Catch-and-release fish handling
How to properly handle fish when catch-and-release fishing.
S. J. Casselman
Fisheries Section
Fish and Wildlife Branch
Ontario Ministry of Natural Resources
July 2005
This report should be cited as follows: Casselman, S. J. 2005. Catch-and-release angling: a review with guidelines for proper fish handling practices. Fish & Wildlife Branch. Ontario Ministry of Natural Resources. Peterborough, Ontario. 26 p.
Printed in Ontario, Canada
(0.3 k P. R. 05 07 15)
(MNR 51968)
(ISBN 0-7794-8590-4)
Single copies of this publication are available from:
Fisheries Section
Fish and Wildlife Branch
Ontario Ministry of Natural Resources
300 Water Street, Peterborough
Ontario, K9J 8M5
Cette publication hautement spécialisée n’est disponible qu’en anglais en vertu du Règlement 411/97, qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec le ministère des richesses naturelles en Ontario au
Executive summary
The use of catch-and-release practices by anglers is increasing. This increase is a result of both anglers viewing the process as a conservation technique and also because catch-and-release practices are being mandated by fisheries managers. Despite the widespread use of catch-and-release, there is generally a lack of understanding regarding the mortality caused by the practice and how variation in catch-and-release techniques may affect the level of mortality.
Fortunately, the increase in catch-and-release practice by anglers has coincided with an increase in research examining catch-and-release practices. While most of the studies to date have been species specific, there are general recommendations that can be made based on the available information.
While catch-and-release is physiologically stressful, stress and therefore mortality can be minimized by following some general catch-and-release guidelines. Gear should be appropriate for the species being angled, allowing for quick retrieval. The use of barbless hooks and circle hooks should be considered to reduce the amount of time required to release fish. Air exposure should be minimized and fish should be released quickly. Depth of capture, hooking location and bleeding should be taken into account when deciding on whether or not to release a fish.
When performed correctly, catch-and-release can be successful with minimal harm to the fish and should be encouraged. However, due to the variation among species in response to catch-and-release techniques, it is recommended that further research is needed to create species-specific guidelines.
Introduction
Over the last several decades catch-and-release has become a common practice among anglers. In a review of recreational fishing in Ontario, which was conducted in 2000, only 5% of anglers surveyed reported that they did not practice catch-and-release to some extent (OMNR, 2003). Catch-and-release may be practiced either voluntarily or because it is mandated. In Ontario, size limits are used as a management technique in many waters for a variety of fish species. Fish may be required to be released if they are under a minimum size limit, over a maximum size limit or within a protected slot size. Additionally, anglers may voluntarily practice catch-and-release as a conservation technique.
One of the key components to the increased use of catch-and-release practices, both by anglers and fisheries managers, is the assumption that fish which are released actually survive the experience. This assumption comes from the observation that when fish are released after being caught they generally swim away, apparently unharmed. However, research indicates that most mortality occurs some time after release (Muoneke and Childress, 1994), thus fish that appear healthy upon release may later exhibit injuries or distress caused by catch-and-release practices. Given the potential impact of mortality on the success of catch-and-release as a management practice, there is an increased demand to understand the level of mortality caused by catch-and-release and determine how various factors may affect catch-and-release survival.
The impact of mortality caused by catch-and-release practices is often underestimated by both anglers and fishery managers. From a review of 118 catch-and-release studies (Appendix 1), which, in total, involved over 120,000 fish, the average mortality associated with catch-and-release angling was 16.2%. Thus, while many anglers may assume that by practising catch-and-release they are having no impact on the fish population, a significant number of released fish may die. Additionally, many anglers will continue to fish after they have caught their limit under the premise that they will release all further fish caught, however they often do not take into consideration the number of fish which will inadvertently be killed as a result of this practice.
The purpose of this review is to synthesize current knowledge related to catch-and-release angling and provide some guidelines to minimize mortality caused by catch-and-release practices. While tournament angling is increasing in Ontario, this review does not examine the special issues related to tournament practices. However, in some instances, findings from research focused on tournaments are presented, when they can be applied to non-tournament angling situations. Given the special nature of tournament angling, and their increase in popularity, a review of tournament practices should be conducted.
Influencing variables for effective catch-and-release
Physiological response
A number of studies have attempted to determine the physiological response to catch-and-release procedures (e.g. Beggs et al., 1980; Gustaveson et al., 1991; Tufts et al., 1991; Ferguson and Tufts, 1992; Cooke et al., 2003a). From these studies a number of general responses can be identified. Extended play time can result in exhaustion, this is characterised by marked acidosis due to the release of protons into the extra-cellular fluid from poorly perfused white muscle (Tufts et al., 1991). Specifically this causes an increase in blood lactate levels and a decrease in extra-cellular pH (Tufts et al. 1991). Once the fish is landed, air exposure causes the gill lamellae to collapse, causing an almost complete loss of gas transfer. This results in an increase in blood CO2 levels and a decrease in blood O2 levels (Ferguson and Tufts, 1992). Exhaustive exercise and air exposure have been shown to produce an increase in cardiac output, with a decrease in stroke volume and an increase in heart rate (Cooke et al., 2003a). While the physiological response of fish to catch-and-release practices is relatively well understood, little is known about the cumulative impact of these sub-lethal stressors.
Some effects of sub-lethal stress caused by catch-and-release are reduced growth, impaired reproductive success and increased susceptibility to disease and pathogens. Clapp and Clark (1989) found that the growth of smallmouth bass was related to the number of hooking events, such that hooking reduced subsequent growth. Mason and Hunt (1967) examined the survival and growth of deeply hooked rainbow trout over a four month period. They found that, of the fish that survived to the end of the experiment, there was no significant decrease in the growth of fish that were released, even for fish in which hooks were left embedded. In examining the effects of catch-and-release on reproductive success, Booth et al. (1994) found that there was no significant difference in the egg survival of angled and non-angled Atlantic salmon. Conversely, Cooke et al. (2000) found that in largemouth bass, which provide parental care to eggs, fish that were angled incurred increased brood predation and increased likelihood of brood abandonment. Similarly, smallmouth bass have been found to have reduced ability to defend their broods after being angled from their nest (Suski et al., 2003). Thus, for some species at least, evidence exists that catch-and-release may result in reduced growth and reproductive success.
In addition to sub-lethal physiological stress, catch-and-release practices could cause injury, which, although initially does not cause mortality, may have detrimental effects. For example, hooks may physically damage gills, jaw, esophagus and eyes. These injuries may inhibit locomotion, feeding or reproduction, all of which may effectively remove previously healthy fish from the population.
Hook type
Although considerable variation exists between species in the effects of gear type on catch-and-release mortality, several generalizations can be made. While there is some variation among species, the use of circle hooks tends to reduce mortality. Circle hooks differ from traditional J-style hooks in that the point of the hook is generally perpendicular to the shank (Figure 1). Circle hooks have been found to be less susceptible to becoming deeply embedded; however, there is some evidence that, in bluegill, the incidence of eye injuries may be greater (Cooke et al., 2003b). In a review of the effectiveness of circle hooks, Cooke and Suski (2004) found that, the use of circle hooks reduced overall mortality rates by approximately 50%, but that there was variation among species.
Figure 1. Schematic of two circle hook designs (a,b) and a conventional J-style hook (c)
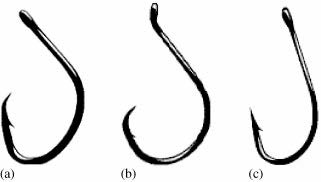
(from Cooke and Suski, 2004).
