Eastern Small-footed Myotis Recovery Strategy
This document is the recovery strategy for Eastern Small-footed Myotis, a species of bat at risk in Ontario.

Cover photo by Brock Fenton
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. Recovery strategies are required to be prepared for extirpated species only if reintroduction is conasidered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of Natural Resources and Forestry Species at Risk webpage.
Recommended citation
Humphrey, C. 2017. Recovery Strategy for the Eastern Small-footed Myotis (Myotis leibii) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources and Forestry, Peterborough, Ontario. vii + 76 pp.
© Queen’s Printer for Ontario, 2017
ISBN 978-1-4606-9787-0 (HTML)
ISBN 978-1-4606-9785-6 (PDF)
Content (excluding the cover illustration) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n'est disponible qu'en anglais en vertu du Règlement 411/97 qui en exempte l'application de la Loi sur les services en français. Pour obtenir de l'aide en français, veuillez communiquer avec recovery.planning@ontario.ca.
Author
Christy Humphrey – Natural Resource Solutions Inc., Waterloo, Ontario.
Acknowledgments
The author would like to thank Sue Russell and Leanne Jennings, Ministry of Natural Resources and Forestry (MNRF), for their assistance with the preparation of this document, as well as Andrew Ryckman, David Stephenson and Charlotte Teat of Natural Resource Solutions Inc. (NRSI) for their suggestions, input and review on various drafts. Thanks also to Heather Fotherby, Lillian Knopf and Mark Docken (NRSI) for their invaluable assistance with the Recovery Strategy Workshop; the GIS department at NRSI for contributing the maps; and Brock Fenton (University of Western Ontario) and Alan Hicks (Vesper Environmental) for contributing the photographs.
In addition, I would like to thank all of those who provided technical assistance. This includes the following individuals who provided information on the distribution of the species in Ontario: Amanda Adams (Texas A&M University), Burton Lim (Royal Ontario Museum), Matt MacPherson (University of Guelph), Derek Morningstar (Golder Associates Ltd.), Kristin Jonasson (University of Western Ontario), Lesley Hale and Mark Browning (MNRF), Liam McGuire (Texas Tech University); Sandy Dobbyn (MNRF), and Toby Thorne. Thank you also to Simon Dodsworth and Don Sutherland (MNRF) for their insights on hibernacula in the province and assistance with historical records, which greatly helped inform the distribution map. I would also like to thank those who provided information relating to the species' ecology, including: Carl Herzog (NYS Department of Environmental Conservation), John Chenger and Bryan Butler (Bat Conservation and Management Inc.), Joy O'Keefe (Indiana State University), Greg Turner (Pennsylvania Game Commission), Dave Yates and Tim Divoll (Biodiversity Research Institute) and Paul Moosman, Jr. (Virginia Military Institute) for his contributions, experiences and photographs for the workshop.
I would like to thank all of those who provided very helpful comments on the draft Recovery Strategy during the jurisdictional review: Sue Russell, Vivian Brownell, Jay Fitzsimmons, Amanda Fracz, Glenn Desy, Adam Gryck, Sandy Dobbyn, Chris Risley, Lesley Hale, Laura Bruce, Don Sutherland (MNRF); Judith Girard, Angela Darwin (Canadian Wildlife Service); Kaleigh Norquay (University of Winnipeg), Jordi Segers (Canadian Wildlife Health Cooperative) and Kirk MacGregor (Toronto Caving Group).
Acknowledgement and thanks are extended to all of those who participated in the Recovery Strategy Workshop held in Cambridge, Ontario on October 21, 2015. Their names and affiliations are as follows:
- Alan Hicks (Environmental Consultant)
- Carl Herzog (New York State Department of Environmental Conservation)
- Cynthia Robinson (Ontario Stone, Sand, and Gravel Association)
- Derek Morningstar (Environmental Consultant)
- Heather Fotherby (Natural Resource Solutions Inc.)
- Jordi Segers (Canadian Wildlife Health Cooperative)
- Kaleigh Norquay (University of Winnipeg )
- Kirk MacGregor (Toronto Caving Group)
- Kristin Jonasson (University of Western Ontario)
- Vivian Brownell (Ministry of Natural Resources and Forestry)
- Chris Hamblin (Ministry of Northern Development and Mines)
- Chris Risley (Ministry of Natural Resources and Forestry)
- Laura Bruce (Ministry of Natural Resources and Forestry)
- Lesley Hale (Ministry of Natural Resources and Forestry)
- Mark Browning (Ministry of Natural Resources and Forestry)
- Morgan Roblin (Escarpment Biosphere Conservancy)
- Sandy Dobbyn (Ministry of Natural Resources and Forestry)
- Sue Russell (Ministry of Natural Resources and Forestry)
- Tom Levy (Canadian Wind Energy Association)
Declaration
The recovery strategy for the Eastern Small-footed Myotis was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all of the individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdiction
Ontario Ministry of Natural Resources and Forestry
Executive summary
The Eastern Small-footed Myotis (Myotis leibii) is Ontario’s smallest bat, with yellow-brown or brown fur and a prominent black face mask, and is the rarest and least known bat species in the province. It overwinters in caves and abandoned mines. There are only 12 known overwintering sites (one of which is no longer suitable) in the province; all are located south of a line extending from the northeast shore of Lake Superior to the Ontario-Québec border. Summer habitats used by the species in Ontario are poorly understood, but elsewhere in its range it primarily roosts in open, sunny rocky habitats and, occasionally, in buildings.
Eastern Small-footed Myotis is primarily threatened by white-nose syndrome (WNS), a disease caused by the fungus Pseudogymnoascus destructans, which has killed well over 5 million cave-hibernating bats in eastern North America since the winter of 2006-2007. The fungus was first detected in Ontario hibernacula in 2010. In 2014, Eastern Small-footed Myotis was listed on the Species at Risk in Ontario (SARO) List, as endangered, affording it species and habitat protection under the Endangered Species Act, 2007 (ESA). The fungus was first detected on Eastern Small-footed Myotis in Ontario in the late winter/spring of 2016.
The recovery goal for Eastern Small-footed Myotis is to maintain a self-sustaining provincial population and to maintain sites currently and historically used for swarming, hibernation or maternity roosting in the province, unless habitat is no longer suitable. The protection and recovery objectives to meet the goal are to:
- inventory and monitor abundance of known populations in the province;
- conduct surveys in suitable habitat for the species to identify its distribution, range and abundance in Ontario;
- increase understanding of the species' ecology through inventory, monitoring, and research;
- monitor the impacts of threats on the populations and increase understanding of the effects of WNS on the species;
- protect and maintain, and enhance or restore, suitability of summer and winter habitats; and
- promote stewardship, education, and increased awareness of Eastern Small-footed Myotis, other rare and at-risk bat species, and their habitats and threats.
The short-term recovery approaches should focus on accurately identifying baseline information for Ontario on distribution, population and habitats used by the species, and, at the same time, mitigate potential threats, actively conduct research and develop long-term management activities to ensure the goal will be achieved.
It is recommended that foraging areas, hibernacula and swarming sites and maternity sites be prescribed as regulated habitat for the species.
Foraging Habitat: Based on the lack of data available for Ontario and the broad nature of foraging habitat identified elsewhere in the range of Eastern Small-footed Myotis, it is not currently possible to identify specific areas of foraging habitat used by this species. As a result, suitable area to allow appropriate foraging resources during critical life cycle periods, such as hibernation and the maternity period, has been included within the recommended area for the habitat regulation for hibernacula/swarming sites and maternity sites.
Hibernacula and swarming sites: All sites known to have been used as hibernacula and swarming sites for Eastern Small-footed Myotis should be prescribed as habitat in a habitat regulation. This is recommended because of: (1) the importance of hibernacula and swarming sites and activities associated with them; (2) the limited availability of suitable caves and abandoned mines in the province; (3) the longevity of use (decades or longer); and (4) the difficulty in identifying potential crevice-based hibernacula. It is further recommended that foraging and roosting resources within 610 m of a hibernaculum and/or swarming site be identified as habitat in the habitat regulation. This distance is based on the average core use area of home ranges identified between July and October in a study in Maine that may represent the area most relied upon by individuals during swarming and prior to hibernation. The area within 610 m of a hibernaculum or swarming site is equivalent to 117 ha, based on a point centroid with a circular buffer. The area should extend 610 m from all known or suspected entrances of a hibernaculum, or total underground extent of a hibernaculum, if known, and/or the concentrated area of swarming activity.
Maternity Sites: Maternity habitat should be identified based on the contiguous ecosite or contiguous anthropogenic site up to 100 ha in size, containing roosting adult female and juvenile Eastern Small-footed Myotis between May 15 and July 31. Areas surrounding maternity sites should also provide adequate foraging resources to support the associated population. As a result, it is recommended that the area within 565 m of the boundary of a maternity site be identified as habitat in the habitat regulation. The total area size should be no greater than 100 ha, including the maternity roosting ecosite and the supporting area of foraging resources. The distance of 565 m is based on two studies: one describes the maximum home range size of adult females emerging from hibernation in Maryland; the other describes minimum home range sizes for juveniles in the summer and fall in Maine.
Future research on roosting and foraging habitats used by the species, particularly during the maternity period, dispersal distances, and home range sizes, as recommended in this recovery strategy, may inform a larger or smaller extent of area that should be included in the habitat regulation.
1.0 Background information
1.1 Species assessment and classification
Table 1. Species assessment and classification of the Eastern Small-footed Myotis (Myotis leibii). The glossary provides definitions for the abbreviations above and for other technical terms in this document
|
Assessment |
Status |
|---|---|
|
SARO list classification |
Endangered |
|
SARO list history |
Endangered (2014) |
|
COSEWIC assessment history |
Not Assessed |
|
SARA schedule 1 |
Not applicable |
|
Conservation status rankings |
GRANK: G4 NRANK: N2N3 SRANK: S2S3 |
1.2 Species description and biology
Species description
Eastern Small-footed Myotis (Myotis leibii) is a small brown bat, with smooth, glossy brown fur and black ears. Males and females of the species are similar in appearance (Banfield 1974). The dorsal fur is black at the base and light yellow-brown or brown at the tips, often appearing yellowish-brown overall. Ventral fur is lighter, often whitish, light gray or pale tan in colour. It has black ears and wings, a black interfemoral membrane between the legs and tail and a prominent black face mask. Additional distinguishing features are its small feet (8mm) and keeled calcar (the cartilage that supports the interfemoral membrane). It is the smallest bat species in Ontario, with a forearm length ranging from 29 to 36 mm and a weight of 3 to 6 g (Barbour and Davis 1969, Banfield 1974, Hall 1981, van Zyll de Jong 1985, Dobbyn 1994, Best and Jennings 1997, NatureServe 2015). The species generally flies slowly and close to the ground (Barbour and Davis 1969, Best and Jennings 1997).
Little Brown Myotis (Myotis lucifugus) is sometimes mistaken for Eastern Small-footed Myotis (Figure 1) but can be distinguished by:
- its larger size, with a forearm length of 33 to 41mm and a foot length of 8 to 12 mm (Banfield 1971, Fenton and Barclay 1980, Hall 1981, van Zyll de Jong 1985);
- its face mask, if present, is less prominent than in Eastern Small-footed Myotis; and
- it generally lacks a keeled calcar (Fenton and Barclay 1980, van Zyll de Jong 1985).
