Proud Globelet recovery strategy
Read the recovery strategy for the Proud Globelet, a mollusc species at risk in Ontario.

About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act, 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species’ persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of the Environment, Conservation and Parks Species at Risk webpage.
Recommended citation
S. Wyshynski and Nicolai, A. 2018. Recovery Strategy for the Proud Globelet (Patera pennsylvanica) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ministry of the Environment, Conservation and Parks, Peterborough, Ontario. vi + 32 pp.
© Queen’s Printer for Ontario, 2018
ISBN 978-1-4868-2784-8 (HTML)
ISBN 978-1-4868-2785-5 (PDF)
Content (excluding the cover illustration) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n’est disponible qu’en anglais en vertu du Règlement 411/97 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec recovery.planning@ontario.ca.
Authors
Sarah Wyshynski – Ecological Consultant
Annegret Nicolai – Université Rennes, Station Biologique de Paimpont, UMR-CNRS 6553 EcoBio/OSUR, 35380 Paimpont, France
Acknowledgments
This recovery strategy was greatly improved by guidance from Tanya Pulfer. She reviewed the draft and made suggestions to complete the content. We thank Dwayne Lepitzki (COSEWIC Molluscs Specialist Subcommittee) and Michael Oldham (MNRF) for reviewing gastropod ecology in relation to recovery. Rebecca Rundell (SUNY-ESF) and Cody Gilbertson (SUNY-ESF) shared their experience on reintroduction and ex-situ rearing of the very rare Chittenango ovate amber snail in the US with us. In addition, Russ Jones (AMEC) and Karen Cedar (Naturalist, Ojibway Prairie Nature Centre) gave us information regarding the protection of Black Oak Heritage Forest. Robert Forsyth (Molluscs Specialist) provided species information and the cover photograph.
Declaration
The recovery strategy for the Proud Globelet was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all of the individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The recommended goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ministry of the Environment, Conservation and Parks
Environment and Climate Change Canada – Canadian Wildlife Service, Ontario
Executive summary
Proud Globelet (Patera pennsylvanica), is a terrestrial land snail in the family Polygyridae. This species, with a yellowish round shell (15-20 mm diameter), lacks a tooth-like protuberance at the shell opening, unlike other species of the genus Patera. Proud Globelet ranges from southwestern Ontario to Iowa and Missouri and east to Pennsylvania. No living individual of this species has ever been documented in Ontario. The sole known population in Ontario was determined based on the presence of empty, fresh shells in 1992 and 1996, and empty weathered shells in 2013. Proud Globelet in Ontario may be restricted to the Black Oak Heritage Forest and a formerly built up industrial site adjacent to this forest, within the City of Windsor. Whether the species is still extant in Ontario remains unclear. The species is currently listed as endangered on the Species at Risk in Ontario (SARO) List under the Endangered Species Act 2007, (ESA).
While little is known specifically about Proud Globelet habitat requirements, they are thought to be specialized to exposed woodland or edge habitat (forest/grassland), which is extremely limited in southwestern Ontario. It is believed that, in the case of the single known Canadian population, the grassland next to the oak forest, where shells have been found, is most likely used as a feeding ground and the forest is most likely used for shelter and egg laying. Food requirements are unknown but may be fungi, leaf litter and fresh plant material. Terrestrial snails rely heavily on moisture and specific micro-climatic conditions, for egg laying and shelter against drought, and low temperature extremes. A greater understanding of the habitat requirements would aid in the protection and recovery of this species.
Proud Globelet is faced with many direct and indirect threats such as: habitat loss and degradation, human intrusion and disturbance, competition from and presence of non-native species, environmental contamination through soil, air and water pollution, in addition to severe weather and climate change. These threats are compounded by limiting factors such as: low dispersal capacity, small population size, relatively long generation time, and slow adaptation to changing conditions in its environment. The extent of any threats to Proud Globelet populations currently remains unknown and requires further investigation.
The recommended recovery goal is to ensure the persistence of Proud Globelet in Ontario by maintaining and protecting existing habitat, reducing known threats, and filling knowledge gaps that will allow for more specific actions to be undertaken, such as threat mitigation and potentially, reintroduction. The recommended protection and recovery objectives are to:
- Confirm the presence/absence/distribution of Proud Globelet in Ontario by 2025;
- Protect, maintain and improve the quality of habitat in and around the Black Oak Heritage Forest, where the species occurs/occurred;
- Protect any newly discovered population(s) and supporting habitat, if found;
- Address knowledge gaps related to biology, habitat requirements, and threats that may assist in recovery efforts; and
- Reintroduce Proud Globelet to suitable habitat if deemed feasible.
As snail populations are usually composed of several hundred individuals, heterogeneously distributed over a habitat, and recognizing the cryptic nature of Proud Globelet, it is recommended that the entire Ecological Land Classification (ELC, Lee et al. 1998) ecosite polygon currently occupied by a population of Proud Globelet, and/or historically occupied by a population of Proud Globelet, be prescribed as habitat in a habitat regulation. Observations that have not been reconfirmed in more than 20 years should be considered historic (Hammerson et al. 2008). In addition, it is recommended that a buffer of 100 m be added to the ELC ecosite polygon, where suitable dispersal and edge habitat are present, to account for dispersal into neighbouring edge habitat. Information on spatial limits of habitat used by Proud Globelet is lacking. Defining habitat by using a contiguous ecological area plus a buffer increases the likelihood that all habitat elements required by Proud Globelet are included.
1.0 Background information
1.1 Species assessment and classification
| Assessment | Status |
|---|---|
| SARO List classification | Endangered |
| SARO List history | Endangered (2016) |
| COSEWIC assessment history | Endangered (2015) |
| SARA schedule 1 | No schedule, no status |
| Conservation status rankings (NatureServe 2017) |
GRANK: G4 (2009) NRANK: N1 (2015) SRANK: S1 |
1.2 Species description and biology
Species description
Proud Globelet is a member of the family Polygyridae with a thin shell which is yellowish olive and has 5¾ to 6 spirals in adults (Figure 1). The lip of the opening is white and narrowly reflected, and the central part of the underside of the shell is completely covered by the lip (Pilsbry 1940). Adult shells measure 15 to 20 mm in diameter. Morphologically, Proud Globelet is unlike any other species of the genus Patera, because it lacks a tooth on the shell wall in the opening and the last spiral is more markedly descending at the opening (Grimm et al. 2010). There is only one other species of the genus Patera, apparently introduced, in Ontario (Flat Bladetooth, Patera appressa, Forsyth et al. 2015).
Proud Globelet forms a single population in Ontario. No data are available on the population structure.

Species biology
Proud Globelet is an air-breathing (pulmonate), simultaneous hermaphrodite snail where both members of a mating pair exchange sperm and produce eggs (Pilsbry 1940). Mating in Polygyridae occurs in fall or early spring, egg laying in spring to late summer (clutch size: 20-80 eggs), and hatching 20 to 60 days after egg laying, depending on temperature and moisture (van Cleave and Foster 1937, Blinn 1963, Steensma et al. 2009).
In terrestrial snails, growth occurs only during periods of activity (spring to fall), and species of the size of Proud Globelet usually reach their adult shell size after one to two years (Barker 2001). Polygyridae sexual maturity is reached after 2 to 3 years and lifespan has been estimated to range between 3 and 5 years (Stiven and Foster 1996, Steensma et al. 2009).
Hibernation in Polygyridae extends from early-October until mid-April. The snails close the shell opening with a calcareous epiphragm (Blinn 1963) and stay, with the shell opening up (Carney 1966), in shallow depressions in the forest floor covered with leaf litter or at soil depths of 5 to 10 cm (Pearce and Örstan 2006).
In general, terrestrial snails require calcium from soil, bedrock or plants for shell formation, reproduction (Barker 2001), and physiological processes, e.g. heat resistance in eggs (Nicolai et al. 2013).
