Black Tern Management Plan
This document advises the ministry on ways to ensure healthy numbers of Black Tern, a species of special concern, return to Ontario.
Management plan prepared under the Endangered Species Act, 2007
June 2013
About the Ontario management plan series
This series presents the collection of management plans that are written for the Province of Ontario and contain possible approaches to manage species of special concern in Ontario. The Province ensures the preparation of the management plans meet its commitments to manage species of special concern under the Endangered Species Act, 2007 (ESA, 2007) and the Accord for the Protection of Species at Risk in Canada.
What is a species of special concern?
A species is classified as special concern if it lives in the wild in Ontario, is not endangered or threat-ened, but may become threatened or endangered due to a combination of biological characteristics and identified threats.
What is a management plan?
Under the ESA, 2007, a management plan identifies actions that could be taken to ensure, at a minimum, that a species of special concern does not become threatened or endangered. The plan provides detailed information about the current species population and distribution, their habitat requirements and areas of vulnerability. The plan also identifies threats to the species and sets a clear goal, possible strategies, and prioritized activities needed to address the threats.
Management plans are required to be prepared for species of special concern no later than five years of the species being added to the Species at Risk in Ontario list as a special concern species.
What’s next?
Nine months after the completion of a management plan a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the plan and the government priorities in taking those actions. The implementation of the management plan depends on the continued cooperation and actions of various sectors, government agencies, communities, conservation organisations, land owners, and individuals.
For more information
To learn more about species of special concern in Ontario, please visit the Ministry of Natural Resources Species at Risk webpage at: www.ontario.ca/speciesatrisk
Recommended citation
Peter S. Burke. 2012. Management Plan for the Black Tern (Chlidonias niger) in Ontario. Ontario Management Plan Series. Prepared for the Ontario Ministry of Natural Resources (OMNR), Peterborough, Ontario. vi + 47 pp.
© Queen’s Printer for Ontario, 2013
ISBN 978-1-4606-2029-8 (PDF)
Cette publication hautement spécialisée « Management plans prepared under the Endangered Species Act, 2007 », n'est disponible qu'en anglais en vertu du Règlement 411/97 qui en exempte l'application de la Loi sur les services en français. Pour obtenir de l'aide en français, veuillez communiquer avec le ministère des Richesses naturelles au 1-800-667-1940.
Author
Peter S. Burke
Acknowledgments
For their contributions to the preparation of this report, thanks are given to: Irene Mazzocchi (New York State Department of Environmental Conservation), Douglas C. Tozer (Bird Studies Canada) who kindly prepared the figures using Marsh Monitoring Program data 1995-2011 for this report, D.V. Chip Weseloh (Canadian Wildlife Service (CWS) Downsview), Richard R. Russell (CWS Nepean), Dave Moore (CWS Burlington), J.D. McCracken (Bird Studies Canada), Erica H. Dunn (CWS Ottawa), R.D. McRae (Field Biologist, Consultant), Andrew Couturier (Bird Studies Canada), Colin D. Jones (Ontario Ministry of Natural Resources (OMNR)), Donald A. Sutherland (OMNR), Dawn M. Burke (OMNR), Michael Richardson (consultant), Alan Wormington (consultant), Don Tyerman (OMNR), Kathy Jones (Bird Studies Canada), David Bree (OMNR), Terry Brady (Environmental Officer, Lennox Generating Station, Ontario Power Generation).
Thanks to the official sponsors of the Ontario Breeding Bird Atlas (Bird Studies Canada, Canadian Wildlife Service, Federation of Ontario Naturalists, Ontario Field Ornithologists, and Ontario Ministry of Natural Resources) for supplying Atlas data, and to the thousands of volunteer participants who gathered data for the project.
Data provided by NatureServe in collaboration with Robert Ridgely, James Zook, the Nature Conservancy - Migratory Bird Program, Conservation International – CABS, World Wildlife Fund – US, and Environment Canada – WILDSPACE.
Declaration
The management plan for the Black Tern was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This management plan has been prepared for the Government of Ontario, other responsible jurisdictions and for the many different constituencies that may be involved in managing the species.
The management plan does not necessarily represent the views of all of the individuals who contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The goals, objectives and management approaches identified in the plan are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this plan is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the management of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this plan.
Responsible jurisdictions
Ontario Ministry of Natural Resources,
Environment Canada – Canadian Wildlife Service, Ontario
Parks Canada Agency
Executive summary
The Black Tern is a semi-colonial, pigeon-sized marsh-nesting tern. Its breeding plumage is unmistakable with a black head, neck and underparts, medium-grey dorsal surface (including the wings and tail) and a short notched tail. It is a long-distance migrant that returns to Ontario in early May to breed and departs by early September. It nests in large freshwater marshes of at least 20 hectares with emergent vegetation interspersed with open water but will use smaller wetlands with the same features. Nests are placed on floating or emergent substrates that are often wet, just above the waterline, and in close proximity to the edge of low-density emergent vegetation and open water. The terns are most vulnerable during the egg stage where nests are lost from changing water levels or wave action. Reproductive success can also be low due to predation pressures. However, Black Terns are able to re-nest until late July, and they are adapted to their wet environment by having porous egg shells. In winter, they become almost exclusively marine, forming large flocks along the coasts of northern South America and western Central America.
The Black Tern is a species of special concern in Ontario. It is found in scattered locations across the province, north to Big Trout Lake and Fort Albany. The highest densities occur along the lower Great Lakes coastlines, Bruce Peninsula, Manitoulin Island and the southern edge of the Canadian Shield. Abundance across much of the north is limited by a lack of suitable wetlands for nesting; however, distribution in that region is poorly known due to low monitoring coverage. Small concentrations are found in the Clay Belt, Lake-of-the-Woods and wetlands adjacent to southern James Bay. Breeding Bird Surveys show province-wide and continent-wide declines since 1960. The Marsh Monitoring Program documented a 32 percent decline at coastal Lake Ontario sites and 19.4 percent at coastal Lake Erie and Lake Huron sites from 1995 to 2002. Currently, the Black Tern is listed as endangered in New York, Ohio and Pennsylvania, special concern in Michigan and imperiled in Wisconsin. It is under review in Minnesota. Populations have disappeared from traditional sites and continue to decline at existing sites.
Along with problems of low population density in the province, the species faces uncertainty due to the combined threats of habitat loss, invasive species and increased pressure from human population growth and climate change. Wetlands on the Great Lakes now cover only a fraction of their former area, and those that exist outside of protected areas remain threatened by human development. Lack of suitable nesting habitat in remaining wetlands appears to be a large problem, as populations continue to decline. Stabilized water levels within the last 50 years on Lake Ontario and continual decline of water levels on the other lower Great Lakes have changed the amount of breeding habitat available. Increasing numbers of invasive species, particularly European Common Reed Grass, European Frog-bit, Common Carp and Mute Swan, are associated with declining Black Tern populations and can affect wetland structure, reducing suitability for terns. Climate change models predict that the Black Tern will become extirpated, or nearly so, in adjacent northeastern States due to changes in current breeding habitat.
The goal of this management plan is to maintain or improve the viability of Ontario’s Black Tern population. To reach this goal, a set of objectives has been determined with measurable, time-defined approaches that involve the support and cooperation of interested organizations, landowners, institutions and government. The objectives of the management plan are:
- Determine distribution and abundance of Black Tern populations in Ontario
- Clarify the main threats to Black Tern populations
- Maintain the distribution and abundance of the Black Tern in Ontario by protecting habitat and reducing the impacts of threats
1.0 Species assessment and classification
Common Name: Black Tern
Scientific Name: Chlidonias niger surinamensis
SARO List Classification: Special Concern
SARO List History: Vulnerable (1996)
COSEWIC Assessment History: Not at Risk (1996), No Designation Required (1988)
SARA Schedule 1: Special Concern (formerly Vulnerable) (November 1, 2004)
Conservation Status Rankings:
GRank: G4,
NRank: N4B, NZN,
SRank: S3B
The glossary provides definitions for the abbreviations above.
2.0 Species information
2.1 Species description and biology
Species description
The Black Tern is a small tern, close in size to a Rock Pigeon (Columba livia). It has distinct breeding plumage, adults are unmistakable – solid black on the head, breast and underparts. The long slender wings, short, shallowly forked tail, and back are a smooth medium grey. The lower belly and undertail coverts are white. Leg color is typically dark reddish black and the slender bill is all black. In late summer, adults molt into their winter (basic) plumage in which the underparts and head become mainly white. This transition creates a piebald appearance. In winter, the head is mainly white with a black area around the nape and ear coverts, creating the impression of wearing black headphones. The rest of the body is smooth grey, similar to breeding plumage. The juvenile is similar in coloration to the adults in winter plumage but has warmer, brown tones to the mantle (back and wing coverts), giving it a modestly scaled appearance. Birds in their first full summer of life (i.e. one-year olds) have variable amounts of black on the head and underparts, similar to winter plumage.
Vocalizations include short "kik" calls that serve as a contact between individuals most of the year. In the breeding season, birds also give a longer "kyew-dik" call, which serves as individual recognition, and harsher scolding calls, such as "kreea" when mobbing an intruder near the nest (Heath et al. 2009).
Species biology
Reproduction
The Black Tern is a semi-colonial marsh nester that typically occurs at sites in Ontario in groups of less than 20 pairs (Gerson 1988, Weseloh 2007). In some parts of its core range, such as the Prairie regions, it occurs in much larger group sizes, occasionally reaching well over 100 pairs (Heath et al. 2009). Generally, individual marshes support scattered clusters of terns where key nesting features are present (Mazzocchi et al. 1997, Sandilands 2010, NatureServe 2012). Colony sizes tend to be larger along coastal Great Lakes due to larger marsh sizes than inland, where less favourable or small sites may contain as few as one to three pairs (Austen and Cadman 1994, NatureServe 2012).
Nest placement can vary. Though nests are most often within 5 to 20 m of one another, solitary nesters and nests separated by distances of 20 to 600 m are also common (Cuthbert 1954, Bailey 1977, Dunn 1979, Mosher 1986). Both adults guard an area immediately around the nest sites (within about a two m diameter). Though home ranges are unknown, foraging can occur up to four km away (Heath et al. 2009). However, most feeding tends to occur within 500 m of the nest (Mosher 1986).
Nests are opportunistically placed on a variety of structures. These include floating or anchored dead vegetation, floating boards or logs, cattail rootstalk, Muskrat (Ondatra zibethicus) lodges or feeding platforms, abandoned bird’s nests, raised mud and broken down bulrushes (Cuthbert 1954, Bergmann et al. 1970, Bailey 1977, Dunn 1979, Carroll 1988, Novak 1990, Steen and Powell 2012b). Studies have shown an acceptance of man-made artificial rafts as nesting substrates, usually in the absence of suitable nest sites (Alvo et al. 1998, Shealer et al. 2006). The nest itself is usually a shallow depression in the substrate but may be constructed by the pair on dead vegetation (Cuthbert 1954, Bailey 1977, Heath et al. 2009). Nest cups may be wet or dry (Carroll 1988). Height of the nest is typically two to six cm above water but depends on the substrate used (Dunn 1979, Peck and James 1983). Nests built as high as 20 cm above water have been recorded in New York (Firstencel 1987). Given the low typical nest height, the eggs are generally close to the water line and prone to flooding. The eggs are porous and adapted to wet conditions typical of nesting sites (Weseloh and Jermyn in prep.). If a nest is destroyed, birds will sometimes rebuild at the old site (Cuthbert 1954, Bailey 1977) or choose to re-nest some distance away (up to 42 km) (Mazzocchi and Muller 1993 as cited in Heath et al. 2009).
Clutch size ranges from one to six eggs but is typically two to three (Heath et al. 2009). Three eggs were found in 307 out of 319 nests in a southern Ontario study (Weseloh and Jermyn in prep.). Egg laying begins in the third week of May in southern Ontario (Peck and James 1983, Heath et al. 2009), with an average mean clutch initiation date of 27 May (Weseloh and Jermyn in prep.). It may be up to two weeks later for breeding sites north of the Great Lakes, however, information is lacking. Incubation lasts 19 to 24 days (Bergman et al. 1970, Bailey 1977, Carroll 1988, Mazzocchi et al. 1997) from the laying of the first egg. In Ontario, incubation periods of 19 to 22 days have been recorded (Dunn 1979, Peck and James 1983). Though the Black Tern is single-brooded, birds may lay a replacement clutch 8 to 22 days following nest failure (Bailey 1977, Gerson 1988, Mazzocchi and Muller 1993 as cited in Heath et al. 2009), pushing the incubation period into late-July (Firstencel 1987, Novak 1990, Weseloh and Jermyn in prep.). Both adults incubate the eggs, although the male does less often (Cuthbert 1954, Goodwin 1960, Carroll 1988). Hatching usually occurs from late June to early July (Heath et al. 2009). Chicks are semi-precocial, meaning they are fairly mature and mobile when they hatch. They are capable of swimming within 24 hours (Carroll 1988). They grow quickly for the first 2 to 10 days of life, gaining mass at a rate of 4.18 to 5.18 g/day (Bailey 1977, Dunn 1979, Mosher 1986, Gilbert and Servello 2005a). In the absence of disturbance, both adults bring food to the nest for 7 to 14 days while the young remain flightless (Goodwin 1960, Firstencel 1987, Carroll 1988). Disturbance (e.g., from researchers or predators) may cause the young to leave and scatter earlier before being capable of flight (Goodwin 1960, Firstencel 1987). Adults continue to tend the young after fledging (usually day 20 to 24 after hatching) (Bailey 1977, supported by Dunn 1979, Firstencel 1987, Carroll 1988) and move to open water where they defend feeding territories from other terns as a family group (Heath et al. 2009). Parents may continue to feed them for up to two weeks in the feeding territory (Cuthbert 1954, Mosher 1986).
