American Eel Recovery Strategy
This document advises the ministry on ways to ensure healthy numbers of the American eel, a threatened or endangered species, return to Ontario.

Recovery strategy prepared under the Endangered Species Act, 2007
2013
About the Ontario Recovery Strategy Series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act, 2007 (ESA, 2007) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species' persistence in the wild.
What is a recovery strategy?
Under the ESA, 2007, a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA, 2007 outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. There is a transition period of five years (until June 30, 2013) to develop recovery strategies for those species listed as endangered or threatened in the schedules of the ESA, 2007. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of Natural Resources Species at Risk webpage at: www.ontario.ca/speciesatrisk
Recommended citation
MacGregor, R., J. Casselman, L. Greig, J. Dettmers, W. A. Allen, L. McDermott, and T. Haxton. 2013. Recovery Strategy for the American Eel (Anguilla rostrata) in Ontario. Ontario Recovery Strategy Series. Prepared for Ontario Ministry of Natural Resources, Peterborough, Ontario. x + 119 pp.
Cover illustration: Ontario Ministry of Natural Resources/COA – Jason Mortlock
© Queen’s Printer for Ontario, 2013
ISBN 978-1-4606-3059-4 (PDF)
Content (excluding the cover illustration) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n'est disponible qu'en Anglais en vertu du Règlement 411/97 qui en exempte l'application de la Loi sur les services en français. Pour obtenir de l'aide en français, veuillez communiquer avec Pamela Wesley au ministère des Richesses naturelles au
Authors
Rob MacGregor – (Former) Ontario Ministry of Natural Resources, Great Lakes Branch Peterborough, Ontario
John Casselman – Queen’s University, Kingston, Ontario
Lorne Greig – ESSA Technologies Ltd., Richmond Hill, Ontario
John Dettmers – Great Lakes Fishery Commision, Ann Arbor, Michigan, U.S.A. W.A. (Bill) Allen – Heritage One, Burk’s Falls, Ontario
Larry McDermott – Plenty Canada, Lanark, Ontario
Tim Haxton – Ontario Ministry of Natural Resources, Aquatic Science Unit –Southern Science, Peterborough, Ontario
Acknowledgments
We express our sincere thanks to Elder Dr. William Commanda O.C. (Officer, Order of Canada and Founder of Circle of All Nations), Elder Albert Marshall (Eskasoni Mi'Kmaw First Nation), Elder Murray Whetung (Curve Lake First Nation) and Henry Lickers Akwesasne First Nation) for their dedication and tireless efforts to work toward harmony with the environment. We thank them for their wisdom and for granting permission to share their insights in the recovery strategy. We give particular thanks to Elder Commanda for informing the Governor General and the Queen that "the ancient American Eel, which was once so plentiful in the Ottawa River Watershed, has been placed on the Endangered Species List in Ontario" (Commanda 2009). Thanks to Dr. Cheryl Bartlett for permission to include the Two-Eyed Seeing logo developed by Integrative Science at Cape Breton University and to David Oliver of Skylark Information Systems for assistance with preparation of maps to support our work. We especially appreciate the efforts of Dr. L. Bernatchez and Caroline Cote, Laval University, in preparing the genetics section and thank Dr. Chris Wilson, geneticist for OMNR, for his review of an early draft of it. Thanks also to Dr. Peter Hodson, Queen’s University and Dr. John Fitzsimmons, Fisheries and Oceans Canada (Burlington) for their helpful comments on specific sections of the strategy and to Dale Honeyfield, USGS for making his paper available for use in the strategy. We also appreciate the numerous anonymous comments provided by agencies, First Nations, other stakeholders and the general public; many were very helpful in strengthening the final document.
The partnering efforts by Aboriginal and non-Aboriginal peoples in writing this recovery strategy and the likelihood of continuing long-term work together, has strengthened our relationships with one another and with the American Eel. Collective efforts among government, stakeholders and Aboriginal peoples to recover this species will not only aid in the restoration of lost ecological services, and restore biodiversity, cultural and natural heritage values, but will be a significant milestone in recovering and strengthening relationships among our cultures.
Declaration
The recovery strategy for the American Eel was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all of the individuals who provided advice or contributed to its preparation or the official positions of the organizations with which the individuals are associated.
The goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ontario Ministry of Natural Resources
Fisheries and Oceans Canada
Executive summary
The American Eel (Anguilla rostrata) is the only member of the genus Anguilla found in North America. In Ontario, it is near the northern extremity of its range, which spans fresh and coastal Atlantic Ocean waters of North, Central (Mexico) and northern South America. Aboriginal traditional knowledge, anecdotal (local knowledge from the public), archaeological information, historical documents and old fisheries records tell us that the American Eel was once extremely abundant throughout all tributaries to Lake Ontario and the St. Lawrence River. Declining abundance in most watersheds appears to have been underway by the turn of the twentieth century. More recently, the American Eel has been apparently extirpated from many parts of its Ontario range and is in serious decline where it still exists, leading to its listing as endangered under Ontario’s Endangered Species Act, 2007 (ESA).
Present science considers the American Eel to consist of a single breeding population in which all individuals travel to the Sargasso Sea in the Atlantic Ocean to spawn. From there, young eels drift with ocean currents and most eventually migrate inland into streams, rivers and lakes. Ontario’s eels, being virtually all female and the most fecund within the species' range, are an important segment of the global population.
In Ontario, the American Eel is a highly valued fish for Aboriginal peoples, and was also highly valued by European settlers. It thus forms a strong component of Ontario’s cultural and natural heritage. It is clear that the species has been in decline in Ontario due to anthropogenic effects for a century; the American Eel has been completely extirpated from extensive areas of many Ontario watersheds and is in steep decline in the remainder of the province’s waterbodies.
The cumulative effects of eel mortality during downstream migration due to hydro-electric turbines, reduced access to habitat imposed by man-made barriers to upstream migration, commercial harvesting in jurisdictions other than Ontario, contaminants, and habitat destruction, alteration and disruption are among the most significant threats to the survival and recovery of the American Eel in Ontario. Thiamine deficiency in Lake Ontario eels may pose additional stress to Lake Ontario eels, but research is required to confirm the potential effects.
Recovery of the American Eel in Ontario is a long-term prospect, likely to take many eel generation times to complete in its fullest sense (one generation = approximately 20 years). The recovery goal for the American Eel is to re-establish the species in a wide variety of waters throughout its historical range in Ontario by the year 2150, at abundance levels that: (1) restore cultural relationships and natural heritage values, (2) are consistent with ecosystems of high integrity and function, (3) strengthen the biodiversity of the province’s watersheds, and (4) provide valued ecological services. Achievement of the goal will provide the best opportunity for long-term persistence of the species in Ontario while enabling Ontarians to regain some of the benefits they once derived from the species. Given the extensive time frame (equivalent to seven eel generations) of the recovery goal, the range of presently available mitigation approaches and the potential for development of new approaches over this period, it is the opinion of the American Eel Recovery Team that the goal is reasonable and achievable. Although full recovery of historical abundance may not be feasible, recovery to beneficial levels should be possible in most areas of the historical range. Much progress can be made within one eel generation time. Now that anthropogenic mortality due to fishing in Ontario has been addressed, it is recommended that eel recovery actions emphasize strategic provision of enhanced, adequate and safe upstream and downstream passage. The recovery goal will be achieved through the following recovery objectives.
- Strategically restore access to habitat within the historical range of the American Eel.
- By 2150, restore resilience of the American Eel to anthropogenic stress in Ontario by diversifying habitats available to the American Eel across its historical range in Ontario. This should be accomplished by protecting and strategically restoring access to and use of, both the upper St. Lawrence River/Lake Ontario and the inland watersheds formerly used by the American Eel in Ontario.
- By 2050, increase production and enhance resilience of the American Eel by strategically restoring access to all immediate tributaries of the Ottawa River, Lake Ontario and the upper St. Lawrence River (generally proceeding downstream to upstream). Improvements to downstream passage should be made within 10 years of restoring access to areas where it was formerly prevented.
- Beginning immediately and using the habitat range in 2000 as the baseline, increase American Eel access to habitat by 10 percent every five years, consistent with the draft National Management Plan for the American Eel (Canadian Eel Working Group [CEWG] 2009).
It is recommended that the watershed areas in which to restore access should be strategically determined through the development and implementation of Watershed-based Implementation Plans (WIPs), with full public and Aboriginal consultation.
- Increase escapement and recruitment.
- Increase escapement of silver and large yellow eels from watersheds in their historical range within Ontario.
- By 2050, reduce cumulative mortality rates by 50 percent at the watershed level (the benchmark against which this is to be measured is the 1997-2002 average; CEWG 2009). The intent is to increase the escapement of large, mature female eels from provincial waters to levels targeted in implementation plans for a given watershed. This objective is intended to support increased recruitment of eels. As there is no eel fishing in Ontario, the focus will need to be on cumulative mortalities due to turbines.
- By 2070, increase the number of American Eels annually migrating from Ontario to the ocean to levels consistent with those observed in the early 1980s. Continue to undertake negotiations with power operators to develop options to reduce mortality, increase escapement and enhance recruitment of the American Eel in Ontario. Consult with Aboriginal communities, the public and other stakeholders on the options.
- Enhance recruitment.
- Measured at the Moses-Saunders ladders (Saunders and New York Power Authority ladders combined), achieve recruitment of eels ascending the ladders consistent with the returns observed during the late 1970s and early 1980s at the Saunders ladder (as this was the only ladder in existence during the early 1980s).
- Increase escapement of silver and large yellow eels from watersheds in their historical range within Ontario.
- Reduce anthropogenic mortality of eels in boundary waters managed jointly with other jurisdictions.
- Locate, protect, restore and enhance habitats upon which eels depend.
- Reduce other sources of stress on the American Eel (e.g., contaminants, disease, harmful destruction, alteration or disruption of habitat).
- Use an appropriately coordinated and strategic watershed-based approach to eel recovery across its historical range in Ontario.
- Strengthen the engagement of Aboriginal peoples, stakeholders and other partners in the development and implementation of recovery actions for the American Eel.
- Maintain strong Ontario participation and leadership in the development and implementation of coordinated inter-jurisdictional protection, management and recovery of the American Eel and its habitats at national and bi-national levels.
- Ensure ongoing understanding by scientists, managers, stakeholders, First Nations and the general public of the current status of the American Eel and the efficacy of recovery strategy actions.
- Evaluate potential short-term methods of supporting eel abundance through such means as translocations and eel ladders in key watersheds.
- Address knowledge gaps to enable and enhance protection, conservation and recovery efforts.
The American Eel recovery should occur through coordination and integration of science, management and conservation across the numerous jurisdictions and among the agencies and organizations responsible for eel management in North America. It is important that Ontario continue its strong efforts to encourage the participation of others to reverse the American Eel declines. It also should include a commitment to integrate western science with Aboriginal Traditional Knowledge and community knowledge in the implementation of the recovery strategy.
All migratory corridors (historical and current) for the American Eel should be contained in the habitat regulation. This would include all waters that are tributaries to Ontario’s portions of Lake Ontario, the St. Lawrence River and the Ottawa River.
It is recommended that the habitat regulation should protect the primary habitat in both lentic (still) and lotic (moving) waters, including all waters extending from the high-water mark down to a depth of 10 m for all reaches currently or formerly occupied or used as migratory corridors by the American Eel. This includes all rivers, streams and rivulets, both permanent and ephemeral. It should be noted that potential habitat can be much broader depending on the water body and can extend from the high water mark to any depth. Local knowledge should be used to determine if refinements in particular water courses or reaches are necessary. Otherwise, protecting the primary habitat to a depth of 10 m should be sufficient.
Finally, as the recovery of many aquatic fish species at risk will be prevented by the same anthropogenic impacts, an ecosystem approach should be adopted during the development and implementation of the WIPs wherein other species at risk are given due consideration at the same time.
1.0 Background
Knowledge integration
The American Eel Recovery Team developed this recovery strategy to guide the recovery and facilitate the long-term sustainability of American Eel (Anguilla rostrata)
One of the purposes of Ontario’s Endangered Species Act, 2007 (ESA) is to "identify species at risk based on the best available scientific information, including information obtained from community knowledge and Aboriginal Traditional Knowledge (ATK)." In developing the recovery strategy a strong effort was made to incorporate both community knowledge and ATK. Both have been included wherever possible in development of the recovery strategy.
Cultural differences in how knowledge is obtained, viewed and communicated make integration of ATK with western science a significant challenge – one that is important to surmount, and can be met with ongoing dialogue among those who have a commitment to the guiding principle of Two-Eyed Seeing (Allen 2008a, Allen et al. 2008).
The principle of Two-Eyed Seeing was developed by Elder Albert Marshall of Eskasoni First Nation, who described it as the respectful joint integration of ATK and empirical science. The American Eel Recovery Team adopted and embedded this principle in the process of developing the Recovery Strategy for American Eel in Ontario.
The American Eel Recovery Team views ATK as an integrative 'way of knowing' gained through deep spiritual, physical, emotional and intellectual ties with nature. It reflects intimate, holistic observation of the environment. Aboriginal Traditional Knowledge in this recovery strategy is not considered to be analogous to data that can be collected; however, it is considered to reflect the insight and understanding that arises from analysis in western science. While ATK rests on the foundation of generations of oral knowledge sharing, it is not static and thus not solely traditional. Aboriginal Traditional Knowledge is unique to specific local environments and, as with the continuing refinements of western science, it grows with each generation providing insight into current conditions. Eels have not only been of major importance for Aboriginal peoples as a source or food for thousands of years, they were of substantial cultural, spiritual, material and medicinal significance to Aboriginal peoples (Prosper and Paullette 2002, MacGregor et al. 2008, 2009, 2011, Denny et al. 2012). Consequently, ATK for this species is of special significance.
The joint efforts of Aboriginal and non-Aboriginal members of the recovery team to integrate knowledge from ecology and fisheries science with ATK and community knowledge in developing this recovery strategy has provided a much richer understanding than could have been gained with an ATK or western scientific perspective alone.
Anecdotal information from an earlier time, and early eyewitness accounts provide valuable insight into the past distribution, abundance and importance of the American Eel (Pauly 1995, Pinnegar and Englehard 2008). Clearly, ATK provides especially valuable information from an earlier time. All forms of information, including anecdotal information, early eyewitness accounts and ATK have been and continue to be critically important to piecing together the former status and distribution of eels in Ontario.
Elder William Commanda, founder of a Circle of All Nations, talks in terms of the joint need for very long-range perspectives and vision, saying that we need to "come together in love, peace, reconciliation and unity" (Thumbadoo 2005), and work with "one heart, one mind, one love, and one determination" (Circle of All Nations, undated). He states that, "Today, the plight of the Eel must awaken us to the crucial need to transform our relationship with Mother Earth and All Our Relations, and to awaken us to the pivotal role of Indigenous Peoples in this process" (Commanda, pers. comm. 2008).
The successful restoration of eels to their native habitat across the historical range will be consistent with Canada’s commitment to Aboriginal peoples in the UN Convention on Biodiversity (CBD 2000), and will also restore some benefits lost to residents of Ontario in all parts of the species' range.
Elder Commanda’s perspective has been a hallmark of the development of this recovery strategy. It has been an exercise of strong, unified thinking and consensus among the scientists, resource managers, stakeholders and Aboriginal people representatives on the American Eel Recovery Team (see Appendix 1). As noted in a letter from Chief Kirby Whiteduck, Algonquins of Pikwàkanagàn, eels were a highly important food source for the Algonquin people, and were an important element of their economic, cultural and social way of life (MacGregor et al. 2011). Algonquins are very supportive of efforts to rehabilitate American Eel in its historic range (MacGregor et al. 2011, Algonquins of Ontario, 2012).
Aboriginal peoples participating in the development of the Recovery Strategy for the American Eel in Ontario see the process as one of both healing the damage done to the eel and strengthening the relationships among all involved in the recovery effort. Knowing that American Eel has long been integral to their cultural identity, practices and customs, Aboriginal representatives have resolved to support Ontario and Canadian efforts for recovery of the species (see Appendix 2 and MacGregor et al. 2011).
1.1 Species assessment and classification
Common name:
American Eel
Scientific name:
Anguilla rostrata
SARO List Classification:
Endangered
SARO List History:
Endangered (2008)
COSEWIC Assessment History:
Threatened (2012), Special Concern (2006)
SARA Schedule 1:
No Schedule
Rankings:
G-Rank: G4 N-Rank: N4 S-Rank: S1?
The Glossary provides definitions for technical terms, including the acronyms used in the table.
1.2 Species description and biology
Species description
The American Eel is variously known as the Atlantic Eel, Freshwater Eel, Common Eel, Silver Eel, Yellow Eel, Bronze Eel, Easgann, and Anguille d'Ameriqué, among other names. The Mi'kmaq people called eels Kat (Prosper and Paulette 2002). Eels were called pimizi by the Algonquins (McGregor 1994), bimizi by the Ojibwe (Baraga 1878), and goda:noh by the Seneca (Bardeau 2002).
Juvenile and adult American Eels are yellowish-green or brownish, elongated, serpent-like fish with very small, deeply embedded scales. In Ontario, eels are generally larger (maximum length of about 1.3 m), less dense, slower growing and older (up to 42 years; J. Casselman, unpub. data) than individuals found in the southern part of the range. Casselman (2003, 2008) provides detailed information on the size, age and growth of the American Eel.
The American Eel naturally inhabiting the upper St. Lawrence River, Ottawa River and Lake Ontario watersheds comprise a distinctive sub-population or phenotype. The hallmark of this phenotype is that these eels (when mature) are exclusively large, old and highly fecund females when mature, the most fecund in the species' range (Casselman 2003, Verreault et al. 2003, 2009, COSEWIC 2006, Tremblay 2009). The extremely high reproductive value of large, old female fish has been well described and recognized (Palumbi 2004, Berkeley et al. 2004a,b, Field et al. 2008, Venturelli et al. 2010, Hutchings and Rangeley 2011). The contribution of these individuals to the spawning stock of the American Eel is considered to have been biologically significant (Canadian Science Advisory Secretariat [CSAS] 2011) when eels were more abundant in these waters.
It is important to note that only female eels are typically observed in headwater streams (Goodwin and Angermeier 2003) and that the American Eel body sizes typically increase with distance from the ocean (Lookabaugh and Angermeier 1992, Smogor et al. 1995). As eels in Ontario are at the extremity of the range at the headwaters of the the St. Lawrence River (several thousand km from the ocean), and fecundity increases with body size (Barbin and McCleave 1997), it is not surprising that Ontario eels are all females, the largest and most fecund in the North American Range.
Eel genetics and population structure
Two species of Anguillid eels spawn in the Sargasso Sea: the American Eel and European Eel (Anguilla anguilla). Morphological and genetic studies have established that the American and European Eel are two distinct species, yet they are capable of hybridizing (Albert et al. 2006, van Ginneken and Maes 2005). Molecular genetics data provide evidence both supporting and rejecting the hypothesis that the American Eel is composed of a single, randomly mating (panmictic) population (reviewed in Maes and Volckaert 2007).
Recently however, a very thorough population genetics analysis – based on the genotyping of 18 "neutral" microsatellite markers on over 2,500 individuals from 34 locations and nine year classes – found no significant evidence of genetic differentiation between life history stages (glass versus yellow eels), geographic origin or age classes. This constitutes very strong and definitive support for the panmixia hypothesis (Bernatchez et al. 2011, Côté et al. 2013). Even so, individual eels from this single, randomly mating population are not genetically or phenotypically identical and therefore may not have the same fitness under different environmental conditions.
For example, controlled growth studies conducted by Côté et al. (2009) (see also Bernatchez et al. 2011) showed that eels from the Maritimes (Cape Breton) grew more quickly in both freshwater and brackish water environment than eels from Grande-Rivière-Blanche (Québec). Also, the plastic growth response to both environments differed between eels of both origins. These results suggested that young eels that survive and settle at a given location may be genetically different (within the context of panmixia) from those at other locations (L. Bernatchez, pers. comm. 2010, Bernatchez et al. 2011). These divergent groups are called "clusters" or "contingents" (Secor 1999), rather than "populations", since eels are panmictic and there is no reproductive barrier. Each eel "contingent" is composed of individuals with similar fitness in a particular environment.
Indeed, recent research supported this hypothesis and revealed genetic differences at coding genes under selection between glass eels from different sampling sites along the Atlantic coast (Gagnaire et al. 2012). These authors also isolated surface temperature encountered when approaching the coastal area as a major factor that can induce a form of "genetically structured contingents" (Gagnaire et al. 2012). Regional differences in patterns of survival suggest the possibility of at least partial demographic independence among distinct American Eel contingents. In particular, evidence from research and personal observation suggests that St. Lawrence River/Lake Ontario eels represent a regionally distinct phenotype, and that this phenotype may have a genetic basis (Vladykov and Liew 1982, Bernatchez et al. 2011).
The principal mechanism behind eel contingents is thought to be differential mortality, but differential migratory behaviours can not yet be ruled out. The American Eel within the Upper St. Lawence River-Lake Ontario (USLR-LO) watersheds (including the Ottawa River) represent a small contingent of the global American Eel gene pool that is unique in being most fit for the USLR-LO environment. At spawning, these genes become dispersed into the broader population gene pool. As the American Eel abundance declines, the risk of losing these relatively rare genes increases dramatically. If lost it may not be possible to rescue the phenotype from other sources (L. Bernatchez, pers. comm. 2010, Bernatchez et al. 2011). This could perhaps explain why the recruitment decline in the USLR-LO environment has been more pronounced than in the Maritimes provinces.
Species biology
Life cycle
The American Eel has a complex life history (Figure 1) with stages occurring in oceanic, coastal, estuarine and freshwater environments. The American Eel begins life in the Sargasso Sea and returns to the Sargasso Sea to spawn, the only location where it does so. Spawning, which has never been observed, has been inferred from sampling of young in the Sargasso Sea. Spawning emigration begins in May from the Richelieu River (Québec). Emigration peaks between July and September in Lake Ontario and the St. Lawrence River waters and may continue into November.
Eggs hatch into larvae that are called leptocephali because of their transparent and willow-leaf-like form. The larvae drift in the Gulf Stream system for 7 to 12 months and transform into glass eels once they reach 55 to 65 mm in length. Glass eels have the typical elongate and serpentine form of the species and become progressively pigmented as they move across continental shelves to the shoreline. Once pigmented, they are considered elvers. The elver stage lasts from 3 to 12 months, during which time some migrate upstream into fresh water. In Atlantic Canada, timing of elver migration varies geographically. On the north shore of the Gulf of St. Lawrence, arrival occurs in July when elvers reach 60 to 70 mm in length. As elvers grow, they become known as yellow eels and after a number of years, they mature into silver eels. Additional information on the complicated life cycle of the American Eel is available in Tesch (1977) and COSEWIC (2012).
Figure 1. Life cycle of the American Eel (OMNR 2007)
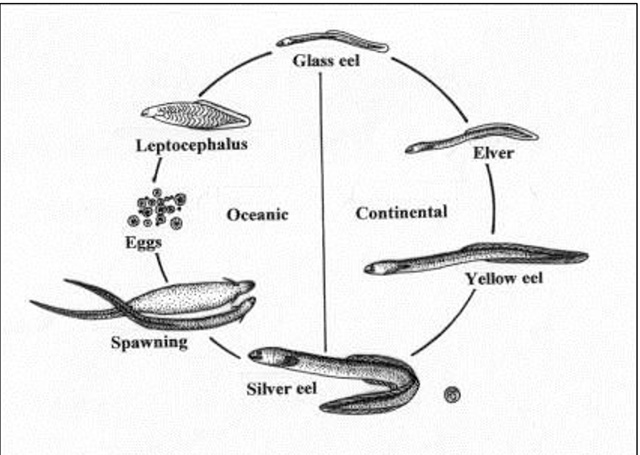
Dispersal of eels into fresh water can be heavily influenced by density-dependent effects (Feunteun et al. 2003), i.e., the higher the density, the stronger the push to continue to move upstream. It can also be somewhat random (Ibbotson et al. 2002, Edeline et al. 2007), especially as eels grow larger. Juvenile eel movements into the Upper St. Lawrence River-Lake Ontario system appear to be both random and density-dependent. Eels tend to be very slow to mature in these waters (Jessop 2010).
The yellow eel stage is most commonly observed in fresh water and is the principal stage in the life cycle that is observed in Ontario. Yellow eels are characterized by thick, tough skin, yellow-green to olive-brown colouration on the belly and darker colouration on the back. Sexual differentiation occurs during the yellow eel stage, the principal growth stage. Yellow eels may continue to travel upstream for many years, with seasonal peaks, usually between June and August in the upper St. Lawrence River. In Canada, eels typically hibernate in mud during winter, entering torpor at temperatures below 5°C, although there are records of eels remaining active during winter. Eel "balling" in the mud in winter has been well documented by Aboriginal peoples and commercial fish harvesters who speared large numbers through the ice (Prosper and Paulette 2002). This practise continues in the Maritimes.
The true silver phase is rarely seen in Ontario waters, although a greying intermediate phase occurs in some of the largest, oldest individuals (Casselman 2003). Silver eels, the mature freshwater phase, are greyish to white ventrally and develop a number of morphological and physiological adaptations for the long migration back to the spawning grounds. These include an enlarged pectoral fin, enlarged eye, modified retinal pigments and increased body fat. Mature eels are considered to spawn (in the Sargasso Sea) between February (peak) and April.
Resilience of the American Eel
Many diadromous fish populations use multiple modes of migration and multiple habitats (McDowall 1996). While eels are typically catadromous (migrating from freshwater to the sea to spawn), this life history strategy is not obligatory, as some eels appear to complete their entire life cycle in marine environments (Lamson et al. 2006). For eels and other fish species, segments of the population that exhibit different life cycle strategies are called "contingents" (Secor 1999, Jessop et al. 2002). In the American Eel, at least two contingents are recognized: (1) eels that complete their life cycle exclusively in marine environments; and (2) eels that migrate into and use freshwater environments to grow and mature before returning to the sea for spawning. Multiple life history strategies can reduce overall variance of population responses to environmental change, thus increasing stability and resilience (MacGregor et al. 2009, Secor 2010). Diversity of life history tactics in fish populations is increasingly recognized as having the effect of offsetting environmental stochasticity and contributing to long-term persistence (Secor 2007). For American Eel, diversity in life cycle strategies has been a hallmark of the species' success and is helpful in understanding the formerly wide distribution. MacGregor et al. (2009) discuss the importance of life cycle diversity to the American Eel resilience, conservation and recovery.
Different life cycle contingents can be differentially vulnerable to exploitation, habitat degradation and climate change (Secor 1999, 2007). For this reason, constituent patterns of life cycle diversity within populations should be regarded as a "portfolio," or a collection of life cycles, which hedges against future environmental uncertainty through mechanisms that permit life cycle diversity to persist generation after generation (Secor and Kerr 2009). Some may argue that marine-resident eels are sufficient to prevent the extinction of the species. Such speculation would be hazardous and risk-prone (McCleave and Edeline 2009). Further losses of freshwater eels may have serious demographic impacts because freshwater eels, by silvering at a larger size than sea eels, have higher fecundity (McCleave and Edeline 2009).
While catadromy in anguillid eels may be facultative, it remains a major life history trait for anguillid eels. Freshwater habitats tend to be more diverse (Secor 2010), less risky in terms of predation and support lower densities than brackish water habitats (Daverat et al. 2006). These features promote a diversity of outcomes (Secor 2010) and a larger diversity of phenotypes than brackish sites characterized by high densities and high natural mortalities (Daverat et al. 2006), thereby confering resilience to the eel population (Secor 2010). Moreover, it has been well documented that for the American Eel, relatively low densities in the USLR-LO system promote the development of juvenile eels as females (J. Casselman, unpub. data, Jessop 2010). Large, fecund females are the dominant form in upstream freshwater habitats (e.g., McCleave 2001). This has been the "trademark" of eels in Ontario (Casselman 2003, Verreault et al. 2003, 2009, COSEWIC 2006, Tremblay 2009). Large size and high fecundity enhance resilience in eels, as does a diverse array of accessible habitats in which to grow and mature. In Ontario, the diverse array of freshwater habitats (rivers, streams, marshes, ponds, lakes, etc.) formerly accessible and extensively used by the freshwater contingent of the American Eel would have promoted varying outcomes (e.g., survival, growth rate), thereby minimizing risks due to future environmental or anthropogenic change (Secor 2010).
Many Ontario eels remain in fresh water for an extremely long time (up to 25 years) before migrating back to sea. Such longevity could span periods of poor oceanic conditions (Secor 2007, Cairns et al. 2009, Secor and Kerr 2009, Secor 2010), thereby conferring further resilience to the population. The fact that naturally recruited mature eels indigenous to Ontario are large, old females would add substantially to the population’s resilience to both environmental variability and exploitation as it has for numerous other fish species (Berkeley et al. 2004, Palumbi 2004, Law 2007, Secor 2007, Anderson et al. 2008, Venturelli et al 2010).
Ecological role
Prior to the 1980s, the American Eel exhibited the largest range of any freshwater fish species in the western hemisphere, and held a dominant position in the fish communities by numbers and biomass in many habitats, often representing more than 50 percent of the biomass in some nearshore and riverine fresh waters (Smith and Saunders 1955, Ogden 1970), and no doubt played a dominant role in the fish community (Casselman 2003). The American Eel has been shown to be a keystone species in some freshwater fish communities. When dominant numerically or in distribution, eels can drive ecosystem processes through structuring of the fish community (Machut et al. 2007). For example, eels have substantially determined the structure of fish communities and energy flow within the habitats of the Hudson River watershed, New York (Schmidt et al. 2006).
The American Eel affects the community structure and energy flow by sequestering nutrients and transporting them upstream as it goes through its life cycle processes, thereby supplementing some stream habitats (Anderson and Schmidt 2006). For example, the Maryland Department of Natural Resources (MdDNR 1999) suggested that, until their decline, the American Eel in the Susquehanna River played an important role in removing excess nutrients from the watershed by using them in growth and production, and then delivering them to the ocean where the eels spawn and die. Similar observations have been made in France and western Europe, where the European Eel is a main fish species acting as a source of organic matter for freshwater systems. As such, eels serve as biotic vectors of organic matter fluxes between marine and freshwater systems and play a significant role in the functioning of these aquatic ecosystems (Lafaille et al. 2000). Eel migrations (ascending elvers and descending silver eels) have been shown to be responsible for a significant net output of carbon from the river to the sea (Lafaille et al. 2000). Lafaille et al. (2000) suggest that increasing eutrophication of freshwater systems in Europe signals a reduced relative contribution of European Eel to organic matter fluxes. Similarly, there are many freshwater systems within the historical range of the American Eel in Ontario where eutrophication is a problem (e.g., nearshore waters of Lake Ontario and many of the Kawartha Lakes; LaMP 2009, Gartner Lee Ltd. 2002). Given the significant decline of eels in Ontario and elsewhere in the St. Lawrence River watershed, the input and net export of organic carbon by this species will have been greatly reduced in recent years, thereby reducing the species' contribution to the functioning of aquatic ecosytems in these waters.
Because eels are top predators, consisting of many immigrating cohorts, and resident in Ontario for long periods of time (an average of 10 to 20 years) before emigrating back to sea, they add important stability to the nearshore fish communities of Ontario. Small yellow eels feed extensively on invertebrates and, as their size increases, they begin to feed intensively on small fish (Ogden 1970). Large yellow eels in the Ottawa River often feed extensively on crayfish (Orconectes spp.) and other invertebrates, and are frequently caught by anglers using worms as bait (K. Punt, pers. comm. 2009). Rapidly maturing eels in Lake Ontario and the upper St. Lawrence River feed heavily on pelagic Alewife (Alosa pseudoharengus) and, to a much lesser extent, Rainbow Smelt (Osmerus mordax), just prior to emigration (J. Casselman, unpub. data).
Their ability to occupy interstitial spaces in the rock suggests that if abundant, they could be significant predators on the young of invasive species such as Round Goby (Neogobius melanostomus) and Rock Bass (Amblopites rupestris) (J. Casselman, unpub. data). Eels are ferocious predators. Small eels often attack food items that are larger than they are, spinning violently to dismember whatever is in their grasp (J. Casselman, pers. comm. 2009).
Eels are also important competitors. Large eels compete directly with other piscivores, such as bass (Micropterus spp.), Northern Pike (Esox lucius), and Walleye (Sander vitreus) that feed on similar prey items. However, this association needs to be quantified.
In addition to being predators and competitors, the American Eel is an important prey fish for Beluga Whales (Delphinapterus leucas) (Hodson et al. 1994). Further, the American Eel is known to be an important host for the mussel Elliptio complanata; the decline of this mussel in the Susquehanna River has been linked to the demise of eels in the same watershed due to blockages caused by the many dams and hydro-electric facilities constructed on this system (Blankenship 2006).
There is little doubt that eels function as an integral component of nearshore fish communities in Ontario. When dominant numerically (as eels formerly were in many parts of their historical range in Ontario), the American Eel functioned as a keystone species, structuring and adding stability to the neashore fish community (Schmidt et al. 2006, Machut et al. 2007), while coexisting harmoniously with other top predators such as walleye.