Terrestrial snails are prone to freezing in winter. Different strategies that are somewhat plastic have evolved to enable survival at sub-zero temperatures (see review by Ansart and Vernon 2003). Mortality during hibernation is around 40% in some species and drives population dynamics (Peake 1978, Cain 1983). Burch and Pearce (1990) suggest refuges with buffered environmental conditions, such as temperature and humidity, may be the most important factor limiting terrestrial snail abundance. Indeed, snails rely on buffered microsites because high temperature variability (temperatures ranging between below and above zero degrees) from fall to spring increases mortality (Nicolai and Ansart 2017).
In temperate regions, many species only aestivate for a short period of time in extreme summer conditions and have developed biochemical stress reactions that protect cells and maintain survival mechanisms, such as membrane fluidity, osmoregulation and enzyme activity. However, unusually long heat and drought periods increase mortality (Nicolai et al. 2011).
Nothing is known of Proud Globelet’s diet. It is potentially herbivorous (feeding on fresh or dead plant material or both) and/or fungivorous (feeding on fungi).
Active dispersal distances and home ranges sizes are unknown for Proud Globelet, but other Polygyridae species of similar size moved between 120 and 220 cm per day within a home range of 80 to 800 m2 over a 100-day study (Pearce 1990). A three-year study showed a maximal dispersal of 32.2 m (Edworthy et al. 2012), whereas a four-year study confirms that snails return to suitable hibernation sites and have home ranges greater than 50 m2 (Blinn 1963).
1.3 Distribution, abundance and population trends
Globally, the Proud Globelet occurs/occurred mainly in the eastern and mid-states of North America, ranging from Ontario, southward to Kentucky, westward to Missouri and eastward to Pennsylvania (Figure 2). Current population size and distribution throughout the United States is unclear at this time. The only known Canadian population of Proud Globelet occurs/occurred in southwestern Ontario.
NatureServe (2017) and CESCC (2016) provides the following ranks:
Global Rank: G4
National Rank (Canada): N1
National Rank (US): N4
Sub-national ranks (S-ranks) in Canada and the USA are as follows:
- Canadian Provinces where Proud Globelet occurs
- Ontario: S1 (CESCC 2016).
- US States adjoining southwestern Ontario
- Michigan: SNR and SC (Michigan Natural Features Inventory 2013)
- Pennsylvania: S2 (Pennsylvania Natural Heritage Program 2017)
- Ohio: SNR (Ohio Department of Natural Resources 2012)
- New York: not present (Hubricht 1985; Schlesinger 2017)
- Other US states where Proud Globelet occurs
- Iowa: SNR (Natural Resource Commission Iowa 2009)
- Illinois: SNR (Cummings and Phillips 2013)
- Indiana: SNR (Indiana Department of Natural Resources 2013)
- Kentucky: S3S4 (Kentucky State Nature Preserve Commission 2013)
- West Virginia: S2 (West Virginia Natural Heritage Program 2016)
- Missouri: SNR (Missouri Department of Conservation 2018)
(G4 – apparently secure, N1 – critically imperiled nationally, N4 – apparently secure. SNR – not ranked sub-nationally, SC – special concern (at the state level), S1 – critically imperiled sub-nationally, S2 – imperiled sub-nationally, S3 – vulnerable sub-nationally, S4 – apparently secure sub-nationally)
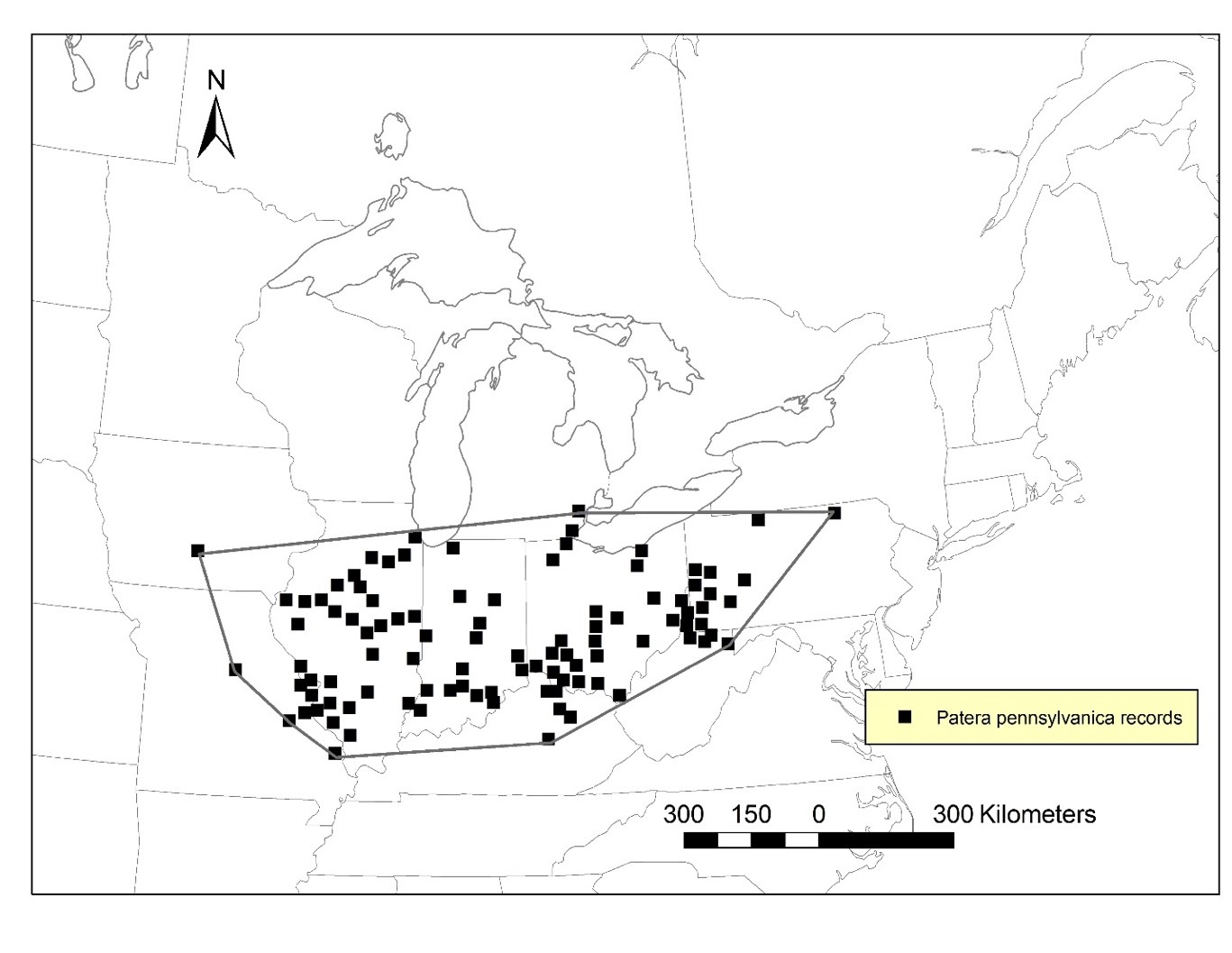
In Ontario, and Canada, Proud Globelet is restricted to the south border of the Black Oak Heritage Forest and to a formerly industrial built-up site adjacent to the south side of this forest in the City of Windsor (Figure 3). The extent of occurrence (EOO) and the Index Area of Occupancy (IAO) are 4 km2 (COSEWIC 2015), representing 0.001% of the global range (COSSARO 2016). This population may be genetically isolated from other populations in the United States. No living individuals of Proud Globelet have ever been documented in Canada. Empty, fresh shells of this species were first found in the Black Oak Heritage Forest in 1992 (collector Oldham, CMNML 096171, COSEWIC 2015). Empty, fresh shells were found again in the same place in 1996 (collector Oldham, CMNML 096170, COSEWIC 2015), indicating recently dead individuals (Pearce 2008), thus an extant population at the time (COSEWIC 2015). However, in a targeted survey at the same location in 2013, only old, weathered shells (15 adults and juveniles that died 5-15 years ago) were found (collector Nicolai, CMNML 096184, COSEWIC 2015). Similarly, in 2013, one old, weathered shell on a formerly industrial built-up area south of the Black Oak Heritage Forest was found (collector Oldham, MJO 41549, COSEWIC 2015). An unverified record of Proud Globelet, with no museum specimens, occurs on Bois Blanc Island, Ontario in the Detroit River (Figure 3, Walker 1906). This occurrence was cited as a Michigan record in both Walker (1906) and La Rocque (1953) and therefore does not appear in COSEWIC (2015).
