., covers, tarpaulins, awnings, tents, webbing and netting, and ropes). PCP is registered for use by the USEPA as an insecticide (termicide), fungicide, herbicide, mollucide, algicide, disinfectant, and as an anti-fouling ingredient in paint. Production of PCP in the European Union (EU) ceased in 1992, and the marketing and use of PCP and its compounds was largely prohibited in 1991. In Canada, PCP is classified as a Tier II substance targeted for reduction in the COA respecting the Great Lakes Basin Ecosystem.
In developing the BMP guidance documents, three reference criteria were considered with respect to final effluent concentrations for harmful substances. The three reference criteria are identified in Table ES.1. Reference Criteria 1 are the most stringent and Reference Criteria 3 are the least stringent. Due to the methodology applied to develop the reference criteria, as elaborated within the main text, two of the three reference criteria are the same in some instances.
| Substance | Reference Criteria 1 (mg/L) | Reference Criteria 2 (mg/L) | Reference Criteria 3 (mg/L) |
|---|---|---|---|
| 1,4-Dichlorobenzene | 0.0005 | 0.017 | 0.47 |
| 3-3-Dichlorobenzidine | 0.0006 | 0.002 | 0.002 |
| Hexachlorobenzene | 0.0000065 | 0.0001 | 0.0001 |
| Pentachlorophenol | 0.0005 | 0.005 | 0.005 |
BMPs are described in this document in four categories: elimination and reduction; operating and housekeeping; education and training; and treatment. The first three categories are considered pollution prevention (P2) measures; treatment is not. P2 is defined as
"the use of processes, practices, materials, products, substances or energy that avoid or minimize the creation of pollutants and waste, and reduce the overall risk to the environment or human health."footnote 1
P2 measures are more effective than treatment in reducing releases of hazardous substances and should, therefore, be implemented in preference to treatment to meet required release reference criteria. Multiple P2 measures can be implemented concurrently.
Table ES.2 identifies the pollution prevention BMPs described inthis document.
Table ES.2
Summary of P2 Measures for 1,4-Dichlorobenzene, 3,3-Dichlorobenzidine, HCB, and PCP
| Substance Addressed | BMP Name | BMP Number |
|---|---|---|
| 1,4-dichlorobenzene | Eliminate 1,4-dichlorobenzene containing space deodorizers | BMP #1 |
| 1,4-dichlorobenzene | Substitute 1,4-dichlorobenzene -containing agents used for moths, moulds, mildews | BMP #2 |
| 3,3-dichlorobenzidine | Substitute 3,3-dichlorobenzidine containing pigments | BMP #3 |
| HCB | Substitute HCB-containing Pigments | BMP #4 |
| HCB | Minimize the Use of Chlorinated Solvents | BMP #5 |
| PCP | Substitution and Prohibition of PCP use in Textiles | BMP #6 |
| Substance Addressed | BMP Name | BMP Number |
|---|---|---|
| All | Modify Cleaning Procedures | BMP #7 |
| Substance Addressed | BMP Name | BMP Number |
|---|---|---|
| All | Management and Staff Training | BMP #8 |
In the case of these four substances, very little information was available in literature regarding their presence in wastewater effluents of the industrial sectors of interest. Similarly, little information was available on the effectiveness of P2 measures to reduce or eliminate the substances. Unlike other BMP documents in this series, there is no analysis of the combinations of P2 measures required to reduce the concentrations of these substances. However, potential treatment scenarios are identified and costs estimated with respect to achieving the required reductions to meet the reference criteria. Potential P2 measures are, nevertheless, identified that could be useful in reducing the presence of these substances in wastewater effluents. Treatment measures required to achieve the three reference criteria (Table ES.1) were developed using estimates and engineering judgment. Cost ranges for treatment capital and operating costs are also estimated. Cost estimates for treatment systems were based on a range of assumed wastewater flow rates.
Capital and annual operational and maintenance (O&M) costs were developed for the reference criteria for each substance using a wastewater flow range of 1 m3/h to 50 m3/h. Table ES.3 presents a summary of the treatment processes determined for each substance to meet the reference criteria, and Table ES.4 presents a summary of the estimated capital and O&M cost data for wastewater treatment.
| Substance | Estimated Treatment Requirement |
|---|---|
| 1,4-Dichlorobenzene: Reference Criteria 1 | Air stripping, sand/mixed media filtration, and GAC |
| 1,4-Dichlorobenzene: Reference Criteria 2 and 3 | Air stripping |
| 3,3-Dichlorobenzidine: Reference Criteria 1, 2 and 3 | Sand/mixed media filtration, GAC, microfiltration, and AOT |
| HCB: Reference Criteria 1, 2 and 3 | Sand/mixed media filtration, GAC, microfiltration, and AOT |
| PCP: Reference Criteria 1 | Sand/mixed media filtration, GAC, microfiltration, and AOT |
| PCP: Reference Criteria 2 and 3 | Sand/mixed media filtration, and GAC |
| Reference Criteria | Capital Cost Range: 1 m³/h | Capital Cost Range: 25 m³/h | Capital Cost Range: 50 m³/h | Annual O&M Cost Range: 1 m³/h | Annual O&M Cost Range: 25 m³/h | Annual O&M Cost Range: 50 m³/h |
|---|---|---|---|---|---|---|
| 1,4-Dichlorobenzene: Criteria 1 | $152,000 | $585,000 | $949,000 | $23,000 | $70,000 | $95,000 |
| 1,4-Dichlorobenzene: Criteria 2 and 3 | $113,000 | $226,000 | $336,000 | $17,000 | $27,000 | $34,000 |
| 3,3-Dichlorobenzidine: Criteria 1 | $360,000 | $1,802,000 | $3,464,000 | $54,000 | $216,000 | $346,000 |
| 3,3-Dichlorobenzidine: Criteria 2 and 3 | $360,000 | $1,668,000 | $3,199,000 | $54,000 | $200,000 | $320,000 |
| HCB: Criteria 1 | $360,000 | $1,802,000 | $3,464,000 | $54,000 | $216,000 | $346,000 |
| HCB: Criteria 2 and 3 | $360,000 | $1,668,000 | $3,199,000 | $54,000 | $200,000 | $320,000 |
| PCP: Criteria 1 | $360,000 | $1,802,000 | $3,464,000 | $54,000 | $216,000 | $346,000 |
| PCP: Criteria 2 and 3 | $70,000 | $438,000 | $748,000 | $10,000 | $53,000 | $75,000 |
Note that estimates are dependent on the incoming concentrations of the pollutants identified prior to P2 measures. Concentrations may be reduced through the effective implementation of P2 measures and may reduce or eliminate the levels of treatment required for each substance resulting in reduced treatment costs. Site-specific wastewater testing is necessary at all facilities to determine compliance with regulations and to implement appropriate measures.
Overview of this document (1)
1.1 Objective and Audience
This document identifies best management practices (BMPs) to eliminate or reduce 1,4-dichlorobenzene, 3,3-dichlorobenzidine, hexachlorobenzene (HCB), and pentachlorophenol (PCP) in wastewater effluents of industrial sectors. The benefits of undertaking BMPs are also described. This BMP document is a guide only; site-specific analysis of each facility is required to identify the most effective pollution prevention and treatment measures.
This document is one in a series of documents to identify BMPs to eliminate or reduce specific harmful pollutants potentially found in wastewater effluents of six industrial sectors in Ontario. Unlike other documents in this series, this guide does not focus on a single industrial sector. Appendix A identifies the other industrial sectors and substances for which similar BMP documents have been developed.
The two primary audiences for this document are:
- Municipal representatives interested in assisting industrial facilities with sewer discharges to eliminate or reduce harmful pollutants.
- Industrial facility representatives interested in implementing BMPs to eliminate or reduce harmful pollutants, and to increase company reputation for implementing 'green policies', specifically industrial facility operations staff and management staff.
The four harmful pollutants addressed in this BMP document have been identified at both the federal and provincial government levels, as part of on-going initiatives to limit the effect of wastewater discharges on receiving waters. Appendix B provides a list of agreements and programs, as well as substances identified by the Ontario MOE to be of particular concern under these or other initiatives.
1.2 Benefits of Implementing Pollution Prevention
In addition to reductions in pollutants released to water, air, and soil, implementation of pollution prevention best management practices and high quality environmental performance has numerous benefits:
- Increased cost-effectiveness and lower long-term costs through implementation of pollution prevention measures in a planned, holistic manner;
- Increased customer satisfaction through meeting expectations for goods and services to be produced in an environmentally responsible manner;
- Social benefits, such as good community relations and potential endorsement of facility efforts and activities;
- Compliance with federal and municipal regulations;
- Reduced risk and liability resulting from regulatory non-compliance, spills, and environmental emergencies;
- Increased innovation through process and materials improvements and supply chain communication;
- Better return on investment for shareholders;
- Health and safety benefits through reduced worker exposure; and
- Higher public approval ratings and improved corporate reputation.
A study of the relationship between environmental performance and financial performance,
Literature references on pollution prevention do not generally quantify benefits and cost savings resulting from implementation of P2 measures. Individual case studies, however, often do identify cost savings and benefits. Refer to Appendix C, Case Study Examples Demonstrating Benefits of P2 Measures for case studies of facilities that have documented the benefits of implementing P2 measures while, at the same time, reducing releases of hazardous substances.
1.3 Methodology
This BMP document was developed by a consultant team with the advice of a Steering Committee of provincial and municipal representatives. A detailed review of literature was conducted by the consultant team to identify available information on specific substancesector combinations. Sector specialists and other representatives identified through the literature review were contacted for additional information and to obtain recent data, where available. Engineering estimates and consultant team expertise also supported the analysis and development of the BMP documents.
For 1,4-dichlorobenzene, 3,3-dichlorobenzidine, HCB, and PCP, limited information was found in literature on pollution prevention for specific industrial processes. For this reason, a more general BMP document that is non-sector specific has been developed to identify pollution prevention measures and treatment options to achieve the reference criteria.
1.4 How to Use This Document
In addition to this introductory section, this BMP document consists of the following sections:
- Section 2, Background, provides information on the use of the substances of interest, reference criteria used to analyze and develop the BMPs and reporting requirements for the substances.
- Section 3, Pollution Prevention, identifies pollution prevention (P2) options, including operating, housekeeping, training and education opportunities and suggestions.
- Section 4, Treatment, identifies the specific combinations of treatment to achieve the three reference criteria levels, including underlying assumptions for the reduction analyses.
- Section 5, References, identifies key reference documents used in the development of this BMP document.
- Section 6, Glossary, defines terminology and acronyms used in the document.
- Appendices provide information on other documents in this series, a list of harmful substances of particular interest, and case studies.
Once having read this document, practitioners are encouraged to:
- Assess the concentration of identified substances in the effluent of their facility versus the three reference criteria analyzed (Section 2.2).
- Identify potential sources of these substances in their effluent and assess pollution prevention and treatment options, as well as broader management practices (Sections 3 and 4).
- Review the assumptions stated in Sections 3 and 4 and information presented in the Tables of Section 4 for an indication of measures that could potentially be implemented to meet the target reference criteria.
- Refer to municipal sewer use by-laws or other requirements applicable to the facility with respect to control requirements for the substances.
- Refer to other relevant sector-specific information in BMPs that have been prepared and may contain applicable and useful information as guidance to potential implementation of P2 and treatment measures.
Background (2)
2.1 Substances of Interest
This BMP addresses the list of substances noted in Table 2.1. Some industrial sectors
| Substance | Sample sectors (others may apply) |
|---|---|
| 1,4-dichlorobenzene |
|
| 3,3-dichlorobenzidine | Chemical Manufacturing Sector (NAICS 325) |
| HCB | Chemical Manufacturing Sector (NAICS 325) |
| PCP |
|
Due to the general uses of some of the products containing the substances listed (e.g., space deodorizers), this BMP may be relevant for industry sectors other than those listed above.
For the purposes of assessing the effectiveness of treatment technologies, representative raw wastewater concentrations of the substances addressed in this document have been estimated as summarized in Table 2.2. The raw wastewater concentrations in Table 2.2 were determined from a review of available data for the sectors identified in Table 2.1. In the data reviewed, concentrations of pollutants in wastewaters varied greatly. Each facility should assess its wastewater components, as the compounds listed in Table 2.2 may be found at higher, lower or negligible concentrations, depending on operating conditions and existing pollution prevention and treatment practices.
| Substance | Concentration in Wastewater (prior to pollution prevention or treatment) (mg/L) |
|---|---|
| 1,4-Dichlorobenzene | 9.7 |
| 3,3-Dichlorobenzidine | 6.1 |
| Hexachlorobenzene | 0.24 |
| Pentachlorophenol | 1 |
This BMP document addresses specifically the compounds listed in Table 2.2. Other compounds that may be present in the wastewater should be identified as they may be reduced by practices identified herein or by other practices.
2.1.1 1,4-Dichlorobenzene
Chlorinated benzenes are produced by chlorination of benzene in the presence of a catalyst. The chlorobenzene isomers produced include 1,2-dichlorobenzene, 1,3-dichlorobenzene, and 1,4-dichlorobenzene. Crystallisation combined with distillation is used to obtain separation of the isomers.
1,4-Dichlorobenzene is a neutral, colourless, flammable solid
1,4-Dichlorobenzene has been used principally as a space deodorant for restrooms and waste containers, and as a fumigant for control of moths, moulds, and mildews. Use of 1,4-dichlorobenzene in the production of polyphenylene sulfide (PPS) resin has increased steadily over recent years. 1,4-Dichlorobenzene is also used as an intermediate in the production of other chemicals and may be used for manufacture of dyes and pharmaceuticals.
1,4-Dichlorobenzene is highly volatile; therefore, most references in the literature are made with respect to air emissions. For example, the 1995 TRI Releases for Textiles Manufacturing Facilities
A summary of the potential sources of 1,4-dichlorobenzene in wastewater from the Chemical Manufacturing sector is presented in Table 2.3.
| Sub-sector | Potential Sources of 1,4-dichlorobenzene |
|---|---|
| Basic Chemical Manufacturing (NAICS 3251) | Intermediate by-product in chemical manufacturing. Production of dyes. |
| Resin, Synthetic Rubber, and Artificial SyntheticFibres, and Filaments Manufacturing (NAICS 3252) | Use in production of polyphenylene sulphide resin. |
| Pesticide, Fertilizer, and Other Agricultural Chemical Manufacturing (NAICS 3253) | Use in pesticide manufacturing. |
| Pharmaceutical and Medicine Manufacturing (NAICS 3254) | Intermediate in synthesis of pharmaceuticals. |
| Paint, Coating, and Adhesive Manufacturing (NAICS 3255) | N/A |
| Soap, Cleaning Compound, and Toilet Preparation Manufacturing (NAICS 3256) | Manufacture of air fresheners and urinal deodorizers. |
| Other Chemical Product and Preparation Manufacturing (NAICS 3259) | Aerosol can manufacturing; intermediate/by-product in chemical manufacturing. |
Note: N/A No information was available regarding potential sources of 1,4-dichlorobenzene in this sub-sector.
Table 2.4 summarizes where 1,4-dichlorobenzene can be found in the dry cleaning and laundry service sector.
| Sub-Sector | Where 1,4-dichlorobenzene May be Found in Process |
|---|---|
| Dry cleaning and laundry services (except Coin-Operated) (NAICS 812320) | Chemicals used in the laundering process. Dyes and moth-proofing material washing off in post treatments. |
| Linen and uniform supply (NAICS 812330) | Chemicals used in the laundering process. Dyes and moth-proofing material washing off in post treatments during processing. |
A summary of the potential sources of 1,4-dichlorobenzene in wastewater from the Textiles Sector is presented in Table 2.5.
| Sub-Sector | Where 1,4-Dichlorobenzene May be Found in Process |
|---|---|
| Fibre, Yarn, Thread Mills (NAICS 3131) | Insecticidal fumigant against clothing moths (accounting for approximately 35 to 55% of the 1,4-dichlorobenzene produced), mildew and mould repellent for textiles finishing.
