Survey protocol for Ontario’s species at risk snakes
This survey protocol provides a standardized, science-based approach for conducting field surveys for species at risk snakes in Ontario.

Recommended citation
Recommended Citation: OMNRF. 2016. Survey Protocol for Ontario’s Species at Risk Snakes. Ontario Ministry of Natural Resources and Forestry, Species Conservation Policy Branch. Peterborough, Ontario. ii + 17 pp.
Cover illustrations: Eastern Hog-nosed Snake (top), snake habitat on Beausoleil Island (bottom left) and Butler’s Gartersnake (bottom right). Photographs by Joe Crowley.
Cette publication hautement spécialisée, protocole de suivi pour les espèces de serpents en péril en Ontario, en Ontario n’est disponible qu’en anglais en vertu du Règlement 671/92 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec le ministère des Richesses naturelles au
Le présent document vise à établir un protocole normalisé et efficace pour la réalisation d’études sur le terrain sur les serpents en péril en Ontario. Ce protocole décrit les aspects de la biologie des espèces qui sont associés à la détectabilité et à l’identification, leurs aires de répartition, les méthodes d’étude qui conviennent, les qualifications de l’expert et les normes de communication des données en Ontario. Il décrit aussi les conditions qui sont nécessaires pour déduire avec suffisamment d’assurance qu’une espèce est absente dans une région donnée. Le protocole vise à éclairer le travail réalisé sur les serpents en péril conformément aux exigences ou aux conditions de la Loi ontarienne sur les espèces en voie de disparition, mais il peut aussi être appliqué dans d’autres situations où des études sur les serpents en péril doivent être entreprises en Ontario.
© Queens Printer for Ontario, 2016
ISBN 978-1-4606-8168-8 (PDF)
1. Introduction
Effective protection and recovery of species at risk (SAR) and their habitat requires comprehensive and up-to-date knowledge of species’ occurrence and distribution. However, there have been few large-scale surveys and inventories for most of Ontario’s species at risk, and recent, detailed occurrence data are not available for many of these species throughout the province. In the absence of existing occurrence data, field surveys are necessary to determine if a species is present at a particular site. However, many species at risk are inherently rare, occur at low densities and are cryptic, making detection of these species difficult. Furthermore, the detection probability of some species varies considerably with time of year, habitat, weather conditions and search method. This survey protocol was developed in response to the need for reliable, science-based survey methods for species at risk in Ontario. This protocol is based on the best available scientific and technical information at the time of publication, including information from several expert Ontario herpetologists, but it may be subject to change should new information become available.
In addition to providing guidance on survey methodology, the protocol also identifies the level of search effort that is necessary to determine, with reasonable confidence, that a snake species is absent from a site. This level of search effort is recommended when survey data are used to inform assessments of species’ absence. This protocol does not provide methodology to determine population abundance or monitor changes over time. For information about determining species abundance, population monitoring and other field methodology for reptiles see McDiarmid et al. (2012).
This survey protocol provides a recommended approach to assess presence / absence at a site. However, determining if section 10 (general or regulated habitat) of the Endangered Species Act (ESA) applies to a site is a complex process that is not limited to presence / absence surveys. For example, even at sites where survey results are negative, general or regulated habitat of a species at risk may still be present at the site based on nearby occurrences of the species (e.g. on an adjacent property) or the manner in which the habitat is defined within a regulation, habitat description or policy.

2. Species information
This protocol is intended to inform surveys for all Ontario species at risk snakes, with the exception of the Queensnake (Blue Racer, Butler’s Gartersnake, Eastern Foxsnake, Eastern Hog-nosed Snake, Eastern Ribbonsnake, Gray Ratsnake, Lake Erie Watersnake, Massasauga, and Milksnake). A separate survey protocol exists for Queensnake (OMNRF 2015). Individuals carrying out surveys for Ontario’s snakes should be familiar with the identification, ecology, habitat use and distribution of the target species. The following resources provide this species-specific information and should be used as core reference material to accompany this survey protocol:
- The snakes of Ontario: Natural History, Distribution, and Status (Rowell 2012)
- Ontario Ministry of Natural Resources and Forestry (OMNRF) habitat regulations and habitat descriptions (available at Ontario Government website)
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC) status reports (COSEWIC website)
- Species accounts in the Ontario Reptile and Amphibian Atlas (Ontario Reptile and Amphibian Atlas)
- Species accounts on the Canadian Herpetological Society website (Canadian Herpetological Society)
Primary scientific literature and consultation with species experts (including OMNRF staff) can also be a valuable resource. Specifically, consultation with local experts and naturalists can be critical in understanding the local species ecology and habitat use, which often varies among regions.
3. Survey considerations
3.1 Surveyor qualifications
Surveyor experience can significantly influence the probability of species detection when surveying for snakes (Black and Parent 1999; British Columbia Ministry of Environment Lands and Parks 1998; Casper et al. 2001), and surveys carried out by inexperienced surveyors are more likely to result in false negatives (Casper et al. 2001). Consequently, reptile surveys should be carried out by individuals who have a general understanding of snake biology and ecology, as well as prior experience with the target species (British Columbia Ministry of Environment Lands and Parks 1998; Casper et al. 2001; Department of Sustainability, Environment, Water, Population and Water, Population and Communities 2011; S. Gillingwater pers. comm. 2012; J. Litzgus pers. comm. 2012). If individuals who are experienced with the target species are not available, it is highly recommended that the lead surveyor have the following qualifications:
- Prior experience conducting wildlife surveys
- Knowledge of the biology, ecology and habitat use of the target species
- Experience and demonstrated competence with other snake species
- Training from someone with expertise in the target species or through a formal training course that includes field techniques for the target species; A person is considered to have expertise with a species if they have carried out research on that species through a university or other academic institution or is generally recognized within the scientific community as having expertise with the target species.
- The ability to distinguish the target species from similar species in Ontario
Surveyors should also have the ability to navigate, record the survey track, and geo-reference observations using a Global Positioning System (GPS) unit.
An authorization under the ESA, 2007 and a Wildlife Scientific Collectors Authorization under the Fish and Wildlife Conservation Act (FWCA), 1997 may be required to carry out surveys for snakes in Ontario, depending on the species and survey methods. Additional permits may be required from Ontario Parks or Parks Canada Agency if surveys are carried out in provincial parks and conservation reserves or national parks, respectively.
3.2 Records review
A records review should be carried out prior to a field survey. Existing occurrence records may help to better scope the field survey or, if extensive data is already available for a site, existing records may eliminate the need for a field survey. The absence of occurrence records from an area does not indicate that the species is absent; suitable habitat must be adequately surveyed before concluding that the species is unlikely to be present. The following sources can be consulted for information on snake distribution and occurrence records within Ontario:
- OMNRF Natural Heritage Information Centre (NHIC) or by email
- Ontario Reptile and Amphibian Atlas (ORAA)
- Local Conservation Authorities
- Status reports from the Committee on the Status of Endangered Wildlife in Canada (COSEWIC); available through the Species at Risk Act (SARA) Public Registry
- Other information sources such as, but not limited to species experts, OMNRF offices, site-related environmental impact or screening reports, published scientific literature and natural history inventories
3.3 Seasonal timing of surveys for snakes
In Ontario, snakes hibernate underground during the late fall, winter and early spring. Consequently, it is necessary to carry out surveys when snakes are active above ground, known as the active season. In Ontario, the active season typically begins in April or May and ends in September or October, depending on the species, latitude and seasonal weather variation. Thus, these dates should be refined for each situation using detailed species information (see section 2 for relevant sources of information) and regional weather data. Spring and early summer surveys are typically most productive because snakes tend to bask more frequently and are more conspicuous at that time of year (see section 3.4).
