Small-mouthed Salamander and Small-mouthed-dependent Unisexual Ambystoma Recovery Strategy
This document is the recovery strategy for the Small-mouthed Salamander and the Small-mouthed-dependent Unisexual Ambystoma, amphibian species at risk in Ontario.


Cover photos by T.J. Hossie
About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act, 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species’ persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of Natural Resources and Forestry Species at Risk webpage.
Recommended citation
Hossie, Thomas, J. 2018. Recovery Strategy for Small-mouthed Salamander (Ambystoma texanum) and Unisexual Ambystoma Small-mouthed Salamander dependent population (Ambystoma laterale - texanum) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources and Forestry, Peterborough, Ontario. vii + 45 pp.
Cover illustration: Small-mouthed Salamander photo from Pelee Island (top) and Unisexual Ambystoma (Small-mouthed Salamander dependent population) photo from Pelee Island (bottom) by T.J. Hossie.
© Queen’s Printer for Ontario, 2018
ISBN 978-1-4868-2163-1 (HTML)
ISBN 978-1-4868-2164-8 (PDF)
Content (excluding the cover illustration) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n’est disponible qu’en anglais en vertu du Règlement 411/97 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec recovery.planning@ontario.ca.
Authors
Thomas Hossie – Trent University
This Recovery Strategy updates and expands the 2015 Recovery Strategy for the Small-mouthed Salamander (Ambystoma texanum) in Ontario which was written by Stewart E. Hamill – Wildlife Biologist, Merrickville.
Acknowledgments
This document builds on a recovery strategy which was prepared by Stuart E. Hamill for the Small-mouthed Salamander in 2015. I would like to acknowledge the extensive work that went into that strategy. In developing the original strategy Stuart E. Hamill wished to acknowledge his contacts at the Ontario Ministry of Natural Resources and Forestry (MNRF) office in Peterborough, Megan McAndrew and Amelia Argue, Species at Risk Biologists who assisted with guidance and information for the project. He also thanked those who reviewed and commented on the drafts as well as the following individuals who provided details on the species, the locations where it occurs and the habitat, and on threats: Karine Bériault, James Bogart, Joe Crowley, Ron Gould, David Green, Michael Oldham, John Urquhart, Allen Woodliffe.
I would like thank those who helped in the drafting of this document by providing data, or through helpful comments and discussion. My understanding of the salamander complex on Pelee Island has improved through discussions with James Bogart, Jeff Hathaway and Dennis Murray. During the development of this document the following individuals assisted by providing data, helping identify or evaluate threats and knowledge gaps, or by increasing my awareness of ongoing restoration activities on Pelee Island: Karine Bériault, James Bogart, Jeff Bowman, Jill Crosthwaite, Joe Crowley, Ron Gould, Dan Lebedyk, Jessica Linton, Peter Sorrill. I would also like to thank those who helped to improve this Recovery Strategy by reviewing and commenting on earlier drafts of this document.
Declaration
The recovery strategy for the Small-mouthed Salamander and Unisexual Ambystoma (Small-mouthed Salamander dependent population) was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all of the individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ontario Ministry of Natural Resources and Forestry
Environment and Climate Change Canada – Canadian Wildlife Service, Ontario
Executive summary
The Small-mouthed Salamander (Ambystoma texanum) is a medium to large salamander with a robust body and a broad head. They are black or dark grey and the flanks and tail are covered with light grey or grey-blue flecking. Compared to other salamanders in this group, Small-mouthed Salamanders have a small head and the snout is short and blunt. This species occurs widely in the USA, but within Canada it is restricted to Pelee Island, a 42 km2; island in Lake Erie, where the species reaches the northern edge of its range. Small-mouthed Salamanders co-occur on Pelee Island with an essentially all-female lineage of Ambystoma salamanders that are reproductively dependent on Small-mouthed and Blue-spotted Salamanders (Ambystoma laterale). These animals are called Unisexual Ambystoma (Small-mouthed Salamander dependent population) and are sometimes referred to as ‘unisexual polyploids’ because an individual may possess anywhere from two to five sets of chromosomes. All Unisexual Ambystoma in this population share nuclear DNA with both Small-mouthed and Blue-spotted salamanders. Yet, Unisexual Ambystoma have mitochondrial DNA (DNA located in mitochondria and inherited only from the mother) that is distinct from any contemporary species indicating that they are not recent hybrids, but instead stem from a distinct ancient lineage. Unisexual Ambystoma (Small-mouthed Salamander dependent population) are intermediate in appearance to Small-mouthed and Blue-spotted salamanders and cannot be readily distinguished from these species without genetic testing. This fact, combined with the logistical challenges associated with accessing Pelee Island in March, has made it difficult to obtain population estimates for these species. However, the permanent loss of historical breeding sites and the severity of ongoing threats has meant that both Small-mouthed Salamanders and Unisexual Ambystoma (Small-mouthed Salamander dependent population) are now listed as Endangered in Ontario. Rejuvenated survey efforts have resulted in the discovery of previously unknown breeding ponds, yet these salamanders remain restricted to six areas across the island.
The major threats facing these species include:
- habitat alteration, loss and fragmentation through drainage, land clearing or development
- road mortality of migrating adults and dispersing juveniles
- predation or habitat modification by a large and possibly increasing population of Wild Turkeys
- emerging pathogens, including salamander chytrid fungus and Ranavirus
- introduction of fish into breeding ponds
- invasive species, including Phragmites australis australis;
- pollution of breeding ponds through agricultural runoff or de-icing salt
- climate change which is expected to make drought conditions more frequent and could cause breeding sites to dry up prematurely
The recommended recovery goal is to ensure the long-term persistence of the Small-mouthed Salamander and Unisexual Ambystoma (Small-mouthed Salamander dependent population) on Pelee Island. The strategy describes protection and recovery objectives for this species in Ontario, including to:
- Protect and maintain or enhance the quality and quantity of habitat on Pelee Island where Ambystoma salamanders occur, and support habitat creation or restoration activities that increase connectivity among populations.
- Implement a monitoring program for salamander populations on Pelee Island that includes assessment of abundance, size or age structure, genetic composition, habitat and screening for emerging pathogens.
- Promote and carry out research to better understand Small-mouthed Salamander and Unisexual Ambystoma habitat needs, genetics, population dynamics and threats.
- Investigate existing, former and potential Ambystoma salamander habitats on Pelee Island to determine if restoration, re-introduction or population interventions would be appropriate.
- Promote stewardship, education and outreach programs for private landowners, residents and visitors on Pelee Island.
The main approaches to recovery should include: (i) implementation of a monitoring program, (ii) studying patterns of habitat use and movement in adults and juveniles, (iii) supporting research on genetics, demographics, and threats and (iv) implementing education and outreach programs to minimize the introduction or spread of invasive species and emerging pathogens. Recent surveys have failed to detect Blue-spotted Salamanders on Pelee Island indicating a possible decline in abundance. Given their importance as sperm donors for Unisexual Ambystoma, additional surveys should seek to determine if and where Blue-spotted Salamanders are still present on Pelee Island.
It is recommended that a habitat regulation for Small-mouthed Salamanders and Unisexual Ambystoma (Small-mouthed Salamander dependent population) include:
- Any wetland, pond or vernal or other temporary pool that is being used by Small-mouthed Salamander or Unisexual Ambystoma, or was used by a Small-mouthed Salamander or Unisexual Ambystoma at any time in the previous ten years.
- An area that is within 300 m of a wetland, pond or vernal or other temporary pool described above that provides suitable foraging, dispersal, migration or hibernation conditions for Small-mouthed Salamander or Unisexual Ambystoma.
- Areas that provide suitable conditions for Small-mouthed Salamander or Unisexual Ambystoma to disperse and are within one kilometer of known Small-mouthed Salamander or Unisexual Ambystoma breeding sites.
- A wetland, pond, vernal or temporary pool that would provide suitable breeding conditions for Small-mouthed Salamander or Unisexual Ambystoma that is within one kilometer of a known Ambystoma salamander breeding site.
While not explicitly included in the recommendation for a habitat regulation, habitat that could facilitate dispersal among known breeding sites that are within three kilometers of one another should be protected and enhanced through stewardship and best management practices, where possible.
1. Background information
1.1 Species assessment and classification
| Assessment | Status |
|---|---|
| SARO List Classification | Endangered |
| SARO List History | Endangered (2008), Endangered – Not Regulated (2005), Threatened (2004) |
| COSEWIC Assessment History | Endangered (2014), Endangered (2004), Special Concern (1991) |
| SARA Schedule 1 | Endangered (2005) |
| Conservation Status Rankings | GRANK: G5, NRANK: N1, SRANK: S1 |
*The glossary provides definitions for the abbreviations within and for other technical terms in this document.
| Assessment | Status |
|---|---|
| SARO List Classification | Endangered |
| SARO List History | Endangered (2017) |
| COSEWIC Assessment History | Endangered (2016) |
| SARA Schedule 1 | No schedule, no status |
| Conservation Status Rankings | GRANK: Not assessed, NRANK: Not assessed, SRANK: Not assessed. |
*The glossary provides definitions for the abbreviations within and for other technical terms in this document.
1.2 Species description and biology
Species description
The Small-mouthed Salamander (Ambystoma texanum) is a typical member of the Mole Salamander family (Ambystomatidae), being a medium to large salamander (maximum length 18 cm) with 13-16 prominent costal grooves, robust limbs and body and a broad head (Harding 1997, Owen and Jutterbock 2013). The head in this species, however, is noticeably smaller than that of other mole salamanders and the snout is short and blunt (MacCulloch 2002). The back is black or dark grey and the belly is dark with a few light spots. The flanks and tail are covered with light grey or grey-blue flecking (Petranka 1998). Unisexual Ambystoma (Small-mouthed Salamander dependent population) are intermediate in appearance between Small-mouthed Salamanders and Blue-spotted Salamanders and cannot be readily distinguished from these species without genetic testing. Colouration of Unisexuals is variable, but most individuals are black or grey with light grey, blue-grey, or blue flecking along the flanks and tail. Unisexual Ambystoma in this population can have anywhere from 12 to16 costal grooves (Hossie, unpubl.). Unisexual adults have proportionally smaller heads than Blue-spotted Salamanders of similar size (Kraus et al. 1991) and the distance between the nares (nostrils) tends to be larger in the unisexuals than that of similarly sized Small-mouthed Salamanders (Downs 1989, Hossie, unpubl.).
Larvae have external gills and measure 7 to 14 mm in total length at time of hatching, but may grow to over 75 mm in total length prior to metamorphosis. They possess a broad head and the upper tail fin extends along the dorsum to just behind the head (Petranka 1998, Mills 2016). Colouration is variable and depends on both the amount of ultraviolet (UV) light exposure and water temperature (Garcia et al. 2003, Garcia et al. 2004). Larvae may be a light yellowy-olive colour with dark blotches or almost black (Harding 1997). A pale yellowish lateral stripe is present along the side, but this becomes less distinct with age. The tail fin is heavily blotched and mottled with black, particularly towards the tip (Owen and Jutterbock 2013, Mills 2016). It is not possible to visually distinguish unisexual larvae from the larvae of coexisting Small-mouthed and Blue-spotted Salamanders.
Species biology
In Canada, the Small-mouthed Salamander is only found on Pelee Island, Ontario where the species reaches the northern limits of its range (Bogart et al. 1985, NatureServe 2017). These salamanders live alongside Blue-spotted Salamanders and Unisexual Ambystoma (Small-mouthed Salamander dependent population). Unisexual Ambystoma vastly outnumber both Small-mouthed and Blue-spotted Salamanders, making up over 80 percent of all the Ambystoma salamanders on the island (Hossie and Murray 2017, see also COSEWIC 2004). These three species were isolated together on Pelee Island roughly 4000 years ago with Small-mouthed Salamanders and Unisexual Ambystoma likely having originated from populations to the south, but Blue-spotted Salamanders originating from the Ontario mainland (Lowcock 1989). Together these species now form a unique salamander ‘complex’ on Pelee Island which has persisted without immigration from outside populations despite intense and widespread changes to the island’s landscape.