Dyes Insecticide and fungicide on crops (and so may be present in incoming raw natural materials used by mills). Deodorant for garbage, as a room deodorizer, and in restroom urinal and toilet bowl blocks. |
| Fabric Mills (NAICS 3132); | Refer to “Fibre, Yarn, Thread Mills” |
| Textile and Fabric Finishing and Fabric Coating (NAICS 3133) | Refer to “Fibre, Yarn, Thread Mills” |
2.1.2 3,3-Dichlorobenzidine
3,3-Dichlorobenzidine is a synthetic chlorinated primary aromatic amine.
The prime use of 3,3-dichlorobenzidine in the Chemical Manufacturing Sector is in the production of pigments for the printing ink, textile, paper, paint, rubber, leather, plastic, and related industries.
A summary of the potential sources of 3,3-dichlorobenzidine in wastewater from the Chemical Manufacturing sector is presented in Table 2.6.
| Sub-sector | Potential Sources of 3,3-Dichlorobenzidine |
|---|---|
| Basic Chemical Manufacturing (NAICS 3251) | Pigment manufacturing. |
| Resin, Synthetic Rubber, and Artificial Synthetic Fibres, and Filaments Manufacturing (NAICS 3252) | Found in certain pigments used for plastics. |
| Pesticide, Fertilizer, and Other Agricultural Chemical Manufacturing (NAICS 3253) | N/A |
| Pharmaceutical and Medicine Manufacturing (NAICS 3254) | N/A |
| Paint, Coating, and Adhesive Manufacturing (NAICS 3255) | Found in certain pigments used for paints. |
| Soap, Cleaning Compound, and Toilet Preparation Manufacturing (NAICS 3256) | n/a |
| Other Chemical Product and Preparation Manufacturing (NAICS3259) | Found in certain pigments used for printing inks. |
Note: N/A No information was available regarding potential sources of 3,3-dichlorobenzidine in this sub-sector.
There is only one manufacturer of 3,3-dichlorobenzidine-based pigments in Canada.
3,3-Dichlorobenzidine-based pigments were being imported in quantities greater than 300 tonnes in 1996 and are still being imported. However, most pigments tend to be non-bioavailable and highly resistant to degradation.
2.1.3 Hexachlorobenzene
Hexachlorobenzene (HCB) is a white, crystalline solid, with minimal water solubility of 5 µg/L at 25°C. It is a persistent, bio-accumulative, and toxic (PBT) pollutant targeted by the USEPA.
HCB was used as a fungicide (pesticide) from the 1940s to the late 1970s.
HCB is also found in certain pigments, which are used for paints, inks, plastics, and coatings.
Currently, USEPA regulates the maximum allowable concentrations of HCB as a contaminant in the following pesticides: atrazine, demethyltetrachloro-terephthalate (DCPA), chlorothalonil, picloram, pentachloronitrobenzene (PCNB), simazine, and lindane. According to information obtained from pesticide manufacturers, HCB concentrations range from 8 to 50 parts per million (ppm) in picloram, from 18 to 26 ppm in chlorothalonil, up to 500 ppm in PCNB, and from 700 to 3,000 ppm in DCPA.
According to the 2002 National Pollutant Release Inventory (NPRI) database, facilities from the resin and synthetic rubber manufacturing sectors discharged HCB in 2002.
| Sub-sector | Potential Sources of Hexachlorobenzene |
|---|---|
| Basic Chemical Manufacturing (NAICS 3251) | Formed as a by-product in the production of chemical solvents, chlorine, and chlorine-containing compounds. Pigment manufacturing. |
| Resin, Synthetic Rubber, and Artificial Synthetic Fibres, and Filaments Manufacturing (NAICS 3252) | Found in certain pigments used for plastics. |
| Pesticide, Fertilizer, and Other Agricultural Chemical Manufacturing (NAICS 3253) | Formed as a by-product in chemical solvent production, chlorine salts, and several currently used pesticides. |
| Pharmaceutical and Medicine Manufacturing (NAICS 3254) | N/A |
| Paint, Coating, and Adhesive Manufacturing (NAICS 3255) | Found in certain pigments used for paints and coatings. |
| Soap, Cleaning Compound, and Toilet Preparation Manufacturing (NAICS 3256) | Use of contaminated products in manufacturing process. |
| Other Chemical Product and Preparation Manufacturing (NAICS3259) | Found in certain pigments used for inks. Use of contaminated products in pyrotechnics manufacture. |
Note: N/A No information was available regarding potential sources of HCB in this sub-sector.
The USEPA 1996 National Toxics Inventory estimates that approximately 2,000 pounds of HCB are released to air each year. Water releases are relatively minor, on the order of 300 pounds per year, according to Toxics Release Inventory (TRI) reports. However, millions of pounds of HCB waste are generated from industrial production processes each year; these wastes are treated and disposed as hazardous waste and are not considered to be entering the environment.
2.1.4 Pentachlorophenol
Pentachlorophenol (PCP) is an organo-chlorine compound (C6HCl5O) that has been mainly used as a fungicide. PCP is a non-flammable solid with both a polar and non-polar form and exists as colourless crystals with a very sharp characteristic odour when hot. PCP does not occur naturally in the environment, but is produced by the chlorination of phenol. It is formed readily from the degradation of its salt, sodium pentachlorophenate (NaPCP), used for similar purposes. At present, PCP has its most extensive use in the production of its ester, pentachlorophenyl (PCPL). These three compounds form a chemical family that should be considered together. They are all toxic, persistent, and liable to bioaccumulate.
The solubility of PCP in water is 14 ppm (20 °C), 18 ppm (25°C), and 20 ppm (30°C). PCP is soluble in acetone, alcohols, ether, and hot benzene. It is slightly soluble in petroleum ether, carbon tetrachloride, and paraffins.
In the United States, PCP is one of the more heavily used pesticides. PCP is registered for use by the USEPA as an insecticide (termicide), fungicide, herbicide, mollucide, algicide, disinfectant, and as an anti-fouling ingredient in paint.
In the past, these three compounds (i.e., PCP, NaPCP, PCPL) have been used in oil-based paints, as glue preservatives (e.g., in leather and toilet paper), as an intermediate product in pharmaceutical synthesis, and as an intermediate product in the manufacture of colouring substances (anthraquinon colorants) in the chemical manufacturing industry. The USEPA is currently reassessing PCP for re-registration as an older pesticide. PCP was one of the most widely used biocides in the U.S. prior to regulatory actions to cancel and restrict certain non-wood preservative uses of PCP in 1987. PCP is a standardized oil-borne preservative. It now has no registered residential uses.
The production of PCP as a wood preservative began in the 1930s. In 1947, the U.S. commercial wood preserving industry had reported usage of nearly 3,200 metric tons of PCP. Prior to the 1987 U.S. Federal Register Notice cancelling and restricting certain non-wood uses, PCP was a registered herbicide, defoliant, mossicide, and disinfectant. As of 2002, approximately 11 million pounds of PCP had been produced.
PCPL is used in the global textiles industry as a fungicide on heavy-duty textiles and fibres subject to attack by fungi and bacteria during storage and use. These textiles include wool, cotton, flax and jute fabrics, and yarns used in covers, tarpaulins, awnings, tents, webbing and netting, and also sisal and manila ropes. NaPCP is used as a fungicide in the maize or rice starch used to stiffen or size the yarn, and this remains in the cloth after weaving.
Production of PCP in the European Union (EU) ceased in 1992. The marketing and use of PCP and its compounds was prohibited in 1991, except for the treatment of wood, impregnation of fibres and heavy-duty textiles not intended for clothing, as an ingredient in chemical synthesis and, under individual authorizations, in situ treatment of buildings of cultural or historic interest.
A summary of the potential sources of pentachlorophenol in wastewater from the Chemical Manufacturing sector is presented in Table 2.8.
| Sub-sector | Potential Sources of Pentachlorophenol |
|---|---|
| Basic Chemical Manufacturing (NAICS 3251) | Production of PCP from PCPL. |
| Resin, Synthetic Rubber, and Artificial Synthetic Fibres, and Filaments Manufacturing (NAICS 3252) | N/A |
| Pesticide, Fertilizer, and Other Agricultural Chemical Manufacturing (NAICS 3253) | Production of PCP as pesticide. |
| Pharmaceutical and Medicine Manufacturing (NAICS 3254) | Historically an intermediate in pharmaceutical manufacturing. |
| Paint, Coating, and Adhesive Manufacturing (NAICS 3255) | Historically used in oil-based paints, glue preservatives. May still be used as anti-fouling ingredient in paint. |
| Soap, Cleaning Compound, and Toilet Preparation Manufacturing (NAICS 3256) | N/A |
| Other Chemical Product and Preparation Manufacturing (NAICS 3259) | N/A |
note: N/A No information was available regarding potential sources of pentachlorophenol in this sub-sector
A summary of the potential sources of pentachlorophenol in wastewater from the Textiles Sector is presented in Table 2.9.
| Sub-Sector | Where PCP May be Found in Process |
|---|---|
| Fibre, Yarn, Thread Mills (NAICS 3131) | Fungicide on heavy-duty textiles and fibres used in covers, tarpaulins, awnings, tents, etc.
Fungicide in maize or rice starch used to stiffen/size yarn Residues on wool products from insecticides on sheep Griege goods (woven fabric just off the loom that has not yet been dyed or finished) |
| Fabric Mills (NAICS 3132); | Refer to “Fibre, Yarn, Thread Mills” |
| Textile and Fabric Finishing and Fabric Coating (NAICS 3133) | Refer to “Fibre, Yarn, Thread Mills” |
2.2 Reference Criteria for Concentrations of Substances of Interest in Discharges to Sewers
This sub-section identifies the reference criteria for substances of concern. In developing the BMP guidance documents, three reference criteria were considered with respect to final effluent concentrations for harmful substances. In Table 2.10, Reference Criteria 1 are the most stringent; that is, Reference Criteria 1 are the lowest reference criteria, whereas Reference Criteria 3 are the least stringent reference criteria. Due to the methodology applied to develop the reference criteria, as elaborated below, two of the three reference criteria are the same in several instances.
| Substance | Designation | Reference Criteria 1 (mg/L) | Reference Criteria 2 (mg/L) | Reference Criteria 3 (mg/L) |
|---|---|---|---|---|
| 1,4-Dichlorobenzene | COA Tier II | 0.0005 | 0.017 | 0.47 |
| 3-3-Dichlorobenzidine | COA Tier II | 0.0006 | 0.002 | 0.002 |
| Hexachlorobenzene | COA Tier I | 0.0000065 | 0.0001 | 0.0001 |
| Pentachlorophenol | COA Tier II | 0.0005 | 0.005 | 0.005 |
The Canadian Environmental Protection Act, 1999 (CEPA) is the cornerstone of the Government of Canada’s environmental legislation aimed at preventing pollution and protecting the environment and human health. CEPA recognizes the contribution of pollution prevention and the management and control of toxic substances and hazardous waste to reducing threats to Canada’s ecosystems and biological diversity. CEPA acknowledges the need to virtually eliminate the most persistent toxic substances that remain in the environment for extended periods of time before breaking down, and bioaccumulative toxic substances that accumulate within living organisms.
From a regulatory perspective, pollution prevention planning becomes one of the tools Environment Canada risk managers can use to address Schedule 1 CEPA toxic substances. Facilities that use Schedule 1 CEPA toxic substances should be aware of the impact that CEPA may have on them.
Reference Criteria 1
Substances identified in Canada-Ontario Agreement respecting the Great Lakes Basin Ecosystem (COA) are either Tier I substances, subject to virtual elimination, or Tier II substances, targeted for reduction. Column 2 of Table 2.10 identifies substances subject to the COA. For substances identified in the COA, Reference Criteria 1 are the more stringent of the Recommended Method Detection Limit (RMDL) or the Provincial Water Quality Objective (PWQO).
For other substances not subject to COA, Reference Criteria 1 are the more stringent of 20 times the PWQO or 20 times the RMDL.
Reference Criteria 2
Reference Criteria 2 were established by the minimum values identified in municipal sewer use by-laws in Ontario for the identified substances. In cases where the sewer use by-law limit was the same as the PWQO or RMDL, Reference Criteria 2 are the same as Reference Criteria 1.
Reference Criteria 3
Reference Criteria 3 were established by the median values identified in municipal sewer use by-laws in Ontario for the identified substances. In cases where only one by-law identified a limit for the substance, or where the same limit was identified in all by-laws, Reference Criteria 3 are the same as Reference Criteria 2.
2.3 Select Regulatory Requirements for the Substances Addressed
Toxic and hazardous substances may be subject to several regulations at the federal, provincial, and municipal levels, in addition to international agreements and protocols. It is incumbent on owners and operators of industrial facilities to be knowledgeable of all requirements for specific substances used in, produced by, transported to and from, or otherwise used at their facilities and operations.
The following section is intended as a guide only regarding specific regulations. Proponents are advised to consult these regulations directly to ensure they have all information they require. Requirements discussed in this section include municipal sewer use by-laws, the NPRI and the federal Environmental Emergency requirements.
Municipal Sewer Use Bylaws
The majority of municipalities in the province of Ontario have municipal sewer use by-laws. A wide range of materials, chemicals, and conditions for discharge are identified in the sewer use by-laws with corresponding objectives that may include the following:
- Protection of the environment;
- Protection of municipal staff and infrastructure;
- Efficient use of the system;
- Prevention of stormwater and 'clear' water from entering the system;
- Protection of sludge or biosolids quality; and
- Protection of public health and safety and protection of public property.
Some municipal sewer use by-laws include an option for entering into over-strength agreements with industrial dischargers, although these agreements are typically limited to substances intended for treatment by the community wastewater treatment facility and do not include the toxic substances addressed in this document. Some municipal sewer use by-laws also require pollution prevention planning and reporting by industrial facilities. Proponents should access the municipal sewer use by-law pertaining to the community sewer system into which they discharge to ensure they are in compliance with all discharge and reporting requirements of the by-law.
Canada’s National Pollutant Release Inventory (NPRI)
The NPRI has several reporting thresholds, including number of employee hours, quantities and concentrations of reportable substances manufactured, processed or otherwise used, with requirements pertaining to specific cases where substances are produced as by-products. Practitioners are encouraged to reference the NPRI website
| Substance | NPRI Reportable Substance | NPRI Part Designation | Reporting Threshold |
|---|---|---|---|
| 1,4-Dichlorobenzene | Yes | Group 1A and Part 5 | 10 tonnes |
| 3-3-Dichlorobenzidine | Yes | Group 1A | 10 tonnes |
| Hexachlorobenzene | Yes | Part 3 | Releases reportable in grams |
| Pentachlorophenol | No | N/A | N/A |
There were no companies reporting releases of 1,4-dichlorobenzene in wastewater effluent in the 2003 NPRI reporting year. This indicates that there were no companies that met the reporting threshold identified in the above table (assuming all sector facilities complied with the regulation).
One chemical company reported a release of 3,3-dichlorobenzidine in wastewater effluent in the 2003 NPRI reporting year; however, the discharge amount reported was actually below the reporting threshold.
One chemical company reported a release of hexachlorobenzene in wastewater effluent in the 2003 NPRI reporting year. Pentachlorophenol is not a reportable substance to the NPRI.
