Colostrum for the dairy calf
Learn about using colostrum for dairy calves. This technical information is for dairy producers.
Introduction
The most important factor in dairy calf health and survival is feeding the newborn calf adequate amounts of high-quality colostrum early in life. The cost of raising replacement dairy animals increases when calf-rearing practices result in high mortality rates or high treatment rates for preventable diseases.
At birth, a calf has a poorly developed immune system. Colostrum is the “first milk.” It is rich with the antibodies that provide the calf protection from diseases until the calf’s immune system develops protective immunity. In addition to providing protection from disease, colostrum is also an important source of nutrients for the newborn calf.
Calf immune system and colostrum
Colostrum has high concentrations of antibodies compared to milk. Antibodies are also known as immunoglobulins (Ig). They are proteins that protect against disease-causing organisms, or pathogens. The three major types of Ig that are typically found in the colostrum of dairy cows are IgG, IgM and IgA. The concentration of antibodies in colostrum begins to decrease after calving but is sustained at a higher concentration than milk for a few days after calving. Antibodies have important roles in the immune system, including identifying and destroying pathogens and preventing pathogens from attaching to membranes and causing disease. Colostrum also contains other important nutrients and factors that contribute to growth, health and gut development in the calf.
As a calf’s immune system develops in the first few weeks of life, its response to pathogens is limited, leaving the calf vulnerable to illness. Antibodies that are transferred from colostrum to the calf in the first few meals after birth, provide protection from disease through this period. This is known as passive immunity and protects the calf until its own immune system becomes protective (Figure 1).
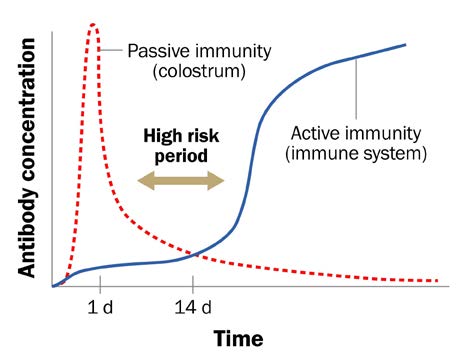
Accessible description of Figure 1
Antibodies that are absorbed by the calf from colostrum feedings will degrade over time. Antibody concentrations will be half of the original concentration by 28.5 days after birth. It is important to have effective colostrum management practices to ensure calves have adequate protection against pathogens for as long as possible while the immune system develops. With proper colostrum management, calf illness and death can be minimized.
When acceptable levels of IgG are fed and absorbed by the calf, successful transfer of passive immunity (TPI) occurs. Successful transfer of passive immunity provides protection for the calf from pathogens. It is also associated with improved growth, health, age to first calving and improved milk production in first and second lactations.
Failure of transfer of passive immunity (FTPI) occurs if the calf is not fed an acceptable quantity and quality of colostrum in a timely manner. This is defined as calves that have IgG serum concentrations of less than 10 g/L. Research has shown that 8%–37% of dairy calves in Ontario suffer from FTPI. This means that many calves have inadequate immunity and are more likely to contract illness.
Feeding colostrum
There are four critical components for consideration in colostrum-feeding management:
- Quantity — How much colostrum the calf receives.
- Quickness — How quickly the calf receives colostrum after birth.
- Quality — The concentration of immunoglobulins in the colostrum.
- Cleanliness — The levels of pathogens in the colostrum.
Quantity
The Code of Practice for the Care and Handling of Dairy Cattle (2023) requires that both male and female calves are fed at least 4 L of high-quality colostrum within 12 hr after birth. Ideally, calves are fed at least 10%–12% of their birth weight at the first feeding. The best practice is to feed the first meal of colostrum within 1–2 hr after birth. Afterwards, it is recommended to feed calves 3-L meals of colostrum, transition milk or a blend of colostrum and milk in 12-hr intervals for 3 days before switching to milk or milk replacer.
