Key air contaminants
Ontario monitors levels of common air pollutants associated with smog formation across the ambient air monitoring network. These pollutants have numerous, diverse and widespread sources, and can have adverse effects on human health, the environment, and property. More information on the key air contaminants, their impacts to health and environment, and their sources is available below.
Nitrogen dioxide
Nitrogen dioxide (NO2) is a reddish-brown gas with a pungent odour, which transforms in the atmosphere to form gaseous nitric acid and nitrates.
NO2 plays a major role in atmospheric reactions that produce ground-level ozone, a major component of smog.
NO2 also reacts with other gaseous contaminants in the air (e.g., ammonia) leading to the formation of PM2.5.
Impacts to health and environment
NO2 can irritate the lungs and lower resistance to respiratory infection. People with asthma and bronchitis have increased sensitivity to NO2.
NO2 chemically transforms into nitric acid in the atmosphere and, when deposited, contributes to the acidification of lakes and soils in Ontario. Nitric acid can also corrode metals, fade fabrics, degrade rubber, and damage trees and crops.
Sources of nitrogen oxides
Combustion or burning of carbon-based materials (e.g., wood, gasoline etc.) in air produces nitrogen oxides (NOx), of which NO2 is a component. The transportation sector is the main source of nitrogen dioxide in Ontario.
Fine particulate matter
Airborne particulate is the general term used to describe a mixture of microscopic solid particles and liquid droplets suspended in air. Particulate matter (PM) includes aerosols, smoke, fumes, dust, fly ash and pollen.
Fine particulate matter, denoted as PM2.5, is less than 2.5 micrometres in diameter, approximately 30 times smaller than the average diameter of a human hair.
Impacts to health and environment
Fine particulate matter can have various negative health effects, especially on the respiratory and cardiovascular systems.
Exposure to fine particulate matter is associated with increased hospital admissions and emergency room visits — and even to death from heart or lung diseases. Both long- and short-term particle exposures have been linked to health problems.
People with heart or lung disease, children and older adults are particularly sensitive to this pollutant.
Sources of fine particulate matter
Fine particulate matter consists of primary and secondary PM2.5. Primary PM2.5 is emitted directly to the atmosphere. Major sources of primary PM2.5 include residential fireplaces and wood stoves, motor vehicles, smelters, power plants, industrial facilities, and agricultural burning and forest fires. Secondary PM2.5 is formed indirectly in the atmosphere through a series of complex chemical reactions of gaseous precursors such as nitrogen dioxide and sulphur dioxide.
Ground-level ozone
Ground-level ozone (O3) is a colourless, odourless gas at typical ambient concentrations, and is a major component of smog.
Ground-level ozone is a gas formed when NOx and volatile organic compounds (VOCs) react in the presence of sunlight. NO2 breaks down in the presence of sunlight to form oxygen atoms (O) and NO. These oxygen atoms then react rapidly with molecular oxygen (O2) in the atmosphere to produce ground-level ozone.
The formation and transport of ozone is strongly dependent on weather conditions and emissions of chemicals that contribute to the formation of ozone, such as NOx and VOCs (also known as ozone precursors).
Schematic formation and scavenging of ozone
Ground-level ozone is a gas formed when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of sunlight. NOx is comprised of NO2 and nitric oxide (NO). NO2 breaks down in the presence of sunlight to form oxygen atoms (O) and NO. These oxygen atoms then react rapidly with molecular oxygen (O2) in the atmosphere to produce ground-level ozone. This series of reactions, however, can be influenced by the presence of other chemicals, such as VOCs. Ground-level ozone is depleted when it reacts with nitric oxide to form NO2 (a process known as scavenging) decreasing ozone concentrations.
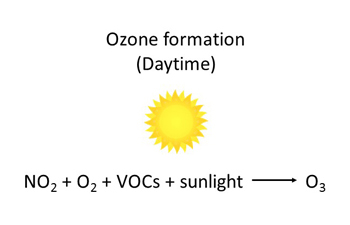
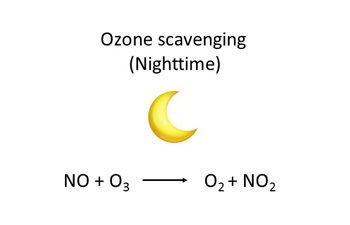
In large urban centres, ozone concentrations are generally lower, because ozone is depleted by reacting with NO emitted by vehicles and other local combustion sources to form NO2. The NO2 reacts with volatile organic compounds in the presence of sunlight to form ozone in downwind suburban and rural areas, resulting in higher ozone levels.