Figure 1. Eastern Small-footed Myotis and Little Brown Myotis

Note the small size, distinct black face mask, and yellow-brown appearance of Eastern Small-footed Myotis (left), compared to Little Brown Myotis (right). (Photo: Alan Hicks)
Species biology
Eastern Small-footed Myotis is the least studied and most poorly understood bat species in Ontario (Fraser et al. 2007, COSSARO 2013). It is a cave-hibernating bat that over-winters in caves and abandoned mines (Hitchcock 1941, 1945, 1949, Davis 1955, Fenton 1972). Seasonal behavioural patterns and timing of reproductively active males coincide with the patterns of other Myotis species found in Ontario, suggesting that the reproductive cycle is similar (Banfield 1974). In the fall, mating takes place during an activity known as "swarming". Swarming sites are locations where males and females congregate to mate and prepare for hibernation, and are usually located at the entrance to hibernacula (Fenton 1972). Swarming likely begins in August and lasts until the onset of hibernation, based on the observation of this species outside of hibernacula at the same time as other swarming bat species (Fenton 1969, S. Dobbyn unpub. data, L. Hale unpub. data, L. McGuire unpub. data). Sperm is stored over-winter by females, with ovulation and fertilization occurring in the spring (Gannon et al. 2012, USFWS 2013). A specific maternity period for this species has not been determined in Ontario. Although species-specific data is limited, it is generally understood that females give birth to only one young per year (Best and Jennings 1997) between mid-May and the end of July. In Ontario, two maternity colonies have been identified, one in early July and one in mid-July. In both of these, at least some juveniles were already volant at the time of discovery (Hitchcock 1955, M. Browning unpub. data).
Bats entering hibernation develop a large fat reserve in order to fuel metabolism during hibernation (Hallam and Federico 2009). In general, juveniles tend to weigh less than adults going into hibernation and can therefore be at greater risk of overwinter mortality (Fenton 1970, Hallam and Federico 2009). Eastern Small-footed Myotis has been observed to hibernate in association with the remainder of Ontario’s cave-hibernating bat species, including Big Brown Bat (Eptesicus fuscus), Little Brown Myotis, Northern Myotis (Myotis septentrionalis) and Tri-colored Bat (Perimyotis subflavus) (Hitchcock 1949, M. Browning unpub. data). Eastern Small-footed Myotis is considered the hardiest cave-dwelling bat in eastern North America (Barbour and Davis 1969). It is known to begin hibernation later in the fall and end hibernation earlier in the spring than other species, generally from late November to early April in eastern Ontario (Fenton 1972). Eastern Small-footed Myotis also tends to hibernate alone or in small groups, often in cracks or crevices, particularly near the entrance to caves or abandoned mines, and will hibernate in a vertical or horizontal position (Hitchcock 1949, Davis 1955, Best and Jennings 1997, Veilleux 2007).
During the winter, bats enter into torpor by lowering their body temperature to that of their surroundings (Barbour and Davis 1969) and reducing their heart rate (Gannon et al. 2012). They will arouse from hibernation at regular intervals to drink water, groom, defecate or urinate, make short flights, crawl around for exercise or even mate with other torpid bats, and also rarely to eat (Gannon et al. 2012). The metabolic requirements to arouse from torpor and undertake activities during these periods of arousal are the greatest source of energy depletion during hibernation (Thomas et al. 1990, USFWS 2013).
Male and female Eastern Small-footed Myotis have been found in almost equal numbers in Ontario hibernacula (Hitchcock 1949). They weigh four to six grams at their heaviest (during early hibernation) with body size and weight generally consistent between males and females. Both sexes also lose the same amount of body weight, an average of 16 percent, over the hibernation period (Fenton 1972). However, the survival rate over seven winters for banded individuals in an Ontario hibernaculum was estimated to be significantly lower in females than in males. The estimated mean annual survival for females was 42.1 ± 7.1 percent and for males, 75.7 ± 11.1 percent (Hitchcock et al. 1984).
Upon emergence from hibernation in the spring, fat reserves are depleted and foraging must occur to improve body condition before the maternity season. Some adult males will continue to spend time during the spring and summer (May through July) at their hibernation sites in Ontario (L. McGuire, unpub. data). Generally however, the species is assumed to travel to summer habitats and return, along with juveniles, to swarming sites and hibernacula in the late summer and fall (August until the onset of hibernation).
Compared with the species' overwintering biology, the summer activities of Eastern Small-footed Myotis are relatively unknown. In the United States, they have been observed roosting alone or in small groups in their summer habitats (Johnson et al. 2011, Thompson 2013, Moosman et al. 2015, D. Yates and T. Divoll, in prep.), typically crevices and cracks associated with rocky sites, similar to their preferences for roosting in crevices and cracks during hibernation. After females give birth to young, they may roost alone with their young, or cluster together in maternity colonies. The exact reasons for clustering behaviour in bats are not well-understood; however, thermoregulation (the regulation of body temperature) is thought to play a large role (Kunz et al. 2009a). Availability of water is likely extremely important during this time, as lactating females of other Myotis species in North America have been found to drink 13 times more frequently than non-reproductive adult females (Adams and Hayes 2008). In natural or artificial (e.g. rip rap, waste rock piles) rocky habitats throughout the species' range, maternity colony size has been found to range from a single adult female with one juvenile to approximately 20 individuals of various ages (Moosman et al. 2015, G. Turner, pers. comm. 2015, M. Browning unpub. data). A colony of "about a dozen" was observed behind a sliding barn door in eastern Ontario (Hitchcock 1955), and a historic building in North Carolina is known to contain a maternity colony of a minimum of 33 individuals, and possibly up to 92 (O'Keefe and Lavoie 2010). A maternity colony of at least 17 individuals was identified in the porch of an historic building in Tennessee (Fagan et al. 2016). A colony of at least 12 individuals (including 5 adult females and 7 volant juveniles) was identified in an old barn in the Golden Horseshoe Area in 2016 (M. Browning unpub. data).
Eastern Small-footed Myotis has been found to change specific roost locations very frequently, even daily, unless foraging on a given night was not possible because of poor weather (Bat Conservation and Management Inc. 2003, Johnson and Gates 2008, Johnson et al. 2011, Thompson 2013). Observations have also been made of Eastern Small-footed Myotis switching roosts within 24 hours by making short flights during the daytime (Moosman et al. 2015). Successive roost site locations have ranged from within 20 m to a distance of 8.5 km away from previous roost sites (Johnson and Gates 2008, Johnson et al. 2011, Thompson 2013). Distance between successive roost sites and frequency of roost-switching may be related to the relative number of suitable roost sites in the area (Lewis 1995, Thompson 2013). Frequent roost-switching is hypothesized to be beneficial in order to reduce the risk of predation and parasite load (Lausen and Barclay 2002, Johnson et al. 2011).
In the periods shortly before hibernation (October in Maine) and shortly after hibernation (March in Virginia), frequency of roost switching may decrease as Eastern Small-footed Myotis have been found to remain within rock roosts for two to three days or more before emerging for short bouts of foraging activity (Moosman et al. 2015, D. Yates and T. Divoll, in prep.).
Eastern Small-footed Myotis is considered an aerial insectivore, pursuing and capturing insects primarily during flight. It has been known to feed on a variety of primarily small, soft-bodied flying insects including moths, flies and beetles, as well as net-winged insects, true bugs, caddisflies and mayflies. The dominance of flying insects in the diet indicates this species feeds primarily by aerial hawking, hunting and capturing insects on the wing. However, the observation of some spiders and crickets in the diet, as well as soil in fecal samples, suggests the species may also glean insects from surfaces including the ground or opportunistically feed on these species while roosting (Johnson and Gates 2007, Moosman et al. 2007, Johnson et al. 2012, Thomas et al. 2012, Dodd et al. 2014). There may be competition for prey with Little Brown Myotis and Northern Myotis, based on similarity of diet determined through captures of all three species at the same sites (Moosman et al. 2007).
Eastern Small-footed Myotis has been known to survive at least 12 years in Ontario (Hitchcock 1965, Paradiso and Greenhall 1967), which is also the current age record for the species within its entire range (USFWS 2013).
Eastern Small-footed Myotis, like other bat species in Ontario, produces high-frequency vocalizations in the form of ultrasound echolocation calls, primarily to gather information about its environment for orientation and to search for prey (Simmons and Stein 1980). This is generally different from songs produced by birds, which are more often used to identify individuals of a species rather than to gain information about surroundings (Barclay 1999). Echolocation call sequences can be recorded through acoustic monitoring, which enables identification of call sequences to species level through analysis of temporal and spectral information. However, a sufficient level of acoustic detail is required to do this (Parsons and Szewczak 2009).
Characteristics of echolocation calls tend to be similar among the Myotis species compared to other genera, for example, the Hoary Bat (Lasiurus cinereus). Hoary Bat is more easily differentiated from other species by its more distinctive call characteristics (Simmons and Stein 1980, Fenton and Bell 1981, Dewar et al. 1985, Barclay 1999). The calls of Myotis species bats are characterized by: (1) high frequency modulation, (i.e., large bandwidth); (2) short duration; and (3) higher "low frequencies", typically around 40 kHz, compared to most other Ontario bat species (Fenton and Bell 1981, Dewar et al. 1985, Murray et al. 2001, Mukhida et al. 2004). Despite these distinguishing call characteristics for Myotis species in general, differentiating the calls of individual species within this genus can be difficult. In a study examining differences in echolocation call characteristics of Little Brown Myotis and Eastern Small-footed Myotis in Ontario, lowest frequency and frequency of maximum energy were determined to be the most important species-discriminating parameters. For Eastern Small-footed Myotis, mean values were determined to be 46.6 ±3.2 kHz and 48.5 ±4.5 kHz, respectively, based on calls from four individuals (Mukhida et al. 2004).
It is important to collect sound from high frequencies as it is useful to distinguish whether a recording was made of an entire bat call or if the quieter, higher frequency portion at the beginning of the call attenuated before it could reach the microphone. High frequency sound tends to diminish in the atmosphere more rapidly than low frequency sound (Griffin 1971), so not only are Myotis species' calls more difficult to record in general, but information at the higher frequency ranges of Myotis calls is often lost unless the bat is close to the microphone.
Another aspect of gathering information from echolocation data is that there can be significant variation in the calls, depending on:
- the individual bat;
- the characteristics of the habitat in which the bat is located (e.g., cluttered versus open);
- the purpose of the call (e.g., searching for prey, approaching prey and immediately prior to capturing prey, or a social call); and
- the presence of other individuals of the same species in the area (Obrist 1995, Barclay 1999).
The physics of sound travel through the environment can also interfere with the ability to collect all of the desired information from a bat echolocation call. Factors such as distance of the bat from the microphone, echoes from other surfaces, refraction of sound through air currents and travel of a bat towards and away from a microphone (Doppler effect) can distort bat calls before they reach the microphone of an ultrasound detector (Parsons and Szewczak 2009).