1.3 Distribution, abundance and population trends
Eels have undergone local extirpations or substantial declines in many regions throughout their North American range (Richkus and Whalen 2000, ASFMC 2000, 2006, Casselman 2003, de Lafontaine et al. 2009a, MacGregor et al. 2009, Weeder and Uphoff 2009, Fenske et al. 2011, NatureServe 2011). Recently, the American Eel has been determined to be 'depleted' by the Atlantic States Marine Fisheries Commission (ASMFC) for American Eel within their authority. The ASMFC has further indicated that that management efforts to reduce mortality of eels in the U.S. are warranted (ASMFC 2012 a,b).
In 2011, the United States Fish and Wildlife Service (USFWS) decided to evaluate listing the American Eel as a threatened species under the Endangered Species Act (USFWS 2011b), but to date their findings have not been made public. Legal action has recently been filed over the delay in releasing official finding (Courthouse News Service 2012).
The American Eel is known to have an exceptional ability to colonize a variety of habitats (Helfman et al. 1987, Moriarty 1987, Wiley et al. 2004). The following reconstruction of the historical range of eels in Ontario clearly demonstrates the plasticity in habitat use patterns that enabled eels to colonise a wide variety of ecosystems (Daverat et al. 2006) in the province. Until dams and hydro-electric facilities were constructed within the historical range of the species, habitats used by the species spanned vast areas of the province ranging from large riverine (e.g., Ottawa River, St. Lawrence River) and large lacustrine habitats (e.g., Lake Temiskaming, Lake Ontario), to small streams, small lakes, ponds, wetlands and damp grassy sloughs. Essentially, eels could be found anywhere within the Ottawa River, St. Lawrence River and Lake Ontario watershed.
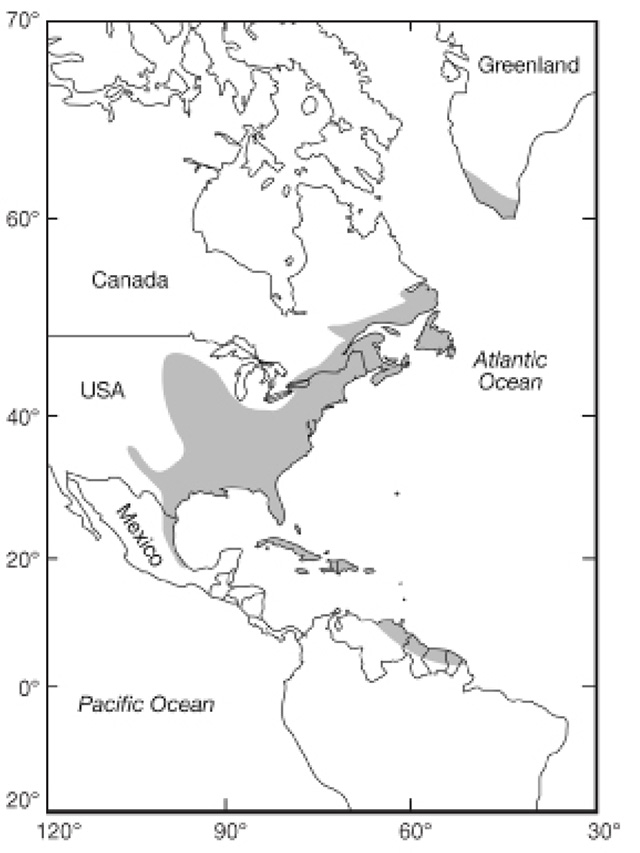
The historical range of American Eel included all accessible freshwater, estuarine and coastal marine waters of the western North Atlantic. It extended from Venezuela in the south through the Gulf of Mexico to Labrador in the north and as far inland as the headwaters of the Mississippi River (U.S.) and, in Ontario, near the extremity of their range, inland as far as Niagara Falls and the headwaters of the Ottawa River (Figure 2).
Figure 2. Geographic distribution of American Eels (modified from Tesch 1977; DFO 2010)
Enlarge Figure 2. Geographic distribution of American Eels
Overview of American Eel distribution in Ontario
At one time, eels accounted for more than 50 percent of the total fish biomass in many freshwater systems (Smith and Saunders 1955, Ogden 1970, Lary et al. 1998), including the nearshore waters of Lake Ontario and the upper St. Lawrence River (Casselman 2003, MacGregor et al. 2009). However in more recent times the population status of American Eel has declined substantially in many areas of its historical range.
Baselines and perceptions of former abundance and distribution in Ontario have shifted over time (MacGregor et al. 2008, 2009), and the following comments of Heidenreich (1971:105) regarding the natural environment of Huronia are equally applicable within the historical range of eels in Ontario:
Relicts of the original forest in Huronia are rare and tell us almost nothing of the species distribution. The same is true of drainage conditions before and after European settlement. Some of the creeks and springs present in the 17th and 18th century are gone today as well as at least four small lakes. In some cases old drainage channels have been obliterated, in other cases water has been diverted, and throughout the area swamps have been drained and the water table has dropped."
Many watersheds have been similarly altered within the historical range of eels in Ontario, making reconstruction of their historical distribution complex. The examination and integration of ATK, archaeological information, historical records and local community knowledge has been especially important in building an understanding of the historical distribution and abundance of eels in the province.
Although eels are at the extremity of the species' range in Ontario, they were once widely distributed, abundant and important in the province (MacGregor et al. 2009). Archaeological records show eel remains extending throughout the Lake Ontario, St. Lawrence River and Ottawa River watersheds. Fish bones in archaeological contexts are preserved in the alkaline soils found in southern Ontario, but not in the acidic soils of the Canadian Shield further north. Figure 3 shows archaeological sites in the southern part of the historical eel range where eel bones have been identified in faunal analyses. Since most archaeological sites in Ontario are not subject to faunal analyses, only a fraction of the known sites provide data on the presence of eels. The site shown on the Ottawa River is within Québec, but close to the provincial border. Some circles represent two sites in close proximity. Most sites have fewer than five eels, often only one eel.
Eel bones have been found at some sites that may be outside historical American Eel range, as in the Lake Simcoe watershed, where the eel may have been transported to the site by human agency. Sites on the St. Lawrence River and Ottawa River have evidence of being used as eel harvesting and/or processing sites for the transport of eels elsewhere. (Data for site locations was provided by W. A. Allen, Heritage One based on the Ontario Ministry of Culture database.)
Two archaeological sites more than 4,000 years old at the base of an Ottawa River rapids yielded substantial eel remains (Clermont and Chapdelaine 1998, Clermont et al. 2003). A complex of stone weirs and pools was documented in 2007 in the rapids just upstream from these sites (W. A. Allen, unpub. data). At this stone weir complex a ground slate tool of a style dating to at least 4,000 years of age also was recovered (W. A. Allen, unpub. data). An association between the harvesting weirs and the nearby archaeological sites is likely.
There are numerous accounts of waters of the St. Lawrence, Ottawa, Mississippi, Clyde and Mattawa Rivers shimmering in the moonlight with young eels during their upstream migration (L. McDermott, pers. comm. 2009, H. Lickers, pers. comm. 2009). These observations reflect high recruitment events into Ontario waters. Early records and ATK reveal high abundance of eels in many inland watersheds of Ontario, sufficient to support local commercial fisheries (MacGregor et al. 2009). For instance, Québec commercial eel harvests from the Ottawa River ranged from 3.4 to 15.0 metric tonnes annually between 1930 and 1937 (Dymond 1939). Commercial harvest records for the North Bay District waters of the Ottawa River show thousands of pounds of eels harvested during the period 1924 to 1938, peaking at 4,027 kg in 1932 (OMNR 1984). When and where abundance was high, the natural tendency of yellow eels to disperse randomly would have been enhanced by density-dependent drivers.
Figure 3. Distribution of archaeological sites in Ontario with known Eel remains
Green circles show the Minimum Number of Individual eels (MNI) at each site. Yellow circles indicate location of sites. Green circles at sites which have extensive eel remains completely fill the yellow circle or extend beyond the circle. After Allen (2010).
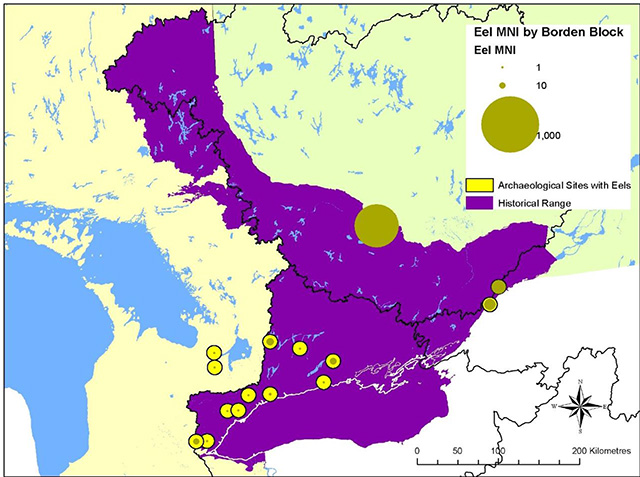
Enlarge Figure 3. Distribution of archaeological sites in Ontario with known Eel remains
While waterpower development in tributary watersheds began in about 1907 at stations such as Galetta on the Mississippi watershed, the development of waterpower facilities spanning the entire mainstem of the Ottawa River began in the middle reach in 1932, with the commissioning of Chats Falls Generating Station. By the late 1940s, commercial harvests of eels in North Bay area waters of the Ottawa River had declined to less than 200 kg annually. This steep decline follows an expected and familiar time lag of 15 or more years after construction of a barrier. However, other factors such as commercial fishing would also have contributed to the steep decline by rapidly depleting the stock once recruitment to the waters was prevented by barriers. This was the likely pattern of range contraction in the province: elimination of recruitment to former habitats by barriers, followed by depletion of the remaining stock due to commercial fishing, turbine mortality, natural mortality and natural emigration during downstream spawning migrations.
The strong contraction in the range of American Eel in Ontario continued into the 2000s (Figure 4). American Eels once were abundant in all accessible tributaries of Lake Ontario and the St. Lawrence and Ottawa River systems and have provided sustenance, material, medicinal and spiritual uses to Aboriginal peoples for thousands of years (MacGregor et al. 2008, 2009). Where eels continue to persist in inland rivers and lakes, their abundance is now very low, and eels are approaching extirpation from all inland watersheds in Ontario. Some 25 years after construction of the Moses-Saunders Generating Station, the abundance of large eels in Lake Ontario also began to decline rapidly (Casselman 2003, MacGregor et al. 2008, 2009); eel abundance is now at extremely low levels and the fisheries have been closed for conservation reasons since 2004 (MacGregor et al. 2008). The collapse of eels in Ontario is due largely to a 99 percent reduction in recruitment (Casselman 2003, Casselman and Marcogliese 2007, MacGregor et al. 2008, 2009). On a more positive note, there has been a very small but nonetheless encouraging increase in recruitment in recent years (Pratt and Mathers 2011).
Figure 4. Contraction of the distribution of American Eel in Ontario
Information used to depict the distribution of American Eel in Ontario was compiled from aboriginal traditional knowledge, local community knowledge, archaeological data and recorded captures via netting.
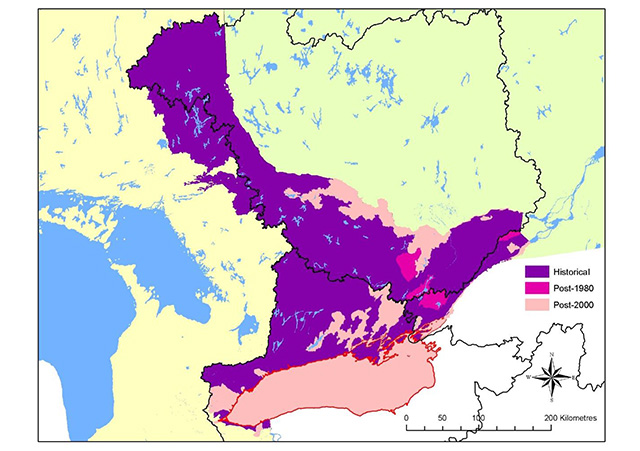
Enlarge Figure 4. Contraction of the distribution of American Eel in Ontario
Ottawa River watershed
Reviews of historical records, as well as anecdotal, ATK and archaeological information, enabled us to piece together the historical distribution of eels in the Ottawa River watershed. This information clearly shows that eels once penetrated as far north in Ontario as Lakes Temagami and Temiskaming (some 580 km from the confluence of the Ottawa River with the St. Lawrence River), and tributaries, such as the Blanche and Montreal Rivers (Purvis 1887, Reading Eagle 1902, Barlow 1907, Livermore 1914, 1915, New Liskeard Speaker 1928, Ville de Temiskaming 1996, MacGregor et al. 2011, S. Ross, pers. comm. 2011). Here eels could be very large (Reading Eagle 1902) and appeared to be most prevalent in these waters prior to the construction of large hydro-electric dams on the mainstem of the Ottawa River, and the repair of the water control structure at the outlet of Lake Temiskaming (R. Bartlett, pers. comm. 2010, D. McLaren, pers. comm. 2011, G. VanLeeuwen, pers. comm. 2011 via G. Davies). Additionally, near the turn of the 20th century, eels still occurred in tributaries of the Montreal River (a large northern tributary of the Ottawa River) as revealed when lakes such as Kerr Lake were drained as a consequence of mining activities in Cobalt, Ontario. Many eels were observed on the mud bottom after the lake was drained (Livermore 1914, 1915, Dumaresq 2006).
Further evidence of widespread distribution in the upper Ottawa River comes from reports of eels actually traversing the height of land connecting the Ottawa and French River watersheds using damp grassy or marshland areas. By these means, or via Lake Temagami/Sturgeon River, eels appeared to disperse and enter Lake Nipissing (MacGregor et al. 2011) when they were abundant in the Ottawa River watershed. An eel was recorded as late as 1969 at Sturgeon Falls at Lake Nipissing. It was thought that its origin in Lake Nipissing was via the Mattawa River/Trout Lake system (part of the Ottawa River watershed) and then overland via lowland wet grassy areas connecting Lake Nipissing to Trout Lake (Young 1970, MacGregor et al. 2011).
Eels once penetrated deeply into several Algonquin Park lakes associated with the Petawawa, Madawaska and Opeongo Rivers (tributaries to the Ottawa River system) (Mandrak and Crossman 2003). The last documented eel caught in the Park was in 1936 (Mandrak and Crossman 2003); however, a few eels have been reported caught by anglers in the Petawawa River near the boundaries of Algonquin Park as recently as 2002 (K. Punt, pers. comm. 2009). Aboriginal traditional knowledge reports several generations of a Bancroft Algonquin family harvesting large and abundant eels in Salmon Trout Lake in the Madawaska subwatershed. Eels were also once abundant in the Muskrat River and Bonnechere Rivers (K. Punt, pers. comm. 2010, MacGregor et al. 2011).
Eels now appear to be extirpated in most of Algonquin Park (Martin and Fry 1973, Mandrak and Crossman 2003) and in the watersheds of Lakes Temiskaming and Temagami, as no verified occurrences have been reported in the last 40 to 50 years or more. However, this needs to be verified by focussed assessment.
A few eels are still caught in research nets and incidentally by anglers in Lac Des Chats on the mainstem of the Ottawa River near Arnprior, Ontario (three dams up from the confluence of the Ottawa River with the St. Lawrence River), but long-time anglers from the area report very strong declines in catches (K. Punt, pers. comm. 2009). Drastic decline, and in many instances extirpation, of eels has occurred throughout tributaries of the middle and upper reaches of the Ottawa River, coinciding with the construction of hydro-electric dams (e.g., see OMNR/Québec MNRF 1999). For instance, eels have not been observed in the mainstem of the Ottawa River above Des Joachims hydro-electric facility at Rolphton for many years (OMNR 2008a, K. Punt, pers. comm. 2009). They also have not been seen in Calabogie Lake since the late 1970s when the development of waterpower production in Arnprior intensified (K. Punt, pers. comm. 2009), and now are considered extirpated from Round Lake, Golden Lake and above Renfrew Power Generation (at Renfrew) on the Bonnechere River (OMNR 2008a, K. Punt, pers. comm. 2009).
The foregoing information indicates that the American Eel used to be an integral and important component of the fish communities in the upper and middle reaches of the Ottawa River watershed. These fish communities included other top predators, such as walleye, which persist to this day, whereas the highly migratory American Eel has disappeared. If access to these reaches were restored, it is reasonable to conclude that eels would find the habitat still suitable for them, given that the less migratory species remain in these reaches. Similarly, there is no reason to doubt that suitable habitat for eels remains within Pembroke District systems (K. Punt, pers. comm. 2010, MacGregor et al. 2011), but hydro-electric facilities constructed in the early to mid-twentieth century in the Ottawa River watershed have severely limited access (i.e., three hydro-electric facilities were constructed downstream of Pembroke District on the mainstem Ottawa River during this timeframe; no other barriers have been constructed on the mainstem downstream of Pembroke District).
Other (non-hydro) barriers have been constructed on some tributaries of the Ottawa River within the district, but it is the mainstem barriers that pose the initial problem to eel passage and access to district waters. Eels are now in extremely low abundance or extirpated in most waters upstream of the mainstem hydro-electric facilities, even though young eels have been observed attempting to traverse obstructions at some mainstem and tributary hydro-electric barriers near Pembroke via old log chutes and sluices (K. Punt, pers. comm. 2009). Given the substantial barriers to migration posed by the mainstem dams in the lower reaches of the Ottawa River (including the Carillon, Chaudier Falls, Chats and Chenaux), the low current abundance of eels in Pembroke waters of the Ottawa River is not surprising.
According to local community knowledge and ATK, hydro-electric facilities pose similar barriers to eel penetration of the Bonnechere River/Round Lake watershed. There have been reports of local extirpations/drastically reduced abundance since the construction of dams associated with hydro-electric facilities within these reaches (OMNR 2008a, OMNR/Québec MNRF 1999, L. McDermott, pers. comm. 2010, 2011, W.A. Allen, pers. comm. 2010, 2011, S. Ross, pers. comm. 2011).
Eels were once numerous in the lower Ottawa River at places such as Chaudier Falls (Reading Eagle 1902), especially during migratory periods and still persist in these waters albeit at very low densities (MacGregor et al. 2009).
Mississippi River subwatershed
The Mississippi River is a large subwatershed of the Ottawa River. The American Eel was once highly abundant in this river and heavily used by Aboriginal peoples and early European settlers. By the 1980s, the species had declined to very low densities due to reduced recruitment (MacGregor et al. 2009, Casselman and Marcogliese 2009, 2010a), exacerbated by the construction of numerous hydro-electric facilities on the main stem Ottawa River and on the Mississippi River. Quantitative electrofishing in the Mississippi River in 2009 and 2010 confirmed that eels were very rare (except more prevalent at the mouth). Casselman and Marcogliese, in an unpublished review and occurrence analysis of their catch data, found that only 1.8 percent of the 112 transects sampled below High Falls produced eels and all were below the first dam at Galetta.
Aboriginal traditional knowledge supports this observation. For example, in the Mississippi River watershed, ATK confirms the presence, abundance and use of the American Eel above High Falls in the headwaters (Mazinaw and Crotch Lakes) up to the mid-20th century. The American Eel was reported present in Gull Lake (well upstream of High Falls) in the 1920s (OMNR 1971). The presence of eels in waters upstream of High Falls is further confirmed by observations that eels were harvested in the early 1900s from Ragged Chute on the Mississippi River (well upstream of High Falls) and shipped via the old K and P railway line to the Kingston fish market (Bennett and McCuaig 1981). Aboriginal traditional knowledge confirms that eels disappeared from these waters in the 1940s, some 20 years after construction of a large downstream hydro-electric facility at High Falls in 1920 (L. McDermott, pers. comm. 2009).
Aboriginal traditional knowledge further indicates that no eels have been observed above the High Falls facility since the early 1950s. Similarly, no eels have been recorded in government netting programs above High Falls since the 1950s, when Ontario began recording fisheries information in these waters. Eels continue to decline in reaches of the Mississippi River downstream of High Falls (T. Haxton, pers. comm. 2009). Extrapolation of declining trap-net catches over the past three decades in five lakes throughout the Mississippi River watershed suggests that eels have now probably disappeared from the upper half of the watershed (J. Casselman, unpub. data).
It should be noted that there are several other hydro-electric facilities on the Mississippi River downstream of High Falls that would affect eel dispersal into the Mississippi River. There is evidence that some eels have managed to find their way around the Mississippi River facilities downstream of High Falls. However, the six m tall High Falls facility is considered to be a complete impasse to further upstream migration by eels because there is no known alternate route around this barrier (Tremblay et al. 2011). As such, the High Falls barrier is considered to be the current upstream limit to eel distribution in the Mississippi River watershed. The remainder of the fish community (e.g., walleye) remains above the High Falls barrier (A. Bendig, pers. comm. 2011), so it is reasonable to conclude that suitable habitat for eels remain upstream of the High Falls facility if access was to be restored.
In recent years, intensive and extensive electrofishing surveys have been conducted in Ontario waters throughout the upper St. Lawrence River and eastern Lake Ontario, the lower Mississippi River and its watershed, and the Ontario and Quebec waters of the Ottawa River below the middle section of Lac du Rocher Fendu. The sampling, conducted at over 30 locations in the Ottawa and Mississippi river watersheds, involved a non-probabilistic index design, which provided not only local density data but also good distributional information. Casselman and Marcogliese, in a simple presence or absence occurrence analysis of their electrofishing catch data (unpublished review), examined eel distribution, assembling and combining 200-m site presence or absence data for 2009 (Casselman and Marcogliese 2010a) and 2010 (Casselman and Marcogliese 2011). These site presence or absence occurrences indicated trends that, in their professional judgment, were indicative of eel abundance throughout the lower Ottawa and Mississippi river watersheds. Below Carillon dam, 4.7 times more sites contained eels than above the dam (27.4% compared with 5.8%). This disparity was even more striking when level of effort was considered, because 4.8 times more sites were electrofished upstream of Carillon dam than below (320 compared with 69), strongly reinforcing the difference. Even though sites were not chosen randomly, electrofishing effort was intense and broadly distributed. This general summary of occurrence by site supports the present evidence of declining upstream density of eels in the Ottawa River watershed, the disproportionately greater occurrence below the first dam (which has no specifically designed facilitated passage), and the fact that eel occurrence and distribution throughout the watersheds are now at record-low levels.
St. Lawrence River and Lake Ontario watersheds
It has been recognized for many centuries that the important eel fisheries in the lower St. Lawrence River benefited, to a great degree, from eels migrating from what today would be called Ontario waters. For instance, in a 1634 Jesuit Relation (Thwaites 1896 – 1901:311, 314), the following was written regarding the eel fisheries in the St. Lawrence River in Quebec and their source from more distant northern waters:
It is wonderful how many of these fish are found in this great river, in the months of September and October, and this immediately in front of the settlement of our French…"
It is thought that this great abundance is supplied by some lakes in the country farther north, which, discharging their waters here, makes us a present of this manna that nourishes us …"
Accounts from the mid-1600s record an Onondaga fisherman of the St. Lawrence Iroquois spearing as many as 1,000 eels in a single night (Thwaites 1896 – 1901), and there are many historical and archaeological references to the large abundance of eels in the St. Lawrence River and its tributaries. In more recent times, Elder Commanda noted that his ancestors and others have talked about eels "creating great silver pathways in the rivers during migration times" (Commanda, pers. comm. 2008). Indeed, prior to the turn of the 20th century, the St. Lawrence River watershed was considered to support the most productive eel fisheries in the world (The New York Times 1880). As late as the mid-1980s, eels from Ontario were still estimated to contribute 67 percent of the eels to the important commercial eel fisheries in Quebec (Verreault and Dumont 2003). Millions of silver eels were harvested from the St. Lawrence River annually in Quebec’s long-standing tidal weir fisheries (average of 431 t annually between 1970 and 1989, COSEWIC 2006)
A large hydro-electric facility, Moses-Saunders Generating Station, was constructed across the St. Lawrence River at Cornwall, Ontario in 1958. Another (Beauharnois Generating Station) was constructed and commissioned in phases between 1930 and 1961, some 80 km downstream in Quebec and again spanned the entire river. While there are locks enabling shipping to continue, these two dams pose major barriers to upstream eel migration. An eel ladder was constructed on the Ontario side of the Moses-Saunders facility in 1974. The ladder was successful, passing as many as one million elvers per year in the early 1980s. Subsequently, two ladders became operational at Beauharnois in 2002 and a state of the art eel ladder was constructed in 2006 on the American side of the Moses-Saunders facility. A productive fishery remained upstream of the Moses-Saunders facility for at least 20 years after its construction. Many of the eels being harvested would have been resident prior to development. Despite assistance provided by the ladders in recent years, eel abundance in Lake Ontario eventually collapsed and the Ontario fisheries were closed in 2004-2005 (Ontario Government 2004, MacGregor et al. 2009).
The contribution of the St. Lawrence River eels to species-level fecundity has been estimated to range between 26.5 percent and 67 percent depending on the method used (COSEWIC 2006), and is considered to be substantial (CSAS 2011). Given the former abundance of eels in Ontario, the large size and fecundity of the province’s all-female population (Casselman 2003, Tremblay 2009), and the projected impact on species-level fecundity by eels from the St. Lawrence River/Lake Ontario, the weight of evidence indicates that Ontario holds a special segment of the global population that once contributed strongly to spawner biomass. Despite market prices well above the long-term mean in the 1970s to 2000s, commercial harvesting statistics indicate that eels have declined substantially from their former abundance in both Ontario (Casselman 2003, MacGregor et al. 2009) and Quebec (MacGregor et al. 2008, 2009, de Lafontaine et al. 2009a). The contribution of eels from the Ontario watersheds to the spawning stock has likely changed significantly as a consequence of their province-wide collapse.
Tributaries of the upper St. Lawrence River and Lake Ontario including the Gananoque (including Charleston Lake), Cataraqui (including Big Clear and Cranberry Lakes), Napanee (including Thirteen Island Lake), Salmon (including Beaver, Bull, Buck, and Kennebec Lakes), Moira (including Moira and Stoco Lakes) and the Trent-Otonabee (including Kawartha Lakes) once supported an abundance of eels (e.g., 2.1 – 11.4 tons harvested annually between 1885 and 1900) (MacGregor et al. 2009). Now eels are rarely found in any of these waters. Eels appear to have been relatively rare in the upper Trent and Otanabee Rivers/Kawartha Lakes waters since the early 1900s, coinciding with the construction of numerous dams and hydro-electric facilities (e.g., Sills Island, Sidney and Frankford Generating Stations) (MacGregor et al. 2009). While the hydro-electric facilities on the Trent River are all associated with locks that form integral components of the Trent-Severn waterway (similar to the facilities on the St. Lawrence River), it is doubtful that the locks provide adequate, safe passage for upstream or downstream migrants. Additionally, downstream migrants will follow the main flow and be subject to high cumulative turbine mortalities.
The last few reports of eels in the Kawartha Lakes region occurred in the mid-1980s. Elder Murray Whetung of Curve Lake First Nation, a carrier of ATK dating back to the 1920s in the Kawartha Lakes region, agrees with this assessment of past and present eel status in these waters (M. Whetung, pers. comm. 2009). However, eels apparently persisted much longer in waters closer to Lake Ontario. For instance, in 1980 anglers mentioned that many people were catching large eels in Round Lake on the Crowe River watershed (which flows into the lower Trent River) (C. McCauley, unpub. data) and eels continued to occur in the Moira, Salmon and Napanee Rivers until the 1970s. Moreover, annual commercial harvests of eels continued in the Cataraqui River until all commercial eel fisheries were closed in 2004. The protracted persistence of eels in the aforementioned watersheds is likely attributable to the low number of hydro-electric facilities on some of these systems (C. McCauley, pers. comm. 2009). Although there are many barriers on the Moira River, most of which were developed at one time to produce hydro-electricity, none are currently active so turbine mortality is not currently an issue in the watershed.
The total annual number of eels migrating up the ladder at Moses-Saunders Dam on the St. Lawrence River represents the longest-term data set on American Eel recruitment (Castonguay et al. 1994, Casselman et al. 1997a, Casselman 2003). After a peak in 1982 to 1983, ladder counts dropped sharply and fell to record low levels in the late 1990s (Figure 5). The few eels that ascended the ladder in the 1990s were much larger and older than typical recruits before the decline (Casselman 2003). Although recruitment has increased slightly in recent years, it still remains at minimal levels (J. Casselman, pers. comm. 2009).
Figure 5. Total number of eels ascending the eel ladder(s) at the Moses-Saunders Dam, Cornwall, Ontario for 1974 – 2011
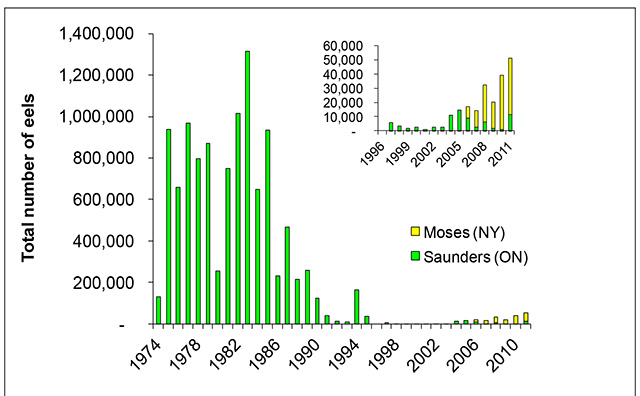
No counts are available for 1996 (A. Mathers, pers. comm. 2009). Moses is on the New York side of the St. Lawrence River, and Saunders is on the Ontario side.
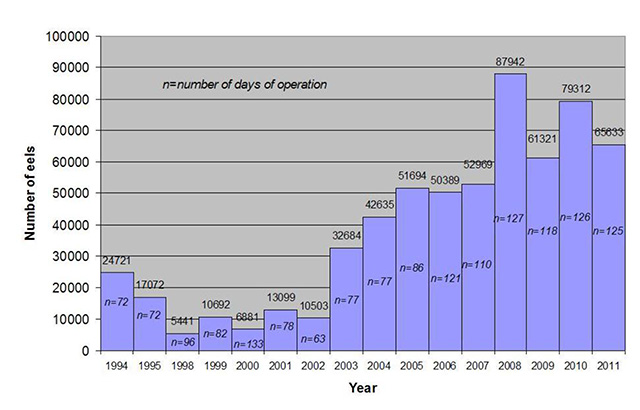
The number of eels ascending the ladders at Beauharnois has increased steadily in recent years, reaching a peak of almost 88,000 at the western ladder in 2008 (Figure 6). The number of eels ascending the Beauharnois ladders declined somewhat in 2011 to 65,633 (Figure 6). Once eels have traversed the ladders at Beauharnois, they enter Lake St. Francis (downstream of the Moses-Saunders Generating Station). Lake St. Francis appears to be the only remaining area in Ontario where eels are still moderately abundant (A. Mathers, pers. comm. 2010). The fact that the number of eels ascending both Beauharnois and Moses-Saunders ladders has been increasing recently (albeit still at extremely low numbers relative to the early 1980s; Figure 5) after the introduction of management actions aimed at reducing, and then eliminating eel fishing in Ontario (MacGregor et al. 2008, 2009), is cause for some optimism for the success of future recovery efforts (Ontario Government 2004).
Figure 6. Total number of eels ascending the western eel ladder on Beauharnois Generating Station, St. Lawrence River, Province of Quebec (1994 – 2008)
Note: counts from 1994 – 2002 represent the number of eels climbing an incomplete ladder, then captured in nets and transported above Beauharnois (after Guillemette, S. and D. Desrochers, 2011).
Niagara watersheds
At the westernmost extremity of American Eel range within Ontario (Niagara Area) eels were once abundant along the Lake Ontario shoreline and within the lower Niagara River. Bartram (1751:92) observed that "Below the Falls in the holes of the rocks, are a great plenty of Eels, which the Indians and French catch with their hands without other means". Gill (1908:121) noted that "at the proper season you will find them [eels] by the cartloads, by millions upon millions", and Goode (1881:83) observed that "the visitor who enters under the sheet of water at the foot of the falls will be astonished at the enormous number of young eels crawling over the slippery rocks and squirming in the seething whirlpools", indicating that they were clearly impeded by the falls. Eels were also plentiful within Martindale Pond and Jordan Harbour and were found in many inland watersheds of the Niagara Peninsula. While eels are now rare in these areas, the occasional eel has been captured over the last two decades in Twelve Mile Creek (MacGregor et al. 2011, A. Yagi, pers. comm. 2009).
Introductions
Niagara Falls apparently is the natural limit of American Eel distribution in the Great Lakes, and the species was considered absent from Lake Erie waters prior to the opening of the Welland Canal in 1829 (Trautman 1981). Eels probably gained access to Lakes Erie, Huron and Superior through the Welland Canal (Scott and Crossman 1973), but have never been very abundant in these waters. While there are reports of some commercial harvests of eel in the upper Great Lakes as early as 1907, and Lakes Erie and St. Clair as early as 1914 (D. Coulson, pers. comm. 2010, K. Punt, pers. comm. 2010), it is unlikely that Lake Erie and the upper Great Lakes formed part of the historical range, given the formidable obstacle posed by Niagara Falls. These harvest reports could be as a result of the following.