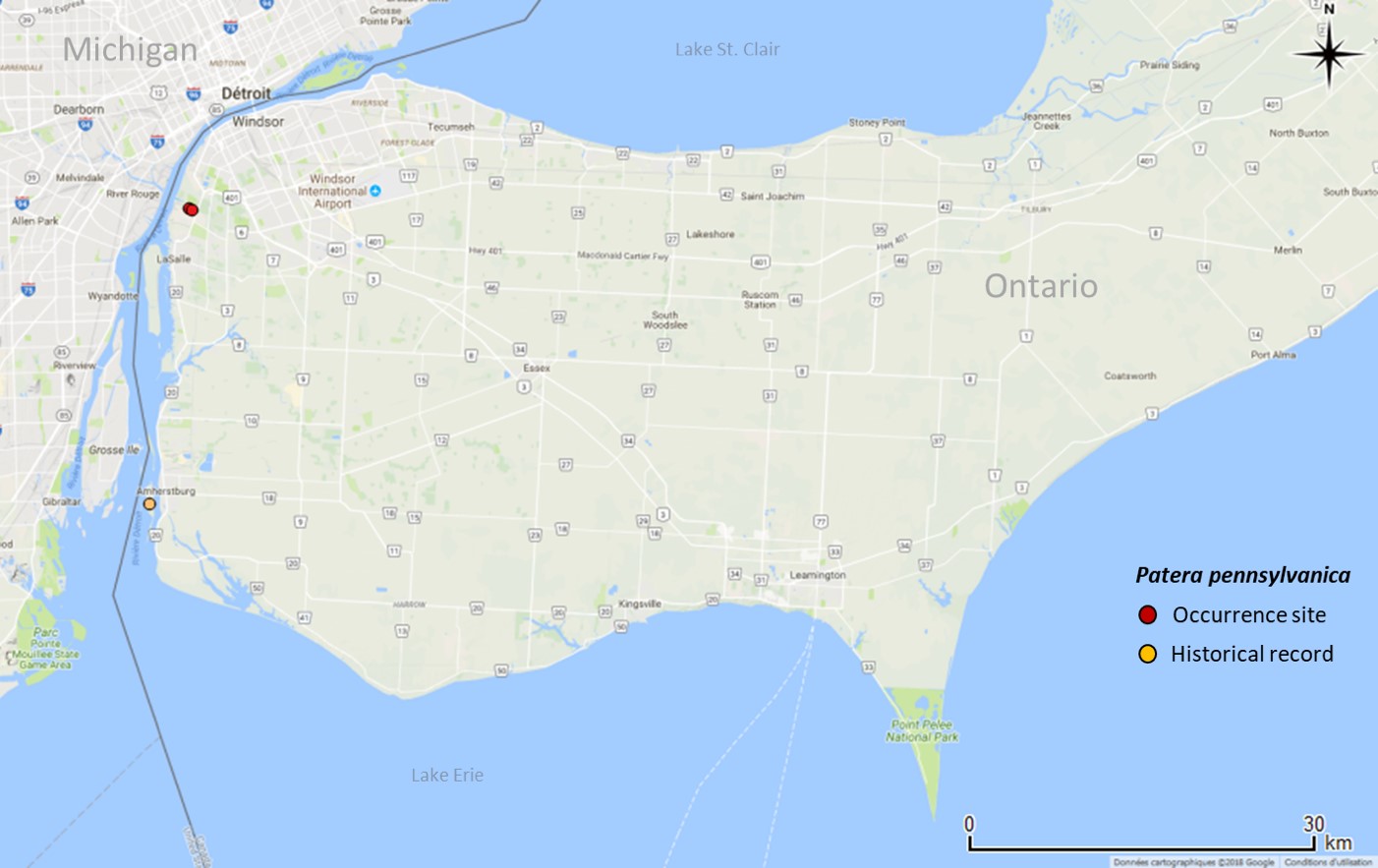
The closest records from outside Canada are in Monroe County, Michigan, United States (Michigan State University Collection of Zoology, MCZ 152070, and Walker 1906), but the exact locations are unknown. It is unclear whether the species is still extant in Michigan because no other, recent records are available.
Large water bodies such as the Detroit River represent natural dispersal barriers for ground dwelling terrestrial snails (Gittenberger 2007), making rescue from outside Canada unlikely.
1.4 Habitat needs
Hubricht (1985) described Proud Globelet’s habitat in the United States as wooded hillsides or ravines, under leaf litter and stones
. The habitat Proud Globelet has been found in, in Canada, is sandy oak forest and disturbed (former industrial site with building rubble) shrubby prairie at the southern border of the sandy oak forest (COSEWIC 2015). From these select observations, it can be inferred that Proud Globelet likely needs exposed (towards the sun) wooded habitat (ravines, hillsides) or forest edge habitat including adjacent grassy or shrubby area. General observations of terrestrial gastropods (e.g. COSEWIC 2014, COSEWIC in press, Nicolai and Ansart 2017) showed that exposed hillside or edge habitat allows the snails to take advantage of sunny and warm moments for activity (usually after a rain or early morning), while being close to shelter against drought and cold (under leaf litter, wood logs, in the sandy and humus-rich soil). Snails in such habitat seem to feed on herbaceous plants that are present in exposed woodland or adjacent grassland, and to use the forest for egg laying in the humus-rich forest soil and for feeding on decaying wood or fungi (if this is part of their diet).
Terrestrial snails, being prone to freezing and drying, rely on three general microhabitat attributes:
- snow cover in winter that buffers cold temperature or temperature variability,
- leaf litter and wood logs that keep moisture in dry conditions during the summer or buffer cold temperature during the spring and fall, when snow is absent (Nicolai and Ansart 2017), and
- humus-rich soil for egg laying to keep eggs in constant moisture and temperature conditions.
1.5 Limiting factors
The Canadian population of Proud Globelet is extremely isolated and small. The habitat in the two occurrence sites is surrounded by a heavily industrialized and urbanized area and the Detroit River. Terrestrial gastropods are generally limited by their low dispersal capacity that can be increased by habitat specialization (Dahirel et al. 2015). Proud Globelet seems to be specialized to exposed woodland or edge habitat (forest/grassland), which is particularly limited in southwestern Ontario. The fidelity of Polygyridae to hibernation sites and the specific requirements for hibernation and aestivation sites might also be limiting factors. Terrestrial snails heavily rely on moisture and specific micro-climatic conditions. Their adaptability to changing climate conditions might be limited (Nicolai and Ansart 2017). Additionally, small population size makes Proud Globelet susceptible to stochastic events and low reproductive potential.
1.6 Threats to survival and recovery
The threats for Proud Globelet were organized following the International Union for Conservation of Nature (IUCN) Threats Classification Scheme (Version 3.2).
Transportation and service corridors
The Detroit River International Crossing project for transportation of goods between Canada and the U.S. will increase traffic volume in the area north of the Black Oak Heritage Forest. Although the road, customs inspection plaza and the bridge construction will not directly affect Proud Globelet habitat, air- and waterborne pollution (e.g. heavy metals and road salt) represents a potential threat to the species (Viard et al. 2004).
