Green Dragon Management Plan
This document advises the ministry on ways to ensure healthy numbers of Green Dragon, a species of special concern, return to Ontario.

Management plan prepared under the Endangered Species Act, 2007
June 2013
About the Ontario management plan series
This series presents the collection of management plans that are written for the Province of Ontario and contain possible approaches to manage species of special concern in Ontario. The Province ensures the preparation of the management plans meet its commitments to manage species of special concern under the Endangered Species Act, 2007 (ESA, 2007) and the Accord for the Protection of Species at Risk in Canada.
What is a species of special concern?
A species is classified as special concern if it lives in the wild in Ontario, is not endangered or threatened, but may become threatened or endangered due to a combination of biological characteristics and identified threats.
What is a management plan?
Under the ESA, 2007, a management plan identifies actions that could be taken to ensure, at a minimum, that a species of special concern does not become threatened or endangered. The plan provides detailed information about the current species population and distribution, their habitat requirements and areas of vulnerability. The plan also identifies threats to the species and sets a clear goal, possible strategies, and prioritized activities needed to address the threats.
Management plans are required to be prepared for species of special concern no later than five years of the species being added to the Species at Risk in Ontario list as a special concern species.
What’s next?
Nine months after the completion of a management plan a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the plan and the government priorities in taking those actions. The implementation of the management plan depends on the continued cooperation and actions of various sectors, government agencies, communities, conservation organisations, land owners, and individuals.
For more information
To learn more about species of special concern in Ontario, please visit the Ministry of Natural Resources Species at Risk webpage.
Recommended citation
Donley, R., J.V. Jalava and J. van Overbeeke. 2013. Management Plan for the Green Dragon (Arisaema dracontium) in Ontario. Ontario Management Plan Series. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. vi + 43 pp.
© Queen’s Printer for Ontario, 2013
ISBN 978-1-4606-2031-1 (PDF)
Content (excluding the cover illustration) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Management plans prepared under the Endangered Species Act, 2007 », n'est disponible qu'en anglais en vertu du Règlement 411/97 qui en exempte l'application de la Loi sur les services en français. Pour obtenir de l'aide en français, veuillez communiquer avec le ministère des Richesses naturelles au 1-800-667-1940.
Authors
Rhonda Donley, Carolinian Canada Coalition
Jarmo Jalava, Carolinian Canada Coalition
Jennifer van Overbeeke, Carolinian Canada Coalition
Acknowledgments
The authors wish to acknowledge and thank the following people and organizations for their assistance: Jennifer van Overbeeke provided substantial assistance with formatting and editing the final document. Daria Koscinski, Carolinian Canada Coalition, prepared the maps. The Natural Heritage Information Centre provided access to their paper files and occurrence data. Jane Bowles, Larry Lamb, and Gerry Waldron shared their considerable knowledge and experience with this species. Allen Woodliffe shared his knowledge of the Aylmer District populations, provided photographs from his collection, and reviewed the draft. Dave Jolly provided his sightings and information on flowering times in Ontario. Sandra Turner gave an update on the status and management of a population at Ruthven Park. Mary Gartshore and Craig Willet shared information on their respective native plant nursery operations. Dr. Jon Lovett-Doust provided information on his previous research and reviewed the draft. Patrick Nantel provided background information on the population viability assessment he conducted on Quebec populations. Randall Van Wagner provided information on the Lower Thames Valley Conservation Authority’s recent vegetation work. Maria Kuzmina and Jeremy de Waard (Biodiversity Institute of Ontario) provided information on the Barcode of Life project activities. Tammy Dobbie and Graham Buck provided contacts for some of the authorities consulted.
Declaration
The management plan for the Green Dragon was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This management plan has been prepared for the Government of Ontario, other responsible jurisdictions and for the many different constituencies that may be involved in managing the species.
The management plan does not necessarily represent the views of all of the individuals who contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The goals, objectives and management approaches identified in the plan are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this plan is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the management of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this plan.
Responsible jurisdictions
Ontario Ministry of Natural Resources
Environment Canada - Canadian Wildlife Service, Ontario
Executive summary
Green Dragon (Arisaema dracontium) is a perennial herb in the Araceae (Arum) family, which also includes the more familiar Jack-in-the-Pulpit (A. triphyllum). Green Dragon was designated as special concern by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) in 1984 and by the Ontario Ministry of Natural Resources (OMNR) in 1988. These designations are a result of Green Dragon’s relatively small number of viable populations and vulnerability to habitat loss and destruction. The species is listed as special concern under Ontario’s Endangered Species Act, 2007.
Green Dragon is found from Texas and Florida north to Wisconsin, Ontario, Quebec and New York. It grows along rivers, creeks and clay floodplains in moist deciduous woods and thickets, typically in seasonally flooded locations that are shaded or partially shaded by surrounding trees. Current data suggest that there are between 60 and 80 extant occurrences of Green Dragon in Ontario. Of these, only a small proportion are considered viable, although there appear to be a number of recently-discovered populations in protected areas. Rothfels and Smith (2003) estimated the Ontario population of Green Dragon to be approximately 11,000 plants, with at least 287 of these producing fruit. No recent population estimates have been made and an accurate estimate is not possible because of the lack of comprehensive data.
The reproductive biology of Green Dragon has received considerable attention because individual plants can change the sex of the flowers they produce. Large, healthy plants produce monoecious (male and female) flower heads, while smaller or damaged plants have only staminate (male) flowers. Seeds are likely spread by small mammals, birds, and flood events. In addition to producing seeds, Green Dragon also reproduces through vegetative offsets which are shed by the parent plant and become physiologically independent. Offsets are dispersed upstream or downstream by flood events.
The main threats to Green Dragon in Ontario are habitat loss and degradation. The species is found in the most heavily populated and modified parts of Ontario where historical impacts on its floodplain habitat have been extensive. Although floodplains are generally protected today through zoning, development on adjacent upland areas may impact hydrology and introduce other threats (e.g., invasive species, trampling along trails), even if the floodplain forest is retained. Hardening of surfaces through paving of roads and building construction results in water level peaks and more severe spring flooding. Because of the species' special adaptations to survival in floodplain habitat, flood control activities by conservation authorities may have contributed to low survival and germination rates. Due to historical and ongoing development, Green Dragon is confined to increasingly small, isolated habitat fragments in Ontario. These populations are vulnerable to stochastic events as well as direct threats.
This management plan offers strategies to maintain existing Green Dragon populations, restore populations that are not self-sustaining, enhance connectivity between populations to facilitate natural dispersal, and allow for population expansion. If successful, the strategies would result in the eventual de-listing of the species. The management approaches to achieve these goals and fill knowledge gaps for Green Dragon in Ontario are grouped into the following five objectives:
- Conduct baseline inventory to determine the size and number of extant sites, site quality, and population health. Implement regular monitoring to track population trends and changes in habitat quality in response to management actions and threats.
- Protect and manage species and habitat at extant sites in Ontario to achieve and maintain viable population levels.
- Address key knowledge gaps relating to life cycle requirements, habitat requirements and prioritization of threats.
- Promote awareness and stewardship of Green Dragon with First Nations, land managers, private landowners, municipalities and key stakeholders.
- Support and implement landscape- and ecosystem-based planning and recovery initiatives to increase the amount of available habitat for Green Dragon, and to enhance habitat connectivity for dispersal and population expansion.
1.0 Species assessment and classification
Common name (population): Green Dragon
Scientific name: Arisaema dracontium
SARO List Classification: Special Concern
SARO List History: Special Concern (2004)
COSEWIC Assessment History: Special Concern (1984)
SARA Schedule 1: N/A (SARA Schedule 3: Special Concern)
Conservation status rankings:
GRANK: G5 NRANK: N3 SRANK: S3
The glossary provides definitions for the abbreviations above.
2.0 Species information
2.1 Species description and biology
Species description
Green Dragon (Arisaema dracontium) is a perennial herb in the Araceae (Arum) family, which also includes the more familiar Jack-in-the-Pulpit (A. triphyllum). Sometimes known as Dragon-root (Rothfels and Smith 2003), Green Dragon usually produces a single compound leaf at the end of a 15 to 90 cm stalk. The leaf is palmately divided into 5 to 21 leaflets; most plants have 7 to 13 leaflets. Jack-in-the-pulpit has only three leaflets. Green Dragon leaflets may be up to 28 cm long and 10 cm wide. The centre leaflet is shorter than the adjacent leaflets. The two leaflets on either side of the central leaflet are the largest; the leaflets become progressively smaller towards the ends of the leaf (Rothfels and Smith 2003, eFloras 2008). The number and arrangement of the leaflets can make the plant appear to have multiple leaves.
Mature plants produce a single flowering stem each year which is usually shorter than the leaf stalk (Rothfels and Smith 2003). Like all species in the Arum family, the flowers are on a spadix (fleshy spike) (Srivastava and Banerji 2012). The flowers are tightly packed around the cylindrical base of the spadix. The flowering part of the spadix is enclosed within a light green, persistent membranous spathe (leaflike bract) that sometimes has purple markings. When the spadix is mature, the top part of the spathe opens to expose the tip of the flower cluster and the entire spadix appendage. The long, tapered, flowerless appendage that tops the spadix is the Green Dragon’s most striking feature. When mature, this appendage is bright orange (sometimes yellow) and extends 3 to 17 cm beyond the top of the spathe.
The Green Dragon spadix may have only male flowers (staminate) or a combination of male and female flowers (monoecious). Completely female (pistillate) flower heads are extremely rare in natural settings (Schaffner 1922, Lovett-Doust and Cavers 1982a, Clay 1993). The gender of flowers on a single plant may change between years. When both male and female flowers are present, the male flowers appear above the female flowers on the spadix (Lovett-Doust and Cavers 1982a, Clay 1993). The female flowers are a green and shaped like inverted cones (Gauvin 1984).
Two to three months after flowering, the female flowers mature into fleshy red or orange berries that contain one to several bean-like seeds (Clay 1993, Srivastava and Banerji 2012). The berry cluster (an infructescence) is highly visible (Clay 1993) and remains at the top of the stem into the fall, after the leaf has wilted (Natural Heritage Endangered Species Program 2009). The berries are larger and fleshier than Jack-in-the-pulpit’s and usually contain more seeds (three to six vs. one to three), but are otherwise very similar to Jack-in-the-pulpit (Rennert 1902).
The plant overwinters as a corm with roots radiating from the top (Cole 1962, eFloras 2008). Although the corm grows continually, it does not exceed eight cm in diameter because the previous year’s growth is sloughed off to release the offset shoots which can root to form a new plant (Cole 1962, Boles 1996, eFloras 2008).
