Bank Swallow General Habitat Description
This document is a technical, science-based description of the area of habitat protected for the Bank Swallow.
A general habitat description is a technical document that provides greater clarity on the area of habitat protected for a species based on the general habitat definition found in the Endangered Species Act, 2007.
General habitat protection does not include an area where the species formerly occurred or has the potential to be reintroduced unless existing members of the species depend on that area to carry out their life processes.
A general habitat description also indicates how the species’ habitat has been categorized, as per the policy “Categorizing and Protecting Habitat under the Endangered Species Act” and is based on the best scientific information available.
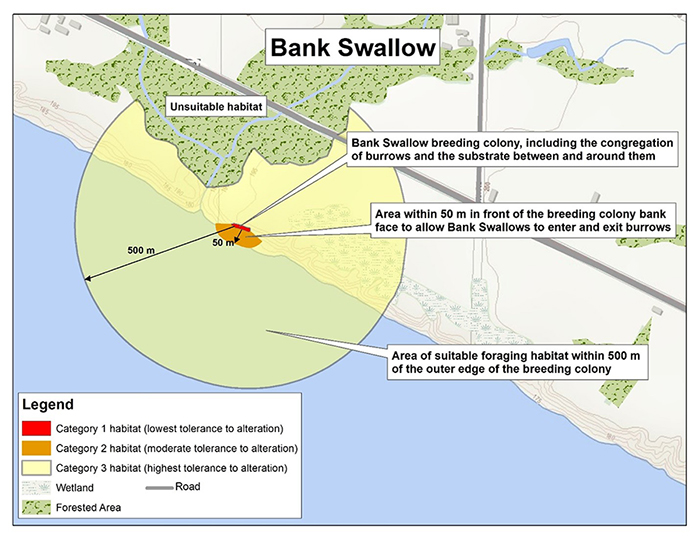
Habitat categorization
- Category 1 (red): The Bank Swallow breeding colony, including the congregation of burrows and the substrate around them.
- Category 2 (orange): The area within 50 m in front of the breeding colony bank face
footnote 1 to allow Bank Swallows to enter and exit burrows. - Category 3 (yellow): The area of suitable foraging habitat within 500 m of the outer edge of the breeding colony.
Category 1
The Bank Swallow breeding colony, including the congregation of burrows and the substrate between and around them, will be considered to have the lowest level of tolerance to alteration. Breeding colonies represent key areas used by members of the colony for reproduction including egg laying, incubation, feeding, resting, and rearing young.
The three main habitat types occupied by Bank Swallows include coastal cliffs, riverbanks, and active sand and gravel pits (Hickling 1959). Breeding colonies occur at discrete locations in vertical banks. Preferred banks are vertical (90 degrees) or slightly inclined (75 degrees) to slightly reclined (105 degrees) (Hjertaas 1984) although Bank Swallows will also use banks with steeper angles from 70 degrees to 110 degrees (M. Cadman pers. comm.). Sites with low tree and shrub cover on the tops of the banks and talus slopes are also preferred (Hjertaas 1984).
The breeding colony is composed of multiple burrows, with nesting chambers constructed at the end of the burrows. Males use their feet and beaks to dig burrows in vertical faces composed of firm but friable soils composed mostly of silt, sand and loamy substrates, which may also contain clay or gravel (Garrison 1999). Burrows that are located higher on cliff faces have greater reproductive success (Garrison 1999).
Burrows are usually excavated 60-90 cm deep, generally over a period of five days, although burrow excavation may take as long as 14 days (Garrison 1999). Males that fail to attract a mate to the burrow will relocate and dig a new burrow to establish a new territory (Kuhnen 1985). It is important to note that approximately 50% of burrows within a breeding colony are occupied during a given breeding season due to a surplus of excavated burrows (Garrison 1999, Cadman and Lebrun-Southcott 2013). Burrows are constructed until an average distance of 27-30 cm between burrows within the colony is reached (Sieber 1980 in Jones 1987). The Bank Swallow is non-territorial, although the burrow and the area immediately in front of the burrow are actively defended (Kuhnen 1985, Garrison 1999).