The likelihood of a given habitat being occupied at a certain time of year is also an important consideration when planning snake surveys. Depending on the species, snake habitat use may also change throughout the active season (e.g. Carfagno and Weatherhead 2006; Harvey and Weatherhead 2006). For example, Massasaugas on the Bruce Peninsula use forested areas in the spring and they move into larger open-canopy habitats (e.g. rock outcrops or alvars) in June (Harvey and Weatherhead 2006).
3.4 Environmental conditions
Snakes are ectotherms and regulate their body temperature through behavioural thermoregulation (e.g. basking in the sun or seeking shelter from the heat). Ontario’s snakes have preferred body temperatures within the range of 25-34 °C, and they select microhabitats that allow them to maintain body temperatures as close as possible to this preferred range (Brown and Weatherhead 2000; Blouin-Demers and Weatherhead 2001b; Row and Blouin-Demers 2006b; Harvey and Weatherhead 2010; Harvey and Weatherhead 2011). Snakes are most likely to bask on sunny days when ambient temperature is lower than preferred body temperature (Row and Blouin-Demers 2006b; Harvey 2008), especially when these conditions follow several days of inclement weather. Harvey (2008) reported that Massasaugas in Ontario are most likely to bask at temperatures between 16 and 26 °C. Other Ontario species prefer lower temperatures (Harvey and Weatherhead 2011) and likely bask under cooler conditions. Basking tends to be highest in the spring due to low environmental temperatures and the need to increase metabolic activity after hibernation, and basking activity is often lowest in the fall (Row and Blouin-Demers 2006b; Harvey and Weatherhead 2011). Being ectothermic also means that metabolic rates and activity levels are dependent on the ambient temperature and the snakes’ ability to thermoregulate (Blouin-Demers et al. 2003; Harvey and Weatherhead 2010). In Ontario, snakes are most active when air temperature is between 15 and 30 °C.
By influencing microhabitat use and activity levels, environmental conditions have a significant effect on detectability. For example, consider how the following environmental conditions affect detectability:
- Cool overcast (or stormy) conditions: snakes cannot warm up and are likely to be inactive and remain hidden (low detectability).
- Warm conditions: snakes can be encountered moving throughout habitat (moderate to high detectability).
- Cool sunny conditions: snakes will select microhabitats that facilitate basking (high detectability).
- Hot sunny conditions: ground temperature can be higher than air temperature and may exceed a species’ upper thermal limit when air temperature is above 25-30 °C. Snakes will seek out cool microhabitats and shelter (low-moderate detectability).
It is essential that environmental conditions at the time of the survey are documented so that survey results can be accurately interpreted. Suitable environmental conditions for snake surveys are described for each survey method below.
3.5 Identification of survey sites
For all snake species with the exception of Gray Ratsnake and the semi-aquatic species (Lake Erie Watersnake and Eastern Ribbonsnake), surveys should generally be concentrated in open-canopy and semi-open habitats such as rock outcrops, forest clearings and edges, fields, meadows, savannah, prairie, the edges of wetlands and shorelines of lakes and rivers (Figures 1). Many snake species demonstrate selection for open, semi-open and forest edge habitat (Blouin-Demers and Weatherhead 2001a; Row and Blouin-Demers 2006a; Row and Blouin-Demers 2006b; Carfagno and Weatherhead 2006; Harvey 2006; Lagory et al. 2009). These habitats provide warmer conditions where snakes can effectively thermoregulate and maintain body temperature closer to their thermal optimal temperature (Blouin-Demers and Weatherhead 2001a; Row and Blouin-Demers 2006b; Harvey and Weatherhead 2010). Within forested landscapes, snakes may select small clearings and areas with low canopy cover rather than moving into larger open habitats (e.g. Harvey and Weatherhead 2010). Thus, in forested areas, it is important to survey small clearings and areas of low canopy cover throughout the forest; large expanses of forest should not be excluded from surveys because it is not open-canopy at a coarse landscape scale. Small forest clearings (< 10 m) are often only detectable through site surveys or the use of high resolution aerial photographs. In addition to a tendency to be more abundant in open habitats, detectability is often higher in these habitats because snakes are more conspicuous when basking.
Gray Ratsnakes utilize forest habitats extensively throughout the active season (Weatherhead and Charland 1985; Blouin-Demers and Weatherhead 2001a; Carfagno and Weatherhead 2006). In addition to open-canopy and edge habitats, surveys for this species should also include closed-canopy forest, particularly forested areas that are in close proximity to open habitats.
Surveys for watersnakes and Eastern Ribbonsnakes should be carried out in wetlands and along the shorelines of lakes, rivers and other aquatic habitats. However, these species regularly bask in adjacent open terrestrial habitats, and surveys should include open-canopy terrestrial habitat within 10 m of the shoreline.
When it is possible to identify potential hibernacula, these habitats should be searched several times during the early spring. Snakes tend to be more conspicuous at these sites because they are often occupied by multiple individuals and because snakes bask regularly after emerging from hibernation. Hibernation habitat varies with species and region, and a thorough review of the species biology and habitat use is required to inform spring emergence surveys. For example, Massasaugas in eastern Georgian Bay tend to overwinter in conifer swamps and other lowland habitats while Gray Ratsnakes in eastern Ontario often make use of south-facing rocky slopes.
Prior to site visits, identify potential habitat (e.g. open-canopy and semi-open habitats, edge habitat) using aerial photographs, orthophotos Ecological Land Classification maps or other high-resolution land cover information. A site visit should be carried out to assess the potential habitat and to confirm the presence of suitable habitat. If detailed maps or other habitat information is not available for a site, the entire site should be thoroughly searched to identify suitable habitat. All suitable habitat should be described or mapped and this information should inform the survey design.






Figure 1. Examples of open-canopy habitats that are used by Ontario’s snakes. Photographs by Joe Crowley
3.6 Other survey considerations
Animal health: Snake Fungal Disease
Snake Fungal Disease (SFD), which is caused by the fungus Ophidiomyces ophiodiicola, is an emerging threat to North American snakes. This pathogen has resulted in mortality and population decline in several snake species (Clark et al. 2011; Lorch et al. 2016; CWHC 2016). SFD has been documented in a wide range of snake species throughout the eastern United States since 2006 (Lorch et al. 2016; NEPARC 2015), and it has been confirmed at three locations in southwestern Ontario (L. Shirose, pers. comm. 2016). The fungus O. ophiodiicola has been confirmed in snakes from several other locations in southern and central Ontario, although it is not known if the fungus has caused clinical symptoms of the disease in those specimens (L. Shirose, pers. comm. 2016). A proactive approach to prevent further spread of SFD is needed to combat this new threat to Ontario’s snakes. Individuals working with snakes in southwestern Ontario should follow appropriate decontamination protocols when travelling between sites. Any field equipment or clothing that has been in contact with snakes should be thoroughly washed and then soaked in a 3% bleach solution for two minutes (CWHC 2016). The fungus can be free-living in the soil (Allender et al. 2015), and boots should also be washed and disinfected between sites.