Human intrusions and disturbances – recreational activities
The Black Oak Heritage Forest has a high trail density and is intensively visited for recreation. Data on visitor numbers and activities are not available. Large trails represent barriers for Proud Globelet movement (Wirth et al. 1999). Moreover, trampling by pedestrians is a known threat for some snail species (Baur and Baur 1990a).
Residential and commercial development
Urban and industrial development in the surrounding area of Proud Globelet habitat can have a negative impact on this species. Construction of industrial facilities adjacent to Proud Globelet habitat can reduce edge habitat, alter soil composition, structure and hydrology, and change vegetation composition thereby reducing food sources (Charrier et al. 2013). Proud Globelet shells were found on a former industrial site, which has partially re-naturalized (no active restoration has taken place). While there are currently no new development proposals for the site, the vacant land is available for redevelopment (Cedar pers. comm. 2018).
Approximately 20 years ago, a wood dump site was created by the City of Windsor on the southern border of the Black Oak Heritage Forest. The dump was created as a place to discard wood from urban forestry practices, such as thinning, pruning and removal of trees on city owned land. The wood dump has reduced edge habitat by occupying former grassland that was adjacent to the forest and was probably used as feeding grounds by Proud Globelet. The dumped wood reaching heights of 4-5 m also changed light and micro-climatic conditions at the forest edge.
Invasive and other problematic species, genes and diseases
There are several highly invasive plants in southern Ontario, including Garlic Mustard (Alliaria petiolata). They have been observed displacing native vegetation and altering soil nutrient cycles, thereby slowing restoration (Catling et al. 2015). Although a positive impact of an invasive plant on land snail diversity has been documented in western Pennsylvania (Utz et al. 2018), invasive plants can also lead to a decrease in endangered snail abundance, as shown in Europe (Stoll et al. 2012).
Non-native earthworms have invaded parts of Canada relatively recently. They have been shown to have major impacts on ecosystems (CABI 2016) and could indirectly affect terrestrial snail communities (Norden 2010, Forsyth et al. 2016). Earthworms, such as the Asian genus Amynthas (Qiu and Turner 2017), already recorded in Windsor (Reynolds 2014), alter forest floor habitats by reducing or eliminating the natural leaf litter layer and digging up and mixing the mineral soil with the organic surface layer. Besides the leaf litter loss, other negative consequences for snails include altering understory vegetation composition (Drouin et al. 2016) by feeding on forest plant seeds (Cassin and Kotanen 2016) or by altering plant-fungi mutualism (Paudel et al. 2016) and thus reducing available food plants and microhabitat.
Competition with exotic terrestrial gastropods is also a potential threat (Whitson 2005, Grimm et al. 2010, Campbell et al. 2014) through aggression (Kimura and Chiba 2010), density effects and/or food competition (Baur and Baur 1990b). Dusky Arion (Arion subfuscus), Grey Fieldslug (Deroceras reticulatum), Grovesnail (Cepaea nemoralis) and White Heath Snail (Xerolenta obvia) are present in the Proud Globelet’s habitat (COSEWIC 2015). It is difficult to estimate an impact on Proud Globelet, because no data are available on inter-specific interactions with terrestrial snails or slugs.
Polygyridae have been noted to be one of the intermediate hosts of the Meningeal Worm (Parelaphostrongylus tenuis) (Rowley et al. 1987). In general, parasitic mites are also common in snails. The infection rate within a population ranges between 45-75%. Depending on the mite species, infections can cause high mortality, reproduction perturbations, and reduced cold hardiness (Baur and Baur 2005). Parasites could therefore be a potential threat, especially in combination with other environmental factors, such as climate change or pollution.
Natural system modifications
Prescribed fire has become an important management tool for prairie and forest conservation in North America (Gottesfeld 1994, Williams 2000), particularly to limit the invasion of exotic species (Brooks and Lusk 2008) and to promote growth and reproduction of native prairie species (Towne and Owensby 1984). Burning directly and indirectly affects survival of ground nesting animals, litter dwelling organisms, and soil invertebrates, including snails (Nekola 2002). Fire reduces and modifies organic substrates and residues, which buffer and shelter these organisms, in addition to being sources of nutrients. Fire also changes microclimates when post-burn bare soil is heated by the sun, thereby increasing soil evaporation (reviewed by Saestedt and Ramundo 1990; Knapp et al. 2009). Fire destroys the upper part of soil habitat, the litter and uppermost humus layer, which is the most important factor affecting survival for litter-soil organisms (Bellido 1987).
Black Oak Heritage Forest has been subjected to prescribed fire (Windsor Star 2008), and may be again in the near future to enhance habitat for species at risk and control for invasive plants. Direct impact of fire on snail populations may be reduced when habitat is widespread and recolonization from unburned areas is possible. When habitat areas are small, larger fires are expected to be detrimental to populations, while fires that are very patchy and restricted to an overall small area would be less harmful.
Pollution
The high degree of industrialization surrounding the Black Oak Heritage Forest suggests some level of soil, water and air pollution that could negatively affect Proud Globelet and/or its habitat. Heavy metals in soil and plants are accumulated in tissues (Notten et al. 2005) and are known to decrease food consumption, growth and fecundity (number of clutches per season) in snails (Laskowski and Hopkin 1996) which can affect population dynamics and maintenance in the area. The exact level of impact on Proud Globelet has not been studied.
The high amount of garbage in Proud Globelet’s habitat can also lead to soil pollution with microplastics that might have a negative impact on leaf litter / soil biota (Duis and Coors 2016).
While there are no agricultural effluents in Proud Globelet’s habitat, invasive plant management very often includes herbicide use. Population level impacts of herbicides on terrestrial snails and slugs were not detected in agricultural (Roy et al. 2003) or forested (Hawkins et al. 1997) landscapes, but laboratory studies have shown that exposure to some herbicides increases mortality of some snail species (Koprivnikar and Walker 2011) and could affect reproduction (Druart et al. 2011). Until now, invasive plants were not controlled in Black Oak Heritage Forest, but a future management plan could consider such measures.
Climate change and severe weather
In temperate regions, climate change will involve increases in both average temperatures and the frequency of extreme weather events such as heat waves, drought and increased precipitation (Della Marta et al. 2007). Abnormal temperature extremes and variations represent a threat to snails (Nicolai and Ansart 2017). Heat waves and drought could cause high mortality to Proud Globelet due to heat or dehydration stresses (Nicolai et al. 2011). High temperature variations during spring and fall or during winter when snow is absent can increase mortality in land snails (Nicolai and Ansart 2017). Windsor has experienced high temperature variations during past years, e.g. March 1998: highest temperature 22.4°C followed by lowest temperature -15.9°C (Climate Canada 2014), but the impact on Proud Globelet has not been studied.
1.7 Knowledge gaps
Proud Globelet is an understudied species with a small Canadian range. Knowledge on species distribution is limited and biology, specifically diet, physiological responses to environmental factors and interaction with exotic species, are unknown, which may hinder the efficacy of protection strategies. Research on the following knowledge gaps would contribute to a more complete understanding for the protection and recovery of the species and its habitat.
- Canadian presence/absence and distribution: It remains unclear as to whether there is an extant population of Proud Globelet in Canada. Southwestern Ontario has been surveyed for terrestrial gastropods of conservation concern; however, additional inventory work in the Black Oak woods along with other areas in Windsor and vicinity would be helpful in confirming presence of any living individuals. Additionally some sites and areas which have not been explored yet that may be good locations to focus efforts include: Bois Blanc Island (historical record in Walker 1906), sandy forests in Norfolk County and the Niagara escarpment.
- Population viability analysis.
- Life history traits: growth, reproduction, life span, dispersal.
- Habitat requirements: diet, physico-chemical parameters in the soil and litter, habitat structure (physical elements, vegetation composition).