Federal Environmental Emergency (EE) Regulation
Environmental Emergency (EE) Regulations under Part 8 of the CEPA 1999 promote prevention and planning for preparedness, response, and recovery. None of the four substances discussed in this document are identified in the federal emergency regulation at this time. Practitioners are encouraged to reference the regulatory requirements at Environment Canada’s website.
MOE Spills Action Centre
When a spill occurs, it is the responsibility of the owner and the person who had control of the pollutant at the time it was spilled to clean up and dispose of the pollutant at the time it was spilled to clean up and dispose of the pollutants and ameliorate any adverse effects in a timely manner. It is the Ministry’s role to ensure that those responsible take preventative measures and use proper clean up, disposal, and amelioration practices. Under the Environmental Protection Act, the Ministry can order those responsible for the spill to clean up the site.
The MOE should be contacted (Spill Action Centre 1-800-268-6060) if the spill is discharged to a storm water system and into the natural environment, migrates off-site, or where the spill occurs off-site. In such a situation, the MOE, the municipality and the controller, and/or owner of the pollutant, if different, are to be notified.
Pollution prevention (3)
Due to the lack of validated P2 measures in the literature for these substances, the focus of this BMP is on wastewater treatment methods for 1,4-dichlorobenzene, 3,3-dichlorobenzidine, HCB, and PCP. Some P2 measures are presented, as substance elimination/reduction is the first priority in the Environmental Management Options Hierarchy (refer to Figure 3.1).
P2 is defined as "the use of processes, practices, materials, products, substances or energy that avoid or minimize the creation of pollutants and waste, and reduce the overall risk to the environment or human health."
Treatment (Section 4) is not a pollution prevention activity. For many substances, treatment moves pollutants from one media to another (e.g., removal of a metal from the water effluent to a solids residue) and does not avoid or minimize the creation of the pollutant or waste.
The best way to improve environmental management issues is to use a systematic approach. One key first step is to develop an environmental policy and strategy that is formally supported through senior management’s commitment to the strategy. An Environmental Management System (EMS) is a tool that organizations in a variety of sectors have implemented to systematically identify, prioritize, and take action to address the environmental impacts of their operations and services. It is recommended that all facilities consider developing, adopting and implementing an EMS. One example of such a system is the ISO 14001 standard. Pollution prevention, product stewardship, and social responsibility are important aspects of a comprehensive, integrated environmental approach. Employee engagement and training, communication throughout the supply chain, and customer education may be appropriate elements for a successful, integrated approach to long-term sustainability.
The following sequence of steps (Figure 3.1) presents a hierarchy of techniques for undertaking pollution prevention and waste minimization:
Figure 3.1 Environmental Management Options Hierarchy
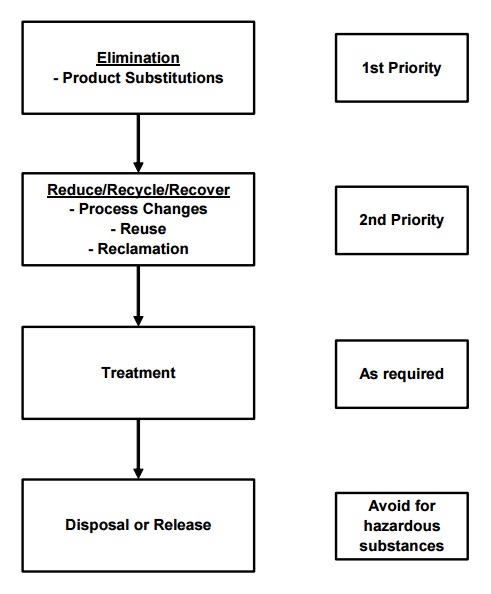
- Eliminate generation of waste streams through process development and design;
- Reduce waste streams at source through process-integrated changes to raw materials, equipment and operating procedures;
- Recycle waste streams by direct re-use or reclamation and re-use;
- Recover any resource value from waste streams; and
- Treat and dispose of waste streams using end-of-pipe techniques.
The sequence of general techniques to prevent and minimize release of water pollutants includes the following steps:
- Identify all wastewater streams and characterize their quality, quantity, and variability;
- Minimize quantity of water used in the process;
- Minimize contamination of process water and washwater contamination with hazardous raw materials, product, or wastes;
- Maximize wastewater re-use; and
- Maximize the recovery and retention of substances from streams unfit for re-use.
3.1 Overview of P2 Measures for 1,4-Dichlorobenzene, 3,3-Dichloro-benzidine, HCB, and PCP
This sub-section provides an overview of the P2 measures discussed in the following three sub-sections: 3.2 Pollution Elimination or Reduction; 3.3 Operating Procedures and Housekeeping; and, 3.4 Education and Training.
Table 3.1 Overview of P2 Measures for 1,4-Dichlorobenzene, 3,3-Dichlorobenzidine, HCB, and PCP
| Substance Addressed | BMP Name | BMP Number |
|---|---|---|
| 1,4-dichlorobenzene | Eliminate 1,4-dichlorobenzene containing space deodorizers | BMP #1 |
| 1,4-dichlorobenzene | Substitute 1,4-dichlorobenzene -containing agents used for moths, moulds, mildews | BMP #2 |
| 3,3-dichlorobenzidine | Substitute 3,3-dichlorobenzidine containing pigments | BMP #3 |
| HCB | Substitute HCB-containing Pigments | BMP #4 |
| HCB | Minimize the Use of Chlorinated Solvents | BMP #5 |
| PCP | Substitution and Prohibition of PCP use in Textiles | BMP #6 |
| Substance Addressed | BMP Name | BMP Number |
|---|---|---|
| All | Modify Cleaning Procedures | BMP #7 |
| Substance Addressed | BMP Name | BMP Number |
|---|---|---|
| All | Management and Staff Training | BMP #8 |
3.2 Pollution Elimination or Reduction
P2 opportunities to eliminate or reduce hazardous substances include material substitutions and process alterations. Changes in operating costs will depend on the cost differential of the substitute in comparison with the hazardous material. Where the cost of the substitute is higher, operating costs will increase; however, where the cost of the substitute is lower, operating costs will decrease. Some capital investment in equipment modifications or replacements to accommodate any differences in properties of the substitute substances may also be required. Alterations to processes to reduce use of hazardous substances may entail changes in operating budget, including possible reductions in costs due to more efficient operations. Capital investment for equipment modification or replacement may also be required.
3.2.1 Elimination and Reduction Measures for 1,4-Dichlorobenzene
BMP #1: Eliminate 1,4-dichlorobenzene containing space deodorizers:
1,4-Dichlorobenzene used to deodorize restrooms and waste containers should be substituted with deodorizers that do not contain 1,4-dichlorobenzene or any other toxic/hazardous substances (e.g., deodorizers may also contain formaldehyde). Facilities should verify product ingredients with product suppliers and through Material Safety Data Sheets (if available).
BMP #2: Substitute 1,4-dichlorobenzene -containing agents used for moths, moulds, mildews:
In the textiles sector, alternatives to 1,4-dichlorobenzene containing agents for moths, moulds, and mildews should be considered. The Australian textile finishing industry has ceased using toxic moth-proofing agents that do not readily biodegrade.
- Clorphenylid: Was used widely, but now has been withdrawn because of environmental problems generated during its manufacture.
- Flucofuron: Not fully evaluated at this time, but is known to be ineffective against certain pests. Evaluations are still underway.
- Cycloprothrin: Has good performance with an aquatic toxicity rating that is approximately three orders of magnitude less than that of permethrin.
- Diphenylurea: Has lower aquatic toxicity than permethrin, but is less biodegradable.
- Cyfluthrin: Effective, but has been withdrawn because of reactions by textile mill workers to the chemical.
- Sulcofuron: Shows low affinity in some application methods and is ineffective against certain pests.
footnote 36
3.2.2 Elimination and Reduction Measures for 3,3-Dichlorobenzidine
BMP #3: Substitute 3,3-dichlorobenzidine-containing pigments:
In the chemical manufacturing sector, product substitution or reformulation could eliminate 3,3-dichlorobenzidine-based pigments from the manufacturing process. The greatest barrier to reformulated paint products is customer specifications. There are yellow pigment substitutes for 3,3-dichlorobenzidine-based pigments, but they are more expensive and less efficient.
3.2.3 Elimination and Reduction Measures for Hexachlorobenzene
BMP #4: Substitute HCB-containing Pigments:
In the chemical manufacturing sector, HCB is used in the manufacture of some pigments. Facilities should ensure their suppliers provide pigments for paint, inks, plastics, and coatings that do not contain HCB or other toxic/hazardous substances.
BMP #5: Minimize the Use of Chlorinated Solvents:
HCB is formed inadvertently as a by-product in the production of chemical solvents and chlorine and chlorine-containing compounds, such as ferric/ferrous chloride. The reduction in the manufacture and use of chlorinated solvents, such as tetrachloroethylene, trichloroethylene, and vinyl chloride, has reduced the releases of HCB resulting from the manufacture and use of these compounds. Further opportunity for reductions should be identified wherever possible.
3.2.4 Elimination and Reduction Measures for Pentachlorophenol
There are a few possible substitutes for PCP in industrial preservation. They include water-borne mixtures commonly of copper, chromium, and arsenic compounds.
BMP #6: Substitution and Prohibition of PCP use in Textiles:
For the impregnation of textiles, substances such as zinc-2-pyridinthiol-N-oxide, 2,2'-dihydroxy-5,5'-dichloro-diphenyl-methane-ester, tributyltinoxide ester, and others can be used instead of PCPL.
- The company Marks and Spencer (2004) prescribes a code of practice for textile production in which the use or presence of pentachlorophenol and its compounds is prohibited.
- In Finland, Sweden, and Norway, the use of PCP in textile impregnation had ceased by the end of the 1970s. In Switzerland, the production, marketing, import and use of PCP, its salts and preparations is prohibited, and also the import of PCP-treated textiles, leather products, and wood is prohibited. According to Commission Directive 1999/51/EC, the impregnation of textiles and fibres with PCPL will cease by December 31, 2008 in France, Ireland, Portugal, Spain, and the UK.
footnote 39 - PCP use for indoor materials is banned in Germany, Japan, and the United States (USEPA). Environment Canada (EC) plans action to ban the import of products containing PCP or its compounds after 2008.
footnote 40
3.3 Operating Procedures and Housekeeping for All Substances
BMP #7: Modify Cleaning Procedures:
In the chemical manufacturing sector, wastewater from this sector is primarily generated by cleaning operations of the process areas and associated equipment. Reducing cleaning needs can assist in reducing the amounts of 1,4-dichlorobenzene, 3,3-dichlorobenzidine, HCB, and PCP discharged in wastewater effluent. Suggested actions include the following:
- Clean interiors of dry formulation equipment with dry carrier prior to any water rinse. The carrier material must be stored and re-used in future formulation of the same or compatible product or properly disposed of as solid waste.
- When performing rinsing of raw material drums, storage drums, and/or shipping containers that contained pollutant, re-use the drum/shipping container rinsate directly into the formulation at the time of formulation or store for use in future formulation of same or compatible product. Alternatively, use a staged drum rinsing station (counter-current rinsing).
- Store the rinsate from interior rinsing (does not include drum/shipping container rinsate) for re-use in future formulation of same or compatible product.
3.4 Education and Training
Education and training are P2 measures that can be implemented concurrently with elimination/reduction BMPs and operating/housekeeping BMPs. Investments in education and training for management and staff can return significant benefits, including improved staff motivation, an improved health and safety record, reduced material losses, improved productivity, and, potentially, improved customer satisfaction. Communication and education of the supply chain, including material and equipment suppliers, can result in improved working relationships, as well as environmental benefits resulting from reduced pollution release.
It is important to keep education and training current and to ensure a management system is in place to maintain the relevance of education and training delivered. As mentioned above, a comprehensive management approach is important for effective reduction of releases of hazardous substances, including reductions through education and training.
Some operating costs may be incurred to initiate education and training practices, for example, time required to discuss improved materials specifications with suppliers. Once implemented, however, these costs can be expected to be off-set by the benefits of education and training. Capital investment is not typically required for implementation of education and training practices.
BMP #8: Management and Staff Training:
- Ensure every employee is fully trained before beginning his or her first employment shift and whenever new equipment is installed or new procedures implemented. They should be familiar with the hazards that accompany the material they are using and be aware of potential sources of contamination. Material safety data sheets (MSDS) should be available for all compounds used at the facility.
- Ensure employees are familiar with the site layout and catch basin locations. Ensure they employ good housekeeping practices and understand proper reporting procedures.
- Ensure all employees are aware of the spill response plan and are properly trained to carry it out.
- Document all employees' training and retain the records for a minimum of two years after the employee ceases employment; e.g., date and location of training, subject(s) covered, test results if applicable, trainer’s name, etc.
3.5 P2 Options and Costs
Due to the limited available information in the literature on these substances, no analysis has been undertaken on the effectiveness of P2 measures in removing these substances. The effectiveness of P2 measures will vary from sector to sector, depending on availability of substitutes and current usage rates of these substances within the sectors. Consequently, sector-specific information on the effectiveness of P2 implementation and the resultant P2 reduction and/or elimination of these substances should be determined.
It should be noted, however, that P2 measures are the most effective means of reducing the release of these substances into the environment and therefore, P2 measures should be adopted by industry with respect to these substances.
Treatment (4)
Treatment is not a P2 measure and it is not as effective as P2 in preventing the release of hazardous substances since it occurs after the hazardous substance has been used or created and subsequently becomes part of the facility’s wastewater. With some treatment, the hazardous substance may be simply transferred from the water to the air or the sludge. Operating and capital costs of treatment can be significant. As a result, treatment should only be considered after P2 measures have been implemented and after all efforts have been taken to reduce or eliminate the substance first through P2 practices.
4.1 Treatment
Treatment measures and BMPs must be assessed and implemented based on specific site and process conditions and characteristics. The following subsections present treatment processes to be considered to meet the reference criteria.
The reference criteria outlined in Section 2.2 are provided for the purpose of assessing the potential for application of select treatment technologies for the select substances identified in this BMP document. Other treatment processes may be more applicable at facilities that have a wastewater stream containing other substances requiring removal.
4.1.1 Treatment Measures for 1,4-Dichlorobenzene
Five treatment processes are potentially applicable to meeting the reference criteria for 1,4-dichlorobenzene outlined in Section 2.2. The treatment processes provided are presented in sequential order of treatment requirements, with the process generally required to achieve the lowest concentration presented last. These treatment processes can be used alone or in combination, depending on specific wastewater properties.
- Air stripping: 1,4-dichlorobenzene is a volatile compound and can be volatilized in an air stripper. After volatilization, the 1,4-dichlorobenzene in the air can be treated using granular activated carbon (GAC) adsorption. See below for more information on GAC.
- Aerobic biological treatment: Biological treatment involves contacting wastewater with a microbial reactor to remove biodegradable organic pollutants. The microorganisms convert the organic material into new microbial cells, which results in a sludge that requires disposal. Aerobic biological treatment involves adding air to the process to facilitate aerobic biodegradation, which is the process required for the contaminants of concern. Treatment can be either a suspended biomass system (such as activated sludge) or an attached growth system (e.g., trickling filters, rotating biological contactors). Both types of systems require a clarification process after the bioreactor. This process requires specific environmental control to operate effectively, e.g., sufficient aeration and a limited pH range. There is the potential for 1,4-dichlorobenzene to inhibit biological treatment due to toxicity of this substance at concentrations above a threshold level (estimated to be approximately 5 mg/L). Higher concentrations may be tolerated through acclimatization of the biomass to this compound. Pilot testing may be required for this process to verify its suitability for a specific wastewater stream. Typically, biological treatment is used for wastewater streams with a 5-day biochemical oxygen demand (BOD5) greater than 100 mg/L.
