Recovery strategy for the Bank Swallow
Read the recovery strategy for the Bank Swallow (Riparia riparia), a species of bird at risk in Ontario.

Cover photo by Tianna Burke.
About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act, 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species' persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of Natural Resources and Forestry Species at Risk webpage.
Recommended citation
Falconer, M., K. Richardson, A. Heagy, D. Tozer, B. Stewart, J. McCracken, and R. Reid. 2016. Recovery Strategy for the Bank Swallow (Riparia riparia) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources and Forestry, Peterborough, Ontario. ix + 70 pp.
© Queen’s Printer for Ontario, 2016
ISBN 978-1-4606-7672-1 (HTML)
ISBN 978-1-4606-7681-3 (PDF)
Content (excluding the cover illustration) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007», n'est disponible qu'en Anglais en vertu du Règlement 411/97 qui en exempte l'application de la Loi sur les services en français. Pour obtenir de l'aide en français, veuillez communiquer avec recovery.planning@ontario.ca.
Authors
Myles Falconer, Bird Studies Canada
Kristyn Richardson, Bird Studies Canada
Audrey Heagy, Bird Studies Canada
Doug Tozer, Bird Studies Canada
Becky Stewart, Bird Studies Canada
Jon McCracken, Bird Studies Canada
Ron Reid, Bobolink Enterprises
Acknowledgments
Funding for the preparation of this recovery strategy was provided by the Ontario Ministry of Natural Resources and Forestry (OMNRF) through a contract to Bird Studies Canada (BSC). Many thanks to Jay Fitzsimmons (OMNRF) and Amanda Fracz (OMNRF) for overseeing project delivery.
We would like to thank everyone who participated in the technical workshops held at Guelph, Ontario on 11 September 2014 and 5 March 2015. Their names and affiliations are as follows:
- Carolyn Zanchetta (Bird Studies Canada)
- Liza Barney (Bird Studies Canada)
- Stephen May (CBM Aggregates)
- Mike Lebreton (CBM Aggregates)
- Greg Mitchell (Environment and Climate Change Canada)
- Mike Cadman (Environment and Climate Change Canada)
- Ben Dopson (G. Tackaberry and Sons Construction Co. Ltd.)
- Sean Male (Hatch Ltd.)
- John Bayliss (Holcim (Canada) Inc.)
- George Antoniuk (Miller Group)
- Jill Crosthwaite (Nature Conservancy of Canada)
- Sonje Bols (Nippissing University)
- Brandon Holden (Ontario Field Ornithologists)
- Ken Burrell (Ontario Field Ornithologists)
- Peter Roberts (Ontario Ministry of Agriculture, Food and Rural Affairs)
- Alison Clark (Ontario Ministry of Natural Resources and Forestry)
- Andrew Chard (Ontario Ministry of Natural Resources and Forestry)
- Chris Risley (Ontario Ministry of Natural Resources and Forestry)
- Jay Fitzsimmons (Ontario Ministry of Natural Resources and Forestry)
- Joe Nocera (Ontario Ministry of Natural Resources and Forestry)
- Mark Browning (Ontario Ministry of Natural Resources and Forestry)
- Stephen Douglas (Ontario Ministry of Natural Resources and Forestry)
- Larry Sarris (Ontario Ministry of Transportation)
- Dan Gibson (Ontario Power Generation)
- Marianne Leung (Ontario Power Generation)
- Cynthia Robinson (Ontario Stone, Sand and Gravel Association)
- Anthony Chegano (Saugeen First Nation)
- Esme Batten (Saugeen First Nation)
- Jo-Anne Harbinson (Saugeen Valley Conservation Authority)
- Paul General (Six Nations)
- Debbie Badzinski (Stantec)
- Karen McDonald (Toronto Region Conservation Authority)
- Erica Nol (Trent University)
- Tianna Burke (Trent University)
- Chris Guigelemo (University of Western Ontario)
Declaration
The recovery strategy for the Bank Swallow was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all of the individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
- Ontario Ministry of Natural Resources and Forestry
- Environment and Climate Change Canada – Canadian Wildlife Service, Ontario
- Parks Canada Agency
Executive summary
The Bank Swallow (Riparia riparia) is a small migratory songbird. Despite being one of the most widespread swallows in the world, it is less familiar to most people than some other swallow species. The Bank Swallow breeds in colonies throughout North America, Europe, and Asia, and overwinters in Central and South America, southern Africa, and southern and southeast Asia. Several subspecies are recognized but only one subspecies, Riparia riparia riparia, breeds in North America. Due to population declines across the northern portion of its North American breeding range, the Bank Swallow is listed as threatened under Ontario’s Endangered Species Act, 2007 (ESA) and has been assessed as threatened in Canada by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC). The decline in aerial insectivorous bird populations, including swallows, flycatchers, swifts and nightjars, has become a major conservation concern in Ontario and elsewhere. However, the mechanisms driving these declines are not well understood.
In Ontario, the Bank Swallow breeds across the entire province, but is most common in southern Ontario. Large colonies (i.e., 1000 or more pairs) occur along the shores of Lakes Erie and Ontario, on the Saugeen River, and in some aggregate extraction pits. The Bank Swallow is sparsely distributed throughout the Canadian Shield and Hudson Bay Lowland regions, where it occurs in aggregate pits, on lakeshores and along large river corridors. Population trends show an annual rate of decline of 6.2 percent and 4.8 percent in Ontario since 1970 and 2002, respectively. The cumulative population loss in Ontario from 1970 through 2012 is about 93 percent. The current population estimate for Bank Swallow in Ontario is 409,000 individuals based on targeted burrow count surveys in aggregate pits and quarries, lake bluffs of Lakes Erie and Ontario, and river surveys on the Saugeen and Nottawasaga Rivers.
Bank Swallow habitat includes nest sites, foraging areas, and nocturnal roost sites. Bank Swallows build nest burrows in eroding vertical banks, such as lakeshore bluffs, riverbanks, and banks or stockpiles created in aggregate pits and construction sites. During breeding and migration Bank Swallows forage in a variety of open terrestrial and aquatic habitats including wetlands, open water, riparian areas, grasslands, and agricultural areas, as well as shrubland. Regions with dense forest cover are generally avoided. Bank Swallows roost at night in large wetlands or shrub thickets in or near water. Roost sites are used mainly during migratory periods or post-breeding, and to a lesser extent while breeding. Migratory stopover sites are usually centred on large marshes where birds roost at night and disperse to forage throughout the day (Turner 2004, Winkler 2006). There is little information available for Bank Swallows in terms of the relative importance or area requirements of these disparate habitats and their proximity to each other.
Numerous factors have been proposed as possible explanations for the recent declines in Bank Swallows, but the information needed to critically evaluate these threats is generally lacking. It is possible that multiple direct and indirect threats at various stages and locations in its life cycle, including factors operating outside of Ontario, are having an additive impact on populations.
Known and potential direct and indirect threats affecting reproduction and survival include: (1) loss of nest site habitat; (2) loss or degradation of foraging habitat; (3) negative effects of environmental contaminants, pesticides and pollution on food supply; (4) reduced nest productivity due to human activities and persecution; (5) habitat loss, disturbance and human persecution at roost sites; and (6) compounding influences due to climate change and severe weather.
To better identify the primary threats to the Bank Swallow in Ontario, knowledge gaps related to: (1) vital rates and population source/sink dynamics; (2) diet and food supply; (3) habitat use, requirements and trends on the breeding grounds; (4) wintering and migration habitat and ecology; (5) Best Management Practices; and (6) climate change effects, must be addressed.
The recovery goal is to maintain a stable, self-sustaining Bank Swallow population of at least 330,000 breeding individuals across the breeding range in Ontario by 2035 (within 20 years). Over the next 10 years, the goal is to reduce the rate of decline and to prevent any further declines by 2035. The implementation of recovery actions over the short-term, such as Best Management Practices in the aggregate industry to increase or maintain reproductive outputs, will help slow the rate of decline. The aim to maintain a stable, self-sustaining population within 20 years is thought to allow sufficient time to address the recovery objectives identified in this strategy including:
- Address knowledge gaps to better understand the magnitude or severity of threats and/or identify biological and socio-economic factors that may impede or assist recovery efforts;
- Protect habitat and reduce or mitigate potential threats through stewardship, communication, education and outreach, and habitat management; and
- Inventory, monitor and report on the state of Bank Swallow populations and habitats in Ontario and elsewhere to track the progress of recovery activities.
It is recommended that until knowledge gaps are addressed, the following areas should be considered in developing a habitat regulation:
- Nest sites occupied at least once within the last three breeding seasons. The nest site encompasses a buffered distance of 50 metres out from the extent of the colony.
- Foraging habitat includes any open terrestrial or aquatic habitats within 1000 metres of a colony that have been used by foraging birds during the breeding season at least once within the last three breeding seasons. Aquatic habitats (e.g., wetlands, lakeshore) within the foraging habitat may be especially significant as a source of emergent aerial insects (i.e., food supply).
- Nocturnal roost sites that are used regularly by any number of Bank Swallows. Regular use would be defined as roosting on more than one night per year in at least two of the past three years. This habitat should be protected throughout the year and should continue to be protected for three years after the last record of use. The extent or boundary of regulated habitat at a roost site should be defined on a case-by-case basis, but should include the areas that are directly used (e.g., as perches or cover) by roosting birds, plus the open air space they use to enter the site. Use of ecosite polygons, as defined by the most current Ecological Land Classification schemes for Ontario or the Ontario Wetland Evaluation System, may be appropriate tools for delineating the boundaries of wetlands associated with roost sites.
1.0 Background information
1.1 Species assessment and classification
Table 1. Species assessment and classification of the Bank Swallow (Riparia riparia). The glossary provides definitions for the abbreviations within, and for other technical terms in this document.
| Assessment | Status |
|---|---|
| SARO list classification | Threatened |
| SARO list history | Threatened (2014) |
| COSEWIC assessment history | Threatened (2013) |
| SARA schedule 1 | No schedule, no status |
| Conservation status rankings | GRANK: G5 NRANK: N5 SRANK: S4B |
1.2 Species description and biology
Species description
The Bank Swallow (Riparia riparia) is the smallest swallow species in the western hemisphere (length: 12 cm, mass: 10 - 18 g). Sexes appear similar in size and plumage. Bank Swallows have a grey-brown head, mantle, rump and wing coverts, contrasting with darker brown flight feathers and white underparts, separated by a well-defined, brown upper breast-band (Figure 1). Plumage is similar throughout the year, but juveniles (younger than 6 months old) can be distinguished from adults by buff-edged upperparts and a buff-pink wash to the throat (Pyle 1997). The Bank Swallow is best distinguished from other swallows by its small size, distinctive breast-band and characteristic flight pattern, in which the bird’s wings are held at a sharper angle towards the tail while giving quicker wing-beats than other swallow species (Garrison 1999).
Figure 1. Bank Swallow in flight (photo credit: Tianna Burke).

The only subspecies recognized as regularly occurring in Canada is R. r. riparia, which also occurs across Europe and much of Asia and Africa (where it is commonly known as the Sand Martin). Recent genetic analysis shows that for R. r. riparia, North American and Eurasian populations have no ongoing intercontinental gene flow (Pavlova et al. 2008). Further genetic studies may be needed to address uncertainty in subspecies designations and genetic relationships across the Bank Swallow’s range. Several other recognized subspecies of the Bank Swallow occupy smaller areas in parts of eastern and southern Asia and northeastern Africa (Turner and Rose 1989).
Species biology
Life cycle and reproduction
The Bank Swallow is a highly social species, nesting in colonies ranging in size from a few (rarely single) nests to several thousand nests. The distribution of colony sizes is usually skewed to many smaller-sized colonies with fewer large colonies, and as such, the median colony size is likely a better measure of central tendency than the mean. Peck and James (1987) report the Ontario mean colony size to be 45 nests, while unpublished data gathered from Ontario studies by Bird Studies Canada, Environment and Climate Change Canada and OMNRF (Appendix B:) suggest mean and median colony sizes of about 130 and 50 nests, respectively. Bank Swallows are socially monogamous, although both sexes pursue extra-pair copulations (Garrison 1999).
In Ontario, the breeding season spans from early May to mid-August, and nesting peaks in June (Peck and James 1987, Cadman unpub. data 2011). Birds can breed in their first year (i.e., by 10 - 11 months of age; Cramp et al. 1988). Older birds often arrive first at colony sites, followed one to two weeks later by first-year breeders (Kuhnen 1985). Males mostly excavate the nest burrow and nest chamber, while females build most of the nest cup using grasses, plant stems, fibers, and feathers. Nest burrow length ranges from 40 to 110 cm (Garrison 1999, Falconer unpub. data 2013). The number of burrows within a colony is almost always more than the number of actual breeding pairs. Many burrows are started, but abandoned due to obstacles (e.g., large roots or rocks), burrow instability, or simply because males are unable to attract a female (Garrison 1999). Some burrows remain intact between years and sometimes will be reused or enlarged; however, new burrows are typically dug each year (Garrison 1999). The mean proportion of burrows occupied by nesting pairs out of the total number of burrows in a colony ranges from 43 to 74 percent and varies annually, seasonally and by habitat characteristics (Garrison 1999). For estimating the number of pairs in a colony, a general assumption of 50 percent burrow occupancy is often used (Wright et al. 2011). This approximates the values observed in two unpublished studies in lake bluff and aggregate pit habitat in Ontario (Appendix B:, Cadman unpub. data 2011).
Bank Swallows are mostly single-brooded in Ontario. Second broods are known throughout Europe (Cramp et al. 1988), but may also occur in North America (including Ontario) based on a small number of nest burrows being reused following successful fledging (Hjertaas 1984, Bull 1985, Peck and James 1987, M.D. Cadman, pers. comm. 2014). Confirmation of double-brooded birds (via banding) is needed. Clutch size is typically five eggs (range: 2 - 7 eggs; Peck and James 1987, Falconer unpub. data 2013, Cadman unpub. data 2012). Eggs are incubated for 14 days (range: 12 - 16 days) mostly by the female. Nestlings fledge at 18 to 22 days of age, but may roost in nest burrows for up to one week after fledging (Garrison 1999).
Predators may reduce productivity by depredating eggs, nestlings, fledglings and/or adult Bank Swallows. Species that have been identified as predators (that occur in Ontario) include ratsnakes, foxsnakes, rats, chipmunks, raccoons, badgers, skunks, weasels, foxes, coyotes, gulls, falcons, hawks, crows and ravens (COSEWIC 2013).
The Belted Kingfisher (Megaceryle alcyon) can usurp Bank Swallow nest burrows (T. Burke, pers. comm. 2014) and several other avian species have been observed occasionally nesting within Bank Swallow colonies (often by enlarging burrows or simply occupying existing burrows); however, it is unknown if interspecific competition over nest sites is a serious threat (see COSEWIC (2013) for species list).
Several flea species (Siphonaptera: Ceratophyllus sp.; Celsus sp.) inhabit Bank Swallow burrows and can reduce nestling mass by about five percent (Alves 1997). Sites with high flea concentrations are generally not reused in subsequent years (Haas et al. 1980). There is at least one species of parasitic mite (Sternostoma tracheacolum) that has been observed in Bank Swallows that is known to cause significant irritation of the lower respiratory tract of some birds, particularly those in captivity (Fain and Hyland 1962, Pence 1975, Knee et al. 2008). The impact, if any, of this mite on free-ranging Bank Swallows is unknown (Fain and Hyland 1962). Several larval blow fly species (Diptera: Calliphoridae) frequently infest colonies, and at least one species, Protocalliphora chrysorrhoea, is restricted almost entirely to inhabiting the nests of Bank Swallows and parasitizing nestlings (Sabrosky et al. 1989). Although P. chrysorrhoea infestations may cause physiological stress in nestlings, nestling mortality rates are unaffected (Whitworth and Bennett 1992). Monitoring parasitic species that are restricted to single species hosts (such as P. chysorrhoea) may be important in determining appropriate conservation measures. In these cases, management or control of parasite populations should be addressed with caution, as coextinction of parasites with their hosts is an overlooked, but potential issue (Colwell et al. 2012, Stringer and Linklater 2014).
Most studies reporting survival rate estimates for Bank Swallows do not control for the confounding effect of dispersal, which likely varies by age, sex and habitat. Thus, estimates should be cautiously interpreted. Moreover, most survival rate estimates come from European populations, and as such may not accurately reflect the demographics of the Ontario population.
Average apparent annual survival is in the range of 33 to 35 percent for juveniles and 40 to 53 percent for adults (Garrison 1999). This is comparable to survival rates of similar species, such as the Barn Swallow (Hirundo rustica) (MacBriar and Stevenson 1976, Freer 1977, Persson 1987). The average age of breeding adults ranges between 1.7 to 2 years, assuming a constant adult annual survival rate in the range of 40 to 50 percent and juvenile (first year) survival of 35 percent (see Appendix A:). The longevity record for this species in the wild is an adult banded in Iowa that lived at least 9 years (Petersen and Mueller 1979).
Males and females have similar annual survival rates (Cowley and Siriwardena 2005). Survival rates fluctuate widely on an annual basis and may be most negatively influenced by droughts on the wintering grounds (Szép 1995, Cowley and Siriwardena 2005) and/or prolonged wet and cold periods on the breeding grounds (Cowley and Siriwardena 2005).