Unisexual Ambystoma (i.e. including all designatable units) are characterized by a unique genetic system and do not fit the conventional biological species concept. The term ‘unisexual’ refers to the fact that nearly all of the salamanders from this lineage are female (Bogart et al. 1985, Bogart and Licht 1986). All Unisexual Ambystoma share mitochondrial DNA that is distinct from any bisexual species (i.e. species with two sexes) indicating that they are not hybrids resulting from recent mating events among contemporary species (Hedges et al. 1992). Instead, all Unisexual Ambystoma, including those on Pelee Island, form a monophyletic lineage that arose 3 to 5 million years ago (Bi and Bogart 2010). All Unisexual Ambystoma do however share nuclear DNA with other co-occurring bisexual species from the genus Ambystoma and individual unisexual salamanders may possess anywhere from two to five chromosome complements (i.e. may be diploid, triploid, tetraploid or pentaploid). At least one chromosome complement in all Unisexual Ambystoma is invariably derived from the Blue-spotted Salamander genome (Bogart et al. 2009, Bi et al. 2008). A shorthand nomenclature is used to refer to various genomotypes of Unisexual Ambystoma depending on which chromosome complements they possess. Specifically, in this nomenclature the number of A. laterale and A. texanum chromosome sets that an individual salamander possesses corresponds to the number of uppercase ‘L’s and ‘T’s, respectively. For example, a diploid unisexual with one chromosome set from A. laterale (L) and one chromosome set from A. texanum (T) would be referred to as an ‘LT’ individual, whereas a triploid unisexual with one chromosome set from A. laterale (L) and two from A. texanum (T) would be an ‘LTT’ individual. In this nomenclature, pure (i.e. diploid, bisexual) Small-mouthed Salamanders are referred to as ‘TT’, and pure (i.e. diploid, bisexual) Blue-spotted Salamanders are ‘LL”.
No other Canadian population of Unisexual Ambystoma coexists with Small-mouthed Salamanders, and all unisexuals in this population possess one or more chromosome complements from the Small-mouthed Salamander genome (COSEWIC 2016). Unisexuals on Pelee Island lack any genetic contribution from Jefferson Salamanders and exhibit a relatively high proportion of diploid and symmetrical tetraploids relative to mainland populations (COSEWIC 2016). Unisexual Ambystoma (Small-mouthed Salamander dependent population) therefore includes all unisexual salamanders on Pelee Island (COSEWIC 2016). The Unisexual Ambystoma (Small-mouthed dependent population) designatable unit includes diploids (LT), triploids (LLT, LTT), tetraploids (LLLT, LLTT, LTTT), and rare pentaploids (e.g. LLTTT, LTTTT) (COSEWIC 2004, COSEWIC 2016, Hossie and Murray 2017).
For all Unisexual Ambystoma, sperm is required to initiate egg development, but typically does not contribute nuclear DNA to the resulting embryo (Bogart and Licht 1987, Bogart et al. 2007). This form of reproduction is termed ‘gynogenesis’ and produces offspring that are genetically identical to the mother (Bogart et al. 2007). Rarely, nuclear DNA from the sperm is incorporated into the egg resulting in an embryo that has an additional set of chromosomes relative to its mother (i.e. increased ploidy). This form of reproduction has been termed ‘kleptogenesis’ and is unique to Unisexual Ambystoma (Bogart et al. 2007). The frequency of sperm incorporation increases with water temperature during reproduction (Bogart et al. 1989). Five bisexual Ambystoma species are known to be viable sperm donors for Unisexual Ambystoma (A. laterale, A. texanum, A. jeffersonianum, A. tigrinum, and A. barbouri; Bogart et al. 2009), however only A. texanum and A. laterale are potential sperm donors for unisexuals in the Small-mouthed Salamander dependent population due to geographic isolation. The process of ploidy-reduction is less well understood, but must be possible for diploid unisexuals to exist. A leading hypothesis suggests that symmetrical tetraploids (e.g. animals that possess two sets of chromosomes from each of A. laterale and A. texanum genomes) are uniquely capable of producing reduced diploid embryos which receive one set of chromosomes from each of the two bisexual genomes (Bogart and Bi 2013). If true, symmetrical tetraploids would be critical to the generation of genetic diversity in unisexual populations. Symmetrical tetraploids can only be produced in systems where there are two sperm-donating species. This is an uncommon situation, but occurs on Pelee Island and appears to have shaped the genetic structure of the Unisexual Ambystoma that occur there (Bogart et al. 1985, COSEWIC 2016).
Adult salamanders from all three species (i.e. Small-mouthed Salamander, Blue-spotted Salamander and Unisexual Ambystoma (Small-mouthed Salamander dependent population)) migrate to fishless vernal pools in early spring. Male Small-mouthed Salamanders may engage in courtship with females prior to depositing small packages of sperm called spermatophores along the bottom of the pond, or may deposit spermatophores randomly (Petranka 1998, Owen and Jutterbock 2013). Courtship of Unisexual Ambystoma by male Small-mouthed Salamanders has also been observed (Licht 1989; Licht and Bogart 1990). Female salamanders, including unisexuals, mount spermatophores and pick up seminal fluid with their cloaca. Females may collect sperm from multiple spermatophores (Garton 1972) and fertilization of eggs is internal. Unisexual Ambystoma on Pelee Island may collect sperm deposited by either Small-mouthed or Blue-spotted Salamanders (Bogart et al. 1985, Bi et al. 2008).
Small-mouthed Salamanders and Unisexual Ambystoma (Small-mouthed Salamander dependent population) deposit eggs either in loose clusters along submerged vegetation or singly along the bottom of the pond roughly 24 to 48 hours after sperm collection. Adults disperse back to the terrestrial habitat shortly after depositing eggs. Small-mouthed Salamanders produce more numerous egg clutches with eggs that are smaller in diameter compared to unisexuals on Pelee Island (Licht 1989). Clutch size for both Small-mouthed Salamander and unisexuals on Pelee Island averages roughly 200 eggs, but may be as numerous as 560 eggs (Licht 1989). The hatching success of eggs produced by Small-mouthed Salamanders and unisexuals on Pelee Island is low (range = 0-63%, mean ± SD = 22.9 ± 25.6, n = 7 egg masses; Bogart and Licht 1986, Licht 1989). Typical hatching success rate of Unisexual Ambystoma eggs in this population is reported to be only about 16 percent (Bogart and Licht 1986), but ranges from 0 to 74.5 percent (Licht 1989). The relatively low hatching success and viability of developing embryos may be attributed to low fertilization rates and developmental abnormalities in unisexual embryos (Licht 1989). Developing egg masses and larvae can survive under ice (Cagle 1942), but development is inhibited below 5°C (Punzo 1983).
Surviving eggs hatch 2 to 8 weeks from deposition depending on water temperature (Downs 1989, Minton 2001) and develop into free swimming larvae with external gills. Larvae feed on various aquatic invertebrates, but may opportunistically consume amphibian larvae and are occasionally cannibalistic (Minton 2001). Breeding sites must hold water continuously from mid-March to late June for larvae to reach metamorphosis, however, larvae may not leave ponds until late August when conditions are favourable. Upon completing metamorphosis juveniles disperse from ponds into the terrestrial habitat on rainy or humid nights. Once on land salamanders spend most of their time under rocks or decaying logs, but may be found under bark, wood boards or sheets of tin. Small-mouthed Salamanders are also known to use burrows made by mammals and crayfish and are occasionally plowed up in agricultural fields (Owen and Juterbock 2013). Juveniles and adults feed primarily on earthworms and small invertebrates (Owen and Juterbock 2013). As summer progresses salamanders move deeper underground and are more difficult to find. In the fall, salamanders move below the frost line where they remain until the following spring.
Small-mouthed Salamanders reach sexual maturity in about two years (Petranka 1998), but Unisexual Ambystoma (Small-mouthed Salamander dependent population) may require more time and probably reach sexual maturity after 2 to 3 years (Licht and Bogart 1989). Adult Ambystoma salamanders from many species do not attempt to breed every year and may forego migration to breeding sites for multiple successive years (Petranka 1998, Pfingsten et al. 2013). The main predators of juveniles and adult salamanders are raccoons, foxes, snakes, and birds, while larvae are fed on by crayfish, insects, birds and snakes (Petranka 1998, Pfingsten et al. 2013). If present, carnivorous fish will rapidly consume developing embryos and larvae (Kats et al. 1998).
1.3 Distribution, abundance and population trends
Global range
Small-mouthed Salamander and Unisexual Ambystoma (Small-mouthed Salamander dependent population) occur in both Canada and the USA. Small-mouthed Salamanders have a global range extent of 200,000 to 2,500,000 square km (NatureServe 2017) and their range extends from southwestern Ontario and Ohio westward to eastern Kansas, and southward to eastern Texas, Louisiana, Mississippi, and Alabama (Petranka 1998). A complete list of states and provinces where the Small-mouthed Salamander occurs and the relevant Conservation Status Ranks can be found in Appendix Table A1. Small-mouthed Salamanders occur on Pelee Island, and have also been found on other islands in Lake Erie, including Kelly’s Island and Middle Bass Island (King et al. 1997). The global population size of Small-mouthed Salamanders is unknown, but probably exceeds 100,000 (NatureServe 2017). Over its entire range Small-mouthed Salamander abundance appears to be relatively stable, but may have experienced small declines (less than 30%) over the short-term (NatureServe 2017).
The Unisexual Ambystoma (Small-mouthed Salamander dependent population) designatable unit is, by definition, restricted to Pelee Island (COSEWIC 2016). However, Unisexual Ambystoma that are dependent on Small-mouthed Salamanders can be found in Michigan, Indiana, and Ohio (COSEWIC 2016), and are known from several Lake Erie islands including, Kelly’s Island (Ohio) and the Bass Islands (Ohio) (COSEWIC 2016). The full range may not be well defined because genetic testing is required to identify these animals and many populations have not yet been tested (COSEWIC 2016). The global population size for Unisexual Ambsytoma (Small-mouthed Salamander dependent population) is not known. Unless specified otherwise, any subsequent reference to Unisexual Ambystoma in this document refers specifically to Unisexual Ambystoma (Small-mouthed Salamander dependent population).
Canadian range
In Canada, Small-mouthed Salamanders and Unisexual Ambystoma (Small-mouthed Salamander dependent population) are restricted to Pelee Island, a 42 km2; island in the western basin of Lake Erie. In 1991, they were known to occupy at least five locations on the island, however two of these breeding ponds were eliminated by development and permanent loss of water by the year 2000 (COSEWIC 2004). Recent work beginning in 2015 has identified six areas on the island that are currently occupied by these species and separated by more than 1 km (COSSARO 2016, Hossie and Murray 2017). This increase reflects renewed survey efforts that included land subsequently acquired by the Nature Conservancy of Canada (NCC) as well as private property where landowners have recently authorized surveys.
In this document “breeding site” refers to a specific pond, wetland, vernal or other temporary pool where salamanders reproduce, and “breeding location” refers to larger areas typically separated by more than 1 kilometer that may include several breeding sites (e.g. several ponds within close proximity). Current breeding locations are composed of a mixture of semi-natural habitat, abandoned livestock ponds, and vernal ponds created through stewardship activities. Specifically:
- ponds isolated from the lake located in Lighthouse Point Nature Reserve;
- a spring-fed abandoned livestock pond located in a patch of secondary succession forest on private property and a flooded woodlot owned by NCC which are located within close proximity to each other;
- multiple small ponds created through stewardship activities on two adjacent parcels of private land;
- a flooded woodlot on a nature reserve jointly owned by Ontario Nature, Essex Region Conservation Authority (ERCA) and the NCC;
- a spring-fed abandoned livestock pond located in a patch of secondary succession forest owned by NCC; and
- a flooded woodlot within the provincial Fish Point Nature Reserve.
One additional breeding location identified in the 2004 COSEWIC Assessment and Update Status Report on the Small-mouthed Salamander is on private land and has not been surveyed in over 10 years because of land access restrictions. No Small-mouthed Salamanders were observed at this location when it was last surveyed, but it did have abundant Blue-spotted Salamanders and various genomotypes of Unisexual Ambystoma (COSEWIC 2004). The current status of this breeding location is not known.
In recent years the NCC has undertaken several wetland creation and restoration activities on Pelee Island (J. Crosthwaite, pers. comm. 2017). While eggs and larvae (including LT, LTT, and LTTT individuals) were observed in one of these new ponds, effective recruitment has not yet been demonstrated (T. Hossie, pers. obs. 2017).
The abundance of Small-mouthed Salamanders and Unisexual Ambystoma on Pelee Island is not currently known. Obtaining populations estimates has been complicated by the cryptic nature of salamanders outside of the breeding season combined with logistical challenges in accessing and working on the island during the breeding season in March. Additional difficulties arise from the fact that genetic methods are required to accurately distinguish among Small-mouthed Salamanders, Blue-spotted Salamanders and Unisexual Ambystoma. A mark-recapture estimate based on four consecutive nights of trapping in one breeding pond in March 2016 estimated the number of salamanders (i.e. both bisexuals and unisexuals) in that pond at the time of sampling to be 789 individuals (95% confidence interval: 430-2367) (Hossie and Murray 2017).