Nest success (at least one egg hatching successfully) has been reported as high as 51.4 percent in Ontario (Weseloh and Jermyn in prep.) and 50 percent in New York (Firstencel 1987). It is typically, around 34 percent in Wisconsin (Bailey 1977), 29 percent in Iowa (Bergman et al. 1970) and 27 percent in Ontario (Dunn 1979). Measurements of chick-to-fledging survival have yielded similar percentages (Heath et al. 2009), indicating most loss occurs prior to hatching. For example, within 19 sites in New York, the number of nests producing fledglings in a colony averaged 20 percent (Novak 1990). The ratio of 'young to adults' observed in a study area in Wisconsin (Mossman 1980 as cited in NatureServe 2012) was 1:4 (25%). Dunn (1979) reported that most losses occur at the egg stage with wind and wave action due to storms, changing water levels and boat vehicle traffic are the leading causes (Bergman et al. 1970, Bailey 1977, Dunn 1979, Mosher 1986, Carroll 1988, Gilbert and Servello 2005b). Earlier nests, larger clutch sizes and nests separated by greater distances from other nests were the most successful in Minnesota (Maxson et al. 2007).
Survivorship and site fidelity
Site fidelity of Black Terns is variable but typically low. Terns may return to previous nesting areas for many years (Carroll 1988) but may suddenly abandon marshes with no visible changes in site characteristics (Gerson 1988). They have also been found to return to a site after an absence of years, presumably having moved to other wetlands in the area or different regions in the interim (Mazzocchi et al. 1997). Site fidelity may be low due to changes that occur in marsh characteristics and/or nesting requirements such as vegetation density, water level and amount of nesting substrate (Bailey 1977, Dunn 1979). Typical of marsh nesting gulls and terns, Black Terns tend to respond to changes in site quality as a group, such that all pairs within the colony may abandon sites which become less suitable (McNicholl 1975). In Ontario, Dunn (1979) reported a return rate of adults of 27 percent at Long Point, at a time when the local population was much higher than present, while Bailey (1977) found the return rate to be 40 percent in Wisconsin. Survivorship remains largely unknown because of fairly low breeding- ground site fidelity (Heath et al. 2009). The maximum age known is eight years, five months (Heath et al.2009), but Servello (2000) proposed an estimated longevity of 17 years based on what is known in other tern species. Nothing is known about survivorship and site fidelity on the wintering grounds (NatureServe 2012). Pairs that return to the same wetland will mate again, but it is thought that majority of birds change mates each year (Heath et al. 2009).
Diet
The Black Tern’s diet is unusual for a tern and includes both invertebrates and fish (compared to most terns that eat only fish (are piscivorous); Cuthbert 1954, Mosher 1986, Dunn 1979, Beintema et al. 2010). Birds forage while flying, taking food at or near the water’s surface, or off of emergent vegetation (Goodwin 1960). One study in Michigan reported that arthropods form 93 percent of the adult diet (Cuthbert 1954). In the breeding season, the chicks are fed insects, spiders, crayfish and small mollusks. Commonly fed insect items include dragonflies and damselflies (Odonata), mayflies (Ephemeroptera), caddisflies (Trichoptera), moths (Lepidoptera), beetles (Coleoptera) and water scorpions (Hemiptera). The proportion of insects versus fish fed to chicks varies according to availability (Gilbert and Servello 2005a). Minnows are commonly taken, sometimes as far as four km away from the nest, with longer flights typically done by the male (Heath et al. 2009). In New York, minnows were brought to the young for 41 percent of feedings (Goodwin 1960). In Ontario on Lake Erie, fish comprised 13 percent of the diet and dragonflies six percent (Dunn 1979). Dunn (1979) estimates fish provide one-third of the protein in the diet of chicks. There is evidence of no relationship between diet composition of fish vs. insects and chick growth rates through the first 12 days in Maine (Gilbert and Servello 2005a). Though elsewhere, in an acidic peat-bog wetland in the Netherlands, chicks fed a diet of strictly invertebrates exhibited calcium deficiencies, resulting in death (Beintema et al. 2010). After fledging, the young take a larger number of fish of increasing sizes (Heath et al. 2009). The winter and migratory diet is not well known but appears to be comprised mainly of small fish supplemented with insects (Heath et al. 2009).
Predation
Predation on nestlings has been reported in a number of studies, however, in most cases the predator identity was unknown. Suspected predators include Mink (Neovison vison), Great Blue Heron (Ardea herodias), Black-crowned Night Heron (Nycticorax nyticorax), Ring-billed Gull (Larus delawarensis), Great Horned Owl (Bubo virginianus), Northern Water Snake (Natrix sipedon) and Snapping Turtle (Chelydra serpentina) (Cuthbert 1954, Goodwin 1960, Firstencel 1987, Novak 1990). A variety of experimental studies have attempted to increase survival by penning in nestlings (Bailey 1977, Dunn 1979, Mosher 1986, Alvo et al. 1998, Gilbert and Servello 2005b, Heath and Servello 2008, Weseloh and Jermyn in prep). An enclosure of protective material such as hardware cloth was placed around the nest in order to restrict movement in and out of the nest site by young and potential predators. As a result of the mixed success rates, (i.e., predation rates ranging from 8 to 61.5 percent between years in one study alone (Bailey 1977) and because of the artificial nature of penning nestlings, these experiments have contributed little to our understanding of predation and nestling survivorship.
2.2 Population and distribution
The Black Tern is Holarctic in its distribution, meaning it can be found throughout the world’s northern continents. The North American subspecies, Chidonias niger surinamensis, nests from British Columbia, southeastern Yukon and southern Northwest Territories eastwards across the Prairie Provinces and Ontario to southern Quebec, southern New Brunswick and western Nova Scotia. It occurs southwards locally into central California, Colorado, Nebraska, southern Illinois, northern Ohio, northwestern Pennsylvania, northern New York, Vermont and eastern Maine. It formerly bred south to Missouri and Kentucky (Heath et al. 2009, NatureServe 2012; Fig. 1). In Ontario, the Black Tern is found in scattered locations across the province, north to Big Trout Lake and Fort Albany (James 1985), with highest densities along the lower Great Lakes coastlines, Bruce Peninsula, Manitoulin Island and the southern edge of the Canadian Shield (Weseloh 2007). The latter area has the highest proportion of wetlands in the Canadian Great Lakes Basin (Detenbeck et al. 1999). Abundance across much of the north is limited by a lack of suitable wetlands for nesting (Dunn 1987, Austen and Cadman 1994, Austen et al. 1996); however, distribution in that region is poorly known due to low survey coverage. Small concentrations are found in the Clay Belt, Lake-of-the-Woods and wetlands adjacent to southern James Bay. The subspecies Chidonias niger niger occurs in northern Europe, Russia and Siberia south to the Mediterranean, Asia Minor, Turkestan and the Caspian and Aral Seas (Health et al. 2009).
Figure 1. Distribution of the Black Tern (Chlidonias niger surinamensis). Taken from Ridgely et al. 2003.

The Black Tern is found in open waters in the wintering grounds, residing offshore of both the Pacific and Caribbean coasts of Panama and northern South America, from Colombia eastwards to French Guiana and northern Brazil, and southwards to northern Peru. It occurs northwards along the Pacific Coast to Guerrero, Mexico, and can be an irregular visitor throughout the winter range (see Fig. 1; Heath et al. 2009).
Spring migrants start to appear in a broad front across North America by April and arrive in southern Ontario by early May (Gerson 1988), though scattered birds may return on the last few days of April (Wormington in prep.). The bulk of birds migrate through the southern US in the first half of May. The arrival of birds at the northernmost breeding sites in the Hudson Bay Lowlands and boreal regions is likely to occur during the third and fourth week of May. Pair formation may begin before arrival on the breeding grounds but much of the courtship is performed during the first 10-14 days after arriving and before the eggs are laid in mid-late May (Heath et al. 2009). Adults and their young begin to stage in favoured feeding areas near the nesting site by late July in southern Ontario and then move onto bigger water bodies, forming larger groups of birds by August (Gerson 1988).
The Black Tern used to gather in large numbers in July and August along eastern Lake Ontario, western Lake Erie (Jermyn and Weseloh in prep., Wormington in prep.) and formerly the upper Niagara River (3000-5500 birds in the late 1960's; Carroll 1988).
Most Black Terns depart Ontario between mid-August and early September (Wormington in prep.) and likely follow a coastal route to the wintering grounds (Heath et al. 2009). Migrants are thought to move singly or in small flocks. Larger flocks start to form on or near the wintering grounds further south along coastal sites in the southern US to Central America (Heath et al. 2009. Loftin (1991) reported that the Black Tern was the most abundant bird in the Gulf of Panama during the boreal winter and that non-breeding birds were also quite numerous during the summer months.
The only estimate of the North American population is between 100,000 and 500,000 birds (Kushlan et al. 2002). This was generated by regional experts and reported numbers in the literature across the tern’s range. The centre of Black Tern abundance is the prairie pothole and aspen parkland regions of the northern US states of North Dakota, Iowa and Minnesota, and the provinces of Manitoba, Saskatchewan and Alberta (Peterjohn and Sauer 1997). The northeastern populations (including Ontario) are considered to have lower densities, due to fewer suitable wetlands (Peterjohn and Sauer 1997). In 1994, Austen and Cadman estimated the Ontario population to be between 2,873 and 14,996 breeding pairs in southern Ontario, and likely a much lower number (due to fewer suitable wetlands) in northern Ontario.
The Black Tern has continuously declined across its global range since the 1950s. Declines in the range of 95 percent have been reported within European populations in the Netherlands (Beintema 1997). In North America, data from the Breeding Bird Survey (BBS) between 1966 and 1996 showed an annual decline of 3.1 percent per year (Peterjohn and Sauer 1997). This represents a decline of 61.1 percent in 30 years (Naugle 2004). The steepest declines occurred during the 1970s, while rates became more gradual through the 1980s and early 1990s. In Ontario, the greatest declines occurred during the period from 1966 to 1979. At that time, the annual rate of decline was 13.2 percent per year (Peterjohn and Sauer 1997). Similarly, the Canadian Wildlife Service (CWS) reported a significant Canada-wide decline of 3.2 percent per year using BBS data from 1970 to 2011.
Declines in Ontario during this time period based on BBS data averaged 4.47 percent per year and regionally they declined 5.52 percent per year in the Boreal Hardwood Transition Zone (Bird Conservation Region 12) and 2.8 percent per year in the Lower Great Lakes/ St. Lawrence Plain Zone (Bird Conservation Region 13). Additional analyses examined trends from 2001 to 2011 and results showed the overall provincial trend declining at 4.69 percent, with the Boreal Hardwood Transition declining at 5.1 percent and the Great Lakes/ St. Lawrence Plain Zone declining at 5.08 percent over this period (low reliability, A. Smith in prep. 2013). However, the BBS is known for data deficiencies for colonial marshbirds like Black Tern. The surveys limited to roadsides, which under-sample the available habitat and the semi-colonial habits of the tern make sampling more difficult. Furthermore, population fluctuations are large and commonplace, due to the often temporary nature of suitable habitat across the landscape (Peterjohn and Sauer 1997). Lastly, not enough routes occur with Black Tern’s present range to make the data reliable (Alvo and Dunn 1996).
Outside BBS data, there is further evidence of Black Tern declines in Ontario. In 1934, P.A. Taverner wrote "no extensive expanse of watery marsh" in southern Ontario was lacking Black Terns (Austen et al. 1994). Populations at Long Point dropped by 80 to 90 percent from 1981 to 1991 (J.D. McCracken, pers. comm. as cited in Austen and Cadman 1994).
