American Chestnut Recovery Strategy
This document advises the ministry on ways to ensure healthy numbers of the American Chestnut a threatened or endangered species, return to Ontario.

Recovery strategy prepared under the Endangered Species Act, 2007
About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species' persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. There is a transition period of five years (until June 30, 2013) to develop recovery strategies for those species listed as endangered or threatened in the schedules of the ESA. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of Natural Resources Species at Risk webpage at: www.ontario.ca/speciesatrisk
Recommended citation
Boland, G.J., J. Ambrose, B. Husband, K.A. Elliott and M.S. Melzer. 2012. Recovery Strategy for the American Chestnut (Castanea dentata) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. vi + 43 pp.
Cover illustration: Allen Woodliffe
© Queen’s Printer for Ontario, 2012
ISBN 978-1-4435-9426-4
Content (excluding the cover illustration) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée Recovery strategies prepared under the Endangered Species Act, 2007, n'est disponible qu'en Anglais en vertu du Règlement 411/97 qui en exempte l'application de la Loi sur les services en français. Pour obtenir de l'aide en français, veuillez communiquer avec Pamela Wesley au ministère des Richesses naturelles au
Authors
Greg Boland - University of Guelph
John Ambrose - Botanical Consultant
Brian Husband - University of Guelph
Ken A. Elliott, RPF - Ontario Ministry of Natural Resources
Melody Melzer - University of Guelph
Acknowledgments
Members of the recovery team wish to acknowledge the numerous landowners who provided access to their properties during various past inventory and monitoring projects. As well, we want to thank the Canadian Chestnut Council (CCC) for their tireless efforts to promote the importance of saving this legendary tree species and for their continuing on-the-ground work in the areas of maintaining, breeding and restoring chestnut trees. We would also like to acknowledge the field work and research conducted by Jeffrey Tindall, John Gerrath and Karen McKendry and the financial support of the World Wildlife Fund (WWF) and Canadian Wildlife Service (CWS) through the Endangered Species Recovery Fund, the Natural Sciences and Engineering Research Council of Canada and the Ontario Ministry of Natural Resources (OMNR). This assistance allowed the team to finalize the first WWF-based Recovery Plan in October 2000 and the researchers were able to finalize the 2001-2003 inventory and first ecological report (2005) under that plan. Finally we would like to thank all of our Advisory Committee Members and the many reviewers and advisors within the OMNR and the CWS who helped us through the numerous drafts and revisions to this strategy.
Declaration
The recovery strategy for the American Chestnut has been prepared in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all of the individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ontario Ministry of Natural Resources
Environment Canada, Canadian Wildlife Service – Ontario Region
Executive summary
American Chestnut (Castanea dentata) was a dominant forest tree species in northeastern North America before populations were devastated by the introduction in 1904 of the fungal pathogen, Cryphonectria parasitica, which causes chestnut blight. By the 1950s, American Chestnut had been devastated throughout its native range. In southwestern Ontario, populations of American Chestnut were reduced to far less than one percent of the original 1.5 to 2.0 million trees estimated to have been present. Recent surveys in 2001 to 2003 confirmed that Ontario has at least 601 mature and immature individuals of American Chestnut, but this estimate likely represents 30 to 70 percent of the total number in Canada. The native range in Ontario accounts for 3.9 percent of the native range of American Chestnut in North America. In 1987, American Chestnut was designated as a threatened species by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) and in 2004 was re-designated as endangered. American Chestnut is listed as endangered on the Species at Risk in Ontario (SARO) List and receives protection under the Endangered Species Act, 2007 (ESA).
American Chestnut’s native range extends from southern New England to the southern Appalachian mountains. It still survives as remnant populations and individuals throughout this range, mainly by resprouting from collars of surviving root systems. During a survey conducted from 1994 to 1997, American Chestnut was identified at 135 sites in southwestern Ontario. Approximately 58 percent of the sites contained only one tree or regenerating clump. Between 2001 and 2003, 601 individuals were located at 94 sites (average of 6.5 per site); nearly 50 percent of these were less than 10 m tall and greater than 10 cm in diameter at breast height. At least 60 of the 601 individuals showed evidence of flowering or producing burs, however, these trees produced no detectable seed. Approximately one half of the sites containing surviving chestnut were located in Elgin, Haldimand and Norfolk counties.
The goal of this recovery strategy is to restore American Chestnut populations in Ontario to a self-sustaining state, whereby natural recruitment results in the maintenance or an increase of current population size throughout the species' native range. The objectives of this recovery strategy are to:
- survey suitable habitat and/or formerly occupied habitat for American Chestnut, and protect and monitor known populations within the species' native range in Ontario;
- promote protection and public awareness of American Chestnut;
- develop and evaluate management measures to control threats; and
- secure Ontario sources of germplasm originating from blight-free trees.
Initiation and/or completion of these objectives will contribute to increased knowledge and conservation of remnant populations of American Chestnut in Canada and assess strategies for improved management of chestnut blight.
Chestnut blight continues to have the greatest negative impact on populations of American Chestnut. Other factors such as loss and degradation of habitat, possible hybridization with other Castanea species, and the possible introduction of oriental gall wasp (Dryocosmus kuriphilus Yasumatsu) from the United States are also of concern.
Until the impact of chestnut blight can be reduced, restoring American Chestnut to a more secure position in the Carolinian forest is unlikely. Therefore, approaches to control chestnut blight are critical. Potential approaches include hypovirulence (a viral infection that weakens the blight fungus), natural resistance to disease and breeding for disease resistance. Although hypovirulence has been successful in controlling blight in Europe, there has been less success using this approach in North America. Further research may identify factors that contribute to increased efficacy. Qualitative or complete resistance to blight has not been observed in surviving populations of American Chestnut, but concerted attempts have been and continue to be made to identify and select quantitative or incomplete resistance. Finally, breeding programs using resistance genes from Asian chestnut species are underway in the United States and more recently in Canada. Here emphasis has been placed on incorporating this resistance into germplasm adapted to environmental conditions within the native range of American Chestnut in southwestern Ontario.
It is recommended that the Ecological Land Classification (ELC) ecosite types where one or more American Chestnut trees currently occur or where one or more individuals were previously documented in written reports or surveys (for example, Ambrose and Aboud 1987, Melzer et al. 2004, Tindall et al. 2004, Natural Heritage Resource Centre database, etc.) be prescribed as habitat within a habitat regulation under the ESA. It is recommended that trees planted for horticulture, landscaping or research be exempt from the habitat regulation but should be individually assessed for genetic conservation value.
1.0 Background information
1.1 Species assessment and classification
Common name (population): American Chestnut
Scientific name: Castanea dentata
SARO List Classification: Endangered
SARO List History: Endangered (2008), Endangered – Not Regulated (2005), Threatened (2004)
COSEWIC Assessment History: Endangered (2004), Threatened (1987)
SARA Schedule 1: Endangered (August 15, 2006)
Conservation status rankings:
GRANK: G4 NRANK: N3 SRANK: S2
The glossary provides definitions for technical terms, including the abbreviations above.
1.2 Species description and biology
Species description
American Chestnut (Castanea dentata) is a member of the Fagaceae or Beech family. There are up to 14 described species of trees and shrubs in the genus Castanea. These species include Chinese Chestnut (C. mollissima), European Chestnut (C. sativa), Japanese Chestnut (C. crenata), Henry Chinquapin (C. henryi), Ozark Chinquapin (C. ozarkensis), Seguin Chestnut (C. seguinii) and Allegheny Chestnut (C. pumila). Only American Chestnut is native to Canada (Farrar 1995). However, Chinese Chestnut and hybrids and to a lesser extent, European Chestnut and Japanese Chestnut have been planted within the range of American Chestnut. Over the past two centuries, American Chestnut was initially considered as a range extension of European Chestnut, then as a variety of European Chestnut, and finally as a distinct North American species (Sudworth 1892).
American Chestnut is a large, deciduous canopy tree, that can grow up to 30 m tall and have a trunk up to 1.5 m in diameter, with smooth dark brown/olive bark that separates into broad flat-topped ridges with age. Leaves are yellowish-green, alternate and simple, 15 to 28 cm long and taper to both ends. Leaves have 15 to 20 veins running parallel on each side and each vein ends in a prominent tooth. American Chestnut is monoecious and self-incompatible with male flowers occurring in catkins and female flowers occurring singly or in small clusters at the base of some catkins. Trees flower in late May to early July and are insect-pollinated (Ambrose and Kevan 1990). One to three nuts are enclosed in a spiny husk, five to eight centimetres across and are edible. Nuts usually mature by first autumn frost and are primarily dispersed by small mammals and birds that cache or bury them. American Chestnut has a faster rate of growth than other associated hardwood species and under good site conditions, mature trees can increase in diameter by up to 2.5 cm per year (Kuhlman 1978).
Species biology
American Chestnut can begin to produce seed as early as eight years of age. The life-cycle of forest canopy trees such as American Chestnut has two critical phases: (1) establishing seedlings in the understory; and (2) attaining a favourable position in the canopy after a disturbance (Paillet, 1994). As it is shade tolerant, American Chestnut typically persists in the understory of relatively open oak-dominated forests but responds rapidly to openings that develop in the canopy. When a chestnut tree is cut or the above ground part dies from blight, the root collar typically survives and gives rise to new sprouts. However, the repeated harvesting or re-infection of stems can weaken and eventually kill the entire tree (Paillet 1994).
Ecology
Although American Chestnut still persists in some areas, it no longer persists in sufficient numbers to fulfill its former ecological role. Many organisms were directly or indirectly influenced by this tree. Most of the species that relied on American Chestnut for food were considered to be generalists including: deer, rodents, insects and bird species. It is thought that these species now browse other nuts such as acorns, walnuts, beech nuts and hickory nuts.
Information on the diversity of phytophagous (plant-eating) insects on Castanea species in North America is not available, particularly before the introduction of chestnut blight. However, chestnut stems and blight cankers were found to harbour a large, diverse insect fauna of at least 495 insect species (Russin et al. 1984), the majority of which were from the Coleoptera and Diptera families. The pandemic of chestnut blight on American Chestnut is thought to have resulted in the decline or extinction of several phytophagous insects (Opler 1979, cited in Harvell et al. 2002). The Lesser Chestnut Weevil (Curculio sayi Gyllenhal) and Larger Chestnut Weevil (Curculio caryatrypes Boheman) are both native to North America but since the decline of American Chestnut, have become less common (Bessin 2003). The Clearwing Chestnut Moth (Synanthedon castanae Busck) was previously thought to have become extinct in the northeastern United States but was rediscovered in Connecticut in 1989 (Anagnostakis et al. 1994). Recent introductions have also occurred. For example, the Chestnut Gall Wasp (Dryocosmus kuriphilus Yasumatsu) was first reported in the United States in 1974 and is known to feed on Castanea species (Rieske 2007).