Nest success is often relatively high across the range of the Bank Swallow. In Ontario, nest success in aggregate pits and lake bluff habitat was 66 and 75 percent, respectively (Tozer and Richmond 2013). Nest success is negatively affected by predation, extended periods of cold and wet weather, and bank collapse (COSEWIC 2013). Some colonies are either destroyed or partially destroyed during operations at aggregate pits (Campbell et al. 1997, M.D. Cadman, pers. comm. 2014) and during road construction (Petersen and Mueller 1979). Collisions with vehicles may be an important source of mortality for Bank Swallows, especially for first-year birds (Mead 1979, Dale 2001).
There is no available information on annual reproductive success, or average annual female fecundity, because it is unknown what proportion of the population breeds on an annual basis (Garrison 1999).
Diet and foraging behaviour
The Bank Swallow is primarily an aerial forager, consuming mostly flying insects. Terrestrial or aquatic insects or spiders are sometimes taken when locally abundant. During the breeding season, flies (Diptera), ants, bees and wasps (Hymenoptera), beetles (Coleoptera), and bugs (Hemiptera) represent 80 to 95 percent of the diet by frequency (Garrison 1999). A stable isotope study from Japan suggested that the main food source for nestlings from a river colony was terrestrial flies (Nakano et al. 2007). Bank Swallows breeding on the Lake Erie bluffs appear to feed on abundant emergent chironomids (i.e., midges) at least during part of the breeding period (M. Falconer, pers. obs.). The diet of Bank Swallows in Ontario has not been studied in any detail.
Bank Swallows generally forage in flocks approximately 15 m above ground (Garrison 1999). As the temperature increases throughout the summer, Bank Swallows tend to forage higher, presumably foraging on dispersing insects (e.g., nuptial ant flights; M. Falconer, pers. obs.). Like other swallows, Bank Swallows tend to forage relatively low over water or land during prolonged cold periods as a result of reduced insect activity (Williams 1961, Taylor 1963, Turner and Rose 1989).
Reports on distances between colonies and feeding sites vary. In aggregate pits in the United Kingdom, Turner (1980) found that feeding sites were within 260 m (mean = 200 m) of the colony when adults were provisioning nestlings and within 690 m (mean = 600 m) during nest building based on observations of colour-marked birds. Radio-tagged breeding birds along the north shore of the Lake Erie bluffs spent most of their time foraging within 1000 m of the colony (Appendix B:). Greater foraging distances from the nest site are likely to occur during periods of low insect abundance caused by colder weather conditions (Turner 1980, Ghilain and Bélisle 2008). Turner (1980) found that Bank Swallows foraged 80 percent further on average from the colony when temperatures were ≤ 16°C compared to ≥ 20°C (500 m and 110 m, respectively).
There is no information regarding diet or foraging behaviour during migration or on the wintering grounds.
Migration and dispersal
Bank Swallows are long-distance, diurnal migrants, travelling from North America to their wintering areas in northern and central South America (Garrison 1999). Compared to European populations, very little is known about Bank Swallow migration and dispersal in Ontario, let alone North America. Band recovery data are limited, although there is one record of an Ontario bird banded as a fledgling and recovered in northern Peru during mid-November of the same year (>5000 km; Brewer et al. 2000). Another band encounter record shows an adult bird that travelled almost 1000 km from southern Ontario to southern Missouri in 22 days during the month of July (Brewer et al. 2000). Based on the frequency of observation records, the main migratory route is likely through the Central America isthmus, although small numbers of birds occur regularly on some Caribbean islands (Garrison 1999).
Bank Swallows generally arrive in Ontario starting in mid- to late April and continue through May, and most depart starting in late July and continue through August and September. The frequency of eBird checklists reported in Ontario (1900-2014) shows the spring migration peak in the second week of May and the fall migration peaking from the first to third week of August (eBird 2015).
Surviving adults generally return to breeding sites (i.e., fidelity rate) at a higher rate than first-year birds (55 - 92%; Petersen and Mueller 1979, Freer 1979, Szép 1990, Szép 1999). The percentage of surviving juveniles returning to their natal area ranges from 46 to 59 percent (MacBriar and Stevenson 1976, Freer 1979, Szép 1990) and is greater for males than females (Freer 1979, Holmes et al. 1987, Szép 1999). In the United Kingdom, juveniles dispersed distances of 10 to 49 km (70%), 50 to 99 km (17%), 100 to 199 km (7%) and >199 km (6%) away from natal colonies (Mead 1979). On a smaller scale in Hungary, juveniles dispersed distances of 0 to 10 (55%), 10 to 25 (31%), and >25 km (14%) (Szép 1990).
Predation, bank collapse, or other events that result in nest mortality have an apparent influence on philopatry in successive years (i.e., adults experiencing nest mortality events do not recolonize). New birds will apparently recolonize these sites in successive years (Freer 1979). Successful breeding at a site has been found to increase the probability that the bank will be recolonized in successive years (Freer 1979). Szabo and Szép (2010) found that although birds were philopatric to colonies, between years neighbouring birds resettled in different areas of the colony as a group, suggesting a non-random settlement pattern with a presumed social implication.
Recent banding studies in Ontario and the Maritimes suggest low return rates (~2%) of adult Bank Swallows to breeding sites, although banding effort was relatively low (i.e., 307 birds banded over 2 years, M.D. Cadman, pers. comm. 2014). In contrast, adult return rates summarized in Freer (1979) range from 4 to 13 percent, but these data are based on initial bandings of tens of thousands of birds over 6 to 17 years. Banding studies with larger sample sizes over more years and sites are needed to determine whether the low return rates are due to high inter-annual dispersal, low survival rates, or both.
About one week post-fledging, juveniles start to form large flocks (called crèches) near colony sites, perching along telephone, hydro and fence wires, and on tree branches, exposed tree roots, cliff sides, and stockpiles of sand (Garrison 1999). In the United Kingdom, fledged juveniles disperse widely (up to several hundred kilometres) and use different nocturnal roost sites on a nightly basis, whereas adults tend to repeatedly use a single roost site close to the breeding colony (Mead and Harrison 1979). Juveniles visit multiple colonies during this dispersion, presumably assessing the suitability of breeding sites for future years. Juveniles also initiate fall migration later than adults (Mead and Harrison 1979). Migratory movements are funnelled through lowland river valleys where foraging opportunities are presumed to be favourable (Mead and Harrison 1979).
1.3 Distribution, abundance and population trends
Distribution
The Bank Swallow has an extensive global distribution, breeding in temperate zones of the northern hemisphere (North America, Europe and Asia) and wintering throughout Central and South America, Arabia, Africa, India, and southeastern Asia (Turner and Rose 1989). In North America, the Bank Swallow breeds across most of Canada and Alaska (south of the treeline) and across the northern two-thirds of the United States.
In Ontario, the Bank Swallow breeds across the entire province; however, it is most common in southern Ontario south of the Canadian Shield, where glacial outwash deposits (e.g., sand plains) are more widespread (Chapman and Putnam 1984). Large colonies (i.e., 1000+ pairs) occur along the shores of the Saugeen River, Lakes Ontario and Erie, and in some aggregate pits (Sandilands 2007). The Bank Swallow is more sparsely distributed throughout the Canadian Shield and Hudson Bay Lowland regions, where it occurs locally in aggregate pits and along large river corridors (Figure 2, Sandilands 2007).
Figure 2. Breeding distribution of the Bank Swallow in Ontario.
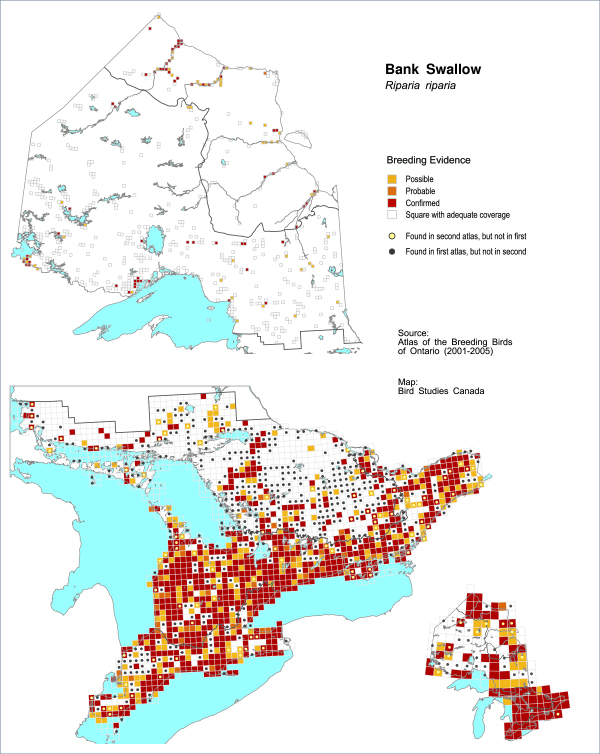
Based on Breeding Bird Atlas data (see Cadman et al. 2007). Coloured squares indicate that Bank Swallow was reported in a 10 km square during both atlas periods (1981-1985 and 2001-2005). Black dots identify squares where Bank Swallow was recorded during 1981-1985, but not during 2001-2005. Yellow dots identify squares where the species was recorded during the second atlas, but not the first. Inset map shows distribution on 100 km block scale.
Historical distribution
It is not known to what extent the distribution and abundance of the Bank Swallow has changed since prior to European settlement. Historically, Bank Swallows nested only in natural habitats created by erosion, including riverbanks and lake bluffs. Over the last ~100 years, several human influenced landscape changes emerged and likely became primary drivers influencing changes in Bank Swallow distribution in Ontario. Changes that have likely increased Bank Swallow distribution include the increase in open foraging habitat resulting from clearing of forests, and availability of nesting opportunities at human-made sites, such as aggregate pits and road cuts (COSEWIC 2013). Changes that have likely reduced Bank Swallow distribution include the loss of nest sites along many waterways in Ontario due to water control structures, channeling of rivers, and erosion control measures (COSEWIC 2013).
Abundance
Information on the abundance of Bank Swallows in Ontario is available from three sources: (1) the North American Breeding Bird Survey (BBS; Environment Canada 2014a); (2) the Ontario Breeding Bird Atlas (Cadman et al. 2007); and (3) Bank Swallow burrow count surveys (Appendix B:, Leung unpub. data 2010, Cadman and Lebrun-Southcott 2013, Browning and Cadman unpub. data 2015). The temporal and geographic scope of these monitoring surveys varies, as does the accuracy of resulting estimates of relative density and abundance.
Estimates based on BBS data from 1998 to 2007 suggest that the Ontario Bank Swallow population was approximately 200,000 individuals representing about 1 percent of the global (19 million), 3 percent of the continental (6 million), and 17 percent of the national (1.4 million) population (Partners in Flight Science Committee 2013). Given that the Ontario Bank Swallow population has been declining 4.8 percent annually over the past decade (see Table 2), the current Ontario population estimate based on BBS data would be in the order of 150,000 individuals. The reliability of the BBS population estimate may be questionable though, as the BBS does not likely sample colonial species precisely enough to confidently estimate population size. The BBS likely over-samples birds nesting in human-made habitat and under-samples colonies in natural habitat, particularly the large populations found along the lower Great Lakes shorelines. For example, no more than 114 Bank Swallows were recorded during BBS surveys between 1995 and 2013 on the two survey routes located closest to the large Lake Erie shoreline population (described below), even though portions of these routes are within 3.5 km (route # 68-202 Springfield) and 0.5 km (route # 68-303 Wallacetown) of the shoreline.
Abundance data from the 2001-2005 Ontario Breeding Bird Atlas include extensive point counts (on- and off-road), and population information was reported for many individual colonies. The Atlas data indicate high densities of birds along the north shores of Lakes Ontario and Erie, as well as large colonies on the Saugeen River (Figure 3, Sandilands 2007). Many Bank Swallow colonies were unreported or under-reported during the Atlas (Sandilands 2007). A population estimate based on Atlas data is not available due to the limitation of using the point count sampling method for deriving population size estimates for a colonial nesting species.
Figure 3. Relative abundance of the Bank Swallow within northern and southern Ontario.
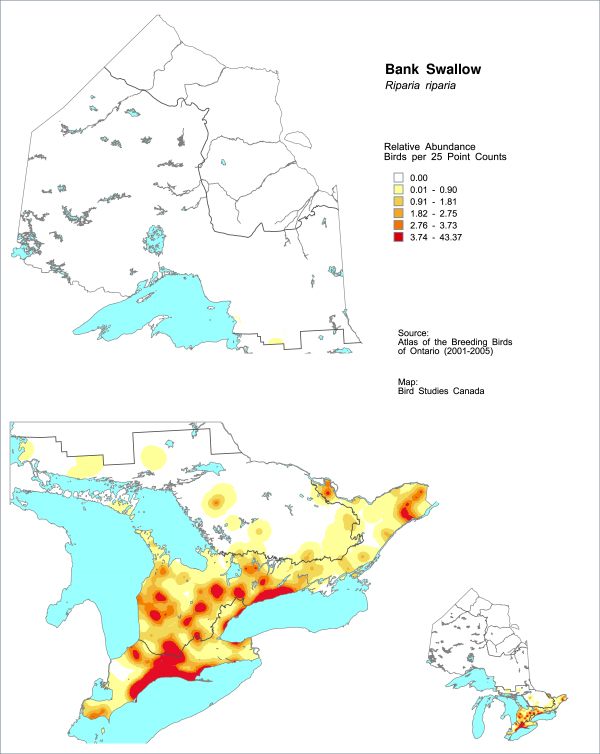
Based on Breeding Bird Atlas point count data collected in 2001-2005 (Cadman et al. 2007).
The most accurate abundance information for this species in Ontario is a series of Bank Swallow burrow count inventories conducted on Lakes Ontario and Erie, Saugeen River and other rivers, and aggregate pits throughout Ontario between 2009 and 2014. The population estimate for breeding birds on Lake Erie derived from these burrow counts is about 110,000 individuals (Appendix B:), while Lake Ontario supports about 20,000 individuals (Leung unpub. data 2010) and the Saugeen River supports at least 2,000 individuals (Cadman and Lebrun-Southcott 2013). The population estimate for Bank Swallows breeding in aggregate pits and quarries in Ontario is about 277,000 individuals (Browning and Cadman unpub. data 2015). This estimate was based on mean abundance of burrows from a sample of 367 pits and quarries throughout Ontario and then extrapolated to the total number of pits and quarries in the province (n = 4056; M. Browning, pers. comm. 2015). All population estimates from burrow count surveys assume a burrow occupancy rate of 50 percent and two adults per occupied burrow (Wright et al. 2011), which is similar to the findings of two unpublished studies in Ontario (Appendix B:, Cadman unpub. data 2011).
Much of northern Ontario has not been thoroughly surveyed. Atlas data indicate there are many (probably small) colonies scattered across northern Ontario, but overall this population likely represents a substantially smaller number of birds compared to southern Ontario (Sandilands 2007). One exception is colonies along the rivers in the Hudson Bay Lowlands, which may be of provincial significance. Confirmed breeding evidence for Bank Swallows was recorded along sections of the Severn, Fawn, Winisk, Ekwan, and Moose Rivers (Figure 2). An assessment of the population size in the Hudson Bay Lowlands is needed to accurately estimate the population size for the province.
The best available information (see above burrow count surveys) indicates that the Ontario Bank Swallow breeding population is in the order of 409,000 individuals. This estimate may be conservative, as it excludes estimates from rivers in the Hudson Bay Lowlands and other areas, and miscellaneous habitats (e.g., construction sites).
Some proportion of individuals in a given population in a given year consists of non-breeding individuals referred to as "floaters" (Kokko and Sutherland 1998). No information exists on floater population dynamics of Bank Swallows. However, the proportion of floaters likely varies temporally and geographically depending on population size (affected by reproductive success in previous year and over-winter survival), and habitat quality and quantity. These relationships are complex, as high quality habitat can produce large numbers of recruits into the floater population, and a large total population decline can occur without a substantial decrease in the number of breeders (Kokko and Sutherland 1998). More study is needed to understand floater population dynamics in the Bank Swallow.
Population trends
Population trend data exist for Bank Swallows in Ontario and the rest of North America for the past four decades from the BBS and Ontario Breeding Bird Atlas projects (Cadman et al. 1987, Cadman et al. 2007, Environment Canada 2014a). Despite large sample sizes, BBS trends are considered only moderately reliable as the survey design for underlying point count data is not well-suited to accurately sample colonial species (Environment Canada 2014a).
According to BBS data from 1970 to 2012, the Bank Swallow population in Canada declined by 95 percent overall or 6.9 percent annually, and the Ontario population declined by 93 percent overall or 6.2 percent annually (Table 2, Figure 4). Greater declines have occurred in more northerly regions, such as Bird Conservation Region (BCR) 8 and BCR 12 compared to BCR 13 (Table 2, Figure 5). In the short-term (2002-2012), the Bank Swallow population has continued to decline significantly, but at a lesser rate in Ontario (-4.8% annually) and also in each BCR (Table 2). BBS data are not available for BCR 7. Since the 1980s, Bank Swallow short-term trends based on BBS data from Ontario show that the severity of the decline has been gradually lessening (A.C. Smith, pers. comm. 2015).
Bank Swallow trends throughout the rest of North America show significant declines in most regions and jurisdictions (Nebel et al. 2010, Sauer et al. 2014). No regions or jurisdictions show significant increases in Bank Swallow populations (Sauer et al. 2014). Recent analysis of aerial insectivore populations using BBS data shows evidence for the initiation of declines during the 1980s for the entire group of swifts, swallows and nightjars across most of North America, although trends and trajectories on either side of the change point vary spatially and temporally both within and across species (Smith et al. 2015, Michel et al. In press). In Europe, several studies report population declines associated primarily with changes in the aggregate industry (e.g., Lind et al. 2002, Heneberg 2013). See section 1.6 for further information on changes in the aggregate industry in Ontario.