- Eelpouts (Lota lota) or Sea Lamprey (Petromyzon marinus) were misreported in commercial catches as American Eel.
- Stocking eels in Lake Erie was carried out by Ohio. Trautman (1981) gives accounts of the Michigan Fish Commission stocking eels from the Hudson River into southern Michigan waters as early as 1878 and of the Ohio Fish Commission stocking eels from the Hudson River beginning in 1882. For more than a decade thereafter eels were liberated into Ohio waters. In 1887, the annual Ohio Fish Commission began to mention the capture of eels in many Ohio localities, especially in the Lake Erie drainage where the species had been formerly rare (Trautman 1981). Anecdotal information reported abundant catches in Maumee Bay and Sandusky River below dams at Fremont. These catches were reported from 1895 and 1910 (Trautman 1981).
- Access was provided by the opening of the Erie and Welland canals. There are several accounts of large (three to four feet long) American Eel being captured in the lower Grand River in the mid to late 1800s well after the Grand River feeder canal was constructed in 1829 to supply water to the original Welland Canal (Dunnville District Heritage Association, pers. comm. 2012).
Occurrences of American Eel in the Great Lakes above Niagara Falls (Lakes Erie, Huron and Superior) apparently are the result of stocking and/or dispersal through the Erie and Welland canals and for now should be considered as introductions outside the historical range (Scott and Crossman 1973, Trautman 1981, COSEWIC 2006). Nevertheless, given their propensity to use damp substrates to surmount obstacles, the possibility that some eels may have found access somewhere over the Niagara Escarpment to Lake Erie and were historically native to Lake Erie and the Upper Great Lakes warrants further investigation. Archaleolgical investigations of the Grand River (a major tributary to Lake Erie), have revealed eel bones but these are interpreted to have been transported by First Nations from Lake Ontario/Niagara River (G. Warrick, pers. comm. 2011). Access by possible routes identified by MacGregor et al. (2011) from the Ottawa River to Lake Nipissing and then via the French River to Lake Huron should also be investigated (see p.15). In any event, any eels above Niagara Falls would likely follow the main flow of the river and be forced to free-fall over Niagara Falls. As the height of the falls is well above the height at which they would survive, in all probability they would die from the fall (see Section 1.6: Mortality During Dowstream Migration – Free Fall). Eels in the upper Great Lakes and Lake Erie therefore would not currently nor in historical times naturally contribute to the spawning population. It is doubtful that there is sufficient flow to attract many downstream migrants into the Welland Canal, but this should be investigated.
While eels were caught in Lake Simcoe from time to time in the mid-late 1900s, they generally were not considered native to the lake. Rather, their presence in the lake was considered to have been facilitated by the development of the Trent-Severn waterway. However, recent field checks in the Balsam Lake area, ATK and archaeological evidence suggest that eels may well have been native to Lake Simcoe (MacGregor et al. 2011). A yellow eel was found in the fall of 2010 in Lake Simcoe. If it arrived naturally, its most likely route was from the Bay of Quinte in Lake Ontario, upstream through the Trent/Kawartha Lakes canal system. To arrive in Lake Simcoe it would have needed to traverse a complex of more than 40 dams and locks (MacGregor et al. 2011). It appears that there were at least two low-lying marshy areas bordering the Talbot River tributary to Lake Simcoe where eels could easily have crossed watershed boundaries (namely at Corson Marsh and Grass Creek Marsh). Balsam Lake is mentioned in an ATK story published in 1914 (George 1914). There are several pre-contact archaeological villages in the Balsam Lake area but potential associations with eels have not been studied. Eel remains have been found in small numbers in an archaeological context at Lake Simcoe (B. Allen, pers. comm. 2010).
Summary
Although eels have virtually disappeared from many inland waters of Ontario, they are still present provincially, primarily in the downstream reaches of some watersheds (lower Ottawa River and its tributaries, lower Trent River, the upper St. Lawrence River, and in Lake Ontario). In all instances, densities are very low (Casselman 2003, Casselman and Marcogliese 2008, 2009, 2010a, 2010b, MacGregor et al. 2009). Lake Ontario and the upper St. Lawrence River remained the last provincial stronghold for eels into the 1980s, and the steep decline of the species in these waters since the mid-1980s has been well documented and publicized (Casselman et al. 1997b, GLFC 2002, Casselman 2003, Dekker et al. 2003, Hoag 2007, Lees 2008, MacGregor et al. 2008).
The precipitous decline of American Eel in Ontario waters is likely a significant threat to the status and recovery of the global population. Ontario’s eels, being virtually all female and the most fecund within the species' range (Casselman 2003, COSEWIC 2006, Tremblay 2009), have formed an especially critical segment of the global population. A growing conservation concern for eels has arisen in part because production from the USLR-LO system has declined to two percent of 1980s levels, and this production consisted entirely of large females whose contribution to the panmictic spawning stock is considered to have been historically significant from a biological perspective (CSAS 2011). Because of its widespread, panmictic nature, the American Eel is considered by NatureServe (2011) to be globally secure (G4) and the species has not been officially listed in the United States as threatened or endangered. However, the species is evidently declining or has been extirpated from parts of several watersheds (e.g., statewide in New Mexico; the Susquehanna River in Maryland and Pennsylvania; the James River in Virginia; most central and western parts of Texas; statewide in Arkansas) (Robison and Buchanan 1988, Chilton 1997, Richkus and Whalen 1999, MacGregor et al. 2009, Natureserve 2011), and is considered highly vulnerable by Natureserve (2011) because of its slow maturation rate and semelparous lifecycle (reproducing only once before death). Additionally, there is strong evidence of overfishing in Chesapeake Bay (Weeder and Uphoff 2009) and major declines in the Potomac River (Fenske et al. 2011). Moreover, there are indications that yellow eel abundance in the eastern United States may be at historically low levels (ASFMC 2006). Because of panmixia, local recruitment may not in some instances be related to local spawner abundance (Avise 2003, Wirth and Bernatchez 2003). However, if a particular region contributes a substantial fraction of total spawner output, then a decreased escapement from that region could affect subsequent recruitment to that region (Chaput and Cairns 2011). Because the dispersal of young eels from the Sargasso Sea can be influenced by large pulses of young eels (Casselman 2003), recovery of abundance and distribution within the distant waters of Ontario may depend significantly on improved production and enhanced density-dependent dispersal of recruits from the Sargasso Sea. This in turn will be influenced by the number of mature female eels that return safely to the Sargasso Sea and spawn successfully.
Detailed mapping of American Eel occurrence in Ontario is available at the Ontario Ministry of Natural Resources District level in MacGregor et al. (2011).
1.4 Habitat needs
Eels spawn in the Sargasso Sea (Schmidt 1922), east of the Bahamas and southwest of Bermuda (25°N; 60°W; McCleave et al. 1987), but habitat requirements for spawning and incubating are poorly understood. Kleckner and McCleave (1988) related the northern limit of spawning by Atlantic eels (Anguilla spp.) in the Sargasso Sea to thermal fronts and surface water masses, with spawning taking place south of east-west thermal fronts that separate southern Sargasso Sea surface water from mixed Subtropical Convergence Zone water to the north.
The American Eel uses a broad diversity of habitats during its growth period (Helfman et al. 1987). These eels occur naturally in perhaps the broadest diversity of habitats of any fish species in the world (Helfman et al. 1987, Moriarty 1987). However, cumulative anthropogenic impacts in fresh water have severely affected their historical freshwater abundance and distribution in North America (MacGregor et al. 2009). During their oceanic migrations, eels occupy salt water, and in their continental phase, they use all salinity zones. In their continental growth phase, marine habitat use appears limited to shallow, protected waters. Survival is affected by environmental conditions in any habitat (oceanic, estuarine, freshwater) occupied during any life cycle phase. Growing eels are primarily benthic, utilizing substrate (rock, sand and mud), bottom and woody debris, and submerged vegetation for protection and cover (Scott and Crossman 1973, Tesch 1977). Vegetation (e.g., eel grass) and interstitial spaces consisting of rock piles, logs and other complex structures are important to eels as cover, particularly during daylight hours, and should be protected as habitat. Given the high abundance of eels often observed in tributaries, tributary waters seem to be a very important component of eel habitat (Machut et al. 2007). Habitat in tributaries is often of high quality and less disturbed than other areas (Machut et al. 2007).
The plasticity in habitat use patterns is a strategy that allows eels to colonize a wide variety of ecosystems at the scale of the species' geographic range, conveying a remarkable "bet-hedging" strategy to the species (Daverat et al. 2006) that maintains strong resilience to anthropogenic or other environmental change. However, obstacles such as dams and hydro-electric facilities constrain habitat use patterns (Cairns et al.2004, Daverat et al. 2006). The construction of dams and hydro-electric facilities in fresh water has grown significantly in Ontario over the past century, as it has in Québec and other regions of North America (Machut et al. 2007, MacGregor et al. 2009), constraining the amount and diversity of habitat available to eels. The impacts of dams and hydro-electric facilities on access to fish habitat is well documented (Hitt et al. 2012); for instance, up to 84 percent of riverine habitat in the U.S. eastern seaboard and Lake Ontario drainages are upstream of dams (Busch et al. 1998) and the situation is similar in Canada (MacGregor et al. 2009).
Our reconstruction of historical distribution suggests that, for the most part, natural obstructions such as waterfalls did not pose complete impasses to upstream colonization by eels in watersheds within the historical range in Ontario, including the Ottawa River, Trent River, and the USLR-LO watersheds, with the likely exception of Niagara Falls. Anthropogenic barriers, on the other hand, occur throughout eel habitat in Ontario; where they occur, these barriers can be complete obstacles to upstream migration and dispersal of eels unless other routes around the structures are available (Tremblay et al. 2011). The ability of eels to overcome obstacles is size-dependent. Small eels (<10 cm long) can creep up damp vertical barriers (Legault 1988), but larger eels generally cannot bypass dams or large waterfalls (McCleave 1980, Barbin and Krueger 1994). Hence, larger eels attempting to move upstream require unobstructed passage or eel ladders (Moriarty 1987). Connectivity among important inland habitats is important to ensure eels are able to disperse effectively and take advantage of the diverse growth and maturing aquatic habitats in the province, thereby strengthening resilience in the sub-population. Additionally, safe and adequate passage to and from the oceanic spawning grounds is required to complete the life cycle.
In fresh water, eels are predominantly sedentary (Feunteun et al. 2003). Otoliths, or ear stones, can provide a chemical environmental history for eels. Casselman (1982) analyzed strontium/calcium ratios in eel otoliths to document migratory history – ocean life, immigration into the St. Lawrence River, and residency in Lake Ontario. Recent investigations using otolith microchemistry (Jessop et al. 2002, Cairns et al. 2004, Thibault et al. 2005) report three main movement patterns related to coastal waters: (1) saltwater residency; (2) freshwater residency; and (3) inter-habitat shifting. In the St. Jean River on the Gaspé Peninsula, some freshwater resident eels exhibit nomadic behaviour, performing very short intrusions into brackish or salt water (Daverat et al. 2006). Otolith chemistry has shown that some eels spend their entire life cycle in the ocean, making it clear that not all eels exhibit catadromous life history strategies and that catdromy in eels is facultative (Tsukamoto et al. 1998, Jessop et al. 2002, Morrisonet al. 2003, Arai et al. 2004). However, the proportion of non-catadromous eels remains un-quantified.
Temperate anguillids are well known for their phenotypic plasticity of habitat use in fresh water (Helfman 1987, Daverat et al. 2006). At large spatial scales, American Eel occurrence does not appear to be strongly related to habitat features (Smogor et al. 1995) and eels are frequently reported as habitat generalists (Bain et al. 1988, Daverat et al. 2006) in freshwater. However, there is a need for more specific information on habitat use at finer scales of resolution. At broad geographic scales, eels generally do not show consistent preferences for habitat type, cover, substrate, water temperature, or density of predators (Hawkins 1995, Smogor et al. 1995), but eel densities are influenced by water depth and velocity (Wiley et al. 2004). However, more is being learned of micro-habitat use by eels from smaller scale studies, and the importance of riparian areas is becoming well known (Machut et al. 2007). For instance, the importance of riparian habitat to species of eels is well documented. Riparian areas provide important sources of allochthonous prey (e.g., terrestrial insects). They are sources of deciduous leaf litter that provide very critical cover for eels seasonally, and riparian embankments that are undercut or overhung with riparian growth and in-stream debris provide critical cover and resting habitat for many anguillids and other fish species in freshwater systems (Merrick and Schmida 1984, Hicks 1997, Glova et al. 1998, Glova 2002, Pusey and Arthington 2003, Baxter et al. 2005, Machut et al. 2007).
Local seasonal movements by eels may also be driven by changing water temperature, oxygen concentration and water quality. Winter habitat requirements are poorly understood (Tesch 1977, Feunteun et al. 2003). Eels in small tributaries such as the Bonnechere River have been observed moving downstream in the fall from hard clay bottoms to areas in the lower reaches with mud or silt bottoms where eels are known to overwinter by burrowing into the mud (K. Punt, pers. comm. 2009).
Yellow eels tend to occupy home ranges in fresh water (Morrison and Secor 2003), and their normal scope of activity is within a relatively restricted area (LaBar and Facey 1983). However, some American Eels have been shown to make seasonal migrations in spring and fall, establishing home ranges in summer. Some eels may inhabit thermal refuge areas in winter (Hammond and Welsh 2009).
Finally, it is important to note that ATK, local community knowledge, archaeological information, historical records and scientific papers all document the remarkable and regular behaviour of large and small eels leaving the water and moving considerable distances along damp substrates such as moss, grass, rocks and cement. Large and small migrating eels often have been found in gardens and wriggling through wet grass alongside many migratory corridors, including the Ottawa River and St. Lawrence River (Meek 1916, Haro et al. 2000, H. Lickers, pers. comm. 2009, K. Punt, pers. comm. 2009), further emphasizing the importance of riparian areas to eels.
Habitat use by eels appears to be extremely diverse and access to a diverse array of habitats is fundamental to the resilience of eels in future environmental or other anthropogenic changes (Secor 2007, 2010, Secor and Kerr 2009, MacGregor et al. 2009). In addition, there may be important micro-habitat requirements that have not been considered. For example, eels typically overwinter in soft substrates where they burrow into the upper layers of sediment (Jessop et al. 2009). These wintering grounds may be quite specific and need to be located and evaluated in Ontario waters where eels are still present.
1.5 Limiting factors
Panmixia and global population changes
According to current science, the American Eel consists of a single genetic population in which all individuals of the species mate randomly at the same spawning site in the Sargasso Sea. As a result, factors (biological, ecological and anthropogenic) outside the range of the eel in Ontario have the potential to limit recovery within Ontario. Some 25 jurisdictions have management responsibilities for American Eel in North America (MacGregor et al. 2008). Hence, the conservation and management of American Eel will require bi-national and inter-jurisdictional cooperation (MacGregor et al. 2008, 2009, Velez-Espino and Koops 2009). However, because Ontario supports a very important sub-population of eels having high reproductive value, Ontario actions alone can significantly affect the status of eels in Ontario regardless of their panmictic nature. If a particular region contributes a substantial fraction of total spawner output, then decreased escapement from that region could affect subsequent recruitment to that region (Chaput and Cairns 2011). Of course, panmixia also means that Ontario eels will benefit as a result of global conservation efforts. Moreover, because of the high reproductive value of the USLR-LO sub-population in Ontario, New York and Quebec, actions in Ontario may negatively or positively affect recruitment to other jurisdictions.
Because eels are panmictic and dispersal of young eels to Ontario waters is somewhat density-dependent, a decline in the global population of American Eel (especially spawners) could lead to reduced density of young eels and hence reduced dispersal of young recruits to Ontario at the extremity of the range where declining recruitment has been most noticeable (Casselman 2003).
Larval dispersal
Leptocephali are not very mobile for a period of time and somewhat dependent at this life history stage on ocean currents for their dispersal from the Sargasso Sea to continental waters. The potential effects of ocean currents on recruitment have been described by Friedland et al. (2007), Bonhommeau et al. (2008) and Miller et al. (2009). However, other factors such as fishing mortality may disrupt the ability of spawners to reach the Sargasso Sea and must be considered as possible contributors to recruitment declines (Miller et al. 2009). Anguillid eel populations can likely survive wide ranging changes in oceanic and continental environmental conditions, considering that Atlantic eel species have survived extreme conditions such as ice ages since their evolution millions of years ago (Miller et al. 2009).
1.6 Threats to survival and recovery
Several threats need to be addressed to achieve recovery of eels in Ontario. The impact of each of these threats on eels has not yet been fully quantified in all watersheds. A model developed to examine the cumulative effects of anthropogenic mortality on eels found that fishing, followed by turbine mortality, were significant factors affecting eels, and that eels were sensitive to the effects of habitat exclusion by dams (Reid and Meisenheimer 2001).
Moreover, declining abundance of female spawners is cause for widespread concern. For instance, female spawner escapement is estimated to have decreased from the Potomoc River in the U.S. by 94 percent between 1980 and 2008 (Fenske et al. 2011). Spawning stock biomass may have decreased in American Eel to levels that impair recruitment (ASFMC 2000, de Lafontaine et al. 2009a), and recently the ASFMC has declared the status of American Eel in its jurisdictions as depleted (ASFMC 2012 a,b,c). Inadequate attention to the spawning population is a common shortcoming in fisheries management (Walters and Maguire 1996, MacGregor et al. 2009). The loss of spawners is a key threat – many of the threats described below describe causes of the decline in the spawning population.
Harvesting
Throughout its range, all continental life stages of the American Eel are harvested. To date, there has been no coordinated attempt to establish a total allowable catch for the North American "stock" as a whole that would be sustainable. Ontario established quotas for eels in the 1980s but they were never achieved largely because the stock was declining so rapidly. Aboriginal peoples have a long association with the species and have harvested eels for millennia, as exemplified by archaeological evidence from Morrison and Allumette Islands in the Ottawa River (Clermont and Chapdelaine 1998, Clermont et al. 2003).
The effects of commercial fishing have been much more severe than Aboriginal fishing, both globally and within the province. Commercial harvest records cover more than a century for the upper St. Lawrence River and Lake Ontario. The total North American harvest increased from an average of 1,215 tons annually between 1950 and 1955 to an unprecedented peak of 2,915 tons in 1978 (Casselman and Marcogliese 2007). This increase in harvest was largely driven by significant increases in market and price for eels (Casselman and Marcogliese 2007, MacGregor et al. 2009). By the early 1990s, North American harvests began to decline. By 2004, eel harvests fell rapidly to 840.4 tons. This decline occurred despite sustained high prices, well above the long-term mean (Casselman and Marcogliese 2007). Overall trends in Ontario commercial harvests parallel those of Canada and the United States (MacGregor et al. 2009).
Between 1950 and 2003, Ontario commercial eel harvests averaged 80.1 metric tonnes, but rose substantially in the 1970s to an unprecedented 228.2 metric tonnes (Casselman and Marcogliese 2007), representing 20 percent of the total Canadian harvest. Ontario harvests plummeted thereafter. As harvests rose rapidly, concerns arose over the sustainability of mortalities due to fishing and turbines (Kolenosky and Hendry 1982). In 1980, an experimental quota of 270 metric tonnes was implemented for Ontario’s portion of the USLR-LO (Kolenosky and Hendry 1982). This was a quota that was set when harvests were at record-high levels. Subsequent harvests never approached this quota, a clear indication that harvests (in Ontario and elsewhere) were not sustainable, particularly in the face of other cumulative anthropogenic sources of stress and mortality.
Ontario harvests declined substantially thereafter, in synchrony with strong harvest declines across North America (MacGregor et al. 2009). There was evidence of recruitment overfishing in the USLR-LO. It is clear that the combined mortalities from commercial harvesting in Ontario and Québec were not sustainable especially when the additional mortalities due to other threats, in particular turbines, were considered (de Lafontaine et al. 2009a, MacGregor et al. 2009, MacGregor et al. in press). The Québec silver eel fisheries have also exhibited severe declines in recent years (COSEWIC 2006, MacGregor et al. 2008, 2009, de Lafontaine 2009a,b).
As noted earlier, there is evidence that over-fishing has occurred for some time in other parts of the species' range such as Delaware and Chesapeake Bay (Clark 2009, Weeder and Uphoff 2009, Fenske et al. 2011). In Ontario, the commercial yellow eel fishery was closed in 2004 and the small recreational fishery for eels was closed in 2005 (MacGregor et al. 2008, 2009). Although recent buy-outs of some eel fishermen may reduce the Quebec harvest, there is no quota in that province and yellow and silver eels are still harvested in the St. Lawrence River system by Québec commercial fishers. Glass eels are harvested by fishers in eastern Canada and the United States. The vast majority of Canada’s glass eel harvest is exported, primarily to Asia (Jessop 1997). Glass eels from Canadian fisheries are the only available source of glass eels for conservation stocking efforts aimed at maintaining and/or producing yellow and silver eels.
In the United States, the American Eel Technical Committee and Stock Assessment Subcommittee of the Atlantic States Marine Fisheries Committee cautioned that "although commercial fishery landings and effort in recent times have declined in most regions (with the possible exception of the glass eel fishery), current levels of fishing effort may still be too high given the additional stressors affecting the stock such as habitat loss, passage mortality and disease, as well as potentially shifting oceanographic conditions. Fishing on all life stages of eels, particularly young‐of‐the‐ year and in‐river silver eels migrating to the spawning grounds, could be particularly detrimental to the stock, especially if other sources of mortality (e.g., turbine mortality, changing oceanographic conditions) cannot be readily controlled." (ASMFC 2012a).
Barriers to migration
Man-made dams threaten the American Eel survival and recovery in several ways: (1) by serving as barriers to upstream migration that limit access to rearing habitats, (2) by causing mortality to mature eels during their downstream spawning migration, (3) by limiting access to tributary headwater streams (thereby reducing the number/proportion of females produced and (4) by increasing the risk of mortality as a result of delayed passage and increased density at the foot of the dams (Krueger and Oliveira 1999, Wiley et al. 2004, Larinier 2008 Machut et al. 2007, McCarthy 2008, Lasne et al. 2008, Hitt et al. 2012). While dams built for any purpose can have these effects, hydroelectric dams pose a significant threat to eels in Ontario due to their height, slope, material, turbine operation and location at the entrance to Ontario watersheds.
Dams are among the most pervasive hydrological alterations of watersheds and their effects on aquatic ecosystems are well known and documented (McCully 1996, Humborg et al. 1997, Vörösmarty et al. 1997, 2010, World Commission on Dams [WCD] 2000, Freeman et al. 2003). When dams include turbines the combined effects of the barrier with turbine mortalities, they become major impediments to eel abundance, distribution, production, survival and recovery (Beauchamp 1908, Adams and Hankinson 1928, Haro 2000, ASFMC 2000, Verreault et al. 2004, Machut 2006, Machut et al. 2007, de Lafontaine et al. 2009a, MacGregor et al. 2008, 2009, 2011, MacGregor et al. in press).
Barriers were the dominant factor in predicting eel abundance in the Hudson River, New York (Machut et al. 2007). Gephard and McMenemy (2004) and Schmidt et al. (2009) noted the importance of fish passage structures in reducing the constraints and impacts to upstream eel movements. Reduced access to headwater streams may also influence stock-recruitment dynamics by decreasing the production of female eels (Krueger and Oliveira 1999).
Within the historical range of eels in Ontario, numerous barriers have led to substantial cumulative loss in access by eels to formerly productive maturing habitat and have limited the capacity of Ontario’s waters to rear large, highly fecund females. Range contraction has been clearly documented within the Ottawa River watershed (MacGregor et al. 2009, 2011, Allen 2010). If eels were still able to access the Ottawa River in sufficient numbers as elvers and also escape the significant cumulative mortalities induced by the series of turbines in the watershed (MacGregor et al 2009), spawner biomass and population-level fecundity of eels from Ontario could improve substantially. This would have significant impact on subsequent recruitment (Russel and Potter 2003, Verreault et al. 2004, MacGregor et al. in press). Barriers to upstream migration had a greater effect on European Eel densities than distance from the ocean (White and Knights 1997). Indeed, in Ontario, local community knowledge and ATK suggest that the dams associated with waterpower facilities in the watershed were the key cause of their disappearance in Round and Golden Lakes and the Bonnechere River (L. McDermott, pers. comm. 2009, K. Punt, pers. comm. 2010).
Barriers to upstream migration
As discussed earlier, preventing access to Ontario’s diverse array of habitats has serious implications for production and resilience of the USLR-LO/Ottawa River subpopulation and by extension, subpopulations in other regions. When no passage way is provided, dams can severely impede upstream dispersal of juvenile eels (Haro et al. 2000). It has been estimated that in the United States 85 percent of freshwater habitat for migratory fish has been lost due to barriers (Lary et al. 1998). In a 1998 study, the U.S. Fish and Wildlife Service determined that eels may have been eliminated from 81 percent of their historic habitat between Connecticut and Maine due to the construction of a large number of dams (ASMFC 2000). Barriers reduced eel densities by at least a factor of 10 on the Hudson River (Machut et al. 2007). While some dams are partially passable, Hitt et al. (2012) suggests that such dams permit only a subset of the total migratory population to move upstream, influencing the sex ratios and fecundity of the population.
In Ontario, where at least 953 dams of various types and sizes exist within the American Eel’s historical range (Figure 7), reduced access to former habitat due to dams appears to be substantial. Of these dams at least 91 are for hydroelectric power generation (Figure 8).
Figure 7. Location of dams, barriers and other water control structures within the historical American Eel range in Ontario
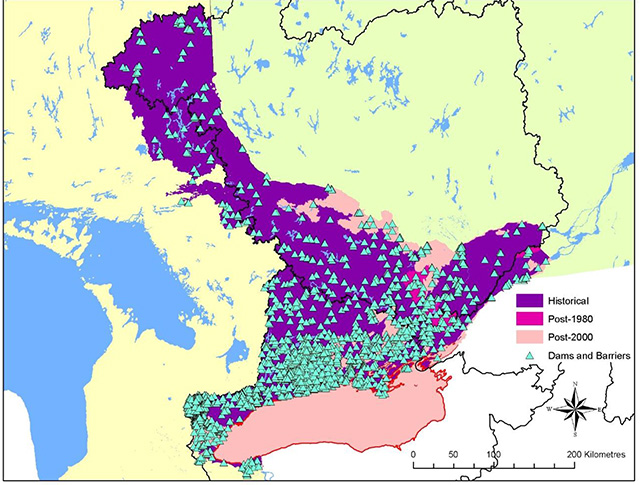
Whereas eels can surmount natural obstacles of considerable height given the right conditions (Haro 2000; Hitt et al. 2012), their capacity to climb decreases with increasing slope and eel size and is of no assistance to surmount vertical walls made of dry and smooth surfaces (Larinier et al. 2006). Concrete vertical dams are generally impassable even when of low height. Dams in Quebec were found to present a great variety of physical characteristics and to be used for all kinds of purposes (Tremblay et al. 2011). Many dams were found to be impassable by these authors, including a fair proportion of low dams (two to three metres in height), although alternates for passage may exist at some low dams. Machut et al. (2007) found that eels had some difficulty passing structures higher than two metres.
Figure 8. Hydroelectric facilities within the Ontario range of American Eel
Not all 90 hydro dams are distinctly visible on this map because the scale is about 1:2,800,000. At this scale, structures as far as one kilometer apart (for example) may not be separable by eye, despite the fact that the turquoise triangular symbol has been chosen to best show the hydroelectric dams at this scale.
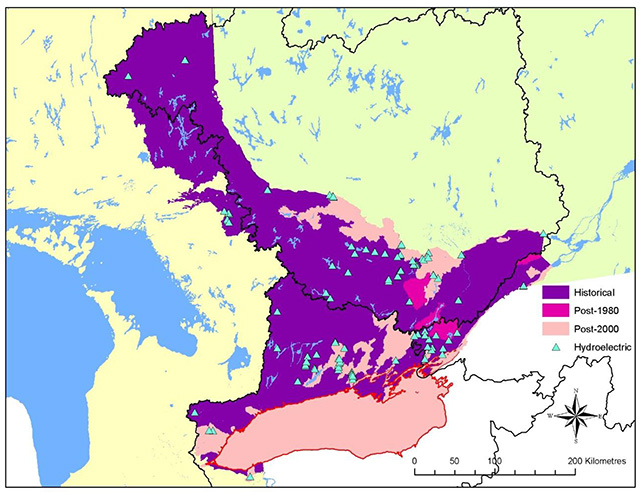
Enlarge Figure 8. Hydroelectric facilities within the Ontario range of American Eel
Data provided to the Recovery Team from the Ontario Provincial Dams Inventory Database
In terms of their height, hydroelectric dams generally pose a greater threat to upstream migration than dams built for other purposes. Hydroelectric facilities, for which there is height data within the Provincial Dams Inventory, range from 1.2 m to 63 m high, and average 9.9 m high (Figure 9). The 721 non-hydroelectric dams, for which we have height data, average 3.64 m in height, ranging from 0.40 to 40 m high (Figure 10). Based on the available data the majority (84%) of hydroelectric dams for which we had height data exceed the three metre height criterion identified by Tremblay et al. (2011) for impassability, while 50 percent of the non-hydroelectric dams are above the three meter criterion.
Figure 9. Height distribution of hydroelectric dams within the Ontario historical range of eels (average, minimum and maximum heights in meters)
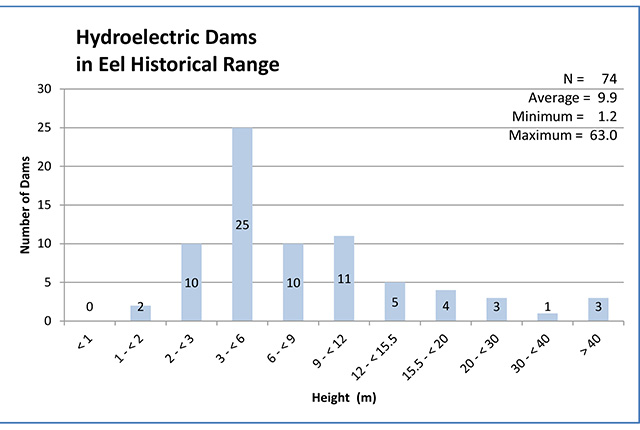
Dams that block upstream passage due to their height threaten access to all habitats upstream of their location. Consequently dams located in the lowest river reaches within a watershed, especially those on the mainstem access routes have the greatest impact. The Ottawa and St. Lawrence River watersheds are the two primary routes of penetration into Ontario’s aquatic habitats and upstream migration on the mainstems of both waterways has been seriously compromised by hydroelectric facilities. On the Ottawa River, downstream of the water control structure at the outlet of Lake Temiskaming (located some 580 km upstream of the confluence of the Ottawa River with the St. Lawrence River), six hydroelectric facilities span the mainstem of this migratory corridor. Below the Lake Temiskaming water control structure, there are no non-hydroelectric dams that span the mainstem of the Ottawa River. Similarly, the twodams that span the St. Lawrence River are both associated with hydroelectric facilities (Beauharnois in Quebec and Moses-Saunders in Ontario).
Figure 10. Height distribution of non-hydroelectric dams within the Ontario historical range of eels
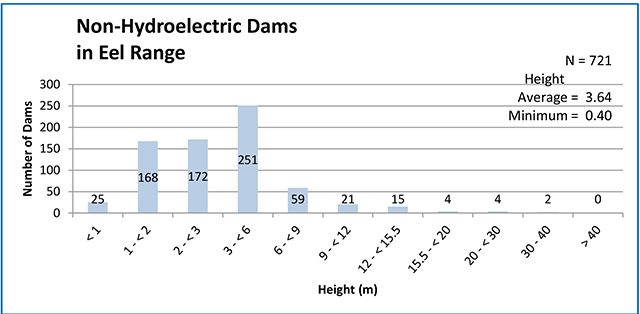
Although navigation locks are associated with the large hydroelectric facilities that are most downstream on the Ottawa River (Carillon Generating Station) and the St. Lawrence River (Moses-Saunders Generating Station), it is unlikely that they provide effective passage for eels. The passage of migratory fish through navigation locks is generally regarded as fortuitous given the low attraction flows (Larinier and Marmulla 2004). The Beauharnois hydroelectric facility was completed in 1960. Between 1960 and 1994 (when the first experimental ladder was installed on the western side of Beauharnois dam), the St. Lawrence Seaway locks at Beauharnois were the only means of passage for eels migrating further upstream (P. Dumont, pers. comm. 2011). However, the seaway locks at Beauharnois would only allow a small proportion of juvenile eel to reach the Beauharnais canal (Desrochers 2000) and the USLR-LO until the eel ladders were installed at Beauharnois.
With the exception of two eel ladders at the Moses-Saunders facility on the St. Lawrence River (one on the Ontario side and one on the American side), as of 2011 no permanent provisions for upstream fish passage for any fish species have been made at any of the hydroelectric stations within the historical range of eels. It is also highly likely that the locks and dam associated with the Beuharnois Generating Station (spanning the St. Lawrence in Quebec), limited recruitment to Ontario’s USLR-LO, at least until two eel ladders were installed at this facility in 2006. Structures associated with Beauharnois Generating Station in Quebec may have affected upstream migration into both rivers, but there are now eel ladders to enhance passage.