Species biology
For floodplain and slough species like Green Dragon, the growing season begins after spring flood levels recede. This often means that the growing season for these species begins later and is shorter than for upland species (Damman 1994). Flowering occurs in late May through late June (Dembinsky 1966). Green Dragon is probably pollinated by thrips (Heterothrips spp.), fungus gnats (Mycetophylla spp.) and other small insects (Huttleston 1953 as cited in Gauvin 1984, Boles 1996). The long, brightly coloured spadix appendage is thought to attract insect pollinators (Lovett-Doust and Cavers 1982a). Insects are necessary for pollination; monoecious flower spikes that were covered to exclude insects withered and did not set seed (Cole 1962). The plants remain in flower for a one to two week period, then quickly go to fruit and lose the flower stalk (Jolly, pers. comm. 2013).
The reproductive biology of Green Dragon and its close relative, Jack-in-the-pulpit, have received considerable attention because of the plant’s ability to change the sex of its flowers. Schaffner (1922) and Cole (1962) demonstrated experimentally that the sexual expression of an individual Green Dragon is dependent on the size and health of the plant, not its age or genetic makeup. This has also been shown to be the case for Jack- in-the-pulpit (Lovett-Doust and Cavers 1982b). In Green Dragon, large healthy plants produce monoecious flower heads, while smaller or damaged plants have only staminate (male) flowers (Schaffner 1922, Cole 1962, Lovett-Doust and Cavers 1982a, Gauvin 1984, Boles 1996). Non-flowing plants are smaller than staminate plants (Schaffner 1922, Rothfels and Smith 2003).
Plant size also influences the number of flowers on staminate plants and the ratio of male to female flowers on the spadix of monoecious plants. Larger staminate plants tend to have more flowers than smaller staminate plants. Larger monoecious plants have fewer male flowers and more female flowers than smaller monoecious plants (Cole 1962, Clay 1993). Clay (1993) determined that Green Dragon is likely to produce pistillate flower heads when the basal stem diameter reaches or exceeds 25 mm. However, basal stem diameter rarely exceeds 20 mm in wild populations.
Fruit production may influence plant size in the subsequent season. Green Dragon plants grown in a greenhouse often produce staminate flower heads the year after producing monoecious flower heads and vice versa (Clay 1993). Clay (1993) speculated that Green Dragon’s high levels of fruit set may reduce the size of monoecious plants, preventing them from growing large enough to become pistillate. Jack-in-the-pulpits are smaller the year after they produce fruit (Lovett-Doust and Cavers 1982b, Bierzychudek 1984 as cited in Rothfels and Smith 2003), but this has not been studied in Green Dragon.
Green Dragon appears to be self-incompatible, as isolated monoecious plants do not set seed and the spadix often withers soon after flowering (Huttleston 1953 as cited in Gauvin 1984, Cole 1962). However, individuals are able to reproduce sexually with their clonal offspring (Boles et al. 1999, Cole 1962), which indicates that the self-incompatibility is due to physical characteristics, not genetics (Rothfels and Smith 2003). Green Dragon may be self-incompatible because the anthers (part of the stamen that holds pollen) of male flowers burst open to release their pollen well before its stigmas (the part of the plant where pollen is received and germinates) become receptive (Huttleston 1953, as cited in Gauvin 1984). They may also be self- incompatible because the pollen remains dormant until it is removed from the spathe and aerated by a pollinator (Boles 1996, Rothfels and Smith 2003). The latter has been observed in other Arisaema species (Galil and Meiri 1992, as cited in Boles 1996), but has not been studied in Green Dragon.
In Ontario, the fruit mature in late summer to fall (Rothfels and Smith 2003). Clay (1993) found that the female flowers next to the male flowers (i.e., the highest female flowers on the spadix) did not produce berries. Ripe berries usually contain one or two plump, ripe seeds and two or three shriveled, undeveloped seeds. Berries containing three or more ripe seeds are extremely rare (Clay 1993). Cole (1962) found that usually less than half of the berries on a spadix become "well filled with pulp and seeds." Older, larger plants produce better-filled berries than younger, weaker plants. Flowering stems on monoecious plants with very few female flowers and staminate plants both wither at the end of the flowering season. They do not produce berries even if the female flowers are fertilized (Schaffner 1922).
The association between plant health and the ability to reproduce sexually may have management implications. At the two Ontario populations studied by Lovett et al. (1982a), staminate plants were three times more abundant than monoecious plants, and many of the more recent observations have similar or even greater ratios of vegetative to flowering plants (NHIC 2012, 2013). The high percentage of non-flowering plants and low percentage of monoecious plants suggests that Ontario populations are stressed to the point where sexual reproduction is reduced.
Small mammals, birds and flood events are the suspected mechanisms for seed dispersal, but direct observations have not been made (Boles et al. 1999, Rothfels and Smith 2003). Germination experiments conducted by Yang et al. (1999) found that physical and chemical scarification decreased the germination time but did not affect the seed’s ability to germinate (germinability). Immersion in phytohormones increased the germinability rate for seeds from all populations studied. These results suggest that Green Dragon has some adaptations to dispersal by vertebrates (Rothfels and Smith 2003).
Green Dragon and Jack-in-the-pulpit exhibit "double dormancy". Seeds with this type of dormancy are often referred to as "two-year seeds" because at least two cold periods (winters) separated by a warm period (summer) are required to complete germination. Green Dragon requires cold/warm/cold stratification (Baskin and Baskin 1998). Yang et al. (1999) found that a cold, wet period is required for most seeds to start germinating, particularly for seeds from the northern part of the range.
Green Dragon exhibits a high dormancy rate. Pre-treatment by wet, cold stratification, immersion in phytohormones, or a combination of these pre-treatments increased the germination rate, especially in seeds from northern populations (including Ontario), but did not cause all viable seeds to germinate. Between 10 and 25 percent of the seeds remained dormant (Yang et al. 1999). Pickett (1913) also found that some viable seeds remained dormant. This persistent dormancy may be caused by a shortage of phytohormones. Shortages of these hormones have been found to suppress seed germination in other species even under favourable environmental conditions (Yang et al. 1999). Prolonged dormancy in a small percentage of seeds could be a useful long term survival strategy for Green Dragon due to its reliance on seasonal and intermittent wetlands (Woodliffe pers. comm. 2013a).
Light is not required to initiate germination and does not affect the percentage of seeds that germinate, but it does have other effects on germination and seedling establishment. Exposure to light delays the onset of germination, but also makes seeds significantly more likely to develop a green leaf during the first growing season. It also promotes the development of additional adventitious roots (extra roots that usually sprout above ground) during the first growing season, especially in seedlings that also produce a green leaf (Yang et al. 1999). These responses to light are likely an adaptation to spread out germination over the course of the growing season and could have implications for management of the species.
Baskin and Baskin (1998), Pickett (1913) and Rennert (1902) provide detailed descriptions and drawings of Green Dragon’s morphological progression from seed to corm. Most seeds germinate after the first cold period but do not produce an above ground shoot until after the second cold period (Pickett 1913, Cole 1962). The embryo grows, a corm and roots are produced, scales form around the stem bud and energy is transferred from the seed to the corm, but the stem and leaves are not produced (blind germination). This is the opposite of Jack-in-the-pulpit, with which most seeds will produce leaves in the first year and only a few exhibit blind germination (Pickett 1913). The Green Dragon germination process is slow, taking two to five months (MacDougal 1901, Pickett 1913), a month longer than Jack-in-the-pulpit’s germination period (Rennert 1902). Yang et al. (1999) found that seeds from Ontario have a shorter, more concentrated germination period (i.e., more seeds germinate at the same time) than those from southern populations. It is unknown whether this difference is caused by genetics. Towards the end of the process, when all of the food has been transferred from the seed to the corm, the tissues connecting the seed and the corm shrivel up and the corm becomes independent. The roots detach and disintegrate before the winter rest period (Picket 1913). This may have important management implications, as corms at this stage of development can easily be transplanted in the fall.
Green Dragon seeds have a thick, hard seed coat and exude a yellow, water-soluble material (possibly a mixture of tannin and/or other water-soluble compounds) when soaked. The embryo is smaller and less developed than the embryo in Jack-in-the-pulpit seeds. These features appear to be the cause the species' prolonged germination period, but further study is needed (Yang et al. 1999).
Green Dragon also reproduces through vegetative offsets produced by the corm. Rothfels and Smith (2003) noted that vegetative reproduction appears to be the main, if not only, means of population maintenance and growth in many Ontario populations, including one of the largest. For management of the species it is important to note that offsets are dispersed upstream or downstream by flood events (Boles 1996).
Larger corms produce more offsets than smaller corms. After two years of development, the offsets are shed by the parent plant and become physiologically independent (Cole 1962). If the offsets remain crowded around the base of the mother plant, most die in the first year after detachment. The surviving offsets produce staminate flowers in the second or third year after they produce leaves (Cole 1962). At the end of 14 years, a single mother plant had produced a patch of 23 flowering individuals (17 staminate and 6 monoecious) and over eighty immature individuals (Cole 1962). As the lower portion of the corm disintegrates, the roots contract to pull the plant deeper into the soil (Cole 1962).
Characteristics of Green Dragon other than its reproductive biology may also be important considerations for its management in Ontario. For example, it is weak stemmed and often falls over fairly early in the summer. Individuals may benefit from the supporting stems of the other species it is commonly associated with (Rothfels and Smith 2003). Also, Green Dragon is easily transplanted (Cole 1962, Rothfels and Smith 2003).
Sanders and Burk (1992) documented a naturally occurring population of Green Dragon and Jack-in-the-Pulpit hybrids in Massachusetts. This is the only known hybrid population, despite the large shared range and affinity for the same or similar habitats.
Members of the Arum family can be long lived. Srivastava and Banerji (2012) report that in some species individuals live for up to 100 years. The lifespan of Green Dragon is unknown, but individuals have survived for twelve years in a garden (Cole 1962). It is likely that the lifespan is longer than Cole observed.
One short-term study in Quebec suggests that a minimum of 160 individuals is required for the long-term viability of a given population (Comité ZIP et MOE 1999, as cited in Rothfels and Smith 2003). However, the population model that was used to derive that value had many shortcomings and was not published nor peer-reviewed (Nantel pers. comm. 2013). Also, the climate, soils and other ecological conditions in the St. Lawrence Lowlands ecoregion are different from those of the Lake Erie Lowland ecoregion (Gauvin 1984, Soil Classification Working Group 1998). The minimum viable population size may be different in Ontario.
2.2 Population and distribution
Green Dragon is found from Texas and Florida north to Wisconsin, Ontario, Quebec, and New York. It is listed as endangered in New Hampshire (New Hampshire Natural Heritage Bureau 2012), threatened in Massachusetts and Vermont (Massachusetts Division of Fisheries and Wildlife 2012, Vermont Natural Heritage Inventory 2012), "menacé" (threatened) in Quebec (Développement durable, Environnement, Faune et Parcs 2012), and "exploitably vulnerable" in New York (Department of Environmental Conservation 2012) and is protected by legislation in these jurisdictions. The species has been reported in Rhode Island but these reports have not been substantiated with specimens (Rothfels and Smith 2003, eFloras 2008). Table 1 summarizes the species' conservation status throughout North American range. The species has also been reported from several locations in eastern Mexico though more study is needed to determine if these plants are in fact Green Dragon.