Massasauga safety
The Massasauga is Ontario’s only venomous snake. This species occurs throughout large areas on the Bruce Peninsula, along the eastern shore of Georgian Bay, in Wainfleet Bog on the Niagara Peninsula, and at one site in the town of Lasalle, near Windsor. The Massasauga is a timid snake that prefers to avoid conflict whenever possible. When a Massasauga feels threatened, it will often rattle to announce its presence or escape into a nearby retreat site (e.g. under shrubs, a crevice in the rock). Massasaugas will typically only strike in defence as a last resort, and their striking distance is about one third to one half of their body length (typically striking distance is less than 40 cm). Further information about this species, including distribution, ecology, conservation and safety considerations, is available at Massasauga website.

When working in Massasauga habitat, surveyors should:
- wear appropriate field gear, including hiking boots and long pants,
- pay careful attention to where they are stepping,
- be aware of their surroundings and listen for the sound of the rattle,
- make sure that an area has been thoroughly scanned for Massasaugas and other potential threats before flipping cover objects,
- never place hands or fingers near areas that cannot be seen, and
- never pick up a Massasauga or unidentified snakes.
If a Massasauga is encountered, surveyors should maintain a distance of at least 2-3 m from the snake to avoid causing undue stress to the snake. If someone is bitten by a Massasauga, call 911. The injured person should remain calm and avoid strenuous activity. It is dangerous, unnecessary and illegal to attempt to catch or kill the snake in question; there is only one venomous species in Ontario and medical personal will be able to determine if envenomation occurred.
Avoiding harm to snakes and sensitive habitats during surveys
There is the potential for surveyors to cause accidental harm to snakes by stepping on them or crushing them under cover objects. Surveyors should pay careful attention to where they are walking, avoid stepping on potential cover objects (rocks, vegetation mats, brush piles, etc.) that have not been searched, and take care not to crush snakes or other wildlife when searching under cover (see discussion on “searching under cover” in section 4.1). Surveyors should also minimize stress to the animals by refraining from capturing and handling snakes unless it is necessary for species identification or research purposes (note that authorizations under the ESA, 2007 and/or FWCA are required to capture most snake species in Ontario).
Since snake surveys often require thorough searches of the habitat, including actively searching under cover, there is a risk of damaging these habitats in the process. Particularly sensitive habitats include overwintering sites, gestation sites, nesting sites and communal basking and/or shedding sites. Invasive techniques that result in the destruction of microhabitat features (e.g. ripping apart a rotting log or stump) should be avoided, and microhabitat features should always be left exactly how they were found (e.g. return rocks and logs to their original position). If surveys occur in sensitive habitats (e.g. shallow sphagnum bogs or alvars), minimize the amount of time spent in these habitats and select a path that will have the lowest risk of damaging sensitive vegetation communities or altering habitat structure.
4. Survey protocols
Several survey methods are discussed in this section. However, visual encounter surveys (VES) are the only survey method that is recommended for assessing presence / absence for all species except the Butler’s Gartersnake; both VES and Artificial Cover Object (ACO) surveys are recommended for assessing presence / absence of the Butler’s Gartersnake. The other survey methodologies are useful for supplementing VES surveys and increasing confidence in the results, or for quickly assessing presence across large areas. However, they are generally not sufficient to assess presence / absence and this is discussed in more detail for each method.
4.1 Visual encounter surveys
A visual encounter survey is a standard, effective method for carrying out presence / absence surveys for snakes (Guyer and Donnelly 2012). This technique is effective for assessing presence / absence of all Ontario SAR snakes; however, the Eastern Hog-nosed Snake is very difficult to detect with any survey method. Combining VES with other techniques, such as road surveys or artificial cover object (ACO) surveys, helps to improve the overall chances of species detection.
Survey technique
Visual encounter surveys are carried out by slowly walking through suitable habitat while watching for basking and foraging snakes, as well as searching under cover objects such as logs, rocks, artificial cover, etc. Surveyors should also listen for the sound of snakes moving through vegetation or leaves, which can often draw attention to an otherwise inconspicuous snake in dense cover. Shed skins may also be encountered during surveys and can provide valuable data on species presence (see Gray 2012 for guidance on identification of shed snake skins in Canada). Although this section provides a general description of how and where to search for snakes, the specific habitat preferences of the target species should be researched in detail prior to carrying out surveys. The reference material in section 2 provides detailed information on species-specific ecology and habitat preferences of Ontario’s snake species.
Snakes favour microhabitats that provide optimal thermal conditions and adequate cover or retreat sites (Row and Blouin-Demers 2006a; Harvey and Weatherhead 2006; Harvey 2008), such as rock piles, dead stumps, low-lying shrubs and other ground vegetation, old building foundations, scrap piles, boards and other human-created structures, and forest edges. Surveyors should target and thoroughly search these key microhabitat features. When surveys are carried out under cool, sunny conditions, surveyors should focus on areas that are receiving sunlight, such as the sunny edges of shrubs, rock piles, etc. or forest edges. As ambient temperature increases throughout the day, surveyors should increasingly look into vegetated or structurally complex areas associated with these features but that are partially or fully shaded. For example, during a sunny afternoon, snakes are more likely to be found under a table rock or a shrub rather than at the edge of these features.
- In open-canopy habitats with lots of ground cover, such as grassy fields, meadows with dense mats of dead grasses or vegetated shorelines, high quality microhabitat is continuously distributed throughout the entire site (e.g. Figure 1d and 1e). Since snakes may be foraging or basking anywhere within the habitat, the entire area should be thoroughly searched by walking evenly-spaced transects. Transects should be close enough that all cover objects and other microhabitat features will be encountered and searched, and any snakes hiding or moving within the habitat would be observed. In most habitats, transect spacing of about 5 m is appropriate. Transects should be used as a general guide, but surveyors should move back and forth between high quality microhabitats or microhabitat features and should not follow a straight line.
- Alternatively, high quality microhabitats may be clustered, such as in the case of a rock barren or alvar with large expanses of flat, open rock interspersed with rock piles, shrubs or forest patches (e.g. Figure 1a). In this case, surveys should be focused on forest edge, around the edges of shrubs, within vegetation patches and near rock piles, dead stumps, junk piles or other notable microhabitat features. Within a forested area, surveys should be focused on clearings, edges and other areas with low canopy cover.
When surveying shallow aquatic habitats, such as coastal fens, surveys should be carried out in evenly spaced transects to cover the entire habitat. When searching for semi-aquatic species around deeper wetlands or along the shorelines of rivers and lakes, surveyors should search the terrestrial area within 10 m of the shoreline, as well as any vegetated shallow (1 m or less) aquatic areas that are accessible. Binoculars should be used to scan ahead to detect basking or swimming snakes before they notice surveyors and retreat under cover or into the water.