Using data obtained from more than 1200 larvae collected on Pelee Island from 1984 to 1991, unisexuals made up 78 percent of the Ambystoma salamander population (COSEWIC 2004). More recent survey efforts on Pelee Island examined more than 830 samples (adults and larvae) collected from 2015 to 2017 and found that unisexuals made up over 95 percent of the sample (Hossie and Murray 2017). This may indicate that the relative abundance of Small-mouthed Salamanders has declined since 1991. Note however that historic and contemporary samples used different sampling methods and were drawn from different localities on Pelee Island which could limit direct comparison. For example, contemporary samples do not include two historical sites that no longer exist or the historical breeding location where Blue-spotted Salamanders were abundant, whereas historic samples did not include samples from two recently discovered sites. A more direct comparison can be made for two breeding locations that were surveyed in both time periods (i.e. 1984-1991 and 2015-2017) and by restricting the comparison to larval samples. Such comparisons reveal a significant decline in relative abundance of Small-mouthed Salamanders at one site where A. texanum was historically abundant (proportion of A. texanum in historic sample: 28.1%, n = 274, contemporary sample: 3.3%, n = 61; χ² = 17.01, DF = 1, P < 0.001), but not the other site where the relative abundance of A. texanum was already low (historic: 1.4%, n = 351, contemporary: 1.2%, n = 169; χ² = 0.05, DF = 1, P = 0.82).
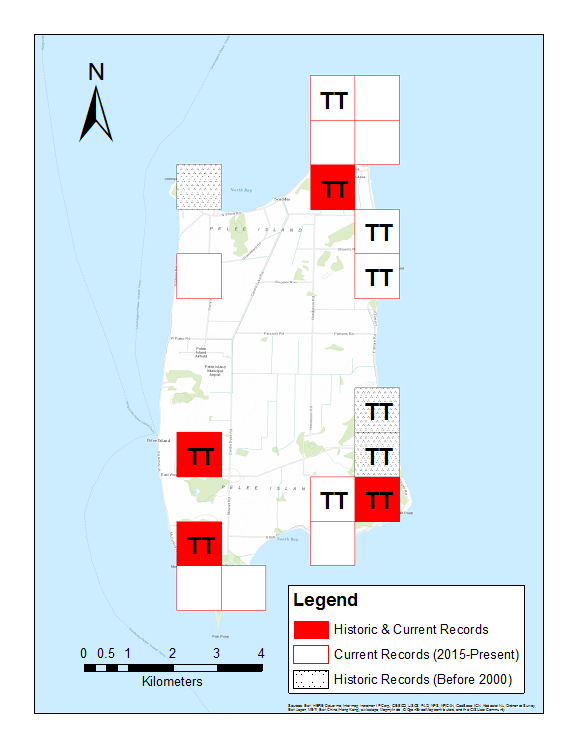
Figure 1. Historical and current distribution of the Small-mouthed Salamander and Unisexual Ambystoma (Small-mouthed Salamander dependent population) in Ontario.Areas where genetically-confirmed Small-mouthed Salamanders have been recorded are indicated with ‘TT’.
1.4 Habitat needs
Breeding habitat
Adults require woodland ponds, wetlands or other fishless water bodies for breeding activities that occur in March. The combination of water connected to suitable terrestrial habitat is therefore essential. Breeding sites are generally ephemeral and may be filled by spring run-off, ground water or by springs. These vernal pools must hold water until at least late June for the aquatic larvae to reach metamorphosis (Ryan 2007, Hossie and Murray 2017). Breeding ponds require egg attachment sites (e.g. submerged sticks and branches or emergent vegetation), as well as a food source (i.e. aquatic invertebrates) for developing larvae. Small-mouthed Salamanders and Unisexual Ambystoma on Pelee Island appear to use water bodies with a wide range of abiotic conditions (Table 3). Studies of pond characteristics in other populations of Small-mouthed Salamander or Unisexual Ambystoma indicate that embryos and larvae tolerate a wide range of water depth, temperature and water chemistry conditions (Pierce and Wooten 1992, Punzo 1983, Bériault 2005). Large increases in embryonic mortality occur as pH drops below 6 and low pH could be problematic if it results in reduced availability of aquatic invertebrate prey (Sandinski and Dunson 1992).
| Month | Dissolved Oxygen (mg/L) | pH | Total dissolved solids | Salinity | Temperature (°C) |
|---|---|---|---|---|---|
| March | 0.5-13.5 | 7.2-9.2 | 220-470 | 130-205 | 2.3-14.9 |
| April | 2.6-15.0 | 7.3-9.0 | 158-373 | 103-244 | 10.0-14.9 |
| May | 0.4-8.9 | 7.2-8.5 | 145-510 | 97-348 | 13.4-24.1 |
| June | 0.3-6.7 | 6.9-8.28 | 185-468 | 128-320 | 17.6-31.5 |
Terrestrial habitat
Outside of the spring breeding season adult Small-mouthed Salamanders and Unisexual Ambystoma generally remain hidden underground in shaded areas with soft moist soil, such as poorly drained swamp woodlands and floodplains, but may also use rocky quarry sites and alvars. In Ontario, Small-mouthed Salamanders are found in several types of moist habitats, including tall-grass prairies, dense hardwood forests and agricultural lands if such areas contain, or are adjacent to, suitable breeding ponds. These habitats also possess soils soft enough to enable adults to find burrows, such as those created by crayfish (Williams 1973, Owen and Juterbock 2013). The habitat needs of Unisexual Ambystoma (Small-mouthed Salamander-dependent population) are assumed to be similar to those of Small-mouthed and Blue-spotted Salamanders (COSEWIC 2016). Their reliance on sperm from male Small-mouthed or Blue-spotted Salamanders for reproduction means that unisexuals inhabit areas where at least one of these species occurs. Petranka (1998) reports that Unisexual Ambystoma with genomes from Small-mouthed Salamanders are restricted to habitats with clay-based soils. In contrast, Blue-spotted Salamanders are more strongly associated with drier, sandy soils (Minton 1954, Jutterbock and Owen 2013), which could mean such habitats are suitable for unisexuals on Pelee Island as well. Salamanders can be found within crayfish burrows, beneath or within rotting logs, and under rocks or leaf litter (Williams 1973, Downs 1989). Preliminary work on the Pelee Island salamander complex indicates that microhabitat selection is influenced by both soil moisture (preferred range = 10-30%) and canopy cover (preferred range = 50-100% cover) (Hossie and Murray 2017). The overwintering ecology of adult Small-mouthed Salamanders and Unisexual Ambystoma is poorly understood, however they presumably retreat below the frost line into deep rock fissures and rodent burrows during the winter.
Adult Small-mouthed Salamanders are thought to undergo less substantial migration compared to other Ambystoma species and remain close to their breeding ponds (i.e. within 50-60 m) (Williams 1973, Parmalee 1993). Treadmill endurance trials recently conducted on adult Ambystoma salamanders in Ohio indicate that Small-mouthed and Blue-spotted Salamanders can walk further than sympatric Unisexual Ambystoma prior to exhaustion (Denton et al. 2017). This work indicated that in a single movement session Small-mouthed and Blue-spotted Salamanders can travel over 150 m, whereas unisexuals can only travel less than 50 m in a single session (mean ± SD: A. texanum: 159.25 ± 86.4 m, n = 14; A. laterale: 161.20 ± 16.09 m, n = 2; unisexuals: 34.47 ± 28.2 m, n = 19). Hoffman (2017) radio-tracked unisexual salamanders (Ambystoma laterale (2) – jeffersonianum) from a population in Maine. Her work indicates that salamanders make large initial migrations away from the pond, then subsequently make few short movements in random directions (Hoffman 2017). Over 94 days these salamanders traveled an average straight line distance of 172 m from the wetland (range 6 - 403 m), and the average distance travelled in one night was 41 m (SD = 51, max = 194 m) (Hoffman 2017). She found that, with 95 percent confidence, a radius of 362 m from the pond would include the average maximum distance moved by unisexual salamanders in the populations she studied. This number should not be confused with the area that includes 95 percent of the salamanders which could be larger or smaller. Similar work conducted by Karine Bériault (2005) in Ontario examined migration distances for unisexuals (mostly Ambystoma laterale – (2) jeffersonianum) and found that the average net distance travelled from the breeding pond was 206.3 ± 134.8 m (range = 37.3-514.4, n = 12). These salamanders were tracked for an average of 53.25 ± 13.4 days (range 8-60) (Bériault 2005). Work by Bériault (2005) did not re-implant transmitters in salamanders after the 8 to 60 day period and may not capture the entirety of their movements away from breeding ponds (K. Bériault, pers. comm. 2017). It therefore remains possible that salamanders move further from breeding ponds in late summer or fall (e.g. towards more distant overwintering sites). Similar radio-tracking work has not been conducted on Small-mouthed Salamanders or Unisexual Ambystoma (Small-mouthed Salamander dependent population).
Small-mouthed Salamanders and Unisexual Ambystoma) also require terrestrial habitat suitable to disperse among breeding ponds. Work by Denton et al. (2017) estimated realized dispersal distance for Small-mouthed Salamanders and Unisexual Ambystoma in Ohio by combining genetic assignment tests with landscape analyses. They found that Small-mouthed Salamanders can disperse further than Unisexual Ambystoma, but both species can disperse more than 3 km from their natal ponds (mean Euclidean distance travelled: A. texanum = 6826 m, n = 13; unisexuals = 3300 m, n = 11) (Denton et al. 2017). These realized dispersal distances are much greater than the typical migration distances and instead probably reflect occasional long-distance dispersal events made by juveniles, possibly over multiple years. Adult Small-mouthed Salamanders do cross roads (COSEWIC 2004) indicating that roads are not an impermeable barrier, although this will depend on the type of road and heavy traffic could reduce survival during migration or dispersal. Greenwald et al. (2009) found that ponds surrounded by agricultural landscapes were associated with more genetically isolated populations, whereas greater amounts of deciduous forest were associated with decreased genetic isolation in three species of pond-breeding Ambystoma. This indicates that dispersal among sub-populations may be hindered when ponds are separated by agriculture opposed to forest. These authors also found that in relatively pristine habitats, genetic isolation increases with distance from neighboring breeding ponds (Greenwald et al. 2009), indicating that effective dispersal among populations decreases with distance. Small-mouthed Salamanders may however be more tolerant of human activities such as agriculture than other Ambystoma species (Owen and Juterbock 2013). This has been inferred based on repeated observations of Small-mouthed Salamanders being plowed up in farm fields (Minton 2001), and from studies which suggest that they can persist in agricultural areas with some degree of habitat fragmentation (Kolosvary and Swihart 1999, Owen and Juterbok 2013).
1.5 Limiting factors
A number of intrinsic or evolved factors probably limit the abundance of both Small-mouthed Salamanders and Unisexual Ambystoma (Small-mouthed salamander dependent population), these include:
- low hatching success of eggs and low viability of developing embryos
- intermittent juvenile recruitment (i.e. occasional years where no, or very few, larvae survive to metamorphosis)
- delayed maturation
- site fidelity to breeding habitat
- limited dispersal ability
- high degree of specificity for limited habitats (i.e. areas where fishless temporary ponds are within or adjacent to forested areas)
In addition to these limiting factors, Unisexual Ambystoma are strongly limited by the abundance of co-occurring Small-mouthed Salamanders and Blue-spotted Salamanders (COSEWIC 2016). Unisexual Ambystoma require sperm from either of these species in order to reproduce (Bogart and Licht 1987, COSEWIC 2016). Both the number of unisexuals that get an opportunity to reproduce and the hatching success of the resulting egg clutches is therefore directly linked to the number of available spermatophores produced by male Small-mouthed Salamanders and Blue-spotted Salamanders co-occurring in those breeding ponds.
1.6 Threats to survival and recovery
Many of the threats to Small-mouthed Salamander and Unisexual Ambystoma (Small-mouthed Salamander dependent population) are poorly or incompletely understood. Direct or indirect evidence is presented to support each identified threat, however additional work is needed to fully evaluate the relative severity of their impact on this salamander complex.
Habitat alteration, loss and fragmentation
The loss or degradation of terrestrial and breeding habitat remains a significant threat to Small-mouthed Salamanders and Unisexual Ambystoma. The entire range for both of these species in Canada is restricted to Pelee Island. Much of the suitable habitat was lost through historical logging and wetland drainage in the late 1800s, followed by the conversion of this land to agriculture. Despite these dramatic changes the salamander complex persists in patches of habitat across the island. These patches of habitat appear to be at least partly isolated from one another by drainage canals, roads and unsuitable habitat, however the degree of such isolation remains poorly understood. Small-mouthed Salamander populations within agricultural landscapes in western Ohio have been shown to be inbred and have limited gene flow among forest patches (Rhoads et al. 2017). The apparent low abundance of Small-mouthed Salamanders in each sub-population on Pelee Island could make gene flow among breeding ponds particularly important for maintaining genetic diversity. Much of the existing habitat occurs within protected areas owned by Ontario Parks, NCC, Ontario Nature, or ERCA. Additional salamander habitat on the island is protected by easement agreements or voluntary land stewardship by private land owners.