There are many methods for feeding colostrum that can result in different levels of transfer of passive immunity. Allowing calves to suckle on the dam may lead to a higher risk of FTPI as quality and quantity of colostrum cannot be measured and timing of the first meal can vary. Feeding an adequate quantity of a high-quality colostrum by either nipple bottle, bucket or esophageal tube helps achieve acceptable levels of passive transfer, with preference for nipple bottle feeding. Refusals can be fed by esophageal tube or by providing an additional feeding within 6 hr. Always clean and sanitize feeding equipment before and after each use.
It is important to get the right amount of colostrum to the calf in an effective, timely manner. Consult with your veterinarian to determine the best method for your calves.
Quickness
Timing is critical to a successful colostrum-feeding management program. The ability of a calf’s small intestine to absorb immunoglobulins drops rapidly over the first few hours of life.
The ability of calves to absorb immunoglobulins decreases by about one-third within 6 hr after birth. By 24 hr of age, a calf absorbs less than 10% of the immunoglobulins it originally could, as shown in Figure 2. For this reason, a calf should receive the first feeding of colostrum within 1–2 hr of birth or as soon possible after birth.
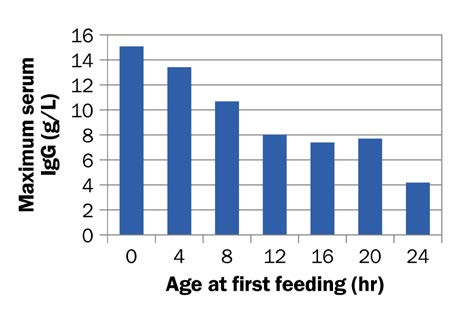
Accessible description of Figure 2
| Transfer of Passive Immunity category | Calf serum IgG category (g/L) | Calf serum equivalent total protein concentration (g/dL) | Calf serum equivalent %Brix | Recommended % calves |
|---|---|---|---|---|
| Excellent | > 25.0 | > 6.2 | > 9.4 | > 40 |
| Good | 18.0–24.9 | 5.8–6.1 | 8.9–9.3 | ~ 30 |
| Fair | 10.0–17.9 | 5.1–5.7 | 8.1–8.8 | ~ 20 |
| Poor | < 10.0 | < 5.1 | < 8.1 | < 10 |
Adapted from Lombard et al., 2020.
Quality
Transfer of passive immunity status is based on circulating IgG concentrations in the calf. Successful transfer of passive immunity can be evaluated in calf blood serum and classified into one of four categories based on new recommendations as outlined in Table 1. The four categories are: excellent, good, fair and poor.
The higher the concentration of IgG or serum total protein levels in the calf 48 hr after birth, the greater the protection the calf has against disease in early life. A study by the U.S. National Animal Health Monitoring System found that a higher percentage of calves in the “poor” and “fair” TPI categories died within the first 60 days of life than calves in the “good” and “excellent” categories (Figure 3).
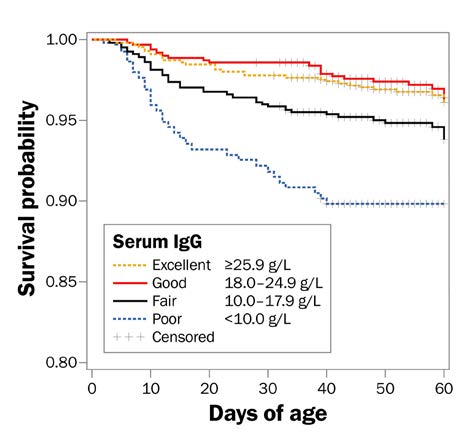
Accessible description of Figure 3
The concentration of antibodies in colostrum decreases rapidly with each milking as the transition from colostrum to milk production occurs in dairy cows. The antibody content in the first colostrum is typically 50–60 g/L but can range from less than 20 g/L to greater than 150 g/L. By the second milking, antibody concentrations are reduced by up to 58% compared to the first milking. By the third and fourth milkings, antibody concentrations are reduced by 85% and 94%, respectively, compared to the first milking. The differences in composition of the first 4 milkings are shown in Table 2.