Barbless hooks are often recommended as an alternative to barbed hooks to decrease catch-and-release mortality. In fact, Manitoba and Alberta have regulated that only barbless hooks may used for angling in those jurisdictions to reduce catch-and-release mortality. Barbless hooks have been demonstrated to reduce handling time through ease of removing the hook, thereby decreasing associated mortality (Cooke et al., 2001). Schaeffer and Hoffman (2002) also demonstrated that the unhooking times of barbless hooks were significantly shorter than barbed hooks, however, the same study indicated that anglers landed 22% more fish using barbed hooks than barbless hooks. Similarly, the use of barbless hooks has been found to significantly reduce mortality in trout (Taylor and White, 1992). It has also been suggested that barbless hooks reduce tissue damage. Thus, while barbless hooks are generally less harmful to fish, anglers may be reluctant to use them because they perceive that catch rates will suffer.
Live vs. artificial baits
The influence of bait type on catch-and-release mortality has also been examined in some detail. Hooking mortality has been found to be significantly greater with natural baits than artificial bait in striped bass (Wilde et al., 2000). Similarly, worm-baited hooks have been shown to be ingested deeper than artificial lures and flies in bluegill, resulting in increased mortality (Siewert and Cave, 1990). In a comparison of hooking mortality of walleye caught on live and artificial leeches, mortality was 10% and 0% respectively, the use of leeches also resulted in deeper hooking (Payer et al., 1989). Results from smallmouth bass also show 11% mortality when using minnows and 0% when using spinner lures (Clapp and Clark, 1989).
Recently the use of scented artificial bait has increased. It is thought that scented artificial baits may be attacked by the fish in a similar manner as live bait, thus increasing mortality. In support of this hypothesis, Schisler and Bergersen (1996) found that hooking mortality was significantly higher when fish were caught on scented bait than when non-scented artificial bait was used. However, Dunmall et al. (2001) found that there was no effect of scented artificial bait on catch-and-release mortality of smallmouth bass. These studies suggest that the use of organic bait, and possibly scented artificial bait, results in deeper hooking which increases the chance of injury during hook removal and increases the length of time that the fish are exposed to air during hook removal. Thus, catch-and-release mortality can be reduced through the use of artificial bait.
Hooking location
The location of hooking has been shown to affect catch-and-release mortality. Catch-and-release mortality of white seabass was directly related to hooking location, and all mortalities involved hook damage to the visceral region (Aalbers et al., 2004). Similar results have been found for largemouth bass in which 56% of fish hooked in the esophagus died, while the mortality of fish hooked in other areas was not significantly different than fish that were not hooked at all (Pelzman, 1978). Dextrase and Ball (1991) found that hooking mortality of lake trout was largely restricted to those fish that were deeply hooked. Mortality in northern pike was also greater in fish that were deeply hooked (Dubois et al., 1994). Schisler and Bergersen (1996) reported that mortality of rainbow trout was significantly greater for fish hooked in the gill arches or esophagus than superficially hooked fish, and this increased mortality was attributed to bleeding intensity associated with hooking location. These studies all point to the fact that fish which are deeply hooked suffer increased mortality.
While the increased mortality associated with deep hooking is understood, it is less clear as to whether it is better to cut the line of deeply hooked fish or try to remove the hook, potentially risking further injury and increased air exposure to the fish. Aalbers et al. (2004) examined the growth and survival of white seabass up to 90 days after catch and found that survival of fish released with hooks left in place was enhanced, as compared to fish with hooks removed, but that growth was reduced. When hooks were removed mortality was 65%, compared to 41% when hooks were left embedded. Of the fish in this study that were released with the hooks left in place, 39% had successfully shed the hooks by the end of the study, however, of the hooks that remained in place there was minimal degradation. These results are similar to those found by Mason and Hunt (1967), who examined the effect of hook removal on the survival of rainbow trout up to four months after release. Two-thirds of the fish released without hook removal survived, while only 11.5% of the fish which had hooks removed survived. Additionally, of the fish that survived with hooks left in place, more than half had shed the hooks by the end of the study. Schill (1996) found that cutting the line on deeply hooked rainbow trout reduced mortality from 58% to 36%, and 60%-74% of fish that were released with hooks left in place had managed to discard the hooks by the end of the study. It has recently been suggested that for species such as bass and walleye, it may be possible to reduce mortality caused by deep hooking by removing the hook through the gills (Strange, 20034). However, to date there have not been any empirical studies which have demonstrated the effectiveness of this technique. Thus, despite the relative few studies which have examined the effect of deep hooking on mortality, it appears as though, for some species, mortality can be reduced if deeply hooked fish are released with the hook left in place.
Bleeding
Myers and Poarch (2000), found that the occurrence of bleeding in hooked largemouth bass was related to both mortality and hooking location. Of 19 bleeding fish, 47% died, whereas only 20% of non-bleeding fish died. Bleeding was observed in 48% of fish hooked in the throat and 50% of fish hooked in the gills, whereas only 1% of fish hooked in the mouth bled. Similarly, results from Arctic grayling show that bleeding intensity was related to hooking location, however, in this study there was no relationship between mortality and bleeding intensity (Clark, 1991). Schisler and Bergensen (1996) found that mortality in rainbow trout was significantly related to bleeding intensity. Their model predicted that the probability of mortality increased from 16% with no bleeding to 40% with heavy bleeding. Mortality has also been found to be significantly related to bleeding in cutthroat trout. Mortality was 6.5% in non-bleeding fish and 52.8% in fish that bleed (Pauley and Thomas, 1993). These studies all show that the chance of mortality increases if fish are bleeding, thus, anglers should consider keeping fish that bleed profusely.
Depth of capture
When fish are caught and retrieved quickly from deep water, injury may result from depressurization. Depressurization can result in over-inflation of the gas bladder, inability to submerge when released, gas embolisms, internal and/or external haemorrhaging and death. Freshwater fish have one of two basic types of swim bladders. Fish, such as carp, esocids, trout and salmon have a duct which connects the swim bladder to the alimentary canal. These fish can expel gas and make buoyancy adjustments more quickly than fish such as, bass, walleye, perch and most panfish which lack a connecting duct and rely on diffusion to deflate their swim bladder. Thus, while susceptibility to depressurization varies among fish species, it has the potential to be a significant source of mortality (Kerr, 2001).
Figure 2. Apparatus used for deep release of lake trout

(photo courtesy of D. Reid, Ministry of Natural Resources, Owen Sound)
To release fish that suffer from depressurization a technique known as “fizzing” has been developed to artificially deflate swim bladders by puncturing the swim bladder with a sharp instrument. In a review of “fizzing”, Kerr (2001) suggested that the practice should be discouraged, as significant damage can result from the procedure, and that fishing deep waters (5-6 m) should be restricted if fish are intended to be released. Kerr (2001) also reviewed several alternatives to “fizzing” for releasing fish caught from deep water. These involved lowering fish back to the depth they were caught at for release, by means of a retrievable weight or submersible cage (Figure 2). While little investigation has gone into determining the effectiveness of these alternatives, they are recommended over fizzing. To prevent potential decompression, catch-and-release angling for species in deep water should be avoided.