Both terrestrial and breeding habitat would be impacted by expansion or intensification of drainage activities if they cause premature drying of breeding ponds or reduce soil moisture of terrestrial habitat. Intensification of drainage activities might also change the composition of forest communities over the long-term (R. Gould, pers. comm. 2017), however the impact of such effects on salamanders is unknown. There is ongoing pressure for development (e.g. new trail systems) on Pelee Island including within areas where salamanders occur (R. Gould, pers. comm. 2017). If trail development entails opening up the canopy it could reduce habitat quality. The impact of trail footprints on terrestrial habitat is exaggerated when visitors (incl. hikers, birders, nature photographers, cyclists) stray from well-defined trails (R. Gould, pers. comm. 2017). The effect of existing trails could be mitigated by adding an elevated boardwalk within an existing trail footprint which could reduce soil compaction and create more habitat for the species beneath the boardwalk, as well as reduce human trampling of the surrounding areas (by restricting people to a boardwalk). More generally, the decline or destruction terrestrial habitat from the clearing of wooded areas has been identified as an important threat to this population (COSEWIC 2016).
Road mortality
Salamanders on Pelee Island may need to cross roads during their spring migrations to or from breeding sites in March, during dispersal of newly metamorphosed juveniles in June to August, or when adults migrate to overwintering sites in the fall. Heavily used or wide roads may constitute a barrier to dispersal and road mortality of breeding adults would strongly impact populations. On Pelee Island, spring migrations to and from breeding ponds generally occur before ferry access to the mainland has begun. As a result, traffic on Pelee Island is generally low during spring migrations. Traffic does increase substantially once the ferry commences operation in April, peaking by mid-summer. Mazerolle (2004) studied amphibian road mortality in New Brunswick and found that Ambytoma salamanders occurred on the road most frequently in the spring (April-May) and late-summer though fall (August-September), but were rarely on the road in mid-summer (June-July). He also observed that road mortality of Ambsytoma salamanders did not vary with traffic intensity within the range he examined (i.e. 5 and 26 vehicles/h) with approximately 40% of salamanders encountered on the road consisting of dead individuals (Mazerolle 2004). Roads may therefore be a source of mortality for adults or juveniles that migrate or disperse late in the summer or fall. Little work has been done to assess this threat, however road mortality has been documented to occur on Pelee Island (R. Gould, pers. comm. 2017) and can strongly impact population viability in pond-breeding salamanders (Gibbs and Shriver 2005). The Township of Pelee has significantly reduced speed limits on most roads in response to the threat that road mortality poses to the many species of endangered wildlife on the island (MNRF 2017). It is not known whether reducing speed limits has an influence on rates of road mortality in salamanders. Construction of new roads, widening of roads or increases in traffic would likely increase mortality and create or amplify barriers to dispersal and remains an important threat.
Wild turkey predation and habitat modification
Wild Turkeys (Meleagris gallopavo) have been identified as a possible threat to salamanders on Pelee Island (COSEWIC 2014a, Hamill 2015, COSEWIC 2016). Wild Turkeys were introduced to the island in 2002 and this population is now actively hunted in the spring. Turkeys are opportunistic predators that scratch to find food and may consume salamanders. The Natural Resources Conservation Service (an agency of the United States Department of Agriculture) lists salamanders as an important food item in the diet of Eastern Wild Turkey (Natural Resources Conservation Service 1999). In addition salamanders are noted as a dietary item of Wild Turkey by McRoberts et al (2014). It remains unclear whether or not salamanders on Pelee Island are well adapted to defend themselves from such predation. Turkeys are highly diurnal and primarily forage during the day when salamanders are concealed under cover, which could minimize predation risk to the salamanders (J. Bowman, pers. comm. 2017). The extent to which predation by Wild Turkeys represents an important threat to the long-term persistence of Small-mouthed Salamanders and Unisexual Ambystoma on Pelee Island depends on many factors including: (i) the extent to which turkeys inhabit areas occupied by salamanders, (ii) the extent to which salamanders are available or accessible to turkeys, (iii) the willingness of turkeys to feed on salamanders, (iv) the age classes and genotypes of salamanders that are consumed, and (v) the overall abundance of turkeys. A separate but related threat posed by Wild Turkeys is the potential for destruction of terrestrial hiding places for salamanders (e.g. decaying logs) when scratching to find food (J. Bogart, pers. comm. 2017).
The turkey population on Pelee Island has grown considerably following the release of 25 birds on the island in 2002. A single flock of over 200 birds was observed in the spring of 2016 (T. Hossie, pers. obs. 2016) and turkeys occur in all areas on the island where salamanders are found (Hossie and Murray 2017). Hunters can purchase up to two turkey tags in the spring and hunt anywhere in Ontario where there is an open season. This currently includes Pelee Island. Other than opening a fall hunting season currently there is no policy mechanism to further increase harvest pressure on Pelee Island. While Pelee Island meets the criteria for considering a fall turkey hunt based on the harvest density in spring, a fall hunt would conflict with the annual pheasant hunt on Pelee Island (J. Bowman, pers. comm. 2017).
Examination of turkey gizzard contents by the Ministry of Natural Resources and Forestry (MNRF), which included animals from Pelee Island, did not find evidence of salamander consumption (J. Bowman, pers. comm. 2017). Techniques such as examining gizzard or stomach contents of captured turkeys are not, on their own, sufficient to determine the level of threat posed by turkeys. Failure to find salamanders (or their DNA) in the gizzard or stomach of turkeys could result from many processes including: (i) sampling turkeys at a time when salamanders are not being consumed, (ii) sampling turkeys in locations where salamanders are scarce or absent, and (iii) salamanders representing a small proportion of the turkey’s diet. Furthermore, even if salamanders represent a small proportion of the Wild Turkey diet on Pelee Island, turkeys could still impose demographically significant mortality in the salamander complex if the turkey population becomes large, turkeys consume large adult salamanders, a large proportion of dispersing juveniles are consumed, or turkeys consume males or females of the less abundant bisexual species (i.e. Small-mouthed or Blue-spotted Salamanders) on which Unisexual Ambsytoma rely. This threat remains poorly understood, but has the potential to severely impact salamander populations.
Research by Trent University has employed artificial (clay) salamanders and trail cameras to examine rates of predation by turkeys and other predators (e.g. raccoons). This work will also shed light on whether salamander behavioural responses to predators (i.e. tail raise) effectively deflect predator strikes (including those by turkeys) away from their head and body. Analysis of this data is ongoing.
Emerging pathogens
There is increasing concern in Canada regarding the possible introduction of Batrachochytrium salamandrivorans (Bsal) which has caused mass die-offs in wild populations of European salamanders (Martel el al 2013, Martel et al. 2014). Bsal is a relative of Batrachochytrium dendrobatidis (Bd) that causes chytridiomycosis in salamanders leading to skin infections that cause lesions, lethargy, anorexia and death (Martel et al. 2013, Martel et al. 2014). Bsal has not yet been reported in Canada or the USA, but both countries are at risk of introduction primarily through the global pet trade (Stephen et al. 2015). Salamanders from the genus Cynops and Paramesotriton are proposed as potential reservoirs for Bsal (Martel et al. 2014). Once introduced to North America, Bsal could become established and impossible to eradicate (Stephen et al. 2015). In January of 2016, the US Fish and Wildlife service issued an interim ruling that lists 201 salamander species as injurious wildlife under the Lacey Act, effectively banning their importation into the USA without a permit. On May 26 2017 Canada followed with a legal import ban on all species of the order Caudata (i.e. all salamanders and newts), living or dead, including any egg, sperm, tissue culture or embryo of such a specimen without a permit for scientific and research purposes (Customs Notice 17-17). These regulations are implemented through amendments to section 5(a) of the Wild Animal and Plant Trade Regulations. Pelee Island is in an area of low-moderate vulnerability to Bsal based on recently produced risk maps (Yap et al. 2015, Richgels et al. 2016) and a Canadian threat assessment produced by Stephen et al. (2015). The susceptibility of Small-mouthed Salamanders and Unisexual Ambystoma to Bsal is unknown (Stephen et al. 2015). Proper decontamination procedures for Bsal and Bd are freely available online from Canadian Herpetofauna Health Working Group (2017) and should help to limit the potential introduction or spread of such pathogens. Unfortunately, such procedures are unlikely to be followed by most users of natural areas on Pelee Island. Specifically, birders, hunters, nature photographers and other tourists frequently travel among locations on the island, but are unlikely to have decontaminated footwear prior to travelling to the island or when moving among locations.
Ranaviruses from the family Iridoviridae have caused major die-offs in wild populations of amphibians in North America. These viruses can also be pathogenic to fish and reptiles (Daszak et al. 1999). Salamanders from the genus Ambystoma are susceptible to some of these viruses and salamander mortalities linked to Ranavirus infection have been documented in Canada (Bollinger et al. 1999, Seburn and Seburn 2000). Larvae are the most susceptible life stage with mortality rates reaching 100 percent and metamorphs can die without overt signs of infection (Seburn and Seburn 2000). Ranavirus may interact with other stressors, such as exposure to agricultural contaminants including Atrazine and nitrates, increasing the susceptibility of salamanders to infection (e.g. Forsan and Storfer 2006). Ranavirus may be able to persist in environments and reservoir species, but such dynamics remain poorly understood (Lesbarreres et al. 2012). Humans can incidentally introduce or spread Ranavirus through contact with infected animals (including salamanders, frogs, turtles, snakes and fish), water or soil. The limited geographic extent and number of sub-populations of Small-mouthed Salamanders and Unisexual Ambystoma on Pelee Island means that the potential impact of Ranavirus is high. Similar concerns regarding lack of decontamination (detailed above) apply equally for Ranavirus and Bsal.
Small-mouthed and Jefferson Salamanders infected by the trematode parasite Clinostomum marginatum have been reported from east-central Illinois (McAllister et al. 2010). The COSEWIC Status Appraisal Summary on the Small-mouthed Salamander Ambystoma texanum in Canada identified the parasite as a possible threat with unknown impact on population viability (COSEWIC 2014a). This parasite is has a complex life cycle which begins with eggs in the water that hatch into miracidia that infect snails where they undergo asexual reproduction. The parasites then become cercaria that leave the snail host and burrow inside the flesh of fish (or amphibians) where they encyst as metacercaria and can remain for years. Finally, these infected hosts are consumed by an aquatic bird (e.g. herons or egrets) and the parasites encyst in their throat then proceed to mature and produce eggs. In 2016 and 2017 Unisexual Ambystoma salamanders with lumps that resemble the cysts from the Clinostomum marginatum were observed on Pelee Island (Hossie and Murray 2017). The identity of these parasites has not yet been confirmed. Further, it remains unclear whether such parasitism, if confirmed, is novel versus previously undetected due to low sampling effort. The apparent incidence on Pelee Island is currently low (i.e. 6 out of 333 salamanders) and the risk posed to the salamander population is unknown.
Fish introduction
The introduction of carnivorous fish to breeding ponds would have rapid and serious effects on the viability of impacted sub-populations. Fish are predators on all life stages of salamanders and can effectively eliminate recruitment. Small-mouthed Salamanders are poorly adapted to avoid fish predation (Kats et al. 1988) and fish reduce the reproductive success of these salamanders (Walston and Mullin 2007). Fish may colonize breeding sites through deliberate or incidental human introduction. Ponds or wetland near the margins of the island could also be colonized if they become temporarily connected to the lake following severe weather or high water levels in Lake Erie. Such colonization events are probably infrequent, but have occurred in the past (R. Gould, pers. comm. 2017). Fish should not be able to persist in breeding ponds that are highly ephemeral, making these sites less susceptible to the long-term effects of fish introduction.
Invasive species
The encroachment of European Common Reed (Phragmites australis ssp. australis) into wetlands and riparian areas on Pelee Island could degrade wetland habitat and reduce the availability of suitable egg placement sites. Although the specific impacts on salamanders are unknown, an analysis by Greenberg and Green (2013) has shown that population decline in Fowler’s Toad (Anaxyrus fowleri) populations is associated with the spread of the European Common Reed. Uncontrolled growth of dense stands of Common Reed stems can effectively eliminate shallow, sparsely vegetated, aquatic areas which are needed by both Fowler’s Toad and Small-mouthed Salamander. European Common Reed forms dense stands around the ‘Lake Henry’ marsh at Lighthouse Point and around Fox Pond at Fish Point, however there is no evidence that either of these water bodies are currently being used for reproduction by Small-mouthed Salamanders or Unisexual Ambystoma. At the present time, European Common Reed is not present at any of the known breeding sites, perhaps because high canopy cover in these areas renders the habitat less suitable for this plant. If the canopy around currently used breeding habitat is opened up, European Common Reed may quickly become an important threat to the persistence and quality of this habitat.