Figure 4. Long-term population indices for Bank Swallows in Ontario during 1970-2012.
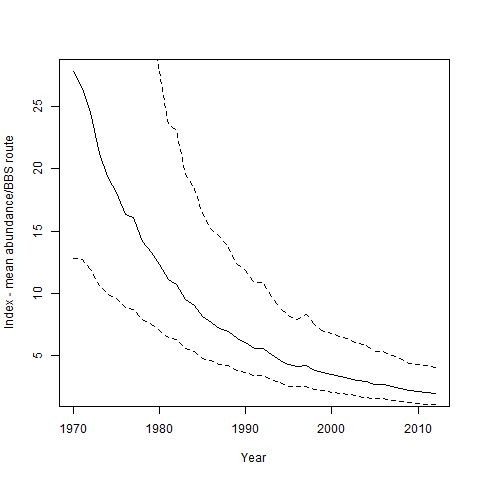
Based on Breeding Bird Survey data (Environment Canada 2014a). Dashed lines depict 95% lower and upper credible intervals.
Table 2. Long and short-term estimates of population change for the Bank Swallow in Ontario and Canada.
| Geographic Region | Time period | Years | Annual trend (%) | Upper Credible Interval | Lower Credible Interval | Overall Reliability | N - routes |
|---|---|---|---|---|---|---|---|
| Canada | Long-term | 1970-2012 | -6.9 | -4.4 | -8.6 | Medium | 479 |
| Ontario | Long-term | 1970-2012 | -6.2 | -4.1 | -9.1 | Medium | 112 |
| BCR-8 ON | Long-term | 1970-2012 | -13.4 | -7.0 | -19.6 | Low | 12 |
| BCR-12 ON | Long-term | 1970-2012 | -8.7 | -6.3 | -11.1 | Medium | 41 |
| BCR-13 ON | Long-term | 1970-2012 | -3.9 | -2.2 | -5.8 | Medium | 59 |
| Canada | Short-term | 2002-2012 | -4.0 | 2.5 | -9.2 | Low | 430 |
| Ontario | Short-term | 2002-2012 | -4.8 | -2.0 | -8.4 | Medium | 95 |
| BCR-8 ON | Short-term | 2002-2012 | -13.4 | -3.4 | -22.9 | Low | 8 |
| BCR-12 ON | Short-term | 2002-2012 | -8.3 | -0.5 | -12.2 | Low | 34 |
| BCR-13 ON | Short-term | 2002-2012 | -4.3 | -1.4 | -8.2 | Low | 53 |
Based on Breeding Bird Survey data (Environment Canada 2014a). Boldface denotes significant trends. Measures of overall reliability of trends are defined by span of geographic coverage, model fit and precision of estimates (see Environment Canada 2014b). Ontario trends are also sub-divided into Bird Conservation Regions (BCR) (see Figure 5).
Figure 5. Bird Conservation Regions in Ontario (Bird Studies Canada and NABCI 2014).
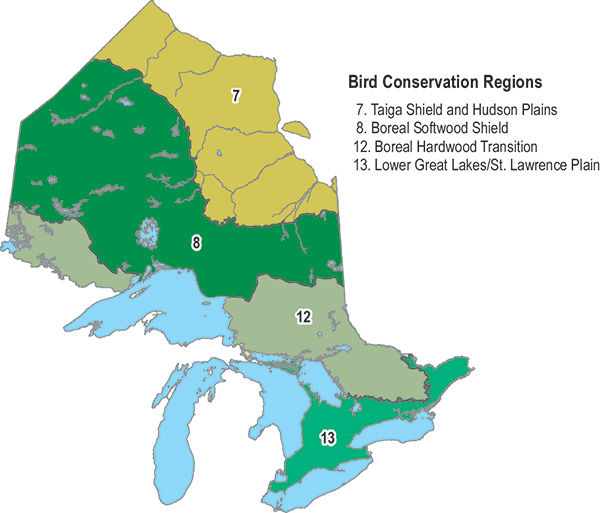
Enlarge Figure 5. Bird Conservation Regions in Ontario map
Figure 6. Ecoregion boundaries used in the Ontario Breeding Bird Atlas analysis.
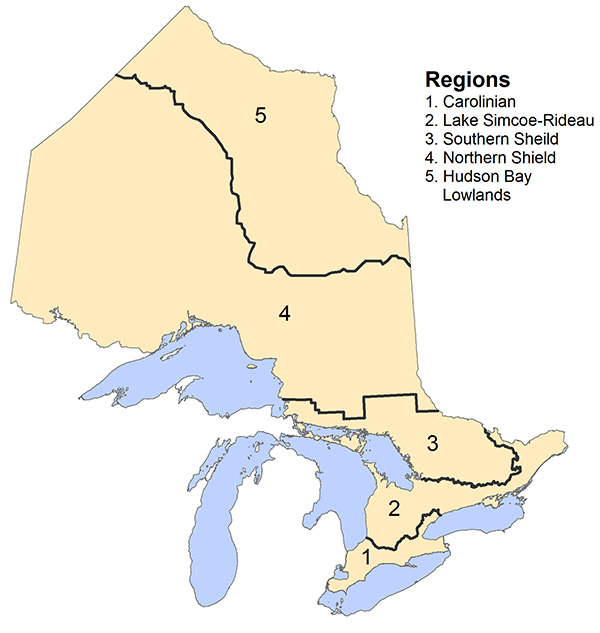
Enlarge Figure 6. Ecoregion boundaries map
Adapted from Figure 1.5 in Cadman et al. (2007).
During the second Ontario Breeding Bird Atlas, Bank Swallows were recorded in 409 (29%) fewer squares across Ontario than in the first atlas (Figure 2). The greatest distributional changes were observed in the Northern Shield (52% fewer squares occupied) and Southern Shield regions (61% fewer squares occupied), despite observer effort being greater in these regions in the second atlas period (Cadman et al. 2007; see Figure 6 for ecoregion boundaries used in the Atlas).
The probability of observation (standardized for 20 hours of observation effort) for the Bank Swallow decreased by 45 percent in Ontario between atlas periods (1981-1985 and 2001-2005; Cadman et al. 2007). Declines in probability of observation were observed in all regions of Ontario, and were most pronounced in the Southern Shield (-69%) and Northern Shield (-65%) regions.
Annual burrow count inventories from 2010 to 2015 of monitored sections of the Lake Erie north shore (between Port Stanley and Long Point, see Appendix B:) indicate large annual variation in numbers, although the trend appears stable (slope = 0, P = 0.8, Figure 7). Similarly, annual burrow counts from 2009 to 2013 on the Saugeen River in Ontario fluctuate considerably and suggest no apparent trend (Cadman and Southcott-Lebrun 2013). These findings are, perhaps, not surprising given the few years of study.
Figure 7. Annual Bank Swallow burrow counts along 64 km of monitored sections from the north shore of Lake Erie.
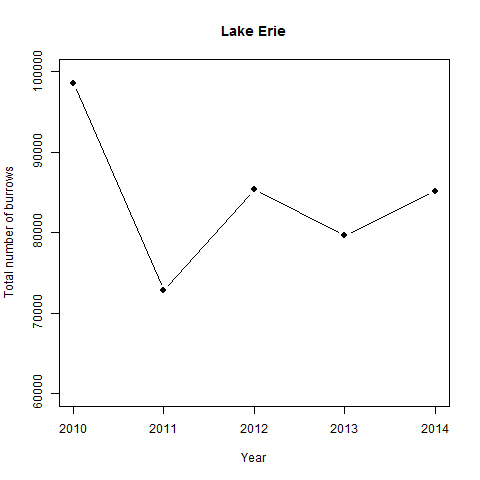
Counts made between Port Stanley and Long Point (2010-2015; Appendix B:).
1.4 Habitat needs
Bank Swallow habitat needs include foraging habitat, nest sites and nocturnal roost sites. Access to suitable foraging areas with a reliable supply of insect prey is necessary throughout their life cycle. Breeding birds require a suitable nest site in proximity to foraging habitat. In addition, Bank Swallows require suitable habitat for roosting at night at all times of the year. As with other swallow species, migratory stopover points are usually centred on large marshes where birds roost at night and disperse to forage throughout the day (Turner 2004, Winkler 2006). There is little information available for Bank Swallows in terms of the importance of area requirements of these disparate habitats and their proximity to each other.
Foraging habitat
Bank Swallows forage in a variety of open terrestrial and aquatic habitats including wetlands, open water, riparian woodlands, grasslands, and agricultural areas, as well as shrubland (Garrison 1999). Regions with dense forest cover are generally avoided at all times of the year. During the pre-migratory period (i.e., August), an Ontario study of farmland birds recorded consistently high numbers of Bank Swallows foraging in apple orchards, whereas corn and soybean fields had no Bank Swallows (Boutin et al. 1999). Grassland habitat may be a preferred foraging habitat type when located in relatively close proximity to a breeding site. In California, increased distances between colonies and nearest grassland habitat was positively related to colony extinction probabilities (Moffatt et al. 2005). Limited information is known about the foraging habitat used by Bank Swallows in Ontario, other than breeding birds mostly forage in open habitats up to 1000 metres from the colony (Turner 1980, Appendix B:). See Diet and foraging behaviour for information on diet and foraging behaviour, including foraging distances.
During migration and on the wintering grounds Bank Swallows forage over a variety of open and aquatic-based habitats, including wetlands, mangrove lagoons, ocean coasts, mudflats, and agricultural areas (Garrison 1999). In Paraguay, wintering Bank Swallows forage over open water habitats more than over fields, marshes, or beaches (Hayes et al. 1990). More information on foraging habitat preferences is needed throughout the life cycle of the Bank Swallow.
Nest sites
During the breeding season (i.e., May through August), nesting Bank Swallows require a vertical or near-vertical bank of a suitable substrate, typically consisting of fine sand or silt. Natural erosion and human-related excavation of material refreshes the vertical profile and keeps the bank suitable for nesting. If the vertical face of a bank is not maintained or "refreshed", it usually slumps and stabilizes within several years, at which point the colony disappears (Garrison 1999, Ghent 2001a, J. Bayliss, pers. comm. 2015). Typical examples of suitable nesting sites include eroding lake bluffs and river banks, extraction faces in aggregate pits, and topsoil piles in construction areas. Some less common sites include woodchip and ash piles, and pre-existing drain holes in concrete structures (e.g., under bridges) (Peck and James 1987, Garrison 1999). Based on Ontario and Quebec nest records scheme data, the percentage of Bank Swallow nests in human-made habitat (65%, e.g., pits) was greater than natural habitat (35%; Erskine 1979). Nest record reports may be biased towards human-made habitats that are easier to access (i.e., sampling artifact). It is unknown if these proportions have changed since the late 1970s, so more study may be necessary.
Attempts to create human-made nesting structures for Bank Swallows have met with varied success (Hopkins 2001, M. Leung, pers. comm. 2014, K. McDonald, pers. comm. 2014). Structures range from cement bunker styles with pre-existing, sand-filled burrows to more natural embankments consisting of layers of clay-sand mixtures. In Ontario, very limited or no success has been achieved with these nesting structures. In most cases, birds have not nested in the structures, despite some birds excavating early in the season. In one case, a colony (with 32 burrows) was established in a human-made bank composed of natural materials, although it was later depredated by a predator (K. McDonald, pers. comm. 2014). In contrast, similar structures created in Europe have been successful (Hopkins 2001). The reason(s) for the discrepancy in occupancy rates of nest structures between Europe and Ontario is currently unknown. More study is needed.
The substrate characteristics of nest sites has received considerable attention (Petersen 1955, Spencer 1962, Hickman 1979, Hjertaas 1984, Jones 1987, John 1991, Heneberg 2001, Lind et al. 2002, Heneberg 2003, Johnson 2006, Heneberg 2009, Silver and Griffin 2009). In general, substrate penetrability and the varying proportions of substrate particle sizes are important for burrowing. Higher proportions of very fine sands (<900 μm) allow birds to excavate deeper burrows that may result in higher reproductive success due to inaccessibility to predators and/or less chance of nest mortality caused by bank collapse (Heneberg 2003). Colony sizes also tend to be greater where the proportion of silt to sand is greatest (Hjertaas 1984, John 1991, Garrison 1999).
Bank length is positively related to bank occupancy, while woody vegetation on the talus slope (below the bank) is negatively related to bank occupancy (Hjertaas 1984, Tozer and Richmond 2013). Open space of at least 60 m out from the bank is needed as Bank Swallows require open flying space for vertical lift when exiting nest burrows (Hjertaas 1984). Bank erosion processes and rates are likely related to bank structure and vegetation characteristics (Garcia 2009), but few studies have examined the effect of erosion on Bank Swallow habitat selection. Garcia (2009) found that sites with high rates of riverbank erosion, which refresh the bank’s vertical face, had the highest levels of colony persistence (i.e., >10 years). Waterbodies and watercourses are often associated, although likely indirectly, with Bank Swallow colonies, since they are often the source of erosion for suitable nesting banks. Notable exceptions include aggregate pits, where banks are created and maintained using heavy machinery. Ghent (2001a) showed that removal of talus to increase bank height to about 2 m resulted in Bank Swallows reoccupying aggregate pit banks. While Bank Swallows generally use taller banks, they have been observed nesting at aggregate sites in Ontario in banks less than one metre high where the face is directly (i.e., within 1 metre) over water (J. Bayliss, pers. comm. 2014). However, smaller banks are typically more accessible to terrestrial predators compared to higher banks.
Roosting sites
Roosting sites, where birds congregate in large numbers at dusk and vacate the site at dawn, are used by Bank Swallows at all times of the year (Winkler 2006). Large wetlands, reed or cane beds, or other dense vegetation over water are typical roosting sites (Winkler 2006). Large aggregations of Bank Swallows and other swallow species use these roosting sites during breeding, post-breeding and migration.
Radio telemetry data from the north shore of Lake Erie shows breeding Bank Swallows may roost as far as 35 km from breeding colonies, even while nests are active (Appendix B:). In Ontario, very few Bank Swallow roost sites are known. The extensive marshes on the north side of the Long Point peninsula on Lake Erie annually host large roosts of Bank Swallows; as many as 45,000 individuals have been recorded on roost monitoring surveys (Falconer unpub. data 2011, D. Bell, pers. comm. 2012). Other existing roost records (i.e., "hundreds") for Bank Swallows in Ontario include a shrubby wetland site in Pembroke (Ross et al. 1984; however, the site no longer exists) and Phragmites reed beds near Port Burwell (B. Bolin, pers. comm. 2013) and Toronto (L. Pady, pers. comm. 2015). It is unknown if changes in the distribution of Phragmites australis, a highly invasive alien plant species of wetland habitat in Ontario that is being actively controlled in some areas, has affected roost site availability or quality for the Bank Swallow.
More information is needed to identify roost sites and examine patterns of use in Ontario.
1.5 Limiting factors
Biological factors influencing successful recovery approaches for the Bank Swallow may include:
- short life span and single-brooded;
- the population is vulnerable to rapid decline if nest mortality is exceptionally high over one or two breeding seasons as most of breeding population consists of young (first- and second-year) birds.
- highly colonial breeder and gregarious behaviour;
- many individuals concentrated at a limited number of breeding, foraging or roosting sites. Factors/threats (e.g., breeding or foraging habitat degradation or loss; stochastic events) during the breeding and non-breeding period could have significant negative impacts in terms of the number of birds affected (potentially thousands at a single site).
- high site-fidelity of adults to breeding sites;
- birds often return to the sites where they bred in previous years, thus loss or degradation of these sites could result in displacement, failure to relocate to suitable habitat, or indirect effects leading to reduced survivorship or recruitment.
- vulnerability to extended bouts of adverse weather or other events that limit the availability of flying insects; and
- attraction to aggregate pits and other human-made habitats for nesting.
- Bank Swallows nest at sites with features shared by aggregate extraction operations. While these human-made sites provide additional nesting habitat which can benefit Bank Swallows, they can also put nests in jeopardy of destruction from aggregate operations. Bank Swallows' attraction to aggregate sites is a limiting factor that necessitates continued cooperation with the aggregate industry for Bank Swallow conservation.
1.6 Threats to survival and recovery
Numerous factors have been proposed as possible explanations for the population declines of Bank Swallows and other aerial insectivores in Canada (Nebel et al. 2010, Calvert 2012, COSEWIC 2013, Smith et al. 2015, Michel et al. In press). However, the information needed to critically evaluate the impacts of these potential threats to Bank Swallows in Ontario is generally lacking (COSEWIC 2013). Critical knowledge gaps that must be addressed to evaluate the severity and magnitude of the many possible threats that affect the survival and recovery of this species are identified in section 1.7.
This summary of human-related threats almost exclusively focuses on threats occurring in Ontario during the breeding and post-breeding periods because: (1) reproductive success is an important demographic factor for this short-lived species; (2) very little is currently known about the nature, extent and severity of threats affecting Bank Swallows during migration and wintering periods; and (3) the focus of this recovery strategy is to identify key practical actions that the Ontario government and other interested parties could undertake or support to promote the recovery of the species in Ontario. It should be noted, however, that implementing recovery actions only in Ontario may be insufficient to recover the population, since threats and subsequent mortality may be too severe on migration and/or the wintering grounds. Another possibility is that the cumulative impact and carry-over effects from threats on the wintering grounds or migration could be hampering reproductive output or survival on the breeding grounds. If birds are exposed to certain threats on the wintering grounds or during migration, which result in poor body condition of adults, negative impacts on breeding output or adult survival may occur on the breeding grounds in Ontario. This makes addressing knowledge gaps and threats related to over-wintering all the more critical to the Ontario population’s recovery.