Table 1. Conservation status of Green Dragon by state and province.
| S Rank | State / Province |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(NatureServe 2012)
In Canada, Green Dragon occurs in the St. Lawrence Lowlands and Lake Erie Lowland ecoregions of the Mixedwood Plains Ecozone (Rothfels and Smith 2003). In Ontario, it occurs southwest of a line from Hamilton to the Maitland River in Huron County. Most records are from a band across the counties of Lambton, Middlesex, and Elgin. Clusters of records also occur in central Haldimand-Norfolk, around the Maitland River in Huron County and in the southwestern portion of Essex County. There are a few scattered records in Brant County, the Municipality of Chatham-Kent and the regional municipalities of Waterloo and Niagara (Figure 1). Green Dragon is not known to occur in the Ontario portion of the St. Lawrence Lowlands ecoregion.
Figure 1. Historical and current distribution of Green Dragon in Ontario
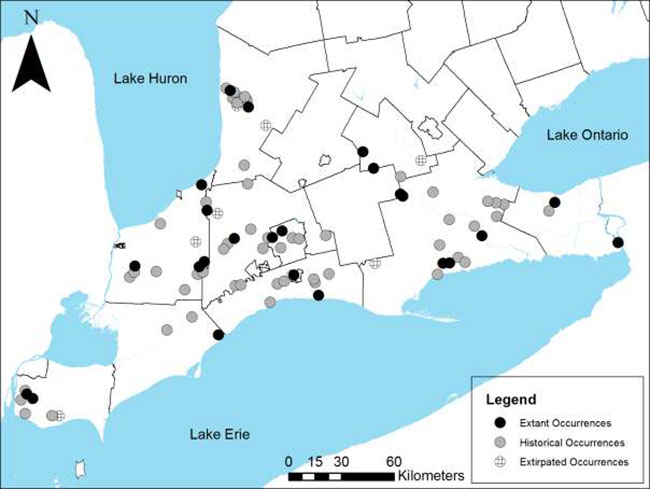
Enlarge Figure 1. Historical and current distribution of Green Dragon in Ontario
Table 2 provides a general summary of Green Dragon occurrences in Ontario. The NHIC estimates that there are 62 extant occurrences of Green Dragon for Ontario. Of these, 11 are considered "viable" (ranked A, B or C), 11 are considered extant (ranked E), 11 are "not viable" (ranked D) and the remainder have not been confirmed or assessed. Another 52 occurrences are considered historical (44) or extirpated (8) (NHIC 2012). The NHIC has nearly 200 unprocessed records for Green Dragon (which include unsuccessful searches for the species) that have not yet been verified and are therefore not included in the above totals (NHIC 2013). However, these observations were reviewed and incorporated into Table 2. Most of the newer records are from conservation authority lands, other protected areas, or First Nations (NHIC 2013). Some of the records may be previously undocumented occurrences or populations; others are likely just additional locations or recent observations within known populations.
Table 2. Summary of Green Dragon Occurrences in Ontario
| County / Upper Tier Municipality | Extant / Historic / Extirpated Occurrences | Comments |
|---|---|---|
| Brant | 1 / 1 / 0 + 1 new extant site |
Probably on private land. One newly-reported occurrence at Six Nations of the Grand River. |
| Chatham-Kent | 1 / 1 / 1 | One large extant occurrence near Chatham (>4000 plants counted in 1993) (Rothfels and Smith 2003). One historical (1987) occurrence on First Nation land probably extant. |
| Elgin | 1 / 9 / 0 | One occurrence on conservation authority land, another in a municipal park. Remainder probably all on private land. Population in Yarmouth Natural Heritage Area was planted as plugs by Catfish Creek Conservation Authority (Jolly, pers. comm. 2012) |
| Essex | 2 / 2 / 0 | Probably two extant occurrences, one of them partly on conservation authority land. |
| Haldimand-Norfolk | 2 / 5 / 1 | One extant occurrence at Ruthven Park National Historic Site on east side of Grand River (two large plants with several smaller plants, plus ~ten small, non-flowering plants about 200m downstream). Another, possibly separate, occurrence on west side of Grand River at Ruthven Park NHS has not been confirmed since 1997 (Turner, pers. comm. 2013). All other occurrences probably on private land. One large occurrence (2737 plants) reported in 1993 (Rothfels and Smith 2003). |
| Hamilton | 2 / 0 / 0 + 1 new extant site |
NHIC (2012) indicate populations to be "historical", however populations were observed in 2002 (Rothfels and Smith 2003). Probably on private land. One newly reported occurrence on Crooks Hollow Heritage Trail in Greensville may be on municipal land (Turner, pers. comm. 2013) |
| Huron | 3 / 7 / 2 | One occurrence at Hullett Provincial Wildlife Area. One occurrence on conservation authority land. Remainder probably private land. |
| Lambton | 3 / 4 / 1 | One possibly extant occurrence at Pinery Provincial Park. Occurrences reported in 2008-2010 from protected areas: >300 plants (>50 flowering) at Walden Tract, 220 plants (60 flowering) at Rock Glen C.A.; also reported from Doherty Tract (NHIC 2013, unpub. data). Remainder probably on private land. |
| Middlesex | 3 / 11 / 1 + ~3 new extant sites |
Several 2009-2010 occurrences on conservation authority land: >100 plants (~30 flowering) at Sadler Tract; 12 fruiting and several vegetative reported for Waun Tract; reported from Strathroy, C.A. and Coldstream C.A. (NHIC 2013, unpub. data). One population, surveyed in 2010, on municipal golf course. Remainder probably on private land. Quinlan (pers. comm. 2013) confirmed that the Green Dragon record in the 2012 watershed report card for The Forks (UTRCA 2012) is likely an error or scale issue, as the species occurs in a natural area just outside the boundaries of this watershed but has not been documented within the watershed. |
| Niagara | 2 / 1 / 0 + 1 new extant site |
One D-ranked occurrence partly on conservation authority land, partly on Crown land and partly on private land. Remainder on private land; one new site with 132 plants (NHIC 2013, unpub. data). |
| Oxford | 1 / 0 / 0 | Probably on private land. |
| Waterloo | 4 / 1/ 1 | One occurrence on naturalist club nature reserve. Remainder probably all on private land. |
Since Green Dragon is conspicuous at its peak and often sought after by botanists, it is unlikely that the species has merely been overlooked at historical sites. It is more likely that such populations are either extirpated, or that they have vague location information and are actually attributable to other element occurrences (Rothfels and Smith 2003).
Rothfels and Smith (2003) estimated the Ontario population of Green Dragon to consist of at least 11,000 plants, with at least 287 of these producing fruit. No current population estimates have been made, and an accurate estimate would not be possible because of the absence of comprehensive data. Trend information for Ontario populations is also not available. Historical records rarely included abundance or demographic information (Rothfels and Smith 2003) and the six sites surveyed by Boles (1996) were not among the 20 sites surveyed by Rothfels and Smith (2003).
2.3 Habitat requirements
Green Dragon is found along rivers, creeks and clay floodplains in mesic (moist) to wet deciduous woods and thickets in eastern North America (Yang et al. 1999, eFloras 2008). The St. Lawrence Lowlands and Lake Erie Lowland ecoregions are characterized by well-drained brunisol and luvisol soils. Poorly drained humic gleysol soils also occur in both of these areas (Gauvin 1984). These soil types and their distribution in Canada are described by the Soil Classification Working Group (1998) and on the University of Saskatchewan’s Soils of Canada website. Within these widespread soil types, Green Dragon is restricted to seasonally inundated floodplains. No site-specific soil assessments have been made at any of the Green Dragon populations (Rothfels and Smith 2003), but two of the occupied St. Lawrence Islands are covered by imperfectly drained gleysol and gleyed podsol soils on either marine clay sediments or alluvium with various textures (Gauvin 1984).
Green Dragon grows in shaded or partly shaded seasonally flooded locations. It is usually found in the narrow transition zone between shoreline areas that remain wet later into the summer and drier uplands. In open riverside plains, which are often dominated by the introduced Reed Canarygrass (Phalaris arundinacea), it occurs where flood debris has accumulated in low spots or by fallen trees. Green Dragon also grows further from the shoreline in wooded floodplains with organic debris from spring floods, often around the edges of depressions or ephemeral ponds, and surrounded by dense stands of Canada Woodnettle (Laportea canadensis) (Rothfels and Smith 2003). In Michigan, it is mostly found along river banks and on small levees formed by seasonal flooding and is found less frequently in forested areas further from the shoreline (Dembinsky 1966).
Rothfels and Smith (2003) found that Green Dragon showed a strong preference for lush creek-side canopy gaps in Ontario. It was common in such openings but rare in the adjacent closed canopy areas. The species generally preferred rich floodplains like those along the Nith River, Twenty Mile Creek and Ausable River over rockier floodplains such as those found along the upper Thames River and Jordan Harbour. Interestingly, Green Dragon was found growing a considerable distance above the Thames River and not on the floodplain (Rothfels and Smith 2003). Rothfels and Smith (2003) provide a detailed summary of species associated with Green Dragon in Canada. These include willows (Salix spp.), ashes (Fraxinus spp.), Canada Woodnettle, Riverbank Grape (Vitis riparia), Virginia Creeper (Parthenocissus quinquefolia), Poison Ivy (Toxicodendron radicans), American Elm (Ulmus americana), other elms (Ulmus spp.), Stinging Nettle (Urtica dioca), Great Ragweed (Ambrosia trifida), Fringed Loosestrife (Lysimachia ciliata), Gray’s Sedge (Carex grayi) and Silky Dogwood (Cornus amomum). These species, along with Silver Maple (Acer saccharinum), are also common associates of Green Dragon in Michigan (Dembinsk 1966). Several provincially significant species, including some special concern and threatened species, were found by at a few Green Dragon sites by Rothfels and Smith (2003), but only American Gromwell (Lithospermum latifolium, S3) and Soft-hairy False Gromwell (Onosmodium molle, S2) appear to be closely linked to the same habitat. Green Dragon has been observed in Green Ash Mineral Deciduous Swamp (SWDM2-2) and Willow Mineral Deciduous Swamp (SWDM4-1) Ecological Land Classification (ELC) vegetation types (Jolly pers. comm. 2013), but ELC is not available for the habitat of most populations.
2.4 Characteristics contributing to vulnerability of species
Green Dragon is a weak-stemmed species that topples over early in the summer (Rothfels and Smith 2003). Many species it is associates with are more robust and grow much taller. Consequently, it can be easily overlooked after its peak. In addition, it can take several years for an individual to achieve flowering size. Young plants are difficult to observe because they blend in well with ground cover vegetation and resemble Jack-in-the-pulpit and other Arisaema species until its leaves are fully out (Jolly pers. comm. 2012). However, the species attracts a lot of interest from botanists and is conspicuous at its peak (Rothfels and Smith 2003). It is unlikely that there are many undiscovered populations in publicly accessible locations. It is possible that additional populations are present in some of the many private woodlands across its range.