The loss of shade due to the death of ash (Fraxinus spp.) trees caused by the Emerald Ash Borer (Agrilus planipennis), an invasive, non-native species, may change wetland or forest conditions, making them less suitable for salamanders. Surveys conducted in 2012 indicated that the Emerald Ash Borer is present at Stone Road Alvar, Lighthouse Point, Fish Point, Red Cedar Savannah, and Sheridan Point (COSEWIC 2014b). Emerald Ash Borer is considered the most significant threat to Blue Ash on Pelee Island, and in 2012 the percentage of infested Blue Ash trees ranged from 4.0-34.5% of trees (COSEWIC 2014b). Damage to ash trees has been heavy in some areas of Pelee Island, however loss of canopy cover is likely to be transient as other tree species appear to be swiftly responding to the gaps in the canopy (Ron Gould, pers. comm. 2017). The resulting change in forest community composition on salamanders in unknown.
Pollution
Salamanders are particularly sensitive to various pollutants which can kill outright or induce sublethal effects in embryos, larvae and adults. Agricultural pesticides are a particular threat as they can reduce survival and metamorphosis of Ambystoma larvae by killing zooplankton thereby reducing food resources (Metts et al. 2005). Nitrogen runoff from agricultural fields or other sources can accumulate in breeding ponds and has been shown to have lethal and sublethal impacts on Ambystoma salamanders (e.g. Marco et al. 1999, Forsan and Storfer 2006, Griffis-Kyle 2007). Many private land owners on Pelee Island practice low-impact farming to support biodiversity and natural heritage, which could limit such impacts. De-icing salt runoff from Pelee Island roads can accumulate in breeding sites and is another pollutant threat. Research has shown that acute exposure (nine days) to experimental concentrations of de-icing salt caused significant reductions in mass of Ambystoma eggs (Karraker and Gibbs 2011).
Climate change
Globally warming temperatures are causing water availability in the Great Lakes area to decrease. Specifically, the Great Lakes area has experienced an increase in evapotranspiration of 0.69 mm/year over the period of 1960 to 2000 (Fernandes et al. 2007). While marginal increases in precipitation are predicted for the Great Lakes area, it may not be enough to offset increasing actual evapotranspiration rates in the region resulting from rising temperatures (Fernandes et al. 2007). Water levels in the Great Lakes themselves, including Lake Erie, are also predicted to decrease over the long term (Moulton and Cuthbert 2000, Mortsch et al., 2006). The salamander communities on islands in Lake Erie, including Pelee Island, may become increasingly imperiled as drought conditions more frequently compromise effective reproduction. Small-mouthed Salamanders are relatively long-lived amphibians. This life history strategy permits multiple attempts at reproduction and buffers populations against occasional years with abnormally warm or dry conditions. Over the long-term, climate change may cause the reduction or loss of suitable breeding habitat for Ambystoma salamanders on Pelee Island. Blue-spotted Salamanders are a cold-adapted species. As conditions on Pelee Island warm the available habitat may become less suitable for both Blue-spotted Salamanders and Unisexual Ambystoma given their shared nuclear genome. Presuming that the genetic model proposed by Bogart and Bi (2013) is accurate (see Species Biology above), the loss of Blue-spotted Salamanders would prevent further generation of symmetrical tetraploids and ultimately prevent Unisexual Ambystoma salamanders on Pelee Island from generating new genetic diversity.
1.7 Knowledge gaps
Population size
The population size of Small-mouthed Salamanders, Unisexual Ambystoma, or Blue-spotted Salamander on Pelee Island remains unknown (COSEWIC 2014a, COSEWIC 2016). Estimates of population size are necessary to determine whether populations are in decline and to assess the long-term viability of the population.
Demographic processes
The demographic mechanisms that influence population size and composition within bisexual-Unisexual Ambystoma salamander complexes (e.g. density-dependence, recruitment, survival, age-to-maturity) are poorly understood, but are key to developing effective species management strategies. The mechanisms underlying low abundance of Small-mouthed Salamanders and Blue-spotted Salamanders relative to Unisexual Ambystoma on Pelee Island are poorly understood, but have important consequences related to the their long-term viability. Research is needed to understand whether and to what extent Small-mouthed Salamander population size is influenced by the abundance of Unisexual Ambystoma and vice versa. The salamander community on Pelee Island has coexisted since isolation from the mainland, however the mechanisms though which this is achieved are not known. Improving our understanding of such mechanisms would inform management of this system.
Genetic composition and processes
Apparent changes in the genetic composition of the salamander complex on Pelee Island over time have now been documented (Hossie and Murray 2017), however the cause of such changes is not known. Such changes could reflect the impact of one or more of the threats listed above. Effective management of Small-mouthed Salamanders and Unisexual Ambystoma will also benefit from both an improved understanding of ploidy reduction and rates of genome exchange. The mechanism for genome exchange proposed by Bogart and Bi (2013) requires empirical validation, but may inform how complex systems involving Unisexual Ambystoma should be managed. For example, if symmetrical tetraploids are key to generating new genetic diversity within Unisexual Ambystoma, and Blue-spotted Salamanders are required to produce symmetrical tetraploids, then protections may need to be extended to Blue-spotted Salamanders. Moreover, managers may need to consider creating corridors that facilitate dispersal of Blue-spotted Salamanders to adjoining sub-populations, given that Blue-spotted salamanders appear to be restricted to a single location on the island (or possibly extirpated).
Movement ecology
Existing radio-telemetry work with salamanders in Ontario has focused on Jefferson Salamanders and Unisexual Ambystoma (Jefferson Salamander dependent population) (e.g. Bériault, 2005). While work from Ohio indicates that the movement ecology of Small-mouthed Salamanders differs from that Unisexual Ambystoma (Denton et al. 2017), to date little is known about the movement ecology of salamanders on Pelee Island. Such information is critical for identifying key habitat areas (such as over summer foraging areas and hibernation sites), determining the appropriate size of habitat buffers and designing habitat corridors to link populations across the island. Radio-telemetry work could also help determine the extent to which roads and drainage canals act as barriers to movement.
Threat posed by wild turkeys
The degree to which Wild Turkeys pose a threat to Small-mouthed Salamanders or the Unisexual Ambsytoma on Pelee Island remains unknown. It is clear however that if turkeys do consume salamanders or influence salamander habitat, then such effects would be of greatest concern when the turkey population grows large. The turkey population size on Pelee Island is unknown. Efforts to assess turkey population size to date have been limited to assessment of harvest records and it is unknown whether these populations will continue to increase given that opportunities for harvest are restricted to the spring. Detailed knowledge of turkey population size, population growth rate, carrying capacity and habitat use would enable researchers or managers to better assess the threat posed by Wild Turkeys.
Value and quality of artificial breeding ponds
Salamanders on Pelee Island have been documented using artificial ponds including abandoned cattle ponds and ponds dug through stewardship activities. There is substantial variation in the use and apparent quality of these breeding ponds, however the source of this variation is not known. It would be valuable to know the biotic and abiotic factors that expedite the use of artificial ponds and facilitates normal hatching success, egg development and larval recruitment.
Blue-spotted salamander abundance and distribution on Pelee Island
Blue-spotted Salamanders act as sperm donors for Unisexual Ambystoma on Pelee Island, but may also be critically important to the maintenance of genetic diversity within unisexual populations (see Species Biology above). From 2015 to 2017 efforts have been made to characterize the genetic composition of the Pelee Island salamander complex. This work has resulted in the collection and genotyping of 655 adult salamanders and 378 larval samples from across the island. Despite this sampling effort no pure Blue-spotted Salamanders have been identified to date, including in areas where they were observed historically. In addition, only about 2 percent of this sample are unisexuals with proportionally more Blue-spotted Salamander DNA (e.g. LLT, LLLT, Hossie and Murray 2017) which is substantially less than was observed historically (COSEWIC 2004). This indicates that Blue-spotted Salamanders may be absent or at least very rare in the areas surveyed. Recent surveys did not include one breeding location on private land that contained the primary breeding site for Blue-spotted Salamanders historically. This location has not been visited in over 10 years. The current viability and quality of terrestrial and breeding habitat at this location is unknown as is population size and genetic composition of the animals that may occur there. Given the importance of Blue-spotted Salamanders to Unisexual Ambystoma on Pelee Island, research is needed to understand why Blue-spotted Salamanders appear to have declined across much of the island.
Historic and additional populations
Several historical populations have not been visited in over 10 years leaving their current viability and genetic composition unknown. In addition, numerous areas around the island have not been surveyed adequately or at all. It is possible that additional sub-populations exist on the island including on both private and protected land.
1.8 Recovery actions completed or underway
Various organizations have undertaken the acquisition and protection of land on Pelee Island in order to maintain species and habitat; Ontario Parks, ERCA, Ontario Nature and the NCC are included. Each provides some level of visitation and surveillance of their properties. The NCC is actively working to restore large portions of habitat on the island, including the creation of a large wetland complex (J. Crosthwaite, pers. comm. 2017). If this wetland complex remains fish free, meets the criteria outlined above for suitable breeding sites and is located close enough to source populations it could provide new habitat. Note however that there is limited evidence to evaluate whether breeding sites created though stewardship activities provide suitable habitat. The NCC actively removes garlic mustard, honeysuckle and European Common Reed from their properties. Removal of European Common Reed likely benefits the salamanders that occur in those areas. Garlic mustard and honeysuckle removal could be beneficial if these species restrict salamander movement, however data is not available to assess whether this is the case. ERCA is not planning any specific management actions relating to Small-mouthed Salamander conservation at this time, but does actively manage their properties to increase and maintain native biodiversity (D. Lebedyk, pers. comm. 2017). Note however that management activities aimed at increasing or maintaining native biodiversity do not necessarily constitute recovery actions for Ambystoma salamanders. Ontario Nature and Ontario Parks have deployed artificial cover boards to help monitor salamanders on their property. Although positive identification based on morphology is not possible for Ambystoma on Pelee Island, the recently launched Ontario Reptile and Amphibian Atlas phone app may help with outreach and education efforts.
Several private land owners on Pelee Island have engaged in stewardship activities to protect or increase the quality of habitat for salamanders including in areas where the presence and reproductive activities of Small-mouthed Salamanders have been confirmed (Hossie and Murray 2017, MNRF 2017). The Township of Pelee has updated waste disposal methods on Pelee Island which has enabled previous retaining ponds to progress into functioning wetlands (MNRF 2017). In addition, the Township of Pelee has significantly reduced speed limits across much of the island to help address road morality concerns for species at risk including the Small-mouthed Salamander (MNRF 2017). It is not known whether reducing speed limits has an influence on rates of road mortality in salamanders.
Hossie and colleagues initiated a long-term individual-based mark-recapture program on Pelee Island in 2015 (Hossie and Murray 2017). To date, over 740 salamanders (including both Small-mouthed Salamanders and Unisexual Ambystoma) have been measured, individually marked and genotyped across various locations on the island. This team has also established standardized survey areas across the island and has begun monitoring spring breeding activities, larval recruitment and habitat. This work has largely been funded by the Species at Risk Stewardship Fund.
2.0 Recovery
2.1 Recommended recovery goal
The recommended recovery goal is to ensure the long-term persistence of the Small-mouthed Salamander and Unisexual Ambystoma (Small-mouthed Salamander dependent population) on Pelee Island.