Temperature
Evidence suggests that catch-and-release mortality is directly related to water temperature, with mortality increasing at extreme temperatures. In a seasonal comparison of hooking mortality of bluegill, Muoneke (1992b) found that mortality was greater in the summer when water temperatures were highest. However, this study did not account for other variables, such as differences in feeding rate or reproductive status, which may have increased mortality during the summer. Similarly, mortality in cutthroat trout has been shown to increase from 0 to 8.6% as water temperature increased from 8°C to 16°C (Dotson, 1982). In a meta-analysis of black bass mortality associated with tournaments, a strong relationship was found between water temperature and both pre-release and post-release mortality (Wilde, 1998). Research from walleye tournaments indicates that mortality increases with water temperature and suggests that tournaments should be limited to the spring and fall (O’Neil and Pattenden, 1992), or when water temperatures are cooler than 15.6°C (60°F) (Boland, 1994). Wilkie et al. (1997) examined the post-exercise physiology of Atlantic salmon at 12°, 18° and 23°C, and found that physiological recovery was slowest at 12°C, however, there was significant mortality at 23°C. This result suggests that warmer temperatures facilitate recovery but that extremely high temperature increases mortality.
Nuhfer and Alexander (1992) found that mortality increased with water temperature in brook trout that were bleeding from the gills or throat area as a result of hooking. Mortality has also been found to increase with water temperature in smallmouth bass (Cooke and Hogle, 2000), largemouth bass (Gustaveson et al., 1991; Meals and Miranda, 1994) and striped bass (Nelson, 1998). Interestingly, Bettoli and Osborne (1998) found that catch-and-release mortality in striped bass was linearly related to air temperature but not water temperature, suggesting the temperature during air exposure may be more important in determining survival than actual water temperature. These studies demonstrate that catch-and-release mortality increases with temperature and special care should be taken with fishing during extremely warm weather.
There has been a similar concern with releasing fish that have been angled during ice-fishing and exposed to cold temperatures. It has been suggested that eyes and gills can be damaged from freezing on extremely cold days. However, studies examining catch-and-release survival of walleye during ice-fishing found no evidence of damage or mortality caused by exposure to cold temperatures (Ellis, 2000). Thus, while brief exposure of fish to cold temperatures may not cause mortality or damage, it is best minimize the time that fish are kept out of the water when ice-fishing.
Type of landing net
Despite the widespread use of landing nets by anglers there has been relatively little investigation into the damage caused by their use or which of the available types of net result in the lowest injury to fish. Generally, it is recommended that the use of landing nets be limited as it is thought to increase fin damage, and remove the protective mucus layer, thus increasing susceptibility to disease. Barthel et al. (2003) examined the effects of landing net mesh type on injury and mortality in bluegill. They quantified the effects of netting for a 168 h period after capture and found that there was zero mortality in fish that were landed without a net while fish that were landed with a net experienced a mortality rate of 4 to 14%. There was also increased pectoral and caudal fin abrasion and dermal disturbance (scale and mucus loss). Of the four types of landing net mesh types compared (rubber, knotless nylon, fine knotted nylon and coarse knotted nylon), the knotted mesh types resulted in greater injury and mortality than rubber or knotless mesh. Thus, injury (and therefore mortality) can be reduced if the use of landing nets is limited to those instances where their use is required to safely land and control fish to prevent mechanical injury. However, when the use of a landing net is required or preferred, it is best to use one made of rubber or knotless mesh.
Figure 3. Muskellunge being handled using a cradle

(photo courtesy of S. Kerr, Ministry of Natural Resources, Peterborough)
To assist in handling large fish (e.g. muskellunge), the use of cradles is often suggested to minimize stress to the fish. Cradles generally consist of mesh strung between two poles to fit the body shape of the fish (Figure 3). The use of cradles enables fish to be restrained in the water while allowing for the removal of hooks, additionally, a tape measure can be incorporated into the construction of the cradle allowing for the fish to be measured while remaining in the water. Although there have been no scientific studies examining the benefit of using cradles for large fish, their use is generally accepted to be beneficial (Smith, 2001).
Air exposure
Ferguson and Tufts (1992) found that there were direct effects of air exposure duration on mortality of rainbow trout. Rainbow trout that were chased for approximately 10 min had a survival rate of 88%, however this fell to 62% for fish that were subsequently exposed to air for 30 s and survival was only 28% for fish exposed to air for 60 s (Ferguson and Tufts, 1992). Cooke et al. (2001) examined the effect of handling time on injury and cardiac disturbance of rock bass. While air exposure did not result in any mortality, bradycardia (decreased heart rate) was observed during air exposure and cardiac output increased after fish were returned to the water. Simulated angling (fish were chased for 30 s) resulted in increased cardiac output and arrhythmia (irregular heartbeat). Fish that had 30 s of air exposure required 2 h for full cardiac recovery while fish that were exposed to air for 180 s required 4 h to fully recover (Cooke et al., 2001). These studies demonstrate the detrimental effects of air exposure, and highlight the need to reduce handling time and air exposure during catch-and-release.
Recovery time
In addition to the immediate effect of catch-and-release, fish may not physiologically recover for some time after being released. Beggs et al. (1980) found that angled muskellunge required 12 to 18 h to recover from acidosis caused by angling. Similar recovery periods have been observed for wild Atlantic salmon, which after being exercised for approximately 10 min, were found to have extracellular acidosis which lasted for about 4 h and blood lactate levels which remained significantly elevated for at least 8 h (Tufts et al., 1991). In a comparison of hatchery and wild rainbow trout, Wydoski et al. (1976) found that hooking induced increased chloride levels in the blood and plasma osmolarity changes which recovered within 8 h (Wydoski et al., 1976). Cooke et al. (2003a) examined the cardiac response of largemouth bass to simulated angling events and found that approximately 135 minutes were required for cardiac variables to return to pre-exercise levels. The length of time required for fish to recover from catch-and-release practices may help explain why mortality is often delayed until after release.
Size of fish
Fish size is thought to be related to catch-and-release mortality because larger fish are more difficult to handle, thus higher mortality may be expected with increased fish size. In support of this hypothesis Meals and Miranda (1994) found that mortality of tournament-caught largemouth bass was significantly greater (29% vs. 9%) in fish greater than 18 inches in length when compared to fish that were between 12 and 14 inches in length. Similarly, in a meta-analysis of mortality associated with black bass tournaments, Wilde (1998) found a non-significant, but positive relationship between fish size and initial mortality. However, the increased mortality observed in larger fish in these studies may be attributed to crowding and increased oxygen consumption while fish are stored in live wells and not to an intrinsic relationship between fish size and mortality. There are also a several studies which have examined the relationship between fish mortality and size and have not found any significant relationship (Titus and Vanicek, 1988; Schill, 1996). It is important to note that the studies discussed here have examined mortality and fish size within species and not between species. Intraspecific studies are difficult to interpret because any observed relationship between fish size and mortality may be attributed to other factors which differ between species, such as feeding behaviour and mouth morphology. However, it may be reasonable to expect that large species, such as muskellunge and pike, may be more susceptible to mortality than smaller species. These large fish are often played for longer periods of time and handled longer for photographs, this results in a larger physiological disturbance after angling. Thus special care should be taken when handling large fish to minimize injury and mortality.
Catch-and-release guidelines
Most catch-and-release research to date has focused on examining species-specific responses to potential factors which affect mortality. However, due to the large number of studies that have been completed to date, a number of general trends are emerging. Thus, while caution should be used when applying species-specific findings to other species, the following recommendations are, given the available knowledge base, general guidelines to be used to reduce catch-and-release mortality for most species.
Angling techniques
- Circle hooks should be used as they will minimize the chance of deep hooking.
- Barbless hooks are recommended as they are easier to remove and therefore reduce handling time.
- The use of live/organic bait should be discouraged as it increases the likelihood of deep-hooking.
- The use of artificial lures should be encouraged.
- Fishing lines must not be left unattended as unattended lines have a greater chance of deeply hooking a fish.