1.3 Distribution, abundance and population trends
The global range of Eastern Small-footed Myotis is found in eastern North America, including southern Ontario and Quebec, and extending southward into the eastern and central United States (largely concentrated in the Appalachian Mountains). In Ontario, the species has been observed in the general area south of a line extending between the northeast shore of Lake Superior and the Ontario-Quebec border, as indicated in Figure 2.
The records mapped in Figure 3 are based on visual confirmation of species identification, i.e., specimens collected, captured, or visually examined (NHIC 2016, S. Dobbyn, unpub. data 1993 – 1994, 2010, L. Hale, unpub. data 2009 – 2012, C. Humphrey and H. Fotherby, unpub. data 2016, K. Jonasson, unpub. data 2014, M. MacPherson, unpub. data 2015, L. McGuire, unpub. data 2007 – 2009, M. Browning unpub. data 2016, B. Lim, D. Riskin and T. Thorne, unpub. data 2016). This is because it is difficult to differentiate Myotis species acoustically (discussed in Section 1.2), and also is because of the general lack of acoustic observations submitted to the Natural Heritage Information Centre (NHIC). Note that not all observations represent element occurrences such as hibernacula, swarming sites, maternity sites or other specific features used by the species.
Figure 2. Distribution of Eastern Small-footed Myotis in North America
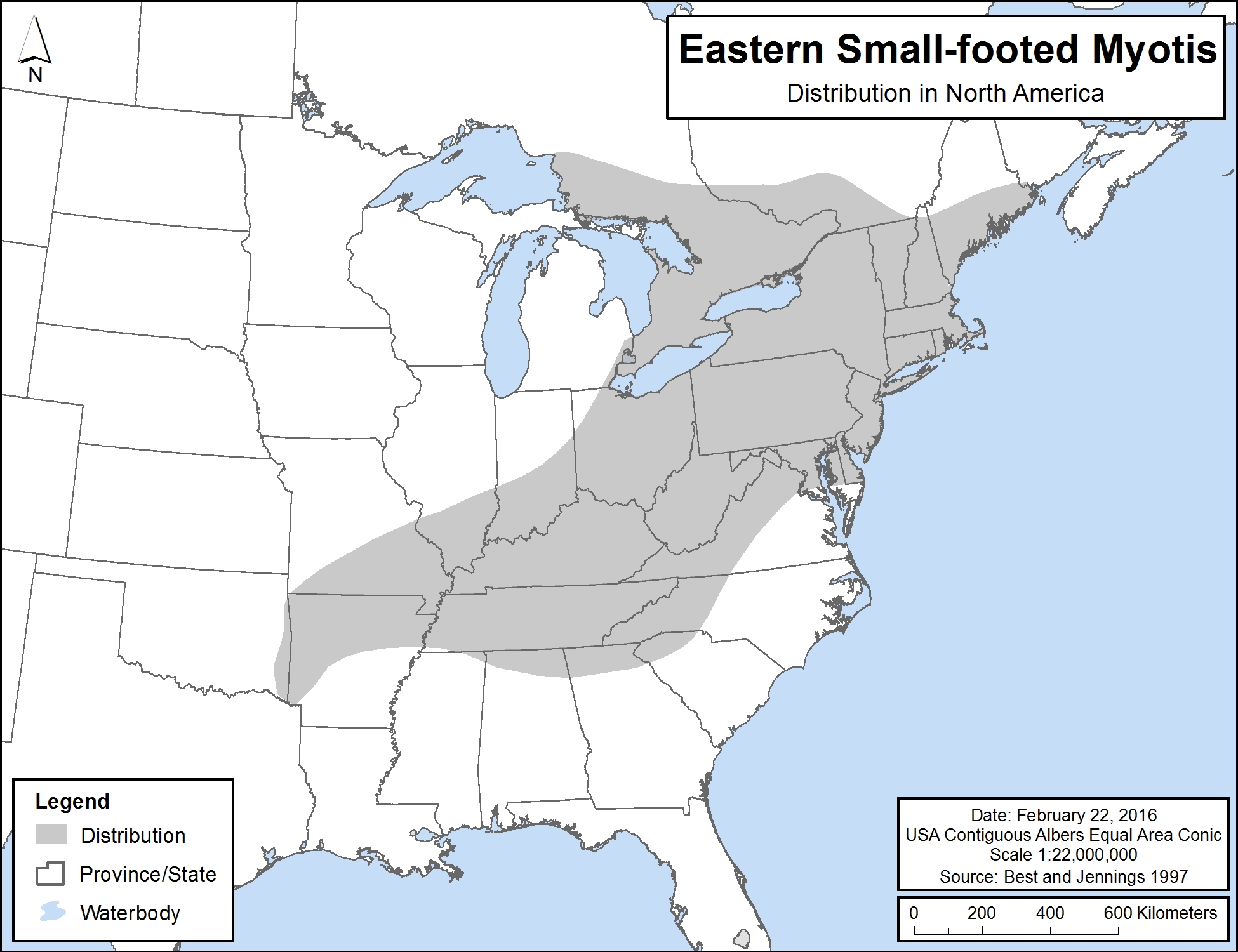
Source: Best and Jennings 1997.
Locations of known hibernacula in Ontario generally coincide with: (1) the Niagara Escarpment; (2) karst areas of eastern Ontario; and (3) abandoned mines in the Canadian Shield of eastern and central Ontario. Observations of the species have generally been concentrated near known hibernacula, although the species has also been observed at additional locations.
Approximately 290 hibernacula have been identified in the species' range in the United States (USFWS 2013), and up to 13 more identified in Ontario. As noted earlier, while Eastern Small-footed Myotis seems to have a greater tolerance for cold conditions during hibernation than other bat species in the province, it has not been recorded from known bat hibernacula located in northern regions of Ontario (Fenton 1972).
Figure 3. Current and historic distribution of Eastern Small-footed Myotis in Ontario
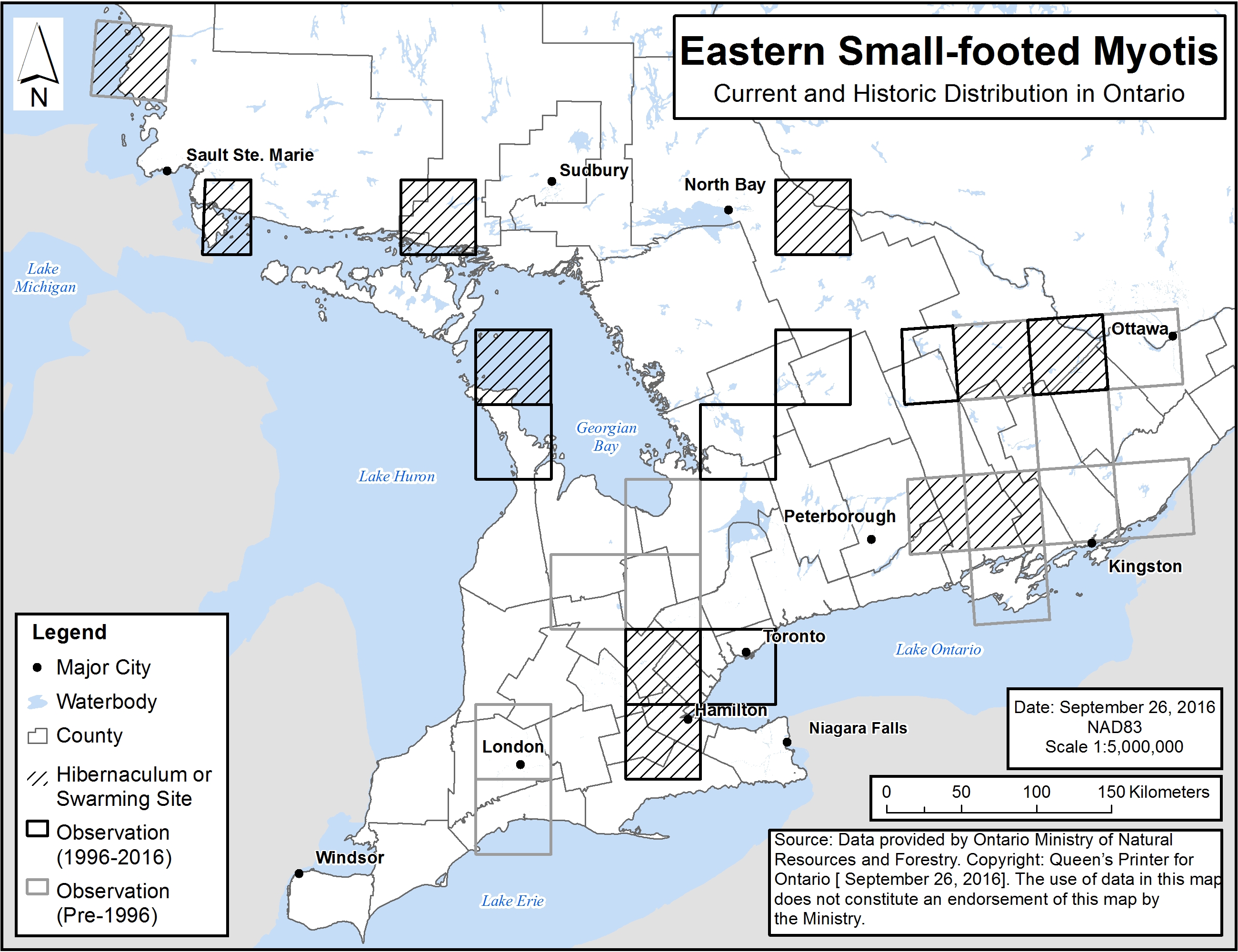
Each 50 km by 50 km standardized UTM grid square contains at least one observation record or element occurrence.
Because Eastern Small-footed Myotis tends to hibernate in crevices, it is possible that bats were overlooked or not visible during previous studies. Also, the species' microhabitat preference for roosting in crevices, often closer to the entrances of caves or abandoned mines, may prevent it from hibernating in northern hibernacula if these microhabitats are too cold to allow torpor. While the potential presence of Eastern Small-footed Myotis at more northern hibernacula should not be ruled out, further research is needed to determine whether the climatic conditions in northern Ontario are a limiting factor in its distribution.
Summer roosts for Eastern Small-footed Myotis are believed to be located in close proximity to their hibernacula (USFWS 2013) for the following reasons:
- Individuals banded at hibernacula and identified in specific summer habitats have been found roosting at distances less than 100 m (Johnson and Gates 2008) and 16 and 19 km away (Hitchcock 1955). Long-distance migrations have not been observed (NatureServe 2015), unlike other similar species such as Little Brown Myotis. Migration distances of more than 800 km between winter hibernacula were recorded for an individual Little Brown Myotis (Fenton 1969).