| Component | Colostrum (Milking 1) | Milking 2 | Milking 3 | Milking 4 |
|---|---|---|---|---|
| Total solid | 26.7% | 18.3% | 14.8% | 13.8% |
| Fat | 5.7% | 4.6% | 4.0% | 3.7% |
| Protein | 15.7% | 8.6% | 5.4% | 4.8% |
| IgG | 94.1 g/L | 39.3 g/L | 13.9 g/L | 6.1 g/L |
| Lactose | 2.4% | 3.7% | 4.1% | 4.1% |
| Milk yield | 5.9 kg | 7.7 kg | 9.7 kg | 12.3 kg |
Adapted from Fischer-Tlustos et al., 2020.
Nutrients in colostrum, such as fat and protein, are also important for a calf’s growth and development. The lactose concentration in colostrum is less than whole milk, which reduces the chance of diarrhea in the newborn calf.
There are multiple factors that can influence colostrum quality including:
- breed
- parity
- genetics
- dry period length
- environmental stress
- nutrition
Multiple-lactation cows usually have higher antibody concentrations than first-lactation cows. Targeting a dry period of greater than 40 days can also increase antibody concentration in colostrum and volume.
Cleanliness
A significant challenge of colostrum feeding is keeping it clean. While feeding colostrum is essential to provide passive immunity for the calf, it is also one of the first ways to potentially expose the calf to pathogens such as E. coli, Salmonella or Mycobacterium avium paratuberculosis.
Pathogens can also cause diseases, such as scours and septicemia, and may interfere with absorption of the antibodies from the gut into the circulatory system. Clean udders, milking equipment and calf-feeding equipment properly before collecting, storing and feeding colostrum. Luminometry swabs are a quick and effective on-site method to measure cleanliness of equipment.
An Ontario study in 2017 found that 59% of colostrum-feeding equipment had a total bacteria count of over 100,000 cfu/mL, and 21% had a total coliform count of over 10,000. This study indicates that dairy producers should pay more attention to equipment cleanliness. In a study conducted in the U.S., it was found that out of 746 colostrum samples, 337 (45.2%) had a total place count of over 100,000 cfu/mL. The study concluded that the source of the bacteria was likely humid climates and an inadequate storage method.
Heat-treating colostrum on farm can significantly reduce the presence of bacteria and other pathogens. It provides a very effective method of improving colostrum cleanliness and can dramatically reduce the potential for diseases to be transmitted through colostrum. It is important to keep heat treatment equipment clean and to follow manufacturer’s directions for both temperature and time of treatment so as not to destroy the antibodies present in the colostrum. Previous research at the University of Minnesota found that colostrum can be heated to 60°C without damaging the antibodies. However, when the colostrum was heated to 63°C, the antibodies were reduced by 34%. To reduce bacterial regrowth, heat-treated colostrum must be properly stored until it is fed.
Storage
Colostrum can be refrigerated at a temperature of 1°C–2°C for up to 3 days or, alternatively, kept frozen at –20°C for up to a year. Avoid using frost-free freezers that will allow the colostrum to thaw and shorten its storage life. Two-litre plastic containers or double-bagged freezer bags are ideal for storage. If using freezer bags, laying them flat in the freezer will speed up freezing and thawing.
After freezing, the best method to thaw the colostrum is using a warm water bath at 50°C. Do not thaw at room temperature, as bacteria will double every 20–30 min. Thawing colostrum in small batches is optimal to reduce the duration of exposure to heat. For all thawing methods, it is important to continuously check the temperature of the colostrum to ensure that it does not exceed 40°C. Do not re-freeze and thaw colostrum. A recent study found that freezing and thawing colostrum resulted in a 7.8% reduction in antibodies.