Within Presq'uile Provincial Park, numbers had declined from 250 pairs in 1948, to 25 to 30 in the 1980s, to 12 to 13 in 1991 (Lisgo 1991 as cited in Austen et al. 1996 ). Numbers increased to 20+ pairs in the mid 1990's (Teeuw 1995, Verreault 1996) before becoming extirpated by the late 1990s (D. Tyerman, pers. comm.). In 1991-92, a CWS survey of the lower Great Lakes and St. Lawrence River found 61 coastal colonies and an estimated 545 nests, including some wetlands within 10 km of eastern Lake Ontario (see Austen et al. 1996). By 2001, a similar study documented widespread declines across lower Great Lakes breeding sites in Ontario. At this time researchers counted 717 individual terns at 40 colonies. Although the data is not directly comparable between the two studies due to different sampling protocols (Weseloh 2007) estimates made of the 1991 population suggested a decline of 35 percent (3.2 percent per year) (Graham et al. 2002 as cited in Weseloh 2007). In the second Breeding Bird Atlas (2001-2005), the probability of observation did not change significantly in the province as a whole, however, it dropped by 41, 34, and 52 percent in the Carolinian, Lake-Simcoe-Rideau, and Southern Shield regions, respectively (Weseloh 2007, see Figure 2). The Black Tern disappeared as a breeding bird entirely from the Niagara Peninsula, several coastal marshes along the north shore of Lake Ontario and some sites on the Canadian Shield (Weseloh 2007, see Fig. 2).
Figure 2. Historical and current distribution of the Black Tern (Chlidonias niger) in Ontario. Taken frorn Weseloh. 2007. Black Tern in Atlas of the Breeding Birds of Ontario.

Figure 3. Model-predicted number of Black Terns observed per survey station between 1995 and 2011 by participants in the Great Lakes Marsh Monitoring Program (coordinated by Bird Studies Canada). Data are shown for the province of Ontario in the Lake Huron-Erie versus Lake Ontario basins (upper panel), coastal versus inland marshes (middle panel), and within Areas of Concern (AOCs) versus outside of AOCs (lower panel). Annual indices were generated via a generalized (Poisson) linear mixed model with year as a categorical variable and route as a random variable; vertical bars are 95-percent credibility intervals.
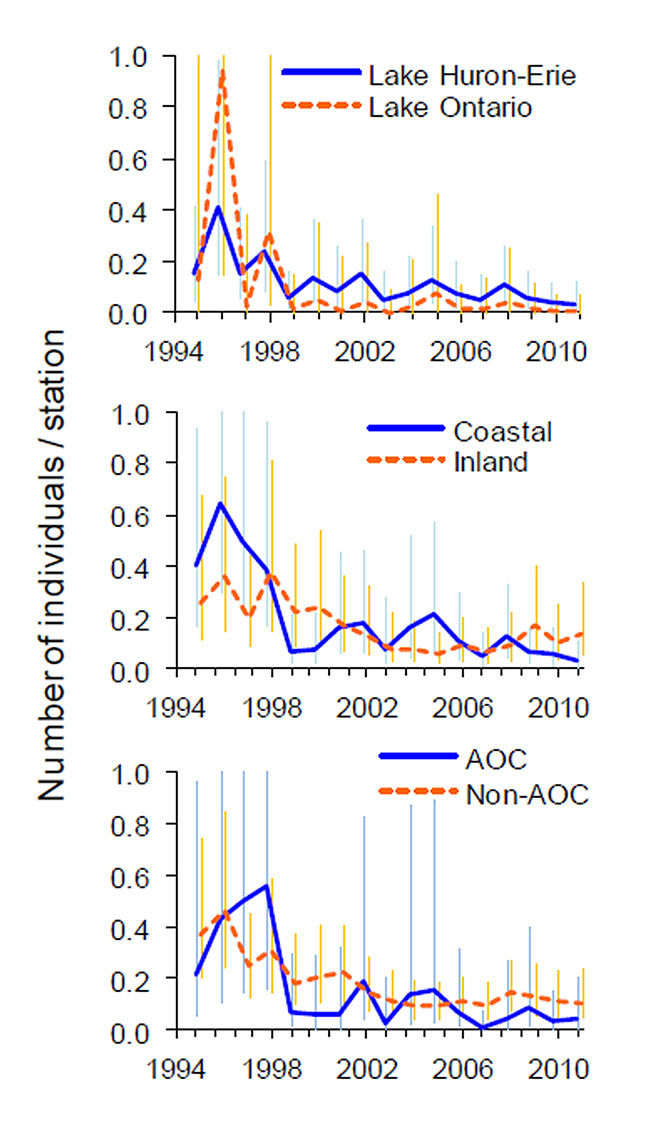
Based on Great Lakes Marsh Monitoring Program (MMP) data collected during the period 1995 to 2002 (Figure 3), Black Tern populations showed declines of 32 percent from the coastal sites on Lake Ontario and 19.3 percent on Lake Erie, and Huron- Michigan (Timmermans et al. 2008). Archer et al. (2009) reported slightly more modest rates of overall decline at 11.4 percent per year (CI -8.4, -14.3, p<0.001) for Black Tern across the Great Lakes Basin using MMP data collected from 1995 to 2007. When only Ontario sites are analyzed for the years 1995 to 2011, we see a decline of 15.6 percent per year (CI -24.2, -5.9, p<0.001) within the Lake Ontario basin and a decline of only 8.8 percent per year (CI -13.4, -4.7, p<0.002) within the Lake Huron-Erie basin. Within the same period in Ontario, populations at coastal sites declined more rapidly (-13.4% per year, CI -17.6, -8.8, p<0.001) than at inland sites (-7.8% per year, CI -11.6, -3.4, p<0.001), although the difference was not statistically significant. An analysis of Areas of Concern (AOC) versus non AOC marshes showed a steeper rate of decline of Black Terns in the AOCs, although, again, the difference was not statistically significant (Fig. 4). AOCs are areas that have experienced environmental degradation and have been identified as failing to meet the objectives of the Great Lakes Water Quality Agreement (US EPA: http://www.epa.gov/glnpo/aoc/).
Figure 4. Locations of Black Tern observations in Ontario between 1995 and 2011 by participants in the Great Lakes Marsh Monitoring Program (data collection, analysis and map by Bird Studies Canada).

Declines elsewhere in the northeast part of the range of the Black Tern are well documented. New York State listed it as special concern in 1983 and began statewide surveys in 1989. By 1999, the status was upgraded to endangered in New York because the population had declined 34.3 percent within 10 years (Mazzocchi and Roggie 2004). The most pronounced declines occurred along Lake Ontario coastal wetlands, where the number of colonies dropped statewide from 28 in 1989 to 14 in 2001 (Mazzocchi 2004). The most recent statewide survey in 2010 found only 10 breeding sites, a drop of 64 percent, since 1989 (Mazzocchi and Kolozsvary in prep.). The Black Tern is currently listed as endangered in Ohio and Pennsylvania, special concern in Michigan and under review in Minnesota (NatureServe 2012). Wisconsin has experienced a nearly 70-percent decline in abundance and site occupancy from 1980 to 2011 (Matteson et al. 2012).
Although published data on the Great Lakes Black Tern population during staging and migration is limited, there is further suggestion of a substantial decline. Declines of Black Terns have been reported during the fall migration along the upper Niagara River in Ontario and New York from the 1960s to the 1970s. The observed numbers averaged 2,000 to 5,000 birds in the 1960s, less than 1,000 in the 1970s, and dropped to less than 100 birds in the mid-and late-1980s (Carroll 1988). Although not as dramatic, the same time period also showed a decline in the Black Tern at Kingston, Ontario (Jermyn and Weseloh in prep.). On the wintering grounds in Panama, the Black Tern has been noted to have reduced numbers since the 1960s (Alvo and Dunn 1996, Heath et al. 2009).
2.3 Habitat requirements
During the breeding season Black Terns inhabit mainly limestone-based, rich, freshwater marshes with an abundance of emergent vegetation along rivers, lakes or inland locations. Fewer sites occur on the Canadian Shield (igneous rock base) in the central and western parts of the province. They are generally considered an area-dependant species, most likely to be found on wetlands in excess of 20 ha (Brown and Dinsmore 1986), although areas as small as 1.6 to 5 ha have been reported used for nesting (Heath et al. 2009, Steen and Powell 2012b). Naugle et al. (2000) demonstrated the importance of broad-scale habitat features on breeding densities of the Black Tern in Iowa. The size of a wetland used by terns was related to characteristics of the larger-scale wetland complex. Terns used smaller wetlands (less than 6.5 ha) most frequently when they occurred in high-density wetland landscapes composed of large (greater than 15.4 ha) and small wetlands, while low-density wetland landscapes which had mainly small wetlands (less than 6.5 ha) were not widely used (Naugle et al. 2000). However, Steen and Powell (2012b) reported that in the prairie pothole region, foraging was directed more by area sensitivity and breeding much less so, with four nests on small ponds 1.6 to 5.0 ha in area. Thus, they recommend that small high-quality wetlands may be useful to nesting terns.
Wetlands used most often by nesting Black Terns feature a roughly equivalent proportion (50:50) of open water interspersed with irregular patches of dense emergent vegetation (Gerson 1988, Carroll 1988, Hickey and Malecki 1997, Linz and Blixt 1997, Maxson et al. 2007). Most often cattail (Typha spp.) and bulrush (Cyperaceae spp.) comprise the vegetation, but also rushes (Juncus spp.), sedges (Carex spp.), bur-reed (Sparganium spp.), spikerushes (Eleocharis spp.), pickerelweed (Pontederia spp.), smartweeds (Polygonum spp.), Reed-canary Grass (Phalaris arundinacea), arrowhead (Sagittaria spp.), spadderdock (Nuphar spp.), water lilies (Nymphaea spp.), Wild Rice (Zizania aquatica) and other plants (Hickey and Malecki 1997, Naugle et al. 2000, Heath et al. 2009). This ratio of emergent plants to open water is often referred to as hemi-marsh (Naugle 2004, Weseloh 2007). Marshes that experience fluctuations in water levels over time or because of Muskrat activity, drought, winter ice damage and fire are most likely to maintain the habitat required by Black Terns (Carroll 1988, Heath et al. 2009). Without these types of disturbances, marshes can develop a thick, uniform cover of emergent vegetation (Gerson 1988). Because of a lack of open water, these sites are eventually rejected (Gerson 1988, Linz and Blixt 1997). Although sensitive to habitat loss, the tern is quick to exploit newly available suitable habitat (Gerson 1988). Black Terns occasionally use sewage lagoons in Ontario (Sandilands 2010) and may also breed sparsely in open fens across the boreal zone of the province (James 1985) and wetlands adjacent to the James Bay shoreline (P .Burke pers. obs.), however, specific information about the characteristics of these sites is lacking.
Nest site features are most influenced by vegetation structure and availability of nest substrate (Bailey 1977, Dunn 1979, Knutson 1991, Maxson et al. 2007). Terns prefer short and dense or sparse and tall emergent vegetation rather than any specific plant species (Naugle et al. 2000). However, they have been recorded to nest in a continuum of vegetation structures from dense stands of emergent plants to open water (Bergman et al. 1970, Dunn 1979, Novak 1990, Hickey and Malecki 1997). Maxson et al. (2007) suggest that Bulrush (Scirpus acutus) and sedge/grass are preferred over Cattail, but cattail tends to be more common, and hence more frequently used for nesting (Dunn 1979, Novak 1990, Gilbert and Servello 2005b). When the nest site is chosen, vegetation height is usually less than 0.25 to 0.5 m, but by the time the eggs hatch it is greater than 1.0 m (Heath et al. 2009). Water depth tends to be 0.5 to 1.2 m but may be less (Cuthbert 1954, Novak 1990, Hickey and Malecki 1997). In Ontario, water depth ranges from 0.075 to 1.22 m (Dunn 1979, Peck and James, 1983). Stabilized water levels during the nesting season are important to nest success (Cooper and Campbell 1997), in order to prevent nests from becoming flooded. Nests are typically located within six m of open water (Cuthbert 1954, Peck and James 1983) but maybe up to 25.3 m away (Novak 1990). At Long Point, nests ranged 0.5 to 12 m (average=4 m) from open water (Dunn 1979). If emergent vegetation is too dense, it prevents access to the surface of the water by the adults, and if it is too sparse, the nest lacks cover from wind and wave action or predators (Mosher 1986). Research has shown high variability of habitat requirements for Black Terns. Despite these, nest placement preferences seem to be given to areas within or at the edge of a low density of emergent vegetation, close to open water.
Black Terns become highly pelagic (preferring open water) after they leave the breeding grounds en route to wintering areas (Gerson 1988, Heath et al. 2009). In Ontario, on the lower Great Lakes, they have used marine buoys and sandbars as roosting sites, but these can be transitory from year to year (Jermyn and Weseloh in prep). In the winter months, they are typically found in marine and marine coastal areas, often in very large groups into the 1000s (Loftin 1991).