- Eastern Small-footed Myotis is the only one of the eight Ontario bat species that has not been documented during months of intensive mist-netting efforts conducted during migration at Long Point on Lake Erie. Long Point is a known migratory stopover point for Silver-haired Bat and is likely a migratory corridor for other species including Hoary Bat (Lasiurus cinereus) and possibly Little Brown Myotis (Dzal et al. 2009, McGuire et al. 2012). Mist-netting was conducted at this location over a combined total of two months of fall migration (Dzal et al. 2009, McGuire et al. 2012) and five and a half months of spring migration (early April through early June) over a period of three years (K. Jonasson, unpub. data). While the general rarity of Eastern Small-footed Myotis could explain the absence of captures from the site, low numbers have been captured at other locations near the north shore of Lake Erie with efforts of no more than a few days (Dewar et al. 1985). Its absence from Long Point is therefore notable, and could indicate the species cannot travel across the roughly 38 km of open water across Lake Erie into New York or Pennsylvania without food or rest. If this is the case, it may restrict the species' ability to disperse widely before needing to give birth and raise young.
- The species' preference for rocky habitats in summer may limit an individual’s home range to those rocky areas which also contain hibernacula (i.e., karst areas and Canadian Shield areas containing abandoned mines with adits). The species' known range overall, based on captures and visual observations, is closely tied to the "cave belt" of eastern North America (J. Chenger, pers. comm. 2015), i.e., concentrated in the Appalachian Mountains. There are additional caves in the karst areas of Ontario and adjacent southwestern Quebec (see Figure 4). This range restriction suggests that the species is not likely to travel far from habitat types containing both hibernacula and suitable summer habitat. Eastern Small-footed Myotis individuals are typically captured within 35 km from locations where the species is known to hibernate.
Figure 4. Distribution of caves and Eastern Small-footed Myotis in North America
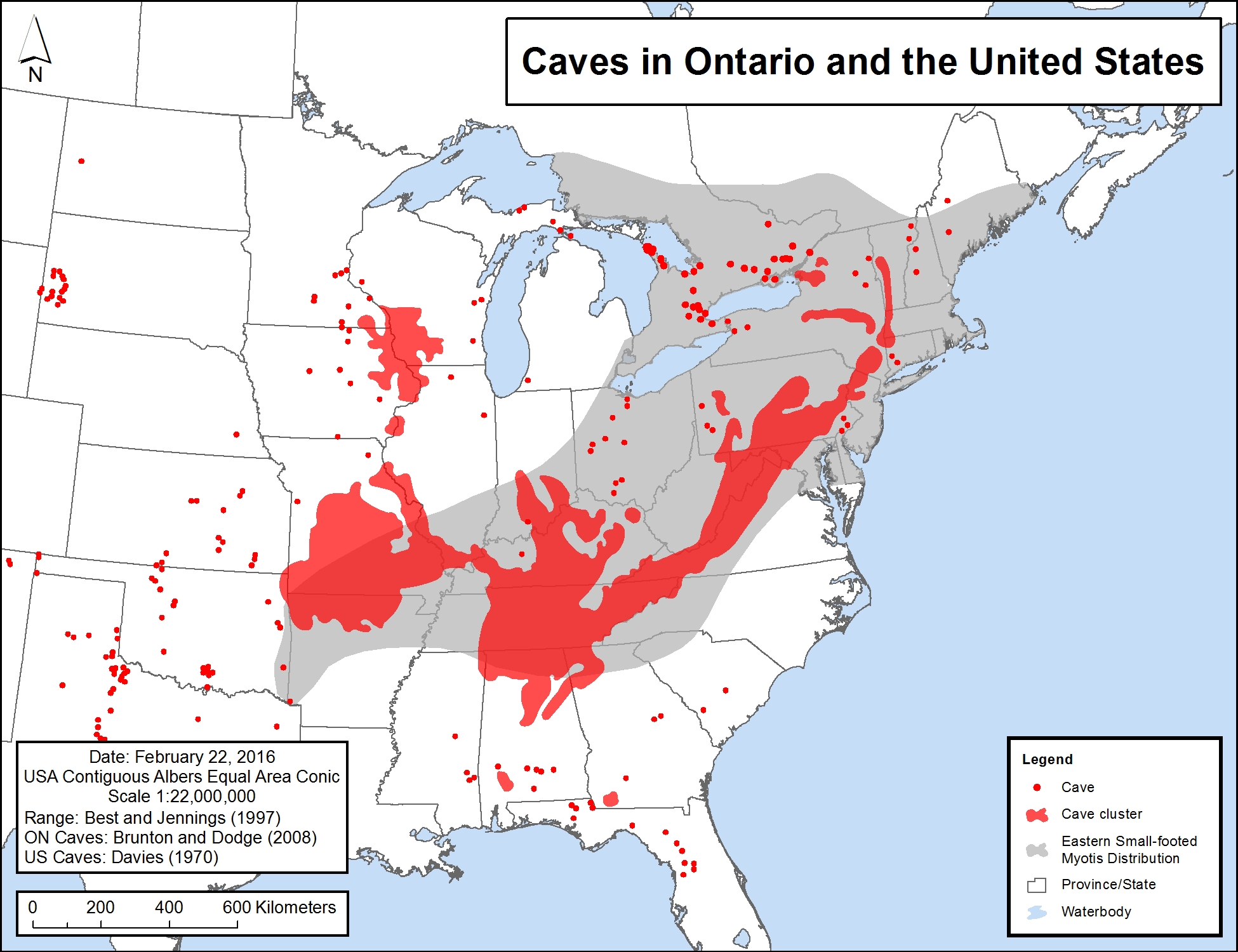
The range is based on Best and Jennings (1997), and caves/cave clusters within the United States are interpreted from Davies (1970). Ontario caves shown are limestone caves, from Brunton and Dodge (2008). A small number of additional (non-limestone) caves are also found in Ontario.
Recent research on summer roosting has resulted in the hypothesis that the species may be able to overwinter outside of traditional cave or abandoned mine hibernacula (e.g., in rock crevices or talus slopes), and perhaps even within the same habitats as summer roosts (Griffin 1945, USFWS 2013, G. Turner, pers. comm. 2015, J. Chenger, pers. comm. 2015, Moosman et al. 2015, D. Yates and T. Divoll, in prep.). It should be noted, however, that this research pertains to locations in the United States that have warmer winter climates than Ontario. It is unknown whether this could also be the case in Ontario.
Despite the above, Eastern Small-footed Myotis individuals have been captured at some locations more than 100 km from the nearest known hibernacula (Dewar et al. 1985, S. Dobbyn, unpub. data, C. Herzog, pers. comm. 2015, M. McPherson, unpub. data, NHIC 2016). In Ontario, these capture sites are primarily located in regions that could contain as-yet unidentified hibernacula (i.e., along the Niagara Escarpment or in areas containing karst or abandoned mines). However, some observations have been made at locations that are also more than 100 km from karst areas or abandoned mines (Davis 1931, Dewar et al. 1985, OMNDM 2016, NHIC 2016,), suggesting the possibility that the species may roost further from hibernacula or hibernate in other types of habitats. For these reasons, protection and recovery of Eastern Small-footed Myotis populations and habitats would benefit from additional research into dispersal ability, particularly in Ontario.
As a result of the difficulty in distinguishing Myotis species acoustically (discussed in Section 1.2), both manual species identification by experienced bat biologists and recent auto-classification software can experience challenges in identifying bat calls to species. Consequently, while data collected from acoustic recordings can be useful to identify likely bat community composition in an area or to identify target areas for further surveys of Eastern Small-footed Myotis, (e.g., crevice searches or mist-netting), it may be more difficult to identify new occurrences of the species from acoustic data, i.e., link the acoustic record to a new hibernaculum, swarming site, maternity site, or other specific feature.
Eastern Small-footed Myotis has always been considered rare throughout its range (Barbour and Davis 1969, Banfield 1974, van Zyll de Jong 1985, Best and Jennings 1997). In Ontario, NHIC has identified just 27 element occurrences in the province, with only 126 total observations tracked. All 27 element occurrences are considered to be Historical.
More recent data indicates that Eastern Small-footed Myotis has been observed or captured at 18 locations in the last 20 years (NHIC 2016, and M. Browning, S. Dobbyn, L. Hale, C. Humphrey and H. Fotherby, K. Jonasson, B. Lim, M. MacPherson, L. McGuire, unpub. data). As a result, four of the 27 tracked element occurrences have had observations of Eastern Small-footed Myotis within the last 20 years, three with data from the last six years, indicating they are extant. Five new occurrences, including two roosting sites, one hibernaculum, one swarming site, and one maternity colony, have also been identified. These data have not yet been incorporated into the NHIC database.
Four additional locations have been confirmed as hibernacula or swarming sites for Eastern Small-footed Myotis, based on new information and review of existing data, and seven new locations have had recent observations, although these may not be hibernacula, swarming sites or maternity colonies. Review of the data has resulted in one additional historical maternity colony, although the exact location is not known. A summary of the number of known hibernacula and/or swarming sites, maternity sites and additional, undetermined locations with records is presented in Table 1.
Table 1. Number of sites with observations of Eastern Small-footed Myotis
|
County or District |
Sites with Observations Pre-1996 |
Sites with Observations 1996-2016 |
Total Sites |
|---|---|---|---|
|
Algoma |
Swarming/Hibernaculum – 1 Undetermined Occurrence – 1 |
Swarming/Hibernaculum – 1 |
3 |
|
Bruce |
Swarming/Hibernaculum – 2 |
Undetermined Observation – 3 |
5 |
|
Elgin |
Undetermined Occurrence – 1 |
1 |
|
|
Frontenac |
Undetermined Occurrence – 3 |
3 |
|
|
Grey |
Undetermined Occurrence – 1 |
1 |
|
|
Haldimand-Norfolk |
Swarming/Hibernaculum – 1 |
1 |
|
|
Haliburton |
Undetermined Occurrence – 1 |
1 |
|
|
Halton |
Undetermined Occurrence – 2 |
Swarming/Hibernaculum – 2 Undetermined Occurrence – 1 |
5 |
|
Hamilton |
Maternity – 1 |
1 |
|
|
Hastings |
Swarming/Hibernaculum – 1 Undetermined Occurrence – 1 |
Swarming/Hibernaculum – 1 |
3 |
|
Leeds and Grenville |
Undetermined Occurrence – 1 |
1 |
|
|
Middlesex |
Undetermined Occurrence – 1 |
1 |
|
|
Muskoka |
Undetermined Occurrence – 1 Undetermined Observation – 1 |
2 |
|
|
Nipissing |
Swarming/Hibernaculum – 1 Undetermined Observation – 1 |
2 |
|
|
Ottawa-Carleton |
Undetermined Occurrence – 1 |
1 |
|
|
Peel |
Undetermined Occurrence – 1 |
Undetermined Observation – 1 |
2 |
|
Renfrew |
Swarming/Hibernaculum – 1 Maternity – 1 Undetermined Occurrence – 3 |
Swarming/Hibernaculum – 1 Undetermined Occurrence – 1 |
7 |
|
Simcoe |
Undetermined Occurrence – 1 |
1 |
|
|
Sudbury |
Swarming/Hibernaculum – 1 |
1 |
|
|
Waterloo |
Undetermined Observation – 1 |
1 |
|
|
Swarming/ Hibernaculum |
6 |
7 |
13 |
|
Maternity |
1 |
1 |
2 |
|
Undetermined Occurrence |
18 |
3 |
21 |
|
Undetermined Observation |
0 |
7 |
7 |
|
Total |
25 |
18 |
43 |
The greatest number of Eastern Small-footed Myotis recorded to be overwintering in an Ontario hibernaculum in one winter was 142, with 198 individuals banded at the site over four winters. All individuals observed were banded between late winter of 1942 and late winter of 1945 (Hitchcock 1945). For decades this was the largest known concentration of the species. However, recent overwintering population counts in New York State have identified a small number of hibernacula for the species that contain 2,000 to over 3,000 individuals (USFWS 2013, C. Herzog, pers. comm. 2015).