- Fishing line used should be appropriate to the species of fish being sought. This will prevent line breaking and reduce playing time.
- Avoid angling during extreme water temperatures, both hot and cold, if you plan on releasing your catch.
Landing a fish
- Angled fish should be retrieved as quickly as possible to prevent fish exhaustion.
- Fish should be landed by hand where possible.
- Where a landing net is required, it should be knotless and preferably made of soft rubber.
- When landing extremely large fish (e.g. muskellunge), the use of landing cradle should be considered.
Handling and photographing a fish
- Keep fish in the water as much as possible to minimize air exposure.
- Never place your fingers through gills or in the eyes.
- Don’t hold heavy fish by the jaw as this may damage the jaw and vertebrae.
- Hold large fish horizontally and support its body to avoid damage to the internal organs.
- Use wet hands or wet cloth gloves to handle the fish.
- Have camera ready prior to landing fish to minimize air exposure.
- If possible, photograph the fish while in water.
Unhooking a fish
- Have longnose pliers available to back the hook out.
- Remove the hook quickly, keeping the fish underwater.
- If the fish is deeply hooked, cut the line and release the fish as quickly as possible.
- Avoid using stainless steel hooks as they take longer to corrode if left in the fish.
Depressurization
- Avoid fishing deeper (5-6 m) waters if you intend to release your catch.
- Consider depth of capture when deciding on whether or not to release a fish.
- Release the fish quickly after it is landed.
- Avoid artificial swim bladder deflation ("fizzing").
Revival
- If there is current, hold the fish upright, facing into the current.
- If there isn’t any current, gently move fish back and forth in the water until gill movements return to normal and it is able to maintain its balance.
- When the fish begins to struggle, let it swim away.
Acknowledgements
I thank Steve Kerr who suggested this project as well as providing assistance and direction in preparation of this report. Cory Suski also provided valuable advice during the preparation of this review. Constructive editorial comments were also provided by Dr. Bruce Tufts.
List of references
Aalbers, S.A., G.M. Stutzer and M.A. Drawbridge. 2004. The effects of catch-and-release angling on the growth and survival of juvenile white seabass captures on offset circle and J-type hooks. North American Journal of Fisheries Management 24: 793-800.
Archer, D.L. and H.A. Loyacano, Jr. 1975. Initial and delayed mortalities of largemouth bass captured in the 1973 National Keowee B.A.S.S. tournament. Proceedings of the Annual Conference of the Southeastern Association of Game and Fish Agencies 28: 90-96.
Barthel, B.L., S.J. Cooke, C.D. Suski and D.P. Philipp. 2003. Effects of landing net mesh type on injury and mortality in a freshwater recreational fishery. Fisheries Research 63: 275-282.
Barwick, D.H. 1985. Stocking and hooking mortality of planted rainbow trout in Jocassee Reservoir, South Carolina. North American Journal of Fisheries Management 5: 580-583.
Beggs, G.L., G.F. Holeton and E.J. Crossman. 1980. Some physiological consequences of angling stress in muskellunge, Esox masquinongy Mitchill. Journal of Fish Biology 17: 649-659.
Bendock, T. and M. Alexanderstottir. 1991. Hook-and-release mortality in the Kenai River chinook salmon recreational fishery. Alaska Department of Fish and Game, Fisheries Data Series No. 91-39.
Bennett, D.H., L.K. Dunsmoor, R.E. Rohrer and B.E. Rieman. 1989. Mortality of tournament-caught largemouth and smallmouth Bass in Idaho lakes and reservoirs. California Fish and Game 75: 20-26.
Bettoli, P.W. and R.S. Osborne. 1998. Hooking mortality and behavior of striped bass following catch and release angling. North American Journal of Fisheries Management 18: 609-615.
Bettoli, P.W., C.S. Vandergoot and P.T. Horner. 2000. Hooking mortality of saugers in the Tennessee River. North American Journal of Fisheries Management 20: 833-837.
Boland, T.L. 1994. The differential return rates of walleye tagged during tournament fishing and Iowa DNR electrofishing on pools 12 and 13, upper Mississippi River, 1991-1993. Iowa Department of Natural Resources, Bureau of Fisheries, Bellevue Fisheries Station, 17 p.
Booth, R.K., J.D. Kieffer, K. Davidson, A.T. Bielak and B.L. Tufts. 1994. Effects of late-season catch and release angling on anaerobic metabolism, acid-base status, survival, and gamete viability in wild Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 52: 283-290.
Bugley, K. and G. Shepherd. 1991. Effect of catch-and-release angling on the survival of black sea bass. North American Journal of Fisheries Management 11: 468-471.
Burdick, B. and R. Wydoski. 1989. Effects of hooking mortality on a bluegill fishery in a western reservoir. In: Catch-and-Release Fishing – A Decade of Experience, pp. 187-196. (Barnhart, R.A. and T.D Roelofs, Eds.). Arcata, California: Humboltd State University, California Cooperative Fisheries Research Unit.
Burkholder, A. 1992. Mortality of northern pike captured and released with sport fishing gear. Alaska Department of Fish and Game, Fisheries Data Series No. 92-3.
Butler, J.A. and R.E. Loeffel. 1972. Experimental use of barbless hooks in Oregon’s troll salmon fishery. Pacific Marine Fisheries Commission Bulletin 8: 23-30.
Childress, W.M. 1989a. Hooking mortality of white bass, striped bass white bass x striped hybrid bass and red drum. Texas Parks and Wildlife Department, Final Report. F-31-R-15.
Childress, W.M. 1989b. Catch-and-release mortality of white and black crappie. In: Catch-and-Release Fishing - A Decade of Experience, pp 175-186. (Barnhart, R.A. and T.D. Roelofs, Eds.). Arcata California: Humboltd State University, California Cooperative Fisheries Research Unit.
Clapp, D.F. and R.D. Clark Jr. 1989. Hooking mortality of smallmouth bass caught on live minnows and artificial spinners. North American Journal of Fisheries Management 9: 81-85.
Clark, R.A. 1991. Mortality of Arctic grayling captured and released with sport fishing gear. Alaska Department of Fish and Game, Fisheries Data Series No. 91-59.
Colvin, M.A. 1991. Evaluation of minimum-size limits and reduced daily limits on the crappie populations and fisheries in five large Missouri reservoirs. North American Journal of Fisheries Management 11: 585-597.
Cooke, S.J. and W. J. Hogle. 2000. Effects of retention gear on the injury and short-term mortality of adult smallmouth bass. North American Journal of Fisheries Management 20: 1033-1039.
Cooke, S.J., K.G. Ostrand, C.M. Bunt, J.F. Schreer, D.H. Wahl and D.P. Philipp. 2003a. Cardiovascular responses of largemouth bass to exhaustive exercise and brief air exposure over a range of water temperatures. Transactions of the American Fisheries Society 132: 1154-1165.
Cooke, S.J., D.P. Philipp, K.M. Dunmall and J.F. Schreer. 2001. The influence of terminal tackle on injury, handling time, and cardiac disturbance of rock bass. North American Journal of Fisheries Management 21: 333-342.
Cooke, S.J., D.P. Philipp, J.F. Schreer and R.S. McKinley. 2000. Locomotory impairment of nesting male largemouth bass following catch-and-release angling. North American Journal of Fisheries Management 20: 968-977.
Cooke, S.J., C.D. Suski, B.L. Barthel, K.G. Ostrand, B.L. Tufts and D.P. Philipp. 2003b. Injury and mortality induced by four hook types on bluegill and pumpkinseed. North American Journal of Fisheries Management 23: 883-893.