American Chestnut also has indirect ecological effects on associated species. Smock and MacGregor (1988) discovered that chestnut leaves altered the consumption rates, growth, and fecundity of shredding macro-invertebrates in headwater streams in the United States. The authors concluded that headwater streams in areas affected by chestnut blight may have experienced subtle changes at the population, community and ecosystem levels due to the demise of chestnut. Other organisms, including a diversity of fungi, bacteria and viruses, were possibly impacted by the decline of American Chestnut but there is little documentation of these possible changes.
Cultural and economic significance
American Chestnut had an important historical role in many rural economies. The nuts were used to fatten livestock and were stored as a winter food source. The nuts were also an important cash crop for many rural families and nuts were sent to major cities over the Christmas season to be roasted and sold by street vendors. One railroad station in West Virginia was reported to have shipped 70,300 kg of chestnuts in 1911 (Giddings 1912 as reported by Kuhlman 1978).
American Chestnut was also an excellent timber tree. Forest-grown trees grew straight and were often free of branches for 50 feet (15 m). The wood was straight-grained, easy to work and rot-resistant. The wood was used for telegraph poles, railroad ties, shingles, panelling, fencing, ship masts, coffins, fine furniture, musical instruments, pulp and plywood. Production of chestnut lumber reached a maximum in 1909 at 663.9 million board feet (Saucier 1973). The United States Forest Service’s estimated value of chestnut timber cut in 1909 was $20 million (Detwiler 1912 as reported by Kuhlman 1978). In 1924, the volume of standing chestnut saw timber was estimated at 19.3 billion board feet in the United States.
Non-timber products derived from this species included tannins extracted from the bark and wood used for tanning leather. In the United States, chestnut was the primary source of tannin for the leather industry. In 1923, over 55 tons (50 tonnes) of tannins were extracted from chestnut wood and bark (Saucier 1973).
Indigenous peoples' use of chestnut ranged from various extractions from leaves, bark, wood and nuts to restore health, to the use of the nuts for food, including soups, puddings and bread (Moerman 2003).
American Chestnut, because of its size and canopy form, was popular in urban plantings as a shade tree. American Chestnut was, and still is, grown in plantations for commercial nut production. There is a small but growing nut industry in Ontario, comprising primarily Chinese and hybrid chestnuts.
1.3 Distribution, abundance and population trends
Global distribution and status
Based on fossil evidence, chestnut species are estimated to have been endemic to North America for at least 17 to 20 million years. Records of chestnut pollen verify that it grew on Long Island 30,000 to 50,000 years ago. Chestnut pollen was also found in 2,000-year-old soil layers in Massachusetts (Anagnostakis and Hillman 1992).
American Chestnut, a dominant climax hardwood, comprised approximately 25 percent of the eastern deciduous forest in the United States before the introduction of chestnut blight. Its native range extended from southern New England to the southern Appalachian mountains and covered more than 80 million hectares of forest (Kuhlman 1978) (Figure 1).
Figure 1. Natural range footnote 1 of American Chestnut (Little 1977)
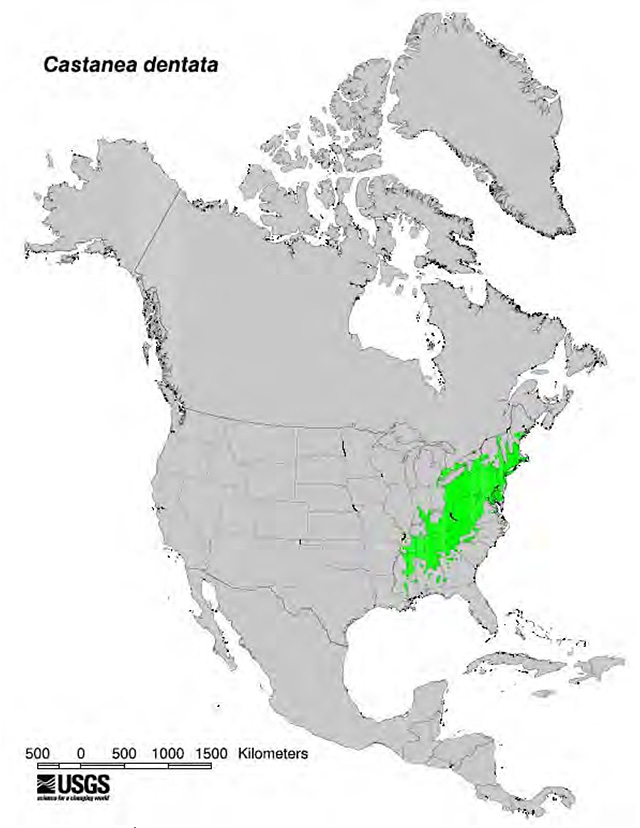
Enlarge Natural range of American Chestnut map
The distribution of American Chestnut has been affected by four important events during the past several thousand years. These events include: (1) a post-glacial migration from south to north; (2) clearing of forests for farming; (3) commercial logging; and (4) introduction of chestnut blight to North America (Hill 1994). Following the most recent glacial retreat, this species migrated north. American Chestnut was considered a slowly dispersing species because evidence of it did not appear in New England until 2,000 years ago. Whereas Eastern White Pine (Pinus strobus), American Beech (Fagus grandifolia), crab apples and elms reached New England 9,000, 7,000 and 4,000 years ago, respectively. However, others believe that chestnut was present in New England in low numbers up to 4,500 years ago (Paillet 1994).
Clearing and logging reduced much of the eastern deciduous forest to only scattered remnants of virgin forest by the time Cryphonectria parasitica, the cause of chestnut blight, was introduced to North America in the early 1900s.
American Chestnut is considered 'apparently secure' with a global conservation status rank of G4. While young shoots of this species are widespread and abundant in the United States, it now seldom reaches reproductive maturity due to the presence of chestnut blight. Presumably there are millions of American Chestnut trees surviving as stumps that produce shoots, but large mature trees are extremely rare and are often isolated or cultivated far from the species' natural range (Table 1). The conservation status of American Chestnut in Canada and Ontario is ranked N3 (vulnerable) and S2 (imperilled), respectively (NatureServe, 2009).
Table 1. Conservation status rankings for American Chestnut (NatureServe, 2009)
| Level | Conservation status | Level | Conservation status |
|---|---|---|---|
| Global | G4 | USA | |
| Canada | N3 | Michigan | S1S2 |
| Ontario | S2 | Mississippi | S1 |
| USA | N4 | Missouri | SNR |
| Alabama | SNR | New Hampshire | SNR |
| Connecticut | SNR | New Jersey | S4 |
| Delaware | SH | New York | S5 |
| District of Columbia | S1S2 | North Carolina | S4 |
| Florida | SX | Ohio | S3 |
| Georgia | S3 | Pennsylvania | S5 |
| Illinois | SX | Rhode Island | SNR |
| Indiana | S3 | South Carolina | SNR |
| Iowa | SNA | Tennessee | S2S3 |
| Kentucky | S1? | Vermont | SNR |
| Maine | S4 | Virginia | S4 |
| Maryland | S2S3 | West Virginia | S4 |
| Massachusetts | SNR | Wisconsin | SNR |
Canadian distribution
American Chestnut naturally occurs below the 43rd parallel in Canada (Fox 1949). This region is generally referred to as the Carolinian zone of southern Ontario and represents the northwestern limits of the native range for American Chestnut in North America.
There appear to be no significant changes in the extent of the natural distribution of American Chestnut in southern Ontario from before the introduction of chestnut blight (Moss and Hosking 1983). American Chestnut occurs in 13 counties along Lake Erie from Windsor to Niagara Falls and north to London. During a survey conducted from 1994 to 1997, American Chestnut was identified at 135 sites in southwestern Ontario. Approximately 58 percent of the sites contained only one tree or regenerating clump. Over one-half of the sites reported in a 2001 to 2003 survey (Tindall et al. 2004) occurred in Elgin, Haldimand and Norfolk Counties. American Chestnut was also reported in Brant, Essex, Halton, Hamilton-Wentworth, Chatham-Kent, Lambton, Middlesex, Niagara, Waterloo and Wellington counties (Ambrose 2004, Tindall et al. 2004). Locations of known occurrence sites are shown on the following map of southern Ontario (Figure 2).
Figure 2. Known occurrence sites of American Chestnut in Ontario (Modified from Tindall et al. 2004)
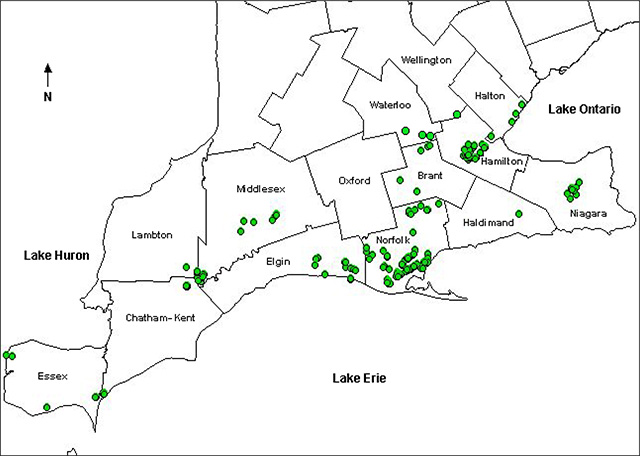
Enlarge known occurrence sites of American Chestnut in Ontario map
Population sizes and trends
It is estimated that there were 1.5 to 2.0 million American Chestnut trees in southern Ontario prior to the introduction of chestnut blight in the 1920s (McKeen 1995, 1997).
The distribution of these populations was estimated to comprise 3.9 percent of the total area of distribution of American Chestnut in North America. The blight entered North America from Asia at New York City around 1904 (Gravatt and Gill 1930). By the mid-1940s, the Ontario populations of American Chestnut were devastated and declined dramatically. In 1947, sprouts that had regenerated from trees killed by blight were "common everywhere" but no living mature trees bearing nuts were found (Fox 1949).
There is little quantitative information on the decline of established populations of American Chestnut since the initial pandemics of chestnut blight. Surveys conducted in recent years are not comparable because of differences in methodologies, but a standardized survey protocol developed in consultation with the American Chestnut Recovery Team, was described by Tindall et al. (2004) and will enable such comparisons in the future. The most recent survey of American Chestnut populations in Ontario located 601 mature and immature individuals (Tindall et al. 2004). In this survey, blight symptoms occurred on 25 percent of all trees and in 48 of the 94 locations inspected. The number of cankers on infected trees averaged 5.7 (ranged from one to 40) and this was often associated with the presence of epicormic shoots. Individuals without blight had significantly smaller mean diameter at breast height (DBH; 12.0 cm) than trees with blight (16.9 cm) (Tindall et al. 2004). Mean height for trees without and with blight was 8.3 m and 9.0 m, respectively. Because Tindall et al. (2004) concentrated heavily on forested public lands and frequently did not sample all trees within any given location, this is likely only 30 to 70 percent of the estimated total population.