2.4 Characteristics contributing to vulnerability of species
There are a number of factors that contribute to the vulnerability of the Black Tern. It has very specific nesting habitat requirements. It is a semi-colonial nester that requires large (20ha +) hemi-marshes where the ratio of open water to emergent vegetation is roughly equal (Gerson 1988, Hickey and Malecki 1997, Naugle 2004, Heath et al. 2009). As the amount of open water in relation to emergent vegetation, water depth and nest site substrate availability can change, sites can become unsuitable (Gerson 1988, Heath et al. 2009). Tern will abandon sites if they become unsuitable. Hickey and Malecki (1997) found that Black Terns selected for highly favourable habitat patches within a marsh that met the criteria listed above (i.e. large hemi-marshes). However, these highly favourable habitats are limited beyond the basic availability of suitable nesting substrate, due to the terns preferences for the presence of conspecifics, and requirements for minimum patch size and patch isolation thresholds (Hickey and Malecki 1997).
Suitable wetlands are naturally patchy in distribution across the breeding range in Ontario (Austen et al. 1994). Nests are placed low to the water’s surface, making them very susceptible to flooding due to wind and wave action, which accounts for the majority of nest failures (Dunn 1979, Gilbert and Servello 2005b). Cold weather could reduce the number of insects that make up the nestling’s diet. Fish could be difficult to capture on windy or cloudy days, because Black Terns only use surface dipping to feed, rather than plunge diving (Beintema 1997). The nestling phase leaves the young vulnerable to predators for up to 20 to 24 days before they are capable of flight (Bailey 1977, Heath et al. 2009) and chick survival may be, in addition to egg-stage loss, an important cause of low breeding success (Heath and Servello 2008). The Black Tern is a single-brooded species, however, it will re-nest until mid-July if a nest failure occurs (Novak 1990, Gerson 1988, Naugle 2004). It is unknown how many attempts to re-nest will be made in a breeding season.
Like other Great Lakes nesting gulls and terns, the Black Tern is susceptible to buildup of contaminants in the ecosystem (Weseloh et al. 1997), not only on its breeding grounds in Ontario, but also on migration and wintering grounds (Heath et al. 2009). On the wintering grounds in the tropics, the tern is closely tied to marine environments vulnerable to changes in food supplies that support large concentrations of birds (Heath et al. 2009). A recent study in Wisconsin reported very low survival of chicks (2%) to breeding age (2 years old) (Shealer 2007 as cited in Matteson et al. 2012). Servello (2000) found that population growth rate was highly sensitive to adult survival, and the same Wisconsin study reported very little adult mortality on the breeding grounds (Matteson et al. 2012). More research is needed to shed light on the threats faced by Black Terns during migration and the wintering grounds and how much they contribute to adult survivorship.
3.0 Threats
Natural ecosystems are continually evolving in response to a variety of forces and factors. But they are limited in their ability to adapt to rapid change, such as that introduced through human activities. Humans sometimes disrupt and degrade biodiversity through habitat loss, introduction of invasive species, population growth, pollution, unsustainable use and climate change. Our growing population combined with our rising levels of resource consumption can threaten biodiversity (OBC, 2011). Recently, an assessment of pressures on Ontario’s biodiversity showed that many threats are increasing (OBC, 2010b).
The following threats have been identified as currently facing the Black Tern:
Habitat loss
The most significant threat to the Black Tern across its breeding range is the loss of wetlands (Gerson 1988, Naugle 2004, Heath et al. 2009, NatureServe 2012). In Ontario, the decline of the Black Tern is due to loss of wetland habitat for breeding (Austen et al. 1994). There has been a 72-percent loss of pre-settlement wetlands in southern Ontario (Ducks Unlimited 2010) due to land conversion for other uses, mainly agriculture (Snell 1987 as cited in Detenbeck et al. 1999) but also residential and industrial development (see population growth) (Ducks Unlimited 2010). The Hurontario Ecoregion, which forms the stronghold of Ontario’s inland Black Tern population, has experienced 40 to 80 percent decline of wetlands in its western portion and a significant loss in the central portion due to cottage development (Snell 1987, as cited in Detenbeck et al. 1999). The Black Tern was not recorded as a breeder on the Niagara peninsula in the second Breeding Bird Atlas, where wetland coverage was reduced to one percent of the landscape by 1996 (Detenbeck et al. 1999).
Coastal wetlands on the lower Great Lakes have historically been a stronghold for Black Tern populations but have experienced the sharpest declines (Carroll 1988, Austen et al. 1996). Water level fluctuation is important for the distribution and abundance in the Black Tern (Mazzocchi et al. 1997, Timmermans 2001, Timmermans et al. 2008). On Lakes Erie, Huron and Michigan water-levels fluctuate year to year due to natural processes.
Lake Ontario and the St. Lawrence River’s water levels have been regulated since 1959, so water levels there do not vary much (Timmermans et al. 2008). Black Terns on Lake Ontario have shown no relationship with water levels for 15 years (Timmermans et al. 2008) and have experienced the greatest declines (32% from 1995-2002) of all the lower Great Lakes. Because the water-level does not change, many of the marshes on Lake Ontario do not have flooding and draw-down events that are important for nutrient cycling and maintaining marsh productivity (Lyon et al. 1986, Mazzocchi and Roggie 2004). As a consequence, many of these are now filled with dense emergent vegetation (Timmermans 2001) and have lost the preferred hemi-marsh stage. As such, Black Tern populations have been reduced or completely lost from many historical sites (Carroll 1988).
Inland marshes, such as those along the southern edge of the Canadian Shield, where the Black Tern is still fairly widespread, appear to be less influenced by larger, landscape-based water level changes such as the amount of run-off from the catchment basin or winter snow accumulations that affect the Great Lakes (Timmermans 2001). Short-term precipitation events during the breeding season may allow marshes to maintain water level features that Black Terns find acceptable for breeding. This has also been found in Maine, where Black Terns were most successful at inland wetlands that were able to maintain water levels that sustained favourable nest site characteristics (Gilbert and Servello 2005b).
Habitat loss for the Black Tern is not only the direct result of outright destruction of marshes due filling in for development, but can also be due to changes in habitat quality in remaining sites (Beintema et al. 2010). Hemi-marsh can change to dense stands of monotypic cattail or invasive European Common Reed Grass (Phragmitis australis). Availability of nest site substrate has been shown to be a limiting factor for Black Terns in New York, Wisconsin and Holland, (Mazzocchi and Roggie 2004, Shealer et al. 2006, Bientema et al. 2010).
In New York State, management of Black Tern habitat has been used at western and northern breeding sites to increase productivity and halt the deterioration of marshes (Hickey and Malecki 1997, Mazzocchi et al. 1997). Many traditional nesting sites have lost Black Terns due to wetland draining, filling in with emergent vegetation, degradation of water quality, invasive plant species or low threshold population densities (Hickey and Malecki 1997, Mazzocchi et al. 1997, Mazzocchi and Roggie 2004). Decreases in populations in coastal wetlands of Lake Ontario may have been caused by increased lake level, destruction of wetlands, human development and recreation (motorized boat traffic) (Carroll 1988, Mazzocchi and Roggie 2004).
Invasive species
Deterioration of nesting habitat due to invasive species is believed to be a contributing factor to the overall decline of the Black Tern in the Great Lakes basin (Hickey and Malecki 1997). The opening of the St. Lawrence Seaway in 1959 resulted in an explosion of invasive plants and animals, which reached an alarming 139 species in the Great Lakes by 1993 (Mills et al. 1993) and 178 species by 2010 (Ontario Biodiversity Council 2010). While many of these species are the result of unintentional releases through ship ballast water being emptied into the Great Lakes, others have been intentionally established (Mills et al. 1993).
Coastal and inland wetlands in the Great Lakes basin used by Black Terns are threatened by a number of invasive plant species which replace native vegetation, cause homogenization (Houlahan and Findlay 2004) and unfavourably alter biotic and abiotic features of nesting sites. Open wetlands are more susceptible to invasive plants than forested wetlands (Detenbeck et al. 1999).
Detenbeck et al. (1999) list eight plant species rapidly expanding in the Great Lakes basin that threaten emergent wetlands.
European Common Reed Grass (Phragmites australis australis) of the non-native strain (haplotype M), has spread rapidly across the Great Lakes basin and poses a major threat to wetlands. Since the period 1995 to 1999, when its abundance increased exponentially at Long Point, European Common Reed Grass has replaced various types of wetlands including cattails, mixed emergent and marsh meadows across the southern Ontario landscape (Wilcox 2003). Black Tern populations at Long Point declined by 90 percent from 1981 to 1991 (J.D. McCracken, pers. comm. in Austen and Cadman 1994) and European Common Reed Grass abundance has been thought to have reduced suitable breeding habitat significantly, possibly limiting the number of breeding sites available.
At Walpole Island First Nation, the extensive channels in the wetlands used to be lined with shallower water with Muskrat activity/houses and other exposed mudflats for potential nesting sites. However, this has changed dramatically in the last two to three decades, as many of the channels are now lined with dense European Common Reed Grass vegetation (A. Woodliffe, pers. comm. 2012). On Lake Michigan at Green Bay in Wisconsin, Reed Grass now covers 75 percent of what was once productive wetland breeding habitat (Matteson et al. 2012).
Evidence exists that although the growth of this plant is tied to shallower patches of a wetland (Herrick and Wolf 2005), the presence of large expanses of European Common Reed Grass may be enough to deter use by Black Terns, and more study is required. Increased abundance of European Common Reed Grass across the Great Lakes is thought to be a result of lower water levels, increases in ambient temperature and both human and natural disturbances (Wilcox et al. 2003).
Another threat is posed by European Frog-bit (Hydrocharis morsus-ranae), which was introduced in the 1930s in Ottawa. It has since spread south and west along the shores of Lake Ontario via the Rideau system, and to Point Pelee on western Lake Erie by 2000, including parts of the Lake Ontario drainage system in central Ontario (Catling et al. 2003).
This free-floating aquatic plant forms dense mats that prevent light from penetrating the water column and can cause declines of submersed native vegetation and other aquatic life due to reduced available oxygen (Catling et al. 2003). A rapid increase in the abundance of European Frog-Bit on Presqu'ile Bay since the 1980s caused many of the open-water backchannels of the marsh to fill in. This has reduced the amount of nesting and foraging habitat formerly occupied by terns and may partly help to explain why the species is currently extirpated from breeding there (R.D. McRae, D. Tyerman, pers. comm. 2012). However, before their extirpation, the terns had moved their nesting areas from the backchannels to the outer marsh where European Frog-bit is not prevalent. At a restored wetland at Bath, Ontario, the area of marsh covered by European Frog-bit was avoided by nesting Black Terns (Alvo et al. 1998). The effects of European Frog-bit on Black Tern nesting habitat in Ontario remains unstudied.
Purple Loosestrife (Lythrum salicaria) is another invasive emergent wetland plant that quickly establishes itself and can out-compete native plant species in a short period of time. It has been reported to have changed the wetland structure of traditional inland nesting sites such as that present in Montezuma National Wildlife Refuge in New York State so that by 1979, 485 ha of Black Tern nesting area had been filled in by Purple Loosestrife (Carroll 1988). However, the rapid expansion of Purple Loosestrife in Ontario occurred mainly after large declines in the Black Tern were noted in the 1980s (Alvo and Dunn 1996). In fact, it has been proposed that Black Terns are not concerned with plant species as much as stem density and water depth for nesting (Alvo and Dunn 1996).
Deterioration of wetlands in the Great Lakes basin due to destruction of submersed vegetation and increased water turbidity by Common Carp (Cyprinus carpio) (Lougheed et al. 1998) and Mute Swan (Cygnus olor) (Petrie and Francis 2003) is well known. Where Carp abundances are high, significant changes to wetlands occur, as their behaviour negatively affects native aquatic vegetation and creates large areas devoid of any plant growth. They disturb the benthic community (the organisms living on or in the sediment at the bottom of a waterbody), increase the turbidity, which spurs declines in zooplankton (Kirk 1991) and affect the entire wetland food chain, reducing the availability of food required by nesting Black Terns.