- Rearing in captivity: the long-term success of rearing in captivity depends heavily on the knowledge of species’ specific diet requirements. Short-term rearing in captivity has been successful with about 30 different species of terrestrial snails in Europe (see Ansart et al. 2014), but it has yet to be tested with Proud Globelet.
- Reintroduction: genetic structure using different markers across the closest U.S. populations to understand genetic variability within the species and determine a potential source population (if within-species genetic variability for the COI gene marker is low, barcoding could help detect the species in citizen science surveys using mucus swabs as described by Morhina et al. 2014).
- Inter-specific interactions: especially the impact of exotic terrestrial gastropods and earthworms through habitat changes or competition for food and shelter (density effects).
- Physiological tolerances and adaptability: heat and cold resistance, responses to pollution, and changes in climatic conditions and soil characteristics.
- Estimation of trampling mortality.
Research on the previously mentioned knowledge gaps would contribute to a more complete understanding for the protection and recovery of the species and its habitat; however, it should be noted that while there is no known living population of Proud Globelet in Canada, research to fill these knowledge gaps may not be possible. Additionally, it is unclear as to whether or not there are any populations of Proud Globelet in the U.S. large enough to conduct research on, and if there are, whether any research findings can be applied to the Ontario population.
1.8 Recovery actions completed or underway
Black Oak Heritage Forest: To-date no management plan has been implemented in the natural area (COSEWIC 2015), but actions, such as closing sections of the forest to the public, have been implemented to avoid further damage to the habitat (Jones pers. comm. 2018). The City of Windsor plans to develop a management plan in the near future (Cedar pers. comm. 2018).
2.0 Recovery
2.1 Recommended recovery goal
The recommended recovery goal is to ensure the persistence of Proud Globelet in Ontario by maintaining and protecting existing habitat, reducing known threats, and filling knowledge gaps that will allow for more specific actions to be undertaken, such as threat mitigation and, potentially, reintroduction.
2.2 Recommended protection and recovery objectives
In order to meet the overall recommended recovery goal, short-term objectives focus on confirming whether Proud Globelet is still extant in Ontario, ensuring that existing habitat, which has become degraded, is improved and protected, and filling any knowledge gaps that could allow for more specific actions that can be taken to reduce threats. Long-term objectives focus on re-establishing or enhancing existing population at historic sites in addition to assisted colonization at sites with suitable habitat.
| Number | Protection or recovery objective |
|---|---|
| 1 | Confirm the presence/absence/distribution of Proud Globelet in Ontario by 2025. |
| 2 | Protect, maintain and improve the quality of habitat in and around the Black Oak Heritage Forest, where the species occurs/occurred. |
| 3 | Protect any newly discovered population(s) and supporting habitat, if found. |
| 4 | Address knowledge gaps related to biology, habitat requirements, and threats that may assist in recovery efforts. |
| 5 | Reintroduce Proud Globelet to suitable habitat if deemed feasible. |
2.3 Recommended approaches to recovery
Table 3. Recommended approaches to recovery of the Proud Globelet in Ontario
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Inventory and Monitoring | 1.1 Develop identification material to aid in accurate recognition of this species and those with which it can be mistaken. |
Knowledge gaps:
|
| Critical | Short-term | Inventory and Monitoring |
1.2 Develop a standardized survey protocol for inventorying and monitoring Proud Globelet populations. Protocol should include:
|
Knowledge gaps:
|
| Critical | Ongoing | Inventory | 1.3 Conduct targeted surveys throughout the Black Oak Heritage Forest and adjacent land where the species has occurred (light industrial area), with focused effort in and around locations where shells have previously been found. |
Knowledge gaps:
|
| Critical | Short-term | Research, Inventory |
1.4 Identify areas of probable habitat for Proud Globelet.
|
Knowledge gaps:
|
| Necessary | Ongoing | Inventory |
1.5 Conduct surveys for new populations of the species, in potentially suitable habitat, by qualified individuals.
|
Knowledge gaps:
|
| Necessary | Short-term | Education and Outreach, Communication, Stewardship, Inventory | 1.6 Develop education and outreach material (e.g. signage, fact sheets) for the general public and staff working in the area around Black Oak Heritage Forest, to raise awareness and aid in the identification of this species. |
Threats:
|
| Beneficial | Ongoing | Inventory |
1.7 Engage volunteers (e.g. local naturalists, land stewards, experts) to undertake surveys for this species to determine presence or absence.
|
Knowledge gaps:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Ongoing | Management, Protection, Education and Outreach, Communication, and Stewardship |
2.1 Assess and implement actions that are needed and appropriate to protect and improve habitat, from human-caused disturbances that include but may not be limited to:
|
Threats:
Knowledge gaps:
|
| Critical | Ongoing | Management, Protection, Education and Outreach, Communication, and Stewardship |
2.2 Assess and implement actions that are needed to protect and improve habitat from threats posed by non-native species.
|
Threats:
Knowledge gaps:
|
| Critical | Ongoing | Management, Protection |
2.3 Identify, protect and/or create refuge areas for Proud Globelet to move into in times of extreme temperatures and/or droughts.
|
Threats:
|
| Critical | Ongoing | Communication, Management, Protection |
2.4 Liaise with City of Windsor on management of habitat.
|
Threats:
Knowledge gaps:
|
| Necessary | Ongoing | Management |
2.5 Identify habitat restoration and/or enhancement opportunities to increase/improve habitat availability in Ontario.
|
Knowledge Gaps:
Threats:
|
| Necessary | Ongoing | Management, Protection | 2.6 As knowledge gaps pertaining to habitat requirements are filled, re-evaluate management and protection actions. |
Threats:
|
| Necessary | Ongoing | Protection | 2.7 As knowledge gaps pertaining to habitat requirements are filled, develop a habitat description or habitat regulation to provide clarity on the area defined as habitat for Proud Globelet in Ontario. |
Threats:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Ongoing | Management, Protection, Monitoring |
3.1 If additional populations are found, assess habitat management and protection needs, as in Objective 2.
|
Threats:
Knowledge gaps:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Management | 4.1 Investigate existing and/or former Proud Globelet habitat in order to gather information on current conditions, human activities and land uses which would be of use when developing and implementing programs for habitat restoration. |
Threats:
Knowledge Gaps:
|
| Critical | Short-term | Research | 4.2 Research the effects of human disturbance on terrestrial snails caused by walking and biking, and estimate potential trampling mortality for Proud Globelet as a result of these activities. |
Threats:
Knowledge Gaps:
|
| Critical | Short-term | Research |
4.3 Engage the academic community to participate in researching knowledge gaps such as:
|
Knowledge gaps:
|
| Critical | Short-term | Research | 4.4 Research the effects of pollution, herbicides and/or insecticides on Proud Globelet. |
Threats:
Knowledge gaps:
|
| Beneficial | Short-term | Research | 4.5 Research adaptive strategies to climate variations including plasticity and evolvability of physiological responses combined with behavior. |
Threats
Knowledge gaps:
|
| Necessary | Ongoing | Research |
4.6 Research the effect of climate change on Proud Globelet.
|
Threats
Knowledge gaps:
|
| Necessary | Ongoing | Research | 4.7 Research impacts of earthworms and non-native gastropods, such as Dusky Arion, Grovesnail and White Heath Snail, on Proud Globelet and its habitat. |
Knowledge gaps:
|
| Necessary | Ongoing | Communication, Research | 4.8 Liaise with researchers and managers in the U.S. (e.g. Iowa, Pennsylvania, Michigan) where Proud Globelet is extant, to share any information regarding life history, habitat parameters, monitoring, global distribution, and threats. |
Knowledge gaps:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Beneficial | Long-term | Research, Management, |
5.1 Evaluate the feasibility of captive breeding to enable augmentation or reintroduction of the species.
|
Knowledge gaps:
|
| Beneficial | Long-term | Management, Protection, Monitoring | 5.2 If feasible, based on population viability analysis, undertake a captive breeding program to enable conservation translocations into the natural environment and to enable research into Proud Globelet biology and ecology. |
Knowledge gaps:
|
| Beneficial | Long-term | Management, Protection, Monitoring |
5.3 Translocate snails into suitable habitat to mimic the natural dispersal of the snail and support existing populations.
|
Knowledge gaps:
|
2.4 Narrative to support approaches to recovery
The first priority in order to meet the overall recommended recovery goal is to determine whether or not Proud Globelet is extant in Ontario. Extensive targeted surveys need to be conducted throughout areas where shells have been found, in addition to areas that may be identified as potentially suitable habitat for Proud Globelet. This would include other areas in Windsor and vicinity, Bois Blanc Island (historical record in Walker, 1906), which has yet to be surveyed, sandy forests in Norfolk County and the Niagara Escarpment.