Ambrose and Aboud (1986) reported seedlings in 7 of 62 sites, whereas Tindall et al. (2004) found none within a 20 m radius of trees in 93 locations. Low recruitment is due, in part, to the fact that few regenerating sprouts survive until reproductive age. The survey by Tindall et al. (2004) found that nearly 50 percent of all trees examined had a DBH greater than 10 cm and 80 percent were less than 20 cm. Only 14 percent were reproductive (i.e., produced catkins or burrs) and no trees were observed with viable seeds (i.e., filled nuts). Low reproductive success in otherwise healthy trees may be related to the fact that these trees are often geographically isolated and therefore, rarely cross-pollinate.
In 1985, McKeen reported that 60 trees, ranging in DBH from 8 to 63 cm, were present within the original range. Other surveys by Ambrose and Aboud (1986) and Boland et al (1997) reported 151 trees over 10 cm DBH, plus numerous uncounted smaller stump sprouts and 297 individuals, respectively. These surveys differed in objectives, search intensity and procedures and, hence, the values estimated from the three studies are not comparable and likely do not reflect a population trend. Derivation of a population estimate for the total number of chestnut stems in North America was precluded by missing data from the United States (McWilliams et al. 2005).
1.4 Habitat needs
American Chestnut occurs in a variety of habitats but is most abundant on well-drained, acidic, sand and gravel soils. In Ontario, American Chestnut most often occurs in regions where the frost-free period ranges from 140 to 180 days, extreme temperatures range from lows of -27 to -29 degrees Celsius and highs of 40 to 41 degrees Celsius, precipitation ranges from 760 to 970 mm of rain plus 89 to 178 cm of snow, with soil pH ranges from four to six (Ambrose and Aboud 1986, Tindall et al. 2004), soil sand content ranges from 50 to 90 percent and elevation ranges from 90 to 290 m (Boland et al. 1997). Most individuals occur in forest or woodland ecosites in which the canopy cover exceeds 70 percent (Tindall et al. 2004). Habitats are most often dominated by oak [predominantly White Oak (Quercus alba) and Red Oak (Q. rubra)] or maple [predominantly Red Maple (Acer rubrum) and Sugar Maple (A. saccharum)], with regular occurrences of species such as: Eastern White Pine, Shagbark Hickory (Carya ovata), Black Cherry (Prunus serotina), Sassafras (Sassafras albidum), White Ash (Fraxinus americana) and American Beech (Ambrose and Aboud 1986, Tindall et al. 2004). Under the Ecological Land Classification (ELC) system (Lee et al. 1998), American Chestnut was found predominantly in three community series: (1) mixed forest; (2) deciduous forest; and (3) treed cliffs (Tindall et al. 2004). The majority (97%) were located in forest or woodland habitats and 79 percent occurred in oak and (or) maple forest ecosites.
1.5 Limiting factors
American Chestnut is a shade tolerant species that has a self-incompatible breeding system (prevents self fertilization) and therefore requires reproductively compatible trees within pollen dispersal range to produce viable seed (Ambrose and Kevan 1990). Due to chestnut blight, single chestnut trees are geographically isolated and thus availability of compatible trees for reproduction is likely a limiting factor.
Chestnut trees produce fruit with high nutritional value that provide an important food source for birds [e.g., Wild Turkey (Meleagris gallopavo) and jays] and for mammals (e.g., squirrels, deer and bears) (Hill 1994). Today, however, due to its low numbers, chestnut is relatively unimportant to wildlife. These wildlife species, however, can be viewed as seed predators which may limit seed dispersal when there are already extremely low numbers. American Chestnuts are long-lived organisms, which limits the rate of recovery to viable, reproductively mature populations. Conversely, the woody perennial life history also allows individual plants to persist as sprouts from surviving root systems well after the initial infection.
Although habitat availability is not a limiting factor for American Chestnut, dispersal to areas that do provide suitable habitat is limited. A large portion of the remaining Carolinian woodlands provide suitable habitat that could be enhanced through management to provide light and good microsites for the establishment and growth of new American Chestnut trees. There are also ongoing programs of habitat restoration that will benefit American Chestnut and other Carolinian species.
1.6 Threats to survival and recovery
The following threats to survival and recovery of American Chestnut are listed in order of importance:
Chestnut blight
Chestnut blight is the single greatest threat to American Chestnut in Canada. The blight was first noticed at the Bronx Zoo in 1904 on nursery stock, but it likely had multiple introductions at that time. The introduction of chestnut blight, caused by the fungus C. parasitica (Murrill) M.E. Barr, devastated the American Chestnut species throughout North America including Ontario. American Chestnut has persisted in southern Ontario by resprouting from the collars of surviving root systems but regenerated sprouts continue to become re-infected by the fungal pathogen. Some trees in southern Ontario are not currently showing blight symptoms. McKeen (1985) reported that 50 percent of trees had no obvious blight and Melzer and Boland (2004) found 41 percent of trees to be free of disease symptoms. In the most recent survey (Tindall et al. 2004), 325 of 459 trees assessed for blight (71%) had no obvious blight symptoms.
Chestnut blight will continue to threaten the remaining small and isolated populations of American Chestnut because it survives on sprouts and on alternative hosts. Cryphonectria parasitica has been observed to kill some alternative hosts but it usually exists on these hosts as a weak pathogen or saprophyte. Alternative hosts of C. parasitica in the Carolinian zone of southern Ontario include: White Oak, Red Oak, Black Oak (Q. velutina), Red Maple, Staghorn Sumac (Rhus typhina), Shagbark Hickory, Bur Oak (Q. macrocarpa), Chinquapin Oak (Q. muhlenbergii), Hop Hornbeam (Ostrya virginiana), Blue Beech (Carpinus caroliniana), Tulip Tree (Liriodendron tulipifera) and Sassafras (Sassafras albidum) (Mooij 1997). Locations for new plantings of American Chestnut for restoration or nut crops should be chosen carefully as they may act as a bridge to connect diseased populations of American Chestnut to isolated populations that have escaped disease.
Loss of individuals
Loss of individuals due to clearing of forests for farming and development continues to be a threat to American Chestnut in Ontario. Several sprout clumps of chestnut occur along roadsides and are repeatedly cut back or sprayed with herbicide so they will not interfere with overhead wires. Several young trees/sprouts have been damaged or killed due to logging and others have been lost due to clearing of forests and fencerows for agriculture and urban development. While many rural land owners practice good forest management and stewardship, exceptions of poorly managed forests including unsustainable logging and even complete clearing to expand other economic activities, are having a negative impact.
Hybridization
Interbreeding between American Chestnut and three introduced chestnut species (Chinese Chestnut, Japanese Chestnut and European Chestnut) may threaten the persistence of American Chestnut in Ontario. This concern stems from the theoretical view that rare species that hybridize with a more abundant relative will by virtue of their small numbers, be assimilated into the more common genome and ultimately cease to exist as a genetically distinct taxon. Although this process has rarely been documented in other plants (Burgess and Husband 2006, Burgess et al. 2008), the potential for hybridization to affect American Chestnut may be significant. From controlled pollinations, it is clear that all four species of chestnut are inter-fertile and can produce viable hybrid offspring. In addition, Chinese Chestnut and to a lesser extent, European Chestnut and Japanese Chestnut are widely distributed and planted in southern Ontario as ornamentals and (or) for nut production. It is likely that these out-plantings are located within pollen-dispersal distance of American Chestnut populations in many locations throughout the native range.
Despite the apparent opportunities for hybridization, the actual measurable risks to American Chestnut may be quite low at this time. Cultivated trees of other Castanea species tend to be clustered together and restricted mostly to the margins (around homes or in nurseries) rather than the interior of American Chestnut habitat. The impact of hybridization would therefore be reduced because members of the same genus do not interact directly and American Chestnut remains in the majority within its own populations. The low occurrence of hybridization was confirmed by a recent genetic analysis of trees in southern Ontario (Gerrath 2006). Gerrath used Randomly Amplified Polymorphic DNA (RAPD) markers to genetically characterize known samples of each species. Then, by comparing these genotypes to those of wild species from the American Chestnut range, trees were screened for hybrid parentage. Sixty trees, many of which were selected as most likely to be hybrids, were sampled from the native range. Only one tree (2% of all trees sampled) was identified as a hybrid, with Japanese Chestnut as the most likely parent. Although many trees have not been assessed, these results indicate that hybridization may not be prevalent in natural populations at the current time and should be considered a low risk to Canadian populations of American Chestnut.
Despite the threat that non-indigenous Castanea species may pose in natural systems, it is the resistance traits that these species have evolved that may provide one of the best solutions for the recovery of American Chestnut in North America. Specifically, backcross breeding programs have been developed to incorporate the resistance component of closely related species of Castanea into the genome of American Chestnut. The details of this method are provided in section 2.3 of this recovery strategy.
Insect pests
Of the insect pests that are known to feed on American Chestnut, little is known about their biology and impacts. They are, therefore, covered in the Knowledge Gaps section (Section 1.7).
1.7 Knowledge gaps
There is sufficient literature on the biology and ecology of American Chestnut to initiate recovery. However, periodic assessment of the status of the species and additional information on the control of chestnut blight are necessary.
The effect of chestnut blight on American Chestnut is ongoing. It has increased the vulnerability of the remaining populations to potential secondary threats such as declines caused by unpredictable population dynamics or environmental disturbances and accumulation of deleterious mutations. Further study and analysis is required to determine which if any, secondary threats are affecting the species and the level and extent of threat they pose.
Hypovirulence associated with fungal viruses as a naturally-occurring biological control strategy has controlled chestnut blight well in some locations in Europe but has failed almost completely in eastern North America (Milgroom and Cortesi 2004). However, some localized results have appeared promising, particularly in Michigan and with the use of hypovirulent isolates from Europe. Research efforts are still underway in the USA to evaluate hypovirulence on a longer-term ecological scale and to identify crucial factors regulating the establishment of hypovirulence in chestnut forests.