Once a pair is established, they expand the end of the burrow upwards and on both sides to create the nesting chamber, in which they construct the nest (Hickman 1979 in Garrison 1999). Nests in Ontario are usually flat, constructed from grasses, straw, and sometimes twigs, other plant stalks, leaves, and rootlets (Peck and James 1987).
Some of the largest breeding colonies with high bird densities are found in bluffs along the shoreline of Lakes Erie and Ontario and the banks along the Saugeen River (COSEWIC 2013). Based on burrow counts and breeding bird survey data, the Ontario population of Bank Swallows has been estimated at 150,000. The largest known colony in Ontario consists of 3,000 pairs on the north shore of Lake Erie (Sandilands 2007). Although a pair of Bank Swallows may nest solitarily, nesting colonies contain 45 pairs on average (Hoogland and Sherman 1976, Peck and James 1987).
A high proportion of breeding colonies in southern Ontario can be found in anthropogenic environments such as sand and gravel pits (Peck and James 1987). Preliminary results of recent research suggest that approximately 50% of sand and gravel pits in southern Ontario have Bank Swallows nesting in them (M. Cadman and M. Browning unpubl. data 2014). Bank Swallows are not as common in sand and gravel pits in other parts of Ontario and appear to decline in abundance with an increase in latitude (M. Browning pers. comm. 2014).
Studies investigating adult nest site fidelity show a range from 56 to 92% of adult birds returning to former breeding colonies (Petersen and Mueller 1979, Szep 1990 in COSEWIC 2013). Swallows may have adapted to the unstable and ephemeral nature of their nesting sites by remaining flexible in the specific colony site they select, while maintaining fidelity to a general breeding area, usually within a few kilometers of the original colony site (Mead 1979). Burrows constructed in relatively stable soils are sometimes reused although Bank Swallows generally prefer to dig new burrows, possibly due to the potential for infection by ectoparasites (Turner and Rose 1989, Garrison 1999, Hopkins 2001, Alves 2008).
An area around the breeding colony is required to ensure bank stability and prevent collapse of the nesting substrate. Disturbance of soils (such as through excavation or operation of heavy machinery) adjacent to the breeding colony has the potential to result in collapse of the nesting substrate (Ghent 2001a, M. Browning unpub. data 2014). The susceptibility to collapse is site-specific and could depend on a variety of factors such as bank age, substrate composition, slope angle, presence of an overhang, and density of burrows. Colonies excavated in loose sand may be subject to higher risk of collapse due to rainfall or nearby activities that disturb the soils in the nesting face (Ghent 2001a).
Mortality caused by substrate collapses impacts Bank Swallows, with 3.6% of mortalities from known causes being attributed to bank collapse in one study (Mead 1979). Another study documented collapse of approximately 5% of Bank Swallow tunnels examined (Hoogland and Sherman (1976) in Ghent 2001a). Freer (1979) found that Bank Swallows did not return to nesting sites if the colony produced few or no young due to substrate collapse or predation. However, new Bank Swallow pairs that were not at the site during one of these events may colonize the site in subsequent years, provided the banks remain suitable (Freer 1979). Conversely, successful breeding at a site increases the probability that adult Bank Swallows will return to a nesting site (Freer 1979).
In natural habitats, mechanisms such as erosion and undercutting of stream banks maintain vertical faces suitable for Bank Swallow nesting (Ghent 2001a). In anthropogenic sites such as sand and gravel pits, Bank Swallows use vertical faces that are maintained by human activities. Without active maintenance, the faces often slump within a few years and become unsuitable for nesting (Freer 1979, Garrison 1999, Ghent 2001b). Hickling (1959) (in Silver and Griffin 2009) found that Bank Swallows preferred a newly eroded vertical face for nesting even though new burrows were required to be dug every year. Therefore, it is recognized that in anthropogenic sites (such as sand or gravel pits), certain activities that occur in the non-breeding season could help maintain the bank face as suitable nesting habitat, provided that the important features of the bank, such as slope, height and substrate composition, are maintained for Bank Swallows for future breeding seasons.
Category 2
The area within 50 m in front of the breeding colony bank face (meaning the vertical face directly associated with, and supporting, the Category 1 habitat) will be considered to have a moderate tolerance to alteration. This area is required to enable unobstructed entry and exit of burrows.