2.2 Recommended protection and recovery objectives
| Number | Protection or recovery objective |
|---|---|
| 1 | Protect and maintain or enhance the quality and quantity of habitat for Small-mouthed Salamanders and Unisexual Ambystoma on Pelee Island where Ambystoma salamanders occur, and support habitat creation or restoration activities that increase connectivity among populations. |
| 2 | Implement a monitoring program for salamander populations on Pelee Island that includes assessment of abundance, size or age structure, genetic composition, habitat and screening for emerging pathogens. |
| 3 | Promote and carry out research to better understand Small-mouthed Salamander and Unisexual Ambystoma habitat needs, genetics, population dynamics and threats. |
| 4 | Investigate existing, former and potential Ambystoma salamander habitats on Pelee Island to determine if restoration, re-introduction or population interventions would be appropriate. |
| 5 | Promote stewardship, education and outreach programs for private landowners, residents and visitors on Pelee Island |
2.3 Recommended approaches to recovery
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Protection |
|
|
| Beneficial | Ongoing | Inventory |
|
|
| Necessary | Ongoing | Protection, Management |
|
|
| Beneficial | Ongoing | Stewardship |
|
|
| Necessary | Ongoing | Protection, Inventory, Monitoring and Assessment |
|
|
| Beneficial | Long-term | Management, Stewardship |
|
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Ongoing | Inventory, Monitoring and Assessment |
|
|
| Necessary | Ongoing | Monitoring and Assessment |
|
|
| Necessary | Ongoing | Monitoring and Assessment |
|
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Short-term | Protection, Management, Research |
|
|
| Beneficial | Short-term | Research |
|
|
| Beneficial | Short-term | Research |
|
|
| Beneficial | Long-term | Research |
|
|
| Beneficial | Long-term | Protection, Management, Research |
|
|
| Beneficial | Short-term | Monitoring and Assessment |
|
|
| Beneficial | Short-term | Research |
|
|
| Beneficial | Long-term | Research |
|
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Beneficial | Long-term | Inventory, Management |
|
|
| Beneficial | Long-term | Management, Research |
|
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Beneficial | Short-term | Stewardship, Education and Outreach, Communication |
|
|
| Beneficial | Short-term | Stewardship, Education and Outreach, Communication |
|
|
| Beneficial | Ongoing | Education and Outreach, Communication |
|
|
2.4 Performance measures
The following performance measures can be used to determine whether recovery actions outlined in this recovery strategy have had beneficial effects on Small-mouthed Salamanders, the Unisexual Ambystoma (Small-mouthed Salamander dependent population) or their habitats. These measures should be used within an adaptive management framework to determine if and when recovery actions outlined in this document should be adjusted. They include:
- population trends (increase/decrease), confirmation of breeding activity and recruitment;
- quantification of new/extirpated populations;
- changes in genetic composition of the salamander complex (e.g. proportion of bisexuals, relative abundance of unisexual genomotypes);
- the number and participation of stakeholders involved in related stewardship and monitoring;
- the number of locations for which identified threats have been reduced, mitigated or eliminated;
- assessment, characterization and monitoring of breeding habitat hydrology;
- increased knowledge of aquatic and terrestrial habitat (e.g. radio-telemetry research); and
- recommendations used to inform the habitat regulation process under the ESA 2007;
2.5 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of Natural Resources and Forestry on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the author will be one of many sources considered by the Minister when developing the habitat regulation for this species.
Habitat protection is perhaps the most important means through which conservation efforts can ensure the long-term persistence of Small-mouthed Salamanders and Unisexual Ambystoma (Small-mouthed Salamander dependent population). Protection of both breeding and terrestrial habitat is required for the species to persist.
Breeding habitat
Given the limited geographic extent of the population in Canada, it is recommended that regulated habitat include all confirmed breeding sites on Pelee Island where Small-mouthed Salamander and Unisexual Ambystoma (Small-mouthed Salamander dependent population) occur. Specifically, a habitat regulation should include any wetland, pond or vernal or other temporary pool on Pelee Island that is being used by a Small-mouthed Salamander or Unisexual Ambystoma or was used by a Small-mouthed Salamander or Unisexual Ambystoma on Pelee Island at any time during the previous ten years. A ten year time period recognizes the cryptic nature of these salamanders, the limited availability of suitable habitat, and the difficulty in conducting surveys on Pelee Island when salamanders are most detectable (e.g. during the March breeding season). Similar rationale has been used to support comparable recommendations for a habitat regulation in other highly cryptic and endangered taxa (e.g. Common Five-lined Skink (Carolinian population), Seburn 2010; King Rail, Kraus 2016). More importantly, a 10 year timeframe would allow for variation in usage of breeding sites from year-to-year, and recolonization of the few remaining breeding sites on the island following local extinction. A ten year window would encompass the eight year generation time estimated for Unisexual Ambystoma (Small-mouthed Salamander dependent population) (COSEWIC 2016), plus a two year time period for new adults to reach sexual maturity. Empirical estimates of local extinction and subsequent recolonization in amphibians range widely, but routinely occur within this timeframe for many species (e.g. see Hecnar 1997, Marsh and Trenham 2001 and references therein). A study of Small-mouthed Salamanders in Indiana found egg masses in two sites that were marked as non-breeding sites ten years earlier (Summitt 2009), suggesting recolonization can occur in ten years or less. That said, recolonization of breeding sites by Ambystoma Salamanders following local extinction may be slow when the nearest source population is further than one kilometer away or when the breeding sites are separated by agriculture. For example, a genetic assessment of Small-mouthed Salamander migration rates within an agricultural landscape in Ohio found low rates of migration among adjacent breeding pools (Rhoads et al. 2017). In a related species (A. macrodactylum), five of six Montana lakes that were inhospitable to salamanders in 1978 had been subsequently colonized prior to 1997/1998 (Funk and Dunlap 1999). A separate study estimated annual breeding site colonization rates of 0.20-0.42 for A. macrodactylum (Hossack and Corn 2007).
Small-mouthed Salamanders generally select breeding sites that are especially shallow and ephemeral by nature (i.e. for courtship, egg laving and larval habitat). As a consequence, important breeding sites may not fill up or hold water long enough to facilitate reproduction every year due to annual variation in precipitation and water level in Lake Erie. While this can result in variable breeding activity and recruitment success from one year to next, even breeding sites that occasionally dry up prematurely remain essential habitat because they provide key breeding habitat during particularly wet years. Moreover, adult Ambystoma may forego migration to breeding sites for multiple successive years (Petranka 1998, Pfingsten et al. 2013).
While salamanders may use created ponds for breeding activities, artificial ponds on their own may not be enough to sustain viable populations (J. Bogart, pers. comm. 2017). That said, created breeding sites do currently provide functional breeding habitat for salamanders on Pelee Island (see Distribution, abundance and population trends). It is unknown whether the creation of new ponds could fully compensate for the loss or degradation of existing breeding habitat. Thus long-term protection of known breeding sites is critical.
Known breeding sites which have not been surveyed for over 10 years should undergo no fewer than three consecutive years of surveys to determine the presence of Small-mouthed Salamander or Unisexual Ambystoma before determining that a given breeding site is not in use. A three year survey requirement to determine salamander presence at a breeding site is consistent with requirements outlined in the Jefferson Salamander Recovery Strategy (Jefferson Salamander Recovery Team 2010).
Suitable breeding sites that are within one kilometer of a known salamander breeding site should also be included in a habitat regulation as these areas may provide important habitat in some years and would allow for population expansion. Small-mouthed Salamanders and Unisexual Ambystoma (Small-mouthed Salamander dependent population) may use vernal pools, woodland pools, deciduous swamps, spring-fed pools, groundwater-supported wetlands, sloughs, old deepened or created ponds or ditches. These sites should have egg attachment sites and hold water from March until late June in some or all years. Small-mouthed and Unisexual Salamanders are vulnerable to predation by fish, and therefore ponds that contain fish that prey on salamander eggs, larvae or adults are not suitable habitat until fish can be removed and recolonization prevented.
Terrestrial habitat
All suitable terrestrial habitat extending radially 300 meters from the edge of a known Small-mouthed or Unisexual Ambystoma breeding site should be included in a habitat regulation. Terrestrial habitat for adults and juveniles includes woodlands, swamps, successional areas, meadows, old fields, agricultural fields and other vegetated areas that provide conditions required for foraging, dispersal, migration, growth and hibernation. Suitable terrestrial habitat will always surround or be adjacent to suitable breeding habitat, and is essential for the survival of Small-mouthed Salamanders as well as Unisexual Ambystoma (Small-mouthed Salamander dependent population). Ambystoma salamanders may cross roads or other open areas such as meadows or agricultural fields when migrating or dispersing and this habitat should be protected or managed in such a way that maintains or augments the ability of Ambystoma salamanders to move though these areas. While the movement ecology of salamanders on Pelee Island remains unstudied, estimates from related systems indicate that salamanders move hundreds of meters from breeding ponds during spring migrations to and from breeding sites (e.g. Bériault 2005, Hoffman 2017, Denton et al. 2017). Telemetry work conducted on mainland Ambystoma salamanders in Ontario guided the Jefferson Salamander Recovery Team to recommend that regulated habitat for Jefferson Salamanders include all suitable terrestrial habitat extending radially 300 m from the edge of the breeding pond (Jefferson Salamander Recovery Team 2010). Hoffmann (2017) found that the 95 percent life zone (i.e. the area we can be 95% sure will include the mean of maximum distances moved by the salamanders, not the area that includes 95% of the salamanders) for Ambystoma laterale (2) – jeffersonianum unisexual salamanders was 362 m when including data from four breeding locations. Natural history literature is limited, but suggests that Small-mouthed Salamanders may make less extensive migrations to and from breeding ponds compared to other Ambystoma species (Downs 1989).
A habitat regulation should also include areas that provide suitable conditions for Small-mouthed Salamander or Unisexual Ambystoma to disperse and are within one kilometer of known Small-mouthed Salamander or Unisexual Ambystoma breeding sites. Such a recommendation is consistent with the regulated habitat for the Jefferson Salamander (Jefferson Salamander Recovery Team 2010). Moreover, this would enable intermittent use of nearby suitable habitat and allow for population expansion.
Corridors (i.e. patches of habitat that provide suitable foraging, dispersal, migration or hibernation) which connect known breeding sites that are within three kilometers of one another will be important for the long-term persistence of Small-mouthed Salamanders and Unisexual Ambsytoma (Small-mouthed Salamander dependent population). Corridor habitat allows for recolonization of old sites following local extinctions and facilitates gene flow (and genetic rescue) among existing sub-populations. Increasing connectivity among sub-populations is particularly important for the Pelee Island salamander complex given that existing populations are already separated by some degree of habitat fragmentation and there is no chance for genetic rescue from populations external to the island. The realized dispersal distances reported by Denton et al. (2017) suggest that, given suitable connecting habitat, Small-mouthed Salamanders and Unisexual Ambystoma possess dispersal capabilities that enable colonization of breeding sites several kilometers (i.e. greater than 3 km) from their natal ponds. At the present time robust delineation of corridor habitat beyond one kilometer is difficult due to the poorly understood movement ecology of Small-mouthed Salamanders and Unisexual Ambystoma combined with unknowns regarding the necessary width of such features. Moreover, corridor habitat linking sub-populations separated by up to three kilometers is likely to include agricultural land and private property. Therefore, areas that provide suitable conditions for Small-mouthed Salamander or Unisexual Ambystoma to migrate or disperse, and would link breeding sites that are separated by up to three kilometers, but are beyond one kilometer from breeding sites should be protected and enhanced through stewardship and best management practices instead of explicit inclusion within a habitat regulation at this time. Wherever possible efforts should be made to increase the connectivity of various sub-populations on the island by protecting or augmenting forested habitat between breeding sites, even when breeding sites are further than one kilometer from one another or separated by roads or agricultural fields. Note that corridors need not be continuous patches of forest, however forested habitats offer the lowest resistance to dispersing and migrating Ambystoma salamanders (Rothermel and Semlitsch 2002, Compton et al. 2007). Efforts to identify and improve corridor habitat should be informed by techniques such as least-cost path analysis employing resistance values from related Ambystoma species, such as those reported by Compton et al (2007).
Until the movement ecology of Ambystoma salamanders on Pelee Island is better documented, it is recommended to enact protection of terrestrial habitat and breeding sites that is no less stringent than existing regulations for Jefferson Salamander habitat outlined in the Endangered Species Act (ESA 2007). However, habitat regulations should also incorporate contemporary knowledge about the migration and dispersal capabilities of Small-mouthed Salamanders and Unisexual Ambystoma (e.g. Denton et al. 2017), and be revised when data specific to populations on Pelee Island becomes available. Namely, it is recommended that the habitat regulation for Small-mouthed Salamander and Unisexual Ambystoma (Small-mouthed Salamander dependent population) include:
- Any wetland, pond or vernal or other temporary pool that is being used by Small-mouthed Salamander or Unisexual Ambystoma, or was used by a Small-mouthed Salamander or Unisexual Ambystoma at any time during the previous ten years.
- An area that is within 300 meters of a wetland, pond or vernal or other temporary pool that is being used by Small-mouthed Salamander or Unisexual Ambystoma, or was used by a Small-mouthed Salamander or Unisexual Ambystoma at any time during the previous ten years, on Pelee Island that provides suitable foraging, dispersal, migration or hibernation conditions for Small-mouthed Salamander or Unisexual Ambystoma.
- Areas that provide suitable conditions for Small-mouthed Salamander or Unisexual Ambystoma to disperse and are within one kilometer of known Small-mouthed Salamander or Unisexual Ambystoma breeding habitat.