Most hibernacula counts across the species' range (including in Ontario) have only identified the presence of a few to tens of overwintering individuals, and numbers tend to fluctuate from year to year (Hitchcock 1941, Hitchcock 1945, Hitchcock 1949, Hitchcock 1965, Fenton 1972, Best and Jennings 1997, Turner et al. 2011). Between 1942 and 1947, recapture of Eastern Small-footed Myotis that were banded by hand at hibernacula in eastern Ontario from one year to the next (on single survey dates) was approximately 25 percent (Hitchcock 1949). It is unknown in this study to what extent individuals not recaptured represent those which: (1) died during the summer; (2) emigrated to other hibernacula permanently or temporarily; or (3) were not visible on the survey dates (e.g., hidden deep within cracks).
Most recently, overwintering data was collected for Myotis species in Ontario between the winters of 2009/2010 and 2012/2013 at two element occurrences (COSEWIC 2013); only one of these sites is a confirmed hibernaculum for Eastern Small-footed Myotis. The surveys identified Myotis species bats only to genus to minimize the time spent in hibernacula and limit disturbance to hibernating individuals (discussed in further detail in Section 1.6). As a result, data for all Myotis species are combined, and include the more common Little Brown Myotis and Northern Myotis. The total estimated Myotis spp. overwintering population sizes for these winters are as follows (COSEWIC 2013):
- 2009/2010 – 14,378
- 2010/2011 – 7,005
- 2011/2012 – 2,638
- 2012/2013 – 2,097
Unfortunately, the largest known overwintering site for Eastern Small-footed Myotis in Ontario is no longer occupied by the species as a result of changes made to the cave in the 1950's (Hitchcock 1965). Currently the site with the largest population is likely an abandoned mine situated approximately 23 km away and within the same county. Thirty-seven individuals were captured and banded here over 15 nights during the swarming season in 2007 (prior to hibernation), with only one recapture. A total of 23 individuals were also captured and banded over 13 nights in 2008, including 11 in May and June (adult males likely remaining at the hibernaculum through the summer) and 12 more of mixed sexes and ages during August swarming (L. McGuire, unpub. data).
1.4 Habitat needs
Eastern Small-footed Myotis utilize different habitats during different periods of their lifecycle: during hibernation, in the summer (especially the maternity period) and when foraging. The species has most frequently been encountered in hibernacula and, as a result, habitat needs in hibernacula are much better understood than for other habitats. Much of the available data on habitat needs of Eastern Small-footed Myotis during hibernation is based on research conducted in Ontario; very little is known about summer habitat use in the province. Most of the available data on summer habitat and maternity colony habitat used by the species are from studies in the United States.
Hibernacula
Eastern Small-footed Myotis in Ontario hibernate in cool caves and abandoned mines with low humidity and temperatures and relatively stable microclimates (Hitchcock 1941, 1945, 1949, 1965, Fenton 1972). They have also occasionally used abandoned railway tunnels in other parts of their range (Johnson and Gates 2007). In Ontario, caves are formed in karst areas where water dissolves soluble rock, creating chambers. Cave hibernacula known to be used by Eastern Small-footed Myotis in Ontario are found primarily in limestone, but also in sandstone. Both caves and abandoned mine shafts may be used by Eastern Small-footed Myotis for hibernation where the entrance remains accessible to flying bats, but remains restricted enough to prevent significant fluctuations in temperature and humidity (OMNR 2000).
Eastern Small-footed Myotis are able to tolerate much colder temperatures, drier conditions and draftier locations than other species (Hitchcock 1949, Fenton 1972). They can tolerate temperatures as low as -9ºC without arousing from torpor (range -9ºC to 4ºC), compared to a low of -4ºC for Little Brown Myotis (range -4ºC to 7ºC Fenton 1972). Humidity levels reported from two Ontario caves containing hibernating Eastern Small-footed Myotis have ranged from 78 to 92 percent on different dates and within different passages (n=4 measurements). However, these measurements were taken from within main or side passages, whereas this species is usually observed hibernating closer to the entrances of hibernacula (Hitchcock 1949). As a result, humidity level at specific locations where the species hibernates is not available. In general, researchers have noted that Eastern Small-footed Myotis is regularly found hibernating closer to the entrances of hibernacula than other species of bats, where they have observed that temperatures are cooler and humidity is lower (Hitchcock 1949, Veilleux 2007).
Eastern Small-footed Myotis hibernate singly or with others, and seem to prefer the cold, dry locations in narrow cracks in the walls or ceiling (Hitchcock 1949), or under rocks or in crevices of the floor (Davis 1955, Tuttle 1964, Martin et al. 1966). This may provide a cooler and more stable thermal zone than hibernating on walls or ceilings (Martin et al. 1966, Fenton 1972), although they do hang on walls and ceilings of passages in hibernacula as well (Hitchcock 1949, Tuttle 1964, Martin et al. 1966, Veilleux 2007).
This species has been found at Ontario hibernacula in lower numbers when snow is present at the end of November than later in the winter, suggesting it may be able to find adequate shelter outside of hibernacula even with snow on the ground (Hitchcock 1949).
Hibernacula can be used by the same individuals in multiple years over their lifetime (Hitchcock et al. 1966) although the extent of migration to other hibernacula within and between years is unknown (Fenton 1972). Because the conditions required for hibernation are found only in specific locations, such as caves and abandoned mines, and these sites are a limited resource on the landscape, they are regularly used by the species over decades or longer (Hitchcock 1941, 1945, 1949, 1965, Fenton 1970, NHIC 2016).
Summer and maternity habitats - Ontario
In Ontario, summer habitats, including sites of maternity colonies, are very poorly understood. Maternity sites are locations that female bats rely upon in order to provide a secure environment in which to give birth and raise young until they are weaned, and are able to fly and effectively forage on their own. Female bats tend to congregate in suitable sites containing similar characteristics to provide this secure environment, and as a result have characteristics such as sufficient protection from predators and a warm environment for thermoregulation. Adult male bats may also be found at some maternity sites.
Historically, only one report of a likely maternity colony of "about a dozen" Eastern Small-footed Myotis in Ontario existed; this was a colony located behind a sliding shed door in Renfrew County in early July of 1953 that dispersed when the door was moved. Species identification was confirmed from two individuals crushed during the process of moving the door. This site was located approximately 16 km from their hibernaculum as one of the crushed individuals had been banded there the winter before (Hitchcock 1955). This is one of the very few records in the entire species' range where both the summer and winter habitats of an individual are known.
In July of 2016, a maternity colony of Eastern Small-footed Myotis was discovered at a cluster of old buildings in Hamilton (county; now the City of Hamilton). The colony was primarily roosting in an old barn, but bats were also captured in mist-nets deployed in the general area of the clustered buildings, and it is unknown if the colony exclusively uses one building or if it uses several in the area. A total of 5 adult females and 7 juveniles were captured in harp traps and mist-nets combined (M. Browning unpub. data). This maternity colony is located approximately 25km from the nearest known hibernaculum for the species. It is found in a known karst area (OMNDM 2016), although surficial rock features are generally absent from the area.
Other observations of Eastern Small-footed Myotis in Ontario outside of the hibernation period (i.e., during the summer) consist entirely of small numbers of individuals, but in a variety of circumstances. The circumstances listed in order of general prevalence of occurrence are as follows:
- captured outside known hibernacula or swarming sites;
- captured while foraging or commuting, in forests, in rocky habitats, or at ponds;
- roosting in or on buildings (barns/sheds or other external sites, including maternity sites and night-roosting or undetermined); and
- crevice-roosting (rock face, cliff, and rock barren).
The extent of the use of summer rock roosts in Ontario has not been determined. Very few observations have been submitted to the NHIC and documentation of diagnostic characteristics necessary to verify reported observations are lacking (i.e., clear photos of the face and calcar as well as measurements of the forearm and foot lengths).
In early July 2015, Eastern Small-footed Myotis was confirmed to be roosting under a rock on exposed Canadian Shield rock barren habitat in Muskoka District (M. MacPherson, unpub. data). This is the first confirmed observation of the species using rocky habitats for summer roosting in the province. One male was also observed roosting in a crevice of a southeast-facing cliff in Halton in late May 2016 (M. Browning and P. Moosman unpub. data). There were two additional reports of bats roosting together beneath loose rock on exposed Canadian Shield rock barren in the summer of 2015, one in Muskoka District (NHIC 2016) and the other in Peterborough County (L. Bruce, unpub. data). These were likely Eastern Small-footed Myotis; however, the species was unable to be confirmed because diagnostic characteristics were not documented. During targeted mist-net surveys undertaken in talus habitats at three sites along the Niagara Escarpment in August and September of 2016, 55% of bats captured were Eastern Small-footed Myotis (n=18, C. Humphrey and H. Fotherby unpub. data). Based on the abundance of rocky habitat types in the province and this observed mist-net capture rate in rocky habitats, it is possible that the species may be found roosting more frequently in these locations if targeted surveys are undertaken.
Eastern Small-footed Myotis has rarely been encountered roosting in buildings (five records to-date in Ontario, including two maternity colonies) compared to other commonly-encountered species, such as Big Brown Bat and Little Brown Myotis. As a result, there are likely to be limiting factors restricting more frequent use of buildings by the species in Ontario, such as a tendency to remain within close proximity to hibernacula. Alternatively, a preference for crevice roosting and the tendency to roost singly or in smaller colonies may explain the lack of observations of the use of voids or enclosed spaces in buildings compared to other species. Observations associated with buildings in Ontario have been limited to barns or sheds, or on exterior walls (Davis 1931, Hitchcock 1955, Fraser and Davy 2004, NHIC 2016, M. Browning unpub. data).
Based on the limited information available, buildings and rocky habitats are known to provide summer habitat, including maternity sites, for Eastern Small-footed Myotis in Ontario. However, summer habitats are very poorly understood and the general lack of data for the species precludes the ability to identify the prevalence of habitat use in the province.
It is also unknown if males and females roost in different habitats outside of hibernation in Ontario, similar to other species such as Little Brown Myotis, or if they use the same habitat types even during the maternity period.
Summer and maternity habitats – range outside of Ontario
Throughout its range, summer and maternity habitats for Eastern Small-footed Myotis have only recently been identified. For example, Best and Jennings (1997) completed a thorough review of the species and, at that time, the only known maternity site was the one behind a shed door in Ontario, as described above, and was the only summer site discussed. Since then, a number of summer habitats, including a few maternity sites, have been identified in the United States.