The Mute Swan is a large species of European waterfowl released into North America around the late 1800s (Petrie and Francis 2003). It has been colonizing wetlands on the Great Lakes since the mid-1960s, with a notable increase in the past 20 years. Petrie and Francis (2003) showed that Mute Swans had increased at a rate of 10 to 18 percent/year from 1971 to 2000 across the lower Great Lakes. The Canadian population was projected to reach 30,000 birds within 30 years at a minimum 10 percent growth rate (Petrie and Francis 2003). During the breeding season, Mute Swans aggressively defend their large territories and chase and even kill Muskrats and wetland-dependant birds and their young. They also consume and uproot large amounts of aquatic vegetation (Ciaranca et al. 1997). They have been shown to cause nest abandonment of terns such as the marsh nesting Forster’s Tern (Sterna forsteri, Ciaranca et al. 1997). In the absence of disease or serious degradation of wetland quality, Mute Swan numbers in Ontario are expected to reach carrying capacity without direct human intervention. At Presqu'ile Provincial Park, Mute Swans began breeding in 1989 and the park now contains approximately 12 breeding pairs (D. Tyerman, pers. comm. 2012), each defending territories as large as six ha (Ciaranca et al. 1997). Numbers of breeding Black Terns declined (as well as Muskrat (R.D. McRae, pers. comm. 2012)) through this period and breeding terns are now extirpated at Presqu'ile (R.D. McRae, pers. comm., D. Tyerman, pers. comm. 2012).
Winter concentrations of Mute Swans have increased quickly since 2000 (D. Bree, pers. comm. 2012) with 916 counted in early January, 2013 (R.D. McRae, pers. comm. 2012). The swans consume and disturb large amounts of wetlands in Presqu'ile and Weller Bay each year. At the Bath Lennox Hydro Generating restored wetland, the number of Black Terns breeding and Muskrats has declined dramatically within the last three to four years in conjunction with a pair of Mute Swans now annually nesting there (T. Brady, pers. comm. 2012). The effects of Mute Swan on Black Tern breeding success and disappearance from nesting sites in Ontario has not been studied and may be important.
Population growth
As Ontario’s human population continues to grow, there is more pressure placed on wetlands and Black Tern breeding habitat. From 2006 to 2011, Ontario’s population grew by 5.7 percent, and most counties within the main distribution of the Black Tern had 25 to 100 people/km2 (Statistics Canada 2012). The expanding population places direct pressure on wetlands in the form of drainage and development for residential, industrial and recreational uses, as well as problems of water level stabilization, sedimentation, contaminants, nutrient inputs and invasive non-native plants and animals (Gerson 1988, Detenbeck et al. 1999, Archer et al. 2009).
Great Lakes coastal wetlands are now just remnants of their former sizes, with sections such as western Lake Erie wetlands down to five percent of their pre-European settlement size (Petrie and Francis 2003). In addition to habitat destruction, human activity can have direct effects on Black Terns. Wakes from motorized boats have been shown to cause nest failure due to drowning of eggs and chicks (Heath et al. 2009). Increasing boater awareness through signage has helped to reduce flooding of nests by boat wakes in New York State (Novak 1990). Wakes from large shipping vessels along the St. Mary’s River caused disturbances to colonies (Scharf 1999 as cited in Kudell- Ekstrum and Rinaldi 2004). Jet skis are capable of accessing shallow marshy areas and caused disruptions to Black Tern nesting areas in Presqu'ile Bay in the 1990s (R.D. McRae, pers. comm. 2012) and at a nesting site in Minnesota (Kudell-Ekstrum and Rinaldi 2004). Black Terns are fairly tolerant of human activity near the nest site, as long as exposure is kept brief (Gerson 1987, Shealer and Haverland 2000). Adult terns will repeatedly show aggression to a human invader near an occupied nest site by repeatedly diving at them through the air in swoops.
On the wintering grounds, increasing human population is likely affecting Black Terns. The stocks of small pelagic fish off the Pacific coast of Panama, a main dietary item where Black Terns winter in abundance, crashed due to overfishing in 1972 and have not recovered since due to continued human fishing pressure (Patterson et al. 1992). This coincided with a period of steep decline across the range of the Black Tern but no causal link was established (Alvo and Dunn 1996).
Pollution
The effects of environmental contamination has been studied in Black Tern populations on the Great Lakes and elsewhere. Evidence that toxic levels in other nesting waterbirds like Caspian Tern (Sterna caspia), Common Tern (S. hirundo) and Forster’s Tern have been shown to be elevated (Weseloh et al. 1997). It is difficult to draw conclusions about the presence of particular contaminants in birds, because their origins cannot be definitively established, especially with a bird that migrates and winters in different parts of the Americas.
Environmental contamination levels in Lake Ontario and the St. Lawrence River Black Tern eggs were not found to be elevated compared to other colonial waterbirds or to contamination studies of Black Terns outside Ontario (Weseloh et al. 1997).
Organochlorines and Mirex were found in eggs and tissues (0.01-0.04 ppm) taken from a Black Tern colony in a coastal wetland in New York. Mirex is known to occur mainly in Lake Ontario and was presumed to have originated, at least in part, from there (Carroll 1988). Eggshell thinning (9% thinning in Ontario) was not enough to cause the reproductive problems that have been experienced by other colonial waterbird species on the Great Lakes (thinning of 15-20% or more). However, Weseloh et al. (1997) cautions that eggshell thicknesses did show declines that warrant further investigation, and an overall better understanding of the effects of contaminants on Black Terns in the Great Lakes is needed.
Black Terns are known to rely much more on insect prey to feed their young than other colonial waterbirds (including Forster’s Tern), which use fish almost exclusively. This may help reduce the amount of pollutant bioaccumulation Black Terns experience. However pesticide use, acid rain and water pollution across southern Ontario’s agricultural landscape may reduce the invertebrate populations that Black Terns require for reproductive success (Carroll 1988, Weseloh et al. 1997, Alvo and Dunn 1996, Heath et al. 2009). These same factors have been associated with losses in Black Tern food supplies for chicks in the Netherlands, much of it caused by eutrophication (excessive nutrients in the water) of wetlands due to agricultural runoff (Beintema et al. 2010).
Furthermore, reduced numbers of Black Terns may be linked to widespread declines of aerial insectivores (swifts, swallows, etc.), that have been documented throughout the Northern Hemisphere over the past 20 years (North American Bird Conservation Initiative Canada 2012). One current hypothesis relates to the increased use of neonicotinoid pesticides, which can persist in aquatic systems and corresponding declines in the availability of insect prey (Tennekes 2010).
Climate change:
Climate change models predict changes in the range of the Black Tern over time (Matthews et al. 2004, Steen and Powell 2012a). Mean global surface temperatures have increased by 0.74°C in the past century and are predicted to increase 1.1 to 6.4°C by 2100 (IPCC 2007 as cited in Steen and Powell 2012a). The magnitude of shifts in temperature or precipitation, along with the changes in their habitat features such as nest sites, food and other resources, will dictate the response of the Black Tern to climate change, as they will need to stay within their physiological tolerance (Wormworth and Mallon 2006 as cited in Steen and Powell 2012a). In the Dakotas, Minnesota and Iowa, Steen and Powell (2012a) predict nearly a complete loss of Black Terns by 2100, due to a decline in available wetlands and hemi-marsh habitat that suffer from drought and wetland disappearance. Matthews et al. (2004) predicted that the eastern United States populations would experience total or nearly complete extirpation.
Climate change is predicted to favour a number of invasive species currently threatening Ontario’s wetlands. Declining water levels and warmer ambient temperatures on Lake Erie promote an abundance of European Common Reed Grass at Long Point and other Great Lakes wetlands (Wilcox et al. 2003) that formerly held much larger breeding Black Tern populations. In Lake St. Clair and western Lake Erie, water level changes have been significant in the last three to four decades. During the higher water of the '70s and into the early '80s, marshes were much more open, replicating the 'hemi-marsh' character. At that time, European Common Reed Grass was present but only in small patches, and mostly of the less problematic native type. However, when water levels lowered, the vegetation, either cattail or reed grass and more often the latter, increased considerably, reducing the openness of the wetlands and affecting the available habitat for the Black Tern (A. Woodliffe, pers. comm. 2012).
Warmer winter temperatures improve survivorship and increase abundance of Mute Swan on the lower Great Lakes, promoting expansion of a growing population into smaller inland wetlands (Petrie and Francis 2003). European Common Reed Grass and Mute Swan, and others (see invasive species section), are suspected to further perpetrate the decline in Black Tern populations in Ontario.
Within the last 20 years, lower water levels on the Great Lakes (with the exception of Lake Ontario), have increased the amount of inner wetland edge which Black Terns find unsuitable, as it lacks the critical habitat features required for nesting (Archer et al. 2009). This is well documented in the changes in marsh vegetation at Long Point (Wilcox et al. 2003). Marshes such as Presqu'ile’s have been expanding outwards at a rapid rate in the last 20 years (R.D. McRae pers. comm. 2012) even though since 2000 Lake Ontario water levels have been above average for eight years and below average for five years (National Oceanic and Atmospheric Administration 2013). Water level manipulation seems a more likely issue for Black Tern on Lake Ontario.
Climate change might also affect the Black Tern on the wintering grounds. It is possible that an increase in the frequency of El Nino Southern Oscillation events (co2science 2013) will affect their marine food resources. A more energetic atmosphere might also produce more frequent and violent storms affecting the birds on their wintering grounds and during migration.
4.0 Management
4.1 Goal and objectives
The goal of this management plan is to maintain or improve the viability of Ontario’s Black Tern population. To reach this goal a set of objectives have been determined with measurable, time-defined approaches that require the support and cooperation of a number of interested organizations, landowners, institutions and government. The key management objectives include further clarification of main threats to Black Tern populations, the development of Best Management Practices to reduce threats and restore habitat and the development of protocols to accurately assess the current status and range of the tern in Ontario. Critical to our understanding of the threats are studies that clarify how development, climate change, invasive species, pollution and increased human recreational activities all cause habitat loss. Monitoring programs should include the study of known populations along with a thorough inventory to accurately assess the status of the provincial population.
Table 1. Management objectives
| Number | Management Objectives |
|---|---|
| 1 | Determine distribution and abundance of Black Tern populations in Ontario. |
| 2 | Clarify the main threats to Black Tern populations. |
| 3 | Maintain the distribution and abundance of Black Tern in Ontario by protecting habitat and reducing the impacts of threats. |
4.2 Management actions completed or underway
Black Terns are protected under the Migratory Birds Convention Act, 1944, c.22 in the United States and Canada and under the Migratory Bird and Game Mammal Treaty between Mexico and the United States (Gerson 1988, Weseloh and Jermyn in prep.). Forest management operations on crown land in Ontario currently identify Black Tern colonies as values and protect them from disturbance (OMNR 2010).
A number of management approaches have been employed in an attempt to stabilize or increase Black Tern populations. The protection of habitat through land acquisition or conservation easement has been aimed at larger wetland complexes (greater than 10 ha) that benefit the wetland bird community in general (Zimmerman et al. 2002). These complexes can accommodate within-season or year-to-year changes in water levels and provide nesting terns with suitable breeding sites in at least some parts of them in a given year (Brown and Dinsmore 1986, Hickey and Malecki 1997).
Restoration of wetlands in areas where loss and degradation have been severe is also a management option. Local-and landscape-levels of suitable habitat for nesting Black Terns are important considerations (Naugle 2000, Zimmerman 2002). The use of flooding/drawdown to manage wetland impoundments for the Black Tern has been used with success in the Tonawanda complex of western New York and the St. Clair National Wildlife Area and is recommended across the tern’s range in the state of New York to increase nesting habitat at inland locations (Mazzocchi and Roggie 2004). Objectives for this method are to provide the appropriate amount of emergent vegetation to open water ratio, stabilized water levels throughout the nesting season and abundant available nesting substrate (Carroll 1988, Hickey and Malecki 1997, Heath et al. 2009). The marshes are drawn down (drained) in May, disked (plowed using a tractor with a disk attachment) in July or August, and then re-flooded. It is recommended that the marshes be placed on a four to six-year cycle of drawdown, with re-flooding in years two and five (Hickey 1997 in Naugle 2004). It is also suggested that water levels be maintained higher than normal in the first year following re-flooding in order to allow Muskrat populations to recover and discourage the growth of invasive plants (Hickey and Malecki 1997, Kudell-Ekstrum and Rinaldi 2004). Impoundments on the Great Lakes are more vulnerable to invasive plant growth due to modified hydrologic regimes and increase in nutrients compared to natural wetlands (Herrick and Wolf 2005). Removal of vegetation by Muskrat herbivory benefits Black Terns by improving the mix of vegetation cover and open water and by increasing the availability of nest substrates (Hickey and Malecki 1997, Alvo et al. 1998). There is a high success rate of Black Terns colonizing impoundments the year following re-flooding, with peak numbers in the second or third year after re-flooding (Hickey and Malecki 1997). Hickey (1997) also recommends providing elevated perches in resting and feeding areas of potential habitat.
Dense cattail stands may be managed by water level control, prescribed burning, disking, good Muskrat populations and application of herbicides in order to promote the ratio of vegetation to open water that Black Terns and other declining wetland birds such as Blue-winged Teal (Anas discors) and Common Gallinule (Gallinula chloropus) prefer (Linz et al. 1994, Hickey and Malecki 1997, Linz and Blixt 1997). Beule (1979 as cited in Zimmerman et al. 2002) described methods for control of dense cattails according to water depth using techniques such as mechanical crushing, black tarps and cutting mature stems below the surface. The relationship between Muskrat populations and Black Tern nesting habitat requires further study as Muskrats may remove too much cattail regrowth if their numbers become too high (Kudell-Ekstrum and Rinaldi 2004, Steen and Powell 2012b).