Boles (1996) and Rothfels and Smith (2003) observed that many Ontario flowering Green Dragon plants do not set seed. While most of the populations surveyed by Rothfels and Smith (2003) in 2002 had some fruit-bearing plants, some populations did not produce any fruit-bearing individuals even though flowering plants were "fairly common". Three of the five Ontario populations with monoecious plants studied by Boles (1996) did not produce any fruit, while the other two had very high (83% and 100%) fruiting rates. The populations in Quebec have never been known to produce fruit (Comité ZIP and MOE 1999, as cited in Rothfels and Smith 2003).
Seeds from Ontario populations tend to be much smaller than seeds from more southerly locations (Boles 1996). Yang et al. (1999) found that seeds from the northern and southern ends of the species' range (Clinton, ON, and Baton Rouge, LA) had significantly lower dormancy rates than seeds from the central part of the range, and that germinability and the relative dormancy rate were inversely correlated with latitude. Seeds from Ontario had the highest mortality rate and were least likely to be viable, but it was unclear whether this was due to lower genetic vigour, small seed size, or the unsuitable conditions. Boles (1996) suggested that the Canadian populations of Green Dragon exhibit low levels of sexual reproduction because they are in marginal
The likely pollinators for Green Dragon are very small animals that generally do not travel more than a few metres. This could lead to little or no sexual reproduction in populations where plants are widely spaced (Boles 1996). It would also restrict or eliminate pollination as a means of gene flow between populations.
The Green Dragon seeds' extended germination period of two to five months (MacDougal 1901, Pickett 1913) could make developing seedlings more vulnerable to disturbance or changes in environment than species with shorter germination periods.
As discussed in the species biology section, Green Dragon exhibits a high dormancy rate, which could be an adaptation to survival in seasonal and intermittent wetland areas. It is unknown how long dormant seeds can remain viable in the seed bank or what conditions are required to break their dormancy. Also, as stated above, a significantly greater percentage of viable seeds from Ontario populations will germinate in the first year after dispersal compared to seeds from populations farther south. This means almost all of a year’s crop of sexually produced seedlings could be wiped out if environmental conditions are not suitable the summer after they are released.
Plants growing in sloughs, depressions, and other parts of the floodplain that trap water are susceptible to summer floods. Water level changes in freely-draining parts of the floodplain are short-lived in and may damage but do not usually kill the above ground parts of plants. Similar flooding in areas that trap water can be inundated for weeks, killing the above-ground portions of plants. At a Connecticut River study site that was flooded by summer storms two years in a row, Green Dragon did not grow during the two years of summer floods or the following year. It made up 1.5% of the plot cover in the second flood-free summer and 4% of the cover in the third flood-free summer. The plants were also much taller in the third flood-free summer than the second flood-free summer (20 cm vs. 12 cm) (Damman 1994).
Arisaema species, including Green Dragon, are susceptible Arisaema rust (Uromyces ari-triphylli) and other naturally occurring diseases and pests (Rennert 1901, Parmelee 1960). One of the populations studied by Schaffner (1922) appeared to be rapidly dying out due to a combination of changing ecological conditions and a severe outbreak of Arisaema rust.
3.0 Threats
Natural ecosystems are continually evolving in response to a variety of forces and factors. But they are limited in their ability to adapt to rapid change, such as that introduced through human activities. Humans sometimes disrupt and degrade biodiversity through habitat loss, introduction of invasive species, population growth, pollution, unsustainable use and climate change. Our growing population combined with our rising levels of resource consumption can threaten biodiversity (OBC, 2011). Recently, an assessment of pressures on Ontario’s biodiversity showed that many threats are increasing (OBC, 2010b).
The Green Dragon faces the following threats.
Habitat loss
Habitat loss and habitat degradation are the most significant threats to Green Dragon in Ontario. Green Dragon occurs in the most heavily populated region of Ontario, where development pressure is high. At the time of the 2003 draft update status report, the Fort Erie population, one of the largest in Ontario, was threatened by a proposed development (Rothfels and Smith 2003). The species' floodplain habitat is usually protected by regulations (Bowles 2013), but conversion to city parks, golf courses, cropland, and other land uses that are allowed in floodplains is a threat. As of 2006, only 3,050 km2 of forest, 222 km2 of wetland, 40 km2 of shrubland and 12 km2 of grassland remained in the 23,805 km2 Lake Erie Lowland ecoregion. The rest of the land base is primarily cropland (Filoso and Larocque 2010). Very little suitable habitat is left in this ecoregion and what remains is under considerable development pressure.
Significant woodlots and provincially significant wetlands receive some protection under the Ontario Planning Act and the Provincial Policy Statement. However, many woodlots and wetlands have never been evaluated and are not protected from development, even though they might meet the criteria. Significant Woodlands are only protected from activities that require an application under the Planning Act; activities such as converting a Significant Woodlot to cropland are not restricted by the designation. Municipal tree-cutting by-laws provide some protection from these types of activities, but Chatham-Kent and Essex do not have such by-laws (Ontario Woodlot Association 2003) and enforcement is not always consistent.
Recent high crop prices have stimulated many farmers to increase the size of the cultivated area on their properties by removing woodlots and hedgerows (Gartshore pers. comm. 2013, Waldron pers. comm. 2013). Such practices have been occurring at an increased rate in recent years, particularly in municipalities such as Chatham-Kent that do not have a conservation by-law, due to high commodity prices and the amalgamation of farms (Jalava, pers. obs.).
Habitat degradation
Changes to the forest canopy structure or to the hydrology of the site may be detrimental to Green Dragon (Rothfels and Smith 2003). Development on adjacent upland areas and tile drainage may alter the hydrology of the area and introduce other threats (e.g., invasive species, trampling from unauthorized recreational trails), even if the floodplain forest is retained.
During their fieldwork, Rothfels and Smith (2003) noted that the Thames River and Jordon Harbour floodplains showed evidence of strong spring scouring. They speculated that this is due to the "hardtopping of much of the surface area in these watersheds, and to the channeling of the main waterways," which would cause sharper water level peaks and more severe spring flooding. In these watersheds, they only found Green Dragon in areas that were higher and further from the shoreline than expected.
Yang et al. (1999) speculated that flood control by conservation authorities may have caused or contributed to the low survival and germination rates of the Ontario seeds in their experiments.
Tile drainage is being installed at a massive scale, notably in Chatham-Kent (Woodliffe pers. comm. 2013b). Tile drainage and other activities alter water levels, flow rates, frequency and intensity of flood events, and floodplain morphology (e.g., ditching, channelization). These activities are likely to impact Green Dragon due to its association with floodplains and reliance on flood events to disperse corm offsets. Study is needed to determine the extent and severity of threat these activities pose to Green Dragon in Ontario.
Habitat fragmentation and population isolation
Due to historical and ongoing development, Green Dragon is confined to increasingly small, isolated habitat fragments in Ontario. Several populations have very few individual plants. Small, isolated populations are vulnerable to stochastic events as well as direct threats (Rothfels and Smith 2003). Isolated populations are often at risk of inbreeding depression and low genetic diversity because gene flow between populations is restricted or prevented. Since the probable pollinators of Green Dragon are insects that generally do not travel long distances, gene flow through pollination is likely limited or not occurring in Ontario. Habitat fragmentation would also limit Green Dragon’s ability to colonize new areas as corm offsets dispersed through flooding are less likely to be deposited in suitable germination habitat. For example, sections of Spencer Creek downstream from the extant Greensville population are constrained in many areas by concrete or "entrenchment", limiting the opportunities for flood-dispersed corms to become established (Turner, pers. comm. 2013).
Two of the Green Dragon populations along Big Creek in the southwest corner of Essex County have extremely low genetic diversity (Boles 1996, Boles et al. 1999). These populations are more geographically and ecologically isolated than the others studied by Boles. This is due to the extreme historical loss of forest cover in the county and the lack of connection to another watershed having Green Dragon populations (Boles 1996). These populations did not produce any fruit, despite having one of the highest numbers of monoecious plants among the six Ontario populations included in the study. Boles (1999) speculated that, although one population was the second largest population in the study and exhibited vigorous clonal reproduction, these two populations will not be self-sustaining in the long term. The four other Ontario populations studied by Boles (1999) had levels of genetic diversity that are more typical of the species throughout its range. Additional research is needed to identify other Ontario populations that exhibit low genetic diversity and may not be self-sustaining.
Recreational trails
Rothfels and Smith (2003) listed recreational trails as a threat but did not elaborate. The two extant patches at Ruthven Park National Historic Site are less than 2.5 ft (0.75 m) from a recreational trail that was mown regularly until staff became aware of the plants in 2011. The trail is no longer mown and signs have been erected near the plants to encourage people to stay on the trail, but the plants are still at risk of trampling by people and pets that venture off the trail (Jolly pers. comm. 2012, Turner pers. comm. 2013). The newly reported population along Spencer Creek in Greensville is next to the heavily used Crooks Hollow Heritage Trail and was being trampled by hikers and cyclists. Concerned citizens have placed logs along the trail near the plants to help protect them, but the number of hikers and mountain bikes using the trail is increasing (Turner pers. comm. 2013). The number of trail users is likely increasing in other areas that are experiencing human population growth.
Logging
In dense, closed-canopy forests, selective logging can temporarily benefit Green Dragon by increasing the light intensity at the forest floor and creating suitable microhabitats by felled tree trunks and branches. At a river levee study site along the Connecticut River, the percent cover of Green Dragon tripled during the first three years after the adjacent plot was clear-cut, but quickly decreased after that (Damman 1994). Damman (1994) did not speculate on the reasons for the rapid drop in vigour. It could be due to the rapid regrowth of woody species, which quickly created a dense thicket in the clear-cut area within the five year study period, or the energy stored in the corms could have been depleted by the two years of rapid growth. Clear-cutting or heavy selective cutting may therefore be a threat to Green Dragon.
Changes to floodplain hydrology
As mentioned above, Green Dragon reproduces through vegetated offsets which are shed by the parent plant and travel upstream or downstream by flooding. Human activities that alter the hydrology of floodplain habitat threaten this form of dispersal. These activities include, but are not limited to, dams, impoundments, other flood control measures, and surface hardening near Green Dragon habitat.
Activities that intensify flood events are likely as much of a threat to the species as activities that prevent or minimize flooding. Turner (pers. comm. 2013) indicated that artificially high water flows due to urban surface hardening and agricultural drainage threaten populations (such as the one at Greenville) as a higher than natural percentage of plants could be exposed and dispersed during a flood. When combined with downstream habitat loss and degradation, a severe flood event that disperses a large percentage of a population could lead to local extirpation.