It is important to note that extensive quantitative electrofishing in the St. Lawrence and Ottawa Rivers in 2009 has shown that there is a disproportionate abundance of eels immediately below Moses-Saunders Generating Station on the St. Lawrence River, and the Carillion and Chats Generating Stations on the Ottawa River (Casselman and Marcogliese 2010a,b). A similar accumulation of eels below the Chambly dam on the Richelieu River in Quebec also occurred prior to retrofitting an eel ladder at the dam (Verdun et al. 2003). Clearly, eels' access to formerly important eel rearing habitat in Ontario continues to be difficult because of the numerous barriers within the historical and current species range, and remains so even where a ladder exists at Moses-Saunders (Casselman and Marcogliese 2010b).
While not all dams pose complete barriers to upstream migration (Haro et al. 2000, Tremblay et al. 2011, Hitt et al. 2012), and the impact is variable depending on the nature of the barrier and species involved, numerous structures on a single watershed substantially impede access to available habitat in many watersheds of North America (McCleave 2001, Goode 2006) and the situation in Ontario is similar (MacGregor et al. 2009). Cumulative effects on eel condition and density occur in the upper reaches of dammed watersheds (Leprevost 2007, Hoffman 2008, Lambert et al. 2011). Verreault et al. (2004) estimated that some 3,700 km2 of suitable habitat (Quebec and Ontario combined) was present before extensive dam construction throughout the Ottawa River watershed.
The loss in accessible habitat due to dam construction in this watershed equates to lost production of approximately 255,000 female silver eels per year (Verreault et al. 2004) and most of the serious impasses were created by the installation of hydroelectric facilities. This is not to imply that other dams within the current and historical range of eels do not create upstream passage problems or that eel ladders should not be placed on some, but for the aforementioned reasons, hydroelectric facilities by far pose the initial, most immediate and greatest threat to upstream migrants in Ontario, despite the fact that more than 50 percent of Ontario’s waterpower facilities are classified as "small hydro".
Mortality During Downstream Migration – Turbines at Hydroelectric Facilities
Hydroelectric facilities in Ontario pose significant challenges to eels (Larinier and Dartiguelongue 1989, Mitchell and Boubée 1992, Desroches 1995, Normandeau Associates Inc. and Skalski 1998, Haro et al. 2000, Dönni et al. 2001, in International Council for Exploration of the Sea [ICES] 2003, McCleave 2001, Allen 2008b), as they impart serious individual and cumulative mortalities to downstream migrants en route to spawn (McCleave 2001, MacGregor et al. 2009, MacGregor et al. in press). Thirty of the hydroelectric facilities in the eel range in Ontario are within the current (post-2000) range and are still affecting eels today (Figure 8). As of 2009, some of the facilities that have been studied recently in the Ottawa River watershed continue to cause annual turbine mortalities of eels and other fish species (including the threatened Lake Sturgeon) (Community Stewardship Council of Lanark County 2010, A. Bendig, pers. comm. 2009, K. Punt, pers. comm. 2009, MacGregor et al. 2011). Many of the hydroelectric facilities on other Ontario watersheds have not yet been studied for turbine mortalities, but turbine mortalities likely occur from time to time on at least the Trent River (MacGregor et al. 2011), and wherever else eels manage to find a way upstream of hydroelectric facilities. With the exception of recent trap and transport and stocking (translocation) efforts at Moses-Saunders (Stanley and Pope 2009, Threader et al. 2010), mortalities due to turbines at Ontario’s hydroelectric facilities continue with no attempt to mitigate them (e.g., Ottawa River, Trent River, Mississippi River) (MacGregor et al. 2011).
When eels were abundant in North American watersheds, entanglement in turbines was sufficient to cause major operational difficulties or complete shutdowns of power plants and mills. These mortalities have been ongoing for decades at many facilities (MacGregor et al. 2009). The following quote from a 1902 newspaper article regarding a large sawmill at Chaudier Falls (a site where hydroelectric facilities now are installed to serve the City of Ottawa) paints a vivid picture of the large number of eels once passing downstream in the Ottawa River at that time:
Hull, Canada: A turbine mill wheel which runs a gang of saws at the Chaudier waterfall stopped suddenly. Upon shutting down the mill and unscrewing the upper cap, it was discovered that the wheel had become packed full of eels. It looked as though there must have been hundreds of thousands of them." Reading Eagle 1902, St. John Daily Sun 1902
Similar situations occurred elsewhere in the Ottawa River watershed as illustrated in the account by Burnett (2007). While modern hydroelectric turbines are much different than the sawmill described above and recognizing that the "hundreds of thousands" mentioned in the quote could have been a colourful exaggeration, it is clear that large numbers of eels once migrated through the Ottawa River and that many forms of turbine mortality have been occurring for decades. The effects of turbine mortality combined with commercial harvesting, have accumulated over the past century to significantly affect spawner biomass.
Cumulative mortalities of eels passing through a series of hydroelectric facilities on smaller watersheds can also be very high, at times approaching 100 percent. For instance, Dönni et al. (2001, in ICES 2003) estimated an average annual mortality of 92.7 percent for European Eel in the River Rhine for a succession of 12 hydroelectric facilities in Germany. This, combined with the evidence provided in MacGregor et al. 2009 and MacGregor et al. in press, suggests that cumulative turbine mortalities imposed by the series of facilities on the Trent and Ottawa Rivers are likely to be very high. While the American Eel has declined substantially in abundance in inland watersheds of Ontario, electrofishing and tailrace surveys in 2009 and photographs submitted to the Ontario Ministry of Natural Resources in 2007/2008 have demonstrated that eels are still being killed by hydroelectric facilities in the Ottawa, lower Trent, and Mississippi Rivers (A. Bendig, pers. com. 2009, Community Stewardship Council of Lanark County 2010, MacGregor et al. 2011). On the St. Lawrence River, the cumulative turbine mortality of eels at just two facilities (Beauharnois and Moses-Saunders) during their downstream spawning migration has been estimated to be 41 percent (Desroches 1995, Normandeau Associates Inc. and Skalski 1998). These mortalities are all of course, large females.
On the Ottawa and Trent River watersheds, there are many facilities that eels would need to traverse during their downstream spawning run (MacGregor et al. 2009). MacGregor et al. (in press) have demonstrated the significant impact of cumulative effects faced by an eel living in Mississippi Lake when she attempts to reach the Sargasso Sea to spawn. The probability of the eel surviving passage through the series of turbines enroute to enter the St. Lawrence River was shown to be as low as 2.8 percent.
Since the closure of commercial and sport fisheries in Ontario in 2004 and 2005 respectively, hydroelectric turbines are the greatest anthropogenic source of eel mortality in the province (Pratt and Mathers 2011). No efforts to address downstream passage issues apparently were required nor attempted at any facility, until pilot trap and transport efforts at Moses-Saunders began in 2007 (OMNR 2008b). The duration of unmitigated impact therefore has been nearly a century at many facilities, and the impacts have accumulated. Additionally, at least 15 proposals for new facilities are known and others may be forthcoming within the historical range of eels in Ontario. If constructed without mitigation they would serve to increase the cumulative mortality. Within the current restricted range this would serve to further reduce the population and impede the potential for recovery.
Ontario’s eels are very susceptible to turbine mortality as a consequence of their large size. This selective mortality exacts a heavy toll on sexually mature downstream migrants (Goode 2006), resulting in far higher mortality rates for female eels than if Ontario’s subpopulation was comprised of a higher proportion of males. As female eels individually contribute far more to recruitment than males and the subpopulation in Ontario is exclusively composed of large, highly fecund females, the cumulative effects of turbine mortalities on spawner biomass and subsequent recruitment could be immense. This is particularly disquieting when one recalls that those individuals that survive the turbines still need to survive the silver eel fisheries in the St. Lawrence River and also natural mortality due to predation.
The overall impact of turbine mortality has not been measured. Only direct, short-term mortality has been considered. Passage through turbines could have other major physical and physiological effects on eels that survive passage. Hence, effects that have been estimated to date should be viewed as conservative estimates, with the understanding that mortalities due to turbines would likely be higher if latent mortality due to injuries could be estimated. While turbines cause a significant mortality on adults, they may also injure and kill non-adult eels entrained in the turbines following a successful passage upstream or as a result of living in the vicinity of the dam.
Now that mortalities due to fishing have been eliminated in Ontario, there is no known source of anthropogenic mortality that is greater than turbine mortalities (Pratt and Mathers 2011). If left unmitigated, mortality caused by hydroelectric turbines will remain the major anthropogenic source of eel mortality in the province to impede recovery of the species in Ontario.
First Nations peoples have added their voice to this concern through Elder Commanda who indicated that hydroelectric facilities affect our watersheds ("Veins of Mother Earth") in a variety of ways and that we must consider their cumulative impacts. He urged caution as we proceed with more of these facilities, in order to respect the life-giving capacity of aquatic ecosystems in their entirety. Elder Commanda expressed deep concern over the impacts of dams and waterpower facilities on eel populations (W. Commanda, pers. comm. 2008).
Mortality during downstream migration – free fall at barriers
Provided that fish fall directly in the water, free-fall of eels over barriers is not thought to cause much mortality until structures reach 13 m (Larinier and Travade 1999, Tremblay et al. 2011 and references therein).
Habitat alteration
Large portions of existing accessible and historical habitat in Ontario remain suitable for phenotypically plastic eels and extensive areas of the historical habitat are protected within parklands (e.g., Algonquin and other provincial parks). Given that the remainder of the fish communities in these waters remains intact it is reasonable to suggest that the main reason eels no longer reside in such waters is because access is prevented by dams and hydro-electric facilities downstream. Portions of the historical and remaining accessible habitat may have been degraded and may be less suitable or productive than a century ago due to industrial effects (e.g., waterpower, pulp and paper mill effluent, etc.), flood and other water control structures, poor land use practices (particularly timber harvest, farming practices and urbanization of watersheds that impair stream quality and riparian zones), imposing additional potential stressors to yellow eels (Machut et al. 2007). For instance, clearing and working land to the shoreline, particularly with no buffer strips, can result in erosion and sedimentation of watercourses, leading to infilling of interstitial spaces important to eels as habitat and loss of important riparian habitat. The detrimental effects of the urbanization of riparian areas are well known, and the importance of protecting and conserving riparian habitat for eels should be apparent (Machut et al. 2007). The invasion of dreissenid mussels (e.g., Zebra Mussel; Dreissena polymorpha) may also have had some impact on eels in some waters (e.g., Lake Ontario), by increasing water clarity and forcing eels into deeper and thermally less preferred waters (J. Casselman, unpub. data).
Operation of water control structures can affect flow and water levels. This could affect the habitat and migration of eels. Water level fluctuations can negatively affect wetland habitat for eels and possibly eels directly during winter drawdown events. Alteration of important wintering habitats has not been assessed, including desiccation of these habitats during winter drawdowns of reservoirs associated with waterpower facilities, flood control and navigation. Winter drawdown of reservoirs can also cause ice scouring and removal of aquatic vegetation in the littoral zone which eels use for cover and protection in other seasons. Creation of reservoirs during the construction of a new manmade barrier can inundate and destroy wetland complexes and wetland habitat for eels.
Additionally, water management regimes can affect fish community structure (Haxton and Findlay 2009). Winter drawdowns of reservoirs can negatively affect available food for juveniles, thereby potentially affecting growth and survival of eels. This has been shown for other littoral zone benthivores such as Lake Sturgeon (Haxton and Findlay 2009); given that eels consume benthic invertebrates, the same situation may apply. Indeed, operating regimes and discharges from reservoirs that alter or reduce summer flows can negatively affect the peak midsummer upstream migration of juvenile eels (J. Casselman, pers. comm. 2009).
Reduced resilience
Reduced resilience of American eel in Ontario results from the cumulative effects of the other threats identified in this section, most notably historical harvesting and ongoing effects of dams which limit production by barring access to habitats and cause mortality during downstream migration. While a result of anthropomorphic threats, it is also a threat in itself. A reduction in resilience (the ability to recover from environmental stresses), especially in threatened species with low abundance, increases the risk of further decline with exposure to future new or ongoing stresses (threats) including natural environmental variations.
As the human population in Ontario is projected to grow rapidly (Ontario Government 2010), anthropogenic impacts can be expected to increase.
Toxicity and contaminants
Contaminants have periodically been problematic in some Ontario waters inhabited by eels, particularly in Lake Ontario and in the lower reaches of some watersheds. Elevated levels of polychlorinated biphenyls (PCBs) have been found in eels that inhabited Lake Ontario. Concentrations of Mirex, a chlorinated hydrocarbon, in Lake Ontario eels enabled researchers to determine the proportion of eels in the St. Lawrence River fisheries that came from Lake Ontario (Dutil et al. 1985). In the 1980s, the commercial eel fishery was closed for a few years because PCB concentration in eels exceeded guidelines. Studies at Queen’s University are currently examining the role that contaminants may play in the eel decline (Science Daily 2007). Recently, some retrospective evidence has been presented that the declines in recruitment of the American Eel to Lake Ontario may have been due in part to historical loadings of polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs) and PCB (Byer et al. 2010).
This does not, however, appear to explain declines in the inland waters of Ontario which began in the very early 1900s prior to the major period of industrial contamination (late 1940s to late 1960s, Cook et al. 2003a). Contaminants such as PCBs may have at one time affected the survival and migration success of eel embryos spawned from adults exposed to Lake Ontario waters and possibly some other localized areas where PCB use became prevalent. However, levels in Lake Ontario have been shown to have declined below toxicity thresholds (Byer et al. 2009) and may no longer be affecting eel recruitment (P. Hodson, pers. comm. 2011).
Similarly, there have been major efforts to eliminate other sources of contamination in other Ontario waters (Montreal Gazette 2005). It is important to take into consideration the fact that eels are a panmictic species and that recruitment and abundance are not likely driven by one single factor at any one location. Moreover, the waters of Lake Ontario have been heavily industrialized and urbanized over the past century and the contaminant loadings have been substantial but are now rapidly declining (LaMP 2009). As another example of water quality issues that may affect eels, substantial quantities of raw sewage still periodically enter the lower reaches of the Ottawa River from the City of Ottawa (Ottawa Citizen 2008). However, despite earlier sources of contaminants, there is little evidence that recovery of other fish species such as Lake Sturgeon (another long-lived species endemic to the Ottawa River) is being impeded by contaminant stress in the Ottawa River (Haxton and Findlay 2008).
While there have been occasional concerns by commercial fishermen over apparent die-offs of eels in the St. Lawrence River (Dutil et al. 1997), and a large oil-spill occurred in 1976 on the St. Lawrence (Save the River 2006), Dutil et al. (1997) found the health of the American Eel in the St. Lawrence was not severely impaired and observed that mortalities in Lake St. Francis might be due to the upstream hydro-electric facility.
Productivity and food-web changes
Profound ecological changes have occurred in Lake Ontario since 1970 (Mills et al. 2003). Forage species are important in production, growth and fecundity of eels, particularly in relation to maturation. Prey species important for eel production in the St. Lawrence River/Lake Ontario system (e.g., Alewife) have been in decline as a result of changes induced by phosphorus control, invasive species (e.g., Zebra Mussel and Quagga Mussel (Dreissena rostriformis bugensis), Round Goby), and other factors (Mills et al. 2003). Changes in prey availability have the potential to affect eel growth and production (J. Casselman, unpub. data). Additionally, high thiaminase levels in alewife (a favoured diet item of Lake Ontario eels) may play a role in eel reproduction and prevent recovery in Lake Ontario (J. Fitzsimmons, pers. comm. 2009, 2011) as they appear to have played a role in inhibiting recovery of other fish species in Lake Ontario (Honeyfield et al. 2012). High thiaminase levels in forage fish break down thiamine in predators that eat them, resulting in low thiamine levels in those predators. There is some evidence that impairment of swimming due to low thiamine levels may adversely affect spawning migration to the Sargasso Sea and impact reproduction if migration is delayed (D. Honeyfield, pers. comm. 2011). However, if thiamine deficiency currently limits the reproductive success of eels, it would only do so for Lake Ontario eels as alewife are not abundant in inland waters of Ontario where native forage species dominate. The effects of thiamine deficiency on the migration and spawning success of Lake Ontario eels needs further review in the context of panmixia.
Changing oceanic conditions
Climate change and other environmental shifts may alter the Gulf Stream, reducing ocean productivity and influencing the production and ability of leptocephali to drift from the Sargasso Sea to continental waters (Friedland et al. 2007, Bonhommeau et al. 2008, Miller et al. 2009).
However, past recruitment indicates that although oceanic conditions influence recruitment, changing conditions should not greatly limit recruitment or restoration. When recruitment was high prior to the mid-1970s, the effects of changing oceanic conditions were undetectable. Since then, recruitment has declined to a point where oceanic effects are now apparent. Historical evidence suggests that if recruitment were high, changing oceanic conditions would be considerably less important (J. Casselman, pers. comm. 2009). If escapement and reproductive capacity were increased, this factor would become less important. Fenske et al. (2011) suggest that declining recruitment and abundance in three distant regions of anguillid eels point to large-scale processes as an important component of eel population dynamics, noting the declines in spawning stock biomass to levels that could impair recruitment and sustainability of fisheries (ASMFC 2000, Casselman 2003, de Lafontaine et al. 2009a,b).
The concern is that the cumulative anthropogenic effects of such factors as over-fishing and turbine mortalities may have destabilized the eel population, making it more sensitive and less resilient to changes in environmental conditions and other perturbations (Bonhommeau et al. 2008, MacGregor et al. 2009). Similarly, poor conditions for survival and growth of other species such as salmon may also have become more common in the marine environment (Friedland et al. 2003; Lawson et al. 2004), thus increasing the impacts of environmental perturbations in fresh water (McCormick et al. 2009). For this reason, continued global warming is an additional concern (Miller et al. 2009).
The foregoing information highlights the importance of reducing human-induced mortality (particularly on large females), and the need to boost production and resilience of the American Eel regardless of the influence of variations in environmental conditions. The following quote is appropriate in this context (Secor 2010):
If we manage for recovery of a freshwater life cycle for eels, which itself shows large diversity then we promote varying outcomes, thereby minimizing risks due to future environmental changes. Here, for instance, we know that freshwater eels often show great longevities that could span periods of poor oceanic conditions."
Environmental effects on recruitment of fishes are well known (e.g., Christie and Regier 1973, Richards et al. 1996), but they are far from predictable (Myers 1998). While there is some thought that changes in oceanic conditions may influence American Eel recruitment (Freidland et al. 2007, Bonhommeau et al. 2008), they are poorly understood and unconfirmed. When correlations with the environment have held up over time for other species, this does not mean that spawning biomass is not important as well. Even if an environmental variable is found to be important, it does not mean it is key to the management of the population. The emphasis on the search for environmental correlations may have led to neglect of other important processes such as the relationship between spawner abundance and recruitment (Myers 1998). Moreover, while humans have little direct control over oceanic and other environmental effects, mortalities due to fishing, turbines and other anthropogenic threats such as contaminants can be reduced to promote adequate spawner escapement (Fenske et al. 2011) and reproductive success (e.g., as in the case of PCB reductions in Lake Ontario).
Both Canada and the European Union have recognized that while shifts in ocean currents may influence annual recruitment to continental waters (Miller et al. 2009), recruitment will be dependent on the biomass of spawners, and there is a clear need to improve production, reduce anthropogenic sources of mortality and enhance escapement of spawners (DFO 2004, EU 2007, CEWG 2009, Brujis et al. 2009, CSAS 2011). While changes in ocean currents may, from time to time, change dispersal patterns of leptocephali to coastal waters, plenty of recruits were available in coastal waters during unfavourable events when spawners were abundant (J. Casselman pers. comm. 2009).
Parasites
An exotic, parasitic bladder worm, Anguillicoloides crassus, that may negatively affect eels has been introduced into United States waters (Fries et al. 1996). This parasite has now been detected in several streams of the Maritime provinces, and very recently three eels in the USLR-LO were found to be infected (A. Mathers, pers. comm. 2010). In the European Eel, the parasite is thought to negatively affect silver eels during spawning migration (Sjoberg et al. 2009 and references therein). Policies and procedures have been implemented to restrict its spread into Ontario during stocking and other transport events (Williams and Threader 2007). By enabling eel access to fresh water through such provisions as eel ladders and dam removal, stocking of parasite-free eels can have the effect of lowering infestation rates (Schmidt et al. 2009). Infestation rates are lower for inland American Eel (Machut and Lindburg 2008) probably because transmission from secondary hosts is reduced (Schmidt et al. 2009).
Cumulative effects
Because eels migrate across an extensive geographic range and have a complex life cycle, the cumulative effects of multiple stressors accrue across the range (MacGregor et al. 2009). The cumulative effects of habitat loss and degradation must have reduced the effective population size of the species in the decades leading up to the declines (Miller et al. 2009). As eels are panmictic, the impacts of commercial fishing, turbine mortalities and lost access to habitat due to dams throughout the species' range, and the effects of those impacts (i.e., lost production due to lost habitat and mortalities due to fishing and turbines), accumulate on one common stock. These cumulative effects have been and continue to be substantial in Ontario and in many regions of North America (Busch et al. 1998, Machut et al. 2007, MacGregor et al. 2009, MacGregor et al. in press, NatureServe 2011). Other factors such as contaminants, ecosystem change and climate change are less understood but may add to the aforementioned effects (Castonguay et al. 1994).
The cumulative effects of reduced access to rearing and maturing habitat, combined with the significant anthropogenic mortalities (fishing and turbines) of Ontario’s large fecund females and with other possible effects such as contaminants and ecosystem change, can depress spawner biomass, reduce population-level fecundity and subsequent production of new juveniles, and reduce resilience to further anthropgenic effects and/or environmental change. This would have the effect of reducing density-dependent dispersal back to Ontario’s female rearing and maturing waters. Indeed, the evidence suggests that the decline in size of the spawning stock exiting the St. Lawrence River was not due to poor recruitment as a result of changes in oceanic conditions, but to large-scale cumulative mortality factors associated with high exploitation in Lake Ontario and to construction of hydro-electric facilities in the 1950s (de Lafontaine et al. 2009a, MacGregor et al. 2009). Certainly, the collapse of eels in Lake Ontario occurred because eels upstream of Moses-Saunders Generating Station were depleted by unsustainable commercial fishing, mortality related to hydro-electric turbines (de Lafontaine et al. 2009a), and a lack of recruitment because of barriers to upstream migration. Contaminants (e.g., PCBs) may also have played a role in the decline of eels in Lake Ontario (P. Hodson, pers. comm. 2011).
As more and more of these pressures are imposed on Ontario’s eels, unless long-overdue mitigation is implemented, recovery of the American Eel will be seriously jeopardized, and the production, escapement and resilience of eels from Ontario will continue to be severely compromised. Without mitigation, Ontario eels are at risk of declining further towards province-wide extirpation (MacGregor et al. in press).
Cumulative effects of dams and hydro-electric facilities on migratory fish species are exacting similar tolls in other jurisdictions. For instance, major rivers in the Gulf of Maine average five or more mainstem dams. The cumulative impacts of these dams are the major reason for the failure of most migratory fish restoration efforts (Goode 2006). Efforts are underway to correct the situation in the Gulf and other jurisdictions in the United States (MacGregor et al. 2011).
Cumulative effects generally have not been considered in the past during project approval processes in Ontario, as no formal mechanism currently exists provincially to include them in project-by-project decision making, or in site- or harvest-specific assessments. This may account, in part, for the decline of the eel despite federal and provincial legislative authorities and mandates to prevent it through provisions in both Canada’s Fisheries Act (Canada Department of Justice 1985), and Ontario’s Lakes and Rivers Improvements Act 1990 (Ontario Government 1990). Implementation of effective conservation measures also has been inhibited by complexities associated with: (a) governance over the lifecycle of the species (life stages of the American Eel span some 25 jurisdictions having management responsibility for the species (MacGregor et al. 2008)), (b) the shifting baseline
The split of management responsibilities between federal and provincial jurisdictions for waters within the province creates a jurisdictional quagmire (MacGregor et al. 2008, MacGregor et al. in press) and complicates conservation governance with respect to American Eel, and the effective assessment of cumulative effects. In light of growing demand for renewable energy and in view of strong human population growth projections for Ontario (Ontario Government 2010), continued lack of consideration/mitigation of the cumulative effects of dams, turbines and fisheries on the American Eel may well be one of the largest single threats to survival and recovery of the species in the province (MacGregor et al. in press). The panmictic, highly plastic life history strategy of the American Eel has enabled the species to be very successful across a wide diversity of habitats. However, panmixia also exposes the American eel to cumulative anthropogenic effects across a wide geographic range, all of which accumulate to negatively impact the single spawning stock. As the future unfolds with growing anthropogenic effects, Ginawaydaganuc (a principle of Algonquin law that acknowledges the web of life and the interconnectedness of all things) serves as a reminder of the possible far reaching effects of cumulative impacts (McDermott and Wilson 2010, MacGregor et al. 2011, MacGregor et al. in press).
1.7 Knowledge gaps
Although it may appear that considerable knowledge about the American Eel exists, there remain aspects of this relatively secretive animal that are poorly understood. Given its unique life cycle, the following information should be acquired to support recovery of this species.
- Abundance and distribution in Ontario under present, as well as historical, conditions require more study. Significant progress has been made with the assistance of local community knowledge and ATK in this recovery strategy (see also MacGregor et al. 2011). Nevertheless, more work in re-discovering information on historical eel distribution and abundance would be beneficial to American Eel protection and recovery in the province. Present and future changes in abundance should be monitored in a quantitative fashion in support of the recovery strategy.
- With the exception of the two generating stations on the St. Lawrence River, the mortality rates specific to individual hydroelectric facilities within the historical range of eels are currently unknown. This information would create a more complete picture of the cumulative effects on the species. This would involve measuring downstream passage at various types and sizes of hydro-electric facilities where eels remain within their historical range. Without such specific information mortality estimates must be based on proxy mortality rates from other facilities, for example as documented by Normandeau and associates (2001), Cooke et al. (2003) and Therrien (1999).
- Downstream passage effects have been assessed only in terms of mortality. No doubt there are less obvious, but possibly equally important, physical and physiological effects on eels that survive entrainment and passage. While a great deal of work is now being carried out on downstream passage, the effects and effectiveness of mitigation options need to be studied in detail. There is much useful information to draw on in New York Power Authority (2009), Stanley and Pope (2009), Ontario Waterpower Association (2010), as well as numerous ongoing activities in the United States and Europe. In particular, light arrays and sound and other guidance mechanisms need further examination, as do trap and transfer programs, by-passes and turbine shut-downs – all of these techniques need to be better understood at different scales and sites.
- While commercial harvest has been stopped in Ontario and reduced in Quebec, the importance of continuing exploitation throughout the range is uncertain in the context of recovery of the species in Ontario.
- Limited upstream and downstream passage at obstructions in Ontario exists at some structures, even when not facilitated. The significance of this and details concerning influencing factors are uncertain.
- Eels are thought to be highly versatile in habitat association and use, but some questions about their specific habitat requirements are outstanding. The general understanding is that eels are adaptable. However, this may well be related to the fact that specific studies on habitat use and requirements have not been adequately carried out. In particular, overwintering habitat is considered to be unique but is not at all well understood. This could be an important factor limiting abundance of the species.
- Information is required on habitat impacts involving changes in behaviour, abundance, growth, survival and production due to alterations by invasive species such as dreissenids, loss of wetlands, and drawdowns caused by water control.
- Despite some significant ongoing research (e.g., at Queen’s University), the importance of contaminants to eel survival, migration, reproductive success and recruitment is poorly understood (particularly in light of panmixia).
- The role of productivity and food-web changes that are underway specifically in Lake Ontario remain poorly understood, particularly the effects of thiamine deficiency and productivity declines in native forage fish communities as they pertain to recovery potential in Lake Ontario.
- Quantification is required of the cumulative impact of habitat loss, in particular blockage of upstream passage on abundance and freshwater production of spawning females, not only in Ontario but throughout the range of the species.
- A better understanding is needed of the stock-recruitment relationships in the American Eel, particularly the role of Ontario and St. Lawrence River eels.
- The ecological, biological and conservation implications of reducing species to extremely low levels require study (e.g., the American Eel in Ontario, Atlantic Cod, Lake Sturgeon in parts of Ontario).
- Climate and environmental conditions are changing. Documentation of habitat availability and use under these changing conditions would support a better understanding. Additionally, the potential effects of global warming on eel recruitment are uncertain.
- Eels are being stocked in Ontario. The abundance of stocked eels, their distribution, possible interaction with other eels, biology, and potential contribution to subsequent recruitment are unknown.
- The potential effects of parasites such as the bladder worm with specific reference to eels in Ontario are not well known.
1.8 Recovery actions completed or underway
In 2004 the Minister of Fisheries and Oceans announced a goal of reducing eel mortality in the USLR-LO system by 50 percent within two years and called on stakeholders and jurisdictions to take the necessary measures to reach this goal. Since that time, a draft National Management Plan has been written, with input from both Ontario and Québec (CEWG 2009). Additionally, both Ontario and Québec have announced plans to undertake mitigation or offsetting measures to reduce mortality and set the scene for recovery of American Eel (OMNR 2008b, MNRF 2009); both plans are now well into implementation.
All of the aforementioned planning and implementation activities were derived from a Decision Analysis exercise carried out in 2005 to 2006 by the Canadian Eel Steering Committee for Downstream Passage and Habitat Issues and documented in Greig et al. (2006). The objective of the Decision Analysis was to develop mitigation measures to increase eel survival in the USLR-LO system. Because of their panmictic nature, MacGregor et al. (2008, 2009) called for a coordinated approach to eel conservation and recovery across jurisdictions. More recently, a proposed regional response based on mortality benchmarks has been proposed in Canada and North America (CSAS 2011, Fenske et al. 2011).
Recovery planning
The American Eel is listed under Ontario’s Endangered Species Act, 2007 (Ontario Government 2007), and under this Act hydro-electric facilities that currently harm eels within the province must ensure they are operating in a manner that complies with the Endangered Species Act and associated regulations. Quebec, Ontario, and Fisheries and Oceans Canada (DFO) have been working on a National Management Plan for the American Eel (CEWG 2009); a draft has been prepared, but finalization has been delayed pending agency comments. The Plan includes a draft framework for eel recovery in the USLR-LO segment of the eel range, and a formal Memorandum of Understanding to develop coordinated management and science approaches for eel conservation across the North American range. The ASMFC in the U.S. has recently determined the status of eels in Atlantic coastal states to be "depleted" and is currently developing an Addendum III which will include a range of options suggested by the American Eel Technical Committee including possible moratoria on glass (elver) and silver eel harvest, reductions in glass and yellow eel catch and effort, seasonal closures and future monitoring requirements (ASMFC 2012c).
Eel fisheries
Ontario commercial eel fisheries were first severely restricted and then closed in 2004 and the recreational fishery for eels was closed in 2005 (MacGregor et al. 2008). This was one of the earliest attempts to reduce mortality and initiate eel recovery in the province. Silver eels escaping from Ontario are still exploited in the Gulf of St. Lawrence fisheries; however, planned reductions in these fisheries now are being implemented as part of the Hydro Québec Action Plan (MNRF 2009). Quebec has closed the historically important Richelieu River fishery, and fisheries in the St. Lawrence have been reduced in recent years by licence retirement. Fisheries regulations (size limits, seasons, etc.) have also been made somewhat more restrictive in the Maritimes (COSEWIC 2006, MacGregor et al. 2008).
Upstream migration
In Ontario, the only actions to provide for upstream passage have been at Moses-Saunders Generating Station on the St. Lawrence River although discussions are currently underway at several other facilities. Here, an eel ladder has been in operation since 1974, and an experimental approach involving glass eel stocking in the USLR-LO has been underway since 2006. In New York, a state of the art eel ladder was recently installed on the U.S. portion of the Moses-Saunders facility.
In the United States, there is much activity to restore passage for migratory fish species (including eels) to the inland waters of many states (Gulf of Maine Council on the Marine Environment [GMCME] 2007, MacGregor et al. in press). For instance, a full migratory fish passage plan for a variety of species including eel has been developed and is now well into implementation for the Susquehanna River in Maryland (Pennsylvania Fish and Boat Commission [PFBC] 2007), and planning is well underway for the Penobscot River in Maine (Penobscot River Restoration Trust [PRRT] 2009). Upstream eel passage on the Oswego River (a New York tributary to Lake Ontario where eels once were highly abundant but disappeared due to hydro-electric installations), (Adams and Hankinson 1928, Henke 1993, see also MacGregor et al. 2009 and references therein) is now required following a Federal Energy Regulatory Commission re-licensing exercise for Brookfield Power at its Varik waterpower facility (Elmer and Murphy 2007).