Cooke, S.J. and C.D. Suski. 2004. Are circle hooks an effective tool for conserving marine and freshwater recreational catch-and-release fisheries? Aquatic Conservation: Marine and Freshwater Ecosystems 14: 299-326.
Dextrase, A.J. and H.E. Ball. 1991. Hooking mortality of lake trout angled through the ice. North American Journal of Fisheries Management 11: 477-479.
Diodata, P.J. 1991. Estimating mortality of hooked and released striped bass. National Marine Fisheries Service, AFC-22.
Dotson, T. 1982. Mortalities in trout caused by gear type and angler-induced stress. North American Journal of Fisheries Management 2: 60-65.
DuBois, R.B., T.L. Margenau, R.S. Stewart, P.K. Cunningham and P.W. Rasmussen. 1994. Hooking mortality of northern pike angled through ice. North American Journal of Fisheries Management 14: 769-775.
Dunmall, K.M., S.J. Cooke, J.F. Schreer and R.S. McKinley. 2001. The effect of scented lures on the hooking injury and mortality of smallmouth bass caught by novice and experienced anglers. North American Journal of Fisheries Management 21: 242-248.
Ellis, G. 2000. Do winter walleye survive release? Ontario Out of Doors 32:46-51.
Faccin, A. 1983. Hooking mortality of fly-caught Duncan River rainbow trout (Salmo gairdneri) in Harper Lake, British Columbia. British Columbia Fish and Wildlife, Fisheries Technical Circular 58.
Falk, M.R. and D.V. Gillman. 1975. Mortality data for angled Arctic grayling and northern pike from the Great Slave Lake area, Northwest Territories. Winnipeg, Manitoba: Canada Department of Environmental Fisheries and Marine Services, Technical Report CEN/D-75-1.
Falk, M.R., D.V. Gillman and L.W. Dahlke. 1974. Comparison of mortality between barbed and barbless hooked lake trout. Winnipeg, Manitoba: Canada Department of Environemtnal Fisheries and Marine Services, Technical Report CEN/T-74-1.
Ferguson, R.A. and B.L. Tufts. 1992. Physiological effects of brief air exposure in exhaustively exercised rainbow trout (Oncorhynchus mykiss): implications for “catch and release” fisheries. Canadian Journal of Fisheries and Aquatic Sciences 49: 1157-1162.
Fielder, D.G. and B.A. Johnson. 1992. Weigh-in, delayed and total mortality of walleyes at two live-release fishing tournaments on Lake Oahe, South Dakota. South Dakota Game and Fish Parks Department, Special Report No. 92-5.
Fletcher, D.H. 1987. Hooking mortality of walleye captured in Porcupine Bay, Washington. North American Journal Fisheries Management 7: 594-596.
Goeman, T.J. 1991. Walleye mortality during a live-release tournament on Mille Lacs, Minnesota. North American Journal of Fisheries Management 11: 57-61.
Gustaveson, A.W., R.S. Wydoski and G.A. Wedemeyer. 1991. Physiological response of largemouth bass to angling stress. Transactions of the American Fisheries Society 120: 629-636.
Harrell, R.M. 1988. Catch and release mortality of striped bass with artificial lures and baits. Proceedings of the Annual Conference of the Southeastern Association of Fish and Wildlife Agencies 41: 70-75.
Hartley, R.A. and J. R. Moring. 1991. Initial and delayed mortality of largemouth and smallmouth basses due to tournaments. In: Warmwater Fisheries Symposium I, pp. 269-272. (Cooper, J.L.and R.H. Hamre, Eds.). Fort Colins, Colorado: U.S. Department of Agriculture, Forestry Service, General Technical Report RM-207.
Hegen, H.E. and A.W. Green. 1983. Handling and tagging survival of hook-caught spotted seatrout held in cages. Proceedings of the Texas Chapter of the American Fisheries Society 5: 39-53.
Hegen, H.E. G.E. Saul and G.C. Matlock. 1987. Survival of hook-caught spotted seatrout. Proceedings of the Annual Conference of the Southeastern Association of the Fish and Wildlife Agencies 38: 488-494.
Hubbard, W.D. and L.E. Miranda. 1991. Mortality of white crappie after catch and release. Proceedings of the Annual Conference of the Southeastern Association of Fish and Wildlife Agencies 43: 49-55.
Hulbert, P.J. and R. Engstrom-Heg. 1980. Hooking mortality of worm-caught hatchery brown trout. New York Fish and Game Journal 27: 1-10.
Hunsaker, D., II., L.F. Marnell and P. Sharpe. 1970. Hooking mortality of Yellowstone cutthroat trout. Progressive Fish Culturist 32: 231-235.
Hysmith, B.T., J.H. Moczygemba and G.R. Wilde. 1992. Hooking mortality of striped bass in Lake Texoma, Texas-Oklahoma. Proceedings of the Annual Conference of the Southeastern Association of Fish and Wildlife Agencies 46: 413-420.
Jackson, J.J. and D.W. Willis. 1991. Short-term mortality of smallmouth bass caught during a live-release tournament at Lake Oahe, South Dakota. Prairie Naturalist 23: 201-204.
Jenkins Jr., T.M. 2003. Evaluating recent innovations in bait fishing tackle and technique for catch and release of rainbow trout. North American Journal of Fisheries Management, 23: 1098-1107.
Kerr, S.J. 2001. A review of “fizzing” - a technique for swim bladder deflation. Ontario Ministry of Natural Resources, Fisheries Section, Peterborough, Ontario, 13 p.
Klein, W.D. 1965. Mortality of rainbow trout caught on single and treble hooks and released. Progressive Fish Culturist 27: 171-172.
Lee, D.P. 1989. Mortality of tournament caught and released black bass in California. In: Catch-and-Release Fishing - A Decade of Experience, pp. 207-216. (Barnhart, R.A. and T.D. Roelofs, Eds.). Arcata, California: Humboltd State University, California Cooperative Fisheries Research Unit.
Loftus, A.J., W.W. Taylor and M. Keller. 1988. An evaluation of lake trout (Salvelinus namaycush) hooking mortality in the upper Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 45: 1473-1479.
Marnell, L.F. and D. Hunsaker, II. 1970. Hooking mortality of lure-caught cutthroat trout (Salmo clarki) in relation to water temperature, fatigue, and reproductive maturity of released fish. Transactions of the American Fisheries Society 99: 684-688.
Martin, J.H., K.W. Rice and L.W. McEachron. 1987a. Survival of three fishes caught on trotlines. Texas Parks and Wildlife Department, Coastal Fisheries Branch, Management Data Serial No. 111.
Martin, J.H., L.W. McEachron, J.F. Doerzbacher, K.W. Rice and J.M. Mambretti. 1987b. Comparison of trotline catches on four bait types in the Laguna Madre during June-August 1985. Texas Parks and Wildlife Department, Coastal Fisheries Branch, Manage. Data Ser. No. 124.
Mason, J.W. and R.L. Hunt. 1967. Mortality rates of deeply hooked rainbow trout. The Progressive Fish-Culturist 29: 87-91.
Matlock, G.C. and J.A. Dailey. 1981. Survival of hook-caught spotted seatrout held in cages. Texas Parks and Wildlife Department, Management Data Serial No. 15.
Matlock, G.C., L.W. McEachron, J.A. Dailey, P.A. Unger and P. Chai. 1993. Short-term hooking mortalities of red drums and spotted seatrout caught on single-barb and treble hooks. North American Journal of Fisheries Management 13: 186-189.