The summer habitat use of Eastern Small-footed Myotis identified elsewhere in its range generally varies from that of other bat species in eastern North America. They have been found to primarily roost in open, sunny rocky habitats, including cracks and crevices in cliffs and boulders, in talus slopes, beneath stones on rock barrens and in rock outcrops containing crevices. Rock type does not appear to be important, as they have been observed to roost within a variety of sedimentary and metamorphic rock types (Best and Jennings 1997, Bat Conservation and Management Inc. 2003, Roble 2004, Steffen et al. 2006, Johnson and Gates 2008, Johnson et al. 2011, Chenger 2012, Moosman et al. 2013, Thompson 2013, Whitby et al. 2013, Moosman, et al. 2015, D. Yates and T. Divoll, in prep.). However, they have also been identified to roost in buildings as well (O'Keefe and Lavoie 2010, Fagan et al. 2016).
Specifically, maternity roosts (locations with pregnant or lactating females and juveniles) have been found in the following locations in published literature.
- An old abandoned cabin, using both crevice roosts (under shingles, behind shutters, boarded windows) and voids (attic and closet) at high elevation in the Great Smoky Mountains in North Carolina (O'Keefe and Lavoie 2010). Up to 92 individuals may have been using the building. Natural rock habitats were sparse within 5 km of the cabin.
- Guardrail crevices in a concrete bridge, where a colony of ≤20 bats was observed (MacGregor, Kentucky Department of Fish and Wildlife Resources, Frankfort, KY, unpub. data as described in O'Keefe and Lavoie 2010).
- Rock outcrops in New Hampshire, where a colony of ≤13 bats was observed ( Veilleux, Franklin-Pierce University, Rindge, NH, unpub. data as described in O'Keefe and Lavoie 2010).
- Rock crevices in sandstone talus slopes and rock fields along transmission lines in the Appalachian Mountains of West Virginia (Johnson et al. 2011), where lactating females were radio-tracked for 4-9 days each between May 26 and July 5. Roosts were in crevices within boulders or within the crevices between rocks. Tracked males primarily roosted in the same talus slopes and rock fields as females and juveniles, but one male also roosted in an adjacent vertical cliff face. Females and juveniles were located closer to the entrances of crevices than males, which roosted deep within crevices.
- Shallow gaps underneath loose rocks on sandstone rock barrens in Illinois (Whitby et al. 2013). One post-lactating female was observed roosting in the same crevice as a juvenile between late July and mid-August, suggesting the site may be used for maternity roosting.
- Talus slopes of sedimentary rock in the Blue Ridge Mountains in Virginia (Moosman et al. 2015), which included males roosting in proximity to females and young during the maternity period, and maternity colonies of up to approximately 20 individuals. Bats were captured by mist net and radio-tracked, observed while conducting visual searches of crevices in plots, or incidentally encountered in crevices while setting up mist nets.
- Three buildings in the Great Smoky Mountains National Park in Tennessee (Fagan et al. 2016), which were all old (85 years) log buildings located in clearings of woods with an abundance of rocky terrain in the vicinity. All three buildings were used by humans on a daily basis in the summer. One building supported a maternity colony of at least 17 individuals, which roosted in three separate exterior locations associated with the rafters and joists of a porch; the interior of the building was modern and airtight. The other two buildings were small, drafty log cabins with doors or windows permanently open; roosting occurred in the main rooms by a small number of individuals.
This species may select open rocky habitats over close-canopied sites as a result of increased solar exposure (Johnson and Gates 2008, Thompson 2013), and has even been observed to bask in the sunlight outside of a roosting crevice (Moosman et al. 2015). Skin temperature of an Eastern Small-footed Myotis roosting in a talus slope closely corresponded to the ambient temperature of the environment, which lends support to this hypothesis (Moosman et al. 2015). The species has only very rarely been observed to roost in dead trees including White Pine (Pinus strobus) snags. Above one roost site on a snag, canopy closure was measured at 25 percent (Thompson 2013).
While the extent to which males and females may select separate roosting locations is unknown, both adult males and adult females (and adult females with juveniles) have been found roosting in the same rocky habitats during the maternity period, even as close as five metres apart (Johnson et al. 2011, P. Moosman, unpub. data). On average, however, within these habitats, females tended to roost in specific locations that were closer to water sources than roosting sites used by males (Johnson et al. 2011).
In the United States, summer roosts have also been found in a variety of anthropogenic sites that mimic natural sunny, rocky habitats. They include large areas of rocky rip rap, crevices in road cuts, waste rock piles, and crevices in concrete bridges and other concrete structures. Summer roosts have also occasionally been found in cracks in old foundations and chimneys, and in quarries with rock piles or active rock faces containing crevices (Bat Conservation and Management Inc. 2003, Johnson and Gates 2008, Chenger 2012, Moosman et al. 2013, Thompson 2013, G. Turner, pers. comm. 2015).
Eastern Small-footed Myotis have also been observed to roost in other anthropogenic features, most frequently associated with buildings (typically in external crevices such as behind shutters or doors, and beneath loose wooden or slate shingles). More rarely, they have been found in enclosed parts of buildings such as attics and porch roofs, in wood and log piles, picnic shelters and even in the crevice of an abandoned bulldozer (Davis 1931, Barbour and Davis 1969, Bat Conservation and Management Inc. 2003, O'Keefe and LaVoie 2010, USFWS 2013, G. Turner, pers. comm. 2015, Fagan et al. 2016, D. Yates and T. Divoll, in prep.). One maternity colony site in an historic building in proximity to hibernacula in North Carolina has been used for at least seven years (O'Keefe and LaVoie 2010, K. Caldwell, pers comm. 2015, M. LaVoie, pers. comm. 2015).
In the eastern United States, Bat Conservation and Management Inc. have tracked seven of approximately 30 radio-tagged Eastern Small-footed Myotis to roosts associated with buildings, with the remaining individuals tracked exclusively to roosts in primarily artificial rocky sites (J. Chenger and B. Butler, pers. comm. 2015). Three of 12 Eastern Small-footed Myotis (two females and one male) were tracked to crevices under slate shingles and flashing in Acadia National Park in Maine, whereas all other individuals were tracked exclusively to roosts in rocks (D. Yates and T. Divoll, in prep).
While many species of bats have been observed to utilize specialized artificial roosts for summer roosting or maternity colonies (such as bat boxes, bat houses, and bat condos), there are no reports of Eastern Small-footed Myotis utilizing these types of artificial roosts (Mering and Chambers 2014). This may be a result of the species' general preference for rock roosts over tree roosts or because these artificial roosts are not usually installed in areas the species occupies or in the vicinity of its preferred habitat. However, Eastern Small-footed Myotis has been observed to roost in a small number of constructed piles of rock in Pennsylvania created exclusively to provide habitat for the species (Pennsylvania Game Commission 2014, G. Turner, pers. comm. 2015).
While it appears that open sunny habitats are the most frequently encountered roosting locations, not all identified roosting sites have been in sunny locations. Radio-tracking studies documenting habitat parameters at individual roost sites have identified percent canopy cover varying between 0 and 90 percent; average canopy cover is reported in studies to be 14 to 85 percent (Johnson and Gates 2008, Johnson et al. 2011, Thompson 2013, D. Yates and T. Divoll, in prep). In less formal studies, Eastern Small-footed Myotis have also been found roosting under rocks in quite shaded locations from time to time (Chenger 2012). The identification of sunny open habitats may represent areas the species is more likely to occupy; however, they may also occasionally occupy shaded crevices nearby.
Foraging habitat
Information on foraging habitat in Ontario is generally lacking. One early report describes the species to have been observed only when foraging infrequently over a peat bog in what is presumed to be Haliburton County (Wright and Simpson 1920). Some observations of the species in Ontario have been mist-net captures, presumably caught while foraging or commuting in forest corridors or over ponds (NHIC 2016, K. Jonasson, unpub. data, B. Lim, D. Riskin and T. Thorne unpub. data).
In the United States, the species has primarily been observed to forage in forests, but also over water bodies and riparian forests (Johnson et al. 2009, Moosman, Jr. et al. 2013, D. Yates and T. Divoll, in prep.) and occasionally in open fields (Bat Conservation and Management Inc. 2003). Eastern Small-footed Myotis may spend more time foraging over terrestrial habitats than Little Brown Myotis (Fenton and Bogdanowicz 2002). In general, the species has been reported to forage between 0.8 km and 13.2 km away from day roosts (Bat Conservation and Management Inc. 2003, Chenger 2008, USFWS 2013).
There are currently two available studies that have identified home range sizes, based on roost locations and surrounding foraging areas; for both studies, radio-telemetry was used to track observations of tagged individuals. In the Maryland study, the home range sizes of four female Eastern Small-footed Myotis, following emergence from hibernacula in the spring, were estimated to be less than 100 ha (10.2 - 99.7 ha), with foraging occurring no further than 1.8 km from roost sites. In this study, suitable rock roosts were located in very close proximity to the hibernaculum (100m, Johnson et al. 2009).
For the other study, conducted on an island in Acadia National Park along the coast of Maine, six juveniles of both sexes and four adult males (10 individuals total) were tracked for approximately 14 days each between July and October (D. Yates and T. Divoll, in prep.). The landscape contains "marshes, old growth forest, ridges, lakes and ponds, and carriage roads" (Divoll 2013, p.9) in generally mountainous terrain, with granite ledges, cliffs, bald mountain tops, occasional rural structures and small towns and villages. Forests were characterized to be dominated by "mature eastern hemlock (Tsuga canadensis), northern white cedar (Thuja occidentalis), spruce (Picea spp.), poplar (Populus spp.), American beech (Fagus grandifolia), birch (Betula spp.), and maple (Acer spp.)" (Divoll 2013, p. 10). In order to characterize home ranges, at least 15 points were collected for each individual and utilization distributions were generated following fixed kernel density methods (Worton 1989). Home ranges were identified as follows (D. Yates and T. Divoll, in prep.).
- Home range sizes (95 % isopleth) for all bats tracked were estimated to be larger in Maine than in Maryland, ranging from 96 ha to 1,489 ha (average size of 652 ha ±488 ha ).
- Core use areas (50 % isopleth) for all bats tracked were 8 ha to 293 ha (average size of 117 ha ±92 ha ).
- Juveniles had both the smallest and largest home range sizes; those of adult males also fell within this range. The resulting average home range size (95 % isopleth) for juveniles was 813 ha (±575 ha; min 96 ha, max 1,489 ha).
- Core use areas (50 % isopleth) for juveniles ranged from 8 ha – 293 ha (average 148 ha ±108 ha).
The difference in home range size between females during the spring and juveniles/adult males during the summer and fall may be related to the need for high site fidelity by females for the maternity period or weak body condition after emerging from hibernation. The study area in Maine is also suspected to contain a possible hibernaculum, based on the presence of the species into mid-October, although this has not yet been confirmed (D. Yates and T. Divoll, in prep).