Stocking of eels
In 2006, as part of an Action Plan negotiated among OMNR, DFO and Ontario Power Generation to begin addressing the substantial turbine mortalities at Saunders Generating Station, a science-based pilot stocking program of eels began in the Ontario portion of the St. Lawrence River and Lake Ontario. Stocking began earlier in Québec. Funding and support for stocking has been provided by Ontario Power Generation, Hydro-Québec and provincial governments. Effectiveness monitoring of the stocking programs has shown that stocking has some promise as a means of maintaining the presence of eels in these waters (good survival and growth). However, disconcerting issues have recently arisen with the program in Lake Ontario that have caused widespread concern among stakeholders, some agency representatives and First Nations. These concerns include: (1) while the proportion varied by year, approximately 40 percent of stocked eels >350 mm in length that could be evaluated were males (Pratt et al. 2011), and (2) there is evidence among stocked eels of very early maturation at a very small size (Verreault et al. 2010). However, this should not be seen as a final answer to the debate surrounding eel stocking or translocations as a conservation measure for depleted stocks. In fact, it raises more questions on the ability of these small migrants to reach the spawning grounds and their real capacity to contribute to stock rebuilding. Their small size implies low individual fecundity and their real benefit to the globally declining abundance for this species is unknown (Verreault et al. 2010). The occurrence of males is an undesirable outcome because recorded history shows that males are unprecedented in Ontario waters (J. Casselman, pers. comm. 2011) and males do not contribute nearly as much to recruitment as do females. The implications of these outcomes need to be thoroughly evaluated before stocking is considered in waters other than the St. Lawrence River and Lake Ontario.
Additionally, stocking was clearly selected as a short-term priority action (Greig et al. 2006). The true contribution of the stocked fish in terms of producing subsequent natural recruitment will, because of the mysteries around spawning, be one of the major drawbacks to the ultimate assessment of their contribution. Nevertheless, as noted by Verreault et al. 2010, the stocking program has provided a good opportunity to learn a great deal in a relatively short period of time. Evaluation of the pros and cons of stocking continues, but because of the foregoing concerns, and concerns regarding possible introduction of the swim bladder parasite, stocking was temporarily discontinued in 2011 pending the results of further research.
Turbine mortality
Negotiations with some power companies in Ontario and Quebec have led to formal action plans to further address and offset turbine-related mortalities at two specific locations on the St. Lawrence River (Beauharnois and Saunders Generating Stations). Where effort has been applied elsewhere, some success has been achieved in reducing downstream mortality (e.g., Boubée et al 2001, Watene and Boubée 2005). One example is the installation of a grid on the water intake at a small hydro-electric dam on the Rimouski River, Québec (G. Verreault, pers. comm. 2009). A trap and transfer program initiated by Ontario Power Generation has shown some promising results (Stanley and Pope 2009), but further evaluation is required to examine its biological effectiveness and the feasibility of full scale implementation (A. Mathers, pers. comm. 2009, T. Pratt, pers. comm. 2010). A single hydro-electric facility located at Appleton on the Mississippi River was redeveloped in the early 1990s with modifications to enable downstream eel passage but the passage actions were not implemented and therefore could not be tested for effectiveness (A. Bendig, OMNR, pers. comm. 2011)
2.0 Recovery
2.1 Recovery goal
The recovery goal for the American Eel is to re-establish the species in a wide variety of waters throughout its historical range in Ontario (Figure 3) by the year 2150, at abundance levels that: (1) restore cultural relationships
Given the extensive time frame (equivalent to seven eel generations) of the recovery goal, the range of presently available mitigation approaches and the potential for development of new approaches over this period, it is the opinion of the American Eel Recovery Team that the goal is reasonable and achievable.
There is little doubt that restoration to former levels of abundance may not be possible or practical in all areas of the province given the extent of hydro-electric facilities on some watersheds, but it is possible to make substantial improvements. Given the strong migratory tendencies of eels, it will not be difficult (by tactical installation of fishways for eels) to return fairly quickly some eels to many lakes or reaches where they have been extirpated or severely depleted for many years. The benefits of doing so have been described elsewhere within this document. Similarly, it will be possible to reduce substantially anthropogenic mortality if there is will and incentives (legal and financial) to do so – the technological, legal and policy tools exist to encourage significant and rapid improvements. Adaptive management and research will enable continuous improvements thereafter but first the "bleeding" must be effectively triaged.
2.2 Protection and recovery objectives
The protection and recovery objectives recommended by the American Eel Recovery Team are presented in Table 1.
Table 1. Protection and recovery objectives
| No. | Protection or Recovery Objective |
|---|---|
| 1 |
Strategically restore access to habitat within the historical range of the American Eel.
|
| 2 |
Increase escapement and recruitment.
|
| 3 | Reduce anthropogenic mortality of eels in boundary waters managed jointly with other jurisdictions. |
| 4 | Locate, protect, restore and enhance habitats upon which eels depend. |
| 5 | Reduce other sources of stress on the American Eel (e.g., contaminants, disease, harmful destruction, alteration or disruption of habitat). |
| 6 | Use a coordinated and strategic watershed-based approach to eel recovery across its historical range in Ontario. |
| 7 | Strengthen the engagement of Aboriginal peoples, stakeholders and other partners in the development and implementation of recovery actions for American Eel. |
| 8 | Maintain strong Ontario participation and leadership in the development and implementation of coordinated inter-jurisdictional protection, management and recovery of the American Eel and its habitats at national and bi-national levels. |
| 9 | Ensure ongoing understanding by scientists, managers, stakeholders, First Nations and the general public of the current status of the American Eel and the efficacy of recovery strategy actions. |
| 10 | Evaluate potential short-term methods of supporting eel abundance through such means as translocations and eel ladders in key watersheds. |
| 11 | Address knowledge gaps to enable and enhance protection, conservation and recovery efforts. |
Rationale and additional context for recovery goal and objectives
Recovery strategies for species that have experienced serious declines require strong, quick and effective action based on the best available science and information if the declines are to be reversed and further localized extirpations prevented. Given the extent of the American Eel decline in Ontario, it will not be sufficient to study the problem without implementing interim management actions at the same time. Research that examines how fish behaviour, habitat, ecology and evolution affect population growth at low abundance should be encouraged and supported (Hutchings and Reynolds 2004). Ongoing research should shed further light on the genetic structure of American Eel within the upper St. Lawrence River – Lake Ontario watershed. As Dekker et al. (2003:28) pointed out:
Research is underway to develop a comprehensive and effective restoration plan. This, however, will require time. The urgent concern is that the rate of decline [of global eel stocks] necessitates swifter protective measures. As scientists in eel biology from 18 countries we unanimously agree that we must raise an urgent alarm now … Precautionary action (e.g., curtailing exploitation, safeguarding migration routes and wetlands, improving access to lost habitats) can and must be taken immediately by all parties involved and, if necessary, independently of each other."
Major risks are posed to fish and fisheries by allowing populations to decline to extraordinarily low levels, and recovery strategies for such species "require the managerial fortitude to place long-term conservation to fish and fisheries ahead of short-term political expediency … Failure to take the conservation biology of marine fishes [such as the American Eel in Ontario] seriously will ensure that … depleted species remain ecological and numerical shadows in the ecosystem where they once dominated" (Hutchings and Reynolds 2004:307).
Due to the panmictic nature of the American eel, threats to eels that migrate to Ontario occur both within and outside the jurisdiction of the province. As documented in Section 1, Ontario’s freshwater eels have constituted a special segment of the American eel population. The abundance of eels in Ontario has collapsed by more than 90 percent, due to anthropogenic threats occurring within the province. The loss of freshwater eels is cause for concern and provides impetus for implementing a precautionary approach to management of the species (McCleave and Edeline 2009). The need for a precautionary approach, with special protection for seaward (downstream) migrating eels also derives from the fact that eels spawn only once (L. Velez-Espino, pers. comm. 2009) and to ensure that the genetic basis for the USLR-LO phenotype is not lost (Bernatchez et al. 2011)
When a particular region contributes a substantial fraction of the total panmictic spawning population, it follows that escapement from that region could affect the subsequent recruitment to the overall population (Chaput and Cairns 2011). It is generally accepted that a prudent management action is to improve escapement for seaward migrating spawners (EU 2007, Chaput and Cairns 2011), providing benefits to the overall population and in this case subsequently increasing recruitment of eels to Ontario. Increasing production and escapement of spawners from Ontario waters appears to be very important to recovery and conservation efforts from the perspective of both the provincial and the overall population.
In Ontario, the cumulative effects of harvest, hydropower and dams have historically been the most significant threats to eels. Now that fishing mortality has been eliminated, the cumulative effects of dams that reduced access to a wide variety of habitats and significant turbine mortality by hydroelectric facilities during seaward migration are the most significant threats to survival and recovery. Thus the two main pillars to this recovery strategy are: (1) strategic enhancement of upstream passage to a diverse array of habitats and, (2) strategically enhanced escapement. Addressing these two pillars effectively is fundamental to recovery of eels in Ontario. This does not diminish the need to address other threats. For instance, it is important that the ongoing pollution abatement programs and efforts to rehabilitate native forage species in Lake Ontario should continue. Both actions will be highly beneficial to eels, the broader ecosystem and to the people that have interests in the lake.
Addressing the cumulative effects of waterpower facilities strategically is especially important given that they represent the most significant threat to eel recovery in Ontario (Section 1.6: Cumulative Effects). Moreover, impending development of several more waterpower facilities within the current and historical range of eels in the province, if not mitigated, will increase the threat to this endangered species. There is sufficient technology and science to justify beginning the strategic, effective and adaptive implementation of improvements to upstream and downstream passage in both the St. Lawrence River and Ottawa River watersheds (the two principal migratory corridors for eels recruiting to and leaving Ontario).
The negative impacts of mainstem dams and hydroelectric facilities on biodiversity and diadromous fish species like eels are well documented (e.g., Larinier 2008, McCarthy 2008, Vorosmarty et al. 2010, Brown et al. 2012, Liermann et al. 2012). In Ontario, waterpower facilities form the first and only major obstacles on the main stems of the two principal migratory routes for eels in the province. The benefits of removing or effectively alleviating the impacts of dams and waterpower facilities on eels are clearly illustrated by McCleave (2001), Hitt et al. (2012) and Howard (2012). Restoring passage on the mainstems lowest in the watershed, will provide the most immediate and significant benefits (McCleave 2001).
The most effective actions in the short term are likely to be rapid, strategic improvements to upstream passage, restoring some level of connectivity within watersheds. Early provision of upstream passage is a widely adopted strategy in numerous North American jurisdictions (Elmer and Murphy 2007, GMCME 2007, PFBC 2007, PRRT 2009), as it is highly feasible and provides numerous benefits (McCleave 2001, Briand et al. 2005, Machut et al. 2007). It is especially effective and important where large females predominate (McCleave 2001, Hitt et al. 2012), despite ongoing turbine mortalities (McCleave 2001). However, Brown et al. (2012) note that a more effective option would be to consider removing mainstem dams and we agree that if for instance, some of the older facilities on the Ottawa River are nearing the end of their lifespan, removal of such facilities in the lower reaches would be a very effective option to consider.
Hitt et al. (2012) found that mitigation of the effects of barriers (in this case dam removal) benefitted eel dispersal over a great distance (150 km). Given the extraordinary propensity of eels to move and colonize new areas at the yellow eel stage, large accumulations of eels below dams are not necessary to stimulate dispersal. Leaving eels to accumulate below dams can lead to reduced growth (J. Casselman, unpub. data) and condition, increased parasite loads and mortality due to competition and predation (Machut 2006, Machut et al. 2007).
Enhancing access to tributary habitat and improving headwater connectivity by improving access may increase the carrying capacity of the entire watershed (Machut et al. 2007), and increase the relative abundance of females which tend to be more common in upstream areas of low density (Krueger and Oliveira 1999, Oliveira and McCleave 2000, Schmidt et al. 2009, Hitt et al. 2012). Consequently, improving upstream passage will enable improvement in production from a diversity of inland watersheds, enhancing over time biodiversity, ecosystem services and resilience to future anthropogenic perturbations in Ontario and elsewhere. This increased resilience will assist eels in enduring the effects of climate change and the planned addition of many more waterpower facilities within the Ontario range of eels (Secor 2010, Venturelli et al. 2010, see also the Supporting Narrative). Indeed, the conservation and improved abundance of females (by restoring connectivity to tributaries and headwater reaches) is a mechanism by which to improve eel abundance throughout their range (Hitt et al. 2012). Vigorous, strategic efforts to protect and restore migratory corridors (see Section 2.5) are required urgently. In addition, there remain pressing needs to identify and protect other habitat features such as riparian areas, wetlands and overwintering habitat (e.g., via Watershed Implementation Plans (WIPs) and development of a habitat regulation). This need is not unique to eels nor to aquatic species in Canada; habitat loss is especially extensive in the most biologically diverse areas of Canada (Kerr and Cihlar 2004), and habitat restoration across broad areas of southern Canada will be necessary for the recovery of most of Canada’s endangered species (Kerr and Deguise 2004).
Because naturally recruited Ontario eels spend one to two decades growing and maturing in fresh water, there will be time to evaluate and implement measures for downstream passage options once upstream passage has been enabled. While it is still early in the eel stocking/translocation projects in the USLR-LO, there is now published evidence that some eels in the upper St. Lawrence River and Lake Ontario may now be maturing and leaving the river system relatively quickly. Some eels have been found to leave in seven to eight years (Verreault et al. 2010). Regardless, if some stocked eels are migrating at an earlier age, there will still be many years before downstream passage mitigation needs to be installed once upstream access is provided.
On-going measures to improve upstream and downstream passage may be required at facilities that currently kill or harm eels. If such measures are implemented, then adaptive management approaches can be employed to achieve improvements over time. There should be no expectation that turbine mortalities can be reduced to zero in the short term, but substantial and ongoing improvements can be made. Eel passage plans should be developed and implemented for all existing waterpower facilities that currently kill or otherwise harm eels. These plans should be ongoing and incorporate adaptive management approaches to improve overall effectiveness over time.
Given the geographic and temporal scope of the strategy, the need for immediate actions and the need to deal with both upstream and downstream passage, an ongoing planning framework, (implemented through watershed-based implementation plans (WIPs)), will be needed to guide implementation. In this regard, we recommend that a strategic watershed approach be used to guide implementation of recovery actions (see page 72 and Appendix 3). If adopted, WIPs will guide the sequencing of recovery actions across the various sub-watersheds within the eels' current and historical range in Ontario. Because of the panmictic nature of eels, it is also important that Ontario continue its strong participation in international and interjurisdictional coordination efforts to manage eels sustainably.
2.3 Approaches to recovery
Table 2. Approaches to recovery of the American Eel in Ontario
1. Strategically restore access to habitat within the historical range of the American Eel
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Critical | Recovery Theme Approach to Recovery | Long-term Management and | 1.1 Using a strategic and phased approach, ensure existing facilities provide upstream passage for the American Eel. |
|
| Critical | Short-term | Management and Protection |
1.2 Develop and implement strategic passage plans for eels on key watersheds.
|
|
| Critical | Ongoing | Management and Protection |
1.3 Ensure all new facilities on watersheds within the native range of eels are designed to allow upstream passage for the American Eel.
|
|
| Critical | Short term | Management and Protection | 1.4 Provide policy and procedure tools to evaluate and address the cumulative impact of numerous water control structures on upstream passage. |
|
| Important | Long-term | Management and Protection | 1.5 Develop a Cybercartographic Atlas in support of the implementation of the recovery strategy. Enhance integration of western science, local community knowledge and ATK. |
|
2. Increase escapement and recruitment
- Increase escapement of silver and large yellow eels from watersheds in their historical range within Ontario.
- Enhance recruitment.
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Critical | Short-term and Long-term | Management and Protection |
2.1 Reduce/eliminate turbine mortality due to hydro-electric facilities on all watersheds within historical range of the American Eel in Ontario.
|
|
3. Reduce anthropogenic mortality of eels in boundary waters managed jointly with other jurisdictions
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Critical | Short-term | Management and Protection | 3.1 Encourage other jurisdictions to reduce commercial harvests of yellow and silver eel (e.g., Ontario should continue to take a leadership role in interjurisdictional science, management and conservation exercises relating to American Eel). |
|
| Critical | Long-term | Management and Protection | 3.2 Encourage other jurisdictions to mitigate turbine mortalities of downstream migrants. |
|
| Critical | Ongoing | Management and Protection | 3.3 Ontario government representatives should become actively involved with the Electric Power Research Institute and HydroNet to ensure research meets Ontario’s needs and priorities. |
|
4. Locate, protect, restore and enhance habitat on which eels depend
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Critical | Ongoing | Management and Protection | 4.1 Ensure no loss of habitat from development and new structures. | Habitat alteration |
| Critical | Ongoing | Management and Protection | 4.2 Identify wetlands of importance to eels (e.g., via WIPs and ensure their protection / restoration. |
|
| Critical | Ongoing | Management and Protection |
4.3 Work in cooperation with all water control boards (including International Joint Commission, Trent-Severn Waterway and Ottawa River Control Board) to identify water management strategies that meet needs for flood control while not detrimentally affecting eels or their habitat.
|
|
| Important | Ongoing | Management and Protection; Research | 4.4 Encourage research to expand understanding of how flows and water levels can be managed to improve habitat for eels. Factors to address include:
|
|
| Critical | Short-term | Research and Monitoring | 4.5 Locate and quantify areas of residual eel abundance. Identify habitat parameters associated with eels. |
|
| Critical | Short-term | Management and Protection | 4.6 Identify additional measures, if any, needed to protect habitats. |
|
5. Reduce other sources of stress on American Eel (e.g., contaminants, disease, harmful destruction, habitat alteration, productivity and food web changes)
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Important | Ongoing | Management and Protection | 5.1 Support (e.g., financial, regulatory and/or institutional support) actions to reduce contaminant and pollution loadings in eel habitats (e.g., Remedial Action Plans, Lakewide Management Plans, Toxics Management Plans, etc.). |
|
| Important | Short-term | Management and Protection Research; Monitoring and Assessment | 5.2 Continue ongoing evaluations of the impact of contaminants on eels. |
|
| Important | Ongoing | Management and Protection | 5.3 Continue ongoing efforts to restore and diversify native forage and other fish communities in Lake Ontario to avoid such effects as thiamine deficiency. |
|
6. Use a coordinated and strategic watershed-based approach to eel recovery across its historical range in Ontario
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Critical | Short-term | Management and Protection | 6.1 Develop upstream and downstream passage strategies and implementation plans. These could form key components of the Watershed Implementation Plans if adopted for American Eel on all key watersheds in Ontario. Begin implementation at downstream facilities and work progressively upstream providing passage overtime. WIPs will refine further as needed. |
|
| Critical | Ongoing | Management and Protection | 6.2 It is recommended that cumulative effects analysis be undertaken in the review of all water power projects and other developments that may affect eels within their historical range in Ontario. |
|
| Critical | Ongoing | Management and Protection |
6.3 Employ decision analysis that builds on existing research to determine priority actions that address the specific threats operating in different parts of the range.
|
|
| Critical | Short-term | Management and Protection | 6.4 Develop a decision support tool to identify and prioritise mitigation actions at hydro-electric installations and other barriers. |
|
| Critical | Short-term | Management and Protection |
6.5 Establish watershed-level escapement targets for silver eels that address cumulative mortalities on each watershed.
|
|
| Critical | Ongoing | Management and Protection |
6.6 In view of the joint federal and provincial interests in the resources of the Trent River and other water bodies under federal jurisdiction, work in close cooperation with the federal government, especially Fisheries and Oceans Canada, to ensure effective implementation of the strategy.
|
|
| Critical | Ongoing | Management and Protection | 6.7 Where appropriate, and consistent with the strategic approach of the recovery strategy, use existing regulatory tools (Ontario’s ESA, the Fisheries Act and the Lakes and Rivers Improvement Act) to mandate upstream and downstream passage at existing facilities. It will be necessary to be strategic and deal with the most significant threats first, low in the watershed, with priority first to those sites where eels still occur. Generally these will be hydro-electric facilities. |
|
| Critical | Short-term | Management and Protection | 6.8 Assess and address cumulative mortalities of eels in Ontario by setting escapement targets at the watershed scale and apportioning reductions in mortality across known sources through the WIPs. |
|
| Critical | Ongoing | Monitoring and Assessment |
6.9 Develop and regularly monitor the following.
|
|
| Critical | Ongoing | Monitoring and Assessment | 6.10 Every 10 years, update/revise the watershed implementation plans as new scientific information regarding the biology and status of the American Eel becomes available. |
|
7. Strengthen engagement of Aboriginal peoples, stakeholders and other partners in the development and implementation of recovery actions for the American Eel
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Critical | Ongoing | Education and Outreach | 7.1 Share and collaborate effectively with Aboriginal communities to integrate ATK into recovery planning and implementation. |
|
| Critical | Ongoing | Education and Outreach | 7.2 Include stakeholders, local community representatives and Aboriginal representatives in the design and implementation processes of education/outreach and recovery planning. |
|
| Critical | Ongoing | Education and Outreach | 7.3 Develop strong and lasting partnerships with Aboriginal communities, industry, other stakeholders and local communities in implementation of the watershed-based implementation strategy. |
|
| Critical | Ongoing | Education and Outreach | 7.4 Where necessary, provide support to enable full participation of appropriate stakeholders, local community representatives and Aboriginal communities in the development and implementation of all aspects of the recovery strategy. |
|
| Critical | Long-term | Education and Outreach |
7.5 Develop education, science-transfer and public-awareness programs:
|
|
8. Maintain strong Ontario participation and leadership in the development and implementation of coordinated interjurisdictional protection, management and recovery of American Eel and its habitats at national and bi-national levels
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Critical | Short-term | Education and Outreach | 8.1 Engage other jurisdictions in developing and implementing interjurisdictional conservation, recovery and management strategies for the American Eel in bi-national and interprovincial boundary waters that address provincial issues. |
|
| Critical | Ongoing | Education and Outreach | 8.2 Continue to direct Ontario resources and expertise towards the development and implementation of coordinated inter-jurisdictional science, management, conservation and protection efforts for the American Eel and its habitat. |
|
| Critical | Short-term | Management and Protection | 8.3. Enable full participation of Aboriginal communities in the development and implementation of interjurisdictional science and conservation exercises. |
|
| Critical | Ongoing | Management and Protection | 8.4 For watersheds managed by other agencies, work in cooperation with the management agencies to protect and improve the status of eels and their habitat. |
|
9. Ensure ongoing understanding by scientists, managers, stakeholders, First Nations and the general public of current status of the American Eel and the efficacy of the recovery strategy actions
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Important | Short-term | Inventory, Monitoring and Assessment | 9.1 Design and implement a monitoring program to provide the necessary information on trends in abundance across the identified key watersheds. Include information from Aboriginal and community knowledge in this assessment, and ensure representation of stakeholders, other interest groups and Aboriginal peoples on monitoring teams. For example, assess recruitment of juvenile eel to the lower Ottawa River downstream of the first barrier. |
|
| Important | Short-term | Inventory, Monitoring and Assessment | 9.2 Integrate and coordinate research among jurisdictions. Support research and assessment that improves understanding of eel population trends and effectiveness of mitigation options. |
|
| Critical | Ongoing | Inventory, Monitoring and Assessment |
9.3 Using best available information and technology, identify recovery opportunities and methods at the watershed level.
|
|
10. Evaluate potential short-term methods of supporting eel abundance through such means as translocations and eel ladders in key watersheds
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Critical | Short-term | Research | 10.1 Evaluate the effectiveness (survival, growth, production of females, etc.) of current stocking efforts in the USLR-LO and elsewhere in the St. Lawrence River watershed where stocking has taken place. |
|
| Critical | Short-term | Research | 10.2 Locate sources of glass eels for stocking, primarily from the St. Lawrence River system or if necessary from elsewhere (areas that produce primarily female silver eels). This will only be required if stocking is scientifically determined to be effective and necessary for eel recovery. |
|
| Critical | Short-term | Research | 10.3 If stocking is scientifically determined to be effective and necessary for eel recovery, work with DFO to ensure the development of a regulation to set aside a portion of the glass eel/elver quota for conservation. This regulation would be analogous to that already developed by the European Union to support eel conservation in Europe. |
|
| Critical | Short-term | Research | 10.4 Evaluate success of stocking programs (survival, growth, production of females etc.). |
|
| Critical | Short-term | Research | 10.5 Explore/evaluate other methods to improve short-term production, e.g., upstream transfer of young eels. |
|
11. Address knowledge gaps to enable and enhance protection, conservation and recovery efforts
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Important | Short-term | Research | 11.1 Work to gain more insights from ATK, and further integrate ATK with western scientific knowledge of eel ecology. |
|
| Critical | Short-term | Research | 11.2 Quantify cumulative mortality estimates and cumulative impacts for each watershed. |
|
| Important | Short-term | Research | 11.3 Develop a population model allowing assessment of the impact of mortality at specific points in the life history on overall abundance, escapement and subsequent recruitment. Use this model to support management decision making. |
|
| Important | Short-term | Research | 11.4 Determine the impact of contaminant loadings and toxicity on the survival and recruitment of eels within a watershed. |
|
| Critical | Short-term | Research | 11.5 Begin immediately to identify timing of migration for recruits and silver eels at existing hydro-electric facilities. Determine how flows and other environmental variables affect movements of eels upstream and downstream. |
|
| Important | Short-term | Research | 11.6 Strengthen the understanding of current and historical distribution of eels by regular monitoring, and by updating the documentation based on historical records, new archaeological finds and ATK. |
|
| Critical | Short-term | Research | 11.7 Assess ecological role and potential ecological impact of reintroducing eels into former habitat. |
|
| Important | Short-term | Research | 11.8 Identify important wetlands for over-wintering eels, locate wintering grounds and wintering eels, describe habitat requirements and evaluate the impact of winter drawdowns on this habitat and on eels. |
|
| Important | Short-term | Research | 11.9 Encourage and support the evaluation of Gulf Stream effects, considering significance of slight increases in recruitment, including the role of regulatory changes on recent slight increases in abundance (e.g., eel ladder numbers). |
|
Supporting narrative
Addressing the threats strategically
In undertaking recovery of eels in Ontario, it is clear that Ontario will need to build strategically from what remains of the uniquely important phenotype of eels native to Ontario. Because of the high individual reproductive value of eels in Ontario, protection of individuals should be a priority. While eels exhibit life history patterns that can be quite resilient (diversity of life cycle contingents, plasticity in habitat use, etc.), they are nonetheless vulnerable to a broad range of threats. Due to the panmictic nature of eels, these threats accumulate across the geographic range of one spawning stock (Verreault and Dumont 2003, Verreault et al. 2004, de Lafontaine et al. 2009a, MacGregor et al. 2009, MacGregor et al. in press).
Addressing the most immediate and largest known sources of mortality are obvious priorities if recovery is to proceed effectively. Identifying and addressing those sources of mortality within provincial boundaries are clearly most important as they are within the direct and immediate control of the province. Commercial and sport fisheries for the American Eel have been closed since 2004 and 2005 respectively, and no longer pose a serious threat to eels in Ontario (Ontario Government 2004, MacGregor et al. 2008, 2009). At that time the Ontario Government realized the importance of continuing with its conservation actions by working with stakeholders to address other anthropogenic sources of mortality including mortality due to turbines (Ontario Government 2004).
When the impacts to upstream migration are considered together with significant mortalities due to turbines, hydro-electric facilities by far pose the most immediate, serious and biggest threat to the continued survival and recovery of eels. This does not diminish the ongoing need to continue reducing contaminant loading and reverse ecosystem changes in Lake Ontario specifically, nor does this discount the effects of other barriers within the province. The effects of non-hydro barriers are generally not as urgent to address initially, because these barriers: (a) are generally far lower in height and hence pose less of an effect on upstream migration, (b) generally do not occur on the mainstems or at the mouths of watersheds, (c) do not cause turbine mortalities and (d) impart only a minimal risk of mortality due to free-fall. The recommended WIPs will further determine the priorities for individual watersheds (see below).
Due to panmixia, the effects of anthropogenic threats (e.g., mortality due commercial fisheries, sport fisheries and turbines) in other jurisdictions may influence the abundance of eels in Ontario and should not be ignored by the province. Continued leadership in interjurisdictional exercises to conserve and restore eels is critically important as effects in the 25 or so jurisdictions accumulate across the range on the single spawning stock (MacGregor et al. 2008, 2009, Feske et al. 2011). In addition to reducing the key sources of anthropogenic mortality, an essential step will be to instill the greatest possible resilience if there is to be a reasonable chance of eels of adapting to a range of anthropogenically induced perturbations including mortalities of spawners and environmental change (Secor 2010, Hutchings and Rangely 2011). As we have seen, resilience is best created in the American Eel by:
- vigorously protecting and ensuring survival of individual eels in Ontario as they are all large, old highly fecund females which confer high reproductive value and resilience in the population (e.g., Field et al. 2008, Venturelli et al. 2010); and
- significantly improving access to a broad range of habitats for the freshwater component of the lifecyle (of which a unique and highly valuable segment is contained in Ontario), thereby promoting varying outcomes and minimizing risks due to future and existing environmental change (Secor 2010).
Watershed-based implementation plans (WIPS)
Since the specific distribution of eel habitat relative to the distribution of hydro-electric facilities and other man-made barriers is unique to each watershed, WIPs are important to planning the implementation of the various recovery actions identified in Table 2.
Development of the WIPs should build and expand upon existing research, analyses and experience (e.g., EPRI 1999, Reid and Meisenheimer 2001, Greig et al. 2006, Goode 2006, Elmer and Murphy 2007, New York Power Authority 2009 and other Federal Energy Regulatory Commission [FERC] relicensing activities and studies, OWA 2010). They are intended to be carried out with strong public consultation and will need to be periodically refreshed as recovery progress is made and new knowledge is gained.
It is during the WIP process that information about the current science, ATK and community knowledge can be exchanged and transferred. The choices should be clearly and transparently outlined and considered holistically. Within these planning exercises, public discussions should take place concerning where in a watershed to allow or restrict access. For instance, in some circumstances eels may prey on or compete with brook trout (O'Connor 1971, Hitt et al. 2012). The two species have co-existed naturally for thousands of years in Ontario watersheds. Nevertheless, it will be important in the later stages of recovery efforts to thoroughly discuss and evaluate the potential ecosystem effects of re-introducing eels to some waters given the time that has passed since the species has been present in some waters.
In developing the Watershed-based Implementation Plans a decision analysis and adaptive management approach is recommended that will:
- strengthen the information needed for planning and decision making;
- develop the short and long-term methods and designs needed to strategically but effectively facilitate passage and minimize migration mortality;
- identify priority habitat areas within each watershed for restoration of access by eels, recognizing the potential plasticity in habitat use by eels and that for the
- most part habitat will likely be suitable; and
- establish reasonable watershed-specific long-term objectives, short-term targets and benchmarks for mortality, recruitment and escapement as a reference against which to measure progress and to help guide the process. Regional approaches for establishing benchmarks have been proposed by Chaput and Cairns (2011) and Fenske et al. (2011).
There will be a strong need for ongoing monitoring, particularly in the key watersheds.
Consistent with the overall strategy, it is expected that WIPs will focus initial recovery efforts within the lower reaches where eels currently still reside. Regardless of currently low densities, and given the exceptionally strong migratory tendencies of eels, it should not be difficult in the short term to enhance eel abundance in many lakes or reaches where they have been severely reduced. In the longer term it should be straightforward to facilitate the return of eels to waters where they have been extirpated, once the decisions are made regarding where within a watershed this would be appropriate.
As with many species that are near extirpation, it will take time and commitment through partnerships to achieve meaningful results. Expectations at the watershed level should always be established mindful of the goal statement of the recovery strategy – it has a 150 year time horizon. Nevertheless, considerable progress towards recovery is possible in the near term with strategic, dedicated commitment at the watershed scale.
Finally, many of the anthropogenic impacts on Ontario eels are similarly constraining or jeopardizing other migratory fish species and mussels in the same watersheds as we identified for eel. We recommend adopting an ecosystem approach within the WIPs, wherein the needs of other aquatic species at risk are considered at the same time. Additional detail about the considerations for WIPs may be found in Appendix 3.
Mitigation
Unlike passage for many other species, techniques for enhancing upstream passage for eels at hydroelectric facilities and other dams are well known and relatively straightforward in many circumstances. Indeed, there are world-renown examples of upstream passage in Ontario, New York and Quebec at the Moses-Saunders and Beauharnois Generating Stations on the St. Lawrence River.
Mitigation actions are feasible and underway in other jurisdictions to address similar impacts at other hydroelectric facilities on freshwater eels (MacGregor et al. 2008, 2011, see also Section 1.8 of this document) and much can be learned by carefully examining these efforts. While there are difficulties in mitigating turbine mortalities at large facilities such as Moses-Saunders Generating Station (New York Power Authority 2009, OWA 2010), some positive results are coming from the trap and transport program at Saunders Generating Station on the St. Lawrence River (Stanley and Pope 2009). Additionally, a number of mitigation projects are underway in other jurisdictions that should be examined (Knights and White 1998, McCarthy et al. 2008, MacGregor et al. 2008, 2011 and references therein).
Recognizing that provision of safe downstream passage will be more problematic than upstream passage, and that some mortality will be inevitable, the key will be to carefully undertake the appropriate research to identify long term downstream passage solutions, while taking immediate interim actions to alleviate turbine mortalities at locations such as the aforementioned facilities. Guidance is available to help determine the best site-specific mitigation options in the recent overviews produced by OWA (2010) and New York Power Authority (2009), but these documents should not be considered to contain the only solutions. More techniques will be developed based on well-studied attempts to mitigate passage on a variety of small, medium and large watersheds in Ontario. Hence, the recovery team recommends that an adaptive management approach should be a cornerstone in implementing this strategy. Options to adjust the operational regime of individual facilities to improve circumstances for eels should be examined as an integral component of the Watershed-based Implementation Plans (WIPs).