If any living individuals are found, they, along with their surrounding habitat, need to be studied. Habitat parameters required by Proud Globelet should be determined by studying any new populations found in Ontario but also by conducting research on Proud Globelet populations in the U.S. Without further understanding of life history traits such as diet, habitat, microhabitat conditions, dispersal, home range size, and minimum population viability, little can be done to recover Proud Globelet in Ontario. Additionally, research needs to be conducted to further understand any identified threats to Proud Globelet and its habitat, in order for the threats to be appropriately mitigated, and habitat protected.
While surveys are ongoing and research is being conducted, it is important to protect and improve any identified Proud Globelet habitat (Black Oak Heritage Forest), using what little is understood, so that the habitat will be available if the population recovers.
Once life history traits and habitat parameters are better understood, the focus of recovery can shift to restoring and enhancing habitat. If an extant population is found in Ontario, it is hoped that restoring and enhancing its habitat along with mitigating threats, will enable the population to re-establish itself. At this time, if no new populations have been found, or any newly found populations are struggling to persist, the possibility of augmenting or re-establishing populations in Ontario can be explored. However without a clear understanding first of the requirements necessary for persistence, there is little point to considering reintroduction. If research has been conducted, and there is a clear understanding of requirements for persistence, and appropriate size and condition of habitat exists and is protected in Ontario, populations should be analyzed to determine if there is a viable source population for reintroduction. If a viable source population is identified, then a captive breeding program can be established to enable the reintroduction program. Snails should be translocated into suitable habitat to mimic the natural dispersal of snails. It is important to monitor the success of the reintroduction.
2.5 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of the Environment, Conservation and Parks on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the author will be one of many sources considered by the Minister when developing the habitat regulation for this species.
Proud Globelet are known to live in wooded hillsides, ravines and in forest/grassland edges. Within these habitats, specific attributes required for the survival or recovery of Proud Globelet are unclear. Once information becomes available and knowledge gaps have been addressed, the area prescribed as habitat should be revised and updated.
As snail populations are usually composed of several hundred individuals, heterogeneously distributed over a habitat, and recognizing the cryptic nature of this species, it is recommended that the entire Ecological Land Classification (ELC, Lee et al. 1998) ecosite polygon currently occupied by a population of Proud Globelet, and/or historically occupied by a population of Proud Globelet, be prescribed as habitat in a habitat regulation. Observations that have not been reconfirmed in more than 20 years should be considered historic (Hammerson et al. 2008). In addition, it is recommended that a buffer of 100 m be added to the ELC ecosite polygon where suitable dispersal and edge habitat are present, to account for dispersal into neighbouring edge habitat, when available. This buffer of 100 m takes into account the longest dispersal distance measured in Polygyridae (32 m) (Edworthy et. al. 2012) plus an additional area to reduce edge effect and maintain microhabitat properties of the edge habitat. Additionally, the 100 m buffer would capture locations where Proud Globelet shells have been found but may not be able to be defined using the ELC, such as the ‘light industrial’ area.
Information on spatial limits of habitat used by Proud Globelet is lacking. It is believed that, in the case of the single known Canadian population, the grassland next to the oak forest is used as a feeding ground and the forest is used for shelter and egg laying. Defining habitat by using a contiguous ecological area plus an additional buffer area increases the likelihood that all habitat elements required by Proud Globelet are included.
Glossary
- Aestivation:
- A period of deep and prolonged sleep or torpor that occurs in the summer or dry season in response to heat and drought.
- Canadian Endangered Species Conservation Council (CESCC):
- The Council was formed in 1998 by federal, provincial and territorial Wildlife Ministers under the Accord for the Protection of Species at Risk in Canada. The Council is responsible for national leadership and direction for preventing wild species from becoming at risk. It has specific responsibilities for overseeing the listing and recovery of species that are at risk nationally, and plays a role in resolving issues under the Accord.
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC):
- The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
- Committee on the Status of Species at Risk in Ontario (COSSARO):
- The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
- Conservation status rank:
- A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. Ranks are determined by NatureServe and, in the case of Ontario’s S-rank, by Ontario’s Natural Heritage Information Centre. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperilled
2 = imperilled
3 = vulnerable
4 = apparently secure
5 = secure
NR = not yet ranked - Ecological Land Classification (ELC):
- A system of classifying and describing land-units based on vegetation.
- Endangered Species Act, 2007 (ESA):
- The provincial legislation that provides protection to species at risk in Ontario.
- Epiphragm:
- A dry layer of calcified phosphate or mucus produced by certain land snails during hibernation which functions to cover the shell opening and prevent desiccation.
- Osmoregulation:
- Maintenance by an organism of an internal balance between water and dissolved materials regardless of environmental conditions.
- Species at Risk Act (SARA):
- The federal legislation that provides protection to species at risk in Canada. This act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
- Species at Risk in Ontario (SARO) List:
- The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
References
Ansart, A., and P. Vernon. 2003. Cold hardiness in molluscs. Acta Oecologica 24:95-102.
Ansart, A., A. Guiller, O. Moine, M-C. Martin, and L. Madec. 2014. Is cold hardiness size-constrained? A comparative approach in land snails. Evolutionary Ecology 28:471-493.
Barker, G.M. 2001. The Biology of Terrestrial Molluscs. CABI Publishing New York, 558 pp.
Baur, A., and B. Baur. 1990a. Are roads barriers to dispersal in the land snail Arianta arbustorum? Canadian Journal of Zoology 68:613-617.
Baur, B., and A. Baur. 1990b. Experimental evidence for intra- and interspecific competition in two species of rock-dwelling land snails. Journal of Animal Ecology 59: 301-315.
Baur, A., and B. Baur. 2005. Interpopulation variation in the prevalence and intensity of parasitic mite infection in the land snail Arianta arbustorum. Invertebrate Biology 124(3):194-201.
Bellido, A. 1987. Field Experiment about direct effect of a heathland prescribed fire on microarthropod community. Revue d’Ecologie et de Biologie du Sol 24:603-633.
Blinn, W.C. 1963. Ecology of the land snails Mesodon thyroidus and Allogona profunda. Ecology 44:498-505.
Brooks, M., and M. Lusk. 2008. Fire Management and Invasive Plants: a Handbook. United States Fish and Wildlife Service, Arlington, Virginia, 27 pp.
Burch, J.B., and T.A. Pearce. 1990. Terrestrial gastropods. Pp. 201-309, in D. L. Dindal (ed.). Soil Biology Guide. John Wiley and Sons, New York.
CABI (CAB International). 2016. Invasive Species Compendium. Datasheet Lumbricus rubellus. [accessed July 2016].
Cain, A.J. 1983. Ecology and ecogenetics of terrestrial molluscan populations. Pp. 597-647 In W.D. Russel Hunter (ed.). The Mollusca, Volume VI. Academic Press, New York. 695 pp.