The need to restore American Chestnut to sustainable population sizes requires the development of methods of increasing blight resistance by screening individuals in natural populations. Conservation and restoration efforts by the Canadian Chestnut Council and The American Chestnut Foundation involve selective breeding programs to enhance resistance of native American Chestnut at a faster rate than that occurring in natural populations. The various programs of ongoing research differ in specific strategy, but share the common feature of starting with an initial cross (F1) between American Chestnut and resistant individuals of Chinese Chestnut. Methods for the inoculation of trees, the identification of resistant parents and progeny and the characterization of resistance genes controlling genetic resistance are needed. This research will hopefully fill the gaps in knowledge associated with blight susceptibility and resistance in American Chestnut populations.
Although hybridization does not currently appear to be a serious threat, its role may change particularly if populations of American Chestnut continue to decline and plantings of introduced chestnut species increase. As a result it will be important to expand the screening for hybrids to other individuals in natural populations (specifically plants with uncharacteristic leaf morphology, growth architecture and reduced blight) and to monitor plant material used in out-plantings.
Another potential threat to the American Chestnut species in Ontario is the Oriental Chestnut Gall Wasp. This wasp was introduced to North America through Georgia during 1974. It is currently found in Alabama, Georgia, Kentucky, Maryland, North Carolina, Ohio, Pennsylvania, Virginia, and Tennessee (Anon. 2009). Galls caused by these wasps suppress shoot elongation, reduce fruiting, and trees with severe infestations often die. It is not known if the Oriental Chestnut Gall Wasp can survive the colder temperatures in the northern portions of the American Chestnut’s native range.
The Chestnut Weevil (Curculio elephas) native to southern and central Europe, may also pose a threat to American Chestnut. Adult female weevils deposit eggs into developing nuts. After hatching, the larvae feed in the nut for several weeks. Infested nuts drop prematurely and larvae chew their way out of the nut after it has fallen. Although there are many introductions of this weevil into North America each year, it has not been observed in the wild (Venette et al. 2003). In commercial nut production, good sanitation, cultural practices and insecticides can effectively control weevils therefore the potential threat is expected to be low.
1.8 Recovery actions completed or underway
Recent surveys of distribution in Ontario were documented by Ambrose and Aboud (1986), Boland et al. (1997) and Tindall et al. (2004). Details of chestnut reproductive biology were elucidated by Ambrose and Kevan (1990). Following the 1986 COSEWIC status report, several studies were conducted in Ontario on select chestnut blight strains that exhibited reduced virulence (Dunn and Boland 1993, McKeen 1995, Boland et al. 1997, Melzer et al. 1997 and Melzer and Boland 1999). These surveys and studies provided a framework from which to develop the recovery objectives outlined in the next section.
Several strategies may show promise for the management of chestnut blight. These strategies include sanitation measures (e.g. removal of dead twigs and stems that act as infection sites, and the removal of infested plant material that acts as sites for sporulation of the pathogen), fungicides, biological control and disease resistance. Diagnostic tests for resistance and early infection will be important for continuing research and management of nursery stock and out-plantings. See Appendix for a description of C. parasitica and symptoms of chestnut blight disease as well as steps that can be taken to prevent disease spread by humans.
Assessment of the status of American Chestnut in Ontario
To assess the population status of American Chestnut trees in southern Ontario, an extensive baseline survey of accessible, known or newly found populations was conducted between 2001 and 2003 using a standardized protocol (see Tindall et al. 2004). A total of 601 mature and immature individuals located in 94 sites across southern Ontario were inventoried, permanently labelled with metal tags, and georeferenced using GPS. The following data were generated from the inventory:
- diameter, height, and reproductive state of each tree;
- health condition of each tree (number and kinds of cankers and degree of tree dieback);
- habitat description, ecosystem type, other species present, canopy cover, slope and soil type, <pH and texture of each site as per the ELC system protocol;
- using sanitary techniques, a very small amount of plant material in the form of leaf, bud and twig samples was collected to serve as herbarium specimens [and possibly for future gene bank (DNA) storage purposes]; and
- taxonomic status and possibility of hybridization, based on morphological, molecular and/or physiological characters.
This survey will be repeated at five to ten year intervals and the results used to assess and monitor the status of known and newly discovered populations within the species' native range in Ontario.
Activities of agencies currently engaged in recovery efforts
The Canadian Chestnut Council founded in 1988, has played an important leadership role in public awareness and in encouraging research on American Chestnut and chestnut blight. Members of the Canadian Chestnut Council have mapped many of the remaining sites of chestnut in southern Ontario and continue to monitor many of these sites. Volunteer members have pollinated and collected nuts from isolated, mature trees and have initiated plantings of chestnut seedlings. In addition the Canadian Chestnut Council initiated a disease resistance breeding program. It incorporates germplasm of American Chestnut from southern Ontario with known intra- and interspecific sources of disease resistance following a similar program of interspecific hybridization being used by The American Chestnut Foundation.
The American Chestnut Foundation was founded in 1983. The mission of the American Chestnut Foundation is to restore American Chestnut as an integral part of the eastern forest ecosystem. It maintains an extensive breeding program for developing resistance to chestnut blight. The goal of this program is to introduce resistance from Chinese Chestnut into American Chestnut while preserving as completely as possible the genome of the American Chestnut. Resistance in Chinese Chestnut appears to be controlled by two or three incompletely dominant genes. Therefore, the goal of this breeding program is to develop chestnuts that are homozygous for both resistance genes. Resistant Chinese Chestnuts are backcrossed to American Chestnuts at least four times resulting in crosses with a genome that is at least 15⁄16ths (94%) of American Chestnut origin. Progeny are tested for resistance by inoculation with virulent isolates of C. parasitica after each backcross. Final selections are intercrossed to produce the first nuts for restoration outplanting. Because the American Chestnut Foundation expects that natural selection has created populations adapted to regional conditions, it has used germplasm of American Chestnut from across the range of American Chestnut. The American Chestnut Foundation maintains breeding programs in Connecticut and Pennsylvania as well as on their main breeding farm in Virginia. The American Chestnut Foundation hopes to have its first resistant line(s) ready for planting in 2010 to 2015.
The Ontario Soil and Crop Improvement Association (OSCIA) initiated a two year project in 1998 to promote interest in the farming community in chestnut recovery and to identify farmers with suitable sites who are willing to set aside up to one acre of land to be planted with American Chestnut seedlings. In 1998 to 1999, the OSCIA coordinated the establishment of 24 demonstration sites with a total of approximately 1,300 American Chestnuts planted in southern Ontario. Ten of the 24 sites are located outside the native range of American Chestnut.
Ongoing research into the potential of using hypovirulence as a biological control strategy is being conducted by Dr. C. McKeen, the Canadian Chestnut Council and Dr. G.J. Boland, University of Guelph. Naturally-occurring healing-type cankers have been observed in southern Ontario and putatively hypovirulent isolates have been recovered from these cankers and their hypovirulence has been confirmed in laboratory tests. Hypovirulent isolates of C. parasitica from Ontario were released at several locations, including an experimental site at Skunk’s Misery in Middlesex and Lambton counties. Hypovirulent isolates compatible with virulent isolates at the site were inoculated around the perimeter of cankers. Expansion of treated cankers was measured in comparison with untreated cankers 15 months after inoculation. For the first one to two years after treatment, statistical differences were detected between treated and untreated cankers and after 15 months, hypovirulent isolates were recovered from 82 percent of the treated cankers. However, visual observations three to five years after inoculation were not as encouraging and many of the treated trees had died from blight. Observations will be continued at this site to see if there are any long-term effects from these treatments.
2.0 Recovery
2.1 Recovery goal
To restore American Chestnut populations in Ontario to a self-sustaining state whereby natural recruitment results in the maintenance or increase of current population size throughout the species' native range.
2.2 Protection and recovery objectives
Table 2. Protection and recovery objectives
| No. | Protection or recovery objective |
|---|---|
| 1 | Survey suitable habitat and/or formerly occupied habitat for American Chestnut and protect and monitor known populations within the species' native range in Ontario. |
| 2 | Promote protection and public awareness of American Chestnut. |
| 3 | Develop and evaluate management measures to control threats. |
| 4 | Secure Ontario sources of germplasm originating from blight-free trees. |
2.3 Approaches to Recovery
Table 3. Approaches to recovery of the American Chestnut in Ontario
- Survey suitable habitat and/or formerly occupied habitat for American Chestnut and protect and monitor known populations within the species' native range in Ontario.
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Inventory, Monitoring and Assessment, Research |
1.1 Survey and monitor status of known and newly discovered populations within the species' native range in Ontario:
|
|
| Necessary | On-going | Inventory, Protection |
1.2 Monitor and maintain planted populations located within the species' native range in Ontario as potential sources of blight-free native germplasm:
|
|
- Promote protection and public awareness of American Chestnut
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Short-term | Communications, Stewardship, Protection | 2.1 Promote protection of known populations of American Chestnut to land management authorities, private landowners and recovery teams |
|
| Beneficial | Long-term | Education and Outreach, Stewardship | 2.2 Promote public awareness of American Chestnut |
|
- Develop and evaluate management measures to control threats
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Research | 3.1 Investigate the effectiveness of various chestnut blight control measures in an experimental setting |
|
| Critical | On-going | Management Monitoring |
3.2 Identify, manage and monitor at least 15 American Chestnut populations of those inventoried within the species' native range in Ontario:
|
|
| Critical | Long-term | Research |
3.3 Develop techniques to decrease species' vulnerability to chestnut blight
|
|
| Beneficial | Long-term | Management | 3.4 Restrict inter-jurisdictional movement of all Castanea species in Canada |
|
- Secure Ontario sources of germplasm originating from blight-free trees
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Beneficial | Short-term | Protection | 4.1 Locate and inventory blight-free American Chestnut stands planted in Ontario outside the species' native range. |
|
| Beneficial | Long-term | Research | 4.2 Monitor and protect at least two blight-free stands planted outside the species' native range in Ontario |
|
Supporting narrative
The approaches described in Table 3 primarily address chestnut blight, the most important threat to the species. The recommendations focus primarily on the need to develop and evaluate blight control methods. If the blight is controlled, the small immature stump-sprout saplings which currently account for a large portion of extant populations may grow to maturity, increasing numbers of fruit-bearing individuals to levels where healthy breeding and seed production can occur.
Until blight can be controlled, the greatest promise for recovery of the species lies in developing and deploying a blight-resistant locally-adapted American Chestnut genotype. As outlined in section 1.6, natural hybridization is considered a potential threat to the recovery of American Chestnut. However since chestnut blight is still the primary cause of endangerment for this species, controlled breeding with close relatives that exhibit a higher degree of resistance such as Chinese and Japanese Chestnut, may be needed to accelerate the evolution of resistance. This research is being pursued by the Canadian Chestnut Council breeding program. The intent of this program involves introducing the disease resistance of Chinese Chestnut into Ontario genotypes of American Chestnut through an initial hybrid cross, backcrossing the hybrids with Ontario genotypes over multiple generations to reduce the Chinese Chestnut genetic contribution to the target level of less than six percent and then selecting crosses that perform well in disease resistance screening tests. It is anticipated that blight-resistant trees that meet these genetic criteria and are phenotypically indistinguishable from naturally occurring genotypes could be produced within 15 years. In addition, naturally occurring individuals with lower blight susceptibility are also being assessed (intraspecific resistance). These efforts would be followed by diligent out-planting efforts to get the resistant genotype established in the network of priority populations. However, there is no information on the durability of resistance as trees mature.