Trap and transfer programs (e.g., McCarthy et al. 2008, Stanley and Pope 2009), spillage, and/or turbine shut-downs at night
Regardless, eels are currently being killed and otherwise affected at hydro-electric facilities in several watersheds and mitigation measures focussed on these threats should be a requirement of any waterpower mitigation plan, agreement or authorization under Ontario’s ESA or the federal Fisheries Act
Addressing new developments
Given the lengthy timelines associated with approvals and construction processes, provisions for passage should be designed and incorporated into the construction specifications for all new facilities within the historical range of eels in the key watersheds identified within the recovery strategy, regardless of their location within the watersheds. Passage designs at facilities in upstream reaches where eels are presently extirpated could be implemented during initial construction, during upgrades to existing facilities or at a later time when downstream access has been restored to the site. When project review opportunities arise at existing facilities and the need for passage at a site has been identified within a WIP, it will also be important to ensure that passage needs at these sites are recognized and incorporated into the approvals processes (an opportunistic approach).
Building resilience
It has been suggested that climate change and environmental variation are reasons to not take action to address eel decline in Ontario, that provincial rehabilitation efforts for eels should be concentrated at Saunders Generating Station (at the outlet from Lake Ontario), and that no mitigation be carried out elsewhere in the province. This would be counter to current knowledge which indicates that such action would be a flawed management strategy (Kraus and Secor 2005, MacGregor et al. 2009). Moreover, this would be inconsistent with the goal and objectives of the strategy. For reasons identified elsewhere, the American Eel Recovery Team considers it unwise and not strategic to focus recovery efforts only on Lake Ontario.
The effects of climate change and other environmental variation is no reason to avoid mitigation of known sources of mortality (MacGregor et al. in press). Rather it is a reason to accelerate mitigation efforts. Strong conservation actions have been shown to be effective for other fish species regardless of fishing-induced or environmentally induced periods of low recruitment and stock declines (Hatch et al. 1987, see also MacGregor et al. in press). Less favourable environmental conditions could further exacerbate the declining sustainability of the species unless mitigating conservation actions (e.g., enhancing spawner escapement) are soon undertaken.
The American Eel Recovery Team strongly opposes concentrating recovery efforts only on Lake Ontario. We have discussed at length the need to build resilience in Ontario’s sub-population of eels by diversifying the range of habitats thereby minimizing the risks from future perturbations. Moreover, recent information pertaining to past effects of contaminants and to the potential effects of ecosystem change/invasive species, including thiamine deficiency in Lake Ontario fish (possibly including eels) underscores the risks associated with concentrating recovery efforts only in Lake Ontario. It is essential to broaden recovery efforts into inland waters where the effects of non-native forage fish (such as alewife which introduce significant quantities of thiaminase when consumed) on eel reproduction are minimized.
The American Eel Recovery Team encourages continued mitigation efforts at Saunders, but not to the exclusion of mitigation on other watersheds. While considerable habitat for eels was once available in Lake Ontario, uncertainties remain regarding its present and future quality given growth projections for the watershed. Also, mitigating the effects of Saunders Generating Station is especially difficult given the problems associated with the magnitude of the St. Lawrence River and Moses-Saunders facility. Leaving eels congregating in the lowest reaches of a watershed leaves them exposed to the most intense and cumulative effects of urbanization, agriculture, industry and other forms of pollution.
The densest human population in Canada surrounds Lake Ontario where contaminants and other pollution issues despite abatement programs, continue to accumulate in many areas of the lake (LaMP 2009). Future growth projections in Ontario predict that the population within the Greater-Toronto Area (GTA) will increase by 50 percent by the year 2130 (Ontario Government 2010). This growth can be expected to lead to additional anthropogenic impacts on Lake Ontario. Lake Ontario is also plagued by profound, ongoing ecosystem change due to the effects of invasive species (SOLEC 2005, LaMP 2009); hence, the need to provide access to other less affected waters is clear. The American Eel Recovery Team strongly recommends that recovery efforts be broadened to include additional waters, to increase the availability and diversity of habitat that is more remote from the cumulative effects of agriculture, urbanization and industrialization, and build resilience in the Ontario’s subpopulation. Increasing available habitat for eels is a worthy goal given the drastic decline in eels (Machut et al. 2007). There is no evidence to suggest that considerably more suitable habitat is not available within many other Ontario watersheds if access were available. This is particularly true in waters where the remainder of the native fish community persists.
Given the historical abundance of eels in the lake, continuation of recovery efforts at Saunders-Lake Ontario is encouraged while uncertainties are investigated and addressed but for the aforementioned reasons, concentrating mitigation and recovery efforts only at Saunders-Lake Ontario is a flawed strategy.
2.4 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of Natural Resources on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the recovery team will be one of many sources considered by the Minister when developing the habitat regulation for this species.
It is recommended that all reaches (Aquatic Resource Areas
It is recommended that within these reaches the prescribed area include primary habitat in both lentic and lotic waters, including all waters extending from the high-water mark
In general, currently or formerly occupied habitat is found in all waters tributary to Ontario’s portions of Lake Ontario, the St. Lawrence River and the Ottawa River. Migratory corridors include (but may not be limited to) all water bodies within the following key watersheds (this includes all associated lakes, rivers, streams, rivulets and waterways, permanent or ephemeral):
- Upper St. Lawrence River-Lake Ontario;
- Ottawa River;
- Mississippi River;
- Bonnechere River;
- Kawartha Lakes;
- Salmon River;
- Moira River;
- Napanee River;
- Credit River;
- Humber River;
- Duffins Creek;
- Bronte Creek;
- Don River;
- Hamilton Harbour and Cootes Paradise;
- Petawawa River;
- Madawaska River;
- Mattawa River;
- Lake Timiskaming (including the Montreal and Blanche Rivers);
- Muskrat River;
- Rideau River;
- Rideau Canal;
- Raisin River;
- South Nation River;
- Gananoque River;
- Trent/Otonabee River;
- Twelve Mile Creek/Martindale Pond;
- Jordan Harbour; and
- Niagara River.
Glossary
Anthropogenic:
Caused by humans.
ATK:
Aboriginal Traditional Knowledge.
Benthivore:
Feeding on bottom-dwelling organisms.
Catadromous:
Going down rivers to the sea to spawn as does the American Eel (Scott and Crossman 1973).
Committee on the Status of Endangered Wildlife in Canada (COSEWIC):
The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
Committee on the Status of Species at Risk in Ontario (COSSARO):
The committee established under Section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
Conservation status rank:
A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or sub-national (S) level. These ranks, termed G-Rank, N-Rank and S-Rank, are not legal designations. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperiled
2 = imperiled
3 = vulnerable
4 = apparently secure
5 = secure
Cybercartographic Atlas:
An integrated and multifunctional system by which the information can be served and shared all over the world in order to play an important role in research and application. Its main functions are logging on, sorting, storage, query, reducing, statistics, mapping, analysis, estimating, and management and exporting geographic information. Potential methods of data representation are maps, text, charts, images, time series animation, 3D visualization, audio and video. Data query, browsing, explore, dynamic mapping, statistical analysis etc. can be realized on the Internet by users.
Density-dependence:
Describes a factor that influences individuals in a population to a degree that varies in response to how crowded (dense) the population is.
Diadromous:
Involves migrations between freshwater and marine ecosystems (McDowall 2009).
Dreissenid:
Small bivalves (clam-like) of the family Dreissenidae. Two species have invaded the Great Lakes (zebra and quagga mussels; Dreissena polymorpha and Dreissena bugensis respectively.
Ephemeral stream:
A watercourse generally without a well-defined channel which flows only in response to rainfall or snowmelt. Ephemeral streams flow for less than 20% of the year during normal rainfall conditions. Includes ephemeral watercourses in urban and agricultural settings.
Endangered Species Act, 2007 (ESA):
The provincial legislation that provides protection to species at risk in Ontario.
Escapement:
That portion of a diadromous fish population that escapes the anthropogenic mortality and reaches the freshwater spawning grounds. The number of eels which have escaped the fisheries and turbines and are available for spawning.
Eutrophication:
Excessive nutrients in a lake or other body of water, usually caused by runoff of nutrients (animal waste, fertilizers, sewage) from the land, which causes a dense growth of plant life; the decomposition of the plants depletes the supply of oxygen. Can also be used to describe the natural aging processes in lakes.
Facultative:
Not compulsory, not restricting.
Fecund:
Producing or capable of producing an abundance of offspring. Egg-laden.
G-Rank:
Global Rank; a rarity rank based on the range-wide conservation status of a species, subspecies or variety
High water mark:
The usual or average level to which a body of water rises at its highest point and remains for sufficient time so as to change the characteristics of the land. In flowing waters (rivers, streams) this refers to the "active channel/bank-full level" which is often the 1:2 year flood flow return level. In inland lakes, wetlands or marine environments it refers to those parts of the water body bed and banks that are frequently flooded by water so as to leave a mark on the land and where the natural vegetation changes from predominately aquatic vegetation to terrestrial vegetation (excepting water tolerant species).
For reservoirs this refers to normal high operating levels (Full Supply Level). For the Great Lakes this refers to the 80th percentile elevation above chart datum as described in DFO's Fish Habitat and Determining the High Water Mark on Lakes (DFO 2005).
Lacustrine:
Of a lake or relating to a lake.
Lentic:
Of, relating to, or living in still waters (as lakes, ponds or swamps).
Leptocephali:
Flat and transparent larva of the eel, marine eels, and other members of the Superorder Elopomorpha. These fishes with a leptocephalus larva stage include the most familiar eels such as the conger, moray eel, and garden eel, and the freshwater eels of the family Anguillidae, plus more than 10 other families of lesser-known types of marine eels. These are all true eels of the order Anguilliformes. The fishes of the other four traditional orders of elopomorph fishes that have this type of larva are more diverse in their body forms and include the tarpon, bonefish, spiny eel, and pelican eel.
Lotic:
Of or relating to or living in actively moving water.
Mitigation:
Elimination or reduction of frequency, magnitude or severity of exposure to environmental, economic, legal, or social risks or minimization of the potential impact of a threat.
N-Rank:
National Rank; refers to the national conservation status rank of an element.
Panmictic:
Describing a population in which mating is entirely random and any two (male and female) individuals are equally likely to mate. Random mating (or panmixis) is one of the assumptions of the Hardy-Weinberg equilibrium. Random mating within an interbreeding population. The American Eel and the Monarch Butterfly are examples of panmictic species.
Piscivore:
Habitually feeding on fish.
Production:
The rate of generation of biomass or new tissue growth in a population. Recruitment: Addition of new members to the aggregate under consideration. In a fishery it is the supply of fish that becomes available at some particular stage in their life history, generally that stage at which the fish first become vulnerable to the gear used in the fishery (Everhart et al. 1975). Addition of new fish to the vulnerable population by growth from among smaller size categories (Ricker 1975).
Resilience:
The magnitude of the population perturbations that the system will tolerate before collapsing into qualitatively different regime (Holling 1973; May 1976).
S-Rank:
Sub-national or Provincial Rank; refers to the provincial conservation status rank of an element, and used to set protection priorities for rare species and natural communities.
S1 Species:
Provincially extremely rare, and high priority for assessment by COSEWIC. An S1 species has very few remaining individuals in Ontario, and is often especially vulnerable to extirpation.
S-Rank-S1?:
The question mark indicates that there is a level of uncertainty associated with the assigned ranking.
Semelparous:
Used to describe an organism that reproduces just once during its lifetime after which its death is inevitable.
Shifting Baseline:
A term used to describe the way significant changes to a system are measured against previous baselines, which themselves may represent significant changes from the original state of the system. The term was first used by the fisheries scientist Daniel Pauly (1995) in his paper "Anecdotes and the shifting baseline syndrome of fisheries". Pauly developed the term in reference to fisheries management where fisheries scientists sometimes fail to identify the correct "baseline" population size (e.g., how abundant a fish species population was before human exploitation) and thus work with a shifted baseline. In this way large declines in ecosystems or species over long periods of time were, and are, masked. There is a loss of perception of change that occurs when each generation redefines what is "natural". The term has become widely used to describe the shift over time in the expectation of what a healthy ecosystem baseline looks like.
Species at Risk Act (SARA):
The federal legislation that provides protection to species at risk in Canada. This act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
Species at Risk in Ontario (SARO) List:
The regulation made under Section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
Stochasticity:
- Involving or containing a random variable or variables: stochastic calculus.
- Involving chance or probability: a stochastic stimulation.
Torpor:
The dormant, inactive state of a hibernating or estivating animal.
USLR-LO:
Upper St. Lawrence River-Lake Ontario
References
Literature
Adams, C.C. and T.L. Hankinson. 1928. The ecology and economics of Oneida Lake fish. Roosevelt Wildlife Annals 1:1-548.
Albert, V., B. Jonsson, and L. Bernatchez. 2006. Natural hybrids in Atlantic eels (Anguilla anguilla, A. Anguilla rostrata): evidence for successful reproduction and fluctuating abundance in space and time. Molecular Ecology 15:1903-1916.
Algonquins of Ontario. 2012. Returning Kichisippi Pimisi – the American Eel - to the Ottawa River Basin, December 2012. Available: http://www.tanakiwin.com/AOO_Returning%20Kichisippi%20Pimisi%20to%20the%20Ottawa%20River%20Basin__Dec2012_20121219.pdf. Accessed September 2013.
Allen, W.A. 2008a. Dbaajmoowin: Dialogue with the elders, in S. Weber and D. Harmon (eds.). Protected areas in a changing world: Proceedings of the 2007 George Wright Society Conference on Parks, Protected Areas, and Cultural Sites. The George Wright Society, Hancock, Michigan.
Allen, W.A. 2008b. American eel: Driving a Shift in Power. Presentation at the A. D. Lattornell Conservation Symposium. November 20, 2008. Nottatwasaga Inn, Alliston, Ontario. Available: http://www.docstoc.com/docs/32725280/The-American-eelDriving-a-Shift-in-Power [link inactive]. Accessed September 2013.
Allen, W.A., L.M. Brady, and P. Decontie. 2008. Manaadjiyindj iyaa manidoo nayaagadjitoodj kije-asin mazinaakobiihiganan – Honouring the Spirits of Sacred Pictographs. Pp. 277-289, in C. Dignard, K. Helwig, J. Mason, K. Nanowin, and T. Stone (eds.). Preserving Aboriginal Heritage: Technical and Traditional Approaches, Proceedings of Symposium 2007. Canadian Conservation Institute, Ottawa, Canada.
Allen, W.A. 2010. Archaeology to the rescue of species at risk. Arch Notes 15(6):5-14, November-December 2010, Ontario Archaeological Society, Toronto.
Anderson, J. and R.E. Schmidt. 2006. Significance of small impoundments to American eel (Anguilla rostrata). Pages 1-20 in W.C. Nieder and J.R. Waldman, editors. Final Reports of the Tibor T. Polgar Fellowship Program, 2005. Hudson River Foundation, New York, New York.
Anderson, C.N.K., C.-H. Hsieh, S.A. Sandin, R. Hewitt, A. Hollowed, J. Beddington, R. M. May, and G. Sugihara. 2008. Why fishing magnifies fluctuations in fish abundance. Nature 452:835-839.
Arai, T., A. Kotake, P.M. Lokman, M.J. Miller, and K. Tsukamoto. 2004. Evidence of different habitat use by New Zealand freshwater eels Anguilla australis and A. dieffenbachia, as revealed by otolith microchemistry. Marine Ecology Progress Series 266:213-225.
ASMFC (Atlantic States Marine Fisheries Commission). 2000. Interstate Management Plan for American Eel. Report No. 36:1-93.
ASMFC (Atlantic States Marine Fisheries Commission). 2006. Stock Assessment Report No. 06-01 of the Atlantic States Marine Fisheries Commission: Terms of References and Advisory Report to the American Eel Stock Assessment Peer Review, January 2006. Atlantic States Marine Fisheries Commission, Washington, D.C.
ASFMC (Atlantic States Marine Fisheries Commission). 2012a. ASMFC Stock Assessment Overview: American Eel. Available: http://www.asmfc.org/speciesDocuments/eel/annualreports/stockAssmts/AmericanEelStockAssessmentOverview_May2012.pdf [link inactive]
ASFMC (Atlantic States Marine Fisheries Commission). 2012b. American Eel Benchmark Stock Assessment. Stock Assessment Report No. 12-01 of the ASFMC. A publication of the Atlantic States Marine Fisheries Commission pursuant to National Oceanic and Atmospheric Administration Award No. NA10NMF4740016. 253 pages + Appendices. Available: http://www.asmfc.org/speciesDocuments/eel/annualreports/stockAssmts/americanEelBenchmarkStockAssessmentReport_May2012.pdf [link inactive]
ASFMC (Atlantic State Marine Fisheries Commission). 2012c. ASMFC American Eel Board Initiates Development of Draft Addendum III to Improve Conservation and Protection of the Stock. ASMFC Newsrelease, August 9, 2012. Available: http://www.savingseafood.org/council-actions/asmfc-american-eel-board-initiates-development-of-draft-addendum-iii-to-improve-conservation-and-protection-of-the-s-2.html [link inactive] and at: http://www.asmfc.org/
Avise, J.C. 2003. Catadromous eels of the North Atlantic: a review of molecular genetic findings relevant to natural history, population structure, speciation, and phylogeny. Pp. 31-48, in K. Aida, K. Tsukamoto, and K. Yamauchi (eds.). Eel Biology. Springer-Verlag, Tokyo.
Bain, M.B., T.J. Finn, and H. Booke. 1988. Streamf!ow regulation and fish community structure. Ecology 69:382-392.
Baraga, F. 1878. A dictionary of the Otchipwe language explained in English, Part 1 English-Otchipwe, Beauchemin & Valois, Montreal, Québec.
Barbin, G.P. and J.D. McCleave. 1997. Fecundity of the American Eel Anguilla rostrata, at 45 degrees N in Maine, USA. Journal of Fish Biology 51:840-847.
Barbin, G.P. and W.H. Krueger. 1994. Behaviour and swimming performance of elvers of the American Eel, Anguilla rostrata, in an experimental flume. Journal of Fish Biology 45:111-121.
Bardeau, P.E.W. 2002. Onondowa'Ga:' Gawe:no, new reference edition. Seneca Nation of Indians, Salamanaca, New York.
Barlow, A.E. 1907. Second Edition of a Report on the Geology and Natural Resources of the Area Included by The Nipissing and Timiskaming Map-Sheets: Comprising Portions of the district of Nipissing, Ontario, and of the County of Pontiac, Quebec. Nabu Press, Feb. 2010. ISBN-13: 978-1145486157. 324 pp.
Bartram, J. 1751. Observations on the inhabitants, climate, soil, rivers, productions, animals, and other matters worthy of notice made by Mr. John Bartram, in his travels from Pennsylvania [sic] to Onondago, Oswego and the Lakes Ontario, in Canada to which annex'd a curious account of the cataracts at Niagara by Mr. Peter Kalm, a Swedish gentleman who travelled there.
Baxter, C.V., K.D. Fausch, and W.C. Saunders. 2005. Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zones. Freshwater Biology 50:201-220. Available: http://warnercnr.colostate.edu/~csaunder/Baxter%20et%20al.%202005.pdf [link inactive]. Accessed September 2011.
Beauchamp, W.M. 1908. Past and Present of Syracuse and Onondaga County, New York: From Prehistoric Times to the Beginning of 1908. Pp. 43-50. S.J. Clark Publishing Co., New York. Available at: http://ia600408.us.archive.org/9/items/pastpresentofsyr01beau/pastpresentofsyr01beau.pdf. Accessed September 2011.
Bennett, C. and D.W. McCuaig. 1981. In search of the K & P.. Renfrew Advance Ltd., Kingston, Ontario. 115 pages.
Berkeley, S.A., C. Chapman, and S.M. Sogard. 2004a. Maternal age as a determinant of larval growth and survival in a marine fish, Sebastes melanops. Ecology 85:1258-1264.
Berkeley, S. A., M. A. Hixon, R. J. Larson, and M. S. Love. 2004b. Fisheries sustainability via protection of age structure and spatial distribution of fish populations. Fisheries 29:23–32
Bernatchez, L., C. Cote, and M. Castonguay. 2011. Genetic structure of the American Eel with emphasis on the St. Lawrence River basin. Great Lakes Fishery Commission, Ann Arbor, Michigan. Available: http://www.glfc.org/research/reports/Bernatchez_2011.htm. Accessed September 2011.
Blankenship, K. 2006. Demise of eels may have doomed Susquehanna mussels, hurt Bay. Chesapeake Bay Journal, July/August 2006. Available: http://www.bayjournal.com/article.cfm?article=2854. Accessed September 2011.
Bonhommeau, S., E. Chassot, B. Planque, E. Rivot, A.H. Knap, and O. Le Pape. 2008. Impact of climate change on eel populations of the Northern Hemisphere. Marine Ecology Progress Series 373:71-80.
Boubée, J.A., C.P. Mitchell, B.L. Chisnall, D.W. West, E.J. Bowman, and A. Haro. 2001. Factors regulating the downstream migration of mature eels (Anguilla spp. at Aniwhenua Dam, Bay of Plenty, New Zealand. New Zealand Journal of Marine and Freshwater Research 35:121-134. Available: http://www.royalsociety.org.nz/publications/journals/nzjm/2001/009/. Accessed September 2011.
Briand, C., D. Fatin, G. Fontenelle, and E. Feunteun. 2005. Effect of re-opening of a migratory pathway for eel (Anguilla anguilla) at a watershed scale. Bulletin Francais de la Peche et de la Pisciculture 378-379:67-86.
Brown, J.Jed., Limburg, K.E.;Waldman, J.R., Stephenson, Kurt; .Glenn. E., Juanes, F., and A. Jordaan. 2012. Fish and hydropower on the U.S. Atlantic coast: failed fisheries policies from half-way technologies. Conservation Letters 0 (2013) 1–7.
Brujis, M.C.M., R.H. Hadderingh, U. Schwevers, B. Adam, U. Dumont, and H.V. Winter. 2009. Managing human impact on downstream migrating European eel in the River Meuse. Pp. 381-390, in J.M. Casselman and D.K. Cairns (eds.). Eels at the edge: science, status and conservation concerns. American Fisheries Society, Symposium 58, Bethesda, Maryland.
Burnett, A. 2007. William (Bill) James Bridgeman—the last miller of Wakefield. Gatineau Valley Historical Society, Gatineau Valley Historical Society Newsletter 2007:04, Chelsea, Québec.
Busch, W.D.N., S.J. Lary, C.M. Castilione, and R.P. McDonald. 1998. Distribution and availability of Atlantic coast freshwater habitats for American Eel (Anguilla rostrata). U.S. Fish and Wildlife Service, Administrative Report 98-2, Amherst, New York.
Byer, J.D., M. Alaee, R.S. Brown, S. Backus, M. Keir, G. Pacepavicius, M. Lebeuf, J. Casselman, S. Kennedy,P.V. Hodson, 2009. Dioxin concentrations in American eel (Anguilla rostrata) captured in Eastern Canada. Organohalogen Compounds 71, 352-356 (Proceedings, Dioxin 2009, 29th International Symposium on Halogenated Persistent Organic Pollutants. Beijing, China, Aug 23-28, 2009).
Byer, J.D., M. Alaee, R.S. Brown, M. Lebeuf, S. Backus, M. Keir, J. Casselman, and P.V. Hodson. 2010. Dioxin related contaminants in Lake Ontario American eel: a likely cause for their decline? Organohalogen Compounds 72:1213-1216.
Cairns, D.K., D.A. Secor, W.E. Morrison, and J.A. Hallett. 2009. Salinity-linked growth in anguillid eels and the paradox of temperate-zone catadromy. Journal of Fish Biology 74:2094-2114.
Cairns, D.K., J.C. Shiao, Y. Iizuka, W.N. Tzeng, and C.D. MacPherson. 2004. Movement patterns of American Eels in an impounded watercourse, as indicated by otolith microchemistry. North American Journal of Fisheries Management 24:452-458.
Canada Department of Justice. 1985. Fisheries Act (R.S., 1985, c. F-14). Available: http://laws-lois.justice.gc.ca/PDF/F-14.pdf. Accessed September 2011.
Casselman, J.M. 1982. Chemical analyses of the optically different zones in eel otoliths. Pp. 74-82, in K.H. Loftus (ed.). Proceedings of the 1980 North American Eel Conference. Ontario Fisheries Technical Report Series #4, Toronto, Ontario.
Casselman, J.M. 2003. Dynamics of resources of the American Eel, Anguilla rostrata: declining abundance in the 1990s. Chapter 18, Pp. 255-274, in K. Aida, K. Tsukamoto, and K. Yamauchi (eds.). Eel Biology. Springer-Verlag, Tokyo.
Casselman, J.M. 2008. Otolith age interpretations of juvenile American Eels ascending the R.H. Saunders Eel Ladder, Moses-Saunders Generating Station, upper St. Lawrence River, 2003-2007. Conducted by AFishESci Inc. for Ontario Power Generation through the Species at Risk Stewardship Program. 13 p. + 5 appendices.
Casselman, J.M. and L.A. Marcogliese. 2007. Long-term changes in American Eel (Anguilla rostrata) commercial harvest and price in relation to declining abundance. Completion report. 94 p. (57 pages text + 4 tables + 22 figures). Prepared for Great Lakes Fishery Commission, Ontario Ministry of Natural Resources, and Department of Fisheries and Oceans by AFishESci Inc., Bath, Ontario.
Casselman, J.M. and L.A. Marcogliese. 2008. Eel abundance in the upper St. Lawrence River and eastern Lake Ontario—quantitative electrofishing index, 2008. September 2008. Conducted for Ontario Ministry of Natural Resources. AFishESci Inc., Bath, Ontario.
Casselman, J.M. and L.A. Marcogliese. 2009. Abundance and Distribution of American Eels (Anguilla rostrata) and Other Fish in the Lower Mississippi and Ottawa Rivers, Fall 2008, as Determined by Quantitative Electrofishing. Conducted by AFishESci Inc. in cooperation with Plenty Canada and Mississippi Valley Conservation for, and with financial support provided by Ontario Species at Risk Stewardship Fund.
Casselman, J.M. and L.A. Marcogliese. 2010a. Abundance and distribution of American eels (Anguilla rostrata) and other fish in the lower Mississippi and Ottawa rivers, 2009, as determined by quantitative electrofishing. Conducted by AFishESci Inc./abbr>, in cooperation with Plenty Canada and Mississippi Valley Conservation for, and with financial support provided by Ontario Species at Risk Stewardship Fund.
Casselman, J.M., and L.A. Marcogliese. 2010b. Abundance and distribution of American eels (Anguilla rostrata) and other fish in the Ontario waters of the upper St. Lawrence River and Charleston Lake, Gananoque River system, 2009, as determined by quantitative electrofishing. Conducted by AFishESci Inc. in cooperation with Plenty Canada and Mississippi Valley Conservation for, and with financial support provided by Ontario Species at Risk Stewardship Fund.
Casselman, J.M., and L.A. Marcogliese. 2011. Abundance and distribution of the American eel (Anguilla rostrata) and other fish in the lower Ottawa River system and tributaries, 2010, as determined by quantitative electrofishing. Conducted by AFishESci Inc. for, and with financial support provided by Ontario Species at Risk Stewardship Fund. February 2011. 30 pages + 2 appendices (325 pages).
Casselman, J.M., L.A. Marcogliese, and P.V. Hodson. 1997a. Recruitment index for the upper St. Lawrence River and Lake Ontario eel stock: a re-examination of eel passage at the R.H. Saunders hydroelectric generating station at Cornwall, Ontario, 1974-1995. Pp. 161-169, in R.H. Peterson (ed.). The American Eel in eastern Canada: Stock status and management strategies. Canadian Technical Report of Fisheries and Aquatic Sciences No. 2196.
Casselman, J.M., L.A. Marcogliese, T. Stewart, and P.V. Hodson. 1997b. Status of the upper St. Lawrence River and Lake Ontario American eel stock—1996. Pp. 106-120, in R.H. Peterson (ed.). The American Eel in eastern Canada: Stock status and management strategies. Proceedings of the Eel Workshop, January 13-14, 1997, Quebec City, Quebec. Canadian Technical Report of Fisheries and Aquatic Sciences, No. 2196.
Castonguay, M., P.V. Hodson, C.M. Couillard, M.J. Eckersley, J.D. Dutil, and G. Verreault. 1994. Why is recruitment of the American Eel, Anguilla rostrata, declining in the St. Lawrence River and Gulf? Canadian Journal of Fisheries and Aquatic Sciences 51:479-488.
CBD (Convention on Biodiversity). 2000. Sustaining Life on Earth: How the Convention on Biological Diversity Promotes Nature and Human Well-Being. Traditional Knowledge, pg. 15. Secretariat, Convention on Biodiversity, Montreal, Canada. http://www.cbd.int/doc/publications/cbd-sustain-en.pdf. Accessed September 2011.
CEWG (Canadian Eel Working Group). 2009. American Eel Management Plan, Draft: February 26, 2009. Fisheries and Oceans Canada, Ontario Ministry of Natural Resources, and Ministère des Ressources naturelles et de la Faune du Québec.
Chapman, D.W. 1978. Production in fish populations. Pp. 5-25, in S. Gerking (ed.). Ecology of Freshwater Fish Production. John Wiley & Sons. New York – Toronto. 520 pp.
Chaput, G. and D. Cairns. 2011. Mortality reference points for the American Eel and an application for evaluating cumulative impacts of anthropogenic activities. Canadian Science Advisory Secretariat, Research Document 2011/053. Fisheries and Oceans Canada. 28 p. Available: http://www.dfo-mpo.gc.ca/Csas-sccs/publications/resdocs-docrech/2011/2011_053-eng.pdf. Accessed September 2011.
Chilton, E.W., II. 1997. Freshwater fishes of Texas. Texas Parks and Wildlife Press. 98 pp.
Christie, W.J. and H.A. Regier. 1973. Temperature as major factor influences reproductive success of fish – two examples. Rapports et Procees Verbaux das Réunions, Conseil Internationale pur l'Exploration du la Mer 164:208-218.
Circle of All Nations. Date unknown. Available: http://www.angelfire.com/ns/circleofallnations/page2.html. Accessed September 2011.
Clark, J.H. 2009. The American Eel fishery in Delaware: recent landings, trends and characteristics of the exploited eel population. Pp. 229-239, in J.M. Casselman and D.K. Cairns (eds.). Eels at the edge: science, status and conservation concerns. American Fisheries Society, Symposium 58, Bethesda, Maryland.
Clermont, N. and C. Chapdelaine. 1998. Île Morrison, Lieu sacré et atelier de l'Archaïque dans l'Outaouais, Paléo-Québec 28, Musée Canadien Des Civilisations et Recherches amérindiennes au Québec, Montréal, Québec.
Clermont, N., C. Chapdelaine, and J. Cinq-Mars. 2003. Île aux Allumettes, L'Archaïque supérier dans L'Outaouais, Paléo-Québec 30, Musée Canadien Des Civilisations et Recherches amérindiennes au Québec, Montréal, Québec.
Collares-Pereira, M.J. and I.G. Cowx. 2004. The role of catchment scale environmental management in freshwater fish conservation. Fisheries Management and Ecology 11:303-312.
Community Stewardship Council of Lanark County. 2010. American Eel Hydro dam tail waters monitoring project 2009: assessment of mortalities on the Ottawa and Mississippi Rivers. Available: http://www.lanarkstewardshipcouncil.ca/pdf/final%20eel%20report%202009.pdf. Accessed September 2011.
Cook, P.M., J.A. Robbins, D.D. Endicott, K.B. Lodge, P.D. Guiney, M.K. Walker, E.W. Zabel, and R.E. Peterson. 2003a. Effects of aryl hydrocarbon receptor-mediated early life stage toxicity on lake trout populations in Lake Ontario during the 20th century. Environmental Science and Technology 37(17):3864-3877.
Cook, T.C., G.E. Hecker, S.V. Amaral, P.S. Stacy, F. Lin, and E.P. Taft. 2003b. Final report – Pilot scale tests Alden/Concepts NREC Turbine - U.S. Department of Energy, Advance Hydropower Turbine Systems Program. Contract No. DE-AC07-99ID13733. 504 pp.