Artificial nest platforms have been utilized as an important tool in management and recovery of Black Terns. They have been accepted by terns as a replacement nesting substrate, especially when natural sites are not available due to flooding. In Europe, artificial platforms have become increasingly valuable in helping to provide nesting substrates (van der Winden and van Horssen 2008). Black Terns readily accepted platforms for nesting (65% occupancy) in Wisconsin in two successive years. In fact, the artificial nests may even achieve higher survival rates and hatching success than nearby natural nests (Shealer et al. 2006). However, artificial platforms have only been shown to be successful where sites retain a good food supply and lack natural nesting opportunities (Beintema et al. 2010). Hickey and Malecki (1997) recommend the use of artificial platforms in the first year following flooding of impoundments. Conversely, where Black Terns nest in wetlands with low spring water levels prone to later flooding, placement of buoyant material under the nest has been used due to the tern’s tolerance of nest manipulation (Gilbert and Servello 2005b). Platforms are more likely to be used by terns if dead vegetation is placed upon them (Heath et al. 2009). Sizes of platforms recommended vary from 12 cm X 20 cm (Mosher 1986) to 81 cm x 81 cm (Kudell- Ekstrum and Rinaldi 2004). Success is increased if platforms have a border that allows chicks to re-enter them and they are moved annually to prevent predators learning their location (van der Winden 2005 as cited in Steen and Powell 2012b).
Studies on the use of artificial platform in Ontario have been limited. A 1995 study in coastal sites along eastern Lake Ontario observed that a number of wetlands had foraging adults but lacked suitable nesting sites that other tern-inhabited marshes possessed. The limited use of decoys and attractants (taped vocalizations) were unsuccessful. It was recommended that artificial nesting platforms be deployed (Richardson 1996) to encourage nesting in more marshes. Artificial nesting platforms have been successfully used by Black Terns at an impoundment at Ontario Hydro’s Lennox Generating Station at Bath (Alvo et al. 1998), and on Amherst Island (Morris 2003). At Presqu'ile Provincial Park the employment of artificial platforms (bare plank, Styrofoam and ABS tube frame style) was used between 1993 and 1996 and increased the local breeding population substantially in 1995 (Gurr 1994, Teeuw 1995, Verreault 1996). Three types of platforms were employed at the large Bath impoundment nesting colony (wire mesh, wooden tray and bare planks) with varied success. Wire mesh was determined to be the best option due to a similar egg hatching success rate to natural nest sites monitored in the study, much better retention of nest characteristics and less attraction to other wildlife that interfered with nest success (Alvo et al. 1998).
Nest enclosures have been employed around individual nests in several studies with varied success. One study in Maine found that most adults accepted them and they resulted in 70-percent success retaining and protecting young until fledging age (Heath and Servello 2008). See Alvo et al. (1998) and Shealer and Haverland (2000) for detailed recommendations on enclosure/exclosure protocols. Dunn (1979) found a 27- percent success rate over two field seasons at Long Point and Bailey (1977) had just 12 percent (3 of 26 chicks) in Wisconsin using enclosures. Nest success at unenclosed nests has been estimated at 15 to 20 percent (Bailey 1977, NatureServe 2012).
4.3 Management plan approaches for action
Table 2. Management plan approaches for action for the Black Tern in Ontario.
1. Determine distribution and abundance of Black Tern populations in Ontario
| Management Theme | Management Approach | Relative Priority | Threats or Knowledge Gaps Addressed | Relative Timeframe |
|---|---|---|---|---|
| Communications | 1.1 Encourage collaboration among relevant agencies such as Ontario Ministry of Natural Resources (OMNR), Environment Canada (EC), Parks Canada Agency (PCA), non-government organizations (such as Bird Studies Canada, Ducks Unlimited) and scientific community to develop and implement habitat protection for the tern. | critical |
|
short-term |
| Research Inventory and Monitoring |
1.2 Address gaps in current known range to better assess the population size and distribution in cooperation with the Marsh Monitoring Program. | necessary |
|
short-term |
2. Clarify the main threats to Black Tern populations
| Management Theme | Management Approach | Relative Priority | Threats or Knowledge Gaps Addressed | Relative Timeframe |
|---|---|---|---|---|
| Inventory and Monitoring | 2.1 Undertake monitoring of known populations, assess viability of populations and identify threats such as: habitat quality, water level control mechanisms, invasive species and human disturbance. | critical |
|
short-term |
| Research | 2.2 Address research needs to help understand breeding success and productivity in Ontario. This includes the effects of water level fluctuations on site tenacity and productivity. | critical |
|
Long-term Ongoing |
| Research | 2.3 Study the effects of invasive species on Black Terns, specifically addressing how they affect breeding success and habitat loss. Include how decreased lake levels and other potential impacts of climate change and invasive species impact Black Tern. | necessary |
|
long-term short-term |
| Research | 2.4 Identifying staging areas, migratory timing and pathways, winter distribution and ecology and threats faced during migration/wintering. | necessary |
|
short-term |
| Communications | 2.5 Engage in communications with researchers studying habitat loss and population decline in Black Tern. Establish partnerships with organizations undertaking control of invasive species (e.g., Ontario Invasive Plant Council). Establish connections with other jurisdictions (e.g., New York, Michigan, Wisconsin) that also contend with threats to their Black Tern populations. | critical |
|
ongoing |
3. Maintain the distribution and abundance of the Black Tern in Ontario by protecting habitat and reducing the impacts of threats
| Management Theme | Management Approach | Relative Priority | Threats or Knowledge Gaps Addressed | Relative Timeframe |
|---|---|---|---|---|
| Protection Stewardship |
3.1 Secure habitat on key sites through acquisition across a representative range in Ontario; especially sites with populations of over five pairs, greater than 20 ha wetlands with hemi-marsh, proximal sites with unoccupied potential habitat, water level control features, minimal threats and stable futures. | critical |
|
short-term |
| Inventory and Monitoring | 3.2 Create a database of sites that are protected. Determine the value of the site to the overall population in Ontario. | critical |
|
short-term |
| Management Inventory and Monitoring |
3.3 Manage prioritized sites to improve/maintain habitat through water level manipulation, use of artificial nests or enclosures when demonstrated to be effective, and removal of invasive species. Assess and monitor impacts of Best Management Practices employed. |
critical |
|
Ongoing |
| Education and Outreach Stewardship |
3.4 Develop educational materials for distribution to landowners, land stewards, conservation authorities, cottager associations and Parks Canada about the Black Tern and threats to its survival. Emphasize threats posed by habitat loss, invasive species and nest flooding due to water vehicle traffic during the breeding season. Encourage management of wetlands through control of water levels to create and maintain Black Tern breeding habitat and other species associated with their habitat. | necessary |
|
short-term |
| Communications Management |
3.5 Ease the effects of population growth/ habitat decline due to human disturbances such as those posed by recreational motorized water vehicles. | critical |
|
short-term |
| Education and Outreach Stewardship |
3.6 Establish partnerships with other governments, landowners, conservation authorities, cottager associations and First Nations to encourage Best Management Practices that are compatible with the needs of the Black Tern and other wetland wildlife (i.e. a multi-species approach) on their lands. | critical |
|
long-term |
| Education and Outreach Communications |
Collaborate with other conservation initiatives with similar habitat and management needs such as Spotted Turtle (Clemmys guttata) and Least Bittern (Ixobrychus exilis) that require permanent to semi-permanent wetlands with an interspersion of open water and emergent vegetation. | critical |
|
long-term |
Supporting narrative
In order to determine the distribution and abundance of the Black Tern in Ontario it is recommended to inventory historical sites where the tern has not been recorded since 2008 for at least two consecutive years, as well as currently known sites. It is also important to identify potential areas occupied by the tern, such as through use of GIS. This approach is especially necessary in the northern parts of the province where Black Terns are sparsely distributed. The time to inventory potential and known sites for terns during the breeding season is from late May to late July. All findings should be reported to the NHIC. Northern breeding sites will likely be hard or impossible to access due to remoteness and cost and will require more time to assess.
In the assessment of the viability of populations and identification of threats at a given site, the following characteristics should be considered:
- habitat quality including the amount of suitable breeding habitat present (hemi- marsh) compared to marsh area (non hemi-marsh)
- water level control mechanisms present (or not) at inland sites
- invasive species such as European Common Reed Grass, European Frog-bit, Purple Loosestrife, Mute Swan, Common Carp or any others detected
- human disturbance due to encroachment of development, recreational water vehicle traffic, etc
Prioritization of risk across sites for those that are in most need of acute management can then be assessed.
The Nature Conservancy (NatureServe 2012) has listed a set a research priorities to better understand the needs of Black Tern, including:
- determine the cause of nest failure and mortality in all age classes at nesting colonies
- continue to evaluate effectiveness of artificial platforms for increasing nest success or population densities with emphasis placed on sites lacking natural nesting substrate or placement in areas with reduced disturbance
- determine nest site fidelity of adults and whether young return to the sites where they hatched; continue to determine effects of contaminants on nesting success, chick development and juvenile and adult survival
- assess effects of disturbance from water vehicle traffic on nesting colonies
Past work on productivity and breeding success in Ontario has shown that Black Terns produce less than one young per nest (Weseloh and Jermyn in prep.) and many of these questions above would require long-term studies. It may be a more productive use of resources to use some parameter estimates generated from other studies (Shealer 2003, Shealer 2007).
Additionally, the importance of Muskrat to the creation and maintenance of suitable Black Tern habitat (Hickey and Malecki 1997) and the potential for water-level manipulation as a management tool for Black Tern conservation needs to be addressed further.
There is also a need to understand the impact of invasive species such as European Common Reed Grass, Purple Loosestrife, European Frog-Bit, Common Carp, Mute Swan and other species that may be affecting Black Tern populations in Ontario.
Very little is known in general regarding staging areas, migratory timing and routes, winter distribution and ecology of the Black Tern’s annual cycle. The potential that some of these factors may be responsible for continuing population declines requires more study.
Because the Black Tern is a species of concern throughout the eastern portions of its range, it has been the focus of much research and thus a large amount of local information exists. It is therefore important to communicate with other jurisdictions (e.g., Quebec, New York, Michigan and Wisconsin) that also contend with threats to their Black Tern populations from factors such as habitat loss and degradation of wetlands. Ontario could participate in the region-wide census of Black Terns already established in surrounding states since 2004 (Mazzocchi and Kolozsvary 2010).
The development of Best Management Practices for the Black Tern through research and communications will help build upon current approaches to maintain species viability as well as benefit other species at risk that share the same habitat. The following approaches could be included in habitat management.
- Develop or improve the ability to regulate water levels and manage habitat for the benefit of breeding terns and other associated at risk marsh species at key wetlands
- Employ the use of artificial nesting platforms at sites that have been recently used by Black Terns when there is a lack of nesting substrate available (usually associated with high water levels). Employ nest enclosures only when there is a demonstrated need due to high predation pressures
- Remove invasive species that pose a risk to the population (following research on invasive species that shows it is justified, see item 2.4). Employ risk management to determine best approach (e.g., combined use of manual and chemical treatment for European Common Reed Grass, European Frog-bit or control of adult/young Mute Swans before tern breeding season) and/or manage water levels and hemi-marsh conditions
In an effort to slow the disappearance of wetlands, especially in southern Ontario where the tern is most abundant, it is important to curb the effects of a growing human population. Effective approaches for the Black Tern could be to engage large groups, such as the cottage and boating industries, to address the needs of Black Tern on public and private lands. This could include providing information on threats posed by habitat loss, invasive species such as European Common Reed Grass, European Frog-bit, Common Carp and Mute Swan, and sensitivity to flooding of nests due to water vehicle traffic during the breeding season. It is also important to provide input to water management plans of Conservation Authorities to improve/create Black Tern nesting habitat and ensure appropriate water levels during breeding season are provided. Use signage or markers to delineate tern nesting areas with an appropriate buffer and require a reduction of vehicle wakes in breeding areas where recreational water vehicles are used.
Glossary
Arthropods: an organism that has an exoskeleton, jointed legs and segmented bodies. Insects, crustaceans (such as crayfish, lobsters), arachnids (spiders, centipedes) are members of this group of animals.
Benthic: Organisms associated with the bottom substrate of aquatic environments such as lakes and rivers.
Bio-accumulation: this occurs in an organism when accumulation of a toxin occurs at a rate greater than which it is lost.