- Granular activated carbon (GAC) or powdered activated carbon (PAC): The GAC process involves conveying wastewater or air containing 1,4-dichlorobenzene vapour through a fixed-bed column containing GAC granules. The GAC adsorbs 1,4-dichlorobenzene. A two-stage system may be required to reduce the concentration to below the concentrations required to meet the reference criteria. The spent GAC is regenerated off-site or on-site in order to recover the adsorbed 1,4-dichlorobenzene and reuse it in the industrial process. The type of pollutants adsorbed and the extent of adsorption are a function of the source material for the GAC and the preparation procedure for the GAC granules. Typically, a sand or mixed media filter is required to remove suspended solids as a wastewater pre-treatment stage for a GAC filter. As an alternative to GAC for wastewater, PAC can be added to a holding tank or to the reactor tank of a suspended biomass biological treatment system. PAC cannot be regenerated and is disposed of as a solid waste.
- Reverse Osmosis (RO): The RO process separates water from dissolved materials in solution by filtering through a semipermeable membrane under pressure. The basic components of an RO system are the membrane, a membrane support structure, a containing vessel, and a high-pressure pump. The permeability of the membrane used, level of wastewater pre-treatment and membrane cleaning are the key criteria for the performance of this process. RO results in a waste stream, or reject, that must be disposed. Filtration using a sand or mixed media filter followed by microfiltration is typically used as a pre-treatment stage for RO.
- Advanced Oxidation (AOT): The AOT process uses ultraviolet (UV) light in conjunction with an oxidant such as ozone or hydrogen peroxide. This combination achieves a significantly greater treatment performance than using the oxidant alone. UV light is used to split the oxidant molecule, producing very reactive hydroxyl radicals. These hydroxyl radicals react quickly with organic pollutants in the water, breaking them down into carbon dioxide and water. The treatment process will break down any organic contaminant; therefore, to treat the organic contaminants of concern, the removal of other general organics may be required before this process is used.
4.1.2 Treatment Measures for 3,3-dichlorobenzidine
Four treatment processes are potentially applicable to meeting the reference criteria for 3,3-dichlorobenzidine outlined in Section 2.2. The treatment processes provided are presented in sequential order of treatment requirements, with the process generally required to achieve the lowest concentration presented last. These treatment processes can be used alone or in combination, depending on specific wastewater properties.
- Aerobic biological treatment: Biological treatment involves contacting wastewater with a microbial reactor to remove biodegradable organic pollutants. The microorganisms convert the organic material into new microbial cells, which results in a sludge that requires disposal. Aerobic biological treatment involves adding air to the process to facilitate aerobic biodegradation, which is the process required for the contaminants of concern. Treatment can be either a suspended biomass system (such as activated sludge) or an attached growth system (e.g., trickling filters, rotating biological contactors). Both types of systems require a clarification process after the bioreactor. This process requires specific environmental control to operate effectively, e.g., sufficient aeration and a limited pH range. There is the potential for 3,3-dichlorobenzidine to inhibit biological treatment due to toxicity of this substance at concentrations above a threshold level. Higher concentrations may be tolerated through acclimatization of the biomass to this compound. Pilot testing may be required for this process to verify its suitability for a specific wastewater stream. Typically, biological treatment is used for wastewater streams with a 5-day biochemical oxygen demand (BOD5) greater than 100 mg/L.
- Granular activated carbon (GAC) or powdered activated carbon (PAC): The GAC process involves conveying wastewater or air containing 3,3-dichlorobenzidine vapour through a fixed-bed column containing GAC granules. The GAC adsorbs 3,3-dichlorobenzidine. A two-stage system may be required to reduce the concentration to below the concentrations required to meet the reference criteria. The spent GAC is regenerated off-site or on-site in order to recover the adsorbed 3,3-dichlorobenzidine and reuse it in the industrial process. The type of pollutants adsorbed and the extent of adsorption are a function of the source material for the GAC and the preparation procedure for the GAC granules. Typically, a sand or mixed media filter is required to remove suspended solids as a pre-treatment stage for a wastewater GAC filter. As an alternative to GAC, PAC can be added to a holding tank or to the reactor tank of a suspended biomass biological wastewater treatment system. PAC cannot be regenerated and is disposed of as a solid waste.
- Reverse Osmosis (RO): The RO process separates water from dissolved materials in solution by filtering through a semipermeable membrane under pressure. The basic components of an RO system are the membrane, a membrane support structure, a containing vessel, and a high-pressure pump. The permeability of the membrane used, level of wastewater pre-treatment and membrane cleaning are the key criteria for the performance of this process. RO results in a waste stream, or reject, that must be disposed. Filtration using a sand or mixed media filter followed by microfiltration is typically used as a pre-treatment stage for RO.
- Advanced Oxidation (AOT): The AOT process uses ultraviolet (UV) light in conjunction with an oxidant such as ozone or hydrogen peroxide. This combination achieves a significantly greater treatment performance than using the oxidant alone. UV light is used to split the oxidant molecule, producing very reactive hydroxyl radicals. These hydroxyl radicals react quickly with organic pollutants in the water, breaking them down into carbon dioxide and water. The treatment process will break down any organic contaminant; therefore, to treat the organic contaminants of concern, the removal of other general organics may be required before this process is used.
4.1.3 Treatment Measures for Hexachlorobenzene
Four treatment processes are potentially applicable to meeting the reference criteria for HCB outlined in Section 2.2. The treatment processes provided are presented in sequential order of treatment requirements, with the process generally required to achieve the lowest concentration presented last. These treatment processes can be used alone or in combination, depending on specific wastewater properties.
- Aerobic biological treatment: Biological treatment involves contacting wastewater with a microbial reactor to remove biodegradable organic pollutants. The microorganisms convert the organic material into new microbial cells, which results in a sludge that requires disposal. Aerobic biological treatment involves adding air to the process to facilitate aerobic biodegradation, which is the process required for the contaminants of concern. Treatment can be either a suspended biomass system (such as activated sludge) or an attached growth system (e.g., trickling filters, rotating biological contactors). Both types of systems require a clarification process after the bioreactor. This process requires specific environmental control to operate effectively, e.g., sufficient aeration and a limited pH range. There is the potential for HCB to inhibit biological treatment due to toxicity of this substance at concentrations above a threshold level. Higher concentrations may be tolerated through acclimatization of the biomass to this compound. Pilot testing may be required for this process to verify its suitability for a specific wastewater stream. Typically, biological treatment is used for wastewater streams with a 5-day biochemical oxygen demand (BOD5) greater than 100 mg/L.
- Granular activated carbon (GAC) or powdered activated carbon (PAC): The GAC process involves conveying wastewater or air containing HCB vapour through a fixed-bed column containing GAC granules. The GAC adsorbs HCB. A two-stage system may be required to reduce the concentration to below the concentrations required to meet the reference criteria. The spent GAC is regenerated off-site or on-site in order to recover the adsorbed HCB and reuse it in the industrial process. The type of pollutants adsorbed and the extent of adsorption are a function of the source material for the GAC and the preparation procedure for the GAC granules. Typically, a sand or mixed media filter is required to remove suspended solids as a pre-treatment stage for a wastewater GAC filter. As an alternative to GAC, PAC can be added to a holding tank or to the reactor tank of a suspended biomass biological wastewater treatment system. PAC cannot be regenerated and is disposed of as a solid waste.
- Reverse Osmosis (RO): The RO process separates water from dissolved materials in solution by filtering through a semipermeable membrane under pressure. The basic components of an RO system are the membrane, a membrane support structure, a containing vessel, and a high-pressure pump. The permeability of the membrane used, level of wastewater pre-treatment and membrane cleaning are the key criteria for the performance of this process. RO results in a waste stream, or reject, that must be disposed. Filtration using a sand or mixed media filter followed by microfiltration is typically used as a pre-treatment stage for RO.
- Advanced Oxidation (AOT): The AOT process uses ultraviolet (UV) light in conjunction with an oxidant such as ozone or hydrogen peroxide. This combination achieves a significantly greater treatment performance than using the oxidant alone. UV light is used to split the oxidant molecule, producing very reactive hydroxyl radicals. These hydroxyl radicals react quickly with organic pollutants in the water, breaking them down into carbon dioxide and water. The treatment process will break down any organic contaminant; therefore, to treat the organic contaminants of concern, the removal of other general organics may be required before this process is used.
4.1.4 Treatment Measures for Pentachlorophenol
Four treatment processes are potentially applicable to meeting the reference criteria for PCP outlined in Section 2.2. The treatment processes provided are presented in sequential order of treatment requirements, with the process generally required to achieve the lowest concentration presented last. These treatment processes can be used alone or in combination, depending on specific wastewater properties.
- Aerobic biological treatment: Biological treatment involves contacting wastewater with a microbial reactor to remove biodegradable organic pollutants. The microorganisms convert the organic material into new microbial cells, which results in a sludge that requires disposal. Aerobic biological treatment involves adding air to the process to facilitate aerobic biodegradation, which is the process required for the contaminants of concern. Treatment can be either a suspended biomass system (such as activated sludge) or an attached growth system (e.g., trickling filters, rotating biological contactors). Both types of systems require a clarification process after the bioreactor. This process requires specific environmental control to operate effectively, e.g., sufficient aeration and a limited pH range. There is the potential for PCP to inhibit biological treatment due to toxicity of this substance at concentrations above a threshold level. Higher concentrations may be tolerated through acclimatization of the biomass to this compound. Pilot testing may be required for this process to verify its suitability for a specific wastewater stream. Typically, biological treatment is used for wastewater streams with a 5-day biochemical oxygen demand (BOD5) greater than 100 mg/L.
- Granular activated carbon (GAC) or powdered activated carbon (PAC): The GAC process involves conveying wastewater or air containing PCP vapour through a fixed-bed column containing GAC granules. The GAC adsorbs PCP. A two-stage system may be required to reduce the concentration to below the concentrations required to meet the reference criteria. The spent GAC is regenerated off-site or on-site in order to recover the adsorbed PCP and reuse it in the industrial process. The type of pollutants adsorbed and the extent of adsorption are a function of the source material for the GAC and the preparation procedure for the GAC granules. Typically, a sand or mixed media filter is required to remove suspended solids as a pre-treatment stage for a wastewater GAC filter. As an alternative to GAC, PAC can be added to a holding tank or to the reactor tank of a suspended biomass biological wastewater treatment system. PAC cannot be regenerated and is disposed of as a solid waste.
- Reverse Osmosis (RO): The RO process separates water from dissolved materials in solution by filtering through a semipermeable membrane under pressure. The basic components of an RO system are the membrane, a membrane support structure, a containing vessel, and a high-pressure pump. The permeability of the membrane used, level of wastewater pre-treatment and membrane cleaning are the key criteria for the performance of this process. RO results in a waste stream, or reject, that must be disposed. Filtration using a sand or mixed media filter followed by microfiltration is typically used as a pre-treatment stage for RO.
- Advanced Oxidation (AOT): The AOT process uses ultraviolet (UV) light in conjunction with an oxidant such as ozone or hydrogen peroxide. This combination achieves a significantly greater treatment performance than using the oxidant alone. UV light is used to split the oxidant molecule, producing very reactive hydroxyl radicals. These hydroxyl radicals react quickly with organic pollutants in the water, breaking them down into carbon dioxide and water. The treatment process will break down any organic contaminant; therefore, to treat the organic contaminants of concern, the removal of other general organics may be required before this process is used.
4.2 Treatment Options and Costs
Treatability information is provided for the individual pollutants specified in the following sections as a guide. The typical treatment processes for the non-sector specific pollutants is discussed in the following sections. Treatment requirements are based on representative wastewater concentrations for each pollutant in wastewater for the chemical manufacturing sector
The proposed treatment strategies identified serve as preliminary guidelines for the level of treatment likely to be required. Site and facility specific information is needed to determine what treatment trains and components are required to achieve the reference criteria. A comprehensive analysis of the wastewater stream is required and bench-scale and/or pilot testing of treatment may be needed to verify the optimum treatment system for a specific facility. The implementation of P2 practices should be first considered before selecting a treatment system for the removal of these substances.
Capital and annual operational and maintenance (O&M) costs were developed for the three reference criteria using a wastewater flow range of 1 m3/h to 50 m3/h. Capital costs include engineering, equipment, piping and instrumentation, electrical and controls, installation, and construction costs.
The annual O&M costs were determined as a function of percentage of capital costs, assuming 15 percent for the 1 m3/h flow condition, 12 percent for the intermediate 25 m3/h flow condition and 10 percent for the 50 m3/h flow condition. Annual O&M costs include a consideration of the following:
- Increased power and energy costs to operate the additional treatment processes;
- Chemical costs for treatment chemicals, where required;
- Additional labour costs for operation;
- Sampling and monitoring costs for the specific substances requiring treatment; and
- Disposal costs for residues and waste streams generated from treatment.
4.2.1 Treatment Options and Costs for 1,4-Dichlorobenzene
Treatability information is provided for 1,4-dichlorobenzene in Table 4.1. Based on an assumption that the BOD5 is less than 100 mg/L
- Reference Criteria 1: Air stripping, sand/mixed media filtration and GAC.
- Reference Criteria 2 and 3: Air stripping.
| Reference Criteria | Assumed Starting Concentration | Treatment Measures | Estimated % Removal Resulting from CombinedTreatment Measures | Estimated Conc. of Substance in Effluent after Treatment |
|---|---|---|---|---|
| Reference Criteria 1 0.0005 mg/L |
9.7 mg/L | Air Stripping¹, filtration² and GAC³ | 99.999% | 0.00005 mg/L |
| Reference Criteria 2 0.017 mg/L |
9.7 mg/L | Air Stripping | 99.9% | 0.01 mg/L |
| Reference Criteria 3 0.47 mg/L |
9.7 mg/L | Air Stripping | 99.9% | 0.01 mg/L |
1 Estimated removal of 99.9%
2 Typically required as a pre-treatment stage for GAC
3 Estimated removal of 99.5%
The estimated capital and O&M treatment costs are presented in Table 4.2. These costs are conceptual level only, normally considered to be accurate to a range of −35% to +50%.
| Reference Criteria | Capital Cost Range: 1 m3/h | Capital Cost Range: 25 m3/h | Capital Cost Range: 50 m3/h | Annual O&M Cost Range: 1 m3/h | Annual O&M Cost Range: 25 m3/h | Annual O&M Cost Range: 50 m3/h |
|---|---|---|---|---|---|---|
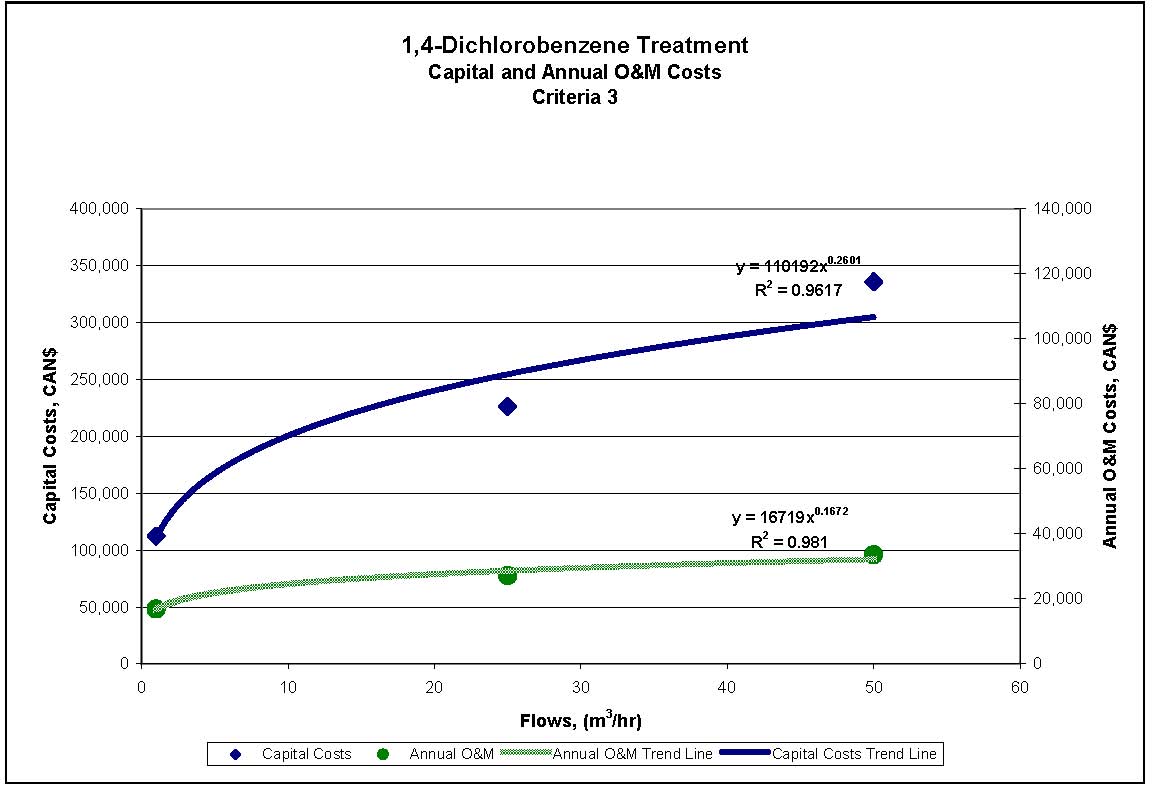
| Criteria 1 | $152,000 | $585,000 | $950,000 | $23,000 | $70,000 | $95,000 |
| Criteria 2 and 3 | $113,000 | $226,000 | $336,000 | $17,000 | $27,000 | $34,000 |
Figures 4.1 to 4.3 show capital and annual O&M costing curves for the estimated cost ranges presented in Table 4.2 for each set of reference criteria.