Bank Swallows rely on open spaces in front of the colonies to provide them with sufficient flying space. In one study, Bank Swallows from most colonies were required to climb less than 1 m as they flew 60 m out from the colony. The same study also found that in other instances, 40 m of open space was required. Sites that required Bank Swallows to climb more than 1 m within 40 m or 2 m within 60 m of the bank face were found to be unsuitable for nesting (Hjertass 1984). The area in front of the nesting face must not be obstructed to ensure that Bank Swallows have sufficient horizontal flying space for entry and exit of the burrows.
Category 3
Category 3 habitat includes suitable foraging habitat from the outer edge of the colony to 500 m and will be considered to have a high tolerance to alteration.Bank Swallows depend on open areas within this distance for foraging on insects, which compose 99.8% of their diet (Garrison 1999). Nearby open areas such as rivers, lakes, wetlands, grasslands, and open fields provide good sources of flying insects. Open terrestrial habitats such as grasslands are preferred for foraging, and forested areas are avoided (COSEWIC 2013, Garrison 1999). Foraging during the breeding season frequently takes place between 200 m and 500 m from the colony (Turner 1980, Garrison 1999, COSEWIC 2013), although Bank Swallows may occasionally range more widely in their search for food (Mead 1979, Andrews and Kinsman 1990).
Temperature plays an important role in the variation of food availability and influences foraging distance. Turner (1980) found that distances traveled by Bank Swallows during the breeding season were greater when temperatures were cooler (for example 502 m ± 197 m at 16° or less), as insect activity drops at lower temperatures.
Activities in Bank Swallow habitat
Activities in general habitat can continue as long as the function of these areas for the species is maintained and individuals of the species are not killed, harmed, or harassed.
Generally compatible activities
- General recreational use of existing trails such as hiking and cycling
- General recreational lake, river, and beach use such as boating and fishing
- Normal use of existing roads
- Removal or disturbance of substrate that does not result in soil instability and/or collapse of Bank Swallow burrows
- In anthropogenic sites such as sand and gravel pits, activities (for example, excavation) in the non-breeding season that maintain the function of the breeding colony bank face as suitable Bank Swallow nesting habitat for future breeding seasons (for example, maintain features such as slope, height and substrate composition)
Generally incompatible activitiesfootnote 2
- Recreational activities such as climbing in sensitive habitat areas (for example, in or adjacent to Category 1 habitat)
- Removal or disturbance of substrate in the breeding season that is likely to result in soil instability and/or collapse of Bank Swallow burrows
- Development activities that result in removal of large tracts of suitable foraging habitat
- Obstruction of the open space within 50 m in front of a breeding colony bank face that results in insufficient flying space for Bank Swallows to enter and exit burrows
Sample application of the general habitat protection for Bank Swallow
- Category 1 habitat (lowest tolerance to alteration): Bank Swallow breeding colony, including the congregation of burrows and the substrate between and around them.
- Category 2 habitat (moderate tolerance to alteration): Area within 50 metres in front of the breeding colony bank face to allow Bank Swallows to enter and exit burrows.
- Category 3 habitat (highest tolerance to alteration): Area of suitable foraging habitat within 500 metres of the outer edge of the breeding colony.
References
Alves, M.A.S. 2008. Effects of ectoparasites on the Sand Martin Riparia riparia nestlings. Ibis 139:494-496.
Andrews, J. and D. Kinsman. 1990. Gravel pit restoration for wildlife. Royal Society for the Protection of Birds, Sandy, Bedfordshire, U.K.
Browning, M., Research Biologist, Ontario Ministry of Natural Resources. Personal communication. June 4, 2014.
Cadman, M., Songbird Biologist, Canadian Wildlife Service, Environment Canada. Personal communication. November 12, 2014.
Cadman, M. and M. Browning. Canadian Wildlife Service and Ministry of Natural Resources and Forestry. Unpublished data. September 11, 2014.
Cadman, M. and Z. Lebrun-Southcott. 2013. Bank Swallow colonies along the Saugeen River, 2009-2013. Ontario Birds 31(3):136-147.