Snakes spend much of their time under cover objects, and targeting these microhabitat features during VES surveys improves the chances of detection. This is especially true of species that are primarily nocturnal during the hot summer months (e.g. Milksnake) and spend most of the day under cover. Even on warm sunny days, snakes may bask under thin cover objects that provide a warm microenvironment while protecting the snake from potential predators. Snakes can be found under a variety of cover objects, including rocks, logs, old stumps, boards and scrap metal. Scrap piles or other discarded items (e.g. old fridge, car hood) may also provide suitable microhabitat and should be searched if it is safe to do so. It is important to investigate small cover objects since snakes can be under cobble-sized rocks as small as 8 cm in diameter. Rocks that are buried in the ground and cannot be easily lifted are less likely to have snakes under them. Cover objects should be searched regardless of weather conditions, since snakes may be using them as retreat sites during inclement weather or for thermoregulation under sunny conditions.
When searching under cover:
- Do not step on rocks or other cover materials before you have checked beneath them. Snakes are regularly crushed or killed under cover objects when people step on or drive over these objects.
- Lift rocks slowly and carefully so that they do not suddenly shift, potentially crushing herpetofauna or other creatures hiding beneath them.
- Use two hands and proper lifting techniques when moving heavy cover objects, and do not lift rocks that are at risk of slipping due to weight.
- All cover materials should be returned exactly how they were found to ensure that previously existing gaps are maintained.
- If an animal is located beneath a cover object, ensure that it moves out of the way before replacing the cover; even seemingly light objects can crush small animals.
- Avoid placing hands or fingers under cover objects; wasp nests and neonate (newborn) Massasaugas can sometimes be encountered under rocks.
Although open-canopy habitat types are utilized for their thermal properties, snakes are often partially concealed within these habitats and are rarely conspicuous even when they are not hiding under cover. For example, in an open-canopy rock outcrop, a snake would likely be located at the base of a shrub, in a dense patch of vegetation or under a rock (Figure 2 and 3); in all cases, the snake would benefit from the warm, open-canopy environment but it would be well-hidden from predators and surveyors alike. Snake surveys require considerable attention to detail and patience since surveyors must move very slowly and carefully search all suitable habitats.
As a general guideline, the search time should be approximately one to two person hours per hectare, depending on the complexity of the habitat. Complex sites with a high density of rocks, ground vegetation or other cover will take more time than sites with very little structure (e.g. closed-canopy forest with few edges or gaps).


Species-specific survey notes
- Massasauga: unlike most other Ontario snakes, Massasaugas are rarely located under cover objects, such as small flat rocks or boards. A four-year study on the northern Bruce Peninsula only documented five Massasaugas under cover boards, despite checking a large network of boards 4262 times. Further, Massasaugas spend considerably more time basking than other Ontario snake species (about 70% of the time; Harvey and Weatherhead 2010), and surveys should focus on detecting basking snakes rather than searching under cover. However, Massasauga basking surveys should still include visual searches for partially or fully-concealed snakes, such as individuals tucked under vegetation or in crevices under large rocks.
- Gray Ratsnake: this species is commonly encountered in trees (Blouin-Demers and Weatherhead 2001a), and it is important to regularly scan the sub-canopy (approx. 1 to 4 m height) when surveying for this species in forested habitats.
Survey timing and environmental conditions
VES for snakes should be carried out under sunny conditions and when air temperature is between 10 and 25 °C or under overcast conditions and when air temperature is between 15 and 30 °C (Casper 2001; EMRT 2005; Harvey 2008). In the spring, surveys can be carried out between 9 am and 5 pm. However, in July and August when daytime temperatures are typically above 25 °C, surveys should be carried out between 8 am and 12 pm or 5 pm and 8 pm. Surveys for basking snakes (e.g. Massasaugas) should not be carried out on days with wind speeds higher than 24 kph (Casper 2001; EMRT 2005); high winds have a cooling effect on microhabitats that would otherwise hold pockets of warm air and encourage basking.
Search effort to determine probable absence
Snakes are cryptic, often occur at low density, demonstrate complex patterns of habitat use (spatial and temporal), and spend much of their time hiding out of sight, making them very difficult to detect during surveys (British Columbia Ministry of Environment Lands and Parks 1998; Casper et al 2001; Harvey 2005; Durso et al. 2011). Harvey (2005) determined that the likelihood of detecting a Massasauga at a known location during surveys was only 1 in 7, despite the snake being visible to surveyors. Given this low detectability and a typical density of two Massasaugas / ha in high quality summer habitat (Harvey 2008), the average detection probability (DP ) of this species would be 0.27 for a one hectare site. Based on data from two sites on the northern Bruce Peninsula, the average DP for Massasauga was 0.21 (Crowley unpublished data). Recent data from a Queensnake study on the Maitland River in Ontario indicate that DP ranged from 0.2 to 0.8 and averaged 0.3 (Aarts and Choquette 2015). Durso et al. 2011 reported DP ranging from 0.03 to 0.46 for several North American aquatic snake species. Determining with reasonable confidence that species with such low DP are absent from a site requires considerable search effort (Casper et al 2001; Durso et al. 2011). Durso et al. 2011 found that between 5 and 61 surveys would be required to determine absence with 95% confidence for a range of North American watersnake species. Assuming a DP of 0.25 to 0.3 for most of Ontario’s snakes (excluding Eastern Hog-nosed Snake), 10 surveys would be required to determine absence with 95% confidence based on the relationship between search effort and detection probability outlined in Casper (2010).
The ten surveys should be spread over the active season, with at least five surveys prior to July 1st. When surveying for Massasaugas, the ten surveys should be split over two years because Massasaugas generally reproduce on a biennial basis and a specific gestation site may not be used every year. When surveys are carried out over multiple years (e.g. for Massasauga), a minimum of five surveys each year are required, with at least three occurring before July 1st in each year.
Eastern Hog-nosed Snakes have much lower DPs than other species at risk snakes in Ontario because populations tend to occur at low density throughout most of Ontario and individuals spend much of their time out of site in inaccessible areas (e.g. underground burrows). Consequently, the search effort necessary to assess presence / absence of this species is considerably higher than the ten surveys recommended for other snake species, and VES are often not a feasible method for assessing presence / absence of this species. Alternatively, assessments of presence / absence can be based on the regional distribution of the species and local habitat suitability. For example, if the species is known to occur within a general area and there is suitable habitat at the site, it can be assumed that the area in question is likely to be inhabited by the species.
Important considerations when assessing absence:
- One survey is the amount of effort required to thoroughly search all suitable habitat (with the recommended effort of approximately 1-2 hours per ha). If the site is large, several site visits or trips may be required to adequately cover the entire area and complete one survey.
- If surveys are not carried out according to the methods outlined in this protocol (e.g. time of year, weather conditions), negative survey results may be inconclusive and lead to a requirement for additional surveys.