Invasive Species
Ontario’s Invasive Species Strategic Plan defines invasive species as "harmful alien species whose introduction or spread threatens the environment, the economy, or society, including human health" (OMNR 2012a). Following that definition, the Ontario Ministry of Natural Resources notes that invasive species may include species native to Ontario that have expanded their range due to human activity and which have become damaging to the new ecosystem (OMNR 2012a).
Invasive species are of particular concern in parts of Ontario where high population densities, strong import-export market and degraded habitat have made the landscape vulnerable to the establishment of new species (OMNR 2012a).
Since floodplains tend to have high levels of natural disturbance, it is not surprising that Rothfels and Smith (2003) found several non-native species to be abundant in areas with Green Dragon. Many sites had Garlic Mustard (Alliaria petiolata), Reed Canarygrass, Moneywort (Lysimachia nummularia), Manitoba Maple (Acer negundo), Dame’s Rocket (Hesperis matronalis), exotic willows, exotic honeysuckles (Lonicera spp.) and Gill-over-the-ground (Glechoma hederacea). Other weedy exotic species were also common. Rothfels and Smith (2003) observed at several sites that Green Dragon seemed to be restricted to the marginal habitat of creek-side locations while the exotics dominated the rest of the floodplain, which is more stable and productive.
Emerald Ash Borer (EAB) has caused changes to forest structure and landowner attitudes toward woodlots that are likely having impacts on Green Dragon, but these impacts have not been investigated. Green Dragon is commonly found in lush, creek- side canopy gaps in Ontario (Rothfels and Smith 2003). EAB has opened up the canopy of affected forests (Woodliffe pers. comm. 2013), creating gaps that could benefit Green Dragon if the species is able to colonize them. However, EAB, Asian Long-horned Beetle (Anoplophora glabripennis) and other exotic forest pests have changed landowner attitudes and activities in woodlots. Landowners may be more likely to allow timber harvest or firewood operations (Woodliffe, pers. comm. 2013). For example, when the first EAB quarantine was established, logging companies implemented advertising campaigns in rural areas of Middlesex County, encouraging landowners to sell the timber in their woodlots while they could. Many woodlots in the county were logged but not completely removed (Donley, pers. obs.). Landowners with woodlots affected by EAB may decide that, since much of the woodlot is already dead, it is time to convert the land to agriculture (Woodliffe, pers. comm. 2013). This may be contributing to the current high rate of woodlot clearing occurring in anticipation of a forest conservation by-law in Chatham-Kent. The impacts of changes in ecology and in landowner attitudes caused by EAB and other exotic pests on Green Dragon needs study.
Exotic earthworms
Sutherland et al. (2011, as cited in Sackett et al. 2012) stated that "non-native earthworms are recognized as an emerging threat to temperate North American forest ecosystems, and are considered one of the most globally important agents of change to biodiversity and associated ecological and evolutionary processes." None of the 19 species of earthworms known to occur in Ontario are native species; 17 were introduced from Europe and two (Sparganophilus eiseni and Bimastos parvus) were introduced from southern US. Another 20 or more species from Asia, Africa, and South America have been introduced to North America but have not yet been confirmed in Ontario (Evers et al. 2012). Earthworms native to the Great Lakes region, including Ontario were likely extirpated during the last glaciation period (Tiunov et al. 2006).
Exotic earthworms were probably introduced accidentally during and after European settlement in soil used for ship ballast or with imported plants in soil (Tiunov et al. 2006) or as cocoons on roots (Schwert 1977). Current accidental introductions are caused by the release of fishing bait worms, escape of worms from vermicomposters (Tiunov et al. 2006) and from the roots of imported plants (Schwert 1977). European settlers may have also deliberately introduced earthworms to "improve" the soil (Evers et al 2012). Once they become established in Ontario, exotic earthworms may be transported to new locations by the movement of construction fill or compost and other gardening materials, in mud on tires of equipment and off-road vehicles (Evers et al. 2012, Tiunov et al. 2006), and, most commonly, by dumped fishing bait (Cameron et al. 2007, Evers et al. 2012). Based on the maps in Evers et al. (2012), 15 of these introduced species are found within the Ontario range of Green Dragon.
Ontario forest species are adapted to the soil structure typical of worm-free environments: thick organic horizons (litter layer and duff layers) on top of mineral soil with low organic content. Earthworms dramatically alter this structure by consuming the duff and by thoroughly mixing the organic and mineral soil layers (Frelich et al. 2006, Tiunov et al. 2006, Evers et al. 2012). Deep burrowing species such as the Night Crawler (Lumbricus terrestis, also commonly known as the Dew Worm) can completely break down the thick duff layer and incorporate it into the soil in just a few years (Sackett et al. 2012). Earthworms also:
- consume the organic content of the mineral soil layers (Sackett et al. 2012);
- make the soil denser, negatively affecting plant roots, by incorporating the thick duff layers into the mineral soil, reducing the thickness of the forest floor and cementing soil particles together (Frelich et al. 2006, Evers et al. 2012);
- reduce soil nitrite and nitrate and phosphorus levels in the soil layers with the highest concentration of fine roots (Frelich et al. 2006, Evers et al. 2012);
- alter soil micro-organism communities and soil nutrient cycling by mixing the soil layers and consuming microbial species (Frelich et al. 2006, Evers et al. 2012);
- alter soil hydrology with their burrows, which greatly increase the water infiltration rate, and by eliminating the duff layer, which normally acts as a buffer against summer drought by absorbing rain water and slowly releasing it into the soil (Evers et al. 2012); and
- disrupt root systems and mychorryzal relations with their burrowing activities, reducing seedling establishment (Evers et al. 2012).
Earthworms can negatively affect species that rely on moister soils, such as Green Dragon. They increase soil density and water infiltration rates and eliminate the duff layer, causing drier site conditions (Frelich et al. 2006, Evers et al. 2012). Green Dragon is usually found on sites with thick layers of organic material from spring floods. Earthworms may incorporate this material into the soil too quickly for seedlings to become established. The loss of the thick organic and duff layer could also affect the winter survival of corms, reducing their insulation to freeze and thaw cycles. Since Green Dragon does not retain its roots over the winter and must expend energy producing new roots each growing season, disruption of its root system by burrowing earthworms could be particularly harmful to this species.
Subsidized/hyper-abundant herbivores and seed predators
The populations of many seed predators and herbivores are being maintained at unnaturally high levels by the abundant food supplies created by human agriculture, waste, and forestry practices (Waller 2008).
Pollution
As a floodplain species, Green Dragon would be exposed to water-borne pollutants, including fertilizers and pesticides from agricultural run-off and golf courses, road salt, sewage from faulty septic systems, excess sediment and industrial effluents. The impact of water pollution on Green Dragon was has not been studied.
Unsustainable use
As a traditional medicinal plant used by aboriginal peoples of North America and an occasionally-planted garden plant, Green Dragon populations may be threatened by overharvesting. The current level of exploitation is unknown. However, no evidence of such impacts was found in the literature, nor in consultations with First Nations (Porchuk, pers. comm. 2013) and local experts. Since many populations have persisted despite being well-known and easily accessible, collection for traditional medicines or horticultural use is likely not a threat to large populations or to the species' continued existence in Ontario. However, a single collection at one of the small populations could cause severe harm or completely eliminate all plants at that site.
Climate change
Canada is seeing rising temperatures, shifting precipitation patterns and increases in extreme weather events as a result of global climate change (Environment Canada 2012, 2013). While projected scenarios vary, most describe increases from 3 to 8 degrees Celsius by the end of the century (Ministry of Environment 2012, Gleeson et al. 2011, Varrin et al. 2007). Strong variability in weather patterns are of particular concern. Extreme weather events such as ice storms, heavy rains, droughts and wind storms are expected to occur more frequently and unpredictably (Gleeson et al. 2011).
Ontario populations of Green Dragon are at risk of the predicted lower water levels due to climate change (Rothfels and Smith 2003). Populations may also be threatened by flash flooding caused by more severe weather events, exacerbated by increased run-off levels due to hardened surfaces associated with urban development.
4.0 Management
4.1 Goal and objectives
- Management Goal: Maintain a viable population of Green Dragon at all currently extant populations. Restore populations that are not self-sustaining. Enhance connectivity between extant populations to facilitate natural dispersal and allow for population expansion to attain the eventual de-listing of the species.
Table 3. Management objectives
| No. | Management Objective |
|---|---|
| 1 | Conduct baseline inventory to determine the size and number of extant sites, site quality and population health. Implement regular monitoring to track population trends and changes in habitat quality in response to management actions and threats. |
| 2 | Protect and manage species and habitat at extant sites in Ontario to achieve and maintain viable population levels. |
| 3 | Address key knowledge gaps relating to life cycle requirements, habitat requirements and prioritization of threats. |
| 4 | Promote awareness and stewardship of Green Dragon with First Nations, land managers, private landowners, municipalities and key stakeholders. |
| 5 | Support and implement landscape- and ecosystem-based planning and recovery initiatives to increase the amount of available habitat for Green Dragon, and to enhance habitat connectivity for dispersal and population expansion. |
4.2 Management actions completed or underway
A number of extant Green Dragon populations occur within protected areas. At least two occurrences are in provincial parks or provincial conservation reserves, and more than a dozen are on properties owned by conservation authorities. More than one is in a private nature reserve, two are in Environmentally Significant Areas and three are in Areas of Natural and Scientific Interest (ANSIs) (NHIC 2012).
One occurrence is at a golf course owned and operated by the City of London. This occurrence was surveyed by city staff and J. Jalava in 2010. Golf course greenskeepers have been made aware of the plants and have been instructed to manage the habitat to conserve the Green Dragon population (Jalava pers. obs. 2010, Bergsma pers. comm. 2013).
In 2013 the City of London and Upper Thames River Conservation Authority will conduct targeted searches for species at risk, including Green Dragon. This will take place during ongoing natural life science inventory work for three Environmentally Significant Areas and for two subwatershed management plan updates (Bergsma, pers. comm. 2013). All of these areas are known or suspected to have Green Dragon populations.
In 2011, Ruthven Park National Historic Site stopped mowing the trail near the Green Dragon patches and erected signs to encourage visitors to stay on the trail (Jolly pers. comm. 2012, Turner pers. comm. 2013). One patch was found immediately adjacent to the trail in 2012. The trail has since been moved 2.5 ft (0.75 m) away from the plants. It cannot be moved farther away from the other patch due to the narrowness of the floodplain at that spot. The Site is pursuing funding opportunities to create boardwalks to protect wet areas along the trail including where the trail cannot be moved, and to protect the plants from trampling. In the interim, logs are placed along the sides of the trail to help keep people on the trail. This is done annually as this section of the trail floods every year. All summer students that work on grounds maintenance are introduced to the species and instructed not to mow in the area. The staff monitor the population as part of their land stewardship program (Turner, pers. comm. 2013).