While recovery of the Bank Swallow in Ontario will depend on minimizing threats to the species wherever they occur, recovery actions to maintain or enhance the productivity of birds in Ontario should increase the probability of successful recovery.
The following assessment of the known and potential threats to Ontario Bank Swallows is based on the best information currently available, including data from unpublished studies and expert opinion gathered during the development of this recovery strategy. It is likely that multiple direct and indirect threats are having an additive or synergistic impact on Bank Swallows (COSEWIC 2013). The significance and severity of these threats should be reassessed as new information becomes available.
Threats are presented in order of decreasing certainty, extent and anticipated importance. The relative severity of these threats is not currently known.
Loss of nest site habitat
Natural habitats
Historically, Ontario Bank Swallows exclusively nested on natural eroding riverbanks and lakeshore bluffs. The availability of these natural nest sites has likely declined due to flood and erosion control measures.
Rivers throughout Ontario have been subject to periodically widespread flooding, especially with rapid snowmelts and ice jams in the spring and/or heavy rainfall events, resulting in riverbank erosion that creates suitable nesting habitat. Throughout the 1940s and 1950s, developed areas of Ontario initiated hazard control programs leading to the creation of numerous flood control dams and erosion control projects (TRCA 2014). While flood and erosion control is beneficial to people and infrastructure in flood hazard zones, it likely results in a reduction in the availability of large eroding nesting banks for Bank Swallows (Garrison 1999, Garcia 2009). Although it is unknown to what extent Bank Swallows nested on rivers prior to the initiation of flood control programs, very few rivers studied to date currently support Bank Swallow colonies in Ontario (M.D. Cadman, pers. comm. 2014, M. Browning, pers. comm. 2015). In other regions, such as California, the principal cause of Bank Swallow decline is thought to be related to erosion control projects (Schlorff 1992, Garrison 1999).
Lakeshore bluffs on Lakes Erie and Ontario support very large Bank Swallow populations, especially in areas with naturally high erosion rates (Falconer unpub. data 2014). Erosion control (shoreline hardening) is widespread on the lower Great Lakes, though it only seems to be effective over small areas (Mickelson et al. 2004). On a large scale, this habitat is likely not threatened because the coastal erosion processes are too powerful to eliminate completely by control efforts. Declining water levels on the Great Lakes could reduce coastal erosion and result in a widespread decline in Bank Swallow habitat. However, water levels on Lakes Erie and Ontario are relatively stable (Gronewold et al. 2013). Further study is needed regarding how lake bluff habitat has been affected by variation in lake water levels and erosion control projects.
Human-made habitats
Over the last ~100 years, Bank Swallows began nesting in a variety of anthropogenic habitats, including aggregate pits and quarries, road cuts, and stockpiles of topsoil and sand at construction sites.
Road cut policy in Ontario has shifted towards stable graded slopes rather than vertical slopes (M.D. Cadman, pers. comm. 2014). No records of Bank Swallows nesting in road cuts have been reported to the Ontario Nest Record Scheme (ONRS 2014) since the 1980s; whereas in the 1930s, one quarter of all nest records were from road cuts (COSEWIC 2013).
Originally, the Ontario aggregate industry consisted of scattered borrow pits across the countryside, but as urban development advanced larger pits were developed near major cities, and this situation remained relatively unchanged until the 1950s (Yundt and Messerschmidt 1979). Aggregate extraction grew substantially during the economic boom of the 1950s and 1960s, and with it, numerous pits were opened (Yundt and Messerschmidt 1979). Triggered in part by this rapid increase in pits, the first provincial pit licensing requirements came into effect in 1971 under the Pits and Quarry Control Act. Pit rehabilitation requirements under this act resulted in slope grading and erosion control practices that eliminate Bank Swallow nesting habitat. Legislation was strengthened with the Ontario Aggregate Resources Act, 1990, resulting in enhanced rehabilitation measures and closure of many pits and quarries (C. Robinson, pers. comm. 2015). Additional legislation (e.g., Greenbelt Act, 2005) has placed stricter requirements on some aggregate operations to implement further progressive rehabilitation measures resulting in continuous removal of nesting habitat within active pits (J. Bayliss, pers. comm. 2015). Land uses at rehabilitated pit and quarry sites now commonly include agriculture and residential or commercial development (J. Bayliss, pers. comm. 2015).
Although aggregate extraction rates have generally increased since regulation, demand for sand and gravel appears to be declining in favour of crushed stone aggregates (Altus Group 2009). Even so, sand and gravel extraction is still widespread in Ontario (S. May, pers. comm. 2015). Below water table extraction of aggregates may be increasing, but this practice generally does not provide large banks for nesting (J. Bayliss, pers. comm. 2015). Similar changes in the aggregate industry have been described in Europe (Lind et al. 2002, Heneberg 2013). The changes in the aggregate industry towards larger, more intensive and more efficient operations are similar to trends in other land use practices on the rural landscape such as agriculture (see below).
Loss or degradation of foraging habitat
Threats to Bank Swallow foraging habitat in Ontario potentially include land cover and land use changes resulting in the loss or degradation of insect-rich, open habitats. Changes in agricultural land use are important, because the majority of the Bank Swallow breeding sites in Ontario exist within agricultural regions. Wetlands and other open aquatic habitats are also important as they support aquatic emergent insects and the species also uses these areas for foraging.
The amount and nature of open country habitat (which includes but is not limited to agricultural lands) in southern Ontario has undergone dramatic changes over the past 200 years (Neave and Baldwin 2011). Open country habitats in southern Ontario prior to European settlement included local areas of native grassland, savannah, alvar and rock barrens, and First Nations agricultural lands. In the 19th century the amount of open country habitat in southern Ontario increased as forested lands were cleared for agriculture (Neave and Baldwin 2011). Over the past century, open country habitat in southern Ontario decreased substantially due to reforestation and succession of marginal farmland (especially in the Southern Shield ecoregion) and urbanization (Blancher et al. 2007, Neave and Baldwin 2011). Since 1971, however, there has been little change in the total amount of open country habitat in southern Ontario (Neave and Baldwin 2011).
While the amount of open country habitat in southern Ontario may have stabilized in recent years, there continues to be major changes to land cover due to changes in agricultural land use that could be affecting Bank Swallows and other wildlife populations (Javorek et al. 2007, Neave and Baldwin 2011). Steady declines in the total amount of farmland in Ontario and the amount of pasture since 1921 have continued through 2011 (Javorek et al. 2007, Statistics Canada 2012). Changes in agricultural land use are driven by socio-economic factors. Changing dietary preferences (e.g., less dairy and beef), changing farm practices and recent high corn and soybean prices have resulted in a general shift from dairy and cattle farming to intensive annual field crop production in the Great Lakes-St. Lawrence region (Latendresse et al. 2008, Jobin et al. 2010).
Studies on Tree Swallows (Tachycineta bicolor) breeding across a gradient of agricultural intensification in Quebec have showed lower nest box occupancy, lower reproductive performance and overall lower numbers (but not biomass) of flies within agro-intensive landscapes (Ghilain and Bélisle 2008, Rioux Paquette et al. 2013, Rioux Paquette et al. 2014). Rioux Paquette et al. (2013) also noted fly abundance across the intensification gradient varied seasonally and annually, suggesting the relationship is more complex than generally assumed and difficult to predict. Studies of insect abundance and biomass on organic and conventional farms have showed mixed results depending on taxa, crop types, regions, etc. (Girard et al. 2014, Kragten et al. 2011). Kragten et al. (2011) found a 70 percent greater abundance of aerial insects in organic farms in the Netherlands. However, Freemark and Kirk (2001) found no difference in Bank Swallow abundance between organic and conventional farms in Ontario. Although more study is needed, it is likely that many factors (e.g., pesticide use, crop type and rotation regimes, amount of natural vegetation nearby) affect the signal and strength of the relationship between bird and/or insect abundance and agricultural intensification.
Changes in the extent and quality of wetlands, riparian areas, and open water habitats could be affecting food availability for Bank Swallows. Throughout southern Canada and especially in densely populated regions, wetlands have undergone tremendous net losses, while cumulative impacts (e.g., drainage, invasive species) continue to exacerbate the degradation of wetland health and function (Bedford 1999, Daigle et al. 2006, Bartzen et al. 2010, FPTGC 2010). In southern Ontario, there has been an estimated 72 percent loss of large (>10 ha) wetlands since European settlement, with the most severe losses seen in southwestern Ontario, parts of eastern Ontario, Niagara and the Toronto area (Ducks Unlimited 2010). From 1982 to 2002, an estimated 3.5 percent loss of large wetlands occurred in southern Ontario and it is likely that smaller wetlands (<10 ha) are declining at a similar or more rapid rate (Ducks Unlimited 2010). Land uses on lands that were historically wetlands include agricultural uses, various urban and rural developed lands and brown fields, hydro right-of-ways, transportation corridors, and forest clearings.
Environmental contaminants, pesticides and pollutants
Environmental contaminants, pesticides and pollutants may directly or indirectly affect the survival and reproductive output of Bank Swallows (and other aerial insectivores) due to:
- poisoning (mortality) or sub-lethal harmful effects caused by exposure to pesticides, heavy metals, endocrine disrupters or other pollutants; and
- reductions or other changes in food supply due to pesticides (particularly insecticides).
Mercury exposure may be a potential threat to the Bank Swallow and/or its food supply. Studies involving the Tree Swallow have shown high mercury concentrations in arthropod prey and adult swallows at mercury-contaminated sites in the northeastern United States (Cristol et al. 2008) and that insectivorous birds, as a guild, have higher mercury concentrations than birds feeding at lower trophic levels (Keller et al. 2014). Mercury has been implicated in a wide range of negative effects for Tree Swallows (and other bird species), including effects on immune and endocrine systems (Hawley et al. 2009, Wada et al. 2009), reduced productivity and survival rates (Brasso and Cristol 2008, Hallinger et al. 2011) and skewing offspring sex ratios towards females (Bouland et al. 2012). The effects of mercury on Tree Swallow reproduction are magnified when early breeding seasons are warmer (Hallinger & Cristol 2011).
Although herbicide and insecticide use, declines in pastured land and increases in farming intensity are generally thought to negatively impact grassland birds, a recent study found that the acute lethal risk (i.e., toxicity to birds) of insecticides used across the United States was the best predictor of grassland bird declines (Mineau and Whiteside 2013). However, ground foraging birds are considered to be at a higher exposure risk to agricultural pesticides compared to aerial insectivores, such as the Bank Swallow (Boutin et al. 1999). The indirect impacts of pesticides and pollution on the food supply or quality of the food supply are potentially more significant threats than direct poisoning for the Bank Swallow, at least on the breeding grounds in Ontario.
The quantity of agricultural pesticides applied in Ontario has declined in recent decades, with a 45 percent reduction in overall agricultural pesticide use between 1983 and 2008, and a 76 percent reduction in agricultural insecticide use in this same period (McGee et al. 2010). A provincial ban on the cosmetic use of pesticides was implemented in 2009. There have also been considerable shifts in the types of pesticides being used in Ontario over time.
Recently there has been considerable concern as to potential biological impacts of neonicotinoid insecticides, a class of insecticide that came into use in the 1990s and is now widely used in Ontario and elsewhere (Douglas and Tooker 2015). Neonicotinoids are systemic insecticides that have been implicated in the decline of non-target arthropods, including bees, other insect pollinators and aquatic macroinvertebrates (Mason et al. 2013, Hallmann et al. 2014, Gibbons et al. 2015, Morrissey et al. 2015, Pisa et al. 2015). There is evidence that this insecticide class could be impacting bird populations due to direct toxicity in some cases (Gibbons et al. 2015), but more commonly as a result of the indirect impact of an overall reduction in insect abundance and/or biomass (Mineau and Palmer 2013, Hallmann et al. 2014). In 2015, the provincial government restricted the agricultural use of neonicotinoids in Ontario.
Reduced nest productivity due to human activities and persecution
Human activities causing mortality is a known threat that can cause severe reductions in local productivity and other adverse effects. The extent and frequency of this threat has not been quantified and it is unknown if this threat has increased over time.
Colonies are sometimes destroyed or partially destroyed during extraction operations at aggregate pits (Campbell et al. 1997, M.D. Cadman, pers. comm. 2014), during road construction (Petersen and Mueller 1979), or during erosion control projects (Environment Canada 2013). However, many aggregate operations in Ontario have improved protection measures for Bank Swallows nesting in pits by managing active bank faces and stockpiles to discourage nesting and reduce incidental take (S. May, pers. comm. 2015). Colonies are also sometimes persecuted by curious children, digging and inserting objects (e.g., tree branches) in burrows (Todd 1963, COSEWIC 2013).
Habitat loss, disturbance and human persecution at roost sites
Habitat destruction or degradation due to activities that disturb roosting birds and land uses that attract predators are potential threats at roost sites. Adjacent urban development, as well as predation by an increasing Merlin (Falco columbarius) population, may have been factors contributing to the abandonment of a Bank Swallow roost in Pembroke, Ontario in the 1990s (Ottawa River Legacy Landmark Partners 2013). The significance and nature of threats to roost sites are unknown, as information on the locations, size, and suitability of Bank Swallow roost sites in Ontario (and elsewhere) is very limited.
Winter roosts in Central and South America may be subject to threats including poisoning or disturbance due to measures taken to control other avian pest species (e.g., Dickcissel [Spiza americana]) (Basili and Temple 1999), or even direct exploitation as a food source, as reported at winter roosts in parts of Asia and Africa (Ewins et al. 1991, Turner 2004).
Climate change
No information exists on the impact of climate change on Bank Swallows and therefore the following hypotheses are speculative.
Migration phenology appears to be shifting with climate change for many bird species (Hurlbert and Liang 2012). Changes in the timing of insect emergence may be occurring as a result of climate change, such that there is a mismatch between the availability and demand in food supply for birds during breeding, migration and/or winter (Both et al. 2010, Jones and Cresswell 2010, but see Dunn et al. 2011). Conditions on wintering grounds (especially droughts) may cause indirect effects on the survival and reproduction for birds. Cooper et al. (2015) give empirical evidence of the effect of food reduction experiments in songbirds on the wintering grounds which lead to poorer body conditions and later migratory departure times of birds, potentially impacting reproductive success the following breeding season.
In the eastern hemisphere, apparent overwintering adult survival of Bank Swallows is reduced in drought years and is negatively related to rainfall levels from the previous breeding season (Szép 1995, Cowley and Siriwardena 2005). Periods of prolonged rainfall can reduce insect availability, increase foraging constraints on adults, and result in bank collapse at colonies (Bryant and Turner 1982, Garrison 1999, Heneberg 2007). In general, swallows are susceptible to periods of low temperatures and high precipitation levels. Severe, prolonged cold snaps during migration and on the breeding grounds can cause mass mortality events of adults and nestlings (Brown and Brown 1998, Newton 2007, Hess et al. 2008). Inclement weather events are considered the primary cause of nestling starvation for Bank Swallows (Turner and Rose 1989). Climate change is also thought to have resulted in increased numbers and intensity of hurricanes, potentially causing high mortality events for some species during fall migration (e.g., Chimney Swift, Chaetura pelagica, Dionne et al. 2008). The effect of hurricane frequency or intensity on Bank Swallow survival during migration has not been studied.
Although the effect of climate change on Bank Swallow populations remains largely speculative, it likely varies geographically and may cause mixed effects, with some beneficial effects and some negative effects, as has been reported for Tree Swallows (Dunn and Winkler 1999, Hussell 2003, Shutler et al. 2012).
1.7 Knowledge gaps
The Bank Swallow has been the focus of some extensive studies in Europe and California. However, much less is known about the ecology and conservation needs of the eastern North American population. The few published studies carried out at breeding colonies in Ontario have been limited in focus, geographic scope and duration (John 1991, Ghent 2001a, Ghent 2001b, Cadman and Lebrun-Southcott 2013).
Fundamental uncertainties that are pertinent to the recovery of this species include:
- demographic processes and factors driving recent declines in Bank Swallow and other aerial insectivores; and
- extent and severity of threats to the Bank Swallow in Ontario and elsewhere.
Uncertainty as to the threats and environmental factors limits our ability to determine what constitutes an achievable long-term recovery goal, prioritize recovery objectives, and predict the efficacy of various recovery approaches.
Knowledge gaps are grouped into six broad themes: (1) vital rates; (2) diet and food supply; (3) habitat requirements and trends in Ontario; (4) wintering and migration habitat and ecology; (5) Best Management Practices (BMPs); and (6) climate change effects.
Vital rates and population source/sink dynamics
Information on the vital rates of the Ontario Bank Swallow population is needed to understand the demographic processes underlying the population decline and to identify where in the life cycle recovery action will be most effective. Determining vital rates will also help determine a sustainable population size required to achieve self-sustaining viability levels (see Recovery Goal).
Comparable demographic data are also needed from other parts of the North American range including regions with varying population trends.