Figure 4.1: 1,4-Dichlorobenzene Treatment for Reference Criteria 1
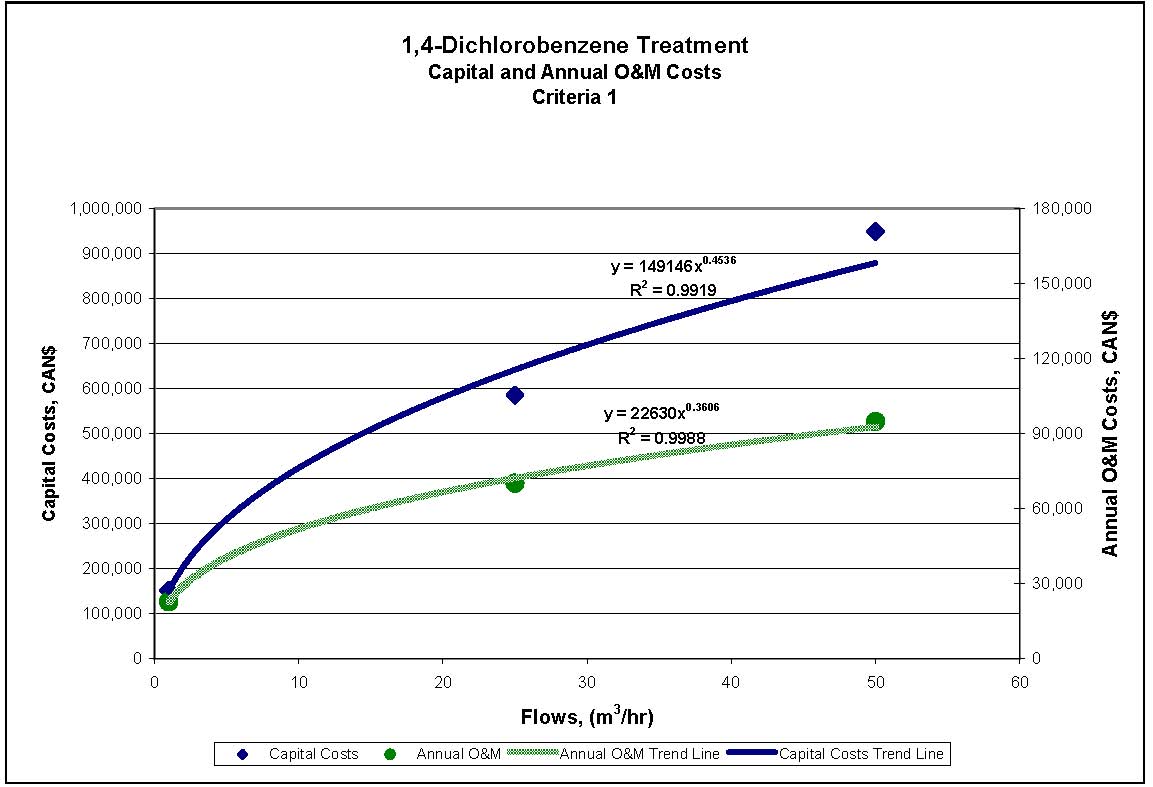
Figure 4.2 1,4-Dichlorobenzene Treatment for Reference Criteria 2
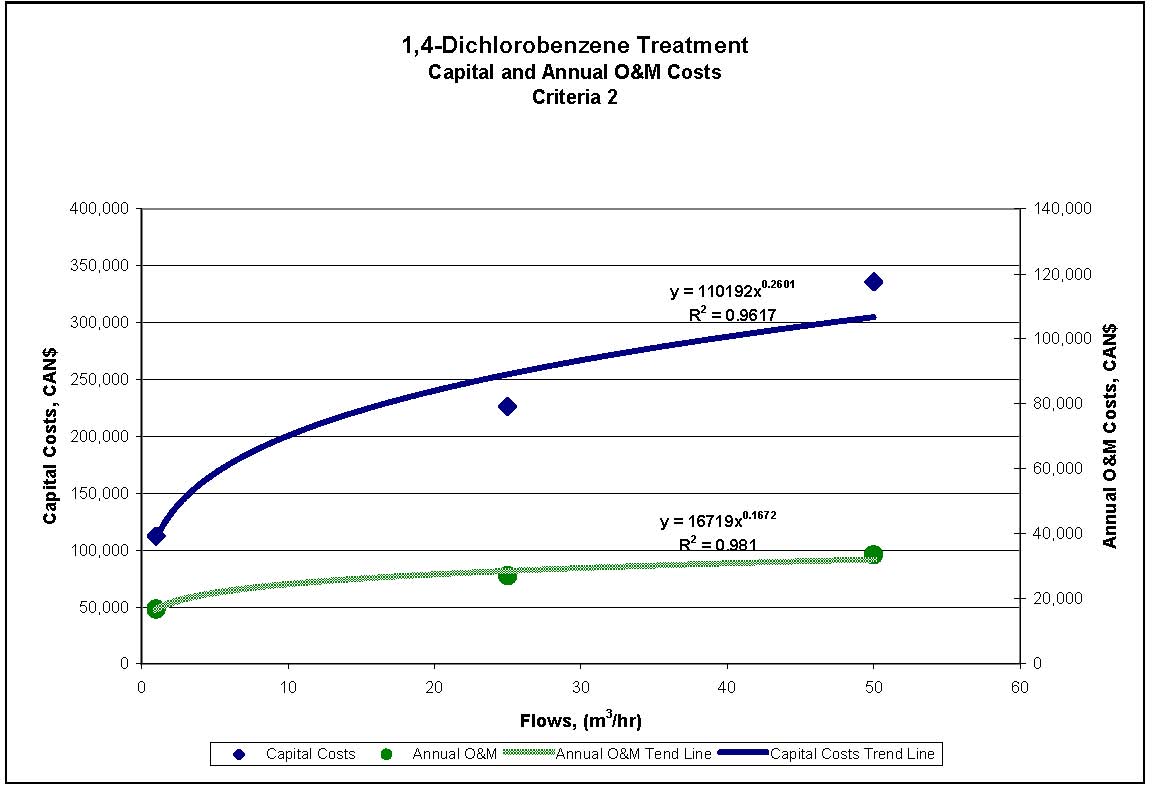
Figure 4.3 1,4-Dichlorobenzene Treatment for Reference Criteria 3
4.2.2 Treatment Options and Costs for 3,3-Dichlorobenzidine
Treatability information is provided for 3,3-dichlorobenzidine in Table 4.3. Based on the assumption that BOD5 is less than 100 mg/L
- Reference Criteria 1, 2 and 3: Sand/mixed media filtration, GAC, microfiltration, and AOT. GAC is used before AOT to reduce the size of the AOT process required.
| Reference Criteria | Assumed Starting Concentration | Treatment Measures | Estimated % Removal Resulting from CombinedTreatment Measures | Estimated Conc. of Substance in Effluent after Treatment |
|---|---|---|---|---|
| Reference Criteria 1 0.0006 mg/L |
6.1 mg/L | Filtration¹, GAC², microfiltration³and AOT4 | 99.99% | 0.0006 mg/L |
| Reference Criteria 2 0.002 mg/L |
6.1 mg/L | Filtration, GAC, microfiltration and AOT | 99.99% | 0.0006 mg/L |
| Reference Criteria 3 0.002 mg/L |
6.1 mg/L | Filtration, GAC, microfiltration and AOT | 99.99% | 0.0006 mg/L |
¹ Sand or mixed media filtration typically required as a pre-treatment stage for GAC.
² Estimated removal of 99.5 %.
³ Typically required as a pre-treatment stage for AOT.
4 Estimated removal of 98%.
The estimated capital and O&M treatment costs are presented in Table 4.4. These costs are conceptual level only, normally considered to be accurate to a range of −35% to +50%.
| Reference Criteria | Capital Cost Range: 1 m3/h | Capital Cost Range: 25 m3/h | Capital Cost Range: 50 m3/h | Annual O&M Cost Range: 1 m3/h | Annual O&M Cost Range: 25 m3/h | Annual O&M Cost Range: 50 m3/h |
|---|---|---|---|---|---|---|
| Criteria 1 | $360,000 | $1,802,000 | $3,464,000 | $54,000 | $216,000 | $346,000 |
| Criteria 2 and 3 | $360,000 | $1,668,000 | $3,199,000 | $54,000 | $200,000 | $320,000 |
Figures 4.4 to 4.6 show capital and annual O&M costing curves for the estimated cost ranges presented in Table 4.4 for each set of reference criteria.
Figure 4.4: 3,3-Dichlorobenzidine Treatment for ReferenceCriteria 1
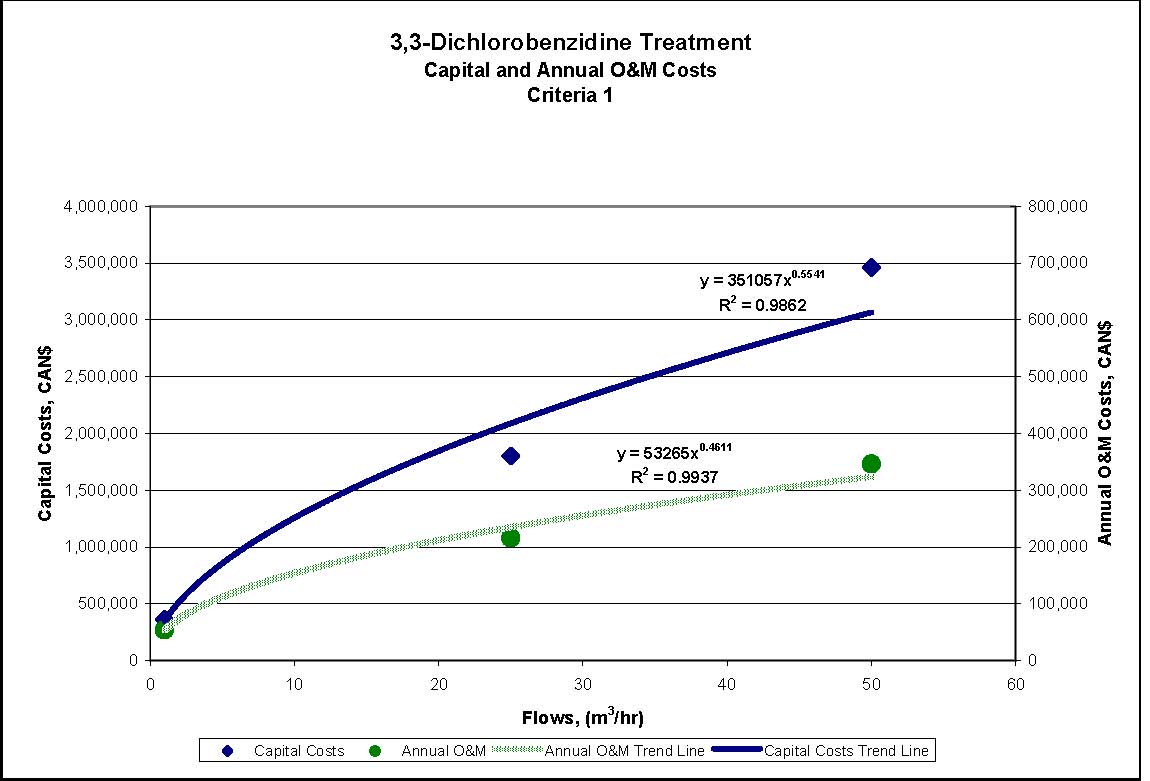
Figure 4.5: 3,3-Dichlorobenzidine Treatment for ReferenceCriteria 2
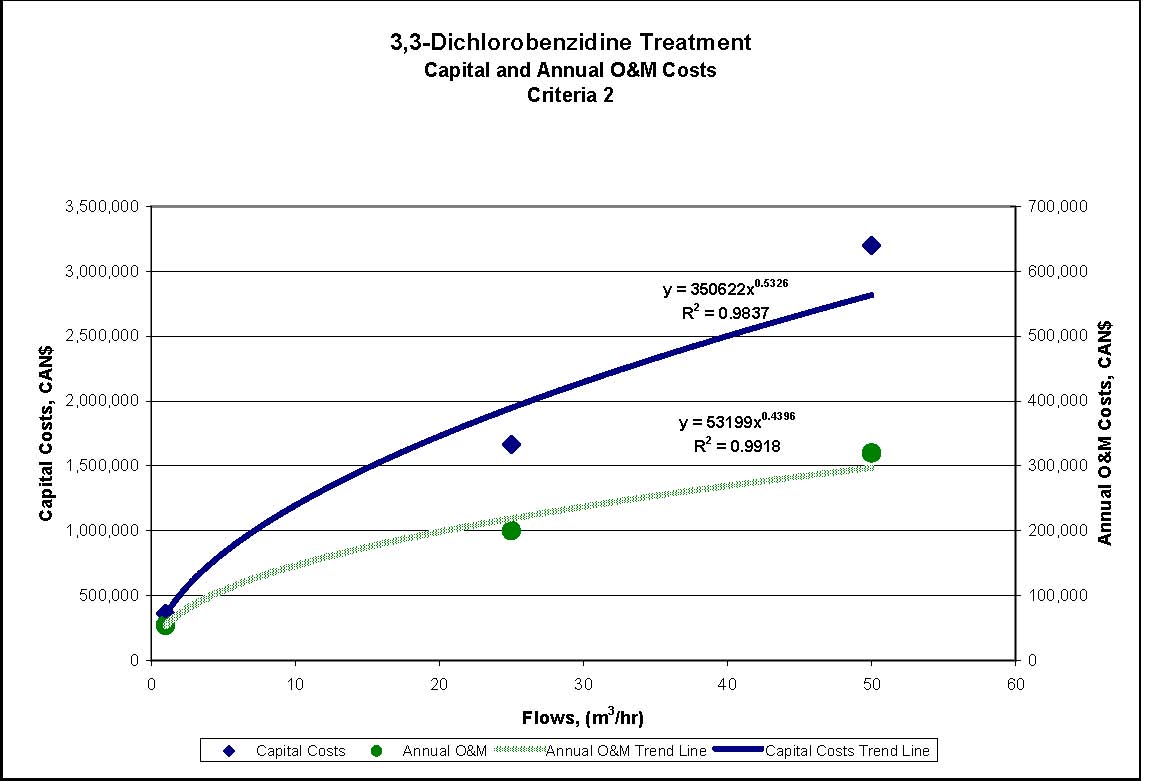
Figure 4.6: 3,3-Dichlorobenzidine Treatment for ReferenceCriteria 3
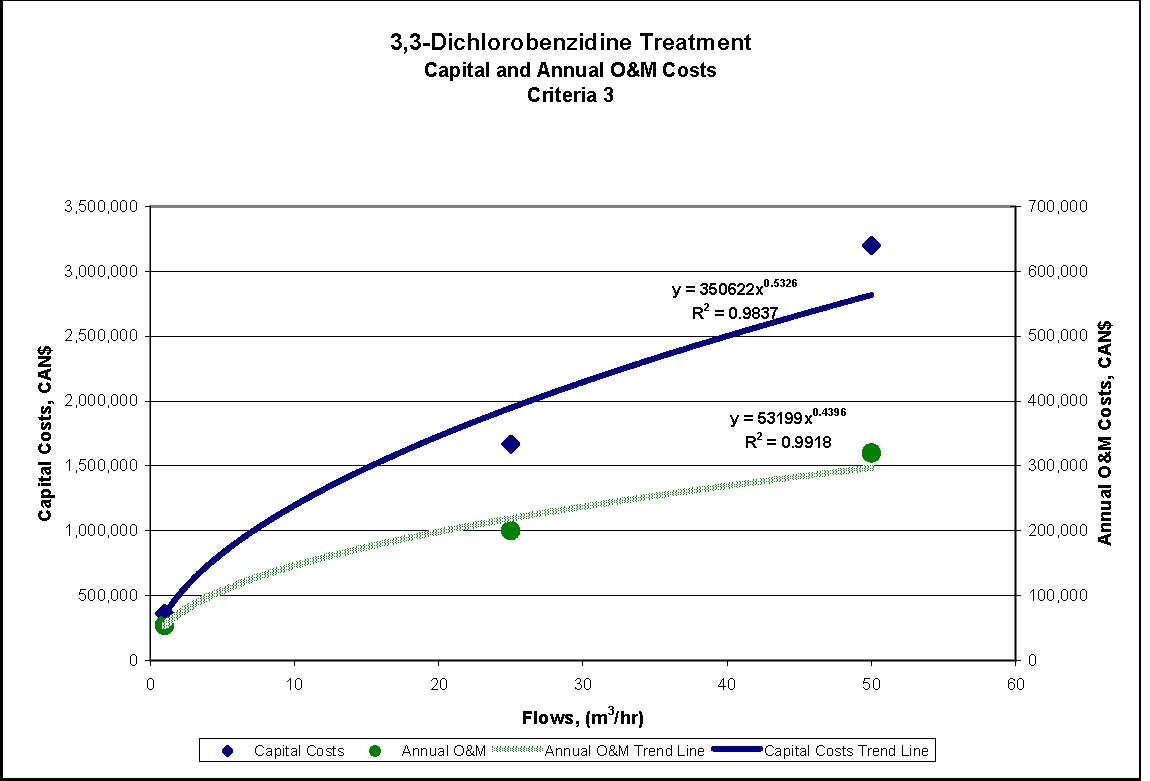
4.2.3 Treatment Options and Costs for HCB
Treatability information is provided for HCB in Table 4.5. Based on the assumption that BOD5 is less than 100 mg/L
- Reference Criteria 1, 2 and 3: Sand/mixed media filtration, GAC, microfiltration and AOT. GAC is used before AOT to reduce the size of the AOT process required.