Thawing colostrum in a microwave is not recommended as it may lead to uneven thawing, clotting and loss of antibodies. If thawing with a microwave is unavoidable, microwave for 14 min on low power and occasionally shake the bag or bottle gently. Continue microwaving until the correct temperature is obtained.
Do not mix colostrum from two or more cows. Pooling colostrum increases disease risk and does not increase antibody concentrations.
Colostrum replacers and supplementation
Colostrum replacers
Colostrum replacement products are available in Canada. Published research supports their effectiveness at increasing immune protection in dairy calves. When purchasing colostrum replacers, make sure to review the amount of IgG delivered per dose and ensure it has a minimum of 100 g of IgG per dose. Follow the manufacturer’s feeding instructions. Ensure mixing and feeding equipment are cleaned before and after use.
Key components to successful use of a colostrum replacer include:
- clean mixing and feeding equipment
- appropriate water temperature
- appropriately measuring powder and water
- mixing until completing combined
- appropriate amount of colostrum fed based on calf birth weight
Colostrum supplements
As a final alternative, if a sufficient amount of high-quality colostrum is not available, commercial colostrum supplements can be a valuable tool for increasing calf immunity. Colostrum replacers contain 100–150 g of IgG per dose while colostrum supplements typically contain 40–60 g of IgG per dose.
Always use products licensed by the Canadian Centre for Veterinary Biologics (CCVB). Colostrum supplements do not contain sufficient antibody concentrations to match those in dam colostrum and are not intended to be a replacement for dam colostrum. Follow the manufacturer’s directions with these products. Ensure mixing and feeding equipment are properly cleaned before and after use.
Evaluating colostrum management
Monitoring your colostrum management program can help to identify and resolve issues with your colostrum-feeding protocols.
Keeping and regularly evaluating records on morbidity and mortality can help determine if colostrum management should be improved. Calves that have FTPI have higher rates of disease and mortality. Work with your veterinarian to solve any issues with morbidity and mortality with your calves.
Passive transfer status can be evaluated by checking blood serum total protein concentrations on a portion of calves at 1–6 days of age. Speak with your veterinarian to discuss training on blood collection.
Regularly review and train staff on standard operating procedures for colostrum feeding. Keep records on colostrum quantity, quickness and quality to identify any trends that may occur.
Testing
The best way to monitor a colostrum management program is by testing IgG or total protein in calf blood serum after 24–48 hr of life. This can be done on a subset of calves each year to reduce labour. The preferred method of testing serum is a serum total protein test. A refractometer is the second-best option for on-farm testing. Evaluate serum readings based on the four categories of passive immunity defined as poor, acceptable, good and excellent.
Monitoring colostrum quality before every feeding can be done. However, the reliability of these readings is low and does not indicate the successful transfer of passive immunity to the calf. Producers should focus on feeding a high-quality, clean colostrum within an acceptable time frame as volume and timing are the most important steps to the successful passive transfer of immunity. Focusing on feeding transition milk can also help improve the outcome of the calf.
Hydrometer
A colostrum hydrometer, often called a Colostrometer, measures specific gravity of colostrum, which has an association with colostrum antibody concentration. However, this can be impacted by other components as well. Accurate measurements require that the temperature of the colostrum be correct, typically, at room temperature (20°C). Higher or lower temperatures can result in incorrect readings. Readings of more than 1.047 are desirable and is indicated on the hydrometer, if designed for testing colostrum. This testing method provides an indication of colostrum quality and does not directly assess IgG concentration.
Refractometer
A Brix refractometer can be used to assess the quality of colostrum by placing a few drops of colostrum onto the instrument and holding it up to the light to get a reading. Colostrum with a Brix value >22% can be given to the calf, however, anything <18% should not be used. Supplementation is required if values are between 18% and 22%. This testing method provides an indication of colostrum quality and does not directly assess IgG concentration.