Among other things, studies are needed to assess:
- breeding productivity;
- adult return rates to colonies;
- recruitment of yearling birds to colonies; and
- survival rates of adults and young at each stage of the annual life cycle (i.e., breeding, post-breeding, and overwintering periods).
This information is needed to address questions regarding population source/sink dynamics such as:
- demographic parameters correlated with nest substrate or colony size; and
- impacts of food shortages, predators, parasites and inter-annual losses of nesting banks on productivity.
Diet and food supply
Widespread declines in aerial insectivore populations has raised concern as to whether there have been large-scale changes in insect populations due to insecticides, environmental contaminants, habitat degradation, climate change or other factors. Indeed, many invertebrate monitoring datasets are showing declines in biodiversity and abundance at greater rates than for vertebrates (Dirzo et al. 2014). For example, studies have noted declines in the benthic chironomids (midges) at study sites in the Great Lakes, coinciding with increases in invasive dreissenid mussels (Soster et al. 2011) and declines in lake water levels (Cooper et al. 2014). However, the uncertainty surrounding even the most basic trends of most insect populations is vast (Cardoso et al. 2011, Collen et al. 2012).
Specific information needs are:
- Bank Swallow diet in Ontario and elsewhere in the life cycle;
- spatial and temporal patterns of prey abundance and diversity among habitats used by Bank Swallows; and
- sources and levels of contaminant loads (e.g., neonicotinoids) impacting food supply.
Habitat use, habitat requirements and habitat trends in Ontario
Specific information that is needed to evaluate the significance of habitat loss and degradation in Ontario as a contributing factor in declines and as a threat to recovery includes the following:
- Nesting sites
- proportion of the population that nests in various habitat types (e.g., lakeshore bluffs, riverbanks, aggregate pits, construction sites) within ecoregion types;
- causal factors driving changes in the availability of nesting habitat in natural and human-made habitats;
- extent of incidental take (nest destruction) in aggregate pits; and
- suitable soil types and structural characteristics to use when creating human-made nesting banks, specifically for guiding aggregate industry BMPs.
- Foraging habitat
- spatial and temporal foraging patterns (e.g., where swallows forage at different times of the year);
- size, habitat type and quality of foraging habitat during the breeding season and how it relates to demographics; and
- spatial and temporal relationships between Bank Swallow populations and changes in land use, agricultural practices, and pesticide use.
- Roost sites
- location and habitat features of significant nocturnal roost sites.
Wintering and migration habitat and ecology
Basic information on migration connectivity is generally lacking and this limits our understanding of the threats to the species' recovery. Studies on the European population highlight the importance of understanding the full life cycle of this long-distance migrant to determine whether, where and what conservation action is needed to address population declines. Key information gaps include:
- location(s) where birds from Ontario spend the winter;
- habitats used for foraging and roosting during winter;
- routes and stopover locations used during spring and fall migration; and
- nature, severity and significance of threats during wintering and migration periods.
Best management practices
Due to their close association with aggregate pits and construction sites, Bank Swallow management issues are a regular occurrence. In addition, conflicts may also occur in shoreline development projects, especially if erosion control measures are involved.
Information is needed to develop BMPs for specific situations including:
- effective and feasible methods for deterring nesting in actively-worked banks, aggregate stock piles and soil piles, while minimizing adverse impact on Bank Swallow productivity;
- methods to minimize adverse effects to Bank Swallows and their nesting habitat within active aggregate operations;
- methods for enhancing occupancy and productivity in human-made nesting banks and/or encouraging nesting in other appropriate locations; methods to assess the effect of human-related disturbance on colonies (e.g., traffic, noise, aggregate extraction); and
- methods to implement erosion control projects that minimize habitat loss or degradation for Bank Swallows.
Climate change
Two aspects of climate change have been identified as relevant to understanding impacts on Bank Swallow populations, as well as other aerial insectivores (Nebel et al. 2010, Calvert 2012):
- potential shifts in phenology of birds and insects related to climate change; and
- potential for increased mortality and/or reduced nesting success due to extreme weather variability, especially droughts on wintering grounds and cold, wet weather on the breeding grounds.
1.8 Recovery actions completed or underway
The decline in Bank Swallow populations and its designation as a threatened species has prompted agencies, organizations and individuals to initiate activities relevant to the recovery of this species. These activities are grouped by recovery theme, with some activities fitting into more than one theme (e.g., several monitoring projects have a research component). Table 3 summarizes recovery actions underway and associated threats and knowledge gaps being addressed.
Inventory, monitoring and assessment of species, habitats or threats
- General bird surveys and monitoring programs that collect information on Bank Swallow populations include:
- BBS: annual road-side bird population survey since 1966 (Environment Canada 2014a);
- Ontario Breeding Bird Atlas: 5-year survey (repeated every 20 years) of bird distribution and abundance across Ontario, last cycle completed 2001-2005 (Cadman et al. 1987, Cadman et al. 2007);
- Ontario Nest Records Scheme/Project Nestwatch (ONRS 2014): ongoing project to compile standardized nest monitoring information from across Ontario in a single
- Targeted burrow count surveys since 2010 have been conducted in a variety of habitats in Ontario including:
- Lake Erie: Bird Studies Canada;
- Lake Ontario: Ontario Power Generation;
- Rivers in southern and northern Ontario: Environment and Climate Change Canada (CWS) and Ontario Ministry of Natural Resources and Forestry; and
- Aggregate pits: Environment and Climate Change Canada (CWS) and Ontario Ministry of Natural Resources and Forestry.
Research
- Tianna Burke (Trent University) is examining source/sink dynamics of Bank Swallows breeding in aggregate pits and natural lake bluff sites.
- Bird Studies Canada is researching breeding season movements and foraging patterns (via radio telemetry), nest survival, and habitat selection of the Lake Erie population in Ontario.
- Environment and Climate Change Canada and OMNRF are conducting randomized surveys of rivers and pits across Ontario to examine habitat selection in relation to surrounding landscape features and bank characteristics, including soil substrate characteristics.
- Environment and Climate Change Canada deployed geolocators on birds at four sites in North America, but recovery of devices was extremely low (1 out of 112 was recovered).
- Dalhousie University researchers (S. Saldanha and T. Imlay) are studying aerial insectivores, including Bank Swallows, in the Maritimes. Activities include stable isotope analysis and geolocator deployment (to determine wintering areas and migration routes), radio telemetry (to determine movements of breeding birds) and biomarkers (i.e., corticosterone and changes in telomere length) to determine environmental stressors present on the wintering grounds and associated carry-over effects on breeding.
Management of species, habitat or threats
- The Forest Management Guide for Conserving Biodiversity at the Stand and Site Scales (OMNR 2010) provides direction for maintaining or enhancing the suitability of special habitat features such as bird nesting sites including swallow colonies.
- Environment Canada, OMNRF, and the Ontario Sand, Stone and Gravel Association developed a fact sheet for pit operators which offers management guidelines for breeding Bank Swallows (OSSGA 2013).
- Aggregate companies are taking initiative in the management of Bank Swallows at their operations by creating new nesting sites and/or deterring birds from nesting in active extraction areas. Some examples include:
- Holcim (Canada) Inc. has experimented with bank creation techniques at some active aggregate pits.
- At Lafarge Canada’s Kirkfield quarry, the slumping faces of storage piles of limestone fines have been refreshed to successfully encourage Bank Swallow nesting.
- Canada Building Materials managers at a gravel pit near Aberfoyle protect active nesting colonies, while discouraging birds from burrowing in active extraction areas by sloping working faces at the end of each day (S. May, pers. comm. 2015).
Protection of species and habitat
- The Bank Swallow (including its nest and eggs) are federally protected under the Migratory Birds Convention Act, 1994.
- This threatened species and its general habitat now receives automatic protection under the provincial ESA, 2007.
- Many wetland habitats that are likely important foraging and/or roosting sites for Bank Swallows are protected as conservation lands and through the Provincial Policy Statement (MMAH 2005).
- Wetlands and shorelines in many parts of southern Ontario also receive protection through the Development, Interference with Wetlands & Alterations to Shorelines and Watercourses Regulations under the Conservation Authorities Act.
Table 3. Summary of recovery actions currently underway and associated threats and knowledge gaps currently being addressed.
| Project | Threat: Loss of nest site | Threat: Loss/degradation of foraging habitat | Threat: Pesticides & pollution | Threat: Human disturbance at nest sites | Threat: Human disturbance at roost sites | Threat: Climate change & weather effects | Knowledge gap: Vital rates | Knowledge gap: Diet & food supply | Knowledge gap: Breeding habitat | Knowledge gap: Winter habitat | Knowledge gap: BMPs | Knowledge gap: Climate change |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC-CWS & OMNRF: Nesting success of BANS in aggregate pits | X | X | X | X | X | |||||||
| EC-CWS & OMNRF: 2014 river and 2013 pit surveys | X | X | X | |||||||||
| OSSGA: Bank Swallow Best Management Practices | X | X | X | X | ||||||||
| Holcim (Canada) Inc.: Techniques implemented in pits with Bank Swallows | X | X | X | X | ||||||||
| Trent Univ.: Productivity of Bank Swallows in aggregate habitats | X | X | X | X | X | X | ||||||
| Bird Studies Canada: Bank Swallow research on Lake Erie | X | X | X | X | X | |||||||
| Dalhousie Univ.: Bank Swallow research in the Maritimes | X | X | X | X | X | X | X |
2.0 Recovery
2.1 Recovery goal
The recovery goal is to maintain a stable, self-sustaining Bank Swallow population of at least 330,000 breeding individuals across the breeding range in Ontario by 2035 (within 20 years). Stabilization will occur by reducing the rate of decline (currently 4.8% per year; Table 2) by 0.5 percent each year over the next 10 years. In northern Ontario where targeted population monitoring does not occur, the short-term goal is to maintain a breeding distribution of at least 20 blocks across northern Ontario by the next atlas period (2021-2025).
Narrative to support recovery goal
The Bank Swallow is native to Ontario and should be maintained in keeping with Ontario’s Biodiversity Strategy (OBC 2011). Maintaining a stable population appears to be a realistic long-term target despite past population declines, as the species continues to be common and widespread in Ontario and the severity of the decline has lessened in recent decades (COSEWIC 2013). The best available information suggests the Ontario population is about 427,000 individuals. A goal of 330,000 individuals acknowledges that further declines are expected over the next 10 years.
The recovery goal consists of a short-term (10 year) and long-term goal (20 year) corresponding to a reduction in the rate of decline and the maintenance of a stable population, respectively. The timeline for slowing the current population decline and achieving a stable population is unknown due to uncertainty from causal factors and the magnitude of current threats. Twenty years is considered a realistic estimate of the time required to complete the extensive multi-year research studies identified in this recovery strategy, and to implement priority recovery actions.
Widespread implementation of state-of-the-art BMPs in Ontario could maintain or increase productivity, survival and the population size of birds breeding in human-made habitats, yielding measurable results (i.e., a slower rate of population decline) within 10 years.
The recovery goal states maintaining the population size "across the breeding range". This is specifically included to address the loss in distribution throughout northern Ontario. Distributional losses are expected to occur primarily in the north, where the species is less abundant. Between the first and second breeding bird atlas periods, there was a 37 percent (51 and 32 blocks, respectively) reduction in the number of confirmed breeding atlas blocks in northern Ontario for Bank Swallows (100 km blocks, see Cadman et al. 2007 and inset map in Figure 2). As the Ontario population size continues to decline up to the next atlas survey period, a further 37 percent loss in northern atlas blocks by 2025 (end of next atlas) is considered a realistic estimate of ongoing distributional change in northern Ontario. Therefore, the short-term goal should be to maintain this distribution at no fewer than 20 blocks across northern Ontario by the next atlas period (2021-2025).
Refined short-and long-term population abundance, distribution and/or trend targets should be established once the factors driving the decline are better understood. Consideration could also be given to developing an additional recovery target for the northern Ontario population that is not well monitored by BBS or burrow count surveys. Specific distribution and/or abundance measures for the Hudson Bay Lowlands (BCR 7) and Northern Shield region (and/or BCR 8) could be developed based on data from Breeding Bird Atlas projects or future species-targeted surveys.
2.2 Protection and recovery objectives
Table 4. Protection and recovery objectives.
| Number | Protection or recovery objective |
|---|---|
| 1 | Address knowledge gaps to better understand the magnitude (or severity) of threats and/or identify biological and socio-economic factors that may impede or assist recovery efforts. |
| 2 | Protect habitat and reduce or mitigate potential threats through stewardship, communication, education and outreach, and habitat management. |
| 3 | Inventory, monitor and report on the state of Bank Swallow populations and habitats in Ontario and elsewhere to track the progress of recovery activities. |
2.3 Approaches to recovery
Table 5. Approaches to recovery of the Bank Swallow in Ontario.
Objective 1: Address knowledge gaps to better understand the magnitude (or severity) of threats and/or identify biological and socio-economic factors that may impede or assist recovery efforts.
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Long-term |
|
1.1 Assess demography of Bank Swallows across a range of habitats, landscapes, regions and years.
|
Threats:
|
| Critical | Long-term |
|
1.2 Coordinate research to examine the link between food supply (insects) and declines in the Bank Swallow population (and other aerial insectivores) in Ontario and elsewhere.
|
Threats:
|
| Critical | Short-term |
|
1.3 Identify, describe and quantify habitat characteristics of nest-sites, foraging areas and roost sites used in Ontario by Bank Swallows at multiple scales.
|
Threats:
|
| Critical | Short-term |
|
1.4 Determine the effects of the quality and/or quantity of foraging habitat on Bank Swallows.
|
Threats:
|
| Necessary | Short-term |
|
1.5 Collaborate with other jurisdictions and organizations to identify wintering areas and migratory routes and assess migratory connectivity and related threats.
|
Knowledge gaps:
|
| Necessary | Long-term |
|
1.6 Assess the effects of climate change and severe weather events on Bank Swallow survival and reproduction.
|
Threats:
|
| Beneficial | Short-term |
|
1.7 Investigate how public policies and socio-economic trends influence Bank Swallow abundance, distribution, habitat, and recovery efforts.
|
Threats:
|
Objective 2: Protect habitat and reduce or mitigate potential threats through stewardship, communication, education and outreach, and habitat management.
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term, but ongoing evaluation |
|
2.1 Work with relevant industries, agencies and organizations to develop, promote, implement, evaluate and refine BMPs for the management of Bank Swallow nesting sites specifically in aggregate pits and quarries, road cut embankments, and construction sites.
|
Threats:
|
| Critical | Short-term |
|
2.2 Coordinate the sharing of information on BMPs and the effectiveness of mitigation measures.
|
Knowledge gaps:
|
| Critical | Short-term |
|
2.3 Develop and evaluate guidelines for structural and material design, placement and management of artificial Bank Swallow nest sites.
|
Threats:
|
| Critical | Ongoing |
|
2.4 Encourage regulatory agencies including the Ontario Ministry of Natural Resources and Forestry, and Environment and Climate Change Canada to recognize and promote stewardship activities, safe harbour agreements, BMPs and incentive programs as an effective approach to the protection of Bank Swallow nest sites and habitats.
|
Threats:
|
Objective 3: Inventory, monitor and report on the state of Bank Swallow populations and habitats in Ontario and elsewhere to track the progress of recovery activities.
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Long-term |
|
3.1 Inventory and assess roost sites used by Bank Swallows (and other swallow species) during the post-breeding period.
|
Threats:
|
| Critical | Short-term |
|
3.2 Complete a baseline assessment of the current state of Bank Swallow breeding habitats, as well as occupancy or use, across Ontario.
|
Threats:
|
| Necessary | Ongoing |
|
3.3 Continue to monitor Bank Swallow population trends in Ontario to determine effectiveness of recovery actions.
|
Threats:
|
| Beneficial | Long-term |
|
3.4 Design and implement a long-term intensive Bank Swallow demographic monitoring program across the Ontario breeding range.
|
Threats:
|
2.4 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of Natural Resources and Forestry on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the author will be one of many sources considered by the Minister when developing the habitat regulation for this species.
Bank Swallow habitat-use varies seasonally in Ontario including breeding habitat and non-breeding habitat. Habitat used exclusively during the breeding season includes the nest (nest burrow and nest cup material), the nest site (bank), and the surrounding open foraging habitat. The span of the breeding season is from about 1 May to 15 August (i.e., records from onset of nest building to latest reported fledging dates; Peck and James 1987, Cadman unpub. data 2011). Non-breeding habitat includes roost sites where Bank Swallows congregate at night, which may be used between about 15 April and 15 September (based on the frequency [>1%] of eBird checklists recording Bank Swallow in Ontario; eBird 2015). Roost sites are especially important as stopover sites during migration.
In developing a habitat regulation for Bank Swallow breeding habitat, the following should be considered.
- Despite severe population declines, the Bank Swallow is still relatively common and widespread in much of Ontario, although the distribution of breeding birds is highly clumped due to the colonial habits of this species (Sandilands 2007).
- About 95 percent of the Bank Swallow population is composed of individuals between one and three years old. Therefore a timeframe of three years is used throughout the descriptions of areas recommended for habitat regulation.
- Available information suggests that the majority of Bank Swallows in Ontario nest in aggregate pits and quarries, and lakeshore bluffs of Lakes Erie and Ontario (Browning and Cadman unpub. data 2015). Very few rivers have suitable nesting banks and therefore relatively few colonies exist in riparian habitat (Browning and Cadman unpub. data 2015). A notable exception is the Saugeen River (Cadman and Lebrun-Southcott 2013).