| Reference Criteria | Assumed Starting Concentration | Treatment Measures | Estimated % Removal Resulting from CombinedTreatment Measures | Estimated Conc. of Substance in Effluent after Treatment |
|---|---|---|---|---|
| Reference Criteria 1 0.0000065 mg/L |
0.24 mg/L | Filtration¹, GAC², microfiltration³ and AOT4 | 99.997% | 0.0000065 mg/L |
| Reference Criteria 2 0.0001 mg/L |
0.24 mg/L | Filtration, GAC, microfiltration and AOT | 99.997% | 0.0000065 mg/L |
| Reference Criteria 3 0.0001 mg/L |
0.24 mg/L | Filtration, GAC, microfiltration and AOT | 99.997% | 0.0000065 mg/L |
¹ Sand or mixed media filtration typically required as a pre-treatment stage for GAC.
² Estimated removal of 99.5 %.
³ Typically required as a pre-treatment stage for AOT.
4 Estimated removal of 99.5%.
The estimated capital and O&M treatment costs are presented in Table 4.6. These costs are conceptual level only, normally considered to be accurate to a range of −35 % to +50%.
| Reference Criteria | Capital Cost Range: 1 m3/h | Capital Cost Range: 25 m3/h | Capital Cost Range: 50 m3/h | Annual O&M Cost Range: 1 m3/h | Annual O&M Cost Range: 25 m3/h | Annual O&M Cost Range: 50 m3/h |
|---|---|---|---|---|---|---|
| Criteria 1 | $360,000 | $1,802,000 | $3,464,000 | $54,000 | $216,000 | $346,000 |
| Criteria 2 and 3 | $360,000 | $1,668,000 | $3,199,000 | $54,000 | $200,000 | $320,000 |
Figures 4.7 to 4.9 show capital and annual O&M costing curves for the estimated cost ranges presented in Table 4.6 for each set of reference criteria.
Figure 4.7 HCB Treatment for Reference Criteria 1
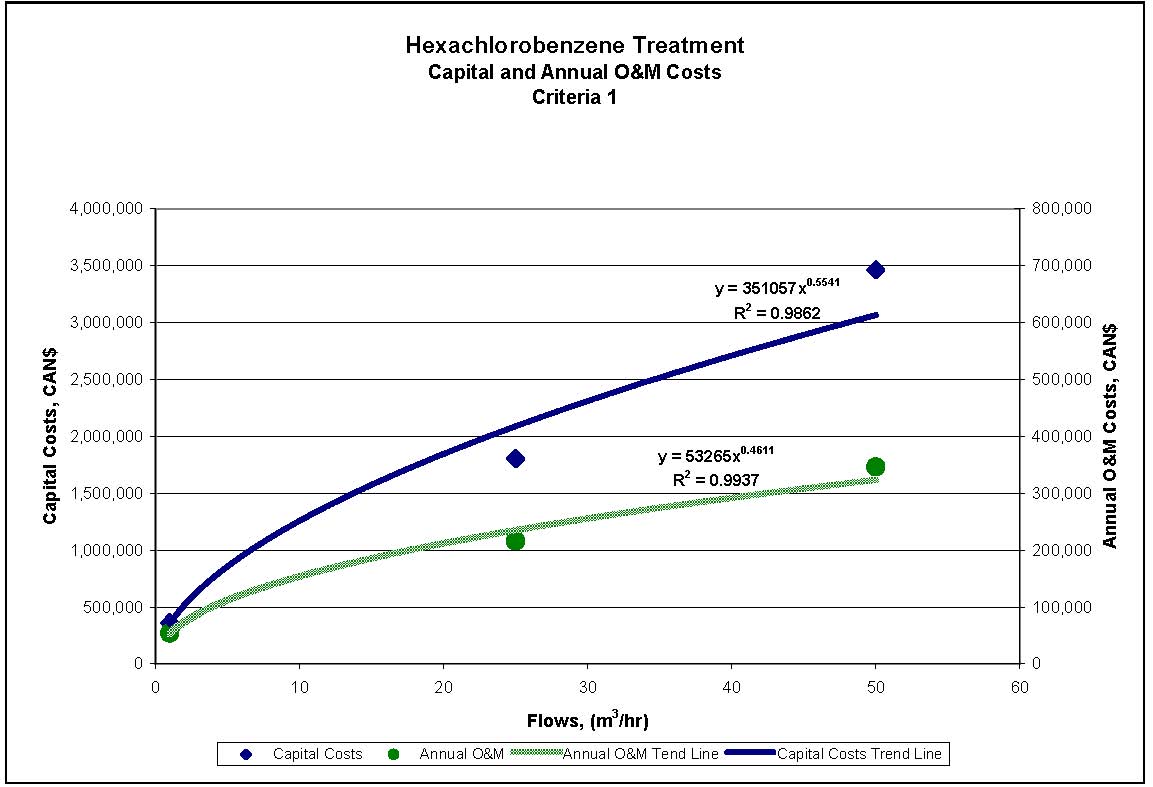
Figure 4.8 HCB Treatment for Reference Criteria 2
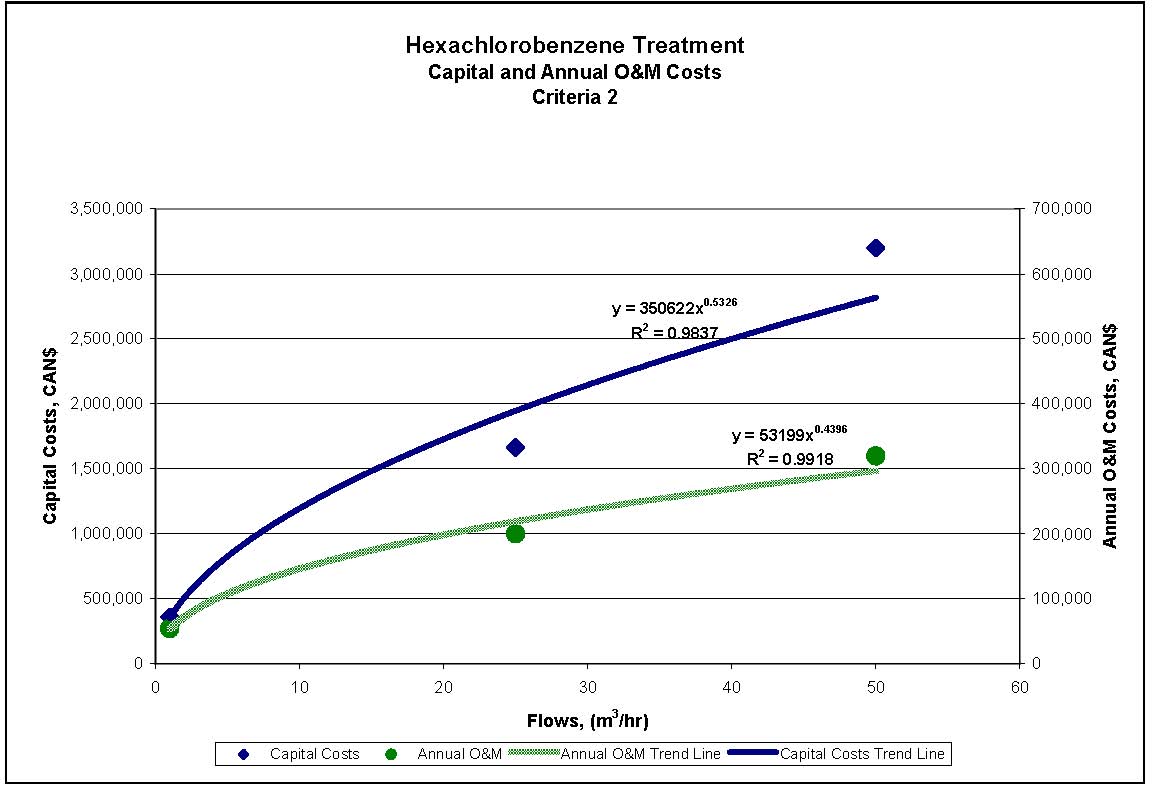
Figure 4.9 HCB Treatment for Reference Criteria 3
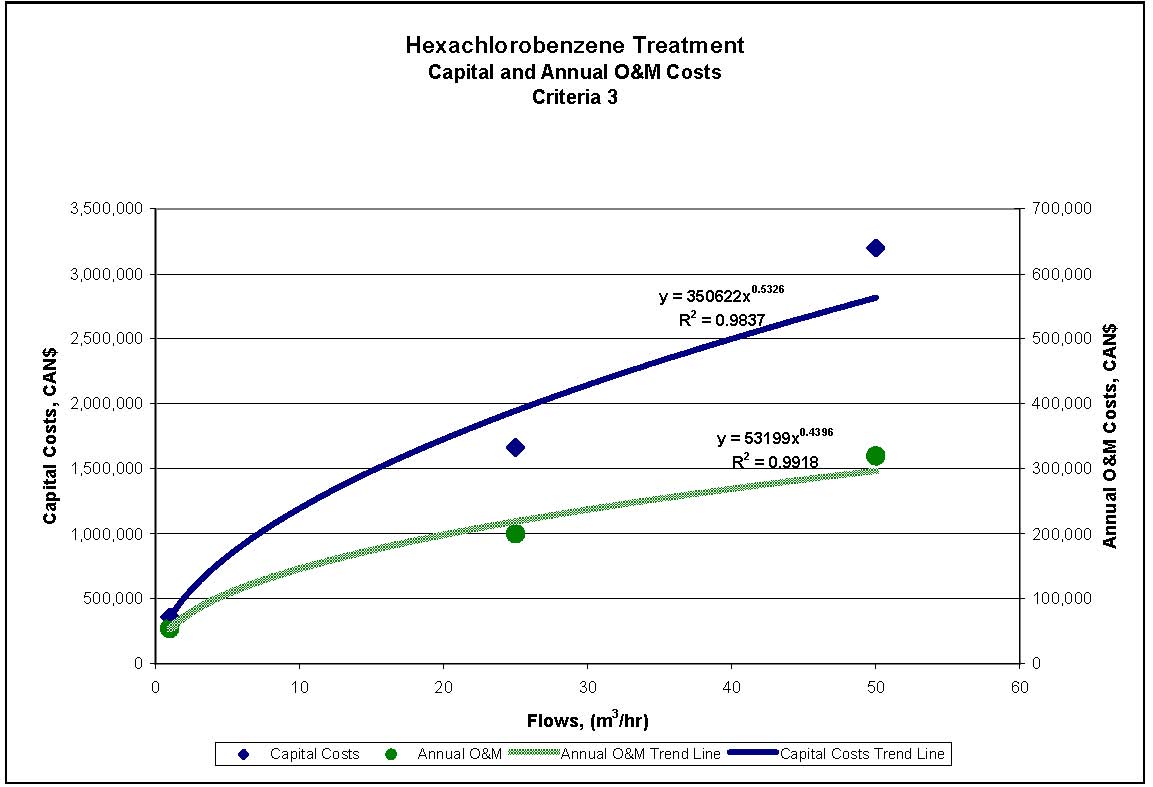
4.2.4 Treatment Options and Costs for PCP
Treatability information is provided for PCP in Table 4.7. Based on the assumption that BOD5 is less than 100 mg/L
- Reference Criteria 1: Sand/mixed media filtration, GAC, microfiltration and AOT. GAC is used before AOT for Criteria 1 to reduce the size of the AOT process required.