Bird Conservation Region: ecologically defined units that have been developed by the North American Bird Conservation Initiative that adhere to ecological, not political boundaries and are employed as a basis for conservation of bird species across the continent.
Carrying capacity: the maximum number of organisms that an environment is able to support given the food, water and habitat requirements of that organism.
Committee on the Status of Endangered Wildlife in Canada (COSEWIC): The committee responsible for assessing and classifying species at risk in Canada.
Committee on the Status of Species at Risk in Ontario (COSSARO): The committee established under section three of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
Conservation status rank: A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. The conservation status of a species or ecosystem is designated by a number from one to five, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperiled
2 = imperiled
3 = vulnerable
4 = apparently secure
5 = secure
Disking: The most intense disturbance of wetland vegetation used in wetland management, often done with a standard agricultural disk. Disking destroys both the erect stems as well as breaking up the extensive rhizome system that keeps plants alive during dry conditions.
Dorsal: the back surface of a bird; including the top sides of the wings and tail.
Ear coverts: the set of feathers on the sides of a bird’s head that cover over the opening of the ear. On some species, such as the Black Tern in juvenile or winter plumage, they are contrastingly colored to create a unique appearance to the head.
Emergent substrates; matter (living or non-living) which protrudes above the surface of the water in which the rest of it lays beneath the surface.
Endangered Species Act, 2007 (ESA): The provincial legislation that provides protection to species at risk in Ontario.
Eutrophication: The response of an ecosystem to the addition of natural or artificial substances such as fertilizers or sewage to an aquatic system.
Fledgling: the stage in the life of a young Black Tern when it is capable of its first sustained flight. Black Terns are semi-precocial and able to leave the nest well before they are capable of flight.
Hemi-marsh: The structure of a freshwater marsh where the proportion of emergent vegetation (e.g., Cattails, Rushes) is equal in proportion (50:50) to open water.
Holarctic: the ecozones of the Northern Hemisphere, includes North America, Asia and Europe.
Hurontario Ecoregion: the strip of the Ontario landscape that runs along the edge of the Canadian Shield from Georgian Bay to eastern Lake Ontario. It is unique in its fauna and flora.
Insectivorous: organism whose diet is mainly comprised of insects.
Invertebrate: an animal that lacks a backbone; examples include insects, crustaceans, spiders, mollusks and worms.
Mantle: The dorsal surface of a bird, including the back and wings.
Marsh Monitoring Program: A volunteer-based program managed by Bird Studies Canada since 1995 where observers annually sample wetlands for birds and calling amphibians to detect trends in populations.
Mollusk: an invertebrate found on land or in water belonging to a diverse group of animals that includes clams and snails.
Organochlorines: a carbon based compound that contains chlorine. These compounds are known for their negative effects on human and other animal life.
Pelagic: A marine-based behavior exhibited by some species of birds that inhabit the open ocean exclusively. Black Terns become pelagic during migration and on the wintering grounds.
Philopatry: The behavior of returning to the same location as an individual’s birthplace.
Physiological: Characteristics of an organism’s healthy functioning.
Piscivorous: an organism whose diet is mainly comprised of fish.
Semi-colonial: The tendency of an organism to breed clumped closely together in looser associations than the short distances exhibited by strictly colonial species which nest side by side. Black Terns will nest as close as two m from one another, but their densities are typically much lower than that.
Semi-precocial: Young that have the characteristics of precocial young in that they are hatched covered with down, eyes open and the ability to leave the nest site but remain until close to adult size.
Single-brooded: during the breeding season, an organism, such as the Black Tern, will attempt to raise one group of young. This may involve more than one attempt if a nest fails before the young are independent. Some species may attempt to raise more than one brood per season and are thus multi-brooded.
Site fidelity: the reuse of a particular area in successive years by a returning individual, often for the purpose of breeding, as in the case of Black Tern.
Species at Risk Act (SARA): The federal legislation that provides protection to species at risk in Canada. This act establishes Schedule one as the legal list of wildlife species at risk to which the SARA provisions apply. Schedules two and three contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule two and three are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule one.
Species at Risk in Ontario (SARO) List: The regulation made under section seven of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
Undertail coverts: the body feathers of a bird that cover the underside of the tail feather bases.
Wetland complex: a series of separate wetlands in close proximity to one another within the landscape.
Zooplankton: Small, sometimes microscopic, animals drifting in the water column that form the basis of the food chain in aquatic ecosystems.
References
Alvo, R. and E. H. Dunn. 1996. Updated Status Report on the Black Tern in Canada. COSEWIC. 10 pp.
Alvo, R., H. Blokpoel and G. McKenna. 1998. Use of natural habitat and artificial nest sites by Black Terns at an impoundment near Kingston, Ontario in 1997. Technical Report Series No. 292. Canadian Wildlife Service, Ontario Region. 32 pp.
Archer, R., H. Wheeler and D.J. Sass. 2009. State of the Great Lakes 2009. Coastal Wetland Bird Communities. US Environmental Protection Agency. www.epa.gov/solec/sogl2009/4507cwbirds.pdf (link no longer active)
Austen, M.J.W. and M.D. Cadman. 1994. The Status of the Black Tern in Ontario. Ontario Ministry of Natural Resources. 29 pp.
Austen M.J.W., M.D. Cadman and R.D. James. 1994. Ontario Birds at Risk. Status and Conservation. Federation of Ontario Naturalists and Long Point Bird Observatory. 165 pp.
Austen, M.J.W., H. Blokpoel and G.D. Tessier. 1996. Atlas of Colonial Waterbirds nesting on the Canadian Great Lakes, 1989-1991. Part 4: Marsh-nesting terns on Lake Huron and the Lower Great Lakes System in 1991. Technical Report Series No. 217. Canadian Wildlife Service. Ontario Region.
Bailey, P.F. 1977. The breeding biology of the Black Tern. Master’s Thesis. Univ. of Wisconsin, Oshkosh.
Bergman, R.D., P. Swain, and M.W. Weller. 1970. A comparative study of nesting Forster’s and Black Terns. Wilson Bull. 82:435-444.
Beintema, A.J. 1997. European Black Terns (Chlidonias niger) in Trouble: Examples of Dietary Problems. Colonial Waterbirds 20(3):558-565.
Beintema, A.J., J. van der Winden, T. Baarspul, J. Pieter de Krijger, K. van Oers, and M. Keller. 2010. Black Terns Chlidonias niger and their dietary problems in Dutch Wetlands. Ardea 98:365-372.
Beule, J.D. 1979. Control and management of cattails in southeastern Wisconsin wetlands. Wisconsin Department of Natural Resources, Madison, Wisconsin. Technical Bulletin No. 112. 39 pp.
Bree, D. 2012. Park Naturalist. Presqu'ile Provincial Park, OMNR. Dec. 19, 2012.
Brady T., 2013. Environmental Officer, Lennox Generating Station, Ontario Hydro, Bath, Ontario. Personal communication, Jan. 8, 2013.
Brown, M. and J.J. Dinsmore. 1986. Implications of marsh size and isolation for marsh bird management. Journal of Wildlife Management 50:392-397.
Carroll, J.R., 1988. Status and Breeding Ecology of the Black Tern (Chlidonias niger) in New York. The Kingbird 38(3):159-172.
Catling, P.M., G. Miltrow, E. Haber, U. Posluszny, and W.A. Charlton. 2003. The Biology of Canadian Weeds. 124. Hydrochorus morsus-ranae L. Canadian Journal of Plant Sciences. 83:1001-1016
Canadian Wildlife Service. 2012. Black Tern. Canadian Bird Trends. http://www.cws-scf.ec.gc.ca/mgbc/trends/index.cfm?lang=e&go=info.bird&speciesid=770. Accessed 11 December, 2012.
Ciaranca, M.A., C.C. Allin, and G.S. Jones. 1997. Mute Swan (Cygnus olor). Account No. 273 in A. Poole and F. Gill, editors. The Birds of North America. Academy of Natural Sciences Philadelphia, Pennsylvania and American Ornithologists' Union, Washington, D.C. USA.
Cooper, J.M. and R.W. Campbell. 1997. Surveys of Selected and Traditional Black Tern (Chlidonias niger) Colonies in British Columbia in 1996. Colonial Waterbirds 20(3):574-581.
Co2science 2013. ENSO (relationship to global warming). http://www.co2science.org/subject/e/summaries/ensogw.php. Accessed 16 March 2013
Cuthbert, N.L. 1954. A nesting study of the Black Tern in Michigan. Auk 71:36-63.
Detenbeck, N.E., S.M. Galatowitsch, J. Atkinson, and H. Ball. 1999. Evaluating Perturbations and Developing Restoration Strategies for Inland Wetlands in the Great Lakes Basin. Wetlands 19(4):789-820.
Ducks Unlimited, 2010. Final Report. Southern Ontario Wetland Conversion Analysis. Ontario Office. Barrie, Ontario. 23 pp.
Dunn, E.H. 1979. Nesting biology and development of young in Ontario Black Terns. Can. Field-Nat. 93:276-281.
Dunn, E.H. 1987. Black Tern in Cadman, M.D., P.F.J. Eagles and F.M. Helleiner. Atlas of the Breeding Birds in Ontario 1981-1985. University of Waterloo. Ontario pp. 192-193.
Firstencel, H. 1987. The Black Tern (Chlidonias niger Linn.): breeding ecology in upstate New York and results of pesticide residue analyses. Master’s Thesis. State Univ. of New York, Rockport.
Gerson, H. 1988. Status Report on the Black Tern (Chlidonias niger). Committee on the Status of Endangered Wildlife in Canada (COSEWIC), Toronto, Ontario. 54 pp.
Gilbert, A.T. and F.A. Servello. 2005a. Insectivory versus piscivory in Black Terns: Implications for food provisioning and growth of chicks. Waterbirds 28(4):436-444.
Gilbert, A.T. and F.A. Servello. 2005b. Water Level Dynamics in Wetlands and Nesting Success of Black Terns in Maine. Waterbirds 28(2):181-187.
Goodwin, R.E. 1960. A study of the ethology of the Black Tern, Chlidonias niger surinamensis (Gmelin). Ph.D. Thesis. Cornell Univ. Ithaca, NY.
Graham D., S.T.A. Timmermans, and J. McCracken. 2002. A comparison of abundance of colonial marsh birds between 1991 and 2001 in the Canadian portions of Lakes Huron, St. Clair, Ontario and Erie. Bird Studies Canada, Port Rowan.
Gurr, M. 1994. Black Tern population study - 1994. Presqu'ile Provincial Park. Ontario Ministry of Natural Resources.
Heath, S.R. and F.A. Servello. 2008. Effects of Predation and Food Provisioning on Black Tern Chick Survival. The Wilson Journal of Ornithology 120(1):167-175.
Heath S.R., E.H. Dunn, and D.J. Agro. 2009. Black Tern (Chlidonias niger), the Birds of North America Online (A. Poole, Ed.). Ithaca: Cornell Lab of Ornithology; Retrieved from the Birds of North America Online: http://bna.birds.cornell.edu.bnaproxy.birds.cornell.edu/bna/species/147
Herrick B.M., and A.T. Wolf. 2005. Invasive Plant Species in Diked versus Undiked Great Lakes Wetlands. Journal of Great Lakes Research 31:277-287.
Hickey, J.M. 1997. Breeding biology and population dynamics of the Black Tern in western New York. M.Sc. Thesis, Cornell University, Ithaca, New York.
Hickey, J.M. and R.A. Malecki. 1997. Nest Site Selection of the Black Tern in Western New York. Colonial Waterbirds 20(3):582-595.
Houlahan J.E. and C.S. Findlay. 2004. Effects of Invasive Plants Species on Temperate Wetland Plant Diversity. Conservation Biology. 18(4):1132-1138.
IPCC.2007.Climate Change 2007: The Physical Science Basis. http://www.ipcc.ch/publications_and_data/publications_ipcc_fourth_assessment_report_ wg1_report_the _physical_science_basis.htm (link no longer active)
James, R. D. 1985. Habitat Management Guidelines for Birds of Ontario Wetlands. Ontario Ministry of Natural Resources.
Jermyn, K. and D.V.C. Weseloh in prep. The Decline of Black Terns at Staging Areas on the Lower Great Lakes. Canadian Wildlife Service. Downsview, Ontario.
Kirk, K.L. 1991. Inorganic particles alter competition in grazing plankton: the role of selective feeding. Ecology 72:915-923.
Knutson, M.G. 1991. Characteristics of Black Tern (Chlidonias niger) nesting habitat at Lakeview Wildlife Management Area, New York. Kingbird 41:228-235.
Kudell-Ekstrum, J. and T. Rinaldi. 2004. Conservation Assessment for Black Tern (Chlidonias niger) Linnaeus. USDA Forest Service, Eastern Region. Milwaukee, Wisconsin. 46 pp.