- Bank Swallows exhibit high site fidelity to nest sites (Garrison 1999) and so damage or destruction of nest sites may be detrimental to reproductive output. Since many nest sites are naturally ephemeral (Garrison 1999), the species presumably also has high dispersal potential. It is unknown how displaced Bank Swallows might disperse and what direct and indirect reproductive impacts are caused by this process.
- Bank Swallow nest sites require a vertical (i.e., at least a 70 degree slope), eroding bank composed of fine sands, silt, loose clay or other erodible substrates (Garrison 1999). Erosion or excavation via machinery maintains nesting banks by refreshing the vertical surface, maintaining bank height and removing talus slumping (e.g., Ghent 2001a). At natural nest sites, erosion control measures (at the nest site or upstream or up-current) or declines in water levels of lakes or rivers at or near nesting banks could result in habitat degradation.
- It is not known if nest site availability is limiting the recovery of this species.
- The nest of the Bank Swallow is placed inside a burrow. Through natural erosion, the nest and burrow is often eroded away between nesting seasons (Garrison 1999, Wright et al. 2011). Therefore a new burrow is generally excavated each nesting season.
- Bank Swallow nests are protected under the federal Migratory Birds Convention Act, 1994 and the Ontario Endangered Species Act, 2007.
- Limited information is known about the foraging habitat used by Bank Swallows, other than breeding birds mostly forage in open habitats up to 1000 metres from the colony (Turner 1980, Appendix B:).
- Unguided attempts to regulate and protect Bank Swallow nests and breeding habitat in aggregate pits and quarries could impede stewardship efforts and recovery for the species. Approaches to foster industry leadership in Bank Swallow conservation may be beneficial.
- Mechanisms are in place in Ontario to ensure that stewardship and socio-economic factors can be considered when implementing habitat regulations (e.g., pits and quarries exemption to ESA Regulation 242/08 through development of BMPs) and through policy decisions regarding the implementation of the ESA legislation.
In developing a habitat regulation for Bank Swallow roosting habitat, the following should be considered:
- The location, size, seasonality, duration and significance of most Bank Swallow roosts in Ontario are not known at present.
- Roost sites are used primarily during the post-breeding and migratory periods.
It is recommended that until knowledge gaps are addressed, the following areas should be considered in developing a habitat regulation:
- Nest sites occupied at least once within the last three breeding seasons. The nest site encompasses a buffered distance of 50 metres out from the extent of the colony.
- Foraging habitat includes any open terrestrial or aquatic habitats within 1000 metres of a colony that have been used by foraging birds during the breeding season at least once within the last three breeding seasons. Aquatic habitats (e.g., wetlands, lakeshore) within the foraging habitat may be especially significant as a source of emergent aerial insects (i.e., food supply).
- Nocturnal roost sites that are used regularly by any number of Bank Swallows. Regular use would be defined as roosting on more than one night per year in at least two years within the past three years. This habitat should be protected throughout the year and should continue to be protected for three years after the last record of use. The extent or boundary of regulated habitat at a roost site should be defined on a case-by-case basis, but should include the areas that are directly used (e.g., as perches or cover) by roosting birds, plus the open air space they use to enter the site. Use of ecosite polygons, as defined by the most current Ecological Land Classification scheme for Ontario or the Ontario Wetland Evaluation System, may be appropriate tools for delineating the boundaries of wetlands associated with roost sites.
Glossary
- Aerial insectivore:
- The suite of bird species that primarily feed on flying insects, especially aerial-foraging birds that catch insects while in flight.
- Borrow pit:
- Term used in construction and civil engineering describing an area where material (usually soil, gravel or sand) has been dug for use at another location. Borrow pits can be found close to many major construction projects or are sometimes used for waste disposal/landfills.
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC):
- The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
- Committee on the Status of Species at Risk in Ontario (COSSARO):
- The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
- Conservation status rank:
- A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. Ranks are determined by NatureServe and, in the case of Ontario’s S-rank, by Ontario’s Natural Heritage Information Centre. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperilled
2 = imperilled
3 = vulnerable
4 = apparently secure
5 = secure
NR = not yet ranked - Credible intervals (lower and upper limits):
- The credible intervals are a statistical measure of the precision of the population trend. In the case of the Breeding Bird Survey trends provided in this report, given the data and the accuracy of the model, there is a 95 percent probability that the average annual trend in the population lies somewhere between the lower and upper credible intervals (i.e., for Bank Swallow in Ontario the BBS data for 2002-2012 indicate there is a 95% probability that the average annual population change is somewhere between -8.42% and -2.03%). The credible interval is similar in concept to the more familiar confidence interval, but based on Bayesian statistics.
- Diurnal:
- Active during the daytime (rather than at night).
- Ecological Land Classification scheme:
- The Ontario Ecological Land Classification (ELC) scheme is a hierarchical system for consistently defining ecological units on the basis of bedrock, climate, geography and vegetation that is widely used for land use and conservation planning in Ontario.
- Endangered Species Act, 2007 (ESA):
- The provincial legislation that provides protection to species at risk in Ontario.
- Fidelity rate:
- Proportion of surviving birds returning to the home (previous year’s nest site) or natal area compared to surviving birds returning elsewhere.
- Incidental take:
- The inadvertent harming, killing, disturbance or destruction of migratory birds, nests and eggs.
- Nest success:
- Successful fledging of at least one individual from a nest.
- Philopatric:
- The tendency of an individual to remain or return to its home area (e.g., a bird that returns to the same area to nest). See return rate.
- Population source-sink dynamics:
- The concept of how spatial variation in habitat quality affects vital rates and the growth or decline of a population at various scales. Population sources are occupied habitats that produce surplus young whereas population sinks are occupied habitats where productivity is insufficient to offset annual mortality. Persistence of sink populations is dependent on immigration.
- Plumage:
- The pattern, colour and arrangement of feathers covering a bird.
- Return rate:
- Proportion of banded birds observed or recaptured in subsequent years at the same site where they were originally banded. Between year dispersal, survival and recapture effort can influence/bias return rate estimates.
- Safe harbour instruments:
- Instruments that enable landowners to assist in the protection or recovery of species at risk by creating (or enhancing in certain cases) an area that is not currently the habitat of a species at risk while providing the legal assurances that they may modify the habitat in the future, provided all conditions of the instrument have been met.
- Socially monogamous:
- Common avian mating system where individuals form pair bond with single member of opposite sex and jointly raise young (including extra-pair young sired by other males).
- Species at Risk Act (SARA):
- The federal legislation that provides protection to species at risk in Canada. This act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
- Species at Risk in Ontario (SARO) List:
- The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
- Survival rate:
- Proportional estimate calculated from the number of birds surviving at the end of a defined time period (usually by year) divided by the number alive at the beginning of the period. This estimate can be modified to incorporate the effects of dispersal and recapture effort.
- Vital rates:
- Demographic statistics that determine population growth including productivity (e.g., number of young produced per female per year or lifetime), survivorship (e.g., proportion of adults and young that survive from one year to the next), immigration (e.g., number of yearlings recruited to a breeding population) and emigration (e.g., number of yearlings that disperse to breed elsewhere).
References
Altus Group. 2009. State of the Aggregate Resource in Ontario Study Paper 1: Aggregate Consumption and Demand. Altus Group Economic Consulting. Toronto. 77 pp.
Alves, M.A.S. 1997. Effects of ectoparasites on the Sand Martin Riparia riparia nestlings. Ibis 139:494-496.
Bartzen, B.A., K.W. Dufour, R.G. Clark, and F.D. Caswell. 2010. Trends in agricultural impact and recovery of wetlands in prairie Canada. Ecological Applications 20:525-538.
Basili, G.D., and S.A. Temple. 1999. Winter ecology, behavior, and conservation needs of Dickcissels in Venezuela. Studies in Avian Biology 19:289-299.
Bayliss, John, pers. comm. 2015. Written communication with M. Falconer (BSC). Comments provided during jurisdictional review of draft recovery strategy. January 2015. Manager, Environment. Holcim (Canada) Inc., Concord, Ontario.
Bedford, B.L. 1999. Cumulative effects on wetland landscapes: Links to wetland restoration in the United States and southern Canada. Wetlands 19:775-788.
Bell, David, pers. comm. 2012. Verbal and email correspondence with M. Falconer (BSC). Several dates between July 2011 and August 2012. Bank Swallow Project Species at Risk Intern (Summer 2011-2012), Bird Studies Canada.
Bird Studies Canada and NABCI. 2014. Bird Conservation Regions. Published by Bird Studies Canada on behalf of the North American Bird Conservation Initiative. [website accessed August 2015].
Blancher, P.J., M.D. Cadman, B.A. Pond, A.R. Couturier, E.H. Dunn, C.M. Francis, and R.S. Rempel. 2007. Changes in bird distributions between atlases. Pp. 32-48 in M.D. Cadman, D.A. Sutherland, G.G. Beck, D. Lepage, and A.R. Couturier (eds.). Atlas of the Breeding Birds of Ontario, 2001-2005. Bird Studies Canada, Environment Canada, Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature, Toronto, xxii + 706 pp.
Bolin, Bruce, pers. comm. 2013. Email correspondence with M. Falconer (BSC). August 2013. Otter Valley Field Naturalists, Port Burwell, Ontario.
Both, C., C.A.M. Van Turnhout, R.G. Bijlsma, H. Siepel, A.J. Van Strien, and R.P.B. Foppen. 2010. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proceedings of the Royal Society B 277:1259-1266.
Bouland, A.J., A.E. White, K.P. Lonabaugh, C.W. Varian‐Ramos, and D.A. Cristol. 2012. Female-biased offspring sex ratios in birds at a mercury contaminated river. Journal of Avian Biology 43:244-251.
Boutin, C., K.E. Freemark, and D.A. Kirk. 1999. Farmland birds in southern Ontario: field use, activity patterns and vulnerability to pesticide use. Agriculture, Ecosystems and Environment 72:239-254.
Brasso R.L., and D.A. Cristol. 2008. Effects of mercury exposure on the reproductive success of tree swallows (Tachycineta bicolor). Ecotoxicology 17:133-141.
Brewer D., A.W. Diamond, E.J. Woodsworth, B.T. Collins, and E.H. Dunn. 2000. Canadian Atlas of Bird Banding - Volume 1: Doves, Cuckoos, and Hummingbirds through Passerines, 1921-1995. Occasional Paper, Canadian Wildlife Service, Environment Canada.
Brown, C.R., and M.B. Brown. 1998. Intense natural selection on body size and wing and tail asymmetry in Cliff Swallows during severe weather. Evolution 52:1461-1475.
Browning, M., and M.D. Cadman. Unpublished data and observations. 2015. Bank Swallow burrow count surveys from a stratified random sample of aggregate pits and quarries across Ontario. (Cadman) Songbird Biologist, Environment and Climate Change Canada, Burlington, Ontario. (Browning) Research Scientist, Ontario Ministry of Natural Resources and Forestry, Peterborough, Ontario.
Browning, Mark, pers. comm. 2015. Written communication with M. Falconer (BSC). Comments provided during jurisdictional review of draft RS and preliminary estimates of Bank Swallow population breeding in aggregate pits and quarries. January and April 2015. Research Scientist, Ontario Ministry of Natural Resources and Forestry. Peterborough, Ontario.
Bryant, D.M., and A.K. Turner. 1982. Central place foraging by swallows (Hirundinidae): the question of load size. Animal Behaviour 30:845-856.
Bull, J. 1985. Birds of New York State. Cornell University Press, Ithaca, NY. 655 pp.
Burke, Tianna, pers. comm. 2014. Verbal communication with M. Falconer (BSC). Bank Swallow workshop, Guelph Arboretum, Guelph, Ontario. September 2014. M.Sc. Candidate, Trent University, Peterborough, Ontario.
Cadman, M.D., Unpublished data and observations. 2011. Bank Swallow aggregate pit surveys in Wellington County 2010 - 2011. Songbird Biologist, Environment and Climate Change Canada, Burlington, Ontario.
Cadman, M.D., Unpublished data and observations. 2012. Bank Swallow nest survival analysis in Wellington County aggregate pits 2011 - 2012. Songbird Biologist, Environment and Climate Change Canada, Burlington, Ontario.
Cadman, M.D., and Z. Lebrun-Southcott. 2013. Bank Swallow colonies along the Saugeen River 2009-2013. Ontario Birds 31:136-147.
Cadman, M.D., D.A. Sutherland, G.G. Beck, D. Lepage, and A. Couturier (eds.). 2007. Atlas of the Breeding Birds of Ontario, 2001-2005. Bird Studies Canada, Environment Canada, Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature, Toronto. xxii + 706 pp.
Cadman, M.D., P.F.J. Eagles, and F.M. Helleiner (eds.). 1987. Atlas of the Breeding Birds of Ontario. University of Waterloo Press, Waterloo, ON. 617 pp.
Cadman, Michael D., pers. comm. 2014. Numerous verbal communications and email correspondence to M. Falconer (BSC). September 2014. Also, communication with M. Falconer at Aerial Forager Workshop at Bird Studies Canada (March 2012) and at the Barn Swallow workshop, Guelph Arboretum, Guelph, Ontario (December 2012) and the Bank Swallow workshop, Guelph Arboretum, Guelph, Ontario (September 2014). Landbird Biologist, Canadian Wildlife Service – Ontario Region, Environment and Climate Change Canada, Burlington, Ontario.
Calvert, A.M. 2012. Research priorities to support the conservation of aerial insectivores in Canada. Unpublished report. Environment Canada and Bird Studies Canada. May 2012. 18 pp.
Campbell, R.W., N.K. Dawe, I. McTaggert-Cowan, J.M. Cooper, and G.W. Kaiser. 1997. The Birds of British Columbia. Vol. 3. University of British Columbia Press, Vancouver. 693 pp.
Cardoso, P., T.L. Erwin, P.A.V. Borges, and T.R. New. 2011. The seven impediments in invertebrate conservation and how to overcome them. Biological Conservation 144:2647-2655.
Chapman, L.J., and D.F. Putnam. 1984. The physiography of southern Ontario, Ontario Geological Survey, Special Vol. 2. Government of Ontario, Canada.
Collen, B., M. Böhm, R. Kemp, and J.E.M. Baillie. 2012. Spineless: status and trends of the world’s invertebrates. Zoological Society of London, United Kingdom. 88 pp.
Colwell, R.K., R.R. Dunn, and N.C. Harris. 2012. Coextinction and persistence of dependent species in a changing world. Annual Review of Ecology, Evolution, and Systematics 43:183-203.
Cooper, M.J., G.A. Lamberti, and D.G. Uzarski. 2014. Spatial and temporal trends in invertebrate communities of Great Lakes coastal wetlands, with emphasis on Saginaw Bay of Lake Huron. Journal of Great Lakes Research 40:168-182.
Cooper, N.W., T.W. Sherry, and P.P. Marra. 2015. Experimental reduction of winter food decreases body condition and delays migration in a long-distance migratory bird. Ecology 96:1933-1942.
COSEWIC. 2013. COSEWIC assessment and status report on the Bank Swallow Riparia riparia in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. ix + 48 pp.
Cowley, E., and G.M. Siriwardena. 2005. Long-term variation in survival rates of Sand Martins Riparia riparia: dependence on breeding and wintering ground weather, age and sex, and their population consequences. Bird Study 52:237-251.
Cramp, S., D.J. Brooks, E. Dunn, R. Gillmor, and J. Hall-Craggs. 1988. The birds of the western Palearctic, Vol. 5: tyrant flycatchers to thrushes. Oxford University Press, Oxford, UK. 1136 pp.
Cristol, D.A., R.L. Brasso, A.M. Condon, R.E. Fovargue, S.L. Friedman, K.K. Hallinger, A.P. Monroe, and A.E. White. 2008. The movement of aquatic mercury through terrestrial food webs. Science 320:335.
Daigle, R., D. Forbes, G. Parkes, H. Ritchie, T. Webster, D. Bérubé, A. Hanson, L. DeBaie, S. Nichols, and L. Vasseur (eds.). 2006. Impacts of sea-level rise and climate change on the coastal zone of southeastern New Brunswick. Project Report. Environment Canada, Dartmouth, Nova Scotia, Canada, ISBN 0662439473, Cat. No.: En8445/2006E, EPSM773 (also available on CDROM). 644 pp. + 5 appendices.
Dale, S. 2001. Necrophilic behaviour, corpses as nuclei of resting flock formation, and road-kills of Sand Martins Riparia riparia. Ardea 89:545-547.
Dionne, M., C. Maurice, J. Gauthier, and F. Shaffer. 2008. Impact of Hurricane Wilma on migrating birds: The case of the Chimney Swift. Wilson Journal of Ornithology 120:784-792.
Dirzo, R., H.S. Young, M. Galetti, G. Ceballos, N.J. Isaac, and B. Collen. 2014. Defaunation in the anthropocene. Science 345:401-406.
Douglas, M.R., and J.F. Tooker. 2015. Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in U.S. field crops. Environmental Science and Technology 49:5088-5097.
Ducks Unlimited. 2010. Southern Ontario wetland conversion analysis: final report. Ducks Unlimited Canada. Barrie, Ontario. 51 pp. [website accessed February 2015].
Dunn, P.O., and D.W. Winkler. 1999. Climate change has affected breeding date of Tree Swallows throughout North America. Proceedings of the Royal Society of London, Series B 266:2487-2490.