Campbell, S.P., J.L. Frair, J.P. Gibbs and R. Rundell, 2014. Coexistence of the endangered, endemic Chittenango Ovate Amber Snail (Novisuccinea chittenangoensis) and a non-native competitor. Biological Invasions, 17(2):711-723.
Carney WP. 1966. Mortality and apertural orientation in Allogona ptychophora during winter hibernation in Montana. The Nautilus 79 (4): 134-136.
Cassin, C.M., and P.M. Kotanen. 2016. Invasive earthworms as seed predators of temperate forest plants. Biological Invasions DOI 10.1007/s10530-016-1101-x
Catling, P.M., G. Mitrow, and A. Ward. 2015. Major invasive alien plants of natural habitats in Canada. 12. Garlic Mustard, Alliaire officinale: Alliaria petiolata (M. Bieberstein) Cavara & Grande. CBA/ABC Bulletin 48(2):51-60.
CESCC (Canadian Endangered Species Conservation Council). 2016. Wild Species 2015: The General Status of Species in Canada. National General Status Working Group: 128 pp.
Cedar, Karen, pers. comm. 2018. Email correspondence to S. Wyshynski. May 2018. Naturalist. Ojibway Prairie Complex, Windsor, Ontario.
Charrier, M., A. Nicolai, M-P. Dabard, and A. Crave. 2013. Plan National d’Actions de Tyrrhenaria ceratina, escargot terrestre endémique de Corse. PNA, Ministère de l’Ecologie, de l’Energie, du Développement Durable et de la Mer, Paris. 92 pp.
Climate Canada. 2014. Monthly Data Report for Windsor 1940-2013. Web site: http://climate.weather.gc.ca/climateData/monthlydata_e.html?timeframe=3&Prov=ON&StationID=4716&mlyRange=1940-01-01|2013-12-01&Year=1996&Month=01&Day=01 [invalid web page].
COSEWIC. 2014. COSEWIC assessment and status report on the Broad-banded Forestsnail Allogona profunda in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 53 pp.
COSEWIC. 2015. COSEWIC Status Report on the land snail Proud Globelet Patera pennsylvanica. Environment Canada, 46 pp.
COSEWIC. In press. COSEWIC assessment and status report on the Striped Whitelip Webbhelix multilineata in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa.COSSARO. 2016. Ontario Species at Risk Evaluation Report for Proud Globelet (Patera pennsylvanica). Committee on the Status of Species at Risk in Ontario, 14 pp.
Cummings, K., and C. Phillips. 2013. Web site: Mollusca of Illinois. Illinois Natural History Survey. Prairie Research Institute. University of Illinois at Urbana-Champaign. [accessed May 2, 2018].
Dahirel M., E. Olivier, A. Guiller, M-C. Martin, L. Madec, A. Ansart. 2015. Movement propensity and ability correlate with ecological specialization in European land snails: comparative analysis of a dispersal syndrome. Journal of Animal Ecology 84:228-238.
Della Marta, P.M., J. Luterbacher, H. von Weissenfluh, E. Xoplaki, M.Brunet, and H. Wanner. 2007. Summer heat waves over western Europe 1880-2003, their relationship to large-scale forcings and predictability. Climate Dynamics 29:251-275.
Drouin, M., R. Bradley, and L. Lapointe. 2016. Linkage between exotic earthworms, understory vegetation and soil properties in sugar maple forests. Forest Ecology and Management 364:113-121.
Druart, C., M. Millet, R. Scheifler, O. Delhomme, and A. de Vaufleury. 2011. Glyphosate and glufosinate-based herbicides: fate in soil, transfer to, and effects on land snails. Journal of Soil Sediments 11:1373-1384.
Duis, K., and A. Coors 2016. Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environmental Sciences Europe 28: 2, DOI 10.1186/s12302-015-0069-y.
Edworthy, A.B., K.M.M. Steensma, H.M. Zandberg, and P.L. Lilley. 2012. Dispersal, home-range size, and habitat use of an endangered land snail, the Oregon forestsnail (Allogona townsendiana). Canadian Journal of Zoology 90(7):875-884.
Forsyth, R.G., M.J. Oldham, and F.W. Schueler. 2015.Patera appressa (Pilsbry, 1926), an introduced land snail in Ontario, Canada (Mollusca: Gastropoda: Polygyridae). Check List 11(2): 1583.
Forsyth, R.G., P. Catling, B. Kostiuk, S. McKay-Kuja, A. Kuja. 2016. Pre-settlement Snail Fauna on the Sandbanks Baymouth Bar, Lake Ontario, Compared with Nearby Contemporary Faunas. Canadian Field-Naturalist 130(2):152-157.
Gittenberger, E. 2007. Islands from a snail’s perspective. In: Renema (ed) Biogeography, time, and place: Distribution, barriers, and islands. p. 347-363.
Gottesfeld, L.M.J. 1994. Aboriginal burning for vegetative management in northwestern British Columbia. Human Ecology 22:171-188.
Grimm, F.W., R.G. Forsyth, F.W. Schueler, and A. Karstad. 2010. Identifying Land Snails and Slugs in Canada: Introduced Species and Native Genera. Ottawa: Canadian Food Inspection Agency. 168 pp.
Hammerson, G.A., D. Schweitzer, L. Master, and J. Cordeiro. 2008. Ranking Species Occurrences – A Generic Approach. NatureServe, Arlington, Virginia.
Hawkins, J.W., M.W. Lankester, R.A. Lautenschlager, and F.W. Bell. 1997. Effects of alternative conifer release treatments on terrestrial gastropods in northwestern Ontario. The Forestry Chronicle 73(1):91-98.
Hubricht, L. 1985. The distributions of the native land mollusks of the Eastern United States. Fieldiana Zoology 24:47-171.
Indiana Department of Natural Resources. 2013. Web site: Indiana’s state endangered species. [accessed May 2, 2018].
Jones, R., pers. comm. 2018. Email correspondence to A. Nicolai. 13 January 2018. Head Naturalist, Ojibway Nature Centre. Department of Parks, Windsor, Ontario.
Kentucky State Nature Preserve Commission. 2013. Endangered, threatened, and special concern plants, animals, and natural communities of Kentucky with habitat description. Web site: http://naturepreserves.ky.gov/pubs/publications/ ksnpc_specieshabitat.pdf [invalid web page].
Kimura, K., and S. Chiba. 2010. Interspecific interference competition alters habitat use patterns in two species of land snails. Evolutionary Ecology 24:815-825.
Knapp, E.E., B.L. Estes, and C.N. Skinner. 2009. Ecological effects of prescribed fire season: A literature review and synthesis for managers. USDA General Technical Report. Albany, California. 80 pp.
Koprivnikar, J., and P.A. Walker. 2011. Effects of the herbicide Atrazine’s metabolites on host snail mortality and production of trematode cercariae. Journal of Parasitology 97(5):822-827.
La Rocque A., 1953. Catalogue of the Recent Mollusca of Canada. National Museum of Canada, Bulletin 129 i-x, 1-406.
Laskowski, R., and S.P. Hopkin. 1996. Effect of Zn, Cu, Pb, and Cd on Fitness in Snails (Helix aspersa). Ecotoxicology and Environmental Safety 34:59-69.
Lee, H., W. Bakowsky, J. Riley, J. Bowles, M. Puddister, P. Uhling, and S. McMurray. 1998. Ecological land classification for southern Ontario: first approximation and its application. Ontario Ministry of Natural Resources, South-central Science Section, Science Development Transfer Branch. 87 pp.
Michigan Natural Features Inventory. 2013. Michigan’s special animals. Michigan State University Extension (ed.) Lansing, Michigan. 16 pp.
Missouri Department of Conservation. 2018. Missouri species and communities of conservation concern. Website: Missouri Department of Conservation 2018_SOCC.pdf [accessed May 2, 2018].