Kushlan, J.A., M.J. Steinkamp, K.C. Parsons, J. Capp, and others. 2002. Waterbird Conservation for the Americas: The North American Waterbird Conservation Plan (Version 1). Waterbird Conservation for the Americas, Washington, D.C.
Lisgo, K. 1991. Black Tern population study - 1991. Presqu'ile Provincial Park. Ontario Ministry of Natural Resources.
Linz, G.M., D.L. Bergman, D.C. Blixt, and W.J. Bleier. 1994. Response of Black Terns (Chlidonias niger) to Glyphosate-Induced Habitat Alterations on Wetlands. Colonial Waterbirds. 17(2):160-167.
Linz, G.M. and D.C. Blixt. 1997. Black Terns Benefit from Cattail Management in the Northern Great Plains. Colonial Waterbirds 20(3):617-621.
Loftin, H. 1991. An annual cycle of pelagic birds in the Gulf of Panama. Ornithologia neotropical 2:85-94.
Lougheed, V.L., B. Crosbie, and P. Chow-Fraser. 1995. Predictions on the effect of Common Carp (Cyprinus carpio) exclusion on water quality, zooplankton, and submergent macrophytes in a Great Lakes wetland. Canadian Journal of Fisheries and Aquatic Sciences. 55:1189-1197.
Lyon, J., R.D. Drobney, and C.E. Olson, Jr. 1986. Effects of Lake Michigan water levels on wetland soil chemistry and distribution of plants in the Straits of Mackinac. Journal of Great Lakes Research. 12(3):175-183.
Matteson, S.W., M.J. Mossman, and D.A. Shealer. 2012. Population Decline of black Terns in Wisconsin: A 30-Year Perspective. Waterbirds 35(2):185-193.
Matthews, S.N., R.J. O'Connor, L.R. Iverson, and A.M. Prasad. 2004. Atlas of Climate Change Effects of 150 Bird Species of the Eastern United States. United States Department of Agriculture. Forest Service. Northeastern Research Station. Pennsylvania. General Technical Report NE-318.
Maxson, S.J., J.R. Fieberg, and M. R.Riggs. 2007. Black Tern nest habitat selection and factors affecting nest success in Northwestern Minnesota. Waterbirds 30(1):1-9.
Mazzocchi, I. M. and S. L. Muller. 1993. Black Tern (Chlidonias niger) survey in New York State, 1992. Unpublished report. New York State Department of Environmental Conservation, Watertown, New York.
Mazzocchi, I.M., J.M. Hickey, and R.L. Miller. 1997. Productivity and Nesting Habitat Characteristics of the Black Tern in Northern New York. Colonial Waterbirds 20(3):596-603.
Mazzocchi, I.M. and M. Roggie. 2004. Black Tern (Chlidonias niger) statewide surveys and observations in 2001. New York State Department of Environmental Conservation, Watertown, New York.
Mazzocchi, I.M. and Kolozsvary M.B. in prep. Black Tern Statewide Count 2010. New York State Department of Environmental Conservation.
McNicholl, M.K. 1975. Larid Site Tenacity and Group Adherence in Relation to Habitat. The Auk 92(1):98-104.
McRae, R.D. 2012. Biological consultant. .Personal communication. December 5, 2012.
Mills E.L., J.H. Leach, J.T. Carlton, and C.E. Secor. 1993. Exotic Species in the Great Lakes – A History of Biotic Crises and Anthropogenic Introductions. Journal of Great Lakes Research. 19(1):1-54.
Morris, M.E., 2003. Analysis of environmental contaminants in Black Terns (Chlidonias niger). M.Sc. Thesis. Royal Military College of Canada. Kingston, ON. 196 pp.
Mosher, B.C. 1986. Factors influencing reproductive success and nesting strategies in Black Terns Ph.D. dissertation. Simon Fraser University Burnaby, BC. 154 pp.
National Oceanic and Atmospheric Administration, 2013. Great Lakes Environmental Research Laboratory. www.glerl.noaa.gov/data/now/wlevels/dbd/ (link no longer active) accessed 4 March 2013.
NatureServe. 2012. NatureServe Explorer: An online encyclopedia of life [web application]. Version 7.1. NatureServe, Arlington, Virginia. Available http://www.natureserve.org/explore. (Accessed: October 17, 2012).
Naugle, D.E. 2004. Black Tern (Chlidonias niger surinamensis): A Technical Conservation Assessment. [Online]. USDA Forest Service, Rocky Mountain Region. Available: http://www.fs.fed.us/r2/projects/scp/assessments/blacktern.pdf [October 21, 2012].
Naugle, D.E., K.F. Higgins, M.E. Estey, R.R. Johnson, and S.M. Nusser. 2000. Local and Landscape-Level Factors Influencing Black Tern Habitat Suitability. The Journal of Wildlife Management 64(1):253-260.
North American Bird Conservation Initiative Canada, 2012. The State of Canada’s Birds, 2012. Environment Canada, Ottawa, Canada. 36 pp.
Novak, P.G. 1990. Population status of the Black Tern in New York State-1989. New York State Department of Environmental Conservation. 32 pp.
Ontario Biodiversity Council (OBC). 2010. State of Ontario’s Biodiversity 2010. A report of the Ontario Biodiversity Council, Peterborough, Ontario [available: http://www.ontariobiodiversitycouncil.ca/index.php/reports].
Ontario Biodiversity Council. 2011. Ontario’s Biodiversity Strategy, 2011: Renewing Our Commitment to Protecting What Sustains Us. Ontario Biodiversity Council, Peterborough, ON.
Ontario Ministry of Natural Resources. 2010. pp. 125-126 in Forest Management Guide for Conserving Biodiversity at the Stand and Site Scales. Toronto: Queen’s Printer for Ontario 211 pp.
Patterson, K.R., J. Zuzunaga, and G. Cárdenas.1992. Size of the South American sardine (Sardinops ysagax) population in the northern part of the Peru upwelling ecosystem after collapse of anchoveta (Engraulis ringens) stocks. Canadian Journal of Fisheries and Aquatic Sciences 49:1762-1769.
Peck, G.K. and R.D. James. 1983. Breeding Birds of Ontario: Nidiology and Distribution. Vol. 1: Nonpasserines. Life Sciences Miscellaneous Publication, Toronto, The Royal Ontario Museum.
Peterjohn, B.G. and J.R. Sauer. 1997. Population Trends of Black Terns from the North American Breeding Bird Survey, 1966-1996. Colonial Waterbirds 20(3):566-573.
Petrie, S.A. and C.M. Francis. 2003. Rapid Increase in the Lower Great Lakes Population of Feral Mute Swans: A Review and a Recommendation. Wildlife Society Bulletin 31(2):407-416.
Richardson, M. 1996. A survey of Black Terns (Chlidonias niger) occurring in Prince Edward County and adjacent areas of Northumberland, Hastings and Lennox and Addington/Frontenac Counties, with observations of habitat and use of nest platforms in the 1995 breeding season. Report prepared for the Canadian Wildlife Service. 38 pp.
Ridgely, R.S., T.F. Allnutt, T. Brooks, D.K. McNicol, D.W. Mehlman, B.E. Young, and J.R. Zook. 2003. Digital Distribution Maps of the Birds of the Western Hemisphere, version 1.0. NatureServe, Arlington, Virginia, USA.
Sandilands, A.P. 2010. Black Tern in Birds of Ontario: Habitat Requirements, Limiting Factors and Status. Volume 2: Shorebirds through Woodpeckers. pp. 151-155. University of British Columbia Press. Vancouver.
Servello, F.A. 2000. Population Research Priorities for Black Terns Developed from Modeling Analyses. Waterbirds 23(3):440-448.
Scharf, W.C. 1999. Black Tern nesting colonies and Habitats in Marshes of the St. Mary’s River, Michigan. Michigan Birds and Natural History. 6(2):65-73.
Shealer, D.A. and J.A. Haverland. 2000. Effects of Investigator disturbance on the Reproductive Behavior and Success of Black Terns. Waterbirds 23(1):15-23.
Shealer, D.A., J.M. Buzzell, and J.P. Heiar. 2006. Effects of floating nest platforms on the breeding performance of Black Terns. Journal of Field Ornithology 77(2):184-194.
Shealer, D.A. 2003. Annual survival, breeding site fidelity, and natal recruitment of Black Terns breeding in southeastern Wisconsin, 1999-2003. U.S. Fish and Wildlife Service, Horicon National Wildlife Refuge, Mayville, Wisconsin.
Shealer, D.A. 2007. Population dynamics of Black Terns breeding in southeastern Wisconsin. 1999-2007. Passenger Pigeon 69:471-479.
Smith, A. 2013. Senior Biostatistician. Species Abundance and Distribution. Canadian Wildlife Service. Ottawa. Personal Communication. Apr. 17, 2013.
Snell, E.A. 1987. Wetland distribution and conversion in southern Ontario. Inland Waters and Lands Directorate, Environment Canada, Ottawa, ON, Canada Table 6. Working Paper 48, En 73-4/48E.
Statistics Canada 2012. 2006 and 2011 Censuses of Canada. Produced by the Geography Division, Statistics Canada, 2012. www.statcan.gc.ca
Steen V. and A.N. Powell. 2012a. Potential Effects of Climate Change on the Distribution of Waterbirds in the Prairie Pothole Region, U.S.A. Waterbirds 35(2):217-229.
Steen V. and A.N. Powell. 2012b. Wetland Selection by Breeding and Foraging black Terns in the Prairie Pothole Region of the United States. The Condor 114(1):155-165.
Teeuw, M. P., 1995. Management efforts for a declining Black Tern (Chilidonias niger) population in Presqu'ile Bay in 1995. Presqu'ile Provincial Park. Ontario Ministry of Natural Resources.
Tennekes, H.A., 2010. The significance of the Druckney-Kupfmuller equation for risk assessment- The toxicity of neonicotinoid insecticides to arthropods is reinforced by exposure time. Toxicology 276(1):1-4.
Timmermans, S.T.A. 2001. Temporal relationships between marsh bird and amphibian annual population indices and Great Lakes water levels: a case study from the Marsh Monitoring Program. Bird Studies Canada. Environment Canada, and U.S. Environmental Protection Agency, Port Rowan, Ontario.
Timmermans, S.T.A., S.S. Badzinski, and J.W. Ingram. 2008. Associations between Breeding Marsh Bird Abundances and Great Lakes Hydrology. Journal of Great Lakes Research. 34:351-364.
Tyerman, D. 2012. Park Naturalist, Presqu'ile Provincial Park, OMNR. Personal communication, Dec. 20, 2012.
van der Winden, J. 2005. Black Tern Chlidonias niger conservation in the Netherlands – a review. Vogelwelt 126:187-193.
van der Winden, J. and P.W. van Horssen. 2008. A population model for the black tern Chlidonias niger in West-Europe. Journal of Ornithology 149:487-494.
Verreault, M. A., 1996. Black Tern population study - 1996. Presqu'ile Provincial Park. Ontario Ministry of Natural Resources.
Weseloh, D.V. 2007. Black Tern in Atlas of the Breeding Birds of Ontario. Editors M.D. Cadman, D.A. Sutherland, G.G. Beck, D. Lepage, and A.R. Couturier. Bird Studies Canada, Environment Canada’s Canadian Wildlife Service, Ontario Nature, Ontario Field Ornithologists and Ontario Ministry of Natural Resources. 728 pp.
Weseloh, D.V.C., J. Rodrigue, H. Blokpoel, and P.J. Ewins. 1997. Contaminant Concentrations in Eggs of Black Terns (Chlidonias niger) from Southern Ontario and Southern Quebec, 1989-1996. Colonial Waterbirds 20(3):604- 616.
Weseloh, D.V.C. and K. Jermyn. in prep. Breeding Biology and Contaminant Levels in Black Terns (Chlidonias niger) in Southern Ontario. Canadian Wildlife Service, Downsview, Ontario.
Wilcox, K.L., S.A. Petrie, L.A. Maynard, and S.W. Meyer. 2003. Historical distribution and abundance of Phragmites australis at Long Point, Lake Erie, Ontario. Journal of Great Lakes Research 29(4):664-680.
Woodliffe, P.A. 2013. Consultant, former District Biologist, Chatham, OMNR. Personal communication, 8 Jan. 2013.
Wormworth, J. and K. Mallon. 2006. Bird species and Climate Change: The Global Status Report. Version 1.1. Report to the World Wildlife Fund. Climate Risk Pty Limited. Fairlight, Australia.
Wormington, A. in prep. Black Tern in The Birds of Point Pelee.
Zimmerman A.L., J.A. Dechant, D.H. Johnson, C.M. Goldade, B.E. Jamison, and B.R. Euliss. 2002. Effects of Management Practices on Wetland Birds: Black Tern. Northern Prairie Wildlife Research Center, Jamestown, North Dakota. 37 pp.