The Park Management Plan for Clear Creek Forest Provincial Nature Reserve was completed in 2012. Although the management plan does not include specific recommendations relating to Green Dragon, it does include provisions to:
- manage invasive species;
- maintain natural shoreline;
- restore vegetation communities;
- improve and restore natural stream flow to Clear Creek;
- prohibit use of motorized vehicles (ATVs);
- protect plant communities from trampling and other adverse impacts;
- manage species at risk;
- manage deer populations; and
- conduct inventory and monitoring programs for species at risk and vegetation restoration activities (OMNR 2012b).
These provisions address many of the threats to Green Dragon and provide a mechanism to fill some of the knowledge gaps.
Green Dragon is included in the Wetland Plant List for the Ontario Wetland Evaluation System (OWES) as an indicator species for swamps in southern Ontario. This is the only plant list that can be used to delineate wetland boundaries using OWES. In addition, for each species at risk and provincially significant species observed during the evaluation, a completed NHIC Rare Species Reporting Form (or sufficient information to complete one) must be submitted with the wetland evaluation file (OMNR 2013). Consequently, wetland evaluators should be aware of the species, actively search for it while conducting wetland evaluations in southern Ontario, and document and report their observations.
Conservation Action Plans (CAPs) are among the primary recovery approaches recommended in the ecosystem-based Carolinian Woodlands Recovery Strategy (Jalava et al. 2009, Jalava and Mansur 2008). Green Dragon is a nested conservation target in the Short Hills CAP (Jalava et al. 2012a), Niagara River Corridor CAP (Jalava et al. 2012b), Upper Thames River CAP (Upper Thames River CAP Team 2009), Ausable River – Kettle Point to Pinery CAP (Jalava et al. 2010), Rondeau – Erie Coast CAP (Jalava et al. 2013) and Essex Forests and Wetlands CAP (EFW NACP/CAP Team 2009) Carolinian Canada biodiversity hotspots. Recommended habitat stewardship and restoration activities, as well as public outreach and education associated with these CAPs are expected to benefit Green Dragon populations in these areas.
4.3 Management plan approaches for action
Table 4. Management plan approaches for action for the Green Dragon in Ontario
1. Conduct baseline inventory to determine the size and number of extant sites, site quality, and population health. Implement regular monitoring to track population trends and changes in habitat quality in response to management actions and threats.
| Management Theme | Management Approach | Relative Priority | Threats or Knowledge Gaps Addressed | Relative Timeframe |
|---|---|---|---|---|
| Management, Inventory, Monitoring and Assessment | 1.1 Conduct inventory and monitoring at all known sites (extant and historical) and areas of suitable habitat, using standard techniques to assess population size and health, reproduction, habitat quality, threats and limiting factors. All areas of suitable habitat on public lands should be identified and surveyed. Areas of suitable habitat on privately owned sites should be identified and surveyed where possible. | Critical | Distribution and abundance; health, status, threats, demography, and size of populations | Short term Ongoing |
| Monitoring and Assessment | 1.2 Conduct ongoing monitoring of invasive species at extant Green Dragon populations. | Necessary | Invasive species | Ongoing |
| Management, Inventory, Monitoring and Assessment, Research | 1.3 Complete the Element Occurrence verification of mapping for all unprocessed records of Green Dragon in the Natural Heritage Information Centre’s database. | Beneficial | Population size and distribution | Short-term |
| Management, Inventory, Monitoring and Assessment, Research | 1.4 Assess each extant population for barriers to natural dispersal. | Necessary | Habitat fragmentation and isolated populations; changes to floodplain hydrology | Short-term |
| Management, Inventory, Monitoring and Assessment | 1.5 Implement special campaign to encourage OWES certified wetland evaluators to search for and report Green Dragon. Campaign should be timed to coincide with baseline inventory and regular monitoring. | Beneficial | Distribution and abundance; health, status, threats, demography, and size of populations | Short-term Ongoing |
| Management, Inventory, Monitoring and Assessment | 1.6 Investigate the applicability of population viability assessment (PVA) for species that rely on vegetative reproduction. If deemed applicable, conduct robust PVA for Ontario populations. Determine minimum viable population size. Implement management actions to restore non-viable populations. | Necessary | Minimum viable population size in Ontario Isolated populations | Long-term |
| Inventory | 1.7 Develop, implement, and maintain a system to track locations of planted SAR plants, including Green Dragon, and the original sources of the planted individuals. Request annual report from municipalities, conservation authorities, and other organizations involved in habitat restoration on special concern species planted (including species, seed source, general planting locations, number of plugs and seedlings planted at each location and survival rates). Reports could be voluntary. Reporting system should be accessible to stewardship organizations to facilitate tracking. | Critical | Number and location of planted populations | Short-term Ongoing |
Protect and manage species and habitat at extant sites in Ontario to achieve and maintain viable population levels.
| Management Theme | Management Approach | Relative Priority | Threats or Knowledge Gaps Addressed | Relative Timeframe |
|---|---|---|---|---|
| Protection, Management | 2.1 Support incorporation of extant Green Dragon populations into site-specific forest and floodplain management planning. | Necessary | Habitat degradation | Ongoing |
| Protection | 2.2 Encourage and support activities to minimize or prevent impacts of trails on Green Dragon and other SAR plants (e.g., relocate or close trails, install boardwalks). | Necessary | Recreational trails | Short-term Ongoing |
| Management, Monitoring | 2.3 a) Implement invasive species management at high priority sites. b) Monitor changes in Green Dragon and invasive species populations in response to management actions; determine effectiveness of management actions. c) Adapt management actions based on results and implement at other sites as necessary. | Critical | Invasive species | Short-term Long-term Ongoing |
| Protection, Management, Stewardship, Education and Outreach | 2.4 Address the increased demand for natural areas and trails in urban intensification policies to ensure sufficient natural areas and parks to serve the growing population and mitigate the overuse of existing parks. | Beneficial | Recreational trails | Long-term Ongoing |
| Management | 2.5 Work with Conservation Authorities to ensure Green Dragon populations are seasonally flooded at appropriate times to stimulate seed dispersal and germination. | Necessary | Habitat degradation | Long-term |
| Management, Monitoring | 2.6 a) Remove or mitigate significant barriers to dispersal at affected populations. b) Monitor and assess effectiveness and impacts of barrier removal / mitigation measures. | Beneficial | Barriers to dispersal; small isolated populations (stochastic events) | Long-term |
| Management, Monitoring | 2.7 a) Where plants occur in dense clumps, transplant young individuals to adjacent suitable habitat to enhance vegetative regeneration. b) Monitor survival and growth of transplanted individuals and source clumps. | Beneficial | Small, isolated populations (stochastic events) | Long-term |
| Stewardship | 2.8 Encourage and support cultivation of locally sourced Green Dragon for use in restoration projects. | Beneficial | Isolated populations (stochastic events, genetic diversity) | Short-term Ongoing |
| Research | 2.9 Support research into impacts of exotic earthworms on Green Dragon and other SAR plants and potential control and/or threat mitigation measures. | Necessary | Impacts of exotic earthworms Exotic earthworms | Long-term |
| Research, Inventory and Monitoring, Education and Outreach | 2.10 Implement the recommendations in Evers et al. (2012) to address the threats and impacts of exotic earthworms. | Beneficial | Exotic earthworms | Long-term Ongoing |
| Protection, Communication | 2.11 Work with the responsible agencies to ensure that the design, construction, and maintenance of drains under the Drainage Act and the Tile Drainage Act and associated regulations protect Green Dragon and other species at risk. | Critical | Habitat degradation | Long-term Ongoing |
Address key knowledge gaps relating to life cycle requirements, habitat requirements, and prioritization of threats.
| Management Theme | Management Approach | Relative Priority | Threats or Knowledge Gaps Addressed | Relative Timeframe |
|---|---|---|---|---|
| Research | 3.1 Support research to determine minimum viable population size requirements. | Critical | Minimum viable population size in Ontario | Short-term |
| Research | 3.2 Identify impacts of invasive species on Green Dragon; prioritize species and populations for intervention. | Critical | Invasive species Impact of invasive species on Green Dragon | Short-term Ongoing |
| Research | 3.3 Support research into the biology of the species to better understand dispersal mechanisms and reproductive success. | Necessary | Human activities such as flood control. Dispersal mechanisms, limiting factors and needs | Long-term |
| Research | 3.4 Support research into the habitat requirements for successful dispersal and reproduction. | Beneficial | Human activities such as flood control. Habitat requirements for reproductive success, dispersal requirements | Long-term |
| Research | 3.5 Support research into genetic diversity of all Ontario populations. Identify populations that may not be self-sustaining due to low genetic diversity. Implement management actions to improve genetic diversity at these sites, monitor and report on results. | Critical | Isolated populations with low genetic diversity Level of genetic diversity in all Ontario populations; populations with low genetic diversity | Long-term |
Promote awareness and stewardship of Green Dragon with First Nations, land managers, private landowners, municipalities and key stakeholders.
| Management Theme | Management Approach | Relative Priority | Threats or Knowledge Gaps Addressed | Relative Timeframe |
|---|---|---|---|---|
| Education and Outreach, Communications | 4.1 Develop and distribute Best Management Practices fact sheets and other information materials to inform land managers and land owners about the significance and biological needs of Green Dragon. | Necessary | Inadvertent habitat destruction or degradation | Ongoing |
| Management, Outreach, Communications, Inventory and Monitoring | 4.2 Promote, facilitate and monitor the use of locally-sourced native seed stock with nurseries, landscaping companies and agencies involved in wetland restoration (e.g., municipalities, conservation authorities, ENGOs). Encourage native plant nurseries to grow locally sourced Green Dragon for use in wetland restoration and Green Dragon recovery projects. | Necessary | Habitat loss and fragmentation (resulting in reduced natural ability to disperse and expand populations) | Ongoing |
Support and implement landscape- and ecosystem-based planning and recovery initiatives to increase the amount of available habitat for Green Dragon, and to enhance habitat connectivity for dispersal and population expansion.
| Management Theme | Management Approach | Relative Priority | Threats or Knowledge Gaps Addressed | Relative Timeframe |
|---|---|---|---|---|
| Management, Outreach, Communications | 5.1 Support initiatives that protect, restore and rehabilitate natural landscape connectivity in watersheds with Green Dragon populations (e.g., municipal natural heritage systems plans, Carolinian Canada conservation action plans, conservation authority watershed plans). | Necessary | Habitat loss, habitat degradation, habitat fragmentation and isolated populations | Ongoing |
| Research, Stewardship, Education and Outreach | 5.2 Investigate short-term and long-term impacts of EAB and other forest pests on woodlot owner attitudes and activities within the range of Green Dragon. Support and implement programs to encourage retention and management of floodplain forests within the range of Green Dragon. | Critical | Impacts of EAB and other exotic pests Habitat loss, habitat fragmentation | Long-term |
| Protection, Education and Outreach, Stewardship | 5.3 Continue to support the Conservation Land Tax Incentive Program and the Managed Forest Tax Incentive Program. Develop, and implement effective communications materials and use a variety of communication platforms to make woodlot owners within the range of Green Dragon aware of these programs. Assist interested landowners with the development and implementation of property plans. Monitor compliance with approved plans. Evaluate and monitor effectiveness of communications materials (e.g., through number of plans completed) and revise as needed. | Critical | Habitat loss, habitat degradation | Long-term Ongoing |
| Management, Stewardship | 5.4 Identify and prioritize sites for establishing new Green Dragon populations to enhance connectivity and facilitate gene flow between populations. Establish new populations and monitor survival. | Necessary | Isolated populations | Long-term |
| Stewardship | 5.5 Support creek and river shoreline naturalization programs in watersheds with Green Dragon populations. | Beneficial | Habitat loss, habitat degradation, isolated populations | Ongoing |
Glossary
Bract: A modified leaf or scale with a flower or flower cluster in its axil (Oxford University Press 2008)
Brunisol: Soils with minimal weathering (Trant and Filoso 2008).
