Black rot of crucifer crops
Learn about the symptoms, crop rotation, weed and insect control, resistant varieties and chemical control for black rot in crucifer crops.
Introduction
Black rot is caused by a bacteria, Xanthomonas campestris pv. campestris, that can infect most crucifer crops at any growth stage. This disease is difficult for growers to manage and is considered the most serious disease of crucifer crops worldwide (Figure 1). The disease can cause significant yield losses when warm, humid conditions follow periods of rainy weather during early crop development. Late infections can provide a wound for other rot organisms to enter and cause significant damage during storage.

Symptoms
Symptoms of black rot vary considerably depending on the host, cultivar, plant age and environmental conditions. The bacteria can enter plants through natural openings and wounds caused by mechanical injury on roots and leaves. Seedborne bacteria infect the emerging seedlings through pores on the margin of the cotyledons and then spread systemically through the seedling. Infected seedlings grown in the greenhouse under cool conditions (below 15–18°C) frequently do not show any symptoms of the disease. When infected seedlings are transplanted to the field and temperatures rise to 25–35°C during periods of high relative humidity (80–100%), they become stunted with dead spots on the cotyledons (Figure 2) and will eventually wilt, and die. In regions with temperate climates (where temperatures remain cool), disease symptoms on infected seedlings may not always be obvious or appear severe. Infected seedlings grown under cool conditions may ooze bacteria from pores and lesions, which then serve as a source of the pathogen for neighbouring plants.

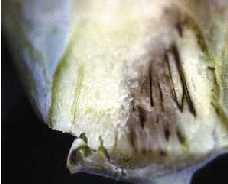
On older plants, the disease symptoms often appear as yellow or dead tissue at the edges of leaves, similar to tip burn, except the lesion frequently progress into a V-shape with the base of the V usually directed along a vein (Figure 3). Close inspection of infected leaves and stems may reveal black veins running through the infected tissue from which the disease gets its name (Figure 4). Lesions on leaves can expand down toward the base of the leaf causing the leaf to wilt and die.




The bacteria produce a sticky polysaccharide called xanthan that eventually plugs the vascular tissue inside the veins causing them to collapse and turn black. The tissue above the plugged, collapsed xylem eventually turns yellow, wilts and dies. During hot humid environmental conditions, the bacteria can move from the leaf into the stem through the xylem. Once inside the stem, the bacteria can move up or down to other parts of the plant including the roots. Systemically infected plants may produce chlorotic areas anywhere on the leaf. Severely infected leafy cole crops such as kale and cauliflower tend to shed their leaves from the bottom up leaving only a tuft of distorted leaves separated from the root system by a scarred barren stem. Symptoms on cauliflower often appear as black flecks or scorched leaf margins. The curds of infected cauliflower heads often become blackened.
Foliar symptoms may not be visible on infected root crops such as rutabaga and radish but blackened vascular tissue can appear inside the edible root tissue rendering the plants unmarketable. Although some infected plants may appear healthy, cutting across infected stems will reveal characteristic blackened vascular tissue. This is a simple method of determining the presence of the disease (Figure 5).

Some symptoms of black rot closely resemble those caused by Fusarium yellows, which causes the vascular tissue to turn brown. Most commercial crucifer cultivars are resistant to Fusarium (Figure 6).

Disease spread
Seed contaminated with black rot bacteria is considered the most important source of the pathogen and significantly contributes to the spread of this disease worldwide. As few as 3 infected seeds per 10,000 (0.03% infected seeds) can result in a black rot epidemic. Seed should be tested and certified to be disease free with less than 1 in 30,000 infected seed.
The organism survives in infected crop tissue left on the soil until the crop tissue rots. However, the bacteria do not survive very long in soil as unprotected free living organisms. The black rot bacteria can also infect and survive on many crucifer weeds. This also contributes to the persistence and spread of the disease. It can grow and multiply on host tissue without infecting or causing disease.
Rain splashed bacteria from contaminated plant residue left on the soil or from neighbouring diseased plants is the primary method of disease spread throughout a field. The bacteria enter and exit through water-secreting glands called hydathodes located at the edges and tips of leaves (Figure 7). Hydathodes often produce a drop of water during periods of high humidity early in the morning. The pathogen spreads very quickly when rain droplets contaminated with bacteria splash onto healthy leaves and enter the hydathodes. The bacteria move into the leaf veins through hydathodes and begin to multiply, rot and plug the veins. Contaminated water droplets that exude out of hydathodes of infected leaves can then be rain- splashed to other plants.
Black rot is more severe and widespread in fields that receive frequent early morning rains, particularly in May and June. Equipment, people, animals and overhead irrigation can further spread the disease. Insects can also spread the bacteria; however, their contribution to the spread of black rot is limited.