- The recommended search effort is based on the assumption that surveys are carried out by experienced surveyors. If surveys are carried out by inexperienced surveyors, additional effort may be required to determine with reasonable confidence that the species is absent.
- In cases where a population may occur at low density and be more difficult to detect than normal, a higher search effort would be necessary to determine with reasonable confidence that the species is absent.
The search effort recommended in this protocol is intended for assessments of presence / absence at sites where the species presence has not been previously documented. The number of surveys recommended in this protocol is not sufficient to conclude that a species has been extirpated from a previously occupied site. It is reasonable to expect that the species may still exist at the site but in low density and, as a result, considerably more effort would be necessary for detection. This is especially true of cryptic species, which can be very difficult to detect when at low density. For example, Casper et al. (2001) recommends 10-15 years of survey effort before concluding that Massasauga populations have been extirpated. Furthermore, when populations occur at low density, not all available habitat will be occupied in a given year, and habitat that is unoccupied in one year may be re-occupied in the following year. Consequently, a significant search effort spanning multiple years is typically necessary to conclude that a snake species no longer occurs at a previously occupied site (see O. Reg. 242/08 (2016) for species-specific survey requirements for removing regulated habitat protection for several of Ontario’s SAR snakes).
4.2 Surveys with artificial cover objects
Artificial cover objects (ACOs) can be used to create suitable microhabitat for snakes that can be easily and systematically searched (Joppa et al. 2009; Godley 2012; Halliday and Blouin-Demers 2015). ACO surveys can be a very effective method of detecting cryptic, difficult-to-survey-for snake species, especially in environments where natural cover is limited or cannot be easily searched. For example, ACOs have been shown to yield high capture rates of Butler’s Gartersnakes in Ontario (Marks pers.comm. 2016). Shed skins may also be encountered under ACOs and can provide valuable data on species presence (see Gray 2012 for guidance on identification of shed snake skins in Canada). However, detectability under cover boards varies considerably between species and between sites, and cover objects are not effective for detecting some of Ontario’s snake species. For example, very few Massasaugas were documented using ACOs of varying designs and materials during an extensive monitoring program on the Bruce Peninsula (Harvey 2008). Given low detection rates in that study, as well as the propensity for Massasaugas to bask in the open, ACOs are not a recommended technique for that species within the Bruce Peninsula and eastern Georgian Bay Massasauga populations. Even when a species typically utilizes ACOs, there can be a considerable lag time of up to several years before a species is detected using the ACOs at a particular site (e.g. Milksnake, Crowley unpublished data). Thus, ACO surveys are best suited for long-term monitoring or augmenting VES surveys at sites where natural cover is limited and, with the exception of Butler’s Gartersnake, should not be used in isolation to assess presence / absence.

Survey technique
ACOs can include a wide range of materials, but flat pieces of metal or wood (typically plywood) are most commonly used for snakes (Harvey 2008; Joppa et al. 2009; Godley 2012; Halliday and Blouin-Demers 2015). Thin (¼ to ¾ inch) plywood boards have been shown to be effective for a wide range of snakes in northeastern North America (Harvey 2008; Joppa et al. 2009; Crowley pers. obs.; Yagi pers. comm. 2015; Halliday and Blouin-Demers 2015), and this material is recommended for ACO surveys in Ontario. Thin metal sheets are also effective as ACOs (Harvey 2008; Halliday and Blouin-Demers 2015), but they can reach lethal temperatures more often than wood boards during hot weather (Harvey 2008), and extreme temperatures under metal ACOs can result in egg mortality when snakes oviposit under them (Porchuk 1996, Yagi pers. comm. 2015). Particle board and very thin plywood should be avoided because these materials warp and disintegrate quickly (Godley 2012; Crowley pers. obs.). Typical sizes of ACOs for snakes are 60-100 cm x 60-150 cm (Harvey 2008; Joppa et al. 2009; Godley 2012; Halliday and Blouin-Demers 2015). When targeting small species, such as gartersnakes and ring-necked snakes, smaller sizes may also be appropriate.
ACOs should be deployed in open and semi-open habitats that receive ample sun exposure (Joppa et al. 2009; Casper and Hecnar 2011; Halliday and Blouin-Demers 2015). ACOs should be in place for a minimum of two weeks prior to beginning surveys (Joppa et al. 2009; Casper and Hecnar 2011), but having them in place the previous fall is ideal. The ACOs should be relatively flush with the ground and placed in areas with little slope or with slopes that have a southerly aspect (Casper and Hecnar 2011; Godley 2012). At least ten ACOs should be deployed for each hectare of habitat being surveyed. ACOs should be numbered and labeled with an organization name and contact information; in some cases it is helpful to include a brief note, such as “research project – please do not remove”. It is usually not necessary to use ACOs to survey for Lake Erie Watersnakes and Eastern Ribbonsnakes because these species typically have high detectability during VES. If using ACOs for these semi-aquatic species, ACOs should be as close to the water as possible, and no more than 10 m from the water’s edge.
Conspicuous ACOs should not be used in areas with public access as they can facilitate illegal collection by poachers or the public. When cover objects are used in areas where the public will encounter them, there is a high risk of cover objects being repeatedly moved or damaged. High public use areas also tend to have elevated populations of subsidized predators, such as skunks and raccoons, and these animals may regularly flip cover objects while they are foraging.
Survey period
Searches under ACOs should be carried out during the spring and early summer (April – early July; Joppa et al. 2009; Casper and Hecnar 2011). ACOs should be checked once a day to once a week. Searches under ACOs may also yield results during the summer months, but surveys should not occur exclusively during this time because detection rates can be much lower (see Survey Timing and Environmental Conditions).
Survey timing and environmental conditions
Cover objects provide an ideal thermoregulatory environment for snakes; they warm up with the surrounding environment, often retain heat longer than their surroundings and offer protection from predation. Detection rate with cover boards is strongly linked to temperature (Joppa et al. 2009; Godley 2012) and is highest when the temperature under cover boards is warmer than the surrounding environment and is between 20-30 °C (Harvey 2008; Joppa et al. 2009). Detection rates are very low in hot (> 30 °C) sunny weather because temperatures under the boards would exceed the preferred temperature range and snakes would overheat (Harvey 2008). Generally, cover boards should be checked in the morning or early evening when air temperature is above 10 °C (Joppa et al. 2009; Casper and Hecnar 2011). However, recent work with the Butler’s Gartersnake in southwestern Ontario indicates that ACO surveys for this species are most productive in the evening between 6-9 pm (S. Marks pers. comm.). For safety reasons, ACO surveys should generally occur before dark. ACOs should not be checked during rainy weather.
Search effort required to determine probable absence
For most of Ontario’s species at risk snakes, ACO surveys should not be used in isolation to assess presence / absence. However, Butler’s Gartersnakes show a strong affinity for artificial cover and can often be detected within a very short time after boards are deployed (Joppa et al. 2009). In the case of Butler’s Gartersnake, ten ACO surveys spread over the active season, with at least five surveys prior to July 1st, should be adequate to assess presence / absence at a site with reasonable confidence. One ACO survey is the amount of effort required to check all of the ACOs at the site.