Utilizing this method for recovery comes at the cost of introducing genes from interspecific crosses with other Castanea species and thus may cause some ambiguity between hybrids developed specifically for the recovery strategy versus those that occur naturally (Jacobs 2007). As indicated in section 1.6, hybridization that occurs naturally is a potential threat to American Chestnut as the genetic component of offspring that are produced is likely to be 50 percent or less American Chestnut. In contrast, the genetic component of hybrids that are produced using the rigorous methods of backcrossing for the recovery strategy will be close to 94 percent or more American Chestnut (Hebard 2005). Therefore, to avoid any ambiguity, reintroduction programs that involve backcrossed American Chestnut should clearly articulate how they differ from naturally occurring hybrids that are a potential threat.
Over time, it is anticipated that selection will favour genotypes with a combination of resistance and local adaptation. Ultimately, the survival of American Chestnut which is affected so severely by blight, may depend on this infusion of genetic variation.
Other efforts include maintaining existing populations in the wild, utilizing management techniques for controlling the blight, maintaining in-situ and ex-situ germplasm through protection and planting. Finally, species recovery efforts continue to benefit from a volunteer network assisting in pollen transfer, seed collection, seed production, tree planting and maintenance.
Approach 1.1
Existing information on the occurrence of surviving individuals and populations of American Chestnut is either incomplete or scattered among various agencies and individuals. A more detailed, standardized and frequent approach to collecting observations on American Chestnut would contribute to a sample and information database. This could possibly be maintained by the Ontario Ministry of Natural Resources or the Canadian Chestnut Council, and would provide more accurate information on the current status of this species and provide a framework for continued recovery efforts.
It is recommended that a protocol be developed for surveying all American Chestnut populations in Ontario every five to ten years, to:
- record number and size of individuals and their state of health;
- record habitat observations (associated species and forest canopy density);
- determine reproductive status of individuals and populations (fruiting and recruitment);
- examine individuals for presence/severity of blight or other threats to health;
- examine individuals for hypovirulent/healing cankers;
- sample chestnut blight populations for culture collection; and
- expand screening for naturally-occurring hybrids
These data will permit estimation of survival and recruitment rates of American Chestnut and the percentage of individuals with chestnut blight, thereby providing a measure of population viability. New observations and reports of American Chestnut will be collected between surveys and added to the survey records.
Approach 1.2
Recovery action of American Chestnut must involve careful consideration of collections and plantings of cultivated American Chestnut trees throughout the native Canadian range. Historically, American Chestnut or cultivars have been planted for the purposes of commercial nut production, landscaping and conservation. Unfortunately, these plantings have been established with little thought about their impact on naturally occurring populations of American Chestnut. There has been little coordination or regulation as to how and where planting should occur. As a result, there is a risk that out-plantings are not true American Chestnut or are not from the best suited local seed sources and that they will serve as conduits for the movement of C. parasitica among populations. At the same time, there is a need for planted trees of known composition to serve as a germplasm reserve for future restoration efforts and for research purposes. The following actions are recommended.
- Identify existing planted populations of chestnut, American or otherwise, planted within the native range of American Chestnut and determine the genetic parentage (species, hybrid) and geographical source where possible.
- Collate and distribute information on existing plantings to the lead recovery agency (Ontario Ministry of Natural Resources) as well as major conservation interests. This document would be used for identifying potential locations for research, for developing a management plan for existing planted populations with the intent of reducing interactions with native populations and for directing and reducing the potential impacts of future planted populations.
- Identify potential locations/sponsors to maintain at least two planted populations of native American Chestnut within the native range. The locations should be located in different parts of the geographic range – such as the southwest and the northeast part of the range – and should be isolated from natural populations by at least 50 kilometres. This distance is recommended as a precautionary approach to avoid blight transference among the natural and planted populations. These planted populations can be used for a variety of purposes including: (1) germplasm reserve for future out-plantings in natural populations and (2) research on genetic variability in native populations, natural blight resistance and blight management. Planted populations used as germplasm reserves should be completely or nearly blight-free.
- Stock the designated planted populations with approximately 40 trees, representing populations from throughout the native range in Canada. These trees should be disease-free and should be characterized genetically to confirm their American Chestnut heritage.
Monitor the state of all planted populations (i.e., incidence of blight; tree age/size and health) with regular updates from owners (using survey methods under approach 1.1).
Approach 2.1
Planning agencies within each municipality in which American Chestnut occurs should be made aware of all known sites within their jurisdiction to be included in their natural heritage mapping. Existing habitats need better protection by land management agencies and private land owners.
Land management authorities
Many of the known sites of surviving American Chestnut are on crown and public lands. However, accurate information is often not communicated directly to agencies and individuals involved with land planning and management. Improved communication can contribute directly to improved management of surviving populations of American Chestnut. It is recommended that planning agencies, conservation authorities, forestry consultants and municipal by-law officers be notified of the status of American Chestnut in Ontario and to work cooperatively with them to protect known populations and their habitats within their jurisdictions. Information and status of regional populations should be made available to these agencies once the inventory is complete.
Private landowners
Some of the known healthy American Chestnut populations are on private land. Consideration should be given to the stewardship or securing of such sites to ensure the protection of these trees. It is recommended that private landowners be contacted to encourage stewardship opportunities. Alternate methods for securing sites could be explored for other lands (such as those where land owners do not reside on the land or are not interested in stewardship). Communication with agencies such as the Nature Conservancy of Canada, local land trusts, and regional stewardship networks is recommended to bring about the securing of land through such mechanisms as landowner stewardship, conservation easements or acquisition. It is important to strive for open dialogue with land owners and assume willingness for good land management and stewardship. However, no action should be taken without their concurrence. Researchers and recovery workers should remember to obtain landowner permission before venturing onto any property.
Recovery teams
Maintaining communication with ecosystem-based recovery teams such as Carolinian Woodlands and watershed-based recovery teams in southern Ontario is recommended.
Approach 2.2
Public awareness of the current status and potential recovery of American Chestnut has been, and will continue to be, an important component of the recovery of this species. It is through such promotion that new sites of chestnut are located, seeds are collected and distributed and much of the enthusiasm and support surrounding this species is generated.
Awareness of the status of American Chestnut by the general public can be increased through communication with farm, forestry, naturalist, and planning organizations. The communication should be periodic highlights of recent findings and improving status of individual sites, landowner stewardship and their actions/activities that have promoted the recovery of this species and opportunities for new participants. It should also include practical information for landowners, such as identifying native chestnuts, chestnut blight cankers and healing cankers.
This outreach can be accomplished using various means including:
- flyers;
- website
- newspaper/magazine articles and news releases;
- booths at community events; and
- community
Approach 3.1
Several methods are professed for the effective short-term control of chestnut blight but little information is available to substantiate these claims. In addition, recent developments in fungicide technology and biological control may present new opportunities for managing this disease and pathogen. A comparative assessment of such practices may identify effective methods and/or products that can be used for future application in recovery efforts as well as by commercial chestnut growers.
It is recommended that the most effective combination of management practices be determined based on existing information and experiment results. Experiments designed to test the following management practices should be conducted in plantings, orchards or natural populations not identified in approach 3.2. Management practices to control chestnut blight might include:
- fungicide treatment of expanding cankers and assessment of canker development and pathogen sporulation;
- mud pack treatment of expanding cankers and assessment of canker development and pathogen sporulation;
- removal of dead uninfected branches that provide infection sites for the pathogen; and
- removal of dead branches, suckers and trees that provide pathogen sporulation sites.
Approach 3.2
Existing populations of American Chestnut are largely fragmented and isolated. This presents an opportunity to manage individual sites more intensively through cultural practices, artificial pollination of trees and out-planting of seedlings. It is recommended that the 15 populations with the highest potential for recovery be identified from those inventoried under approach 1.1, based on some or all of the following criteria:
- size of population – larger populations preferred (over half of the known sites consist of only one individual);
- reproductive status of individuals – reproducing populations preferred;
- ownership – publicly owned land or secured private land is preferred to ensure long-term access and protection;
- blight – sites with, and without blight; and with healed or hypovirulent cankers;
- size of habitat – larger habitats with room for population expansion preferred;
- habitat characteristics – some site characteristics such as soil type have been reported to be conducive to the development of healing cankers;
- geographic location – select populations from across the native range of American Chestnut in southern Ontario; and,
- genetic composition - populations with sufficient spatial separation from known sites of other Casanea or hybrids.
Once the 15 populations have been selected, management measures may be initiated in 10 of the 15 populations. The remaining five populations could initially be unmanaged and serve as experimental controls. The management measures could include: (1) removing dead, sporulating chestnut tissue from the site to reduce inoculum; (2) suppressing canker development using selected treatments; (3) encouraging recruitment of new individuals through pollination; (4) transplanting uninfected individuals from other sites; and (5) thinning or other microhabitat management to improve survival and growth of seedlings. Specific strategies would be based on survey results (see approach 1.1), current research literature and results of experimental investigation of the effectiveness of various chestnut blight control measures (see approach 2.1). The results will be summarized as guidelines to managing sites where chestnut blight is present. As much as possible, recruitment should be encouraged from within each site. Additional sites can be added to the management strategy as deemed necessary.
Finally, the protocol from approach 1.1 should be applied to monitoring of tree health, insect pests and hybridization of these 15 populations every five to 10 years to produce a population health status report.
Approach 3.3
Long-term management strategies to control chestnut blight are critical for the recovery of this species. Currently, there are three techniques with the potential to achieve this goal: (1) spread of hypovirulent strains of chestnut blight; (2) identification of natural resistance in surviving stands of American Chestnut; and (3) breeding for resistance in American Chestnut through hybridization with other Castanea species.
Approaches to the following areas of research are not presented in detail because they are continually evolving and approaches will change as new information is obtained.