Transitioning from colostrum
The milk from milkings 2 to 6 is known as transition milk. Although its antibody levels are not as high as colostrum, transition milk contains other important components for the calf, including nutrients and growth hormones. Its composition compared to colostrum is outlined in Table 2.
Research has shown that feeding transition milk to calves aids in intestinal development. It also results in better health and growth outcomes in the first 3 weeks after birth. Feed transition milk for the first 3 days after birth before transitioning to milk or milk replacer.
Summary
Colostrum is a critical first consideration contributing to the health and survival of newborn calves. The successful transfer of the antibody protection in colostrum from cow to calf is based on four key factors:
- Quantity: Feed calves at least 4 L of colostrum within 12 hr after birth and feed 3-L meals for 3 days after birth. Gradually transition to whole milk or milk replacer.
- Quickness: Feed calves their first meal of colostrum within 1–2 hr after birth.
- Quality: Evaluate a subset of calves for serum total protein and determine the number that scores within the “good” or “excellent” TPI categories.
- Cleanliness: Feed calves colostrum from healthy cows. Keep feeding and milking equipment clean.
Lastly, it is important to work with your veterinarian to establish standard operating procedures for colostrum feeding and review strategies for improving calf health.
References
Buczinski, S., and J.M. Vandeweerd. 2016. Diagnostic accuracy of refractometry for assessing bovine colostrum quality: A systematic review and metaanalysis. J Dairy Sci. 99: 7381–7394.
CalfCare.ca. 2019. First 24 hours: Colostrum. Retrieved from: calfcare.ca.
Chase, C.C.L., D.J. Hurley and A.J. Reber. 2008. Neonatal immune development in the calf and its impact on vaccine response. Vet Clin North Am Food Anim Pract. 24(1): 87–104.
Conneely, M., D.P. Berry, R. Sayers, J.P. Murphy, I. Lorenz, M.L. Doherty and E. Kennedy. 2013. Factors associated with the concentration of immunoglobulin G in the colostrum of dairy cows. Anim. 7(11): 1824–1832.
Davis, C.L., J.K. Drackley. 1998. The development, nutrition and management of the young calf. Iowa University State Press.
Fischer-Tlustos, A.J., K. Hertogs, J.K. van Niekerk, M. Nagorske, D.M. Haines and M.A. Steele. 2020. Oligosaccharide concentrations in colostrum, transition milk, and mature milk of primi-and multiparous Holstein cows during the first week of lactation. J Dairy Sci. 103: 3683–3695
Foley, J.A., D.E. Otterby. 1978. Availability, storage, treatment, composition and feeding of surplus colostrum: A review. J Dairy Sci. 61(8):10331060.
Godden, S.M., J.E. Lombard and A.R. Woolums. 2019. Colostrum management for dairy calves. Vet Clin North Am Food Anim Pract. 35: 535–556.
Johnson, J.L., S.M. Godden, T. Molitor, T. Ames, D. Hagman. 2007. Effects of feeding heat-treated colostrum on passive transfer of immune and nutritional parameters in neonatal dairy calves. J Dairy Sci. 90:5189-5198.7
Lombard, J., N. Urie, F. Garry, S. Godden, J. Quigley, T. Earleywine, S. McGuirk, D. Moore, M. Branan, M. Chamorro, G. Smith, C. Shivey, D. Catherman, D. Haines, A.J. Heinrichs, R. James, J. Maas and K. Sterner. 2020. Consensus recommendations on calf- and herd-level passive immunity in dairy calves in the United States. J Dairy Sci. 103(8): 7611–7624.
Lopez, A.J., and A.J. Heinrichs. 2022. Invited Review: The importance of colostrum in the newborn dairy calf. J Dairy Sci. 105(4): 2733–2749.
McMartin, S., S. Godden, L. Metzger, J. Feirtag, R. Bey, J. Stabel, S. Goyal, J. Fetrow, S. Wells, H. Chester-Jones. 2006. Heat treatment of bovine colostrum. I: Effects of temperature on viscosity and immunoglobulin G level. J Dairy Sci. 89:2110-2118.