May, B.E. 1973. Evaluation of large-scale release programs with special reference to bass fishing tournaments. Proceedings of the Annual Conference of the Southeastern Association of Fish and Wildlife Agencies, 26: 325-329.
May, E. 1990. An evaluation of angler induced mortality of striped bass in Maryland. Maryland Department of Natural Resources, AFC-18-1.
Meals, K. O. and L. E. Miranda. 1994. Size-related mortality of tournament-caught largemouth bass. North American Journal of Fisheries Management 14: 460-463.
Milne, D.J. and E.A.R. Ball. 1956. The mortality of small salmon when caught by trolling and tagged or released untagged. In: Progress Reports of Pacific Coast Salmon Stations, No. 106, pp. 10-12. Nanaimo, British Columbia: Fisheries Research Board of Canada.
Muoneke, M.I. 1991. Seasonal hooking mortality of Guadalupe bass caught on artificial lures. In: Warmwater Fisheries Symposium I, pp. 273-277. (Cooper, J.L. and R.H. Hamre, Eds.). Fort Collins, Colorado: U.S. Department of Agriculture, Forestry Service, General Technical Report RM-207.
Muoneke, M.I. 1992a. Hooking mortality of white crappie, Pomoxis annularis Rafinesque, and spotted bass, Micropterus punctulatus (Rafinesque), in Texas reservoirs. Aquaculture Fisheries Management 23: 87-93.
Muoneke, M.I. 1992b. Seasonal hooking mortality of bluegills caught on natural baits. North American Journal of Fisheries Management 12: 645-649.
Muoneke, M.I. 1993. Seasonal hooking mortality of flathead catfish and blue catfish. Proceedings of the Annual Conference of the Southeastern Association of Fish and Wildlife Agencies 45: 392-398.
Muoneke, M. I. and W.M. Childress. 1994. Hooking mortality: a review for recreational fisheries. Reviews in Fisheries Science 2(2): 123-156.
Myers, R.A. and S.M. Poarch. 2000. Effects of bait type and hooking location on post-release mortality of largemouth bass. Proceedings of the Annual Conference of the Southeastern Association of Fish and Wildlife Agencies 54: 39-45.
Nelson, K. L. 1998. Catch-and-release mortality of striped bass in the Roanoke River, North Carolina. North American Journal of Fisheries Management 18: 25-30.
Newman, D.L. and T.W. Storck. 1986. Angler catch, growth and hooking mortality of tiger muskellunge in small centrarchid-dominated impoundments. American Fisheries Society Special Publication 15: 246-351.
Natural Research Consultants. 1989. Hooking mortality study. Saltonstall-Kennedy Project Quarterly Progress Report, Natural Resources Consultants NA89AB-H-00012.
Nuhfer, A.J. and G.R. Alexander. 1992. Hooking mortality of trophy-sized wild brook trout caught on artificial lures. North American Journal of Fisheries Management 12: 634-644.
Ontario Ministry of Natural Resources. 2003. 2000 Survey of recreational fishing in Ontario: a descriptive analysis. Peterborough, Ontario. 237 p.
Ontario Ministry of Natural Resources and Muskies Canada. 1999. Effective release techniques for muskellunge. Peterborough, Ontario. 7 p.
O’Neil, J. and R. Pattenden. 1992. Walleye mortality at four live-release tournaments in Alberta, 1991. R.L. & L. Environmental Services Ltd. Edmonton, Alberta, 49 p.
Ott, R.A., Jr. and K.W. Storey. 1993. Channel catfish hooking mortality. Proceedings of the Annual Conference of the Southeastern Association of Fish and Wildlife Agencies 45: 399-406.
Parks, J.O. and J.E. Kraai. 1991. Walleye hooking mortality at Lake Meredith. Texas Parks and Wildlife Department, Fisheries Division, Management Data Serial No. 52.
Pauley, G.B. and G.L. Thomas. 1993. Mortality of anadromous coastal cutthroat trout caught with artificial lures and natural bait. North American Journal of Fisheries Management 13: 337-345.
Payer, R.D., R.B. Pierce and D.L. Pereira. 1989. Hooking mortality of walleyes caught on live and artificial baits. North American Journal of Fisheries Management 9: 188-192.
Pelzman, R.J. 1978. Hooking mortality of juvenile largemouth bass, Micropterus salmoides. California Fish and Game 64(3): 185-188.
Rowe, R. and K. Esseltine. 2001. Post catch-and-release survival of Lake Nipissing walleye during ice fishing. Ontario Ministry of Natural Resources, Draft Report. 14 p.
Rutledge, W.P. 1975. Hooking mortality study. Texas Parks and Wildlife Department, Final Report, Federal Aid Project, F-31-R1.
Rutledge, W.P. and D.L. Pritchard. 1977. Hooking mortality of largemouth bass captured by artificial lures and natural bait. In: Catch-and-Release Fishing as a Management Tool, pp. 103-107. (Barnhart, R.A. and T.D. Roelofs, Eds.). Arcata, California: Humboltd State University, California Cooperative Fisheries Research Unit.
Schaefer, W.F. 1989. Hooking mortality of walleyes in a northwestern Ontario Lake. North American Journal of Fisheries Management 9: 193-194.
Schaeffer, J.S. and E.M. Hoffman. 2002. Performance of barbed and barbless hooks in a marine recreational fishery. North American Journal of Fisheries Management 22: 229-235.
Schill, D.J. 1996. Hooking mortality of bait-caught rainbow trout in an Idaho stream and a hatchery: implications for special-regulation management. North American Journal of Fisheries Management 16: 348-356.
Schill, D.J., J.S. Griffith and R.E. Gresswell. 1986. Hooking mortality of cutthroat trout in a catch-and-release segment of the Yellowstone River, Yellowstone National Park. North American Journal of Fisheries Management 6: 226-232.
Schisler, G.J. and E.P. Bergersen. 1996. Post-release hooking mortality of rainbow trout caught on scented artificial baits. North American Journal of Fisheries Management 16: 570-578.
Schramm, H.L., Jr., P.J. Haydt and N.A. Bruno. 1985. Survival of tournament-caught largemouth bass in two Florida lakes. North American Journal of Fisheries Management 5: 606-611.
Schramm, H.L., Jr., P.J. Haydt and K.M. Portier. 1987. Evaluation of pre-release, post-release, and total mortality of largemouth bass caught during tournaments in two Florida lakes. North American Journal of Fisheries Management 7: 394-402.
Seidensticker, E.P. 1977. Mortality of largemouth bass for two tournaments using a “Don’t Kill Your Catch” program. In: Catch-and-Release Fishing as a Management Tool, pp. 99-102. (Barnhart, R.A. and T.D. Roelofs, Eds.). Arcata, California: Humboltd State University, California Cooperative Fisheries Research Unit.
Shetter, D.S. and L.N. Allison. 1955. Comparison of mortality between fly-hooked and worm-hooked trout in Michigan streams. Michigan Department of Conservation, Institute Fisheries Research Miscellaneous Publication No. 9.
Shetter, D.S. and L.N. Allison. 1958. Mortality of trout caused by hooking with artificial lures in Michigan waters. Michigan Department of Conservation, Institute for Fisheries Research Miscellaneous Publication. No. 12.
Siewert, H.F. and J.B. Cave. 1990. Survival of released bluegill, Lepomis macrochirus, caught on artificial flies, worms, and spinner lures. Journal of Freshwater Ecology 5(4): 407-411.
Smith, I. 2001. How to make a cradle release for muskie. Ontario Out of Doors, March: 20-22. Strange, D. 2003. Through-the-gill hook removal. In-Fisherman 28(6): 6-8.