Hypovirulence
The purpose of this technique is to promote the development and spread of hypovirulent strains of chestnut blight amongst existing populations of American Chestnut. Following survey results (see approach 1.1), three or more populations with healing cankers and/or hypovirulent isolates of chestnut blight could be selected to conduct research on the effectiveness of this technique in controlling chestnut blight. The goal for these sites would focus on increasing recruitment of American Chestnut to provide susceptible hosts for the continued growth and possible spread of hypovirulent isolates of chestnut blight. Recruitment of American Chestnut could be increased where possible, through cross-pollination among individuals within a site. Alternatively pollen, seed or seedlings can be imported from other sites with similar characteristics. As much as possible, recruitment should be encouraged from within each site and seedlings should be protected from herbivores. No other blight control measures should be used in these populations so that virulent and hypovirulent isolates can continue to interact on living and dead chestnut tissues.
Other locations in southern Ontario should be monitored for the presence of naturally occurring hypovirulent blight strains. Emphasis should be placed on identifying hypovirulent isolates that are associated with healing and healed cankers and are prevalent or spreading within the native range of American Chestnut. Continuing research will identify additional factors associated with the spread and efficacy of hypovirulent strains of C. parasitica.
Intraspecific breeding for disease resistance
Using species and disease severity information collected from native populations of American Chestnut under approach 1.1 and possibly from populations established outside the species' native range (see approach 3.1), individuals with putative resistance to chestnut blight should be identified for outplanting and/or inclusion in breeding programs.
Where feasible, it is recommended that nurseries of putatively resistant American Chestnut be established and the degree of resistance of these trees to chestnut blight be assessed. Resistant individuals could then be cross-pollinated, to assess the progeny’s degree of blight resistance. Intraspecific breeding may identify individuals of American Chestnut with measurable levels of disease resistance. To date, no significant resistance to chestnut blight has been identified in surviving populations of American Chestnut but differences in susceptibility have been reported.
Interspecific breeding for disease resistance
This technique involves the identification of highly resistant individuals in other Castanea species, such as Chinese Chestnut for use in an on-going backcross breeding program with a representative selection of locally adapted American Chestnut.
It is recommended that efforts be continued to establish nurseries of potentially resistant hybrid Castanea species and assess the degree of resistance to chestnut blight, as well as backcrossing resistant individuals to American Chestnut and assessing resulting progeny for blight resistance. Backcrossing should continue for five or more generations, until the genetic background is at least 94 percent American Chestnut. Such interspecific breeding aims to yield individuals: (1) whose genetic composition is predominantly American Chestnut; (2) have high levels of resistance to chestnut blight; and (3) are adapted to local environmental conditions.
Approach 3.4
To ensure that known sites of American Chestnut outside of the native range of chestnut blight remain free of disease, it is important to prevent the introduction of blight into these regions through the movement of nursery stock of Castanea species. Thus, it is recommended that inter-provincial and international trade of Castanea species be restricted to prevent the introduction and/or spread of chestnut blight from infested/infected seed and/or seedlings of Castanea species.
To that end, a proposal in accordance with the Plant Protection Regulations of the Plant Protection Act should be developed and submitted to the Canadian Food Inspection Agency of Agriculture and Agri-Food Canada regarding the monitoring of nurseries and certification of disease-free stock or restriction of shipments if this cannot be done with certainty. This proposal should also address the introduction of chestnut blight on Castanea species from Ontario to other provinces of Canada or countries where American Chestnut is known to occur.
Approach 4.1:
In a parallel approach to collecting more detailed information and samples from individual sites within the native range of American Chestnut (see approaches 1.1 and 1.2), it is recommended that American Chestnut populations in Canada - but outside of the Ontario native range - also be inventoried. Much of this work would be conducted in collaboration with local organizations and individuals. These populations can serve as ex-situ sources of germplasm for possible transplant into the species native range.
This approach first involves locating populations of American Chestnut occurring outside their native range. Landowners should be contacted before entering sites and offered the opportunity to participate if interested. Collecting information on the origin of plantings is especially important. Once these populations have been located they should be inventoried using the survey protocol outlined in Tindall et al (2004) and summarized in this recovery strategy in section 1.8.
Approach 4.2
Sites of American Chestnut located outside of the native range of southern Ontario represent an important source of germplasm of this species that is located outside of the known distribution of chestnut blight. It is recommended that at least two populations each having a minimum of 40 trees, be selected by 2015. These trees should have origins representative of American Chestnut’s native geographic range in Ontario. Suitable planted populations should be established if they do not currently exist. These plantings should be maintained as an important source of disease-free germplasm for potential future out-plantings. Existing individuals of American Chestnut outside of the native range may also be useful as a source of germplasm if the parentage can be confirmed. Every effort should be taken to keep these planted populations blight-free.
2.4 Performance measures
Table 4. Performance measures for evaluating the achievement of recovery of the American Chestnut in Ontario
| Recovery objectives | Performance measures | Target date |
|---|---|---|
| 1. Survey suitable habitat and/or formerly occupied habitat for American Chestnut and protect and monitor known populations within the species' native range in Ontario. |
|
2015 |
| 2. Promote protection and public awareness of American Chestnut |
|
2015 |
| 3. Develop and evaluate management measures to control threats |
|
|
| 3. Develop and evaluate management measures to control threats |
|
2015 |
4. Secure Ontario sources of germplasm originating from blight-free trees. |
|
2015 |
2.5 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of Natural Resources on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the recovery team will be one of many sources considered by the Minister when developing the habitat regulation for this species.
The area to be prescribed as habitat in a habitat regulation for American Chestnut should include all areas in the counties of Essex, Chatham-Kent, Lambton, Elgin, Middlesex, Norfolk, Brant, Haldimand, Niagara, Hamilton-Wentworth, Waterloo, Wellington and Halton where 1) one or more individuals of the species occur or 2) one or more individuals were previously documented in written reports or surveys (e.g., Ambrose and Aboud 1987, Melzer et al. 2004, Tindall et al. 2004, Natural Heritage Resource Centre database). Research at occupied sites has been conducted by the recovery team to identify which Ecological Land Classification (ELC) ecosites (as defined by Lee et al. 1998) support American Chestnut. With this knowledge, it is recommended that the area prescribed as habitat is restricted to only the contiguous ELC ecosite polygons where there are extant or historic occurrences of American Chestnut. If an individual is close to the polygon edge, a minimum distance of 30 m from the stem of the tree (or sprouting stump) is recommended for inclusion in the area prescribed as habitat in the habitat regulation. This is a precautionary measure to ensure that a minimum distance is met for any ground disturbance that could affect mature trees.
The following ELC ecosite and vegetation classifications were recorded in a status assessment of accessible, known or newly found American Chestnut populations that was undertaken by the University of Guelph between 2001 and 2003 using a standardized protocol (Tindall et al. 2004):
- Treed Cliff (CLT)
- Deciduous Forest (FOD)
- Dry-fresh Oak Deciduous Forest Ecosite (FOD1)
- Dry-fresh Red Oak Deciduous Forest Type (FOD1-1)
- Dry-fresh White Oak Deciduous Forest type (FOD1-2)
- Dry-fresh Oak-Maple-Hickory Deciduous Forest Ecosite (FOD2)
- Dry-fresh-Red Maple Deciduous Forest type (FOD2-1)
- Dry-fresh Oak-Red Maple Deciduous Forest Type (FOD2-2)
- Dry-fresh Poplar Deciduous Forest type (FOD3-1)
- Dry-fresh Deciduous Forest Ecosite (FOD4)
- Dry-fresh White Ash Deciduous Forest Type (FOD4-2)
- Dry-fresh Sugar Maple Deciduous Forest Ecosite (FOD5)
- Dry-fresh Sugar Maple Deciduous Forest Type (FOD5-1)
- Dry-fresh Sugar Maple-Beech Deciduous Forest type (FOD5-2)
- Dry-fresh Sugar Maple-Oak Deciduous Forest Ecosite (FOD5-3)
- Fresh-moist Sugar Maple Deciduous Forest Ecosite (FOD6)
- Dry-fresh Sugar Maple-White Ash Deciduous Forest Type (FOD5-8)
- Dry-fresh Sugar Maple-Red Maple Deciduous Forest Type (FOD5-9)
- Fresh-moist Lowland Deciduous Forest Ecosite (FOD7)
- Fresh-moist Sassafras Deciduous Forest Type (FOD8-2)
- Fresh-moist Oak-Maple-Hickory Deciduous Forest Ecosite (FOD9)
- Mixed Forest (FOM)
- Dry-Oak-Pine Mixed Forest Ecosite (FOM1)
- Dry-fresh White Pine-Maple-Oak Mixed Forest Ecosite (FOM2)
- Dry-fresh Hardwood-Hemlock Mixed Forest type (FOM3-1)
- Coniferous Forest (FOC)
- Dry-fresh Pine Coniferous Forest Ecosite (FOC1)
Prescribing habitat based on the vegetation community will help to preserve the ecological function of the area and the ecological conditions required for the persistence of American Chestnut.
Since the greatest threat to the species is the chestnut blight, isolated planted individuals may be important for maintaining and recovering the species. It is recommended that emphasis be placed on all American Chestnut individuals in natural populations. Trees planted for horticulture, landscaping or research should be exempt from the habitat regulation but can be individually assessed for possible genetic conservation value.
If future scientific studies indicate that additional areas of habitat are necessary to achieve the recovery goals for this species, the habitat regulation should be updated accordingly.
Glossary
Anastomosis: Fusion of two cells or hyphae in contact that reabsorb their walls and fuse into one.
Committee on the Status of Endangered Wildlife in Canada (COSEWIC): The committee responsible for assessing and classifying species at risk in Canada.
Committee on the Status of Species at Risk in Ontario (COSSARO): The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
Conidium: Asexual, non-motile spores of a fungus; they are also called mitospores due to the way they are generated through the cellular process of mitosis.
Conservation status rank: A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperilled
2 = imperilled
3 = vulnerable
4 = apparently secure
5 = secure
H = possibly extinct or extirpated
NA = a conservation status rank is not applicable because the species is not a suitable target for conservation activities
NR = rank not yet assessed
X = presumed extinct or extirpated
Demographic stochasticity: Fluctuations in population growth rates due to random variation in survival and reproduction among individuals.
Endangered Species Act, 2007 (ESA): The provincial legislation that provides protection to species at risk in Ontario.
Environmental stochasticity: Variation in population growth due to fluctuations in environment over time.
Epicormic shoots: Stems that emerge from dormant buds along the trunk of a tree
Ex situ: Not situated in the original, natural or existing place or position.
Extant: In existence; still existing; not destroyed or lost.
GRANK: See "Conservation status rank"
Germplasm: The sum of all genetic material that an individual can transfer to successive generations.
Hypha: A long, branching filamentous cell of a fungus that is the main mode of vegetative growth in fungi.
Hypovirulence: Having less virulent characteristics.
In situ: Situated in the original, natural or existing place or position.