4.3 Road surveys
Road surveying is a well-established survey technique for snakes that takes advantage of the road network to cover large areas and this technique is especially effective for documenting the diversity of species in a particular area (Sullivan 2012). This technique is also a good supplement to VES since road surveys can be carried out in the evening after VES are finished. However, this technique has some limitations. All species are not equally likely to be detected during road surveys, and some species may not be encountered. Species are less likely to be encountered if they are small and difficult to see on the road; are secretive; have small home ranges and are relatively sedentary; or display road avoidance behaviour (Sullivan 2012). Another limitation of this technique is that areas without roads cannot be included in the surveys. For these reasons, road surveys should not be used in isolation to assess presence / absence.
Survey technique
Road surveys use roads as transects and involve walking, biking or driving slowly along roads and documenting the species that are encountered. Surveying on foot or on bike results in higher detection rates (Langen et al. 2007) and is recommended when surveys are limited to a specific site or small geographic area. However, a motor vehicle should be used when the goal of the survey is to sample large geographic areas.
The road surface and the full extent of the shoulders should be searched. Detection rate of road-killed snakes declines rapidly as carcasses are scavenged or obliterated by traffic, and the number of road-killed snakes that are identified beyond 24 hours is low (Antworth et al. 2005; Santos et al. 2011). In order to achieve reasonably high detectability, road surveys should be carried out a minimum of once per day.
When surveys are being carried out in a motor vehicle, surveyors should drive as slowly as possible and should not exceed 45 kph (Langen et al. 2007; Sullivan 2012). Surveys with motor vehicles should be carried out by two people: a driver and a spotter. When an animal is located and if it is safe to do so, the driver should pull onto the shoulder of the road and stop the vehicle so the spotter can identify the species, move it off the road (if it is alive) and record the data. When surveys are being carried out on foot or on a bicycle, the surveyor should walk or cycle along one side of the road and then retrace the route on the other side of the road.
Safety protocols for working on roads should be established prior to conducting road surveys. Surveyors should also be aware of and obey local laws. The following safety precautions, among others, should be taken when carrying out road surveys:
- When stopping, always pull the motor vehicle onto the shoulder and turn on the four-way flashers; never stop in a lane of traffic.
- Wear bright colours (e.g. orange safety vests) or reflectors and carry flashlights at all times during nighttime surveys.
- Be aware of approaching vehicles.
Survey period
In Ontario, road surveys for snakes can be carried out throughout the active season. Some of Ontario’s snakes are more active at certain times of the year, and surveys should be concentrated during the peak activity periods of the target species when those periods are known. For example, Tonge (2006) encountered most Massasaugas on roads during August, which coincides with the breeding season for that species.
Survey timing and environmental conditions
Road surveys for snakes are typically carried out in the evenings (Sullivan 2012; S. Marks pers. comm.). However, daytime surveys have also been reported to be effective in Ontario (Tonge 2006; Stinnisson pers. comm.), and evening surveys may not be possible in the spring and fall due to low nighttime temperatures. In Ontario, road surveys should be carried out between 9 am and 11 pm when air temperature is between 20 and 30 °C to maximize the chances of detecting live individuals or dead individuals before they are scavenged. Morning surveys following a warm evening are sufficient to detect the majority of snakes that were killed the previous day. Road surveys should not be carried out during or immediately following periods of heavy rain.

5. Documentation and reporting
5.1 Documentation
The following information should be documented for each survey (regardless of whether or not target species were observed):
- Names of the surveyors
- Date, time and duration of the survey (beginning and end)
- Number of surveyors and relevant experience with the target species
- A map that delineates survey locations, routes or transects
- Photographs of the habitat
- Weather conditions (cloud cover, wind, air temperature, water temperature; record at the beginning and end of survey)
- Result (positive, negative, number of individuals of each species, etc.)
When a snake is observed, the following information should be collected:
- Name of observer and contact information
- Time and date of observation
- Number of individuals observed
- Photographs of key identification features (e.g. close up of head, belly pattern) to document the observation (including road kills)
- GPS coordinates, including accuracy
- If multiple individuals are observed and are more than ten metres apart, separate GPS coordinates should be submitted for each individual.
- If the GPS location is taken from a point other than where the snake was located, include additional information to allow the point to be mapped accurately (e.g. snake was 20 m NW of GPS location).
- Location description and directions to the site
- A description of the habitat
For ease of documentation, a Survey Form has been provided (Appendix 1).
Note: surveys related to a project or application with the Ministry of Natural Resources and Forestry should not be carried out prior to discussing the specifics of the project with an OMNRF biologist or Ontario Parks zone ecologist.
5.2 Reporting
Species at risk occurrence data should be reported to the Ontario Ministry of Natural Resources and Forestry Natural Heritage Information Centre (Natural Heritage Information Centre website). The NHIC is Ontario’s conservation data centre and maintains the provincial record of Ontario’s species at risk occurrences. Negative survey results should also be submitted to the NHIC. Data should be submitted in digital format (spreadsheet or shape files with associated tabular data) as per instructions on the NHIC website. For questions regarding submission of data to NHIC or access to NHIC data, contact nhicrequests@ontario.ca. The district OMNRF office or the Ontario Parks zone ecologist responsible for the area in question should also be provided with a copy of the data (but please indicate to them if it has already been submitted to NHIC).
Opportunistic observations of other species at risk should also be reported to the OMNRF. Observations of reptiles and amphibians can be submitted to the Ontario Reptile and Amphibian Atlas (Ontario Nature website).
6. References
6.1 Literature cited
Aarts, M. and J. Choquette. 2015. Distribution, Abundance, and Survivorship of Queensnakes (Regina septemvittata) in Huron County. Huron Stewardship Council, Goderich, ON. 38 pp.
Allender, M.C, D.B. Raudabaugh, F.H. Gleason and A.N. Miller. 2015. The natural history, ecology, and epidemiology of Ophidiomyces ophiodiicola and its potential impact on free-ranging snake populations. Fungal Ecology 17: 187-196.
Antworth, R.L., D.A. Pike and E.E. Stevens. 2005. Hit and run: effects of scavenging on estimates of roadkilled vertebrates. Southeastern Naturalist 4(4): 647-656. doi: Hit and Run: Effects of Scavenging on Estimates of Roadkilled Vertebrates
Black, R. and C. Parent. 1999. Assessment and mitigation of the effects of highway construction on eastern massasauga rattlesnakes. Technical report to the Ministry of Natural Resources, Parry Sound District, Ontario, Canada. 13 pp. + figs.
Blouin-Demers, G. and P.J. Weatherhead. 2001a. Habitat use by Black Rat Snakes (Elaphe obsoleta obsoleta ) in fragmented forests. Ecology 82 (10): 2882-2896.
Blouin-Demers, G. and P.J. Weatherhead. 2001b. Thermal ecology of black rat snakes (Elaphe obsoleta) in a thermally challenging environment. Ecology 82: 3025-3043.
Blouin-Demers, G.P., J. Weatherhead and H.A. McCracken. 2003. A test of the thermal coadaptation hypothesis with black rat snakes (Elaphe obsoleta) and northern water snakes (Nerodia sipedon). Journal of Thermal Biology 28: 331-340.