Morrill, K.M., K.E. Robertson, M.M. Spring, A.L. Robinson, H.D. Tyler. 2015. Validating a refractometer to evaluate immunoglobulin G concentration in Jersey colostrum and the effect of multiple freeze-thaw cycles on evaluating colostrum quality. J Dairy Sci. 98: 595–601.
Murphy, J.M., J.V. Hagey and M. Chigerwe. 2014. Comparison of serum immunoglobulin G half-life in dairy calves fed colostrum, colostrum-replacer or administered with intravenous bovine plasma. Vet Immunol Immunopathol. 158(3): 233–237.
National Farm Animal Care Council. 2023. Code of Practice for the Care and Handling of Dairy Cattle. Retrieved from: National Farm Animal Care Council: Dairy cattle.
PennState Extension. 2023. Colostrum Management Tools: Hydrometers and Refractometers. Retrieved from: PennState Extension: Colostrum Management Tools.
PennState Extension. 2022. Feeding the Newborn Dairy Calf. Retrieved from: PennState Extension: Feeding the Newborn Dairy Calf.
Renaud, D.L., D.F. Kelton, S.J. LeBlanc, D.B. Haley, A.B. Jalbert and T.F. Duffield. 2017. Validation of commercial luminometry swabs for total bacteria and coliform counts in colostrum-feeding equipment. J Dairy Sci. 100(11): 9459–9465.
Renaud, D.L., M.A. Steele, R. Genore, S.M. Roche and C.B. Winder. 2020. Passive immunity and colostrum management practices on Ontario dairy farms and auction facilities: A crosssectional study. J Dairy Sci. 103(9): 8369–8377.
Robbers, L., R. Jorritsma, M. Nielen and A. Koets. 2021. A Scoping review of on-farm colostrum management practices for optimal transfer of immunity in dairy calves. Front Vet Sci. 8.
Van Soest, B., M. Weber Nielsen, A.J. Moeser, A. Abuelo and M.J. VandeHaar. 2022. Transition milk stimulates intestinal development of neonatal Holstein calves. J Dairy Sci. 105(8): 7011–7022.
Wiking, L., and R.E. Pedersen. 2008. Effects of heating colostrum in a microwave oven on Immunoglobulin G concentration. Acta Agriculturae Scandinavica. Section A — Animal Science, 59(1): 66–69.
This factsheet was originally written by Brian Lang, OMAFRA, in 2008. It was updated in 2024 by Marlene Paibomesai, Dairy Specialist, OMAFRA, and Andrea Bajus, Technology Transfer Specialist, OMAFRA.
Accessible image descriptions
Figure 1. Maternal antibodies in colostrum protect calves from disease-causing pathogens while their own immune system develops (Penn State)
A line graph showing a calf’s antibody concentration throughout the first 2–4 weeks after birth. The red line depicts passive immunity gained through maternal antibodies. The blue line shows when a calf’s own immune system begins to develop. A calf relies on passive immunity through maternal antibodies in colostrum to protect them from disease-causing pathogens for the first 2–4 weeks of life.
Figure 2. Over the first 24 hours of life, a calf’s ability to absorb immunoglobulins rapidly declines.
A bar graph showing the decline in immunoglobulins absorbed over the first 24 hours of a calf’s life. Calves fed within an hour after birth absorb the highest immunoglobulin concentrations (g/L). For every additional hour waited to feed colostrum, immunoglobulin concentrations (g/L) absorbed by the calf decreases.
Figure 3. The probability of calf survival by serum IgG concentration for pre-weaned dairy heifer calves (Lombard et al., 2020).
The graph shows that calves with poor or fair TPI had a lower chance of survival to 60 days of age than calves with good and excellent TPI. Graph shows that calves with lower immunoglobulin survive for a shorter number of days than calves with higher immunoglobulin.