Committee on the Status of Endangered Wildlife in Canada (COSEWIC): The committee responsible for assessing and classifying species at risk in Canada.
Committee on the Status of Species at Risk in Ontario (COSSARO): The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
Conservation status rank: A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. Where there is some uncertainty in the conservation status rank, the numbers may be combined (e.g., S3S4) - need to verify this addition to definition. The numbers mean the following:
1 = critically imperilled
2 = imperilled
3 = vulnerable
4 = apparently secure
5 = secure
NR = unranked, conservation status not yet assessed
Corm: A rounded underground storage organ of plants, consisting of a swollen stem base covered with scale leaves (Oxford University Press 2008)
Dormancy rate: The number of un-germinated but viable seeds divided by the number of seeds initiated, then multiplied by 100 (Yang et al. 1999). Inverse of germinability rate (i.e., subtract the germinability rate from 100).
Element Occurrence (EO): An element occurrence (EO) is an area of land and/or water in which an element (a unit of natural biological diversity) is or was present. These are defined and stored by the Natural Heritage Information Centre (NHIC). With immobile plants, a distance of 1 km is often used to separate occurrences.
A - Excellent predicted viability
B - Good predicted viability
C - Fair predicted viability
D - Probably not viable
E - Verified extant
F - Failed to find
H - Historical
X - Extirpated
Endangered Species Act, 2007 (ESA): The provincial legislation that provides protection to species at risk in Ontario.
Germinability rate: The number of germinating seeds divided by the number of seeds initiated, then multiplied by 100 (Yang et al. 1999). Inverse of the dormancy rate (i.e., subtract the dormancy rate from 100).
Humic gleysol: Gleysolic soils develop under prolonged periods of intermittent or continuous saturation with water and are characterized by reduced levels of iron and other elements (Soil Classification Working Group 1998, Trant and Filoso 2008). Humic gleysols have at least 3.5 % organic matter (grass, sedge, moss, or forest vegetation) in the surface horizon and have either a dark, organically rich A horizon over 10 cm thick or a mixed surface horizon over 15 cm thick. They are commonly found in poorly drained areas associated with brunisolic, luvisolic, and a few other soil types (Soil Classification Working Group 1998).
Infructescence: An aggregate fruit (Oxford University Press 2008).
Luvisol: Temperate-region soil with clay-rich sublayers (Trant and Filoso 2008).
Mesic: An environment or habitat containing a moderate amount of moisture (Oxford University Press 2008).
Monoecious: An individual that has both male and female reproductive organs; a hermaphrodite (Oxford University Press 2008). In the case of plants, has male and female flowers on the same plant or flower head.
Offset: A shoot that develops laterally at the base of a plant, often rooting to form a new plant.
Physical dormancy: Germination is prevented at time of dispersal due to physical characteristics of the seed. It is usually due to water impermeability of seed coat of fruit, but may also be cause by the shape of seed or the shape and position of embryo (Baskin and Baskin 1998).
Phytohormone: A hormone-like substance produced by a plant.
Relative dormancy rate: The number of un-germinated but viable seeds divided by the number of viable seeds initiated, then multiplied by 100 (Yang et al. 1999). Inverse of relative germinability rate (i.e., subtract the relative germinability rate from 100).
Relative germinability rate: The number of germinating seeds divided by the number of viable seeds initiated, then multiplied by 100 (Yang et al. 1999). Inverse of relative dormancy rate (i.e., subtract the relative dormancy rate from 100).
Scape: A leafless flower stalk growing directly from the ground.
Scarification: The slitting or softening of the outer coat of seeds to assist germination.
Spadix: A flower spike with a thickened, often succulent, central column; usually completely surrounded by a spathe (Oxford University Press 2008).
Spathe: A large bract growing from the base of a spadix and completely enclosing the spadix like a sheath (Oxford University Press 2008).
Stratification: Configuring in layers.
Species at Risk Act (SARA): The federal legislation that provides protection to species at risk in Canada. This act establishes Schedule 1 as the legal list of wildlife species at risk to which the SARA provisions apply. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
Species at Risk in Ontario (SARO) List: The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
Stochastic: Randomly determined; having a random probability distribution or pattern that may be analyzed statistically but may not be predicted precisely (Oxford University Press 2008).
References
Baskin, C.C. and J.M. Baskin. 2001. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination. Academic Press, San Diego, CA. 666 pp.
Bergsma, B. 2013. Email communication with R. Donley and J. Jalava. April 2013. Ecologist, The City of London.
Bierzychudek, P. 1984. Determinants of gender in Jack-in-the-pulpit; the influence of plant size and reproductive history. Oecologia 65:14-18
Boles, R.L. 1996. Genetic structure and sexual reproductive potential of the perennial aroid Arisaema dracontium. M.Sc. thesis, Faculty of Graduate Studies, University of Windsor, Windsor, Ontario.
Boles, R.L., J. Lovett-Doust, and L. Lovett-Doust. 1999. Population genetic structure in green dragon (Arisaema dracontium, Araceae). Canadian Journal of Botany 77:1401–1410
Bowles, J. 2013. Email correspondence with R. Donley. January 2013. Director of the Sherwood Fox Arboretum, Curator of University of Western Ontario Herbarium, and Adjunct Professor of Biology and Geography, University of Western Ontario, London, Ontario.
Cameron, E.K., E.M. Bayne, and M.J. Clapperton. 2007. Human-facilitated invasion of exotic earthworms into northern boreal forests. Ecoscience 14(4): 482-490
Clay, K. 1993. Size-dependent gender change in Green Dragon (Arisaema dracontium; Araceae). American Journal of Botany 80(7): 769-777.
Cole, E.J. 1962. Green Dragon (Arisaema dracontium). The Michigan Botanist 1:56-59
Commité ZIP du lac Saint-Pierre et ministère de l'Environnement (MOE). 1999. Plan de conservation de l'arisème dragon (Arisaema dracontium) du Quebec 1999-2003. Gouvernement du Québec, ministère de l'Environnement, Direction du patrimoine écologique et du développement durable, Québec. 41 pp.
Damman, A.W.H. 1994. JHR 94-236 Connecticut River Flood Plain Vegetation as an Indication of Fluctuations in Water Level and Sedimentation; Final Report for Project 88-4. University of Connecticut, Storrs, CT . 44 pp.
Dembinsky, M.L. 1966. Notes on the distribution of Arisaema dracontium in Michigan. The Michigan Botanist 5:114-117.
Department of Environmental Conservation. 2012. 6 NYCRR Part 193.3 Protected native plants. New York State Department of Environmental Conservation, Albany, NY. Web site http://www.dec.ny.gov/regs/15522.html [Link no longer active] [Accessed 14 January 2013].
Développement durable, Environnement, Faune et Parcs. 2012. Liste des plantes vasculaires menacées. Web site: Menacees Floristiques PDF[Accessed 15 January 2012].
eFloras. 2008. Flora of North America. Missouri Botanical Garden, St. Louis, MO & Harvard University Herbaria, Cambridge, MA. Web site: efloras.org[Accessed 9 January 2013]
EFW NACP/CAP (Essex Forests and Wetlands Natural Area Conservation Plan / Conservation Action Plan) Team. 2009. Essex Forests and Wetlands Conservation Action Plan. Carolinian Canada Coalition, London. X + 63 pp.
Environment Canada. 2012. Climate Trends and Variations Bulletin - Annual 2011, Environment Canada. Website: http://www.ec.gc.ca/adsc-cmda/default.asp?lang=En&n=77842065-1 [Link no longer active] [accessed April 2013].
Environment Canada. 2013. Climate Change Science and Research, Environment Canada. Website: Climate Change Science and Research[accessed April 2013].
Evers, A.K., A.M. Gordon, P.A. Gray, and W.I. Dunlop. 2012. CCRR-23: Implications of a Potential Range Expansion of Invasive Earthworms in Ontario’s Forested Ecosystems: A Preliminary Vulnerability Analysis. Ontario Ministry of Natural Resources, Peterborough, Ontario. 31 pp.
Filoso, G. and H. Larocque. 2010. Ecoregion profile: Lake Erie Lowland. Catalogue no. 16-002-X EnviroStats 4(1):8-12.
Frelich, L.E., C.M. Hale, S. Scheu, A.R. Holdsworth, L. Heneghan, P.J. Bohlen, and P.B. Reich. 2006. Earthworm invasion into previously earthworm-free temperate and boreal forests. Biological Invasions 8:1235-1245.
Galil, J. and L. Meiri. 1992. Effect of wetting on the germinability of the pollen of Arisaema vulgare. Targ.- Tozz. Flora 186:145-152.
Gartshore, M. pers. comm. 2013. Email correspondence to R. Donley. January 2013. St. Williams Forestry & Ecology Centre, St. Williams, ON.
Gauvin, C. 1984. COSEWIC status report on the green dragon Arisaema dracontium in Canada. Committee on the Status of Endangered Wildlife in Canada. 40 pp.
Gleeson, J., P. Gray., A. Douglas, C.J. Lemieux and G. Nielsen. 2011. A Practitioner’s Guide to Climate Change Adaptation in Ontario’s Ecosystems. Ontario Centre for Climate Impacts and Adaptation Resources, Sudbury, Ontario. 74 pp.
Huttleston, D.G. 1953. A taxonomic study of the temperate North American Aracaea. Ph.D dissertation, Cornell University, Ithaca, New York.
Jalava, J.V. and P. Mansur. 2008. National Recovery Strategy for Carolinian Woodlands and Associated Species at Risk, Phase II: Part 1 – Implementation. DRAFT 3, May 9, 2008. Carolinian Canada Coalition, London, Ontario. vii + 124pp.
Jalava, J.V., J.D. Ambrose and N. S. May. 2009. National Recovery Strategy for Carolinian Woodlands and Associated Species at Risk: Phase I. Draft 11 – August, 2009. Carolinian Canada Coalition and Ontario Ministry of Natural Resources, London, Ontario. viii + 75 pp.
Jalava, J.V. and J.D. Ambrose. 2012a. Recovery Strategy for the Drooping Trillium (Trillium flexipes) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. vi + 20 pp.