1.5 Limiting factors
Habitat specificity and availability
Overwintering habitat is considered to be an important limiting factor for populations of cave-hibernating bats (Fenton 1970, Dewar et al. 1985, OMNR 2010) as the specific conditions required by bats to survive through the winter without adequate food resources are likely limited to suitable caves and abandoned mines (Furey and Racey 2016). Suitable cave hibernacula are limited in Ontario because they are generally restricted to karst areas in the province. Abandoned mines are found throughout central and northern Ontario (OMNDM 2016), some of which are also hibernacula for other bat species (Environment Canada 2015). However, the absence of any records of Eastern Small-footed Myotis in the northern regions containing known bat hibernacula may indicate that conditions are not suitable for the species in these areas (see Section 1.3).
Availability of summer roost sites has also been identified to limit the distribution of other similar species of bats in Ontario (Fenton 1970). If Eastern Small-footed Myotis also prove to be most commonly found in rocky habitats in Ontario during the summer, it is unknown if this type of habitat will be a limiting factor. Large rocky areas containing abundant crevices, such as those found within talus slopes or otherwise associated with mountainous areas, are extremely limited in Ontario. There are, however, large areas of exposed Precambrian igneous and metamorphic bedrock on the Canadian Shield in the province. Eastern Small-footed Myotis was recently confirmed to roost under loose rocks in exposed glacier-scoured rock barren Shield habitats in Ontario (M. MacPherson, unpub. data). However, it is unknown to what extent this habitat type is used and what population density it can support. Because of the abundance of potential habitat on the Canadian Shield, further study is needed to determine the summer habitats used by the species, and to what extent, in order to identify whether summer habitat availability is a truly limiting factor in the province.
Survival rate
Eastern Small-footed Myotis is identified to have a lower estimated survival rate, with a mean annual survival of 42.1 ± 7.1 % for females and 75.7 ± 11.1 % for males (Hitchcock et al. 1984), than other species such as Little Brown Myotis, which has a mean annual survival of 70.8 ± 2.2 % for females and 81.6 ± 1.0 % for males (Keen and Hitchcock, 1980). This may be due in part to its preference for colder and draftier hibernation sites. Combined with its tendency to hibernate alone or in smaller groups, this may increase heat and water loss during hibernation (Boyles et al. 2008, Boratyński et al. 2014), which could lead to more frequent arousals from torpor and more frequent trips out of hibernacula in mid-winter (Hitchcock 1984). The species also has a shorter hibernation period than others. All of these factors may combine to deplete fat reserves more quickly and expose the species to greater perils from inclement weather and predation (Hitchcock et al. 1984). The factors may also explain why, despite the apparent cold-tolerance of the species, it has not been found in more northern regions of the province where temperatures outside of hibernacula are likely colder and of longer duration (Fenton 1972). These biological traits could combine to limit the natural abundance and distribution of the species in the province.
In addition, survival of banded Eastern Small-footed Myotis females has been found to be considerably lower than that of banded males (Hitchcock et al. 1984), even though body weights have not been shown to vary significantly between sexes at the start or end of hibernation (Fenton 1972). This may be a result of heavier demands on females related to reproduction, including: gestation and giving birth; greater activity associated with rearing; greater exposure to ectoparasites in maternity colonies; or increased cost of body temperature regulation as a result of smaller maternity colonies and lack of clustering (Hitchcock et al. 1984).
The timing of entering into and exiting from hibernation, as well as the frequency and duration of arousals, is critical to ensure not only overwinter survival of hibernating bats, but also that they are in sufficient condition to promote survival and reproduction (Czenze and Willis 2015).
Dispersal ability
Additional study is needed on the dispersal ability of this species; however, a preference to move short distances between winter and summer habitats will further restrict the distribution of Eastern Small-footed Myotis to those areas where suitable winter and summer habitats occur in close proximity.
Birth rate
Similar to other Myotis species, it is likely that Eastern Small-footed Myotis give birth to only one young per year. Combined with a low female survivorship, this may also help to explain the historically uncommon status of the species (van Zyll de Jong 1985).
1.6 Threats to survival and recovery
White-nose syndrome
The greatest threat to cave-dwelling bats in Ontario is a disease called white-nose syndrome (WNS), caused by the fungus Pseudogymnoascus destructans (Pd). The fungus likely originates from Europe (Blehert 2012, Pikula et al. 2012, Warnecke et al. 2012, Leopardi et al. 2015), and not only thrives in the cool (1° -19 ºC) humid environments of caves and abandoned mines, but lives and reproduces in the external tissues of bats while in these environments (Lindner et al. 2011, Verant et al. 2012). The first evidence of WNS in North America was from bats hibernating in a cave in New York State in February of 2006 (Blehert et al. 2009).
White-nose syndrome presents as a white substance on the muzzles, ears and wings of infected hibernating bats (see Figure 5) where Pd causes lesions in the skin, particularly the wing membrane (Meteyer et al. 2009). It is believed this wing damage disrupts water balance, causing dehydration and loss of electrolytes, and subsequently, excessive arousal of infected bats from torpor (Cryan et al. 2010, Frick et al. 2010, Warnecke et al. 2012, Verant et al. 2014). The prevalence of Pd on bats increases over the winter (Langwig et al. 2015). Bats infected with Pd are less active during bouts of arousal and exhibit reduced clustering behaviour (Wilcox et al. 2014). Despite this, infected bats are sometimes seen flying around or on the ground outside of hibernacula in mid-winter (Cryan et al. 2010, Carr et al. 2014). Excessive arousals use up critical body fat supplies before bats are able to forage in spring, resulting in overwinter mortality (Blehert et al. 2009, Cryan et al. 2010, Frick et al. 2010, Blehert 2012, Warnecke et al. 2012). Bats that do survive until spring may show signs of lesions on the wings, including scar tissue and holes, physiological stress or reduced reproductive success (Meteyer et al. 2009, Reichard and Kunz 2009, Environment Canada 2015).
The presence of Pd on individual bats decreases over summer, likely due to warmer body temperature, and the ambient temperatures and drier conditions of summer habitats, which are unfavourable for fungus growth (Langwig et al. 2015). It is unknown whether bats pass the fungus onto their offspring during the maternity period. Given that the prevalence of Pd on bats drops drastically over the summer and does not increase as a result of the seasonal birth "pulse," when new individuals are added to the population (Langwig et al. 2015), it is not likely that transmission from mothers to young is an important source of Pd transmission among bats (Lorch et al. 2011, Lorch et al. 2013). However, Pd can persist for long periods in the absence of bats, suggesting that bats re-entering a hibernaculum containing Pd could be subject to re-infection (Lorch et al. 2013, Hoyt et al. 2014).
Figure 5. Eastern Small-footed Myotis showing signs of infection from white-nose syndrome in New York State

(Photo: Alan Hicks).
Since its initial discovery, Pd has spread widely throughout eastern North America, and it is estimated that WNS has killed well over 5 million bats (USFWS 2012). Fungal spores are spread between hibernacula by individual bats themselves; the spread may also be facilitated by humans entering infected hibernacula and carrying spores to uninfected sites (Frick et al. 2010, Kunz and Reichard 2010, Blehert 2012, Lorch et al. 2011, Lorch et al. 2013, Shelley et al. 2013). White-nose syndrome was confirmed to be affecting Ontario bats in 2010 (OMNRF 2015b), and three-year declines of Myotis species at hibernacula in Ontario have ranged from 85 to 99 percent (COSEWIC 2013). The fungus continues to spread northward, southward and westward throughout North America, and also has the potential to spread eastward to Newfoundland (J. Segers, pers. comm. 2016a). As shown in Table 2, Pd has been confirmed in all 28 provinces and states comprising the range of Eastern Small-footed Myotis, and WNS has affected individual bats (of any species) in 26 of these (Environment Canada 2015, CWHC 2016, USFWS 2016).
Table 2. Distribution of white-nose syndrome in the range of Eastern Small-footed Myotis (ESFM). Information Sources: USFWS 2016, CWHC 2016, A. Ballmann, pers. comm. 2016, J. Segers, pers. comm. 2015, 2016b
|
Province or State in ESFM Range |
Fungus (Pd) Detected |
White-nose Syndrome Observed in Bats |
White-nose Syndrome Observed in ESFM |
|---|---|---|---|
|
Ontario |
X |
2009/10 |
Suspect (≥1 clinical sign and Pd detected) |
|
Quebec |
X |
2009/10 |
|
|
Alabama |
X |
2011/12 |
|
|
Arkansas |
X |
2013/14 |
|
|
Connecticut |
X |
2007/08 |
|
|
Delaware |
X |
2011/12 |
|
|
Georgia |
X |
2012/13 |
|
|
Illinois |
X |
2012/13 |
|
|
Indiana |
X |
2010/11 |
|
|
Kentucky |
X |
2010/11 |
|
|
Maine |
X |
2010/11 |
|
|
Maryland |
X |
2009/10 |
|
|
Massachusetts |
X |
2007/08 |
|
|
Mississippi |
X |
||
|
Missouri |
X |
2011/12 |
|
|
New Hampshire |
X |
2008/09 |
|
|
New Jersey |
X |
2008/09 |
|
|
New York |
X |
2006/07 |
Confirmed |
|
North Carolina |
X |
2010/11 |
|
|
Ohio |
X |
2010/11 |
|
|
Oklahoma |
X |
||
|
Pennsylvania |
X |
2008/09 |
Confirmed |
|
Rhode Island |
X |
2015-16 |
|
|
South Carolina |
X |
2012/13 |
Confirmed |
|
Tennessee |
X |
2009/10 |
|
|
Vermont |
X |
2007/08 |
Suspect (field report) |
|
Virginia |
X |
2008/09 |
|
|
West Virginia |
X |
2008/09 |
Suspect (field report) |
|
Total |
28 |
26 |
3 (6) |
The Canadian Wildlife Health Cooperative (CWHC) conducts WNS testing and maintains a national database and mapping of its extent in Canada (CWHC 2016). White-nose syndrome was determined to be Suspect (CWHC 2014) in a sample submitted from an Eastern Small-footed Myotis from Ontario in the late winter of 2016 (J. Segers pers. comm. 2016b). This is the first and only record of WNS associated with the species in Canada; however, prior to the winter of 2015-16, there had only been one submission of this species for testing, and this was prior to the confirmation of WNS in the country (J. Segers, pers. comm. 2015). Elsewhere in its range Eastern Small-footed Myotis has been confirmed to be affected by WNS (New York, Pennsylvania, and South Carolina, A. Ballmann, pers. comm. 2016). The species was likely exposed to Pd in 2006, the first winter that signs of WNS were observed in New York State, although the first observations of WNS symptoms in Eastern Small-footed Myotis were in 2008 (C. Herzog, pers. comm. 2015) and first confirmed in the lab in 2009 (A. Ballmann, pers. comm. 2016). Because of the lack of overwintering data for Eastern Small-footed Myotis in Ontario, the degree to which the species is affected by the fungus in the province is unknown. However, Eastern Small-footed Myotis may be less susceptible to WNS than other species such as Little Brown Myotis based on: (1) their tendency to hibernate alone or in smaller groups, so that transmission may be less frequent; and (2) their tendency to hibernate in colder, drier parts of the cave where conditions for WNS are less favourable (Langwig et al. 2012, COSSARO 2013).