- A wetland, pond, vernal or temporary pool that would provide suitable breeding conditions for Small-mouthed Salamander or Unisexual Ambystoma that is within one kilometer of a wetland, pond or vernal or other temporary pool that is being used by Small-mouthed Salamander or Unisexual Ambystoma, or was used by a Small-mouthed Salamander or Unisexual Ambystoma at any time during the previous ten years.
Exclusions
Existing houses, buildings, and structures that are within 300 meters of a breeding pond should not be included within the habitat regulation. Open areas such as agricultural fields that are within 300 meters of a breeding pond, but do not serve as corridors to forested terrestrial habitats and/or other breeding areas should also be excluded. The ESA (2007) describes species exemptions for pits and quarries that include the Small-mouthed Salamander. Where possible opportunities for stewardship and collaboration with pit and quarry operators should be sought.
Newly discovered occurrences
There are numerous locations on Pelee Island that have not yet been adequately surveyed to determine whether Small-mouthed Salamanders or Unisexual Ambsytoma are present. Surveys of breeding sites may take up to three years to sufficiently document the presence or absence of these salamanders.
Glossary
- Cercaria
- A free-swimming larval stage of a parasitic flatworm (also known as trematodes or flukes) during which it passes from an intermediate host (e.g. a snail) to another intermediate host or to the final vertebrate host. In some species, the cercaria will encyst in the intermediate host and rest as a metacercaria.
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC)
- The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
- Committee on the Status of Species at Risk in Ontario (COSSARO)
- The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
- Conservation status rank
- A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. Ranks are determined by NatureServe and, in the case of Ontario’s S-rank, by Ontario’s Natural Heritage Information Centre. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperilled
2 = imperilled
3 = vulnerable
4 = apparently secure
5 = secure
NR = not yet ranked - Cloaca
- A common outlet into which the intestinal, urinary, and genital tracts open. It is located at the base of the tail in salamanders and becomes swollen in male Ambystoma during the breeding season.
- Costal grooves
- Indentations in the skin corresponding to the location of ribs along the sides of salamanders.
- Designatable unit
- Following COSEWIC criteria, a designatable unit is any wildlife species, subspecies or variety that is eligible for status assessment (see full details in COSEWIC’s Guidelines for Recognizing Designatable Units, Appendix F5). Briefly this includes species or groups that are genetically distinct, separated by a major range disjunction, or biogeographically distinct. Normally these species must also be considered a native wildlife species that regularly occurs in Canada
- .Endangered Species Act, 2007 (ESA)
- The provincial legislation that provides protection to species at risk in Ontario.
- Genetic rescue
- The natural or facilitated transfer of genes to inbred populations which results in an overall increase in genetic diversity and biological fitness (e.g. fertility, survival, longevity) in that population. Inbred populations have low genetic diversity, typically resulting from reproduction among closely related individuals in small populations, and often experience a reduction to their biological fitness.
- Genome
- A set of chromosomes containing the genetic material of an organism.
- Hybrid
- An offspring of two individuals of different species.
- Life zone
- The area around a breeding pond that we can be 95% sure will include the mean of maximum distances moved by the salamanders migrating away from that pond. This is not equivalent to the area that includes 95% of the salamanders. See Hoffman (2017) for more details.
- Metacercaria
- See cercaria above.
- Metamorphosis
- Change of physical form, structure or substance, such as the change from larva to adult.
- Monophyletic lineage
- A group of organisms that consists of all the descendants from a common ancestor. For example, genetic work using mitochondrial DNA has revealed that all Unisexual Ambystoma descend from a common ancestor, thus Unisexual Ambystoma form a monophyletic clade despite relying on various sperm donors and having nuclear DNA that is similar to their local sperm donor species.
- Ploidy
- The number of sets of chromosomes an organism possesses (e.g. diploid – two sets, triploid – three sets of chromosomes, tetraploid – four sets of chromosomes, pentaploid – five sets of chromosomes).
- Realized dispersal distance
- The distance that an individual has travelled to from its natal population.
- Species at Risk Act (SARA)
- The federal legislation that provides protection to species at risk in Canada. This Act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
- Species at Risk in Ontario (SARO) List
- The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
References
Bériault, Karine, pers. comm. 2017. Telephone conversation with T. Hossie. July 2017. Management Biologist, Ministry of Natural Resources and Forestry, Parry Sound, ON.
Bériault, K.R.D. 2005. Critical Habitat of Jefferson Salamanders in Ontario: An Examination through Radiotelemetry and Ecological Surveys. M.Sc. Thesis, University of Guelph, 69 pp.
Bi, K., and J.P. Bogart. 2010. Time and time again: unisexual salamanders (genus Ambystoma) are the oldest unisexual vertebrates. BMC Evolutionary Biology 10:238.
Bi, K, J.P. Bogart, and J. Fu. 2008. The prevalence of genome replacement in unisexual salamanders of the genus Ambystoma (Amphibia, Caudata) revealed by nuclear gene genealogy. BMC Evolutionary Biology 8:158.
Bogart, Jim, pers. comm. 2017. Email correspondence with T. Hossie. July 2017. Professor Emeritus, University of Guelph, Guelph, ON.
Bogart, J.P., and K. Bi. 2013. Genetic and genomic interactions of animals with different ploidy levels. Cytogenetic and Genome Research 140: 117-136.
Bogart, J.P., L.E. Licht, M.J. Oldham, and S.J. Darbyshire. 1985. Electrophoretic identification of Ambystoma laterale and Ambystoma texanum as well as their diploid and triploid interspecific hybrids (Amphibia: Caudata) on Pelee Island, Ontario. Canadian Journal of Zoology 63: 340-347.
Bogart, J.P., R.P. Elinson, and L.E. Licht. 1989. Temperature and sperm incorporation in polyploidy salamanders. Science 246:1032-1034.
Bogart, J.P., K. Bi, J. Fu, D.W.A. Noble, and J. Niedzwieki. 2007. Unisexual salamanders (genus Ambystoma) present a new reproductive mode for eukaryotes. Genome 50:119-136.
Bogart, J.P., J. Bartoszek, D.W.A. Noble, and K. Bi. 2009. Sex in unisexual salamanders: discovery of a new sperm donor with ancient affinities. Heredity 103:483-493.
Bogart, J.P., and L.E. Licht. 1986. Reproduction and the origin of polyploids in hybrid salamanders of the genus Ambystoma. Canadian Journal of Genetics and Cytology 28: 605-617.
Bogart, J.P., and L.E. Licht. 1987. Evidence for the requirement of sperm in unisexual salamander hybrids (genus Ambystoma). Canadian Field Naturalist 101:434-436.
Bollinger, T. K., J. Mao, D. Schock, R. M. Brigham, and V. G. Chinchar. 1999. Pathology, isolation and preliminary molecular characterization of a novel iridovirus from tiger salamanders in Saskatchewan. Journal of Wildlife Diseases 35: 413–429.
Bowman, Jeff, pers. Comm. 2017. Email correspondence with T. Hossie. July 2017. Research Scientist. Wildlife Research & Monitoring Section. Ontario Ministry of Natural Resources and Forestry, Peterborough, ON.
Cagle, F.R. 1942. Herpetological fauna of Jackson and Union counties, Illinois. American Midland Naturalist 28: 164-200.
Compton, B.W., K. McGarigal, S.A. Cushman, and L.R. Gamble. 2007. A resistant-kernel model of connectivity for amphibians that breed in vernal pools. Conservation Biology 21: 788–799.
Canadian Herpetofauna Health Working Group. 2017. Decontamination Protocol for Field Work with Amphibians and Reptiles in Canada. 7 pp + ii.
Crosthwaite, Jill, pers. comm. 2017. Email correspondence with T. Hossie. July 2017. Coordinator, Conservation Biology, Southwestern Ontario subregion, Nature Conservancy of Canada, London, ON.
COSEWIC. 2004. COSEWIC assessment and update status report on the Small-mouthed Salamander Ambystoma texanum in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. v + 20 pp.
COSEWIC. 2014a. COSEWIC status appraisal summary on Small-mouthed Salamander (Ambystoma texanum) in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 10 pp (i-x).
COSEWIC. 2014b. COSEWIC assessment and status report on the Blue Ash Fraxinus quadrangulata in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xiii + 58 pp.
COSEWIC. 2016. COSEWIC assessment and status report on the Unisexual Ambystoma, Ambystoma laterale, Small-mouthed Salamander–dependent population, Jefferson Salamander–dependent population and the Blue-spotted Salamander–dependent population, in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xxii + 61 pp. (COSEWIC Assessment and Status Report on the Unisexual Ambystoma in Canada).
COSSARO. 2016. Ontario Species at Risk Evaluation Report for Unisexual Ambystoma (Ambystoma laterale), Small-mouthed Salamander–dependent population, Jefferson Salamander–dependent population and the Blue-spotted Salamander–dependent population. Committee on the Status of Species at Risk in Ontario. 29 pp.
Daszak, P., L. Berger, A.A. Cunningham, A.D. Hyatt, D.E. Green, and R. Speare. 1999. Emerging infectious diseases and amphibian declines. Emerging Infectious Diseases 5: 735–748.
Denton, R.D., K.R. Greenwald, and H.L. Gibbs. 2017. Locomotor endurance predicts differences in realized dispersal between sympatric sexual and unisexual salamanders. Functional Ecology 31: 915-926.
Downs, F. 1989. Ambystoma jeffersonianum. Pp. 87-172, in R.A. Pfingsten and F.L. Downs. (eds.). Salamanders of Ohio. Ohio Biological Survey Bulletin, New Series Vol. 7, No. 2.
Fernandes, R., V. Korolevych, and S. Wang. 2007. Trends in land evapotranspiration over Canada for the period 1960–2000 based on in situ climate observations and a land surface model. Journal of Hydrometeorology 8: 1016-1030.
Forsan, D.D., and A. Storfer. 2006. Atrazine increases ranavirus susceptibility in the tiger salamander, Ambystoma tigrinum. Ecological Applications 16: 2325-2332.
Funk, W.C., and W.W. Dunlap. 1999. Colonization of high-elevation lakes by long-toed salamanders (Ambystoma macrodactylum) after the extinction of introduced trout populations. Canadian Journal of Zoology 77: 1759-1767.
Garton, J.S. 1972. Courtship of the small-mouthed salamander, Ambystoma texanum, in southern Illinois. Herpetologica 28: 41-45.
Garcia, T.S., R. Straus, and A. Sih. 2003. Temperature and ontogenetic effects on color change in the larval salamander species Ambystoma barbouri and Ambystoma texanum. Canadian Journal of Zoology 81: 710-715.
Garcia, T.S., J. Stacey, and A. Sih. 2004. Larval salamander response to UV radiation and predation risk: color change and microhabitat use. Ecological Applications 14: 1055-1064.
Gibbs, J.P., and W.G. Shriver. 2005. Can road mortality limit populations of pool-breeding amphibians? Wetlands Ecology and Management 13:281-289.
Gould, Ron, pers. comm. 2017. Telephone conversation and e-mail correspondence with T. Hossie. July and November 2017. Zone Ecologist, MNRF, Aylmer, ON.
Greenberg, D.A., and D.M. Green. 2013. Effects of an invasive plant on population dynamics in toads. Conservation Biology 27:1049-1057.
Greenwald, K.R., J.L. Purrenhage, and W.K. Savage. 2009. Landcover predicts isolation in Ambystoma salamanders across region and species. 142: 2493-2500.
Griffis-Kyle, K.L. 2007. Sublethal effects of nitrite on eastern tiger salamander (Ambystoma tigrinum tigrinum) and wood frog (Rana sylvatica) embryos and larvae: implications for field populations. Aquatic Ecology 41: 119-127.
Hamill, S.E. 2015. Recovery Strategy for the Small-mouthed Salamander (Ambystoma texanum) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources and Forestry, Peterborough, Ontario. vi + 18 pp.
Harding, J.H. 1997. Amphibians and Reptiles of the Great Lakes Region. University of Michigan Press, Ann Arbor, 378 pp.
Hecnar, S.J. 1997. Amphibian pond communities in southwestern Ontario. Pp. 1-15. in D.M. Green (ed.). Amphibians in decline: Canadian studies of a global problem. Society for the study of Amphibians and Reptiles. Saint Louis, Missouri, U.S.A.
Hedges, S.B., J.P. Bogart, and L.M. Maxson. 1992. Ancestry of unisexual salamanders. Nature 356:708-710.
Hoffman, K. 2017. Breeding Ecology and Habitat Use of Unisexual Salamanders and their Sperm-Hosts, Blue-Spotted Salamanders (Ambystoma laterale). Ph.D. Thesis, University of Maine, 128 pp.
Hossack, B.R., and P.S. Corn. 2007. Responses of pond-breeding amphibians to wildfire: short-term patterns in occupancy and colonization. Ecological Applications 17: 1403-1410.