COSEWIC. 2013. COSEWIC assessment and status report on the Bank Swallow Riparia riparia in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. ix + 48 pp. (Species at risk public registry).
Freer V.M. 1979. Factors affecting site tenacity in New York Bank Swallows. Bird-Banding 50(4):349-357.
Garrison, B.A. 1999. Bank Swallow (Riparia riparia), The Birds of North America Online (A. Poole, Ed.). Ithaca: Cornell Lab of Ornithology; Retrieved from the Birds of North America Online: (http://bna.birds.cornell.edu.cat1.lib.trentu.ca:8080/bna/species/414doi:10.2173/bna.4) [accessed 14 May 2014].
Ghent, A.W. 2001a. Regular spatial patterns of Bank Swallow (Riparia riparia) tunnel entrances, with some possible evolutionary implications. American Midland Naturalist 146:414-423.
Ghent, A.W. 2001b. Importance of low talus in location of Bank Swallow (Riparia riparia) colonies. American Midland Naturalist 146:447-449.
Hickling, R.A.O. 1959. The burrow-excavation phase in the breeding cycle of the Sand Martin Riparia riparia. Ibis 101:497–500.
Hickman, G. R. 1979. Nesting ecology of Bank Swallows in interior Alaska. Master's Thesis. Univ. of Alaska, Fairbanks, 78 pp.
Hjertaas, D. G. 1984. Colony site selection in Bank Swallows. Master's Thesis. Univ. of Saskatchewan, Saskatoon, 129 pp.
Hoogland, J.L. and P.W. Sherman. 1976. Advantages and Disadvantages of Bank Swallow (Riparia riparia) Coloniality. Ecological Monographs 46:33–58.
Hopkins, L. 2001. Best practice guidelines. Artificial bank creation for Sand Martins and Kingfishers. (London's BAP priority species) [accessed May 30, 2014].
Jones, G. 1987. Colonization patterns in Sand Martins (Riparia riparia). Bird Study 34(1):20-25.
Kuhnen, K. 1985. On pair-formation in the Sand Martin, Riparia riparia. Journal of Ornithology 126:1-13.
Mead, C.J. 1979. Colony fidelity and interchange in the Sand Martin. Bird Study 26(2): 99-106.
Peck, G.K. and R.D. James. 1987. Breeding birds of Ontario: nidiology and distribution, Vol. 2: passerines. Royal Ontario Museum. Life Sciences Misc. Publication. Toronto, 387 pp.
Petersen, C. and A.J. Mueller. 1979. Longevity and Colony Loyalty in Bank Swallows. Bird-Banding 50(1): 69-70.
Sandilands, A.P. 2007. Bank Swallow, pp. 394-395 in Cadman, M.D., D.A. Sutherland, G.G. Beck, D. Lepage, and A.R. Couturier, eds. Atlas of the Breeding Birds of Ontario, 2001-2005. Bird Studies Canada, Environment Canada, Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature, Toronto, xxii +706 pp.
Sieber, O. 1980. Causal and functional aspects of brood distribution in Sand Martins (Riparia riparia L.). Zeitschrift für Tierpsychologie 52:19-56.
Silver, M. and C.R. Griffin. 2009. Nesting habitat characteristics of Bank Swallows and Belted Kingfishers on the Connecticut River. Northeastern Naturalist 16(4):519-534.
Szep, T. 1990. Estimation of abundance and survival rate from capture-recapture data of Sand Martin (Riparia riparia) ringing. Ring 13:205-214.
Turner, A.K. 1980. The use and time and energy by aerial feeding birds. Ph.D. dissertation, University of Stirling, Stirling, United Kingdom. 347 pp.
Turner, A.K. and C. Rose. 1989. Swallows and martins an identification guide and handbook. Houghton Mifflin Co., Boston, 258 pp.
Footnotes
- footnote[1] Back to paragraph The breeding colony bank face is the vertical face that is directly associated with, and supports, the Category 1 habitat (meaning the Bank Swallow breeding colony).
- footnote[2] Back to paragraph If you are considering an activity that may not be compatible with general habitat, please contact SAROntario@ontario.ca for more information.