- Reference Criteria 2 and 3: Sand/mixed media filtration andGAC.
| Reference Criteria | Assumed Starting Concentration | Treatment Measures | Estimated % Removal Resulting from CombinedTreatment Measures | Estimated Conc. of Substance in Effluent after Treatment |
|---|---|---|---|---|
| Reference Criteria 1 0.0005 mg/L |
1 mg/L | Filtration¹, GAC², microfiltration³ and AOT4 | 99.95% | 0.0005 mg/L |
| Reference Criteria 2 0.005 mg/L |
1 mg/L | Filtration and GAC | 99.5% | 0.005 mg/L |
| Reference Criteria 3 0.005 mg/L |
1 mg/L | Filtration and GAC | 99.5% | 0.005 mg/L |
¹ Sand or mixed media filtration typically required as a pre-treatment stage for GAC.
² Estimated removal of 99.5 %.
³ Typically required as a pre-treatment stage for AOT.
4 Estimated removal of 90%.
The estimated capital and O&M treatment costs are presented in Table 4.8. These costs are conceptual level only, normally considered to be accurate to a range of −35% to +50%.
| Reference Criteria | Capital Cost Range: 1 m3/h | Capital Cost Range: 25 m3/h | Capital Cost Range: 50 m3/h | Annual O&M Cost Range: 1 m3/h | Annual O&M Cost Range: 25 m3/h | Annual O&M Cost Range: 50 m3/h |
|---|---|---|---|---|---|---|
| Criteria 1 | $360,000 | $1,802,000 | $3,464,000 | $54,000 | $216,000 | $346,000 |
| Criteria 2 and 3 | $70,000 | $438,000 | $748,000 | $10,000 | $53,000 | $75,000 |
Figures 4.10 to 4.12 show capital and annual O&M costing curves for the estimated cost ranges presented in Table 4.8 for each set of reference criteria.
Figure 4.10 PCP Treatment for Reference Criteria 1
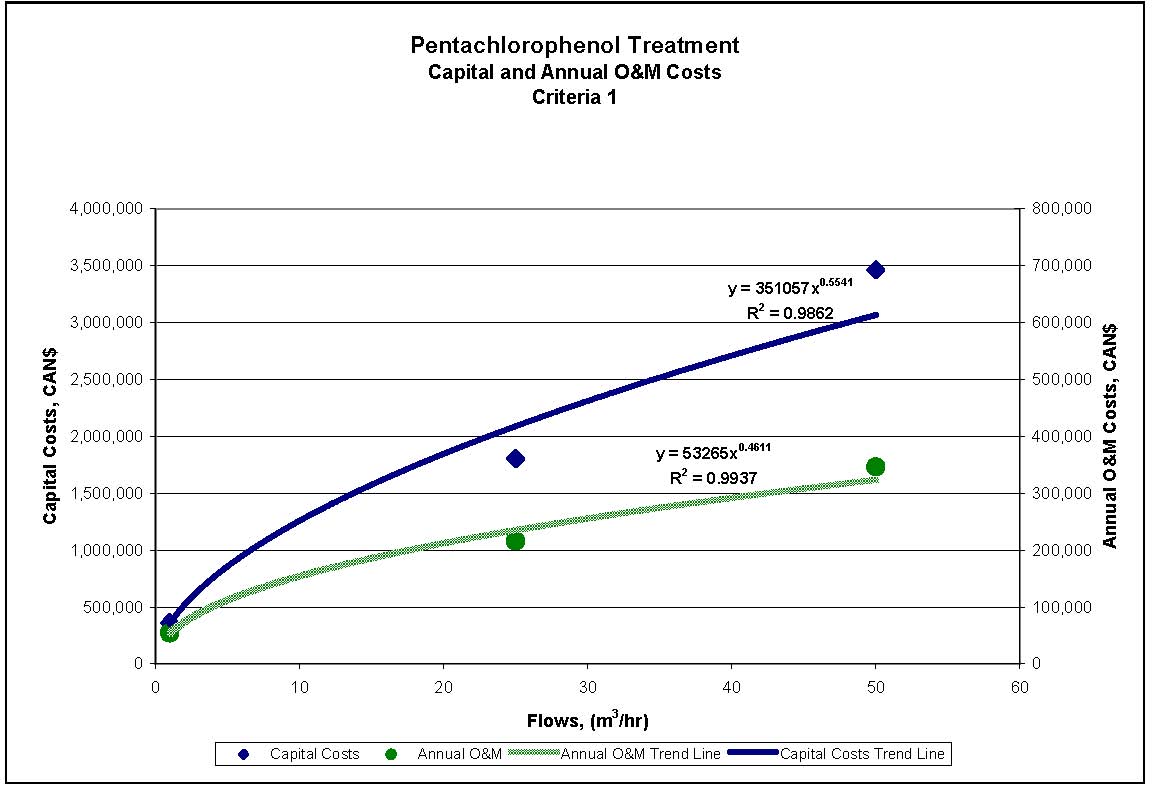
Figure 4.11 PCP Treatment for Reference Criteria 2
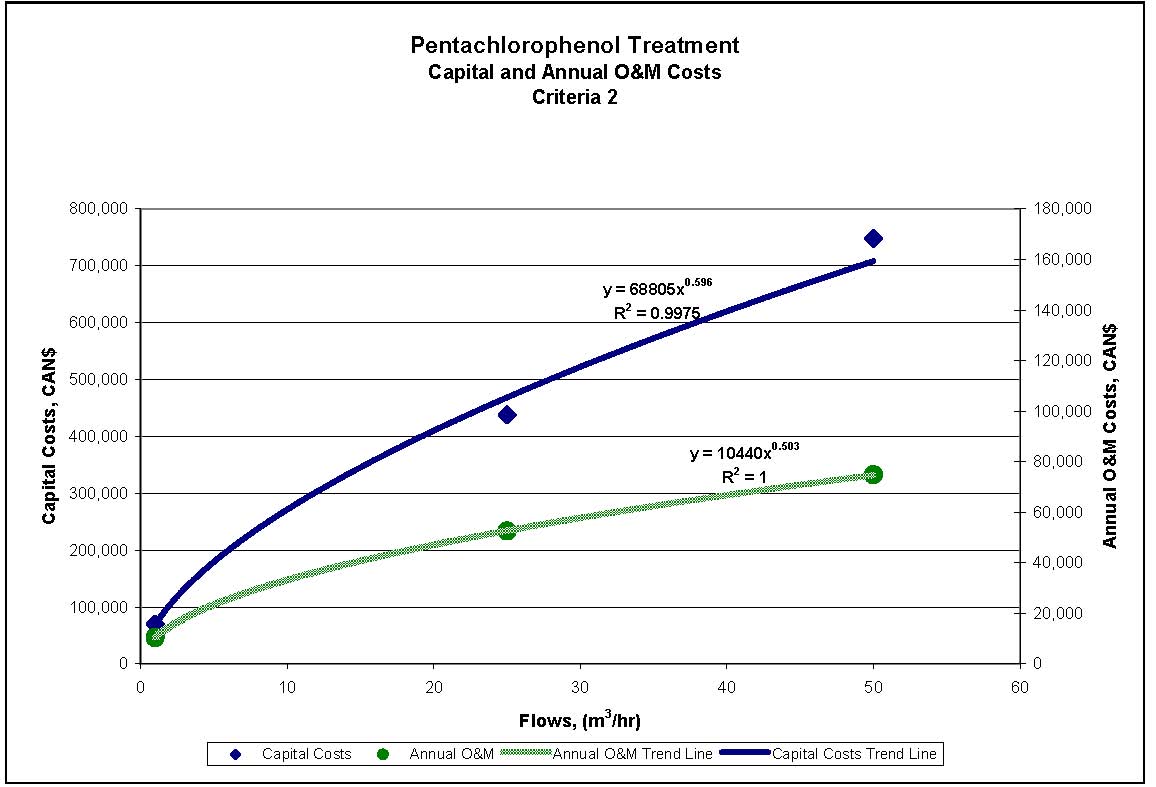
Figure 4.12 PCP Treatment for Reference Criteria 3
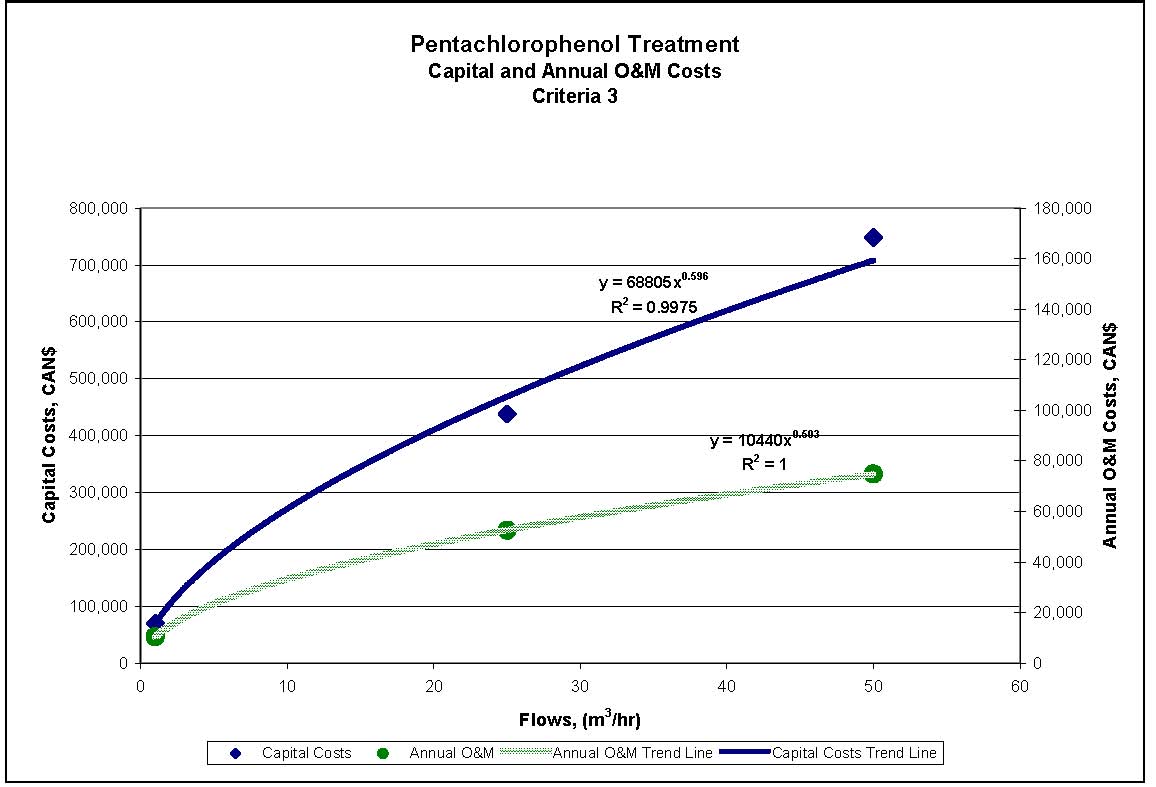
Key References (5)
The following documents were key in preparing this BMP:
- AUS EPA, 1998. Environmental Guidelines for the Textile Dying and Finishing Industry. June 1998. Environment Protection Authority, State Government of Victoria, Australia.
- Benzaon, 1999. Hexachlorobenzene Emissions/Release Inventory for the Province of Ontario, 1988, 1998, and 2000 Draft Report. Submitted to Toxics Prevention Division, Environmental Protection Branch, Environment Canada. April. Benzaon Environmental Inc.
- CEPA, 1993. Priority Substances List Assessment Report, 1,4-Dichlorobenzene. Canadian Environmental Protection Act, 1993. Research 2005 CD provided by MOE.
- Cohen, M. A., S. A. Fenn, S. Konar, 1997. Environmental and Financial Performance: Are They Related? Vanderbilt University, Nashville, TN, 1997
- Environment Canada (a), 2005. Pentachlorobenzene (QCB) and Tetrachlorobenzenes (TeCBs) Proposed Risk Management Strategy. January 2005. Environment Canada.
- Environment Canada (b), 2005. Strategic Options for the Management of Toxic Substances Benzidine and 3,3-Dichlorobenzidine. May, 2005. Environment Canada.
- European Commission (a), 2003. Integrated Pollution Prevention and Control (IPPC) Reference Document on Best Available Techniques for the Textiles Industry. July 2003. European Commission.
- European Commission (b), 2003. Reference Document on Best Available Techniques in the Large Volume Organic Chemical Industry. February 2003.
- Illinois, 1994. Pollution Prevention for Chemical Processes: A Handbook with Solved Problems from the Refining and Chemical Processing Industries. Illinois Waste Management and Research Center. August 1994.
- Marbek, 2005. Review of Existing Municipal Wastewater Effluent (MWWE) Regulatory Structures in Canada. Marbek Resource Consultants for the Canadian Council of Ministers of the Environment (CCME), 2005.
- Marks & Spencer, 2004. Environmental Code of Practice Dyeing, Printing and Finishing. Issue No. 5. Marks & Spencer. September 2004.
- OECD, 2004. OECD Series on Emission Scenario Documents No. 7, Emission Scenario Document on Textile Finishing Industry. June 24, 2004. Organization for Economic Co-operation and Development (OECD), Environment Directorate, Joint Meeting of the Chemicals Committee and the Working Party on Chemicals, Pesticides and Biotechnology.
- OSPAR, 2001. Convention for the Protection of the Marine Environment of the North-East Atlantic OSPAR Priority Substances Series: Pentachlorophenol. 2001. OSPAR Convention.
- US EPA, 2005. Pentachlorophenol, Topical Fact Sheet.
- US EPA, 1996. Best Management Practices for Pollution Prevention in the Textile Industry. United States Environmental Protection Agency. September 1996.
- US EPA, 1997. Profile of the Plastic Resin and Manmade Fiber Industries. September 1997. United States Environmental Protection Agency.
- US EPA, 2000. Technical Development Document for the Final Action Regarding Pre-treatment Standards for the Industrial Laundries Point Source Category. United States Environmental Protection Agency. Revised March 2000.
- US EPA, 1986. Locating and estimating air emissions from sources of chlorobenzenes. 1986. United States Environmental Protection Agency. Office of Air Quality. EPA-450/4-84-007m. 135pp
- Washington, 2002. Paint and Coatings Manufacturing Sector: A Pollution Prevention Assessment and Guidance. November 2002. Washington State Department of Ecology.
- Wisconsin, 2001. Clearinghouse Rule 98-198. March 2001. State of Wisconsin, Department of Natural Resources.
Glossary (6)
- Best Management Practices (BMPs)
- to reduce or eliminate pollutants encompass a wide range of activities including changes to materials or processes, operating procedures, housekeeping activities, and treatment techniques. BMPs may also include management activities, such as education and training, record-keeping and reporting, information systems, and communication with stakeholders, customers, and supply chain partners. BMPs can also include management approaches such as loss control programs and environmental management systems.
- Canadian Environmental Protection Act 1999 (CEPA 1999)
- is federal legislation that was first created in 1988 and consolidated various pieces of 1970s environmental legislation.
footnote 46 In addition, CEPA 1999 added many new Ministerial authorities and obligations, including new requirements for risk assessment and risk management of toxic substances and a strengthened pollution prevention approach. - Criteria
- are the reference criteria identified for analysis. There are three reference criteria, with Reference Criteria 1 being the most stringent and Reference Criteria 3 the least stringent.
- Environmental Management System (EMS)
footnote 47 - refers to management systems focussed on the minimization of harmful effects on the environment caused by corporate activities. Management systems in general are part of an organization’s structure for managing its processes or activities that transform inputs of resources into a product or service, which meet the organization’s objectives, such as satisfying the customer’s quality requirements, complying with regulations, or meeting environmental objectives. Environmental management is what the organization does to minimize harmful effects and to achieve continual improvement of its environmental performance.