British Columbia Ministry of Environment, Lands and Parks (BCMELP). 1998. Inventory Methods for Snakes. Standards for Components of British Columbia’s Biodiversity, No. 38. Resources Inventory Branch, Ministry of Environment, Lands and Parks, Vancouver, BC. 50 pp.0772634874
Brown, G.P. and P.J. Weatherhead. 2000. Thermal ecology of northern water snakes, Nerodia sipedon : population patterns and variation in relation to sexual size dimorphism. Ecological Monographs 70: 311-330.
Canadian Wildlife Health Cooperative (CWHC). 2016. Snake Fungal Disease (fact sheet). Available at: Snake Fungal Disease
Carfagno, L.F. and P.J. Weatherhead. 2006. Intraspecific and interspecific variation in use of forest-edge habitat by snakes. Canadian Journal of Zoology 84: 1440-1452.
Casper, G.S., T.G. Anton, R.W. Hay, A.T. Holycross, R.S. King, B.A. Kingsbury, D. Mauger, C. Parent, C.A. Phillips, A. Resetar, R.A. Seigel and T.P. Wilson. 2001. Recommended standard survey protocol for the Eastern Massasauga, Sistrurus catenatus catenatus. US Fish and Wildlife Service. Fort Snelling, MN. 9 pp. Available at: Eastern Massasauga Survey Protocol
Casper, G. 2010. Confidence levels for a given detection probability and effort. Article produced for the University of Wisconsin-Milwaukee Field Station. 2 pp.
Casper, G.S. and S.J. Hecnar. 2011. Standard operating procedure for: cover board surveys for snakes in the Lake Superior basin. Version 1.0. Available at: Standard Operating Procedure for: Cover Board Surveys for Snakes in the Lake Superior Basin
Clark, R.W., M. N. Marchand, B.J. Clifford, R. Stechert, and S. Stephens. 2011. Decline of an isolated timber rattlesnake ( Crotalus horridus) population: Interactions between climate change, disease, and loss of genetic diversity. Biological Conservation 144: 886-891.
Department of Sustainability, Environment, Water, Population and Communities (DSEWPC). 2011. Survey Guidelines for Australia’s Threatened Reptiles; Guidelines for detecting reptiles listed as threatened under the Environmental Protection and Biodiversity Conservation Act 1999. 104 pp.
Durso, A.M., J.D. Willson and C.T. Winne. 2011. Needles in haystacks: Estimating detection probability and occupancy of rare and cryptic snakes. Biological Conservation 144: 1508-1515.
Eastern Massasauga Recovery Team (EMRT). 2005. Guidelines for Identifying Significant Habitat, and Significant Wildlife Habitat, for the Massasauga in Eastern Georgian Bay and Bruce Peninsula Populations, Ontario. Version 1.0.
Godley, J.S. 2012. Sampling with Artificial Cover in McDiarmid, R.W., M.S. Foster, C. Guyer, J.W. Gibbons, and N. Chernoff (Eds.). 2012. Reptile Biodiversity: Standard Methods for Inventory and Monitoring. Berkeley: University of California Press.
Gray, B.S. 2012. Guide to the Identification of the Shed Skins of the Snakes of Canada. Available at: Guide to the Identification of the Shed Skins of the Snakes of Canada.
Guyer, C and M. Donnelly. 2012. Visual Encounter Surveys in McDiarmid, R.W., M.S. Foster, C. Guyer, J.W. Gibbons, and N. Chernoff (Eds.). 2012. Reptile Biodiversity: Standard Methods for Inventory and Monitoring. Berkeley: University of California Press.
Halliday, W.D. and G. Blouin-Demers. 2015. Efficacy of cover boards for sampling small northern snakes. Herpetology Notes 8: 309-314.
Harvey, D.S. 2005. Detectability of a large-bodied snake (Sistrurus c. catenatus) by time constrained searching. Herpetological Review 64: 413-415.
Harvey, D.S. 2008. Massasauga monitoring – analysis and recommendations v. 1.2. Reported submitted to the Bruce Peninsula National Park / Fathom Five National Marine Park, Parks Canada Agency, Ontario.
Harvey, D.S. and P.J. Weatherhead. 2006. A test of the hierarchical model of habitat selection using eastern massasauga rattlesnakes. Biological Conservation 130: 206-216.
Harvey, D.S. and P J. Weatherhead. 2010. Habitat selection as the mechanism for thermoregulation in a northern population of massasauga rattlesnakes ( Sistrurus catenatus). Ecoscience 17(4): 411-419.
Harvey, D.S. and P.J. Weatherhead. 2011. Thermal ecology of Massasauga Rattlesnakes (Sistrurus catenatus) near their northern range limit. Canadian Journal of Zoology 89: 60-68.
Joppa, L.N., C.K. Williams, S.A. Temple and G.S. Casper. 2009. Environmental factors affecting sampling success of artificial cover objects. Herpetological Conservation and Biology 5: 143-148.
Lagory, K.E., L.E. Walston, C. Goulet, R.A. Van Lonkhuyzen, S. Najjar and C. Andrews. 2009. An examination of scale-dependent resource use by Eastern Hognose Snakes in southcentral New Hampshire. Journal of Wildlife Management 73: 1387-1393.
Langen, T.A., A. Machniak, E.K. Crowe, C. Mangan, D.F. Marker, N. Liddle, and B. Roden. 2007. Methodologies for surveying herpetofauna mortality on rural highways. Journal of Wildlife Management 71(4): 1361-1368. doi: Methodologies for Surveying Herpetofauna Mortality on Rural Highways
Lorch, J.M., S. Knowles, J.S. Lankton, K. Michell, J.L. Edwards, J.M. Kapfer, R.A. Staffen, E.R. Wild, K.Z. Schmidt, A.E. Ballmann, D. Blodgett, T.M. Farrell, B.M. Glorioso, L.A. Last, S.J. Price, K.L. Schuler, C.E. Smith, J.F. X. Wellehan Jr, and D.S. Blehert. 2016. Snake fungal disease: an emerging threat to wild snakes. Philosophical Transactions of the Royal Society B. 371: 20150457. Snake fungal disease: an emerging threat to wild snakes
McDiarmid, R.W., M.S. Foster, C. Guyer, J.W. Gibbons, and N. Chernoff (Eds.). 2012. Reptile Biodiversity: Standard Methods for Inventory and Monitoring. Berkeley: University of California Press.
Northeast Partners in Amphibian and Reptile Conservation (NEPARC). 2015. Snake Fungal Disease: frequently asked questions (fact sheet). Available at Snake Fungal Disease: Frequently Asked Questions
OMNR. 2013. Reptile and Amphibian Exclusion Fencing: Best Practices, Version 1.0. Species at Risk Branch Technical Note. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. 11 pp.
OMNR. 2015. Survey Protocol for Queensnake (Regina septemvittata) in Ontario. Ontario Ministry of Natural Resources and Forestry, Species at Risk Branch. Peterborough, Ontario. ii + 16 pp.