Disease management
Black rot management begins with the identification of potential disease sources and utilising an Integrated Pest Management (IPM) strategy including host resistance, planting disease free seed, avoiding spreading the disease and proper sanitation. Sanitation is the main method that reduces, excludes or eliminates the initial sources of disease. General sanitation practices include crop rotation, disinfecting seed, rouging diseased plants, elimination of refuse piles and eradication of alternative hosts.
Seed treatment
Seedborne inoculum significantly contributes to the spread of black rot bacteria. Growers should only plant tested certified seed < 1 infected seed in 30,000 or 0.003% contamination. When the infection level of seed is not known or disease-free seed is not available, seed should be treated to eliminate the bacteria. Growers who purchase transplants should request proof the seedlings were grown from disease-free or treated seed. During transplanting, diseased seedlings should not be planted in the field.
Seed treatments do not always eliminate 100% of the bacteria on or in the seed, and may adversely affect seed germination and vigour. Soaking seeds in hot water at 50°C for 25–30 min. is the most effective treatment for seedborne blackrot control. Weak seed, seed stored for several years and seed of certain crucifer crops; such as, cauliflower, kohlrabi, kale, rutabaga and summer turnip, may be damaged by hot water treatment; soak for 15 min. at 50°C only.
The effect of the hot water seed treatments on every variety of each individual crucifer crop has not been investigated. Growers are encouraged to treat a small portion of seed and plant in pots to determine the effect of the seed treatment on germination and vigour, prior to treating the entire seed lot.
Avoid disease spread
Use new seed trays each year to avoid contaminating this year’s crop with residual black rot bacteria from the previous year. If purchasing new trays each year is not economically feasible, used trays can be sterilised with steam, boiling water or chemical disinfectants to eliminate potential contamination. Destroy infected seed trays immediately to prevent disease spread to other seedling trays.
Avoid soaking crates or bundles of transplant seedlings in tubs of water before transplanting. The black rot bacteria can spread from diseased to healthy seedlings by infecting leaf scars and wounds on roots when soaked in water.
Black rot bacteria can contaminate the surface of clothing, equipment, tools and water sources. Reducing seeding rates and densities to promote good air circulation, facilitating the quick drying of plants, timing irrigation when plants will dry quickly and restricting field activities until later in the day when fields are dry will help reduce disease spread. Working in diseased fields last will also avoid disease spread from infected to non-infected fields. Wash and disinfect equipment before moving from one field to another.
Field selection
Field selection is very important due to the distance the pathogen can spread. Whenever possible, select fields as far away from fields grown to crucifer crops the previous year. Select fields that are well drained and will not receive run-off water from areas or fields where crucifers have been grown previously. Well drained, light soils are best for crucifer production because they can be worked early in the season and facilitate earlier planting of transplants. Planting early can help avoid disease because environmental conditions are usually not conducive for the development and spread of black rot bacteria.
Crop rotation
Planting disease-free, treated seed or seedling transplants does not necessarily ensure a disease free crop in the field. Crop rotation is also an important management tool. Black rot bacteria can survive in infected crop tissue in soil until the crop tissue breaks down and rots. The time required for crucifer crop debris to rot varies between regions depending on the temperature, amount of soil moisture and soil type. For example, in the states of Georgia and Washington, which experience long, warm summers, it has been estimated that free-living bacteria can survive in infested soil for about 60 days, and up to 615 days in infested host debris. The bacteria can survive longer in soil during cool, wet seasons than during hot, dry seasons. In Ontario, a 3-year rotation is recommended.
Weed control
Black rot bacteria can infect and survive on many crucifer weeds including bird rape (Brassica campestris), Indian mustard (B. juncea), black mustard (B. nigra), shepherd’s purse (Capsella bursa-pastoris), globe-podded hoary cress (Cardaria pubescens), pepper grass (Lepidium densiflore) and wild radish (Raphanus raphanistrum). Disease symptoms on weeds vary from small yellow V-shaped lesions on leaf margins to no visible symptoms. The pathogen can spread up to 30 m from infected plants (including weed hosts) to healthy plants. The pathogen not only infects and spreads from weeds to cruciferous crops, it can also survive on weed seeds and can grow and multiply on weed leaves without infecting or causing disease. Good weed control within fields will aid disease management; however, careful attention to weed control in ditches and along fencerows is also important.
Insect control
The crucifer flea beetle (Phyllotreta cruciferae) can transmit black rot bacteria from infected plants to healthy ones; however, their importance in the spread of the disease is limited. Wounds caused by insects provide an entry point for the disease to infect plants during heavy dews or periods of rain. Insect control will help reduce the spread and severity of disease.
Cull pile management
Infected refuse or cull piles left in the field, provides an excellent source of the black rot bacteria. Fresh cull piles left near fields can result in severe disease epidemics during the growing season. Prepare cole crops for market away from fields, and immediately chop and bury the diseased tissue cut from plants.
Resistant varieties
The development of crop varieties with disease resistance or tolerance to black rot has been the focus of many cole crop breeding programs worldwide. Resistance to black rot was first identified in the Japanese cabbage cultivar, Early Fuji. Today, many crucifer hybrids with black rot tolerance are available for both fresh and processing commercial production.
Chemical control
Soil fumigation can significantly reduce black rot bacteria. Soil fumigation is expensive and alternative methods for managing plant pathogenic bacteria are needed. For more information on chemical control options refer to OMAFRA Publication 363, Vegetable Production Recommendations.
Crop nutrition
The effect of plant nutrient management on the susceptibility of host crops to black rot infection is not fully understood. A balanced nutrient program may reduce the susceptibility of plants to disease infection. Excess nitrogen promotes lush vegetative growth and may increase plant susceptibility. Micronutrients may also be involved with the disease defence mechanisms of crucifer crops.