COSEWIC 2006. COSEWIC assessment and status report on the American Eel Anguilla rostrata in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 71 pp. Available: http://dsp-psd.pwgsc.gc.ca/Collection/CW69-14-458-2006E.pdf [link inactive]. Accessed September 2011
COSEWIC. 2012. COSEWIC assessment and status report on the American Eel Anguilla rostrata in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xii + 109 pp. (http://www.registrelep-sararegistry.gc.ca/default_e.cfm). Available: http://publications.gc.ca/collections/collection_2013/ec/CW69-14-458-2012-eng.pdf. Accessed August 2013
Côté C. L., M. Castonguay|, G. Verreault and L. Bernatchez. 2009. Differential effects of origin and salinity rearing conditions on growth of glass eels of the American Eel Anguilla rostrata: implications for stocking programmes. Journal of Fish Biology (2009) 74, 1934–1948. Available: ftp://ftp.mrnf.gouv.qc.ca/Public/DEFH/Publications/2009/Cote%20et%20al%202009_Differential%20effects%20of%20origin%20and%20salinity%20rearing.pdf. Accessed August 2013
Côté C.L., Gagnaire P-A, Bourret V, Verreault G, Castonguay M, Bernatchez L. 2013. Population genetics of the American Eel (Anguilla rostrata): FST = 0 and NAO effects on demographic fluctuations of a panmictic species. Molecular Ecology. 22: 1763-1776.
CSAS (Canadian Science Advisory Secretariat). 2011. Status Of American Eel and Progress on Achieving Management Goals; Canadian Science Advisory Secretariat Science Advisory Report 2010/062; Fisheries and Oceans Canada, National Capital Region.
Daverat, F., K.E. Limburg, I. Thibault, J.C. Shiao, J.J. Dodson, F. Caron, W.N. Tzeng, Y. Iizuka , and H. Wickström. 2006. Phenotypic plasticity of habitat use by three temperate eel species, Anguilla anguilla, A. japonica and A. rostrata. Marine Ecology Progress Series 308:231-241.
Dekker W., J.M. Casselman, D.K. Cairns, K. Tsukamoto, D. Jellyman, and H. Lickers. 2003. Worldwide decline of eel resources necessitates immediate action: Québec declaration of concern. Fisheries 28:28-30.
de Lafontaine, Y., M. Lagace, F. Gingras, D. Labonte, F. Marchand, and E. Lacroix. 2009b. Decline of the American Eel in the St. Lawrence River: effects of local hydroclimatic, in D.K. Cairns (ed.). Eels at the Edge: Science, Status, and Conservation Concerns. American Fisheries Society Symposium 58:207-228.
de Lafontaine, Y., P. Gagnon, and B. Côté. 2009a. Abundance and individual size of American Eel (Anguilla rostrata) in the St. Lawrence River over the past four decades. Hydrobiologia 647:185-198.
Denny, S., A. Denny and P. Tyson. 2012. Kataq. Mi'kmaq ecological knowledge : Bras d'Or lakes eels. Unama'ki Institute of Natural Resources, Eskasoni, N.S. 24 p. Available : http://www.uinr.ca/wp-content/uploads/2012/02/Eel-MEK-WEB.pdf
Desroches, D. 1995. Suivi de la migration de l'anguille d'Amerique (Anguilla rostrata) au complexe Beauharnois, 1994. Rapport préparé par Milieu & Associes inc. Pour la vice-presidence Environnement, Hydro-Québec, Montréal, Québec.
Desrochers, D. 2000. Passe migratoire à anguille (Anguilla rostrata) au barrage de Chamby et Étude de la migration des anguilles juvéniles du Saint-Laurent. January 2000. Milieu Inc.
DFO (Fisheries and Oceans Canada). 2004. Fisheries and Oceans Canada news release September 17, 2004, DFO and provinces release American Eel management strategy. Available: http://www.dfo-mpo.gc.ca/media/npress-communique/2004/hq-ac79-eng.htm. Accessed September 2011. [link inactive]
DFO (Fisheries and Oceans Canada), 2005. Fish Habitat & Determining the High Water Mark on Lakes: Fact Sheet T-6. Her Majesty the Queen in Right of Canada. ISBN: 0-662-40041-0
DFO (Fisheries and Oceans Canada). 2007. Isolated or dry open-cut stream crossings. Available: http://www.dfo-mpo.gc.ca/habitat/what-quoi/os-eo/qc/pdf/stream-eng.pdf. [link inactive] Accessed September 2011.
DFO (Fisheries and Oceans Canada). 2010. Underwater world. American Eel. Available: http://www.dfo-mpo.gc.ca/science/publications/uww-msm/articles/eel-anguille-eng.htm. Accessed September 2011. [link inactive]
Dönni, W., K-J. Maier, and H. Vicentini. 2001. Bestandsentwicklung des Aals (Anguilla anguilla) im Hochrhein. Mitteilung Zur Fischeri, BUWAL, Bern, Germany. du Creux, F. 1664.
Dumaresq, C. 2006. Cobalt mining legacy. Available: http://cobaltmininglegacy.ca/mininghistory.php. Accessed September 2011.
Dutil, J.-D., B. Legare, and C. Desjardins. 1985. Discrimination d'un stock de poisson,I'anguille (Anguilla rostrata), basee sur la presence d'un produit chimique de synthese, le mirex. Canadian Journal of Fisheries and Aquatic Sciences 42:455-458.
Dutil, L., D. Belanger, and C.M. Couillard. 1997. A telephone survey of eel fishermen regarding external lesions and mortalities of American eels (Anguilla rostrata) from Lake Ontario and the St. Lawrence River basin, Canada. Preventative Veterinary Medicine 31(1-2):33-49.
Dymond, J.R. 1939. The fishes of the Ottawa region. Contributions of the Royal Ontario Museum of Zoology15, Toronto, Ontario.
Edeline, E., L. Beaulaton, R. le Barb, and P. Elie. 2007. Dispersal in metamorphosing juvenile eel Anguilla anguilla. Marine Ecology Progress Series 344:213-217.
Elmer, J.D. and S.P. Murphy. 2007. Improving fish habitat in the Oswego River. Hydro Review, September 2007:16-21.
Environment Canada. 2005. How much habitat is enough? Available: http://www.on.ec.gc.ca/wildlife/factsheets/fs_habitat-e.html. Accessed September 2011.
EPRI (Electric Power Research Institute). 1999. American eel (Anguilla rostrata) scoping study: a literature and data review of life history, stock status, population dynamics, and hydroelectric impacts. Report TR-111873, Palo Alto, California.
EU (European Union). 2007. Council Regulation (EC) No. 1100/2007 of 18 September 2007 establishing measures for the recovery of the stock of European eel. Official Journal of the European Union, 22.9.2007, L 248/17. Available: http://eur-lex.europa.eu/LexUriServ/site/en/oj/2007/l_248/l_24820070922en00170023.pdf. [link inactive] Accessed September 2011.
Everhart, W.H., A.W. Eipper, and W.D. Youngs. 1975. Principles of Fishery Science. Cornell University Press. Ithaca and London. 288 pp.
Fenske, K., M.J. Wilberg, D.H. Secor and M.C. Fabrizio. 2011. An age- and sex-structured assessment model for Amercian eels (Anguilla rostrata) in the Potomac River, Maryland. Canadian Journal of Fisheries and Aquatic Sciences 68:1024-1037.
Feunteun, E., P. Laffaille, T. Robinet, C. Briand, A. Baisez, J.M. Olivier, and A. Acou. 2003. A Review of upstream migration and movements in inland waters by anguillid eels: toward a general theory. Pp. 191-213, in K. Aida, K. Tsukamoto, and K. Yamauchi (eds.). Eel biology. Springer Verlag, Tokyo.
Field, J., C.L. Moloney, L. du Buisson, A. Jarre, T. Stroemme, M.R. Lipinski and P. Kainge. 2008. Exploring the BOFFFF hypothesis using a model of Southern African deepwater hake (Merluccius paradoxus). Pp. 17-26, in K. Tsukamoto, T. Kawamura, T. Takeuchi, T. D. Beard, Jr. and M.J. Kaiser (eds.). Fisheries for Global Welfare and Environment, 5th World Fisheries Congress 2008.
Freeman, M.C., C.M. Pringle, E.A. Greathouse, and B.J. Freeman. 2003. Ecosystem-level consequences of migratory faunal depletion cause by dams. American Fisheries Society Symposium 35: 255-266.
Friedland, K.D., D.J. Reddin, J.R. McMenemy, and K.F. Drinkwater. 2003. Multidecadal trends in North American Atlantic salmon (Salmo salar) stocks and climate change trends relevant to juvenile survival. Canadian Journal of Fisheries and Aquatic Sciences 60(5):563-583.
Friedland, K.D., M.J. Miller, and B. Knights. 2007. Oceanic changes in the Sargasso Sea and declines in recruitment of the European eel. ICES Journal of Marine Science 64:519-530.
Fries, L.T., J.T. Williams, and S.K. Johnson.1996. Occurrence of Anguillicola crassus, an exotic parasitic swim bladder nematode of eels, in the southeastern United States. Transactions of the American Fisheries Society 125:704-797.
Gagnaire, P.A., C.L. Côté, M. Hansen, M. Castonguay, and L. Bernatchez. The Genetic Consequences of Spatially Varying Selection in the Panmictic American Eel (Anguilla rostrata. Genetics. 2012 February; 190(2): 725–736
Gartner Lee Ltd. 2002. Shoreline Environmental Studies in Support of Official Plan Policies. Prepared for the Corporation of the City of the Kawartha Lakes. August 2002. Available: http://www.city.kawarthalakes.on.ca/residents/planning-building/studies-reviews/report.pdf. Accessed September 2011 [link inactive].
George, J. (Washaghezik). 1914. Monsters. Certain Ojibwa Myths by Col. Geo. E. Laidlaw, in 26th Annual Archaeological Report Ontario Being an Appendix to the Report of the Minister of Education Ontario. King’s Printer. Printed by Order of the Legislative Assembly, Toronto, Ontario.
Gephard, S., and J. McMenemy. 2004. An overview of the program to restore Atlantic salmon and other anadromous fishes to the Connecticut River, with notes on the current status of these species in the river. Pp. 287-317, in P.M. Jacobson, D.A. Dixon, W.C. Leggett, B.C. Marcy, Jr., and R.R. Massengill (eds.). The Connecticut River ecological study (1965-1973) revisited: ecology of the lower Connecticut River, 1973-2003. American Fisheries Society, Monograph 9, Bethesda, Maryland.
Gibson, R.B. 2005. Sustainability Assessment Criteria and Processes. Earthscan, London, U.K.
Gill, T. 1908. Life history of the common eel. Transactions of the American Fisheries Society 37(1):115-121.
GLFC (Great Lakes Fishery Commission). 2002. Position statement on American Eel. Available: www.glfc.org/lakecom/loc/eelposition.htm. [link inactive] Accessed September 2011.
Glova, G.J. 2002. Density effects on juvenile shortfinned eel (Anguilla australis) cover preferences in replicate channels. New Zealand Journal of Marine and Freshwater Research 36(3):483-490. Available: http://dx.doi.org/10.1080/00288330.2002.9517103. Accessed September 2011.
Glova, G.J. D.J. Jellyman, and M.L. Bonnet. 1998. Factors associated with the distribution and habitat of eel (Anguilla spp.) in three New Zealand lowland streams. New Zealand Journal of Marine and Freshwater Research 32(2): 255-269. Available: http://www.tandfonline.com/doi/pdf/10.1080/00288330.1998.9516824. Accessed September 2011.
GMCME (Gulf of Maine Council on the Marine Environment). 2007. American Eels: restoring a vanishing resource in the Gulf of Maine. 12 pp. Available: http://www.wildlife.state.nh.us/marine/marine_PDFs/American_Eels_GulfOfMaine.pdf. Accessed September 2011. [link inactive]
Goode, A. 2006. The plight and outlook for migratory fish in the Gulf of Maine. Journal of Contemporary Water Research and Education 14:23-28. Available: http://www.ucowr.org/updates/134/5.pdf. [link inactive] Accessed September 2011.
Goode, G.B. 1881. The eel question. Transactions of the American Fisheries Society 10(1):81-124.
Goodwin, K. R., and P. L. Angermeier. 2003. Demographic characteristics of American eel in the Potomac River drainage, Virginia. Transactions of the American Fisheries Society 132:524–535.
Government of Canada. 2012. Parliament of Canada: Bill C-38. Available: http://www.parl.gc.ca/HousePublications/Publication.aspx?DocId=5524772
Greig, L., I.J. Parnell, and D.R. Marmorek. 2006. Developing an action plan for American Eels in the St. Lawrence River – Lake Ontario Region: Decision analysis. Prepared by ESSA Technologies Ltd., Richmond Hill, Ontario, for Hydro-Québec, Fisheries and Oceans Canada, Ontario Ministry of Natural Resources, Ontario Power Generation, and the U.S. Fish & Wildlife Service, on behalf of the Passage and Associated Habitat Subcommittee of the Canadian Eel Working Group. 155 pp.
Guillemette, S. and D. DesRochers. 2011. Suivi des passes migratoires à anguille à la centrale de Beauharnois et au barrage de Chambly – 2011, [par] Milieuinc., [pour] l'unité Gestion des actifs et conformité réglementaire, division Production Hydro-Québec, 81 p. English title: Monitoring of the eel ladders at the Beauharnois Power Facility and at the Chambly Dam – 2011
Hammond, S.D. and S.A. Welsh. 2009. Seasonal movements of large yellow American Eels downstream of a hydroelectric dam, Shenandoah river, West Virginia. Pp. 309-323, in J.M. Casselman and D.K. Cairns (eds.). Eels at the edge: science, status, and conservation concerns. American Fisheries Society, Symposium 58, Bethesda, Maryland.
Haro, A.J., W. Richkus, K. Whalen, A. Hoar, W.D. Busch, S. Lary, T. Bush, and D. Dixon. 2000. Population decline of the American Eel: implications for research and management. Fisheries 25(9):7-16.
Hatch, R.W., S.J. Nepszy, K.M. Muth, and C.T. Baker. 1987. Dynamics of the recovery of the western Lake Erie walleye (Stizostedion vitreum vitreum) stock. Canadian Journal of Fisheries and Aquatic Sciences 44(Suppl. 2):15-22.
Hawkins, C.M. 1995. Environmental habitat quality requirements/guidelines for American Eel Anguilla rostrata. Document prepared for Habitat Management Division, Department of Fisheries and Oceans, Maritimes Region, Dartmouth, Nova Scotia.
Haxton, T.J. and C.S. Findlay. 2008. Variation in large-bodied fish community structure and abundance in relation to water management regime in a large regulated river. Journal of Fish Biology 74:2216-2238.
Heidenreich, C.E. 1971. The natural environment of Huronia and Huron seasonal activities. Marburger Geographische Schriften. Helft 50. Marburg/Lahn 1971.
Helfman, G.S., D.E. Facey, L.S. Hales, Jr., and E.L Bozeman, Jr. 1987. Reproductive ecology of the American Eel. Pp. 42-56, in M.J. Dadswell, R.L. Klauda, C.M. Moffitt, R.L. Saunders, R.A. Rulifson, and J.E. Cooper (eds.). Common strategies of anadromous and catadromous fishes. American Fisheries Society Symposium 1, Bethesda, Maryland.
Henke, J. 1993. Tales of Oneida Lake. North County Books. Utica, New York.
Hicks, B.J. 1997. Food webs in forest and pasture streams in the Waikato region, New Zealand: a study based on analyses of stable isotopes of carbon and nitrogen, and fish gut contents. New Zealand Journal of Marine and Freshwater Research 31:651-664.
Hitt, N. P., S. Eyler and John E. B. Wofford. 2012. Dam Removal Increases American Eel Abundance in Distant Headwater Streams, Transactions of the American Fisheries Society, 141:5, 1171-1179. Available: http://dx.doi.org/10.1080/00028487.2012.675918
Hoag, H. 2007. A slippery slope. The Globe and Mail, March 31 2007.
Hodson, P.V., M. Castonguay, C.M. Couillard, C. Desjardins, E. Pelletier, and R. McLeod. 1994. Spatial and temporal variations in chemical contamination of American eels, Anguilla rostrata, captured in the estuary of the St. Lawrence River. Canadian Journal of Fisheries and Aquatic Sciences 51:464-478.
Hoffmann, M. 2008. Modélisation de l'impact des ouvrages sur les densités d'anguilles dans le bassin Loire-Bretagne. Master II, Institut Universitaire Européen de la Mer de Brest.
Holling, C.S. 1973. Resilience and stability of ecological systems. Annual Review of Ecology and Systematics 4:1-23.
Honeyfield, D.C., M.E. Daniels, L.R. Brown, M.T. Arts, M.G. Walsh, and S.B. Brown. 2012. Survey of four essential nutrients and thiaminase activity in five Lake Ontario prey fish species. Journal of Great Lakes Research 38(1): 11-17.
Howard, B. C. 2012. American Eels: Freshwater species of the week. Water Currents, National Geographic, August 3, 2012. Available: http://newswatch.nationalgeographic.com/2012/08/03/american-eels-freshwater-species-of-the-week/
Humborg, C., V. Ittekkot, A. Cociasu, and B. Bodungen. 1997. Effect of Danube River dam on Black Sea biogeochemistry and ecosystem structure. Nature (London) 386:385-388.
Hutchings, J.A. and J.D. Reynolds. 2004. Marine fish population collapses: Consequences for recovery and extinction risk. Bioscience 54(4):297-309.
Hutchings, J.A. and R.W. Rangeley. 2011. Correlates of recovery for Canadian Atlantic cod (Gadus morhua). Canadian Journal of Zoology 89:386-400.
Ibbotson, A., J. Smith, P. Scarlett, and P. Aprahamian. 2002. Colonization of freshwater habitats by the European eel Anguilla anguilla. Freshwater Biology 47:1696-1706.
ICES (International Council for Exploration of the Sea). 2003. Report of the ICES/EIFAC Working Group on Eels. International Council for Exploration of the Sea, ICES CM 2003/ACFM:06, Copenhagen, Denmark.
Jessop, B.M. 1997. American Eel elvers and their fishery in the Scotia-Fundy area of Atlantic Canada: an overview. Pp. 134-143, in R.H Petersen (ed.). The American Eel in eastern Canada: stock status and management strategies, Canadian Technical Report of Fisheries and Aquatic Sciences 2196, Québec City.
Jessop, B.M. 2010. Geographic effects on American Eel (Anguilla rostrata) life history characteristics and strategies. Canadian Journal of Fisheries and Aquatic Sciences 67:326-346.
Jessop, B.M., J.C. Shiao, and Y. Iizuka. 2009. Life history of American Eels from Western Newfoundland. American Fisheries Society 138:861-871.
Jessop, B.M., J.C. Shiao, Y. Iizuka, and W.N. Tzeng. 2002. Migratory behaviour and habitat use by American Eels Anguilla rostrata as revealed by otolith microchemistry. Marine Ecology Progress Series 233:217-229.
Kerr, J.T. and I. Deguise. 2004. Habitat loss and the limits to endangered species recovery. Ecology Letters 7:1163-1169.
Kerr, J.T. and J. Cihlar. 2004. Patterns and causes of species endangerment in Canada. Ecological Applications, 14(3), 2004, pp. 743–753
Kleckner, R.C. and J.D. McCleave. 1988. The northern limit of spawning by Atlantic eels (Anguilla spp.) in the Sargasso Sea in relation to thermal fronts and surface water masses. Journal of Marine Research 46:647-667.
Knights, B. and E.M. White. 1998. Enhancing immigration and recruitment of eels: the use of passes and associated trapping systems. Fisheries Management and Ecology 4:311-324.
Kolenosky, D.P. and M.J. Hendry. 1982. The Canadian Lake Ontario fishery for American Eel (Anguilla rostrata). Pp. 8-16, in K.H. Loftus (ed.). Proceedings of the 1980 North American Eel Conference. Ontario Ministry of Natural Resources, Fisheries Branch, Ontario Fish Technical Series No. 4, Toronto.
Kraus, R. T. and D.H. Secor. 2005. Application of the nursery-role hypothesis to a marine fish. Marine Ecology Progress Series 291:301-305.
Krueger, W.H., and K. Oliveira. 1999. Evidence for environmental sex determination in the American Eel, Anguilla rostrata. Environmental Biology of Fishes 53:381-389.
LaBar, G.W. and D.F. Facey. 1983. Local movements and inshore population sizes of American Eels in Lake Champlain, Vermont. Transactions of the American Fisheries Society 112:111-115.
Laffaille, F., E. Feunteun, A. Acou, and J.C. Lefeuvre. 2000. Role of European eel (Anguilla anguilla L.) in the transfer of organic matter between marine and freshwater systems. Internationale Vereinigung fűr Theoretische und Angewandte Limnologie Verhandlungen 27:616-619.
Lambert, P., G. Verreault, B. Lévesque, V. Tremblay, J.-D. Dutil, and P. Dumont. 2011. Détermination de l'impact des barrages sur l'accès de l'anguille d'Amérique (Anguilla rostrata) aux habitats d'eau douce et établissement de priorités pour des gains en habitat. Rapport technique canadien des sciences halieutiques et aquatiques 2921: x + 43 pages.
LaMP. 2009. The Beautiful Lake: A binational biodiversity strategy for Lake Ontario. Prepared by the Lake Ontario Biodiversity Conservation Strategy Working Group In cooperation with the U.S. – Canada Lake Ontario Lakewide Management Plan. April 2009 (Updated July 2009).
Lamson, H.M., J.-C. Shiao, Y. Iizuka, W.-N. Tzeng, and D.K. Cairns. 2006. Movement patterns of American Eels (Anguilla rostrata) between salt-and freshwater in a coastal watershed, based on otolith microchemistry. Marine Biology 149:1567-1576.
Larinier, M. and F. Travade. 1999. La dévalaison des migrateurs: problèmes et dispositifs. Bulletin Français de Pisciculture 353/354:181-210.
Larinier, M. and J. Dartiguelongue. 1989. La circulation des poissons migrateurs: le transit à travers les turbines des installations hydroélectriques. Bulletin Français de la pêche et de la Pisciculture 312/313:1-87.
Larinier, M. and G. Marmulla. 2004. Fish passes: Types, principles and geographic distribution, an overview. In Proceedings of the Second International Symposium on the Management of Large Rivers for Fisheries Volume II. Pp. 283-205, in R. Welcomme and T. Petr (eds.), FAO Regional Office for Asia and the Pacific, Bangkok, Thailand. RAP publication 2004/17. Available: http://www.fao.org/docrep/007/ad526e/ad526e0g.htm#bm16. Accessed October 2011.
Larinier, M. 2008. Fish passage experience at small-scale hydro-electric power plants in France Hydrobiologia (2008) 609:97–108 DOI 10.1007/s10750-008-9398-9
Larinier, M., M. Chanseau, C. Rigaud, P. Steinbach. 2006. Éléments d'aide à la définition d'une stratégie de restauration des axes de migration de l'anguille. Rapport du Cemagref. 23 p.
Lary, S.J., W.-D. Busch, C.M. Castigione, and R. MacDonald. 1998. Distribution and availability of Atlantic Coast freshwater habitats for American Eel (Anguilla rostrata). U.S. Fish and Wildlife Service, Draft Administrative Report: 98-02
Lasne E, A. Acou, A. Vila-Gispert, P. Laffaille. European eel distribution and body condition in a river floodplain: effect of longitudinal and lateral connectivity. Ecology of Freshwater Fish 2008
Law, R. 2007. Fisheries-induced evolution: present status and future directions. Marine Ecology Progress Series 335:271-277.
Lawson, P.W., E.A. Logerwell, N.J. Mantua, R.C. Francis, and V.N. Agostini. 2004. Environmental factors influencing freshwater survival and smelt production in Pacific Northwest coho salmon (Oncorhynchus kisutch). Canadian Journal of Fisheries and Aquatic Sciences 61(3):360-373.
Lees, D. 2008. Eels on wheels. The Walrus. December 2008:42-57.
Legault, A. 1988. Le franchissement des barrages par l'escalade de l'anguille: étude en Sèvre Niortaise. Bulletin Français de la Pêche et de la Pisciculture 308:1-10.
Leprevost, G. 2007. Développement d'un indicateur pour caractériser l'impact migratoire sur le stock d'anguille européenne à l'échelle des bassins. Mémoire technique ONEMA-IAV, Rennes.
Liermann C.R., C. Nilsson, J. Robertson, and R.Y. Ng. 2012. Implications of dam obstruction for global freshwater fish diversity. BioScience 62, 539-548.
Livermore, R. 1914. Methods and Plant Employed in Pumping 400,000,000 Gallons of Water and Mud to Drain Kerr Lake, Cobalt, Ont. Engineering and Contracting 42:140-143. Available: http://www.archive.org/stream/engineeringcontr42chicuoft#page/140/mode/2up. Accessed September 2011.
Livermore, R. 1915. Draining Kerr Lake, Cobalt, Ont. Transactions of the American Institute of Mining Engineers XLIX: 328-342
Lookabaugh, P. S., and P. L. Angermeier. 1992. Diet patterns of American eel, Anguilla rostrata, in the James River drainage, Virginia. Journal of Freshwater Ecology 7:425–431.
MacGregor, R.B., A. Mathers, P. Thompson, J.M. Casselman, J.M. Dettmers, S. LaPan, T.C. Pratt, and W.A. Allen. 2008. Declines of American Eel in North America: Complexities associated with bi-national management. Pp. 357-381, in M.G. Schechter, W.W. Taylor, and N.J. Leonard (eds.). International governance of fisheries ecosystems: learning from the past, finding solutions for the future. American Fisheries Society, Bethesda, Maryland.
MacGregor, R.B., J.M. Casselman, W.A. Allen, T. Haxton, J.M. Dettmers, A. Mathers, S. LaPan, T.C. Pratt, P. Thompson, M. Stanfield, L. Marcogliese, and J.-D. Dutil. 2009. Natural heritage, anthropogenic impacts and bio-political issues related to the status and sustainable management of American Eel: A retrospective analysis and management perspective at the population level. Pp. 713-739, in A.J. Haro, K.L. Smith, R.A. Rulifson, C.M. Moffitt, R.J. Klauda, M.J. Dadswell, R.A. Cunjak, J.E. Cooper, K.L. Beal, and T.S. Avery (eds.). 2009. Challenges for Diadromous Fishes in a Dynamic Global Environment. American Fisheries Society, Symposium 69, Bethesda, Maryland.
MacGregor, R.B., L. Greig, J.M. Dettmers, W. Allen, T. Haxton, J.M. Casselman, L. McDermott. 2011. American Eel in Ontario: Past and Present Abundance, Principles, Approaches, Biological Feasibility and Importance of Recovery. Available: http://www.glfc.org/fishmgmt/AmericanEelinOntario.pdf. Accessed August 2011. [link inactive]
MacGregor, R.B., T. Haxton, J. Casselman, L. Greig, W.A. Allen, and L. McDermott (in press). The demise of American Eel in the Upper St. Lawrence River, Lake Ontario and associated watersheds: Implications of regional cumulative effects. Pp. XX-XX, in B. Sadler, N. Fisher, and A. Rose (eds.). Fish habitat management: policy, science, and practice. American Fisheries Society, Symposium 78, Bethesda, Maryland.
Machut, L.S. 2006. Population dynamics, Anguillicola crassus infection, and feeding selectivity of American Eel (Anguilla rostrata) in tributaries of the Hudson River, New York. M.Sc. Thesis, State University of New York, College of Environmental Science and Forestry, Syracuse, NY. 177 pp.
Machut, L.S. and K.E. Lindburg. 2008. Anglocolla crassus infection in Anguilla rostrata from small tributaries of the Hudson River, New York, USA. Diseases of Aquatic Organisms 79:37-45.
Machut, L.S., K.E. Limburg, R.E. Schmidt, and D. Dittman. 2007. Anthropogenic impacts on American Eel demographics in Hudson River tributaries, New York.
Maes G.E. and F.A.M. Volckaert. 2007. Challenges for genetic research in European eel management. ICES Journal of Marine Science 64:1463-1471.
Mandrak, N.E. and E.J. Crossman. 2003. Fishes of Algonquin Provincial Park, Friends of Algonquin Park, Whitney, Ontario.
Martin, N.V. and F.E.J. Fry. 1973. Lake Opeongo: the ecology of the fish community and of man’s effects on it. Great Lakes Fishery Commission, Technical Report 24, Ann Arbor, Michigan.
May, R.H. 1976. Models for two interacting populations. Pp. 49-70, in R.H. May (ed.). Theoretical Ecology: Principles and Applications. W.B. Saunders Company. Philadelphia – Toronto. 317 pp.
McCarthy, T.K., P. Frankiewicz, P. Cullen, M. Blaskowski, W. O'Connor, and D. Doherty. 2008. Long-term effects of hydropower installations and associated river regulation on River Shannon populations: mitigation and management. Hydrobiolgia 609:109-124.
McCleave, J.D. 1980. Swimming performance of European eel (Anguilla anguilla (L.)) elvers. Journal of Fish Biology 16:445-452.
McCleave, J.D. 2001. Simulation of the impact of dams fishing weirs on the reproductive potential of silver-phase American Eels in the Kennebec River basin. North American Journal of Fisheries Management 21:592-605.
McCleave, J.D. and E. Edeline. 2009. Diadromy as a conditional strategy: patterns and drivers of eel movements in continental habitats. Pp. 97-119, in A.J. Haro, K.L. Smith, R.A. Rulifson, C.M. Moffitt, R.J. Klauda, M.J. Dadswell, R. Cunjak, J.E. Cooper, K.L. Beal, and T.S. Avery (eds.). Challenges for diadromous fishes in a dynamic global environment. American Fisheries Society, Symposium 69, Bethesda, Maryland.
McCleave, J.D., R.C. Kleckner, and M. Castonguay. 1987. Reproductive sympatry of American and European eels and implications for migration and taxonomy. Pp. 186-297, in M.J. Dadswell, R.L. Klauda, C.M. Moffitt, R.L. Saunders, R.A. Rulifson, and J.E. Cooper (eds.). Common strategies of anadromous and catadromous fishes. American Fisheries Society, Symposium 1, Bethesda, Maryland.
McCormick, S.D., D.T. Lerner, M.Y. Monette, K. Nieves-Puigdoller, J.T. Kelly, and B.T. Bjornosson. 2009. Taking it with you when you go: How perturbations to the freshwater environment, including temperature, dams and contaminants, affect marine survival of salmon. Pp. 195-214, in A.J. Haro, K.L. Smith, R.A. Rulifson, C.M. Moffitt, R.J. Klauda, M.J. Dadswell, R. Cunjak, J.E. Cooper, K.L. Beal, and T.S. Avery (eds.). Challenges for diadromous fishes in a dynamic global environment. American Fisheries Society, Symposium 69, Bethesda, Maryland.
McCully, P. 1996. Silenced Rivers: The Ecology and Politics of Large Dams. Zed Books, London (U.K.). 350 pp.
McDermott, L, and P. Wilson. 2010. Ginawaydaganuk: Algonquin law on access and benefit sharing. Policy Matters 17: 205211.
McDowall, R.M. 1996. Diadromy and the assembly and restoration of riverine fish communities: a downstream view. Canadian Journal of Fisheries and Aquatic Sciences 53 (Supplement 1):213-236.
McDowall, R.M. 2009. Making the best of two worlds: diadromy in the evolution, ecology and conservation of aquatic organisms. Pp. 1-22, in A.J. Haro, K.L. Smith, R.A. Rulifson, C.M. Moffitt, R.J. Klauda, M.J. Dadswell, R.A. Cunjak, J.E. Cooper, K.L. Beal, and T.S. Avery (eds.). Challenges for diadromous fishes in a dynamic global environment. American Fisheries Society, Symposium 69, Bethesda, Maryland.
McGregor, E. 1994. Algonquin Lexicon, Kitigan Zibi Education Council, Maniwaki, Québec. 407 pp.
MdDNR (Maryland Department of Natural Resources). 1999. American Eel: Past, Present, and Future. An Eye on Maryland Streams. Maryland Biological Stream Survey Newsletter, March 1999, Vol 6 (1). Available: http://www.dnr.state.md.us/streams/news/march99/eel.html. Accessed September 2011. [link inactive]
Meek, A. 1916. The Migrations of Fish. London, Edward Arnold.
Merrick, J.R. and G.E. Schmida. 1984. Australian Freshwater Fishes: Biology and Management. Griffin Press, Netley, Australia.
Miller, M.J., S. Kamura, K.D. Friedland, B. Knights, H. Kim, D.J. Jellyman, and K. Tsukamoto. 2009. Review of ocean-atmosphere factors in the Atlantic and Pacific oceans influencing spawning and recruitment of Anguillid eels. Pp. 231-249, in A.J. Haro, K.L. Smith, R.A. Rulifson, C.M. Moffitt, R.J. Klauda, M.J. Dadswell, R.A. Cunjak, J.E. Cooper, K.L. Beal, and T.S. Avery (eds.). Challenges for Diadromous Fishes in a Dynamic Global Environment. American Fisheries Society, Symposium 69, Bethesda, Maryland.
Mills, E.L., J.M. Casselman, R. Dermott, J.D. Fitzsimmons, G. Gal, K.T. Holek. J.A. Hoyle. O.E. Johannsson, B.F. Lantry, J.C. Makarewicz, E.S. Millard, I.F. Munawar, M. Munawar, R. O'Gorman, R.W. Owens, L.G. Rudstram, T. Schaner, and T.J. Stewart. 2003. Lake Ontario: food web dynamics in a changing ecosystem (1970-2000). Canadian Journal of Fisheries and Aquatic Sciences 60:471-490.
Mitchell, C.P. and J.A.T. Boubée. 1992. Impacts of turbine passage on downstream migrating eels. New Zealand Freshwater Fisheries Miscellaneous Report No.112. Fisheries Research Centre, Rotorura.