Jalava, J.V. and J.D. Ambrose. 2012b. Recovery Strategy for the Heart-leaved Plantain (Plantago cordata) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. vi + 27 pp.
Jalava, J.V., M. Andreae, J.M. Bowles, G. George, K. Jean, A. MacKenzie, M. McFarlane and P. Scherer. 2010. Ausable River – Kettle Point to Pinery Conservation Action Plan (CAP). The Ausable River – Kettle Point to Pinery Conservation Action Planning Team (Ausable Bayfield Conservation Authority, Carolinian Canada Coalition, University of Western Ontario, Chippewas of Kettle and Stony Point First Nation, Ontario Parks, Nature Conservancy of Canada, Municipality of Lambton Shores, Lambton Federation of Agriculture). Carolinian Canada Coalition, London, ON. ix + 69 pp.
Jalava, J.V., M. Fletcher, D. Koscinski, and the Rondeau – Erie Coast CAP Development Team. 2013. Carolinian Canada Coalition, London, Ontario. 49 pp. + appendices.
Jolly, D. pers. comm. 2012. Email correspondence with R. Donley. November 2012. Senior Biologist, EARTHQUEST Environmental Consultants, Aylmer, Ontario.
Lovett-Doust, J., and P.B. Cavers. 1982a. Resource allocation and gender in the green dragon Arisaema dracontium (Araceae). American Midland Naturalist 108:144-148.
Lovett-Doust, J, and PB. Cavers. 1982b. Sex and gender dynamics in jack-in-the-pulpit, Arisaema triphyllum (Araceae). Ecology 63: 797-808.
MacDougal, D.T. 1901. Seedlings of Arisaema. Torreya 1:2
Massachusetts Division of Fisheries and Wildlife. 2012. Massachusetts List of Endangered, Threatened and Special Concern Species. Web site: http://www.mass.gov/dfwele/dfw/nhesp/species_info/mesa_list/mesa_list.htm#PLANTS [Link no longer active] [Accessed 11 January 2013].
Ministry of Environment. 2012. Climate Change Progress Report. Queen’s Printer for Ontario, 49 pp.
Nantel, P. pers. comm. 2013. Email correspondence with R. Donley. April 2013. Office of the Ecosystem Chief Scientist, Parks Canada.
Natural Heritage Endangered Species Program. 2009. Green Dragon Arisaema dracontium (L.) Scott. Massachusetts Division of Fisheries and Wildlife, Westborough, MA.
Natural Heritage Information Centre (NHIC). 2012. Element Summary Report for Arisaema dracontium. Ontario Ministry of Natural Resources, Peterborough, Ontario. Web site https://www.biodiversityexplorer.mnr.gov.on.ca/nhicWEB/nhicIndex.jsp [Link no longer active] [Accessed: November 19, 2012].
Natural Heritage Information Centre (NHIC). 2013. Unpublished observation data and shapefiles for Arisaema dracontium. Ontario Ministry of Natural Resources, Peterborough, Ontario.
NatureServe. 2012. NatureServe Explorer: An online encyclopedia of life [web application]. Version 7.1. NatureServe, Arlington, Virginia. Available NatureServe.org [Accessed: November 15, 2012].
New Hampshire Natural Heritage Bureau. 2012. Rare Plant List for New Hampshire, General Copy. Division of Forests & Lands, New Hampshire Natural Heritage Bureau, Concord, NH. 16 pp.
Ontario Biodiversity Council (OBC). 2010b. State of Ontario’s Biodiversity 2010. A report of the Ontario Biodiversity Council, Peterborough, Ontario available: http://www.ontariobiodiversitycouncil.ca/index.php/reports [Link no longer active].
Ontario Biodiversity Council. 2011. Ontario’s Biodiversity Strategy, 2011: Renewing Our Commitment to Protecting What Sustains Us. Ontario Biodiversity Council, Peterborough, ON.
Ontario Ministry of Natural Resources (OMNR). 2012a. Ontario Invasive Species Strategic Plan. Toronto: Queen’s Printer for Ontario. 58 pp.
Ontario Ministry of Natural Resources. 2012b. Clear Creek Forest Park Management Plan. Queen’s Printer for Ontario. iii + 17 pp.
Ontario Ministry of Natural Resources. 2013. Ontario Wetland Evaluation System, Southern Manual, 3rd edition, version 3.2. Queen’s Printer for Ontario. viii + 283 pp.
Oxford University Press. 2008. The New Oxford American Dictionary, 2nd edition. Oxford University Press, Inc. on Mac iOS 6.0.1.
Parmelee, J.A. 1960. The Fungi of Ontario, I., Uredinales. Publication 1080. Research Branch, Canada Department of Agriculture, Ottawa, ON.
Pickett, F.L. 1913. The germination of seeds of Arisaema. Proceedings of the Indiana Academy of Science 23:125-128
Porchuk, B., pers. comm. 2013. Correspondence with J.V. Jalava. January 2013. Ecological Consultant, Ontario.
Quinlan, C., pers. comm. 2013. Email correspondence with R. Donley. January 2013. Terrestrial Biologist, Upper Thames River Conservation Authority, London, ON.
Rennert, R.J. 1901. Teratology of Arisaema. Bulletin of the Torrey Botanical Club 27(4):247-250.
Rennert, R.J. 1902. Seeds and Seedlings of Arisaema triphyllum and Arisaema dracontium. Bulletin of the Torrey Botanical Club 29(1):37-54
Rothfels, C. and T. Smith. 2003. Update COSEWIC Status Report on Green Dragon, Arisaema dracontium (L.) Schott. [Review draft]. Manuscript. 48 pp
Sackett, T.E., S.M. Smith and N. Basiliko. 2012. Indirect and direct effects of exotic earthworms on soil nutrient and carbon pools in North American temperate forests. Soil Biology & Biochemistry, 57:459-467.
Sanders, L.L., and C.J. Burk. 1992. A naturally-occurring population of putative Arisaema triphyllum subsp. Stewadsonii x A. dracontium hybrids in Massachusetts. Rhodora 94: 340-347.
Schaffner, J.H. Control of the sexual state in Arisaema triphyllum and Arisaema dracontium. American Journal of Botany 9:72-78
Schwert, D.P. 1977. The first North American record of Aporrectodea icterica (Savigny, 1826) (Oligochaeta, Lumbricidae), with observations on the colonization of exotic earthworm species in Canada. Canadian Journal of Zoology 55:245-248.
Sutherland, W.J., S. Bardsley, L. Bennun, M. Clout, I.M. Côté, M.H. Depledge, L.V. Dicks, A.P. Dobson, L. Fellman, E. Fleishman, D.W. Gibbons, A.J. Impey, J.H. Lawton, F. Lickorish, D.B. Lindenmayer, T.E. Lovejoy, R.M. Nally, J. Madgwick, L.S. Peck, J. Pretty, S.V. Prior, K.H Redford, J.P.W Scharlemann, M. Spalding, and A.R. Watkinson, 2011. Horizon scan of global conservation issues for 2011. Trends in Ecology & Evolution 26:10-16.
Soil Classification Working Group. 1998. The Canadian System of Soil Classification, 3rd ed. Agriculture and Agri-Food Canada Publication 1646. NRC Research Press, Ottawa, ON. 188 pp.
Srivastava, P., and B.K. Banerji. 2012. Gender biasing in Arisaema - a unique and rare phenomenon. Current Science 102(2): 189-193.
Trant, D. and G. Filoso. 2008. Canada’s ecozones and population change, 1981 to 2006. Catalogue no.16-002-X EnviroStats 2(2):21-25. Web site: http://www.statcan.gc.ca/pub/16-002-x/16-002-x2008002- eng.pdf [Link no longer active] and Canada’s ecozones and population change, 1981 to 2006 [Accessed 20 January 2013].
Tiunov, A.V., C.M. Hale, A.R. Holdsworth, T.S. Vsevolodova-Perel. 2006. Invasion patterns of Lumbricidae into the previously earthworm-free areas of northeastern Europe and the western Great Lakes region of North America. Biological Invasions 86:1223–1234
Turner, S. pers. comm. 2013. Email correspondence with R. Donley. April 2013. Stewardship Coordinator, Ruthven Park National Historic Site, Cayuga, ON.
Upper Thames River CAP (Conservation Action Planning) Team. 2009. DRAFT Upper Thames River Conservation Action Plan. Carolinian Canada Coalition, London, ON. 31 pp.
Upper Thames River Conservation Authority (UTRCA). 2012. 2012 Upper Thames River Watershed Report Cards. Upper Thames River Conservation Authority, London, ON. v + 78 pp + appendices.
Varrin, R., J. Bowman and P.A. Gray. 2007. The Known and Potential Effects of Climate Change on Biodiversity in Ontario’s Terrestrial Ecosystems: Case Studies and Recommendations for Adaptation. Ontario Ministry of Natural Resources, Queen’s Printer for Ontario. Ontario. iv + 50 pp.
Vermont Natural Heritage Inventory. 2012. Threatened and Endangered Plants of Vermont. Vermont Natural Heritage Inventory, Vermont Fish & Wildlife Department, Montpelier, VT. 5 pp.
Waldron, G.W., pers. comm. 2013. Telephone conversation with R. Donley, January 2013. Consultant, Gerry Waldron Consulting Ecologists, Amherstburg, ON.
Waller, D.M. 2008. White-tailed deer impacts in North America and the challenge of managing a hyperabundant herbivore. Pp 135-147 in Gaston, A.J., T.E. Golumbia, J.L. Martin, and S.T. Sharpe (eds.) 2008. Lessons from the Islands: introduced species and what they tell us about how ecosystems work. Proceedings from the Research Group on Introduced Species 2002 Symposium, Queen Charlotte City, Queen Charlotte Islands, British Columbia Canadian Wildlife Service, Environment Canada, Ottawa
Woodliffe, P.A. pers. comm. 2013a. Comments on draft Green Dragon management plan submitted to R. Donley. April 2013. (Retired) Area Biologist, Ministry of Natural Resources.
Woodliffe, P.A. pers. comm. 2013b. Email Correspondence with R. Donley. January 2013. (Retired) Area Biologist, Ministry of Natural Resources.
Yang, J., J. Lovett-Doust, and L. Lovett-Doust. 1999. Seed germination patterns in green dragon (Arisaema dracontium, Araceae). American Journal of Botany 86(8): 1160-1167.
Footnotes
- footnote[1] Back to paragraph Information presented in this table should not be considered definitive or comprehensive, as it is based in part on observations from NHIC (2013) that have not undergone the standard verification process used by NHIC to prepare element occurrence records. Several additional recent observations from NHIC (2013) with imprecise location data are not included in the totals but are mentioned in the comments.
- footnote[2] Back to paragraph Boles (1996) used the term "margin" to refer to areas where the species "is more sparsely distributed and where unfavourable environmental conditions are also present". The margin may or may not correspond with the geographic boundaries of the species range.