- Hazardous Substances
- refers to substances that are potentially harmful to the environment or human health and safety. Hazardous substances include substances considered toxic under the Canadian Environmental Protection Act 1999, as well as other substances of interest subject to international agreement and reporting requirements. Refer to the Appendices for a list of substances of particular interest in this series of BMP documents.
- Industrial Facility Representatives
- may include any industrial employee or contractor of an industrial sector with responsibility, for example, for facility operations, facility design, public relations, compliance.
- National Pollution Release Inventory (NPRI)
- is a database of information on annual releases to air, water, land, and disposal or recycling from all sectors - industrial, government, commercial, and others.
footnote 48 The NPRI is a national reporting system legislated under the Canadian Environmental Protection Act1999. - Municipal Representatives
- may include any municipal employee or contractor with responsibility, for example, wastewater quality, wastewater infrastructure management, industrial sewer use programs, industrial relations, public outreach, and/or by-law enforcement.
- NAICS Code
- is the North American Industry Classification System (NAICS), which assigns numerical codes to industrial sectors and sub-sectors in North America. This system has replaced an older system of classification, known as the U.S. Standard Industrial Classification (SIC) system. Statistics Canada uses the NAICS classification system in its analysis of industrial activities in Canada.
- Pollution Prevention (P2)
- is "the use of processes, practices, materials, products, substances or energy that avoids or minimizes the creation of pollutants and waste, and reduces the overall risk to the environment or human health."
footnote 49 - Reference Criteria
- are the maximum desired final effluent concentrations for the harmful substances identified. Three reference criteria were identified for analysis in terms of pollution prevention measures and treatment measures required to achieve the reference criteria.
- Rules of Thumb
- are sets of engineering estimates based on similar or related datasets, professional judgement, and stated assumptions. Rules of Thumb are applied where specific information is not available. In the absence of specific information, Rules of Thumb can be used to develop reasonable ranges of potential outcomes or effects resulting from actions taken (such as implementation of certain P2 or treatment measures, for example).
- Substances of Interest
- are the potentially hazardous substances or toxic substances examined within this series of best management practices. Refer to the Appendices for a list of substances of particular interest in this series of BMP documents.
- Supply Chain
- refers to the network of organizations that provide materials, products, and services to industrial sectors in order that the industry can produce, market, and sell its products. The supply chain can include organizations selling raw materials, organizations selling semi-finished and finished goods, retail outlets, customers, etc.
- Treatment
- in this document refers to wastewater treatment processes used to remove or transform pollutants in the wastewater stream. Treatment is not considered a pollution prevention measure since it occurs after pollutants have been introduced or used in a process; pollutants that are present in a wastewater stream indicate that opportunities to prevent pollution have passed and treatment must therefore be used to reduce release of the pollutants to the environment.
Appendix A: Best Management Practices Documents
Table A.1 identifies the available Best Management Practices Documents in this series, and the industrial sectors and harmful pollutants which are addressed in each.
| Document Name | Sector and Sub-Sector Titles and NAICS Codes | Harmful Pollutants |
|---|---|---|
| Best Management Practices. Textiles Sector: Nonylphenol and its Ethoxylates and Chromium | Textiles Sector (313)
|
|
| Best Management Practices. Fabricated Metal Product Manufacturing: Cadmium, Lead and Copper | Fabricated Metal Product Manufacturing (332)
|
|
| Best Management Practices. Motor Vehicle Parts Manufacturing: Cadmium and Nonylphenol and its Ethoxylates | Motor Vehicle Parts Manufacturing (3363)
|
|
| Best Management Practices. Automotive Repair and Maintenance: Cadmium and PAHs | Automotive Repair and Maintenance (8111)
|
|
| Best Management Practices. Dry Cleaning and Laundry Services: Nonylphenol and its Ethoxylates, Cadmium, and Mercury | Dry Cleaning and Laundry Services (8123)
|
|
| Best Management Practices. Chemical Manufacturing Sector: Cadmium, Chromium, Copper, Mercury, Zinc, Nonylphenol and its Ethoxylates, and Vinyl Chloride | Chemical Manufacturing Sector (325)
|
|
| Best Management Practices. Chemical Manufacturing Sector: Resin, Synthetic Rubber, and Artificial and Synthetic Fibres and Filaments Manufacturing: Cadmium, Chromium, Copper, Mercury, Zinc, Nonylphenol and its Ethoxylates, and Vinyl Chloride | Chemical Manufacturing Sector (325)
|
|
| Best Management Practices. Chemical Manufacturing Sector: Pesticide, Fertilizer, and Other Agricultural Chemical Manufacturing: Cadmium, Chromium, Copper, Mercury, Zinc, and Nonylphenol and its Ethoxylates | Chemical Manufacturing Sector (325)
|
|
| Best Management Practices. Chemical Manufacturing Sector: Paint, Coating, and Adhesive Manufacturing: Cadmium, Chromium, Copper, Mercury, Zinc, and Nonylphenol and its Ethoxylates | Chemical Manufacturing Sector (325)
|
|
| Best Management Practices. 1,4-Dichlorobenzene, 3,3-Dichlorobenzidine, Hexachlorobenzene, and Pentachlorophenol: Non-Sector Specific Practices | Not applicable. |
|
Appendix B: Agreements for toxic reduction and substances of concern
Following is the list of agreements and programs identified by the Ontario MOE to be of particular concern. These agreements and programs were the impetus behind the development of this series of BMP documents.
- The 2002 Canada-Ontario Agreement respecting the Great Lakes Basin Ecosystem (COA), which identifies the goal of virtual elimination Tier I substances, reductions of Tier II substances and virtual elimination of 17 PAHs.
- The Canadian Environmental Protection Act, 1999 (CEPA).
- The 1997 Bi-National Toxics Strategy (BNTS), signed by Environment Canada and the USEPA.
- The Ontario government’s commitment to implement recommendation #32 of Commissioner O'Connor’s Report on the Walkerton Inquiry Part 2 to support major wastewater plant operators to identify practical methods to reduce or remove heavy metals and priority organics that are not removed by conventional treatment.
The following hazardous substances are subject of the agreements identified above and/ or subject of potential concern due to environmental and human health effects. (Note that not all of these substances have been addressed in the series of BMP documents for the six sectors.)
| Substance | COA | CEPA | BNTS |
|---|---|---|---|
| 1,4-dichlorobenzene | Tier II | n/a | Level II |
| 3,3-dichlorobenzidine | Tier II | Schedule 1 | Level II |
| alkyl-lead | Tier I | n/a | Level I |
| cadmium | Tier II | n/a | Level II |
| chromium | n/a | n/a | n/a |
| copper | n/a | n/a | n/a |
| dioxins and furans | Tier I | n/a | Level I |
| hexachlorobenzene | Tier I | Schedule 1 | Level I |
| hexachlorobutadiene/hexachloro-1,3-butadiene | n/a | Schedule 1 | Level II |
| hexachlorocyclohexane | Tier II | n/a | Level II |
| lead | n/a | Schedule 1 | n/a |
| mercury | Tier I | Schedule 1 | Level I |
| nonylphenol and ethoxylates | n/a | Schedule 1 | n/a |
| octachlorostyrene | Tier I | n/a | Level I |
| polynuclear aromatic hydrocarbons (PAHs) | Tier II | Schedule 1 | Level II |
| pentachlorophenol | Tier II | n/a | Level II |
| vinyl chloride | n/a | Schedule 1 | n/a |
| zinc | n/a | n/a | n/a |
Appendix C: Case study examples demonstrating benefits of P2 measures
The following case studies pertain to facilities among the six industrial sectors of interest for this BMP series. The case studies demonstrate the reduction effectiveness of P2 measures for specific applications while, at the same time, demonstrating the benefits of undertaking P2 measures. Reference information is provided for further investigation of the case study experience.
Proponents are encouraged to document their experience with P2 measures for publication as case studies. Several organizations recognize leadership in Canada in the area of P2 implementation, including the Canadian Council of Ministers of the Environment (CCME).
Case Study for P2 Measure: Material Substitution
Hafner °Inc., with four facilities in Granby, Quebec, is the largest Canadian manufacturer of furniture fabric and stretch knitted fabric. Material substitution enabled the company to reduce its nonylphenol and nonylphenol ethoxylated derivatives load from 6,800 kilograms in 2001 to 68 kilograms in 2003. The chemical oxygen demand (COD) of the wastewater was reduced from 210,000 kilograms per year to 110,000 kilograms per year. The reduction in COD reduced the annual effluent disposal costs by $15,000.
Source:Environment Canada’s Pollution Prevention Success Stories: Hafner Inc.
Case Study for P2 Measure: Process Modification
Monsanto Company, Muscatine, Iowa Plant, is a large agricultural herbicide manufacturing facility. Through internal recycling and process modifications, the facility reduced wastewater biochemical oxygen demand (BOD) loading by 97 %. For further information, see the U.S. Environmental Protection Agency’s National Environmental Performance Track website: Performance Track Case Study Monsanto Company Muscatine, Iowa Plant.
Case Study for P2 Measure: Operating Procedures and Housekeeping
Hendersons Automotive Group, a major supplier of seating components, has implemented several good housekeeping measures which have helped raise pollution prevention consciousness among the 180 employees at the company’s Melrose Park plant in South Australia. Cleaner production measures introduced have resulted in annual savings of $270,000. The measures cost a total of $309,000 and paid for themselves in only 18 months after implementation.
Source:Australian Department of the Environment and Heritage’s Eco-Efficiency and Cleaner Production: Hendersons Automotive Group Cleaner Production – Continuous Improvement Programs
Case Study for P2 Measure: Process Modification
Monroe Australia is a leading Adelaide-based manufacturer of shock absorbers and strut suspension units for the automotive industry. The company has implemented a major waste minimization strategy that has enabled it to process liquid waste, reduce water usage, reduce chemical and waste disposal costs, and eliminate pollution. It installed new equipment which treats wastewater to remove emulsified fats and oils, grease, heavy metals and all forms of suspended, colloidal and some dissolved solids. Monroe’s mains water usage has been reduced by over 10 mL per year; wastewater discharge to sewer has been reduced by 50 percent. The new technology has produced a savings of $250,000 per year with total outlay of $530,000 for a payback period of approximately two years.
Source: Australian Department of the Environment and Heritage’s Eco-Efficiency and Cleaner Production: Monroe Australia Pty Ltd Cleaner Production – Waste Minimisation Strategy
Case Study for P2 Measure: Process Modification and Operating Procedures
Specific Plating is a small metal finishing company where parts are plated with metals such as copper, nickel, zinc, silver, and gold. Specific Planting has dramatically reduced its sewer discharges of copper and nickel through pollution prevention efforts including both modifications of industrial processes and improved waste handling and treatment techniques. After the completion of the P2 projects, a reduction of approximately 88% for copper discharges and 85% for nickel discharges was achieved. Wastewater discharge flow has been reduced 27% and off-site sludge disposal has been reduced 53%.
Installation of equipment or changes in operating procedures required an investment of $63,000. Annual savings of $30,000 was realized with the payback period ranging from 1.5 years to just under 3 years. For more information, see City of Palo Alto: Pollution Prevention at Specific Plating Company.
Footnotes
- footnote[1] Back to paragraph Definition from Guidelines for the Implementation of the Pollution Prevention Planning Provisions of Part 4 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), National Office of Pollution Prevention, Environment Canada, 2001
- footnote[2] Back to paragraph Cohen, M.A., et al., 1997
- footnote[3] Back to paragraph Industrial Sectors are referenced by their North American Industry Classification System (NAICS) number.
- footnote[4] Back to paragraph US EPA, 1986
- footnote[5] Back to paragraph CEPA, 1993
- footnote[6] Back to paragraph Database of 1,4-Dichlorobenzene.
- footnote[7] Back to paragraph ATSDR (Agency for Toxic Substances and Disease Registry), 2004. Draft Toxicological Profile for Dichlorobenzenes.
- footnote[8] Back to paragraph U.S. National Safety Council
- footnote[9] Back to paragraph Euro Chlor, February 1999, Euro Chlor Risk Assessment for the Marine Environment: 1,4-Dichlorobezene
- footnote[10] Back to paragraph US EPA Sector Notebook, September 1997
- footnote[11] Back to paragraph International Programme on Chemical Safety
- footnote[12] Back to paragraph Environment Canada, 2005
- footnote[13] Back to paragraph US National Safety Council
- footnote[14] Back to paragraph Environment Canada (b), 2005
- footnote[15] Back to paragraph Environment Canada (b), 2005
- footnote[16] Back to paragraph US EPA
- footnote[17] Back to paragraph Draft PBT National Action Plan for Hexachlorobenzene for Public Review
- footnote[18] Back to paragraph Toronto Society for Coatings Technology, Environmental Update
- footnote[19] Back to paragraph Euro Chlor, Hexachlorobenzene – Sources, environmental fate and risk characterization, January 2005
- footnote[20] Back to paragraph Benazon, 1999
- footnote[21] Back to paragraph OSPAR Commission, 2001
- footnote[22] Back to paragraph EXTOXNET: Extension Toxicology Network - Pentachlorophenol. September 1993
- footnote[23] Back to paragraph US National Safety Council
- footnote[24] Back to paragraph USEPA, 2005
- footnote[25] Back to paragraph OSPAR, 2001
- footnote[26] Back to paragraph Marks & Spencer, 2004
- footnote[27] Back to paragraph US EPA, 1996
- footnote[28] Back to paragraph Marbek, 2005
- footnote[29] Back to paragraph NPRI website
- footnote[30] Back to paragraph Environment Canada EE Regulatory Requirements website
- footnote[31] Back to paragraph Definition from: Guidelines for the Implementation of the Pollution Prevention Planning Provisions of Part 4 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), National Office of Pollution Prevention, Environment Canada, 2001
- footnote[32] Back to paragraph Note that MSDSs may not identify substances comprising less than 1% of the product
- footnote[33] Back to paragraph See Zymoco company website (no longer active).
- footnote[34] Back to paragraph See product MSDS at Zymoco company website (no longer active).
- footnote[35] Back to paragraph AUS EPA, 1998
- footnote[36] Back to paragraph US EPA, 1996
- footnote[37] Back to paragraph OSPAR, 2001
- footnote[38] Back to paragraph US EPA, 1989
- footnote[39] Back to paragraph OSPAR, 2001
- footnote[40] Back to paragraph OSPAR, 2001
- footnote[41] Back to paragraph Refer to Section 2.1
- footnote[42] Back to paragraph Should BOD5 concentrations be higher than 100 mg/L, biological treatment may be required. This would be more costly than GAC treatment but may eliminate the need for GAC
- footnote[43] Back to paragraph Should BOD5 concentrations be higher than 100 mg/L, biological treatment may be required. This would be more costly than GAC treatment but may eliminate the need for GAC. The subsequent AOT treatment process would still be required.
- footnote[44] Back to paragraph Should BOD5 concentrations be higher than 100 mg/L, biological treatment may be required. This would be more costly than GAC treatment but may eliminate the need for GAC. The subsequent AOT treatment process would still be required.
- footnote[45] Back to paragraph Should BOD5 concentrations be higher than 100 mg/L, biological treatment may be required. This may be more costly than GAC treatment but may eliminate the need for GAC. The subsequent AOT treatment process would still be required to meet Reference Criteria 1.
- footnote[46] Back to paragraph Refer to the CEPA 1999 Environmental Registry
- footnote[47] Back to paragraph Definition adapted from definitions by the International Organization for Standardization
- footnote[48] Back to paragraph See the NPRI website
- footnote[49] Back to paragraph Definition in Guidelines for the Implementation of the Pollution Prevention Planning Provisions of Part 4 of the Canadian Environmental Protection Act, 1999 (CEPA 1999), National Office of Pollution Prevention, Environment Canada, 2001