Isolate: A strain or an individual selected from a population of a micro-organism, often maintained in pure culture in laboratory conditions.
Monoecious: Individuals with male and female flowers on the same plant but borne separately.
Mutation accumulation: Rise in frequency of deleterious mutations in small populations due to chance
Mycelium: The entire mass of hyphae that constitutes the vegetative body or thallus of a fungus
NRANK: See "Conservation status rank"
Phytophagous: Feeds on plants
SRANK: See "Conservation status rank"
Self-incompatible: Self-pollinations do not yield seed owing to a physiological rejection.
Species at Risk Act (SARA): The federal legislation that provides protection to species at risk in Canada. This act establishes Schedule 1 as the legal list of wildlife species at risk to which the SARA provisions apply. Schedules 2 and 3 contain lists of species that at the time the act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
Species at Risk in Ontario (SARO) List: The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
Thallus: The vegetative body of a fungus.
Virulent: The degree or measure of pathogenicity of a microbe; the relative ability of a microbe to cause disease
References
Ambrose, J.D. 2004. Assessment and Update Status Report on American Chestnut (Castanea dentata) in Canada. Committee on the Status of Endangered Wildlife in Canada, Ottawa. 19 pp.
Ambrose, J.D. and S.W. Aboud. 1987. Status Report on the American Chestnut, Castanea dentata in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa,.18 pp.
Ambrose, J.D. and P.G. Kevan. 1990. Reproductive biology of rare Carolinian plants with regard to conservation management. Pages 57-63 in G.M. Allen, P.F.J. Eagles, and S.D. Price, eds., Conserving Carolinian Canada: Conservation Biology in the Deciduous Forest Region. University of Waterloo Press, Ontario.
Anagnostakis, S.L. and B. Hillman. 1992. Evolution of the chestnut tree and its blight. Arnoldia 52:2-10.
Anagnostakis, S.L., K.M. Welch, J.W. Snow, K. Scarborough and T.D. Eichlin. 1994. The rediscovery of the Clearwing chestnut moth, Synanthedon castaneae (Busck) (Lepidoptera: Sesiidae) in Connecticut. Journal of the New York Entomological Society 102: 111-112.
Anonymous. 2009. Oriental chestnut gall wasp. University of Missouri Extension.
Bessin, R. 2003. Nut Weevils. University of Kentucky, Cooperative Extension Service, EntFact 206.
Boland, G.J., M.S. Melzer and D.L. Mooij. 1997. Biological Control of Chestnut Blight with Hypovirulence in Southern Ontario. Ontario Forest Research Institute, Ontario Ministry of Natural Resources.
Brewer, L.G. 1995. Ecology of survival and recovery from blight in American chestnut trees (Castanea dentata (Marsh.) Borkh.) in Michigan. Bulletin of the Torrey Botanical Club 122:40-57.
Burgess, K.S. and B.C. Husband. 2006. Habitat differentiation and the ecological cost of hybridization. Journal of Ecology 94:1061-1069.
Burgess, K.S., M. Morgan and B.C. Husband. 2008. Interspecific seed discounting and the fertility cost of hybridization in an endangered species. New Phytologist 177:276-284.
Dunn, M.M. and G.J. Boland. 1993. Hypovirulent isolates of Cryphonectria parasitica in southern Ontario. Canadian Journal of Plant Pathology 15:245-252.
Farrar, J.L. 1995. Trees in Canada. Fitzhenry and Whiteside Ltd., Markham, Ontario. 502 pp.
Fox, W.S. 1949. Present state of the chestnut, Castanea dentata (Marsh.) Borkh. In Ontario. The Canadian Field-Naturalist 63:88-89.
Gerrath, J. 2006. Detection of Hybridization Between Castanea dentata (Marsh.) Borkh. and Non-native Congeners in Ontario. M.Sc. Thesis. University of Guelph, Guelph, Ontario.
Gravatt, G.F. and L.S. Gill. 1930. Chestnut Blight. U.S.A. Department of Agriculture Farmers' Bulletin No. 1641. 18 pp.
Griffin, G.J. 1989. Incidence of chestnut blight and survival of American chestnut in forest clearcut and neighboring understory sites. Plant Disease 73:123-127.
Griffin, G.J. 2000. Blight control and restoration of the American Chestnut. Journal of Forestry 98(2):22-27.
Griffin, G.J., H.C. Smith, A. Dietz and J.R. Elkins. 1991. Importance of hardwood competition to American chestnut survival, growth, and blight development in forest clearcuts. Canadian Journal of Botany 69:1804-1809.
Harvell, C.D., C.E. Mitchell, J.R. Ward, S. Altizer, A.P. Dobson, R.S. Ostfeld and M.D. Samuel. 2002. Climate warming and disease risks for terrestrial and marine biota. Science 296:2158-2162.
Heald, F.D. and M.W. Gardner. 1914. Longevity of pycnospores of the chestnut blight fungus in soil. Journal of Agricultural Research 2:67-75.
Heald, F.D., M.W. Gardner and R.A. Studhalter. 1915. Air and wind dissemination of ascospores of the chestnut blight fungus. Journal of Agricultural Research 3:493-526.
Hebard, F.V. 2005. The backcross breeding program of the American Chestnut Foundation. In, Proceeding of Conference on Restoration of American Chestnut to Forest Lands. Steiner, K.C. and J.E. Carlson (eds.). http://www.acffarms.org/papers/Reprints/The%20Backcross%20Breeding%20Program%20of%20TACF%2C%202005.pdf [link inactive]
Hill, J.M. 1994. Wildlife value of Castanea dentata past and present, the historical decline of the chestnut and its future use in restoration of natural areas. Pages 186-193 in M.L. Double and W.L. MacDonald, eds., Proceedings of the International Chestnut Conference. West Virginia University Press, Morgantown.
Ingram, D.S. 1998. Biodiversity and plant pathogens and conservation. Presentation at 7th International Congress of Plant Pathology, Edinburgh, Scotland. http://www.apsnet.org/online/feature/biodiver/ingram.html [link inactive]
Jacobs, D.F. 2007. Toward development of silvical strategies for forest restoration of American chestnut (Castanea dentata) using blight-resistant hybrids. Biological Conservation 137:497-506
Kuhlman, E.G. 1978. The devastation of American chestnut by blight. Pages 1-3 in W.L. MacDonald, F.C. Cech, J. Luchoc and C. Smith, eds., Proceedings of the American Chestnut Symposium. West Virginia University Books, Morgantown, West Virginia. 122 pp.
Lee, H.T., W.D. Bakowsky, J. Riley, J. Bowles, M. Puddister, P. Uhlig and S. McMurray. Ecological Land Classification for Southern Ontario: First Approximation and Its Application. Ontario Ministry of Natural Resources, Southcentral Science Section, Science Development and Transfer Branch. SCSS Field Guide FG-02.
Little, E.L., Jr. 1977, Atlas of United States Trees, Volume 4. Minor Eastern Hardwoods. U.S.A. Department of Agriculture Miscellaneous Publication 1342. Washington, D.C. 17 pp. 230 maps.
McKeen, C.D. 1995. Chestnut blight in Ontario: past and present status. Canadian Journal of Plant Pathology 17:295-304.
McKeen, C.D. 1985. Chestnut blight and the survival of Castanea dentata in southern Ontario. Canadian Journal of Plant Pathology 7:446
McWilliams, W.H., T.W. Lister, E.B. LaPoint, A.K. Rose and J.S. Vissage. 2005. Current status of chestnut in eastern US forests. In Proceedings of the Conference on Restoration of American Chestnut to Forest Lands. Steiner, K.C. and J.E. Carlson (eds).
Melzer, M.S. and G.J. Boland. 1999. CHV3-type dsRNAs and the GH2 genotype in a population of Cryphonectria parasitica in Ontario. Canadian Journal of Plant Pathology 21:248-255.
Melzer, M.S. and G.J. Boland. 2004. Survey of American chestnut and chestnut blight in Ontario. Canadian Plant Disease Survey 84:118-119.
Melzer, M.S., M. Dunn, T. Zhou and G.J. Boland. 1997. Assessment of hypovirulent isolates of Cryphonectria parasitica for potential in biological control of chestnut blight. Canadian Journal of Plant Pathology 19:69-77.
Milgroom, M.G and P. Cortesi. 2004. Biological control of Chestnut blight with hypovirulence: A critical analysis. Annual Review of Phytopathology 42: 311-338.
Moerman, D. Native American Ethnobotany Database. 2003. http://herb.umd.umich.edu/ [link inactive]
Moss, M.R. and P.L. Hosking. 1983. Forest associations in extreme southern Ontario ca 1817: a biogeographical analysis of Gourlay’s statistical account. Canadian Geographer 27:184-193.
NatureServe. 2009. NatureServe Explorer: An online encyclopedia of Version 7.1. NatureServe, Arlington, Virginia.
Opler, P.A. 1979. Insects of American Chestnut: Possible importance and conservation concern. Pages 83-85 in: Proceedings of the American Chestnut Symposium, W. McDonald, ed. University of West Virginia Press, Morgantown, West Virginia.
Paillet, F. 1994. Ecology and paleoecology of American chestnut in eastern North America. Pages 178-183 in: Proceedings of the International Chestnut Conference. M.L. Double and W.L. MacDonald, eds., West Virginia University Press, Morgantown, West Virginia.
Rieske, L.K. 2007. Success of an exotic gallmaker, Dryocosmus kuriphilus, on chestnut in the USA: a historical account. OEPP/EPPO Bulletin 37:172–174.
Russin, J.S., L. Shain and G.L. Nordin. 1984. Insects as carriers of virulent and cytoplasmic hypovirulent isolates of the chestnut blight fungus. Journal of Economic Entomology 77 (4):838-846.
Saucier, J.R. 1973. American Chestnut…an American wood. FS-230, U.S.A. Department of Agriculture, Forest Service. Washington, D.C. 6 pp.
Smock, L.A. and C.M. MacGregor. 1988. Impact of the American chestnut blight on aquatic shredding macroinvertebrates. Journal of the North American Benthological Society 7:212-221.
Sudworth, G.B. 1892. On the name of the American chestnut. Bulletin of the Torrey Botanical Club 19:152-154.
Tindall, J.R., J.A. Gerrath, M. Melzer, K. McKendry, B. Husband and G.J. Boland. 2004. Ecological status of American chestnut (Castanea dentata) in its native range in Canada. Canadian Journal of Forest Research 34:2554-2563.