Stringer, G.E. 1967. Comparative hooking mortality using three types of terminal gear on rainbow trout from Pennask Lake, British Columbia. Canadian Fish Culturist 39: 17-21.
Suski, C.D., S.S. Killen, S.J. Cooke, J.D. Kieffer, D.P. Philipp and B.L. Tufts. 2004. Physiological significance of the weigh-in during live-release angling tournaments for largemouth bass. Transactions of the American Fisheries Society 133: 1291-1303.
Suski, C.D., J.H. Svec, J.B. Ludden, F.J.S. Phelan and D.P. Philipp. 2003. The effect of catch-and-release angling on the parental care behaviour of male smallmouth bass. Transactions of the American Fisheries Society 132: 210-218.
Taylor, M.J. and K.R. White. 1992. A meta-analysis of hooking mortality of nonanadromous trout. North American Journal of Fisheries Management 12: 760-767.
Tilyou, G.A. and C.E. Hoenke. 1992. Evaluation of unattended yo-yos and triggers. Proceedings of the Annual Conference of the Southeastern Association of Fish and Wildlife Agencies 46: 505-509.
Titus, R.G. and C.D. Vanicek. 1988. Comparative hooking mortality of lure-caught lahontan cutthroat trout at Heenan Lake, California. California Fish and Game 74(4): 218-225.
Tufts, B.L., Y. Lang, K. Tufts and R.G. Boutilier. 1991. Exhaustive exercise in “wild” Atlantic salmon (Salmo salar): acid-base regulation and blood gas transport. Canadian Journal of Fisheries and Aquatic Sciences 48: 868-874.
Vincent-Lang, D., M. Alexandersdottir and D. McBride. 1993. Mortality of coho salmon caught and released using sport tackle in the Little Susitna River, Alaska. Fisheries Reseach 15:339-356.
Warner, K. 1976. Hooking mortality of landlocked Atlantic salmon, Salmo salar in a hatchery environment. Transactions of the American Fisheries Society 105: 365-369.
Warner, K. 1978. Mortality of lake-dwelling landlocked Atlantic salmon, Salmo salar. Transactions of the American Fisheries Society 107: 518-522.
Warner, K. 1979. Mortality of landlocked Atlantic salmon hooked on four types of fishing gear at the hatchery. Progressive Fish Culturist 41: 99-102.
Warner, K. and P.R. Johnson. 1978. Mortality of landlocked Atlantic salmon (Salmo salar) hooked on flies and worms in a river nursery area. Transactions of the American Fisheries Society 107: 772-775.
Weidlein, W.D. 1989. Mortality of released sublegal-sized smallmouth bass, catch-and-release implications. In: Catch-and-Release Fishing – A Decade of Experience, pp 217-228. (Barnhart, R.A. and T.D. Roelofs, Eds.). Arcata, California: Humboltd State University, California Cooperative Fisheries Research Unit.
Welborn, T.L., Jr., and J.H. Barkley. 1974. Study on the survival of tournament released bass on Ross R. Barnett Reservoir, April 1973. Proceedings of the Annual Conference of the Southeastern Association of Game and Fish Agencies 27: 512-519.
Wertheimer, A., A. Celewycz, H. Jaenicke, D. Mortensen and J. Orsi. 1989. Size-related hooking mortality of incidentally caught chinook salmon, Oncorhynchus tshawytscha. Marine Fisheries Review 51(2): 28-35.
Wertheimer, A.C. 1988. Hooking mortality of chinook salmon released by commercial trollers. North American Journal of Fisheries Management 8: 346-355.
Wilde, G.R. 1998. Tournament-associated mortality in black bass. Fisheries 23(10): 12-22.
Wilde, G.R., M.I. Muoneke, P.W. Bettoli, K.L. Nelson and B.T. Hysmith. 2000. Bait and temperature effects on striped bass hooking mortality in freshwater. North American Journal of Fisheries Management 20: 810-815.
Wilkie, M.P., M.A. Brobbel, K. Davidson, L. Forsyth and B.L. Tufts. 1997. Influences of temperature upon the post-exercise physiology of Atlantic salmon. Canadian Journal of Fisheries and Aquatic Sciences 54: 503-511.
Wydoski, R.S., G.A. Wedemeyer and N.C. Nelson. 1976. Physiological response to hooking stress in hatchery and wild rainbow trout (Salmo gairdneri). Transactions of the American Fisheries Society 5: 601-606.
Personal communications
Esseltine, K.R. Department of Biology, Queen’s University, Kingston, Ontario, Canada. Suski, C.D. Harkness Laboratory of Fisheries Research, Aquatic Research and Development Section, Ontario Ministry of Natural Resources, Peterborough, Ontario, Canada.
Appendix 1: Summary of findings from catch-and-release studies
| Species | N | Days | % Mortality | Reference |
|---|---|---|---|---|
| Blue catfish | 52 | 3 | 5.1 | Muoneke, 1993 |
| Channel catfish | 214 | 3 | 19 | Ott and Storey, 1993 |
| Channel catfish | 704 | 6 | 33 | Rutledge, 1975 |
| Channel catfish | 14 | <1 | 0 | Tilyou and Hoenke, 1992 |
| Flathead catfish | 52 | 3 | 11.5 | Muoneke, 1993 |
| Yellow bullhead | 20 | <1 | 0 | Tilyou and Hoenke, 1992 |
| Muskellunge | 3.5 | 30 | Beggs et al., 1980 | |
| Northern pike | 242 | 5-16 | 0-4.8 | Burkholder, 1992 |
| Northern pike | 94 | 4-10 | 6.4 | Falk and Gilman, 1975 |
| Northern pike | 185 | 2 | 1-33 | Dubois et al., 1994 |
| Tiger muskellunge | 217 | 1 | 9.7 | Newman and Storck, 1986 |
| Artic grayling | 180 | 2 | 0.6 | Clark, 1991 |
| Artic grayling | 158 | 4-10 | 5.1 | Falk and Gilman, 1975 |
| Atlantic salmon | 300 | 10-14 | 0.3-5.7 | Warner, 1976 |
| Atlantic salmon | 149 | 5 | 13 | Warner, 1978 |
| Atlantic salmon | 177 | 2-5 | 4-35 | Warner and Johnson, 1978 |
| Atlantic salmon | 1221 | 3-14 | 5.1 | Warner, 1979 |
| Atlantic salmon | 20 | 0 | Booth et al., 1994 | |
| Brook trout | 550 | 7-10 | 1-57 | Shetter and Allison, 1955 |
| Brook trout | 806 | 1 | 2.6 | Shetter and Allison, 1958 |