Impacts to health and environment
Ozone irritates the respiratory tract and eyes. Exposure to ozone in sensitive people can result in chest tightness, coughing and wheezing. Children who are active outdoors during the summer, when ozone levels are highest, are particularly at risk. Individuals with pre-existing respiratory disorders, such as asthma and chronic obstructive pulmonary disease, are also at risk. Ozone is associated with increased hospital admissions and premature deaths.
Sources of ground-level ozone
Major sources of ozone precursors, NOx and VOCs, include the transportation and industrial sectors and general solvent use.
Sulphur dioxide
Sulphur dioxide (SO2) is a colourless gas that smells like burnt matches. It can also be oxidized in the atmosphere to form sulphuric acid aerosols. In addition, SO2 is a precursor to sulphates, one of the main components of airborne secondary PM2.5.
Impacts to health and environment
Health effects caused by exposure to high levels of SO2 include breathing problems, respiratory illness, and the exacerbation of respiratory and cardiovascular disease.
People with asthma, chronic lung disease or heart disease are the most sensitive to SO2.
Sulphur dioxide damages trees and crops and reduces visibility.
Sources of sulphur dioxide
Major sources of SO2 include smelters, industrial processes and coal-fired electric power generation from outside Ontario.
Volatile organic compounds
Volatile organic compounds (VOCs) are organic chemical compounds that may evaporate under normal ambient conditions of temperature and pressure, such as benzene and toluene. VOCs are precursors of ground-level ozone and PM2.5. VOCs are emitted into the atmosphere from a variety of sources, including vehicles, fossil fuel combustion, steel-making, petroleum refining, fuel-refilling, industrial and residential solvent use, paint application, manufacturing of synthetic materials (e.g., plastics, carpets), food processing, agricultural activities and wood processing and burning.
Benzene
Benzene is primarily used in the production of plastics and other chemical products. Large quantities of benzene are produced from petroleum-related processes, either by direct extraction from certain types of crude oils or by chemical treatment of gasoline. Benzene is classified as a human carcinogen (USEPA, 2016).
Toluene
Toluene is used to make chemicals, explosives, dyes and many other compounds. It is also applied as a solvent for inks, paints, lacquers, resins, cleaners, glues and adhesives. Toluene is found in gasoline and aviation fuel. Studies indicate that toluene affects the central nervous system of humans and animals; however, there is little evidence to classify it as a carcinogen (USEPA, 2016).
Ethylbenzene
Ethylbenzene is a colourless liquid that smells like gasoline and is mainly used in the manufacture of styrene. Exposure to ethylbenzene occurs from the use of consumer products, fuel, pesticides, solvents, carpet glues, varnishes and paints. In humans, acute exposure results in respiratory and neurological effects and chronic exposure is associated with developmental and neurotoxic effects. Limited information is available on the carcinogenic effects of ethylbenzene (USEPA, 2016).
Xylene
Xylene is a colourless, sweet-smelling liquid or gas occurring naturally in petroleum, coal and wood tar; it is also used as a solvent in the printing, rubber, paint and leather industries. Xylene, also referred to as mixed xylenes, is a mixture of three isomers: ortho-, meta- and para-xylene, commonly known as o-, m- and p-xylene, which have the same molecular formula but different chemical structure, meaning the arrangement of their atoms is different. In humans, acute and chronic exposures result in respiratory and neurological effects. There is no information on the carcinogenic effects of mixed xylenes on humans (USEPA, 2016).
1,3-Butadiene
1,3-Butadiene is a colourless gas with a mild gasoline-like odour. It is released into the air through motor vehicle exhaust, manufacturing and processing facilities, forest fires or other combustion. Acute exposure to 1,3-butadiene by inhalation in humans results in irritation of the eyes, nasal passages, throat and lungs; in addition, 1,3-butadiene is carcinogenic in humans by inhalation (USEPA, 2016).