Morinha, F., P. Travassos, D. Carvalho, P. Magalhães, J.A. Cabral and E. Bastos. 2014. DNA sampling from body swabs of terrestrial slugs (Gastropoda: Pulmonata): A simple and non-invasive method for molecular genetics approaches. Journal of Molluscan Studies 80: 99-101.
Natural Resource Commission Iowa. 2009. Endangered and threatened plant and animal species. IAC 571, Chapter 77. Web site: https://www.legis.iowa.gov/docs/ ACO/chapter/571.77.pdf [accessed May 2, 2018].
NatureServe. 2017. NatureServe Explorer: An online encyclopedia of life [web application]. Version 7.1. NatureServe, Arlington, Virginia. Available http://explorer.natureserve.org. (Accessed: January 19, 2018).
Nekola, J.C. 2002. Effects of fire management on the richness and abundance of central North American grassland land snail faunas. Animal Biodiversity and Conservation 25(2):53-66.
Nicolai, A., J. Filser, R. Lenz, C. Bertrand, and M. Charrier. 2011. Adjustment of metabolite composition in the haemolymph to seasonal variations in the land snail Helix pomatia. Journal of Comparative Physiology B 181:457-466.
Nicolai, A., P. Vernon, R. Lenz, J. Le Lannic, V. Briand, and M. Charrier. 2013. Well wrapped eggs: Effects of egg shell structure on heat resistance and hatchling mass in the invasive land snail Cornu aspersum. Journal of Experimental Zoology A 319:63-73.
Nicolai, A., and A. Ansart. 2017. Conservation at a slow pace: Terrestrial gastropods facing fast changing climate. Conservation Physiology 5 (1): 007, doi: 10.1093/conphys/cox007.
Norden, A.W. 2010. Invasive earthworms: a threat to eastern North American forest snails? Tentacle 18:29-30.
Notten, M.J.M., Oosthoek, A.J.P., Rozema J., and Aerts, J. 2005. Heavy metal concentrations in a soil-plant-snail food chain along a terrestrial soil pollution gradient. Environmental Pollution 138:178-190.
Ohio Department of Natural Resources. 2012. Wildlife that are considered to be endangered, threatened, species of concern, special interest, extirpated, or extinct in Ohio. Publication 5356 (R1012). 10 pp.
Paudel, S., T. Longcore, B. MacDonald, M.K. McCormick, K. Szlavecz, G.W.T. Wilson, and S.R.Loss. 2016. Belowground interactions with aboveground consequences: Invasive earthworms and arbuscular mycorrhizal fungi. Ecology 97:605-614.
Peake, J. 1978. Distribution and Ecology of the Stylommatophora. Pp. 429-526 In V. Fretter, and J. Peake (eds.). Pulmonates. Academic Press, London. 540 pp.
Pearce T.A. 1990. Spooling and line technique for tracing field movements of terrestrial snails. Walkerana 4(12):307-316.
Pearce, T.A., and A. Örstan. 2006. Terrestrial gastropoda. Pp. 261-285. In C.F. Sturm, T.A. Pearce, and A. Valdés (eds.). The Mollusks: A Guide to Their Study, Collection, and Preservation. American Malacological Society, Pittsburgh, Pennsylvania. 445 pp.
Pearce, T.A. 2008. When a snail dies in the forest, how long will the shell persist? Effect of dissolution and micro-bioerosion. American Malacological Bulletin 26:111-117.
Pennsylvania Natural Heritage Program. 2017. Species of Special Concern Lists. Web site: http://www.naturalheritage.state.pa.us/species.aspx [accessed May 2, 2018].
Pilsbry, H.A. 1940. Land Mollusca of North America (North of Mexico). Volume 1. Part 2. The Academy of Natural Sciences of Philadelphia, Philadelphia. 1113 pp.
Qiu, J., and M.G. Turner. 2017. Effects of non-native Asian earthworm invasion on temperate forest and prairie soils in the Midwestern US. Biological Invasions 19:73-88.
Reynolds, J.W. 2014. A checklist by counties of earthworms (Oligochaeta: Lumbricidae, Megascolecidae and Sparganophilidae) in Ontario, Canada. Megadrilogica 16:111-135.
Rowley M.A., E.S. Loker, J.F. Pagels, and R.J. Montali. 1987. Terrestrial gastropod hosts of Parelaphostrongylus tenuis at the National Zoological Park’s Conservation and Research Center, Virginia. Journal of Parasitology 73:1084-1089.
Roy, D.B., D.A. Bohan, A.J. Haughton, M.O. Hill, J.L. Osborne, S.J. Clark, J.N. Perry, P. Rothery, R.J. Scott, D.R. Brooks, G.T. Champion, C. Hawes, M.S. Heard, and L.G. Firbank. 2003. Invertebrates and vegetation of field margins adjacent to crops subject to contrasting herbicide regimes in the Farm Scale Evaluations of genetically modified herbicide-tolerant crops. Philosophical Transactions of the Royal Society London. B 358:1879-1898.
Saestedt, T.R., and R.A. Ramundo. 1990. The influence of fire on below ground processes of Tallgrass prairie. Pp. 99-117, in S.L. Collins and L.L. Wallace (eds.). Fire in North American tall Grass Prairies. University of Oklahoma Press, Norman.
Schlesinger, M.D. 2017. Rare Animal Status List January 2013. New York Natural Heritage Program. Web site: www.nynhp.org. [accessed May 2, 2018].
Steensma, K.M.M., P.L. Lilley, and H.M. Zandberg. 2009. Life history and habitat requirements of the Oregon forestsnail, Allogona townsendiana (Mollusca, Gastropoda, Pulmonata, Polygyridae), in a British Columbia population. Invertebrate Biology 128:232-242.
Stiven, A.E., and B.A. Foster. 1996. Density and adult size in natural populations of a southern Appalachian low-density land snail, Mesodon normalis (Pilsbry). American Midland Naturalist 136 (2):287-299.
Stoll, P., K. Gatzsch, H. Rusterholz, and B. Baur. 2012. Response of plant and gastropod species to knotweed invasion. Basic and Applied Ecology 13:232-240.
Towne, G., and C. Owensby. 1984. Long–term effects of annual burning at different dates in ungrazed Kansas tallgrass prairie. Journal of Range Management 37:392-397.
Van Cleave, H.J., and T.D. Foster. 1937. The seasonal life history of land snail Polygyra thyroidus (Say). Nautilus 51:50-54.
Viard, B., F. Pihan, S. Promeyrat and S.J-C. Pihan. 2004. Integrated assessment of heavy metal (Pb, Zn, Cd) highway pollution: bioaccumulation in soil, Graminaceae and land snails. Chemosphere 55:1349-1359.
Utz, R.M., T.A. Pearce, D.L. Lewisa, and J.C. Manninoa. 2018. Elevated native terrestrial snail abundance and diversity in association with an invasive understory shrub, Berberis thunbergii, in a North American deciduous forest. Acta Oecologica 86:66-71.
Walker, B. 1906. An illustrated catalogue of the Mollusca of Michigan. Part 1. Terrestrial Pulmonata (land snails). State Board of Geological Survey. 531 pp.
West Virginia Natural Heritage Program. 2016. Rare, threatened, and endangered animals. Web site: http://www.wvdnr.gov/Wildlife/PDFFiles/RTE_Animals_2016 .pdf [invalid link].
Whitson, M. 2005. Cepaea nemoralis (Gastropoda, Helicidae): The invited invader. Journal of the Kentucky Academy of Science 66:82-88.
Williams, G.W. 2000. Reintroducing Indian type fire: implications for land managers. Fire Management Today 60(3):40-48.
Windsor Star. 2008. Controlled burn at Black Oak Heritage Park. Web site: Video of Controlled Burn [accessed May 2, 2018].
Wirth, T., P. Oggier, and B. Baur. 1999. Effect of road width on dispersal and population genetic structure in the land snail Helicella itala. Journal of Nature Conservation 8:23-29.