Dunn, P.O., D.W. Winkler, L.A. Whittingham, S.J. Hannon, and R.J. Robertson. 2011. A test of the mismatch hypothesis: How is timing of reproduction related to food abundance in an aerial insectivore? Ecology 92:450-461.
eBird. 2015. eBird: An online database of bird distribution and abundance [web application]. eBird, Cornell Lab of Ornithology, Ithaca, New York. [website accessed February 2015].
Environment Canada. 2013. Enforcement Notifications. 2012. Yacht Club Fined for Destruction of Bank Swallow Nests. [website accessed October 2014].
Environment Canada. 2014a. North American Breeding Bird Survey - Canadian Trends Website, Data-version 2012. Environment Canada, Gatineau, Quebec. Web site: Breeding Bird Survey Results [accessed October 2014].
Environment Canada. 2014b. Definitions - North American Breeding Bird Survey. Environment Canada, Gatineau, Quebec. [website accessed February 2015].
Erskine, A.J. 1979. Man’s influence on potential nesting sites and populations of swallows in Canada. Canadian Field-Naturalist 93:371-377.
Ewins, P.J., P.D. Round, and D.R. Bazely. 1991. Urban roosting of Barn Swallows Hirundo rustica wintering in Thailand. Forktail 6:68-70.
Fain, A., and K.E. Hyland. 1962. The mites parasitic in the lungs of birds. The variability of Sternostoma tracheacolum Lawrence, 1948, in domestic and wild birds. Parasitology 52:401-424.
Falconer, M., Unpublished data and observations. 2011. Counts of roosting Bank Swallows at Long Point, Ontario during July 2011. Project Biologist, Bird Studies Canada, Port Rowan, Ontario.
Falconer, M., Unpublished data and observations. 2013. Bank Swallow nest survival analysis on Lake Erie bluffs 2011 - 2013. Project Biologist, Bird Studies Canada, Port Rowan, Ontario.
Falconer, M., Unpublished data and observations. 2014. Bank Swallow burrow count analysis from Lake Erie 2010 – 2014. Project Biologist, Bird Studies Canada, Port Rowan, Ontario.
Falconer, C.M., G.W. Mitchell, P.D. Taylor, and D.C. Tozer. In press. Prevalence of disjunct roosting in nesting Bank Swallows. Wilson Journal of Ornithology.
FPTGC (Federal, Provincial and Territorial Governments of Canada). 2010. Canadian Biodiversity: Ecosystem Status and Trends 2010. Canadian Councils of Resource Ministers. Ottawa, Ontario. vi + 142 pp.
Freemark, K.E., and D.A. Kirk. 2001. Birds on organic and conventional farms in Ontario: partitioning effects of habitat and practices on species composition and abundance. Biological Conservation 101:337-350.
Freer, V.M. 1977. Colony structure and function in the Bank Swallow, Riparia riparia L. Ph.D. thesis. State University of New York, Binghamton. 312 pp.
Freer, V.M. 1979. Factors affecting site tenacity in New York Bank Swallows. Bird-Banding 50:349-357.
Garcia, D. 2009. Spatial and temporal patterns of the Bank Swallow on the Sacramento River. Master’s Thesis. California State University, Chico, CA. 94 pp.
Garrison, B.A. 1999. Bank Swallow (Riparia riparia), The Birds of North America Online (A. Poole, Ed.). Ithaca: Cornell Lab of Ornithology; retrieved from the Birds of North America Online. doi:10.2173/bna.414 [website accessed October 2014].
Ghent, A.W. 2001a. Importance of a low talus in location of Bank Swallow (Riparia riparia) colonies. American Midland Naturalist 146:447-449.
Ghent, A.W. 2001b. Regular spatial patterns of bank swallow (Riparia riparia) tunnel entrances, with some possible evolutionary implications. American Midland Naturalist 146:414-423.
Ghilain, A., and M. Bélisle. 2008. Breeding success of Tree Swallows along a gradient of agricultural intensification. Ecological Applications 18:1140-1154.
Gibbons, D., C. Morrissey, and P. Mineau. 2015. A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environmental Science and Pollution Research 22:103-118.
Girard, J., P. Mineau, and L. Fahrig. 2014. Higher nestling food biomass in organic than conventional soybean fields in eastern Ontario, Canada. Agriculture, Ecosystems and Environment 189:199-205.
Gronewold, A.D., A.H. Clites, J.P. Smith, and T.S. Hunter. 2013. A dynamic graphical interface for visualizing projected, measured, and reconstructed surface water elevations on the earth’s largest lakes. Environmental Modelling and Software 49:34–39.
Haas, G.E., T. Rumfelt, and N. Wilson. 1980. Fleas (Siphonaptera) from nests and burrows of the Bank Swallow (Riparia riparia) in Alaska. Northwest Science 54:210-215.
Hallinger K.K., and D.A. Cristol. 2011. The role of weather in mediating the effect of mercury exposure on reproductive success in tree swallows. Ecotoxicology 20:1368-1377.
Hallinger K.K., K.L. Cornell, R.L. Brasso, and D.A. Cristol. 2011. Mercury exposure and survival in free-living tree swallows (Tachycineta bicolor). Ecotoxicology 20:39-46.
Hallmann, C.A., R.P.B. Foppen, C.A.M. van Turnhout, H. de Kroon, and E. Jongejans. 2014. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 511:341-343.
Hawley, D.M., K.K. Hallinger, and D.A. Cristol. 2009. Compromised immune competence in free-living tree swallows exposed to mercury. Ecotoxicology 18:499-503.
Hayes, F.E., S.M. Goodman, J.A. Fox, T.G. Tamayo, and N.E. Lopez. 1990. North American bird migrants in Paraguay. Condor 92:947-960.
Heneberg, P. 2001. Size of sand grains as a significant factor affecting the nesting of bank swallows (Riparia riparia). Biologia Bratislava 56:205-210.
Heneberg, P. 2003. Soil particle composition affects the physical characteristics of Sand Martin Riparia riparia holes. Ibis 145:392-399.
Heneberg, P. 2007. Sand martin (Riparia riparia) in the Czech Republic at the turn of the millenium. Biologiezentrum Linz 39:293-312.
Heneberg, P. 2009. Soil penetrability as a key factor affecting the nesting of burrowing birds. Ecological Restoration 24:453-459.
Heneberg, P. 2013. Burrowing bird’s decline driven by EIA over-use. Resources Policy 38:542–548.
Hess, P.J., C.G. Zenger, and R.A. Schmidt. 2008. Weather-related tree swallow mortality and reduced nesting effort. Northeastern Naturalist 15:630-631.
Hickman, G.R. 1979. Nesting ecology of Bank Swallows in interior Alaska. Master’s Thesis. University of Alaska, Fairbanks. 78 pp.
Hjertaas, D.G. 1984. Colony site selection in Bank Swallows. Master’s Thesis. University of Saskatchewan, Saskatoon. 129 pp.
Holmes, P.R., S.E. Christmas, and A.J. Parr. 1987. A study of the return rate and dispersal of Sand Martins Riparia riparia at a single colony. Bird Study 34:12-19.
Hopkins, L. 2001. Best practice guidelines: artificial bank creation for sand martins and kingfishers (link no longer active). The Environment Agency, London, UK. 29 pp. [website accessed October 2014].
Hurlbert, A.H., and Z. Liang. 2012. Spatiotemporal variation in avian migration phenology: citizen science reveals effects of climate change. PLoS One 7:e31662.
Hussell, D.J.T. 2003. Climate change, spring temperatures, and timing of breeding of Tree Swallows (Tachycineta bicolor) in southern Ontario. Auk 120:607-618.
Javorek, S.K., R. Antonowitsch, C. Callaghan, M. Grant, and T. Weins. 2007. Changes to wildlife habitat on agricultural land in Canada, 1981–2001. Canadian Journal of Soil Science 87:225-233.
Jobin B., C. Latendresse, M. Grenier, C. Maisonneuve, and D. Sebbane. 2010. Recent landscape change at the ecoregion scale in Southern Quebec (Canada): 1993-2001. Environmental Monitoring and Assessment 164:631-647.
John, R.D. 1991. Observations on soil requirements for nesting bank swallows, Riparia riparia. Canadian Field-Naturalist 105:251-254.
Johnson, S.L. 2006. Song learning and syntax patterns in the American Robin and the soil characteristics of Bank Swallow nest sites. Ph.D. Thesis. University of Massachusetts, Amherst. 127 pp.
Jones, G. 1987. Colonization patterns in Sand Martins, Riparia riparia. Bird Study 34:20-25.
Jones, T., and W. Cresswell. 2010. The phenology mismatch hypothesis: are declines of migrant birds linked to uneven global climate change? Journal of Animal Ecology 79:98-108.
Keller, R.H., L. Xie, D.B. Buchwalter, K.E. Franzreb, and T.R. Simons. 2014. Mercury bioaccumulation in Southern Appalachian birds, assessed through feather concentrations. Ecotoxicology 23:304-316.
Knee, W., H. Proctor, and T. Galloway. 2008. Survey of nasal mites (Rhinonyssidae, Ereynetidae, and Turbinoptidae) associated with birds in Alberta and Manitoba, Canada. Canadian Entomologist 140:364-379.
Kokko, H., and W.J. Sutherland. 1998. Optimal floating and queuing strategies: consequences for density dependence and habitat loss. American Naturalist 152:354-366.
Kragten, S., W.L. Tamis, E. Gertenaar, S.M. Midcap Ramiro, R.J. van der Poll, J. Wang, and G.R. de Snoo. 2011. Abundance of invertebrate prey for birds on organic and conventional arable farms in the Netherlands. Bird Conservation International 21:1-11.
Kuhnen, K. 1985. On pair-formation in the Sand Martin, Riparia riparia. Journal of Ornithology 126:1-13.
Latendresse, C., B. Jobin, A. Baril, C. Maisonneuve, C. Boutin and D. Côté. 2008. Dynamique spatiotemporelle des habitats fauniques dans l'écorégion des Basses terres du fleuve Saint-Laurent, 1950-1997. Série de rapports techniques no 494, Environnement Canada, Service canadien de la faune, région du Québec, Québec. 83 p. et annexes.
Leung, M., Unpublished data and observations. 2010. Bank Swallow burrow count surveys on the north shore of Lake Ontario 2010. Environmental Advisor, Ontario Power Generation, Pickering, Ontario.
Leung, Marianne, pers. comm. 2014. Verbal communication with M. Falconer (BSC). Guelph Arboretum, Guelph, Ontario. September 2014. Environmental Advisor, Ontario Power Generation, Pickering, Ontario.
Lind, B-B., J. Stigh, and L. Larsson. 2002. Sediment type and breeding strategy of the Bank Swallow Riparia riparia in western Sweden. Ornis Svecica 12:157-163.
MacBriar, Jr., W.N., and D.E. Stevenson. 1976. Dispersal and survival in the Bank Swallow (Riparia riparia) in southeastern Wisconsin. Milwaukee Public Museum Contributions in Biology and Geology, no. 10. 14 pp.
Mason, R., H. Tennekes, F. Sánchez-Bayo, and P.U. Jepsen. 2013. Immune suppression of neonicotinoid insecticides at the root of global wildlife declines. Journal of Environmental Immunology and Toxicology 1:3-12.
May, Stephen, pers. comm. 2015. Written communication with M. Falconer (BSC). Comments provided during jurisdictional review of draft recovery strategy. January 2015. Lands Manager, Canada Building Materials, St. Mary’s, Ontario.
McDonald, Karen, pers. comm. 2014. Verbal communication with M. Falconer (BSC). Bird Studies Canada Annual General Meeting, Port Rowan, Ontario. September 2014. Project Manager, Toronto and Region Conservation Authority, Toronto, Ontario.
McGee, B., H. Berges, and D. Beaton. 2010. Survey of pesticide use in Ontario, 2008. Ontario Ministry of Agriculture, Food and Rural Affairs. Toronto, Ontario. 18 pp.
Mead, C.J. 1979. Mortality and causes of death in British Sand Martins. Bird Study 26:107-112.
Mead, C.J., and J.D. Harrison. 1979. Sand Martin movements within Britain and Ireland. Bird Study 26:73-86.
Michel, N.L., A.C. Smith, R.G. Clark, C.A. Morrissey, and K.A. Hobson. In press. Differences in spatial synchrony and interspecific concordance inform guild-level population trends for aerial insectivorous birds. Ecography. DOI: 10.1111/ecog.01798.
Mickelson, D.M., T.B. Edil, and D.E. Guy. 2004. Erosion of coastal bluffs in the Great Lakes. In: Hampton, M.A., and G.B. Griggs (eds.). Formation, evolution, and stability of coastal cliffs: status and trends. U.S. Geological Survey Professional Paper 1693, pp. 7-38.
Mineau, P., and C. Palmer. 2013. The impact of the nation’s most widely used insecticides on birds. Report prepared for the American Bird Conservancy, March 2013. 97 pp.
Mineau, P., and M. Whiteside. 2013. Pesticide acute toxicity is a better correlate of U.S. grassland bird declines than agricultural intensification. PLoS One 8:e57457.
MMAH. 2005. Provincial Policy Statement. Ministry of Municipal Affairs and Housing, Toronto, Ontario. 38 pp.
Moffatt, K.C., E.E. Crone, K.D. Holl, R.W. Schlorff, and B.A. Garrison. 2005. Importance of hydrologic and landscape heterogeneity for restoring Bank Swallow (Riparia riparia) colonies along the Sacramento River, California. Restoration Ecology 13:391-402.
Morrissey, C.A., P. Mineau, J.H. Devries, F. Sanchez-Bayo, M. Liess, M.C. Cavallaro, and K. Liber. 2015. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: a review. Environment international 74: 291-303.
Nakano, D., T. Akasaka, A. Kohzu, and F. Nakamura. 2007. Food sources of Sand Martins Riparia riparia during their breeding season: insights from stable-isotope analysis. Bird Study 54:142-144.
Neave, E., and D. Baldwin. 2011. Mixedwoods Plain and Southern Boreal Shield Open Country Birds Habitat Assessment: History and Trends. Unpublished report to Environment Canada, Canadian Wildlife Service – Ontario Region. Downsview, Ontario. 75 pp.
Nebel, S., A.M. Mills, J.D. McCracken, and P.D. Taylor. 2010. Declines of aerial insectivores in North America follow a geographic gradient. Avian Conservation and Ecology - Écologie et conservation des oiseaux. 5:1 [website accessed October 2014].
Newton, I. 2007. Weather-related mass-mortality events in migrants. Ibis 149:453-467.
OBC. 2011. Ontario’s Biodiversity Strategy, 2011: Renewing our Commitment to What Sustains Us. Ontario Biodiversity Council, Peterborough, Ontario. [website accessed October 2014].
OMNR. 2010. Forest management guide for conserving biodiversity at the stand and site scales. Ontario Ministry of Natural Resources, Toronto, Ontario. 211 pp. [website accessed February 2015].
ONRS. 2014. Ontario Nest Record Scheme (electronic database). Royal Ontario Museum and Bird Studies Canada. [website accessed October 2014].
OSSGA. 2013. Bank Swallow fact sheet: guidance for aggregate producers. Ontario Stone, Sand, and Gravel Association. 2 pp.
Ottawa River Legacy Landmark Partners. 2013. Pembroke: Canada’s Capistrano. Canada Heritage River - Ottawa River. [website accessed March 2013].
Pady, Lynn, pers. comm. 2015. Email correspondence with M. Falconer (BSC). February 2015. Toronto Field Naturalists, Toronto, Ontario.
Partners in Flight Science Committee. 2013. Population Estimates Database, version 2013. [website accessed October 2014].
Pavlova, A., R.M. Zink, S.V. Drovetski, and S. Rohwer. 2008. Pleistocene evolution of closely related sand martins Riparia riparia and R. diluta. Molecular Phylogenetics and Evolution 48:61-73.
Peck, G.K., and R.D. James. 1987. Breeding birds of Ontario: nidiology and distribution, Vol. 2: passerines. Royal Ontario Museum. Life Sciences Misc. Publication. Toronto, Ontario. 387 pp.
Pence, D.B. 1975. Keys, species and host list, and bibliography for nasal mites of North American birds (Acarina: Rhinonyssinae, Turbinoptinae, Speleognathinae, and Cytoditidae). Special Publications of the Museum Texas Tech University 8:1-148.
Persson, C. 1987. Age structure, sex ratios and survival rates in a south Swedish Sand Martin (Riparia riparia) population, 1964 to 1984. Journal of Zoology Series B 1:639-670.
Petersen, A.J. 1955. The breeding cycle in the Bank Swallow. Wilson Bulletin 67:235-286.
Petersen, P.C., and A.J. Mueller. 1979. Longevity and colony loyalty in Bank Swallows. Bird-Banding 50:69-70.
Pisa, L.W., V. Amaral-Rogers, L.P. Belzunces, J.M. Bonmatin, C.A. Downs, D. Goulson, D.P. Kreutzweiser, C. Krupke, M. Liess, M. McField, C.A. Morrissey, D.A. Noome, J. Settele, N. Simon-Delso, J.D. Stark, J.P. Van der Sluijs, H. Van Dyck, and M. Wiemers. 2015. Effects of neonicotinoids and fipronil on non-target invertebrates. Environmental Science and Pollution Research 22:68-102.
Pyle, P. 1997. Identification guide to North American birds: Part I Columbidae to Ploceidae. Slate Creek Press, Bolinas, CA. 732 pp.
Rioux Paquette, S., D. Garant, F. Pelletier, and M. Bélisle. 2013. Seasonal patterns in tree swallow prey (Diptera) abundance are affected by agricultural intensification. Ecological Applications 23:122-133.