Ontario Regulation 242/08: 2016. General. Regulation under the Ontario Endangered Species Act, 2007. Available at O. Reg. 242/08
Porchuk, B. 1996. Ecology and conservation of the endangered Blue Racer snake (Coluber constrictor foxii) on Pelee Island, Canada. M.Sc. Thesis. University of Guelph, Guelph, Ontario.
Row, J.R. and G. Blouin-Demers. 2006a. Thermal quality influences habitat selection at multiple spatial scales in milksnakes. Ecoscience 13 (4): 443-450.
Row, J.R. and G. Blouin-Demers. 2006b. Thermal quality influences effectiveness of thermoregulation, habitat use, and behaviour in milk snakes. Oecologia 148: 1-11.
Rowell, J. 2012. The snakes of Ontario; Natural History, Distribution, and Status. pp. vi + 411
Marta Rzadkowska, M. M.C. Allender, M. O'Dell, and C. Maddox. 2016. Evaluation of common disinfectants effective against Ophidiomyces ophiodiicola , the causative agent of snake fungal disease. Journal of Wildlife Diseases 52:759-762.
Santos, S.M., F. Carvalho and A. Mira. 2011. How long do the dead survive on the road? carcass persistence probability and implications for road-kill monitoring surveys. PLOS ONE 6(9). Available online at How Long Do the Dead Survive on the Road? Carcass Persistence Probability and Implications for Road-Kill Monitoring Surveys
Sullivan, B.K. 2012. Road Riding in McDiarmid, R.W., M.S. Foster, C. Guyer, J.W. Gibbons, and N. Chernoff (Eds.). 2012. Reptile Biodiversity: Standard Methods for Inventory and Monitoring. Berkeley: University of California Press.
Tonge, Melissa. 2006. Eastern Massasauga Monitoring and Protocol Development Progress Report. Technical report prepared for the Bruce Peninsula National Park, Tobermory, Ontario. 32 pp.
Weatherhead, P.J. and M.B. Charland. 1985. Habitat selection in an Ontario population of the snake, Elaphe obsoleta. Journal of Herpetology 19: 12-19.
6.2 Authorities cited
Gillingwater, S. 2012. E-mail correspondence with Joe Crowley. Species at Risk Biologist, Upper Thames River Conservation Authority.
Litzgus, J. 2012. E-mail correspondence with Megan Rasmussen and verbal correspondence with Joe Crowley. Professor, Laurentian University.
Marks, S. 2013, 2016. Correspondence with Joe Crowley. SAR reptile specialist, AMEC.
Robinson, S. 2012. E-mail correspondence with Joe Crowley. Species at Risk Biologist, Ontario Ministry of Natural Resources and Forestry.
Shirose, Lenny. 2016. Email correspondence to Joe Crowley on April 13, 2016. Wildlife Biologist, Canadian Wildlife Health Cooperative, Pathobiology, OVC, University of Guelph.
Stinnisson, T. 2014. Correspondence with Joe Crowley. M.Sc. student, Trent University.
Yagi, A. 2015. E-mail correspondence with Joe Crowley. Management Biologist, Ontario Ministry of Natural Resources and Forestry
7. Acknowledgements
This survey protocol was prepared by Joe Crowley, Herpetology Species at Risk Specialist with the Ontario Ministry of Natural Resources and Forestry. Many OMNRF and Ontario Parks staff, as well as several Ontario snake experts, also kindly took the time to review and comment on previous drafts of this document.
Appendix 1: Snake survey form
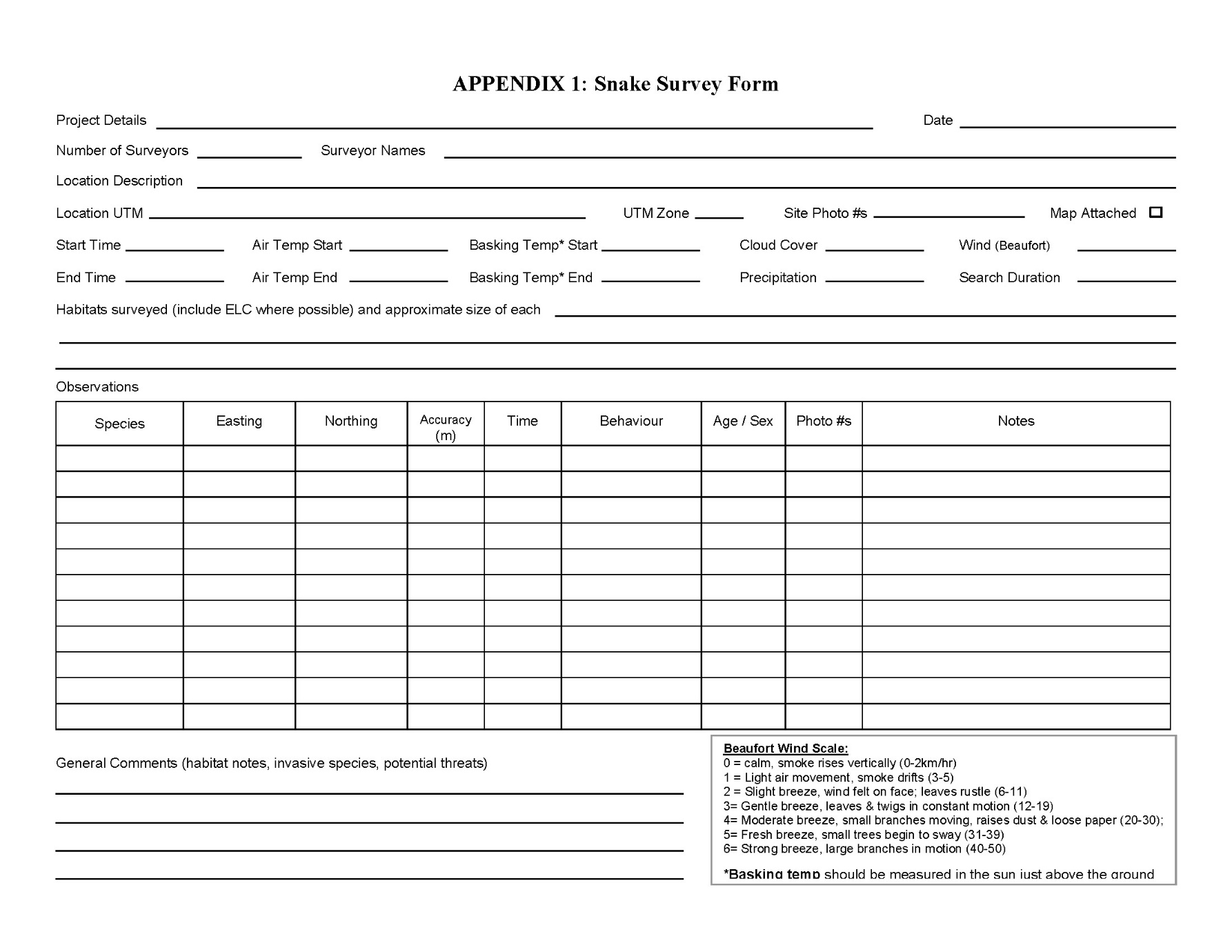
Enlarge a printer-friendly version of the snake survey form (PDF)