MNRF (Ministère des Ressources naturelles et de la Faune). 2009. Une nouvelle mesure pour assurer le renouvellement de l'anguille d'Amerique. Communique de press, le 17 mars 2009. Cabinet du ministre delegue aux Ressources naturelles et à la Faune.
Montreal Gazette. 2005. Tembec pays $1 million dollars for polluting: Forest products firm Tembec Inc. has agreed to pay a $1-million fine for polluting the Ottawa River, the largest penalty ever slapped on any company under Quebec’s environment laws. November 10, 2005. Available: http://www.canada.com/montreal/montrealgazette/news/business/story.html?id=ae880800-e520-4dc7-93fe-b1f5ebc0559c [link inactive]
Moriarty, C. 1987. Factors influencing recruitment of the Atlantic species of Anguillid Eels. Pp. 483-491, in M.J. Dadswell, C.M. Moffit, R.L. Saunders, R.A. Rulifson, and J.E. Cooper (eds.). Common strategies of anadromous and catadromous fishes, American Fisheries Society Symposium 1, Maryland.
Morrison, W.E. and D.H. Secor. 2003. Demographic attributes of yellow-phase American Eels (Anguilla rostrata) in the Hudson River. Canadian Journal of Fisheries and Aquatic Sciences. 60:1487-1503.
Morrison, W.E., D.H. Secor, and P.M. Piccoli. 2003. Estuarine habitat use by Hudson River American Eels as determined by otolith strontium: calcium ratios. Pp. 87-99, in D.A. Dixon (ed.). Biology, Management, and Protection of Catadromous Eels. American Fisheries Society Symposium 33, Missouri.
Myers, R. A. 1998. When do environment-recruitment correlations work? Reviews in Fish Biology and Fisheries 8:285-305.
NatureServe. 2011. NatureExplorer: An online encyclopedia of of life [web application]. Version 7.1. NatureServe, Arlington, Virginia. Available: http://www.natureserve.org/explorer. Accessed August 2011.
New Liskeard Speaker. 1928. Temiskaming fishermen have successful season. Vol. 23(35):1. October 25, 1928.
New York Times, The. 1880. Catching Canadian eel: the fisheries of the St. Lawrence River. The New York Times, October 18.
Normandeau Associates Inc. 2001. Review and Reanalysis of Entrainment Studies at Dam No.4 Hydrostation (FERC#2516) Including an Analysis of Potential Entrainment/Mortality at Dam No. 5 Hydrostation (FERC #2517) and Impacts to Downstream Passage of American eel, Potomac River, Maryland/West Virginia. Available: http://esm.versar.com/pprp/bibliography/282001-0001/282001-0001-04.pdf. Accessed September 2011.
Normandeau Associates Inc. and J.R. Skalski. 1998. Draft final report. Estimation of survival of American Eel after passage through a turbine at the St. Lawrence-FDR power project. Prepared for New York Power Authority, White Plains, New York.
New York Power Authority. 2009. Review of technologies for guiding, capturing, holding, transporting and monitoring outmigrating eels. A report prepared for the New York Power Authority by Versar Inc. 384 pp.
O'Connor, J.F. 1971. Ecology of Brook Trout, American Smelt and American Eel in two lakes in the Matamek River system, Quebec. M.Sc. Thesis, University of Waterloo, Ontario, Canada. 80 pp + appendices.
Ogden, J.C. 1970. Relative abundance, food habits, and age of the American Eel, Anguilla rostrata (LeSueur), in certain New Jersey streams. Transactions of the American Fisheries Society 99:54-59.
Oliveira, K., and J.D. McCleave. 2000. Variation in population and life history traits of the American Eel, Anguilla rostrata, in four rivers in Maine. Environmental Biology of Fishes 59:141-151.
OMNR (Ontario Ministry of Natural Resources). 1971. Bancroft District Records, Big Gull Lake Files.
OMNR (Ontario Ministry of Natural Resources). 1984. Commercial fish production historic records, Northern Inland Waters 1924-1982. Written correspondence from K.J. Chambers to the Regional Directors North Central Region, Northern Region and Northeastern Region, Ontario Ministry of Natural Resources.
OMNR (Ontario Ministry of Natural Resources). 2007. American Eel in Ontario, Status of Resource Report, Ontario Ministry of Natural Resources. Available: http://www.mnr.gov.on.ca/stdprodconsume/groups/lr/@mnr/@sorr/documents/document/stel02_166010.pdf. Accessed September 2011. [link inactive]
OMNR (Ontario Ministry of Natural Resources). 2008a. Fisheries Management in Renfrew County: A State of the Resource Report and a Focussed Review of Fisheries Issues. January 2008. 100 pp.
OMNR (Ontario Ministry of Natural Resources). 2008b. Partners Support Action Plan to Bring Back American Eel: Native Species Important Part of Great Lakes Ecosystem. News release June 20, 2008. Available: http://news.ontario.ca/mnr/en/2008/06/partners-support-action-plan-to-bring-back-american-eel.html. Accessed September 2011.
OMNR/Québec MNRF (Ontario Ministry of Natural Resources/Ministère des Ressources Naturelles et de la Faune du Québec). 1999. A strategic fisheries management framework for the Ottawa River. Ontario Ministry of Natural Resources, Pembroke, Ontario.
Ontario Government. 1990. Lakes and Rivers Improvement Act. Available: http://www.e-laws.gov.on.ca/html/statutes/english/elaws_statutes_90l03_e.htm. Accessed September 2011.
Ontario Government. 2004. McGuinty government protects vanishing American Eel. Press Release April 2004. Available: http://www.waterkeeper.ca/2004/04/09/mcguinty-government-protects-vanishing-american-eel/. Accessed September 2011. [link inactive]
Ontario Government. 2007. Endangered Species Act, 2007. Ontario Government, Toronto. http://www.ontla.on.ca/web/bills/bills_detail.do?locale=en&BillID=1498&detailPage=bills_detail_the_bill. Accessed September 2011.
Ontario Government. 2008. O. Reg. 242/08. To establish new regulatory provisions under the Endangered Species Act, 2007 to allow certain activities to continue. Available: /laws/regulation/080242. Accessed September 2011.
Ontario Government. 2010. Ontario’s long-term report on the economy. Available: http://news.ontario.ca/mof/en/2010/01/positioning-ontario-for-long-term-economic-growth.html. Accessed September 2011.
Ottawa Citizen. 2008. City charged over raw sewage spill. The City of Ottawa Has Been Slapped With Two Water Resources Act Charges for a 15-Day Sewage Spill Into the Ottawa River, and if the City Is Convicted, the Fines Could Range From a Minimum of $25,000 to a Maximum of $6 Million for Each Day. August 1, 2008.
OWA (Ontario Waterpower Association). 2010. Best Management Practices: Guide for American Eel and Waterpower in Ontario. 120 pp.
Palumbi, S. 2004. Why mothers matter. Nature 430 (August 5 2004): 621-622.
Pauly, D. 1995. Anecdotes and the shifting baseline syndrome in fisheries. Trends in Ecology and Evolution 10:420.
PFBC (Pennsylvania Fish and Boat Commission). 2007. Migratory fish restoration and passage on the Susquehanna River. Available: http://www.fish.state.pa.us/pafish/shad/migratory_fish.pdf. Accessed September 2011. [link inactive]
Pinnegar, J.K. and G.H. Engelhard. 2008. The 'shifting baseline' phenomenon: a global perspective. Reviews in Fish Biology and Fisheries 18:1-16.
Pratt, T.C and A. Mathers. 2011. 2010 Update on the status of American Eel (Anguilla rostrata) in Ontario. Canadian Science Advisory Secretariat, Research Document 2011/050, Central and Arctic Region, Fisheries and Oceans Canada. 18 pp. Available: http://www.dfo-mpo.gc.ca/Csas-sccs/publications/resdocs-docrech/2011/2011_050-eng.pdf. Accessed September 2011.
Pratt, T.C., L.M. O'Connor, and R.W Threader. 2011. American Eel stocking effectiveness monitioring on the Upper St. Lawrence River and Lake Ontario. Draft Report prepared for the OPG Action Plan Executive Committee.
Prosper, K. and M.J. Paulette. 2002. The Mi'kmaq Relationship With Kat (American Eel) Scientific Name: Anguilla rostrata, Paqtnkek Fish and Wildlife Commission, Afton First Nation, Antigonish, Nova Scotia.
PRRT (Penobscot River Restoration Trust). 2009. Project details. Available: http://www.penobscotriver.org/. Accessed September 2011.
Purvis, F. 1887. Field Notes of Subdivision Survey of the Township of Hilliard. Field Note Book 1300. Available: Crown Surveys Office, Ontario Ministry of Natural Resources, Peterborough.
Pusey, B.J. and A.H. Arthington. 2003. Importance of the riparian zone to the conservation and management of freshwater fish: a review. Marine and Freshwater Research: 54:1-16. Available: http://www98.griffith.edu.au/dspace/bitstream/10072/6041/1/23131_1.pdf. Accessed September 2011. [link inactive]
Reading Eagle. 1902. Mysteries About Eels: enormous numbers in a turbine wheel-sudden disappearance. July 19:4.
Reid, K.B. and P. Meisenheimer. 2001. The decline of American Eel (Anguilla rostrata) in the Lake Ontario/St. Lawrence Ecosystem: A modelling approach to identification of data gaps and research priorities. Report Prepared for Lake Ontario Technical Committee by Beak International Incorporated, Ref.: 21852.10. Available: http://www.glfc.org/lakecom/loc/eel.pdf. Accessed September 2011. [link inactive]
Richards, A., F. Fogarty, S. Clark, D. Schick, P. Diodati, and B. O'Gorman. 1996. Relative influence of reproductive capacity and temperature on recruitment of Pandalus borealis in the Gulf of Maine. ICES C.M. K:13.
Richkus, W.A., and K.G. Whalen. 1999. American eel (Anguilla rostrata) scoping study report. Final report, March 1999 by Versar, Inc., prepared for Electric Power Research Institute.
Richkus, W.A., and K.G. Whalen. 2000. Evidence for a decline in the abundance of the American Eel, Anguilla rostrata (LeSueur), in North America since the early 1980s. Dana 12:83-97.
Ricker, W.E. 1975. Computation and interpretation of biological statistics of fish populations. Bulletin of the Fisheries Research Board of Canada 191.
Robison, H.W. and T.M. Buchanan. 1988. Fishes of Arkansas. The University of Arkansas Press, Fayetteville, Arkansas. 536 pp.
Russell, I.C. and E.C.E. Potter. 2003. Implications of the precautionary approach for the management of the European eel, Anguilla anguilla. Fisheries Management and Ecology 10:395-401.
Save the River. 2006. The Devastating 1976 NEPCO 140 Oil Spill. Fact Sheet. Available: http://www.savetheriver.org/docs/76_NEPCO_spill_fact_sheet.pdf. Accessed September 2011.
Schmidt, J. 1922. The breeding places of the eel. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences 211:179-208.
Schmidt, R.E, C.M. O'Reilly, and D. Miller. 2009. Observations of American Eels using an upland passage facility and effects of passage on the population structure. North American Journal of Fisheries Management 29:715-720.
Schmidt, R.E., R. Petersson, and T.E. Lake. 2006. Hudson River tributaries in the lives of fishes with emphasis on the American Eel. Pp. 317-330, in J.R. Waldman, K.E. Limburg and D. Strayer (eds.). Hudson River fishes and their environment. American Fisheries Society Symposium 51, Bethesda, Maryland.
Science Daily. 2007. Scientist on Quest for Disappearing Eel. December 27, 2007. Available: http://www.sciencedaily.com/releases/2007/12/071221173633.htm. Accessed September 2011.
Scott, W.B. and E.J. Crossman. 1973. Freshwater fishes of Canada. Bulletin 184, Fisheries Research Board of Canada, Ottawa, Canada.
Secor, D.H. 1999. Specifying divergent migrations in the concept of stock: the contingent hypothesis. Fisheries Research 43:13-34.
Secor, D.H. 2007. The year-class phenomenon and the storage effect in marine fishes. Journal of Sea Research 57:91-103.
Secor, D.H. 2010. Is otolith science transformative? New views on fish migration. Environmental Biology of Fishes 89(3-4):209-220.
Secor, D.H. and L.A. Kerr. 2009. Lexicon of life cycle diversity in diadromous and other fishes. Pp. 537-556, in A.J. Haro, K.L. Smith, R.A. Rulifson, C.M. Moffitt, R.J. Klauda, M.J. Dadswell, R.A. Cunjak, J.E. Cooper, K.L. Beal, and T.S. Avery (eds.). Challenges for diadromous fishes in a dynamic global environment. American Fisheries Society, Symposium 69, Bethesda, Maryland.
Sjoberg, N.B., E. Petersson, H. Wickstrom, and S. Hansson. 2009. Effects of the swimbladder parasite Anguillicola crassus on the migration of European silver eels Anguilla anguilla in the Baltic sea. Journal of Fish Biology 74:2158-2170.
Smith, M.W. and J.W. Saunders. 1955. The American Eel in certain fresh waters of the Maritime provinces of Canada. Journal of the Fisheries Research Board of Canada 12(2):238-269.
Smogor, R.A., P.L. Angermeier, and C.K. Gaylord. 1995. Distribution and abundance of American Eels in Virginia streams: Tests of null models across spatial scales. Transactions of the American Fisheries Society 124:789-803.
SOLEC (State of the Lake Ecosystem Conference). 2005. What are the current pressures impacting Lake Ontario? Available: http://www.epa.gov/solec/indicator_sheets/ontario.pdf. Accessed September 2011. [link inactive]
St. John Daily Sun. 1902. Mysteries About Eels: Enormous numbers in a turbine wheel – a sudden disappearance.
Stanley, D. and G. Pope. 2009. Research into trap and transport as a potential mitigation using traditional fisheries methods. OPG Action Plan for Offsetting Turbine Mortality of American Eel at the R.H. Saunders Generating Station (2006-2011) and OMNR/OPG Saunders ESA Agreement Monitoring
Requirements Part D. OPG Hydro Business, Environment Division, Niagara-on-the-Lake, Ontario. Final Draft.
Tesch, F.W. 1977. The eel: biology and management of anguillid eels. Chapman and Hall, London.
Therrien, J. 1999. Evaluation du taux de survie d'anguilles adultes passant par la centrale hydroélectrique de Saint-Lambert en 1998. Rapport du Groupe-Conseil Génivar inc. Pour Hydraska (St-Lambert) Inc. 24 pp. + annexes.
Thibault, I., J. Dodson, F. Caron, J.C. Shiao, Y. Iizuka, and W.N. Tzeng. 2005. Alternative migratory behavior of the American Eel (Anguilla rostrata) in the Saint-Jean River, Gaspé (Québec). Fish and Diadromy in Europe: Ecology, Management and Conservation Symposium, held March 29-April 1, Bordeaux, France. Preliminary results.
Threader, R.W., L. Blimke, and D. Groman. 2010. Taking stock in stocking – stocking of American Eel Anguilla rostrata in the Upper St. Lawrence River and Lake Ontario. Final Draft.
Thumbadoo, R.V. 2005. Learning from a kindergarten dropout – William Commanda – Ojigkwanong Cultural Sharings and Reflections. Circle of All Nations. Kanata, ON. 192 pp.
Thwaites, R.G. 1896 – 1901. The Jesuit Relations and Allied Documents. 73 Volumes. Burrows Bros., Cleveland, Ohio.
Trautman, M.B. 1981. The Fishes of Ohio. Ohio State University Press. 782 pp.
Tremblay, V. 2009. Reproductive strategy of female American Eels among five subpopulations in the St. Lawrence River watershed. Pp. 85-102, in J.M. Casselman and D.K. Cairns (eds.). Eels at the edge: science, status and conservation concerns. American Fisheries Society, Symposium 58, Bethesda, Maryland.
Tremblay, V., C. Cossette, J.-D. Dutil, G. Verreault and P. Dumont. 2011. Assessment of upstream passability for eel at dams. Canadian Technical Report of Fisheries and Aquatic Sciences 2912. Fisheries and Oceans Canada. 73 pp.
Tsukamoto, K., I. Nakai, and W.V. Tesch. 1998. Do all freshwater eels migrate? Nature 396(6712):635-636.
USFWS (United States Fish and Wildlife Service). 2011a. The American Eel: Considering endangered species act protection. Available: http://www.fws.gov/northeast/newsroom/eels.html. Accessed September 2011.
USFWS (U.S. Fish and Wildlife Service). 2011b. Endangered and threatened wildlife and plants; 90-day finding on a petition to list the American eel as threatened. Federal Register 76:189 (29 September 2011):60431–60444.
van Ginneken, V.J. and G.E. Maes. 2005. The European eel (Anguilla anguilla, Linnaeus), its lifecycle, evolution and reproduction: a literature review. Reviews in Fish Biology and Fisheries 15:367-398.
Velez-Espino, L.A. and M.A. Koops. 2009. A synthesis of the ecological processes influencing variation in life history and movement patterns of American Eel: towards a global assessment. Reviews in Fish Biology and Fisheries 20:163-186. Available: http://www.springerlink.com/content/g5662h2487154p72/. Accessed September 2011.
Venturelli, P.A., C.A. Murphy, B.J. Shuter, T.A. Johnston, P.J. Van Coeverden de Grot, P.T. Boag, J.M. Casselman, R. Montgomerie, M.D. Wiegland, and W.C. Leggett. 2010. Maternal influences on population dynamics: evidence from an exploited freshwater fish. Ecology 91(7): 2003-2012.
Verdun, R., D. Desrochers, and P. Dumont. 2003. Recruitment of American Eels in the Richelieu River and Lake Champlain: Provision of Upstream Passage as a Regional-Scale Solution to a Large-Scale Problem. American Fisheries Society Symposium 33: 125-138.
Verreault, G. and P. Dumont. 2003. An estimation of American Eel escapement from the upper St. Lawrence River and Lake Ontario in 1996 and 1997. Pp. 243-251, in D.A. Dixon (ed.). Biology, management and protection of catadromous eels. American Fisheries Society, Symposium 33, Bethesda, Maryland.
Verreault, G., P. Dumont, and Y. Mailhot. 2004. Habitat losses and anthropogenic barriers as a cause of population decline for American Eel (Anguilla rostrata) in the St. Lawrence watershed, Canada. International Council for the Exploration of the Sea. CM Document 2004/S:04, Copenhagen, Denmark. Available: http://www.ices.dk/products/CMdocs/2004/S/S0404.pdf [link inactive]
Verreault, G., P. Dumont, J. Dussureault and R. Tardif. 2010. First record of migrating silver American Eels (Anguilla rostrata) in the St. Lawrence estuary originating from a stocking program. Journal of Great Lakes Research 36:794-797.
Verreault, G., P. Pettigrew, R. Tardif, and G. Pouliot. 2003. The exploitation of the Migrating Silver American Eel in the St. Lawrence River Estuary, Québec, Canada. Pp. 225-234, in D.A. Dixon (ed.). Biology, management, and protection of catadromous eels. American Fisheries Society, Symposium 33, Bethesda, Maryland.
Verreault G, W. Dargere, and R. Tardif. 2009. American Eel (Anguilla rostrata) movements, growth and sex ratio following translocation. Pp. 129-136, in J.M. Casselman and D.K. Cairns (eds.). Eels at the edge: science, status and conservation concerns. American Fisheries Society, Symposium 58, Bethesda, Maryland.
Vladykov, V.D. and P.K.L. Liew. 1982. Sex of Adult American Eels (Anguilla rostrata) Collected as Elvers in Two Different Streams Along the Eastern Shore of Canada, and Raised in the Same Freshwater Pond in Ontario. Proceedings of the 1980 North American Eel Conference; Ontario Fisheries Technical Report Series 4:6.
Vörösmarty, C.J., K.P. Sharma, B.M. Fekete, A.H. Copeland, J. Holden, J. Marble, and J.A. Lough. 1997. The storage and aging of continental runoff in large reservoir systems of the world. Ambio 26(4): 210-219.
Vörösmarty, C.J., P.B. McIntyre, M.O. Gessner, D. Dudgeon, A. Prusevich, P. Green, S. Glidden, S.E. Bunn, C.A. Sullivan, C. Reidy Liermann, and P.M. Davies. 2010. Global threats to human water security and river biodiversity. Nature 467:555-561.
Walters, C. and J.-J. Maguire. 1996. Lessons for stock assessment from the northern cod collapse. Fish Biology and Fisheries 6:125-137.
Watene, E.M. and J.A.T. Boubée. 2005. Selective opening of hydroelectric dam spillway gates for downstream migrant eels in New Zealand. Fisheries Management and Ecology 12(1):69-75.
WCD (World Commission on Dams). 2000. Dams and Development: A new framework for decision-making. Report of the World Commission on Dams. earthscan Publications, London and Sterling VA. 404 pp.
Weeder, J.A. and J.H. Uphoff Jr. 2009. Are American Eel Harvests in Maryland’s Chesapeake Bay Sustainable? Pp. 347-358, in J.M. Casselman and D.K. Cairns (eds.). Eels at the edge: science, status and conservation concerns. American Fisheries Society, Symposium 58, Bethesda, Maryland.
White, E.M. and B. Knights. 1997. Dynamics of upstream migration of the European eel Anguilla anguilla (L.), in the rivers of Severn and Avon, England, with special reference to the effects of man-made barriers. Fisheries Management Ecology 4:311-324.
Wiley, D.J., R.P. Morgan II, R.H. Hilderbrand, R.L. Raesly, and D.L. Shumway. 2004. Relations between physical habitat and American Eel abundance in five river basins in Maryland. Transactions of the American Fisheries Society 133:515-526.
Williams, B. and R. Threader. 2007. A review of the proceedings and outcomes of the workshop on the American Eel, Anguilla rostrata, stocking in Canadian waters. Montreal, Canada, March 27-28, 2007. Report developed for Ontario Power Generation, Renfrew, Ontario.
Wirth, T. and L. Bernatchez. 2003. Decline of North Atlantic eels: a fatal synergy? Proceedings of the Royal Society of London, Series B: Biological Sciences 270:681-688.
Young, J.K. 1970. Lake Nipissing-Temagami Fisheries Management Unit Report on the fish populations of Lake Nipissing: the growth, distributions and habits (a preliminary report). Available at the Ministry of Natural Resources office in North Bay, Ontario.
Personal communications
Allen, W.A., pers. comm. 2010 and 2011. Heritage One.
Bartlett, R., pers. comm. 2010. Former Lake Temiskaming commercial fisherman. Bendig, A., pers. comm. 2009 and 2011. Ontario Ministry of Natural Resources. Bernatchez, L., pers. comm. 2010. Université Laval.
Casselman, J., pers. comm. 2009 and 2010. Queens University.
Commanda, W., pers. comm. 2008. Manoshkadosh the American Eel. Written statement by Elder W. Commanda concerning the American Eel: on file with the Ontario Ministry of Natural Resources and Plenty Canada.
Commanda, W., pers. comm. 2009. William Commanda’s reflections in correspondence to the Governor General and to the Queen regarding his appointment to Officer of the Order of Canada and his work. Correspondence on file with the Ontario Ministry of Natural Resources and Plenty Canada.
Coulson, D., pers. comm. 2010. Ontario Ministry of Natural Resources.
Dumont, P., pers. comm. 2011. Ministère des Ressources naturelles et de la Faune. Dunnville District Heritage Association, pers. comm. 2012. Huntin' and Fishin': Excerpts from the Reform Press, Dunnville Gazette and Dunnville Chronicle 1884-1909., 20 pp. Unpublished Manuscript. On File at the Dunnville District Heritage Association, Dunnville Public Library, Dunnville, Ontario.
Fitzsimmons, J., pers. comm. 2009 and 2011. Fisheries and Oceans Canada.
Haxton, T., pers. comm. 2009. Ontario Ministry of Natural Resources.
Hodson, P., pers. comm. 2011. Queen’s University, Department of Biology and School of Environmental Studies.
Honeyfield, D., pers. comm. 2011. US Geological Survey. Lickers, H., pers. comm. 2009. Akwesasne First Nations.
Mathers, A., pers. comm. 2009 and 2010. Ontario Ministry of Natural Resources. McCauley, C., pers. comm. 2009. Ontario Ministry of Natural Resources. McDermott, L., pers. comm. 2009. Plenty Canada and Algonquin First Nations. McLaren, D., pers. comm. 2011. Former Lake Temiskaming commercial fisherman. Pratt, T., pers. comm. 2010. Fisheries and Oceans Canada.
Punt, K., pers. comm. 2009 and 2010. Ontario Ministry of Natural Resources. Ross, S., pers. comm. 2011. Algonquin First Nations.
VanLeeuwen, G., pers. comm. 2011. Ontario Ministry of Natural Resources, via G.
Davies, former owner of Young’s Camp, Mowat Landing, Lake Temiskaming. Velez-Espino, L., pers. comm. 2009. Fisheries and Oceans Canada.
Verreault, G., pers. comm. 2009 and 2010. Québec Ministère des Ressources naturelles et de la Faune.
Warrick, G., pers. comm., 2011. Laurier University. Whetung, M., pers. comm. 2009. Curve Lake First Nation.
Yagi, A., pers. comm. 2009. Ontario Ministry of Natural Resources.
Recovery strategy development team members
| Name | Affiliation |
|---|---|
| Rob MacGregor (chair) | OMNR (retired) |
| Alastair Mathers | OMNR |
| Amy Boyko | DFO |
| Anne Bendig | OMNR (retired) |
| Anne Yagi | OMNR |
| William Allen | Heritage One, Burk’s Falls |
| Brad Steinberg | OMNR |
| Cam Mccauley | OMNR |
| Jeff Beaver | Plenty Canada, Lanark |
| John Casselman | Queens University, Kingston |
| Rebecca Geauvreau | OMNR |
| Kevin Reid | Ontario Commercial Fisheries Association |
| Kirby Punt | OMNR |
| Larry McDermott | Shabot Obaadjiwan First Nation; Plenty Canada, Lanark |
| Lorne Greig | ESSA Technologies Ltd., Richmond Hill |
| Marie-Andree Carriere | OMNR |
| Sarah Nugent | OMNR |
| Stuart Niven | DFO |
| Thomas Hoggarth | DFO |
| Tim Haxton | OMNR |
| Tom Pratt | DFO |
| John Dettmers | GLFC |
| Jason Borwick | OMNR |
| Krista Coppaway | Curve Lake First Nation and Plenty Canada |
| Henry Lickers | Akwesasne First Nation |
Appendix 1. Strengthening our relationship
The collaborative effort to develop this strategy through integration of our shared knowledge is a strong example of current efforts to work together to ensure sustainable use of shared resources.
Elder William Commanda carries a Wampum Belt
The recovery team adhered to the agreement recorded in the Welcoming Belt throughout the preparation of this recovery strategy. This strengthening of our relationship is a process that Aboriginal people have anticipated for many generations as foretold in the Sacred Seven Fire Prophecy Wampum Belt, which dates from the late 1400s and which Elder William Commanda also carries for the people.
The seventh prophet talked about a time of choice-making for all – for continued exploitation of land and peoples, or for a renewed respect for Mother Earth and reconciliation between indigenous peoples and the newcomers. The double diamond at the centre of this eight-diamond belt reflects the hope for unity to emerge out of the duality. ."Thumbadoo 2005
Elder William Commanda wrote that "the prophesy tells us that humanity is now at a cross roads, and that we urgently need to evaluate and transform our relationship with Mother Earth and each other" (Commanda 2007). The emergence of concern for the status of endangered species such as American Eel is understood to reflect the unfolding of the seventh prophesy.
Appendix 2. Aboriginal peoples' american eel resolution
The following resolution was created and endorsed during a November 2008 workshop with Aboriginal peoples convened by Fisheries and Oceans Canada and the Ontario Ministry of Natural Resources. The purpose of the meeting was to discuss the listing of American Eels in Ontario as endangered under Ontario’s ESA, the participation of Aboriginal peoples in the development of a Recovery Strategy for the American Eel in Ontario as required by legislation and to seek input on the federal government’s draft National Management Plan and proposed listing of American Eel as special concern under the federal Species at Risk Act. The workshop was attended and endorsed by representatives of the Algonquin and Mi'kmaq First Nations as well as some from Curve Lake Reserve on the Kawartha Lakes. The following is the resolution written and signed by all First Nations in attendance:
We, the Aboriginal people who have attended the Eastern Ontario-Western Québec workshop November 22-24, 2008 appreciate the guidance of our Elders who directed us on the way to address the decline of the American Eel, support the National Management plan guiding principles as amended during this workshop.
We wish to communicate the following, to ensure this ancient fish remains in the full historical range of its habitat and returns to waters from which it has been extirpated.
It was the unanimous decision that the status of the American Eel must be listed nationally as threatened under the SARA.
Our collective Aboriginal responsibilities with the American Eel remain vitally important to us even though our relationship with the eel has been put into jeopardy.
We also reaffirm our responsibilities to our Aboriginal brothers and sisters whose strong relationship with the American Eel is impacted by decisions made in our respective territories.
All development and fisheries management decisions must be guided by the precautionary principle and cumulative impacts must be assessed both on a watershed basis and on the basis that the American Eel, Anguilla rostrata, comes from one genetic stock.
Recognizing that if the American Eel is to recover, both habitat and recruitment issues must be addressed. Therefore, ambitious plans must be implemented immediately to enhance fish passage, reduce harvest and increase recruitment.
The Glass eel fishery for export must be closed, in order to achieve the objective for increased recruitment. Glass eels must be made available for conservation stocking, but only as a temporary measure until long term solutions are achieved to address declining abundance and recruitment.
Aboriginal peoples' ways of knowing and western science must be integrated equally in a full and respectful way in the decision making and implementation of the management plan.
Appendix 3. Considerations for watershed-based implementation plans
Watershed-based implementation plans for American Eel should include but not be limited to the following considerations.
- Use a GIS-based decision support tool and a decision analysis process in determining the best options for mitigation and recovery on each key watershed.
- Establish watershed-specific performance measures in the Watershed Implementation Plan for the following metrics:
- mortalities at hydro-electric facilities;
- recruitment; and
- As fish passage provisions are key to the success of mitigating threats and setting the scene for recovery, the implementation plans must consider the following.
- Adhere to goals and objectives of this strategy.
- Undertake assessment to confirm residual or relict presence/absence and abundance of eels within the historical range.
- Identify existing means of passage where passage enhancements may be most efficiently and effectively implemented.
- Identify strategic sites for mitigation of passage issues on a watershed basis:
- Develop a phased, strategic approach and timelines for implementation.
- Adopt an adaptive management approach where uncertainties are high.
- Identify the need/opportunities to accommodate passage for other fish species at the same time (e.g., American Shad Alosa sapidissima).
- If there are concerns over invasive species entering a watershed upon provision of passage, it should be noted that an eel ladder is a very specialized device and that typically no other species use it but eels. Therefore, the risk of invasive species entering after an eel ladder is installed typically should be minimal. Concerns over sea lamprey using the ladders also appear to be minimal as lamprey migration periods in March to early April occur at a time when American Eel ladders would not be operational.
- Understand that mitigation options for passage will often be quite site-specific.
- Consider the cumulative effects of a series of dams and hydro-electric facilities within a watershed on eels when issuing instruments for these facilities.
- Develop thresholds and benchmarks for success (e.g., escapement and recruitment targets).
- Ensure adequate effectiveness monitoring when issuing legal instruments for undertakings with the potential to have adverse effects on eels.
- Learn from the numerous experiences of other jurisdictions implementing fish passage initiatives at the watershed scale.
- Integrate the needs for power production, navigation and flood control into fish passage designs.
- Adopt an ecosystem approach that considers the needs of other aquatic species at the same time.
Footnotes
- footnote[1] Back to paragraph Throughout this document references to "eels" are references to American Eel.
- footnote[2] Back to paragraph Data provided by H. Taylor, OMNR May 2011.
- footnote[3] Back to paragraph While it is theoretically possible that freefall mortality occurs at some dams in Ontario due to their height, we have heard of no such mortalities and we raise it only because free fall mortality may be occuring but remains undetected.
- footnote[4] Back to paragraph See glossary for definition of shifting baseline.
- footnote[5] Back to paragraph This modification was installed when the dam was rebuilt in the early 1990s on the recommendations of J. Casselman and H. VonRosen (J. Casselman pers. comm. 2010).
- footnote[6] Back to paragraph Includes the concept of fairness.
- footnote[7] Back to paragraph While up to 25% of eels in the St. Lawrence River for example may migrate during the day (OWA 2010), 75% still migrate at night and nightly turbine shutdowns should enhance escapement to a level that significantly exceeds the status quo
- footnote[8] Back to paragraph It is unclear whether eels in Ontario will continue to be protected under the Fisheries Act given recent changes to the Act as a consequence of Bill-C38 (Government of Canada 2012).
- footnote[9] Back to paragraph Aquatic Resource Areas are aggregations of stream segments with similar physical and biological characteristics.
- footnote[10] Back to paragraph See glossary for definition of high-water mark.
- footnote[11] Back to paragraph Wampum belts created from quahog shell beads document agreements, stories and prophesies. The belts serve both as a living record of a commitment, and also as a means to recall the detailed messages embedded in the design (Thumbadoo 2005).