| Brook trout | 630 | 2 | 4.3 | Nuhfer and Alexander, 1992 |
| Brown trout | 490 | 14 | 13.5 | Hulbert and Engstrom-Heg, 1980 |
| Brown trout | 107 | 1 | 0.9 | Shetter and Allison, 1958 |
| Brown trout | 197 | 0-28 | Shetter and Allison, 1955 | |
| Brown trout | 215 | 10 | 3-7 | Barwick, 1985 |
| Chinook salmon | 888 | 4-6 | 22.1 | Wertheimer et al., 1989 |
| Chinook salmon | 506 | 5 | 21-25 | Wertheimer, 1988 |
| Chinook salmon | 100 | 1-5 | 10 | Bendock and Alexandersdotitir, 1991 |
| Chinook salmon | 245 | 5 | 6-11 | Bendock and Alexandersdotitir, 1991 |
| Chinook salmon | 3618 | 11.8 | Butler and Loeffel, 1972 | |
| Chinook salmon | 66 | 2 | 9.1 | Natural Research Consultants, 1989 |
| Coho salmon | 85 | 35 | 42-55 | Milne and Ball, 1956 |
| Coho salmon | 147 | 2 | 6.8 | Natural Research Consultants, 1989 |
| Coho salmon | 4861 | 18.4 | Butler and Loeffel, 1972 | |
| Coho salmon | 384 | 69.3 | Vincent-Lang et al., 1993 | |
| Cutthroat trout | 652 | 30 | 5.11-5.5 | Marnell and Hunsaker, 1970 |
| Cutthroat trout | 690 | 30 | 3.8 | Dotson, 1982 |
| Cutthroat trout | 509 | 10 | 5-73 | Hunsaker et al., 1970 |
| Cutthroat trout | 72698 | 0.3 | Schill et al., 1986 | |
| Cutthroat trout | 578 | 4 | 1.37-48.5 | Titus and Vanicek, 1988 |
| Lake trout | 129 | 4-10 | 6.98 | Falk et al., 1974 |
| Lake trout | 67 | 2 | 14.9 | Loftus et al., 1988 |
| Lake trout | 50 | 2 | 10 | Dextrase and Ball, 1991 |
| Rainbow trout | 100 | 120 | 95 | Mason and Hunt, 1967 |
| Rainbow trout | 1000 | 3 | 1-10 | Klein, 1965 |
| Rainbow trout | 159 | 11-35 | Shetter and Allison, 1955 | |
| Rainbow trout | 300 | 120 | 34.5-82 | Mason and Hunt, 1967 |
| Rainbow trout | 38 | 10 | 5-39 | Barwick, 1985 |
| Rainbow trout | 574 | 2 | 5.7-36 | Stringer, 1967 |
| Rainbow trout | 65 | 1-2 | 20 | Faccin, 1983 |
| Rainbow trout | 346 | 1 | 5.2 | Shetter and Allison, 1958 |
| Rainbow trout | 900 | 28 | 2.1 | Jenkins, 2003 |
| Rainbow trout | 281 | 29-34 | 16 | Schill, 1996 |
| Striped bass | 576 | 3 | 1.87-70.39 | May, 1990 |
| Striped bass | 307 | 3 | 38.1 | Hysmith et al., 1992 |
| Striped bass | 113 | 3 | 0-69 | Childress, 1989a |
| Striped bass | 464 | 14 | 16-17 | Harrel, 1988 |
| Striped bass | 215 | 30-40 | 15-29 | Diodati, 1991 |
| Striped bass | 89 | >3 | 14-67 | Bettoli and Osborne, 1998 |
| Striped bass | 153 | 3 | 6.4 | Nelson, 1998 |
| Palmetto bass | 89 | 3 | 1-29 | Childress, 1989a |
| White bass | 122 | 3 | 0.8 | Childress, 1989a |
| Yellow bass | 5 | <1 | 60 | Tilyou and Hoenke, 1992 |
| Black sea bass | 64 | 2 | 4.7 | Bugley and Shepherd, 1991 |
| Crappie | 15 | <1 | 0 | Tilyou and Hoenke, 1992 |
| Black crappie | 202 | <1 | 19-77 | Childress, 1989b |
| White crappie | 226 | 6-11 | 3 | Hubbard and Miranda, 1991 |
| White crappie | 69 | 18 | 29 | Childress, 1989b |
| White crappie | 43 | 3 | 9.3 | Muoneke, 1992a |
| White crappie | 13 | ≤504 | 15.4 | Colvin, 1991 |
| Bluegill | 170 | 3 | 1.1-25.3 | Muoneke, 1992b |
| Bluegill | 210 | 3 | 0-18 | Burdick and Wydoski, 1989 |
| Bluegill | 75 | 10 | 30-88 | Siewert and Cave, 1990 |
| Bluegill | 200 | 7 | 4-14 | Barthel et al., 2003 |
| Bluegill | 685 | 3 | 1.3 | Cooke et al., 2003b |
| Pumpkinseed | 175 | 3 | 0 | Cooke et al., 2003b |
| Rock bass | 80 | 5 | 0 | Cooke et al., 2001 |
| Black bass | 5 | Lee, 1989 | ||
| Largemouth bass | 1106 | 1-2 | 3-16 | Bennett et al., 1989 |
| Largemouth bass | 3283 | <1 | 14 | Schramm et al., 1985 |
| Largemouth bass | 3129 | 28 | 32 | Seidensticker, 1977 |
| Largemouth bass | 261 | 14 | 19.4 | Archer and Loyacano, 1975 |
| Largemouth bass | 1351 | 6 | 38 | Rutledge and Pritchard, 1975 |
| Largemouth bass | 1422 | 7-23 | 30 | May, 1973 |
| Largemouth bass | 1863 | 19 | 14.3 | Welborn and Barkley, 1974 |
| Largemouth bass | 14-21 | 26.7 | Schramm et al., 1987 | |
| Largemouth bass | 285 | 60 | 11.2 | Pelzman, 1978 |
| Largemouth bass | 2 | 3.2 | Hartley and Moring, 1991 | |
| Smallmouth bass | 70 | 7 | 0-11 | Clapp and Clark, 1989 |
| Smallmouth bass | 634 | 20 | 4.2-47.3 | Weidlein, 1989 |
| Smallmouth bass | 2 | 8.9 | Hartley and Moring, 1991 | |
| Smallmouth bass | 458 | 0-8.5 | Bennett et al., 1989 | |
| Smallmouth bass | 61 | 2 | 4.9 | Jackson and Willis, 1991 |
| Smallmouth bass | 238 | 3 | 0 | Dunmall et al., 2001 |
| Guadalupe bass | 85 | 3 | 2.4 | Muoneke, 1991 |
| Spotted bass | 47 | 3 | 8.5 | Muoneke, 1992a |
| Walleye | 180 | 12 | 1.1 | Fletcher, 1987 |
| Walleye | 865 | 5 | 40 | Goeman, 1991 |
| Walleye | 47 | 3 | 0 | Parks and Kraai, 1991 |
| Walleye | 2357 | 3 | 21 | Fielder and Johnson, 1992 |
| Walleye | 14-28 | 5-16 | Payer et al., 1989 | |
| Walleye | 240 | 2 | 0.8 | Schaefer, 1989 |
| Walleye | 123 | 1 | 23 | Rowe and Esseltine, 2002 |
| Sauger | 74 | <1 | 4 | Bettoli et al., 2000 |
| Black drum | 19 | <1 | 0 | Martin et al., 1987b |
| Black drum | 325 | 0 | Martin et al., 1987a | |
| Red drum | 171 | <1 | 0 | Martin et al., 1987b |
| Red drum | 121 | 3 | 4.13 | Matlock et al., 1993 |
| Red drum | 38 | 3 | 44.7 | Childress, 1989a |
| Red drum | 968 | 0.21 | Martin et al., 1987a | |
| Spotted seatrout | 401 | 7 | 37 | Hegen et al., 1983 |
| Spotted seatrout | 43 | <1 | 20-70 | Martin et al., 1987b |
| Spotted seatrout | 52 | 7-9 | 0-56 | Matlock and Dailey, 1981 |
| Spotted seatrout | 7 | 17-27 | Hegen et al., 1987 | |
| Spotted seatrout | 124 | 3 | 7.29 | Matlock et al., 1993 |
| Spotted seatrout | 127 | 16.54 | Martin et al., 1987a | |
| White seabass | 221 | 90 | 10 | Aalbers et al., 2004 |
0.3 k P. R. 05 07 15
MNR 51968
ISBN 0-7794-8590-4