Hossie, T.J., and D. Murray. 2017. Assessing the population size, genetic structure, critical habitat, and predation threats in Small-mouthed Salamanders. Prepared for the Ontario Ministry of Natural Resources and Forestry, Ontario. 80 pp.
Jefferson Salamander Recovery Team. 2010. Recovery Strategy for the Jefferson Salamander (Ambystoma jeffersonianum) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. vi + 27 pp.
Jutterbock, J.E., and P.C. Owen. 2013. Blue-spotted salamander, Ambystoma laterale (Hallowell 1856) Pp. 101-112, in R.A. Pfingsten, J.G. Davis, T.O. Matson, G.L. Lipps, Jr., D. Wynn and B.J. Armitage (eds.). Amphibians of Ohio. Ohio Biological Survey. Columbus, Ohio. 899 pp.
Karraker, N.E., and J.P. Gibbs. 2011. Road deicing salt irreversibly disrupts osmoregulation of salamander egg clutches. Environmental Pollution 159:833-835.
Kats, L.B., J.W. Petranka, and A. Sih. 1998. Antipredator defences and the persistence of amphibian larvae with fishes. Ecology 69: 1865-1870.
King, R.B., Oldham, M.J., and W.F. Weller. 1997. Historic and current amphibian and reptile distributions in the island region of western Lake Erie. American Midland Naturalist 138: 153-173.
Kolozsvary, M.B., and R.K. Swihart. 1999. Habitat fragmentation and the distribution of amphibians: patch and landscape correlates in farmland. Canadian Journal of Zoology 77: 1288–1299.
Kraus, F. P.K. Ducey, P. Moler, and M.M. Miyamoto. 1991. Two new triparental Unisexual Ambystoma from Ohio and Michigan. Herpetologica 47: 429-439.
Kraus, Talena. 2016. Recovery Strategy for the King Rail (Rallus elegans) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources and Forestry, Peterborough, Ontario. v + 8 Pp. + Appendix.
Lebedyk, Dan, pers. comm. 2017. Email correspondence with T. Hossie. July 2017. Biologist/Ecologist, Essex Region Conservation Authority, Essex, Ontario.
Lesbarreres, D., A. Balseiro, J. Brunner, V.G. Chinchar, A. Duffus, J. Kerby, D.L. Miller, J. Robert, D.M. Schock, T. Waltzek, and M.J. Gray. 2012. Ranavirus: past, present and future. Biology Letters 8: 481-483.
Licht, L.E. 1989. Reproductive parameters of Unisexual Ambystoma on Pelee Island, Ontario. Pp. 209-217, in R. Dawley and J.P. Bogart (eds.). Evolution and Ecology of Unisexual Vertebrates. New York State Museum Bull. 466. Albany, New York.
Licht, L.E., and J.P. Bogart. 1989. Growth and sexual maturation in diploid and polyploidy salamanders (genus Ambystoma). Canadian Journal of Zoology 67:812-818.
Licht, L.E., and J.P. Bogart. 1990. Courtship behavior of Ambystoma texanum on Pelee Island, Ontario. Journal of Herpetology 24:450-452.
Lowcock, L.A. 1989. Biogeography in hybrid complexes of Ambystoma: the interpretation of unisexual-bisexual genetic data in space and time. Pp. 180-207, in R. Dawley and J.P. Bogart (eds.). Evolution and Ecology of Unisexual Vertebrates. New York State Museum Bull. 466. Albany, New York.
MacCulloch, R.D. 2002. The ROM Field Guide to Amphibians and Reptiles of Ontario. Royal Ontario Museum, Toronto, ON.
Marco, A. C. Quilchano, and A.R. Blaustein. 1999. Sensitivity to nitrate and nitrite in pond-breeding amphibians from the pacific northwest, USA. Environmental Toxicology and Chemistry 12: 2836-2839.
Marsh, D.M., and P.C. Trenham. 2001. Metapopulation dynamics and amphibian conservation. Conservation Biology 15: 40-49.
Martel, A., A. Spitzen-van der Sluijs, M. Blooi, W. Bert, R. Ducatelle, M.C. Fisher, A. Woeltjes, K. Chiers, F. Bossuyt, and F. Pasmans. 2013. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proceeding of the National Academy of Sciences of the United States of America 110: 15325–15329.
Martel, A., M. Blooi, C. Adriaensen, P. Van Rooij, W. Beukema, M.C. Fisher, R. A. Farrer, B.R. Schmidt, U. Tobler, K. Goka, K.R. Lips, C. Muletz, K.R. Zamudio, J. Bosch, S. Lötters, E. Wombwell, T.W.J. Garner, A.A. Cunningham, A. Spitzen-van der Sluijs, S. Salvidio, R. Ducatelle, K. Nishikawa, T.T. Nguyen, J.E. Kolby, I. Van Bocxlaer, F. Bossuyt, and F. Pasmans. 2014. Recent introduction of a chytrid fungus endangers Western palearctic salamanders. Science 346: 630–631. (DOI:10.1126/science.1258268)
McAllister, C.T., C.R. Bursey, J.A. Crawford, A.R. Kuhns, C. Shaffer, and S.E. Trauth. 2010. Metacercariae of Clinostomum (Trematoda: Digenea) from three species of Ambystoma (Caudata: Ambystomatidae) from Arkansas and Illinois, USA. Comparative Parasitology 77:25-30.
McRoberts, J.T., M.C. Wallace, and S.W. Eaton. 2014. Wild Turkey (Meleagris gallopavo), version 2.0. In P. G. Rodewald (ed.). The Birds of North America. Cornell Lab of Ornithology, Ithaca, New York, USA. Wild Turkey Information - Birds of North America [accessed: November 10, 2017].
Metts, B.S., W.A. Hopkins, and J.P. Nestor. 2005. Interaction of an insecticide with larval density in pond-breeding salamanders (Ambystoma). Freshwater Biology 50:685-696.
Mills, P.B. 2016. Metamorphosis: Ontario’s amphibians at all stages of development. Published by P.B Mills. Printed and bound by SLG Group, Brampton, ON. 104 pp.
Minton, S.A. 1954. Salamanders of the Ambystoma jeffersonianum complex in Indiana. Herpetologica 10:173-179.
Minton, S.A. Jr. 2001. Amphibians and reptiles of Indiana (Revised Second Edition). Indiana Academy of Science. Indianapolis, Indiana, 404 pp.
Mortsch, L., J. Ingram, A. Hebb, and S. Doka (eds.). 2006. Great Lakes Coastal Wetland Communities: Vulnerability to Climate Change and Response to Adaptation Strategies. Final report submitted to the Climate Change Impacts and Adaptation Program, Natural Resources Canada. Environment Canada and the Department of Fisheries and Oceans, Toronto, Ontario. 251 pp. + appendices.
MNRF. 2017. Draft Government Response Statement to the Recovery Strategies for Blue Racer, Lake Erie Watersnake and Small-mouthed Salamander in Ontario. i + 15 pp.
Moulton, R.J., and D.R. Cuthbert. 2000. Cumulative impacts/risk assessment of water removal or loss from the Great Lakes-St. Lawrence River system. Canadian Water Resources Journal 25:181–208.
Natural Resources Conservation Service (1999). Wild Turkey (Meleagris gallopavo). Fish and Wildlife Habitat Management Leaflet. Number 12. United States Department of Agriculture. 12 pp.
NatureServe. 2017. NatureServe Explorer: An online encyclopedia of life [web application] Version 7.1. NatureServe, Arlington, Virginia. Web site: [accessed: July 25, 2017].
Owen, P.C., and J.E. Jutterbock. 2013. Small-mouthed salamander, Ambystoma texanum (Mathes 1855). Pp. 114-155, in R.A. Pfingsten, J.G. Davis, T.O. Matson, G.L. Lipps, Jr., D. Wynn and B.J. Armitage (eds.). Amphibians of Ohio. Ohio Biological Survey. Columbus, Ohio. 899 pp.
Parmalee, J.R. 1993. Microhabitat segregation and spatial relationships among four species of mole salamanders (genus Ambystoma). Occasional Papers of the Museum of Natural History, University of Kansas 160: 1-33.
Petranka, J.W. 1998. Salamanders of the United States and Canada. Smithsonian Institution Press, Washington and London, 587 pp.
Pfingsten, R.A., J.G. Davis, T.O. Matson, G.L. Lipps, Jr., D. Wynn, and B.J. Armitage (eds.). 2013. Amphibians of Ohio. Ohio Biological Survey. Columbus, Ohio. 899 pp.
Pierce, B.A. and D.K. Wooten. 1992. Acid tolerance of Ambystoma texanum from central Texas. Journal of Herpetology 26: 230-232.
Punzo, F. 1983. Effects of environmental pH and temperature on embryonic survival capacity and metabolic rates in the Smallmouth Salamander, Ambystoma texanum. Bulletin of Environmental Contamination and Toxicology 31 467–473.
Rhoads, E.A., P.K. Williams, and C.M. Krane. 2017. High inbreeding and low connectivity among Ambystoma texanum populations in fragmented Ohio forests. Ecology and Evolution DOI: 10.1002/ece3.3637.
Richgels K.L.D., R.E. Russell, M.J. Adams, C.L. White, and E.H.C. Grant. 2016 Spatial variation in risk and consequence of Batrachochytrium salamandrivorans introduction in the USA. Royal Society Open Science 3: 150616. Online Scientific Paper: Spatial variation in risk and consequence of Batrachochytrium salamandrivorans introduction in the USA
Rothermel, B.B., and R.D. Semlitsch. 2002. An experimental investigation of landscape resistance of forest versus old-field habitats to emigrating juvenile amphibians. Conservation Biology 16: 1324-1332.
Ryan, T.J. 2007. Hydroperiod and metamorphosis in small-mouthed salamanders (Ambystoma texanum). Northeastern Naturalist 14: 619-628.
Sandinski, W.J., and W.A. Dunson. 1992. A multilevel study of effects of low pH on amphibians in temporary ponds. Herpetologica 26:413-422.
Seburn, D.C. 2010. Recovery strategy for the Common Five-lined Skink (Plestiodon fasciatus) – Carolinian and Southern Shield populations in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. vi + 22 pp.
Seburn, D., and C. Seburn. 2000. Conservation Priorities for the Amphibians and Reptiles of Canada. Prepared for World Wildlife Fund Canada and the Canadian Amphibian and Reptile Conservation Network. 92 pp.
Stephen, C., M.J. Forzan, T. Redford, and M. Zimmer. 2015. Batrachochytrium salamandrivorans: a threat assessment of salamander chytrid disease. Canadian Wildlife Health Cooperative. 31 pp.
Summitt, S.D. 2009. Determination of dispersal patterns of the Small-mouthed Salamander (Ambystoma texanum) in Eagle Creek Park (Indianapolis, IN). Undergraduate Honors Thesis, Butler University, 26 pp.
Walston, L.J., and S.J. Mullin. 2007. Responses of a pond-breeding amphibian community to the experimental removal of predatory fish. The American Midland Naturalist 157: 63-73.
Williams, P.K. 1973. Seasonal movements and population dynamics of four sympatric Mole Salamanders, genus Ambystoma. Ph.D. Thesis. Indiana University, Bloomington, IN.
Yap, B.T.A., M.S. Koo, R.F. Ambrose, D.B. Wake, and V.T. Vredenburg. 2015. Averting a North American biodiversity crisis. Science 349, 481–482.
List of abbreviations
- COSEWIC: Committee on the Status of Endangered Wildlife in Canada
- COSSARO: Committee on the Status of Species at Risk in Ontario
- CWS: Canadian Wildlife Service
- ERCA: Essex Region Conservation Authority
- ESA: Ontario’s Endangered Species Act, 2007
- ISBN: International Standard Book Number
- MNRF: Ontario Ministry of Natural Resources and Forestry
- NCC: Nature Conservancy of Canada
- SARA: Canada’s Species at Risk Act
- SARO: Species at Risk in Ontario
Appendix
| Jurisdiction | Conservation Status Rank |
|---|---|
| Global | G5 |
| Canada | N1 |
| Ontario | S1 |
| USA | N5 |
| Alabama | S3 |
| Arkansas | S5 |
| Illinois | S5 |
| Indiana | S4 |
| Iowa | S3 |
| Kansas | S5 |
| Kentucky | S5 |
| Louisiana | S5 |
| Michigan | S1 |
| Mississippi | S5 |
| Missouri | S5 |
| Nebraska | S1 |
| Ohio | SNR |
| Oklahoma | S5 |
| Tennessee | S5 |
| Texas | S5 |
| West Virginia | S1 |
*See Conservation Status Rank in the Glossary for the meaning of each ranking level.