Rioux Paquette, S., F. Pelletier, D. Garant, and M. Bélisle. 2014. Severe recent decrease of adult body mass in a declining insectivorous bird population. Proceedings of the Royal Society of London B: Biological Sciences 281(1786):20140649.
Robinson, Cynthia, pers. comm. 2015. Written communication with M. Falconer (BSC). Comments provided during jurisdictional review of draft recovery strategy. January 2015. Environment and Education Manager. Ontario Sand, Stone, and Gravel Association. Mississauga, Ontario.
Ross, R.K., W.R. Clark, and J.M. Bouvier. 1984. Survey of a major swallow roost in Pembroke. Ontario Birds 2:34-37.
Sabrosky, C.W., G.F. Bennett, and T.L. Whitworth. 1989. Bird blow flies (Protocalliphora) in North America (Diptera: Calliphoridae) with notes on the Palearctic species. Smithsonian Institute Press, Washington, D.C. 312 pp.
Sandilands, A. 2007. Bank Swallow. Pp. 394-395 in M.D. Cadman, P.F.J. Eagles, and F.M. Helleiner (eds.): Atlas of the Breeding Birds of Ontario. Bird Studies Canada, Environment Canada, Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature, Toronto. xxii + 706 pp.
Sauer, J.R., J.E. Hines, J.E. Fallon, K.L. Pardieck, D.J. Ziolkowski, Jr., and W.A. Link. 2014. The North American Breeding Bird Survey, Results and Analysis 1966 - 2012. Version 02.19.2014 USGS Patuxent Wildlife Research Center, Laurel, MD.
Schlorff, R.A. 1992. Recovery Plan: Bank Swallow (Riparia riparia). Nongame Bird and Mammal Section Wildlife Management Division, Report 9302. California Department of Fish and Game: Sacramento, CA. 16 pp.
Shutler, D., D.J.T. Hussell, D.R. Norris, D.W. Winkler, R.J. Robertson, F. Bonier, W.B. Rendell, M. Bélisle, R.G. Clark, R.D. Dawson, N.T. Wheelwright, M.P. Lombardo, P.A. Thorpe, M.A. Truan, R. Walsh, M.L. Leonard, A.G. Horn, C.M. Vleck, D. Vleck, A.P. Rose, L.A. Whittingham, P.O. Dunn, K.A. Hobson, and M.T. Stanback. 2012. Spatiotemporal patterns in nest box occupancy by Tree Swallows across North America. Avian Conservation and Ecology 7:3.
Silver, M., and C.R. Griffin. 2009. Nesting habitat characteristics of Bank Swallows and Belted Kingfishers on the Connecticut River. Northeastern Naturalist 16:519-534.
Smith A.C., M-A.R. Hudson, C.M. Downes, and C.M. Francis. 2015. Change points in the population trends of aerial-insectivorous birds in North America: synchronized in time across species and regions. PLoS ONE 10(7):e0130768.
Smith, Adam C., pers. comm. 2015. Email correspondence with Myles Falconer (BSC). Discussion of the gradually lessening of the severity of short-term BBS trends for Bank Swallow in Ontario. April 2015. Senior Biostatistician, Environment and Climate Change Canada, Ottawa, Ontario.
Soster, F.M., P.L. McCall, and K.A. Herrmann. 2011. Decadal changes in the benthic invertebrate community in western Lake Erie between 1981 and 2004. Journal of Great Lakes Research 37:226-237.
Spencer, S.J. 1962. A study of the physical characteristics of nesting sites used by Bank Swallows. Ph.D. Thesis. Pennsylvania State University, University Park. 105 pp.
Statistics Canada. 2012. 2011 Census of Agriculture: Farm and farm operator data, release date May 10, 2012. [website accessed October 2014].
Stringer, A.P., and W. Linklater. 2014. Everything in moderation: principles of parasite control for wildlife conservation. BioScience 64:932-937.
Szabó, Z.D., and T. Szép. 2010. Breeding dispersal patterns within a large Sand Martin (Riparia riparia) colony. Journal of Ornithology 151:185-191.
Szép, T. 1990. Estimation of abundance and survival rate from capture-recapture data of Sand Martin (Riparia riparia) ringing. Ring 13:205-214.
Szép, T. 1995. Survival rates of Hungarian Sand Martins and their relationship with Sahel rainfall. Journal of Applied Statistics 22:891-904.
Szép, T. 1999. Effects of age- and sex-biased dispersal on the estimation of survival rates of the Sand Martin Riparia riparia population in Hungary. Bird Study 46:169-177.
Taylor, L.R. 1963. Analysis of the effect of temperature on insects in flight. Journal of Animal Ecology 32:88–117.
Todd, W.E.C. 1963. The Birds of the Labrador Peninsula and Adjacent Areas. University Toronto Press, Toronto. 819 pp.
Tozer, D.C., and S. Richmond. 2013. Bank Swallow research and monitoring: 2013 final report. Unpublished report to Ontario Power Generation. 27 pp.
TRCA. 2014. History of flood control in the TRCA. Toronto and Region Conservation Authority, Toronto. [website accessed October 2014].
Turner, A. 1980. The use of time and energy by aerial feeding birds. Ph.D. Thesis, Department of Biology, University of Stirling, UK. 353 pp.
Turner, A.K. 2004. Family Hirundinidae (Swallows and Martins). Pp. 602-685 in Del Hoyo, J., A. Elliott, and D.A. Christie (eds). Handbook of the Birds of the World. Volume 9. Cotingas to pipits and wagtails. Lynx Edicions, Barcelona.
Turner, A.K., and C. Rose. 1989. Swallows and martins an identification guide and handbook. Houghton Mifflin Co., Boston. 258 pp.
Wada, H., D.A. Cristol, F.M.A. McNabb, and W.A. Hopkins. 2009. Suppressed adrenocortical responses and thyroid hormone levels in birds near a mercury-contaminated river. Environmental Science and Technology 43:6031-6038.
Whitworth, T.L., and G.F. Bennett. 1992. Pathogenicity of larval Protocalliphora (Diptera: Calliphoridae) parasitizing nestling birds. Canadian Journal of Zoology 70:2184-2191.
Williams, C.B. 1961. Studies in the effect of weather conditions on the activity and abundance of insect populations. Philosophical Transactions of the Royal Society London Series B 244:331–378.
Winkler, D.W. 2006. Roosts and migrations of swallows. Hornero 21:85-97.
Wright, D.H., H. Lomeli, P.S. Hofmann, and C. Nguyen. 2011. Burrow occupancy and nesting phenology of Bank Swallows along the Sacramento River. California Fish and Game 97:138-147.
Yundt, S.E., and B.P. Messerschmidt. 1979. Legislation and policy mineral aggregate resource management in Ontario, Canada. Minerals and the Environment 1:101-111.
Appendix A: Mean age calculation
Calculation of the mean age in a hypothetical cohort population of 100 breeding individuals (sum of column D) based on annual survival rate assumptions of 0.35 for birds in their first year and 0.4 for birds after their first year. Column A is the age (years) that individuals survive to; Column B is the initial number of birds in the population (e.g., 172 fledglings); Column C is the annual survival rate of individuals; Column D is the number of individuals surviving (i.e., Column B multiplied by Column C); Column E is the number of birds surviving (Column D) multiplied by their age (Column A). Any discrepancies in calculated values are the result of rounding. Adjusting adult survival from 0.4 to 0.5 will result in a mean population age of 2 years.
| A) Age (years) | B) Initial number of birds | C) Survival rate | D) Number of birds surviving to A) | E Number of birds (age-weighted) |
|---|---|---|---|---|
| 1 | 172 | 0.35 | 60 | 60 |
| 2 | 60 | 0.4 | 24 | 48 |
| 3 | 24 | 0.4 | 10 | 29 |
| 4 | 10 | 0.4 | 4 | 15 |
| 5 | 4 | 0.4 | 2 | 8 |
| 6 | 2 | 0.4 | 1 | 4 |
| 7 | 1 | 0.4 | 0 | 2 |
| 8 | 0 | 0.4 | 0 | 1 |
| 9 | 0 | 0.4 | 0 | 0 |
| 10 | 0 | 0.4 | 0 | 0 |
| SUM | 100 | 167 |
Mean age of population = 167 / 100 = 1.67
Appendix B: Supporting information from unpublished data collected by Bird Studies Canada
This appendix includes supporting information about unpublished data sources from Bird Studies Canada. In 2010, Bird Studies Canada began research activities on Bank Swallows along the north shore of Lake Erie. Various components of the research included burrow count inventories, burrow occupancy estimates, nest survival monitoring (Tozer and Richmond 2013), and radio telemetry studies.
Burrow count surveys
The main goal of this research component was to determine the abundance, distribution, and annual variation in the density of burrows along a 64 km section of bluffs along the north shore of Lake Erie. We inventoried all burrows in Bank Swallow colonies from mid-June to mid-July during 2010-2015. We conducted boat surveys using a 24-foot steel work boat and a hired boat operator and surveyed only during calm weather (i.e., winds less than 15 km/hr). During burrow counting surveys, we travelled 5-10 km/hr approximately 50-100 m from shore identifying colonies by the presence of burrows in the bluff faces.
Since many colonies appeared continuous over several hundred metres, but fragmented to some degree, we arbitrarily separated colonies where a >20 m break (i.e., absence of burrows) in the colony existed. All colonies were photographed and geo-referenced with a handheld GPS unit (Etrex Vista HCx, Garmin 2007).
Procedure for burrow counting varied depending on colony size because larger colonies presented practical constraints. Burrows in small colonies (2-50 burrows) were counted individually. However, burrows in moderate- to large-sized colonies (50+) were counted using blocking estimates, where we counted burrows in groups of 10 at a time. At dense colonies (e.g., >500 burrows), we stopped the boat to ensure adequate time to count burrows. A single observer (MF) counted burrows in all years.
Figure 8. Map of the study area showing the density of burrows across the north shore of Lake Erie.
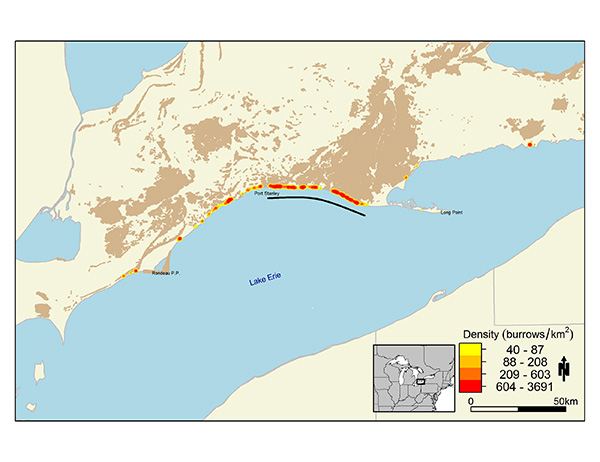
The black line shows the extent of the main 64-km study area where surveys have occurred annually (2010-2014). The shaded brown regions depict sand plain physiography.
Burrow occupancy
The main goal of this research component was to assess the occupancy level of nest burrows to determine an appropriate 'correction' or adjustment for estimating population size because not all burrows are occupied by nesting birds. Wright et al. (2011) suggest a 50 percent rule, based on burrow occupancy levels in California where the researchers inspected nest contents. Most nest burrows on the Lake Erie bluffs are inaccessible, so we assessed occupancy levels by video recording colonies for a specific duration and modeling abundance of active burrows based on removal (capture-recapture) models. We assessed burrow occupancy levels in the mid to late nesting period (15-30 June) of 2010 and 2011. Active burrows were defined as burrows with visible young, adults making feeding visits to the burrow or adults fully entering the burrow (i.e., we excluded observations of adults only perching at the burrow terminal). We recorded video footage at colonies for a duration of 15-min. during the late nesting period (1436 burrows from 15 colonies) and in 2011, we used video recordings (15-min. periods) during three nesting stages; early (30 May-3 June), mid (15-22 June) and late (27-30 June). We recorded activity at 1605 individual burrows at each visit (from 9 sites), excluding 101 burrows destroyed by erosion and/or predators between visits. In both years, we recorded the "time-at-first-detection" for each active burrow during video playback and categorized detections into 1-min. intervals over the 15-min. period (i.e., removal sampling; see White et al. 1982). In some cases, we adjusted the start times of videos because our presence while setting up the video camera at the colony briefly deterred the normal behaviour of birds. To deal with this, we adjusted video start times to begin when the first bird returned to its burrow.
We modeled active nest burrow abundance using "time-at-first-detection" probability with a multinomial-Poisson mixture model in the R package "unmarked". We then calculated the proportion of active nest burrows (from the model) to total burrows in the sample. We examined occupancy levels at 1436 burrows from 15 colonies in 2010 and 1605 burrows from 9 colonies in 2011. Estimated burrow occupancy was 0.57 (CI: 0.53, 0.61) in 2010 and 0.53 (CI: 0.47, 0.60) in 2011.
Radio telemetry
Some of the details of this work are included in Falconer et al. (In press). Additional information related to foraging distances of radio tagged birds is included here.
In this study, a total of 24 adults were tagged at two colonies and were manually tracked using a standardized scanning method at 24 stations located throughout the study area ranging from about 100 m to 1500 m away from each colony.
Figure 9. Study design for radio telemetry scanning stations at one of two Bank Swallow colonies on Lake Erie.
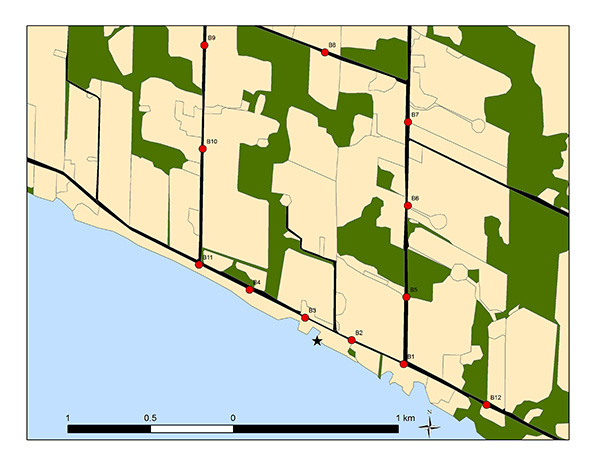
Enlarge Figure 9. Study design map
The arrangement of stations at the second study site was similar. The map shows survey stations (red circles) in proximity to a breeding colony (black star), as well as roads (black lines), forest cover (green) and other habitats (khaki).
At each scanning station, surveyors conducted a 2 min. scan starting in the north position and rotating the antenna position by 45 degrees (i.e., NE, E, etc.) every 15 seconds. Receiver gain control (sensitivity) was set at 50 decibels using a Lotek SRX600 unit. During scans we recorded signal strengths (an indicator of how close birds are to the antenna) and birds' tag identifications. Birds were tracked on 19 survey days (all 24 stations sampled) through June and early July. We estimated the locations of tagged birds detected in scans by assuming signal strength was correlated with distance. To assess the relationship between signal strength and the distance between transmitter and antenna, we attached transmitters to large balloons raised between 15-50 m in the air and recorded signal strengths at a range of distances (50-160 m). We found that transmitter distances beyond 200 m were not detectable with our equipment and settings. We modeled distance using a general linear model based on the calibration data:
- distance to transmitter = -0.706 x (signal strength) +196.6
Only one location (strongest signal) was used for each individual per 2 min. station scan. Thus, the greatest signal strength per individual and the corresponding bearing was used to estimate the location of detected birds at each station. We amassed a total of 410 bird detections from 23 birds.
Our analysis suggests that Bank Swallows tend to fly near their breeding colony, with few flights venturing beyond 1000 metres. We modeled the likelihood of a bird’s location (i.e., relative hazard) relative to the distance from their breeding colony using a Cox proportional hazards model (i.e., discrete choice habitat model). We controlled for random individual effects using the cluster term. Our preliminary analysis suggests that breeding birds spend most of their time (presumably foraging) very close to the breeding colony. For example, birds were about four times more likely to be encountered at the colony compared to 500 metres away.
Figure 10. Predicted likelihood (relative hazard) of a breeding Bank Swallow flying various distances relative to the breeding colony.
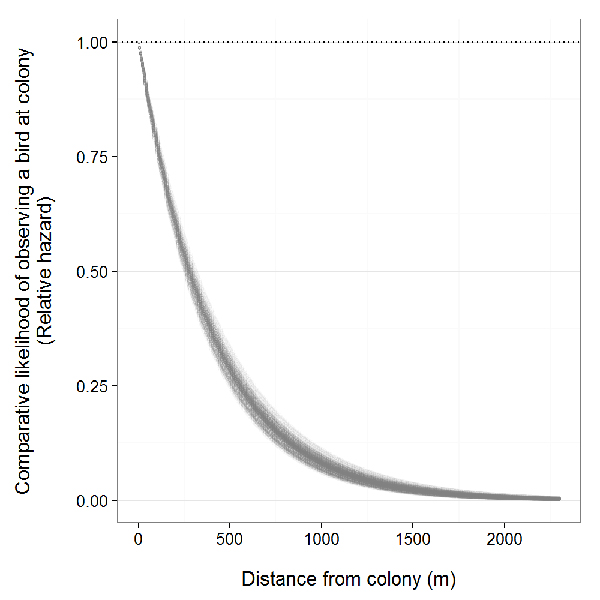
Analysis was based on 410 detections from 23 individuals. The width of the grey line corresponds to 95 percent confidence envelopes.