Venette, R.C., E.E. Davis, H. Heisler and M. Larson. 2003. Mini risk assessment Chestnut weevil, Curculio elephans (Gyllenhal). http://www.aphis.usda.gov/plant_pest_info [link inactive]
Recovery strategy development team members
Recovery strategy development team
| Name | Affiliation and location |
|---|---|
| John Ambrose (Co-chair) | Botanical Consultant, Guelph, Ontario |
| Greg Boland (Co-chair) | University of Guelph, Guelph, Ontario |
| Ken A. Elliott, RPF | Ontario Ministry of Natural Resources, London, ON |
| Brian Husband | University of Guelph, Guelph, Ontario |
| Melody Melzer | University of Guelph, Guelph, Ontario |
Advisors
| Name | Affiliation and location |
|---|---|
| Tannis Beardsmore | Canadian Forest Service - Atlantic Forestry Centre |
| Barb Boysen | Forest Gene Conservation Association, Peterborough, Ontario |
| Paul Catling | Agriculture and Agri-food Canada, Ottawa, Ontario |
| Christopher Cunliffe | Society of Ontario Nut Growers (SONG) |
| Andrew Graham | Ontario Soil and Crop Improvement Association (OSCIA) |
| Colin McKeen | Canadian Chestnut Council |
| Martin Neumann | Grand River Conservation Authority |
| Lindsay Rodger | Parks Canada, Gatineau, Quebec |
| Gerry Waldron | Consulting Ecologist, Harrow, Ontario |
Appendix 1. Chestnut blight
Description of Cryphonectria parasitica and symptoms of chestnut blight
The chestnut blight fungus, Cryphonectria parasitica, has orange mycelium
The asexual spores of C. parasitica, termed conidia, are wet spores and are dispersed by rain, insects, birds, and mammals. Conidia can survive freezing, drying and flooding. In the drip zone of infected trees there can be up to several million viable conidia per gram of soil. Conidia in soil are replenished with each rainfall. Numbers of conidia gradually decrease between periods of rain and conidia survive up to four months of desiccation in soil. These results suggest that there are always viable conidia present in soil under infected trees (Heald and Gardner 1914).
The sexual spores of C. parasitica, termed ascospores, are dry spores and are predominantly wind dispersed. Ascospores are released during periods of rain and for up to several hours after rain has ended. Ascospores released during rain are predominately washed to the ground but those released after rain are wind dispersed. In one study, 23 to 50 ascospores per square inch were counted from water traps exposed for 5 days 300 to 400 feet (91-122 m) from the nearest ascospore source (Heald et al. 1915). Infections by ascospores and conidia
Insect transmission
Many insects have been implicated in the transmission of chestnut blight through non- specific transferral of conidia between trees. One post-epidemic study confirmed that chestnut stems and blight cankers harboured a large, diverse insect fauna (Russin et al. 1984). The majority of 495 captured insect species were from the Coleoptera and Diptera families, and C. parasitica was isolated from 69 insect species (mostly Coleoptera) representing four orders. To date we have not found any evidence that American Chestnut had a strong connection to any individual pollinator species and that no pollinator was solely dependent on chestnut flowers.
Precautions to prevent disease spread by humans
Extreme care must be taken not to move chestnut blight between populations of American Chestnut. All surfaces in the drip zone of the tree are potentially covered with spores of the pathogen, especially the trunk and forest floor. Vehicles should be parked at least 20 metres from the nearest chestnut tree. When approaching a tree, prior to entering the drip zone, shoe/boot covers should be placed over footwear. Disposable gloves should be worn if any contact is made with any surface in the drip zone of the tree. Care should be taken not to allow clothing to contact surfaces, especially if other trees will be visited before the clothing is laundered. Disposable coveralls may be necessary. Just outside of the drip zone, equipment that has touched surfaces must be disinfected with 0.5% sodium hypochlorite. Gloves and shoe covers should be removed and placed in a plastic bag for disposal. Gloves and shoe covers should only be used once.
Disease management strategies
Several strategies have shown promise for the management of chestnut blight. These strategies include sanitation measures, fungicides, biological control, and disease resistance.
Sanitation measures include the removal of dead twigs and stems that act as infection sites, and the removal of infested plant material that acts as sites for sporulation of the pathogen. In Europe, these measures are primarily practiced in chestnut orchards grown for nut production (Milgroom and Cortesi 2004). They are considered to reduce the amount of inoculum of the pathogen but, alone, has a relatively small effect on disease progress and is most effective when used within an integrated management program.
In North America, little sanitation of infected plant material is practiced in natural forests. Previous attempts at using such practices met with relatively limited success, particularly during the height of the pandemic when inoculum of the pathogen was abundant within populations of chestnut. However, the surviving populations of chestnut and chestnut blight have become fragmented and isolated, and many sites no longer appear to contain the pathogen. Therefore, cultural practices that reduce the number of infection sites on susceptible trees or reduce populations of the pathogen may prove more effective now.
Thinning around sprouting chestnuts may promote vigorous growth and reduce their susceptibility to blight infection (Griffin 2000). A recent survey in Ontario also showed a reduced amount of blight infection where the canopy was more open (Tindall et al. 2004). In contrast, removal of the overstory resulted in an increase of disease from 5 percent to 100 percent within five years (Paillet 1994). When thinning around American Chestnut, great care must be taken not to cause wounding because the blight pathogen is a wound pathogen. In sites where alternative hosts are present, thinning of such species within a 20 to 40 metre radius should be considered, particularly if any symptoms of blight are present.
The application of selected fungicides for management of blight cankers has met with limited success. Difficulties in selecting fungicides and formulations that can penetrate to the site of infection in the vascular cambium of woody tissues, and the development of resistance to selected fungicides, appear to be the primary limiting factors to efficacy. Emphasis in previous studies was placed on slowing canker development and/or eliminating the pathogen from infected tissues. Recent developments in fungicide chemistry and formulations may have identified new opportunities for management of plant diseases associated with woody cankers. In addition, the use of fungicides for suppressing sporulation by the pathogen on the surface of diseased tissues has not been examined. Such an epidemiological approach to the management of chestnut blight could contribute to a reduction in the populations of the pathogen over time. Fungicides are regulated compounds in Canada and, if available for use, would be most suitable for protecting individual trees considered to be of high-value, such as orchard trees being used for nut production, grafted trees, etc., and would primarily be effective for relatively brief periods of time. Application of fungicides is not practical in forest settings.
Hypovirulent isolates (i.e., isolates with reduced virulence
Hypovirulence has not been as effective in North America as in Europe, despite the presence of hypovirulent isolates in various regions of the United States and Canada. Several of these isolates have been studied extensively in the United States but there is little evidence that they have successfully reduced the severity of chestnut blight (Milgroom and Cortesi 2004). In Ontario, promising hypovirulent isolates of C. parasitica were characterized from several locations, and assessment of these isolates for biological control efficacy was initiated. Results from field inoculations to date have not been encouraging but additional study and intervention into processes affecting the spread and distribution of hypovirulence may identify factors restricting the efficacy of this approach in North America.
Critical analyses of using hypovirulence for biological control of chestnut blight have concluded that effective control has been observed in Europe and in Michigan but that almost all other attempts in North America have failed, particularly at the population level (Milgroom and Cortesi 2004). Medium or large-scale experiments have been completed in West Virginia, Connecticut, Virginia and Wisconsin where up to hundreds of trees and thousands of cankers were inoculated with hypovirulent isolates, with little evidence of effective biological control of blight. Various characteristics of the fungal viruses, the pathogen, and the trees are thought to determine the success or failure of hypovirulence (Milgroom and Cortesi 2004). Knowledge of these factors, such as tree, site, and climate characteristics, and their influence on the epidemiological aspects of blight are often poorly understood (Griffin 1989, Griffin et al. 1991, Brewer 1995).
Hypovirulent isolates have been found in various locations in North America, but the only region where hypovirulence has been effective is in Michigan where it occurs naturally and in some places trees are remarkably healthy (Milgroom and Cortesi 2004). Populations of chestnut and blight in Michigan are similar to those in Ontario, where one hypovirulence-associated virus, CHV-3, has been associated with healing cankers and infected isolates of the pathogen (Melzer and Boland 1999). However, the role of hypovirulence in Ontario is less clear than in Michigan.
Other strategies for the biological control of chestnut blight have also been evaluated. The use of mud packs directly on cankers is thought to be effective because of the activity of micro-organisms in the soil that affect growth and development of the pathogen in the canker. These micro-organisms may offer an opportunity for alternative approaches to biological control.
There is considerable interest in the potential for identifying or breeding American Chestnut that is resistant to chestnut blight. Evolutionary theory suggests that some resistant trees may be present in an otherwise susceptible population of a species, and that these resistant trees may survive in remnant populations following pandemic diseases. Naturally-occurring resistant trees would be an important discovery for the recovery of this species, and differences in susceptibility have been observed among some individuals (Griffin 2000). Unfortunately, it can be difficult to distinguish between resistant trees and trees that have simply escaped disease and there have been no confirmed examples of American Chestnut that are resistant to chestnut blight. The relatively high proportion of trees in southern Ontario that do not have symptoms of chestnut blight is encouraging.
There is also considerable interest in breeding resistant American Chestnut trees through interspecific hybridization with Chinese and Japanese Chestnuts, followed by recurrent back-crossing to the American species and selection of resistant individuals. It is anticipated that this procedure will result in progeny that are highly resistant to chestnut blight and are at least 94 percent American Chestnut in other characteristics. While this is expected to produce blight resistant trees for planting, there is no information on the durability of resistance as trees mature. The American Chestnut Foundation has a large and established program in breeding for disease resistance in chestnut and outplanted seeds from their breeding program to test for blight resistance in three national forests in 2008. Breeding programs are also established at several universities and government research stations in the United States. The Canadian Chestnut Council has initiated a disease resistance breeding program in southern Ontario that hopes to build on the efforts of the American Chestnut Foundation and other institutions and to incorporate germplasm that is adapted to this region with disease resistant American Chestnut breeding material. In addition to this traditional approach to breeding resistant chestnut, scientists in the United States are evaluating the potential for genetic engineering of American Chestnut with disease resistance genes from other organisms.
Footnotes
- footnote[1] Back to paragraph Note: This range is the current and pre-blight range. The natural range of American Chestnut has not significantly changed since the arrival of chestnut blight; however, the number of trees within the natural range has declined.
-
footnote[2]
Back to paragraph
The entire mass of hyphae that constitutes the vegetative body or thallus of a fungus
Hypha: a long, branching filamentous cell of a fungus, and is the main mode of vegetative growth in fungi - footnote[3] Back to paragraph Asexual, non-motile spores of a fungus; they are also called mitospores due to the way they are generated through the cellular process of mitosis occur at wounds or branch scars.
- footnote[4] Back to paragraph The relative ability of a pathogen to cause disease
- footnote[5] Back to paragraph Fusion of two cells or hyphae in contact that reabsorb their walls and fuse into one