In the United States it has been difficult to establish reliable population estimates of Eastern Small-footed Myotis, even in hibernacula, as a result of the species' tendency to hibernate in crevices that are inaccessible or hard to find. This has proven a challenge for monitoring the effects of WNS on U.S. populations (Langwig et al. 2012, USFWS 2013). Counts of the species at hibernacula across the species' range have historically fluctuated from year to year, typically ranging from zero or a few individuals to tens of individuals (Hitchcock 1941, 1945, 1949, 1965, Fenton 1972, Turner et al. 2011, USFWS 2013, C. Herzog, pers. comm. 2015). While populations have decreased since the onset of WNS at some hibernacula in the United States, increases have also been seen in some years. For example, a study evaluating pre-WNS and post-WNS population estimates using comparable methods at 26 Eastern Small-footed Myotis hibernacula in New York, Pennsylvania, Vermont, Virginia, and West Virginia identified that the number of this species observed fluctuated, with percent changes ranging from -100 percent to +1200 percent with a mean of +78.68 % ±296 % (Turner et al. 2011). Several other examples of population fluctuation are provided in USFWS 2013. This has made it more difficult to establish the degree to which the species is affected by the fungus compared to other cave-dwelling bats in eastern North America (USFWS 2013, C. Herzog, pers. comm. 2015). In the United States, population data has not reliably shown a decline in the species (USFWS 2013). For this reason, surveys of summer habitats and maternity colonies are being undertaken in the species' range to determine whether this may produce more reliable population estimates for the species that can be tracked over time (e.g. Moosman et al. 2015).
In Ontario, most bat hibernacula are confirmed or suspected to be infected with Pd (CWHC 2016, L. Hale, pers. comm. 2015). Hibernacula surveys conducted since the appearance of WNS identified Myotis species bats only to genus to minimize the time spent in hibernacula and limit disturbance to hibernating individuals (discussed in further detail below). As a result, data for all Myotis species are combined (COSEWIC 2013). There is only one hibernaculum in Ontario (Renfrew County) known to contain overwintering Eastern Small-footed Myotis where pre- and post-WNS count surveys were done. Three-year declines in Myotis species of 85 percent from the winter of 2009-10 (when the fungus was first detected) until the winter of 2012-13 were documented here (COSEWIC 2013). One additional known bat hibernaculum in Renfrew County had only summer records of Eastern Small-footed Myotis, which could indicate the potential presence of previously undetected overwintering populations. At this site, three-year declines of 96 percent of Myotis species were documented over the same timeframe (COSEWIC 2013).
White-nose syndrome is identified as the primary reason for the classification of Eastern Small-footed Myotis as endangered (COSSARO 2013), and Pd has now been detected on the species in the province, along with at least one clinical or field sign associated with WNS in the bat (CWHC 2014,J. Segers pers. comm. 2016b). Protection and recovery of Eastern Small-footed Myotis populations in the province would benefit from submission (following CWHC undated) of any dead Eastern Small-footed Myotis observed in the province to CWHC diagnostic labs and additional monitoring of hibernacula. These actions would provide important additional information on the occurrences and prevalence of WNS in the species in Ontario.
Alteration of habitat
Changes to the structure of caves and abandoned mines can alter specific microclimates within hibernacula, including air temperature, humidity and air currents. One of the first recorded examples of cave alteration threatening suitability for bat hibernation in eastern North America was in Ontario. In the 1950's, the hibernaculum for the largest known colony of Eastern Small-footed Myotis at the time was commercialized, including alterations to the cave and its entrance to allow for tourism. This is described to have resulted in the elimination of a cold air current through the particular passage where the bats primarily hibernated. No Eastern Small-footed Myotis have been documented at that site since, despite continued searches into the early 1960's (Hitchcock 1965, NHIC 2016).
Access control to bat hibernation sites, such as the installation of gates or doors, or capping of abandoned mine sites, is known to result in direct mortality of hibernating individuals if installed during the hibernation period, and also has the potential to eliminate habitat for future use (USFWS 2013). However, installation of properly constructed, bat-friendly gates can increase the use of a site by the species (USFWS 2013).
Other alterations to hibernacula have been identified as threats to Eastern Small-footed Myotis and other bats in Ontario and other portions of its range (Hitchcock 1949, Amelon and Burhans 2006, USFWS 2013). These include:
- reclamation or resumption of activities in previously abandoned sites (especially mines);
- new ground works (mining, quarrying, etc.) in proximity to hibernation sites;
- cave or abandoned mine passage collapse; and
- ice damming, snow build-up, or other debris build-up at the entrance, which can eliminate air-flow or access, or even cause flooding.
Industrial activities, such as quarrying, mining, agriculture, construction, or forestry that take place near caves or abandoned mines could cause or contribute to changes in microclimate, airflow, or hydrology within these features, even if the activities are not occurring directly within or above the site (Environment Canada 2015). However, there is a lack of specific data linking some of these activities to these types of changes (e.g. agriculture, forestry).
While few summer habitats have been identified in Ontario, they may also be altered or destroyed through a variety of human activities. The USFWS (2013) reported the removal of Eastern Small-footed Myotis habitat in the United States because of highway construction, commercial development, construction of wind energy projects, surface coal mining, and natural gas extraction. Mined-land reclamation processes (removal of rock habitats such as rock piles and cliffs from previously mined lands) may also modify or destroy roosting habitat. The quality of foraging sites in close proximity to Eastern Small-footed Myotis roosting sites may also be altered through forest removal and management activities, e.g., changes in insect prey availability, as well as the availability and characteristics of roosting sites, although these potential effects on the species are not well known (Hayes and Loeb 2007, USFWS 2013).
Additionally, the USFWS (2013) reports one incident of vandalism destroying a portion of an Eastern Small-footed Myotis roost in the species' range.
Exclusion of a colony from an occupied building or demolition of buildings containing colonies also poses a threat to bats, both through direct mortality of adults and juveniles, and habitat loss. This threat is increased when it occurs during the maternity season as flightless juveniles may also be at risk of starvation when nursing mothers are excluded. The number of potential roost sites altered or destroyed, and potential effects of these impacts on Eastern Small-footed Myotis populations, are largely unknown across the species' range (USFWS 2013).
Alteration of summer habitat is known to have resulted in both direct mortality and elimination of a roost site for the only suspected maternity colony in the province when a door normally kept in the open position was moved (Hitchcock 1955). Other sites have generally not been documented, or if documented, not assessed; the level of risk to these sites is unknown.
Interactions with wind turbines
Bat fatalities at wind energy facilities are documented worldwide (Arnett and Baerwald 2013), and certain projects or regions have documented high bat mortality as a result of interaction with wind turbines (Arnett et al. 2008, Baerwald and Barclay 2009, Frick et al. 2010, Rydell et al. 2010, Arnett and Baerwald 2013, Bird Studies Canada et al. 2014, Arnett et al. 2016). Bat fatality as a result of interaction with wind turbines is caused by direct collision with wind turbines and trauma to the lungs associated with barotrauma (a sudden change in air pressure in proximity to turbine blades) (Baerwald et al. 2008, Grodsky et al. 2011, Rollins et al. 2012). Bats are thought to be attracted to wind turbines (Horn et al. 2008), but the exact reasons for this attraction are still not well-known (Arnett and Baerwald 2013, Arnett et al. 2016).
Wind energy projects have been installed throughout the range of Eastern Small-footed Myotis (USFWS 2013), including throughout the species' range within Ontario (OMECC 2015). While there are no published reports of mortality of Eastern Small-footed Myotis resulting from interaction with wind turbines in the United States (Arnett and Baerwald 2013), a very small number of fatalities of Eastern Small-footed Myotis have been reported from wind energy projects in North America (USFWS 2013) including Ontario (COSSARO 2013, Bird Studies Canada et al. 2014, NHIC 2016). Two fatalities of the species are reported in USFWS 2013 from unknown sources in the United States, and four have been reported in Ontario (Bird Studies Canada et al. 2014, NHIC 2016).
Fatality of Eastern Small-footed Myotis is reported to be less than 0.1 percent of total observed bat mortality at wind energy facilities in Ontario, with four individual mortalities reported (Bird Studies Canada et al. 2014, NHIC 2016). While reported as Eastern Small-footed Myotis, these individuals were not able to be subsequently verified as a result of the lack of diagnostic characteristics in the data and photographs provided. As a result, the reports of fatalities of Eastern Small-footed Myotis in Ontario (from Bruce County and Chatham-Kent) have not been included in the distribution of the species shown in Figure 3.
It is possible that fatalities of this species associated with wind turbines may be under- or over-represented based on errors in species identification, and in particular, the difficulty in identifying highly decomposed carcasses; however, this error rate has not been quantified. Improving the accuracy in identifying bat carcasses resulting from interaction with wind turbines may aid in the understanding of the actual impact of wind turbines on this species in the province. The accuracy in identification of Eastern Small-footed Myotis can be improved by identifying and recording diagnostic characteristics of the species (forearm and foot length measurements and photographs of the calcar and face), or through the use of DNA-based identification (e.g. barcoding) in the case of advanced decomposition and/or traumatic injury.
Regardless, the level of impact to the species to-date has likely been relatively low based on the overall dominance of long-distance migrants in the composition of bat fatalities at wind energy facilities in North America and Ontario (Arnett and Baerwald 2013, Bird Studies Canada et al. 2014, Arnett et al. 2016). The notable size difference between Eastern Small-footed Myotis and other short-distance migrants, such as Big Brown Bat or Little Brown Myotis, also aids in identification. The majority of wind turbines installed in Ontario to-date are located in the southwestern portion of the province (OMECC 2015), where observations of the species have generally been lacking.
The tendency for Eastern Small-footed Myotis to fly slowly and close to the ground may make the species less susceptible to wind turbine mortality (USFWS 2013). In addition, its nature as a crevice-roosting, short-distance migrant may also explain the low numbers of Eastern Small-footed Myotis fatalities resulting from collision with wind turbines, compared to effects on tree- and foliage-roosting long-distance migrants such as Eastern Red Bat (Lasiurus borealis), Hoary Bat, and Silver-haired Bat (Arnett et al. 2008, Bird Studies Canada et al. 2014).
However, the threat to Eastern Small-footed Myotis from wind turbines may be increased if wind energy projects are situated in close proximity to or between suitable summer and winter habitats. In particular, mortality of bats has been documented to be highest during late summer and early autumn (July to September in the U.S. and Canada) when bats are moving between summer and winter habitats (Bird Studies Canada et al. 2014, Arnett et al. 2016). Long-distance migrant species may drive this trend, comprising 78.4% of dead bats collected in the U.S. and Canada (Arnett et al. 2016) and 70.1% collected in Ontario (2006 to 2012, Bird Studies Canada et al. 2014). However, preliminary analyses indicate that mortalities of resident bat species not normally performing long-distance migrations, including Little Brown Myotis and Big Brown Bat, may also be highest between July and September (Bird Studies Canada et al. 2014), even when corrected to account for varying monitoring periods (A. Ryckman, unpub. data).
Human disturbance
Even during some of the first population counts, human interference at hibernacula has been noted as a threat to the species (Hitchcock 1949). Human disturbance at hibernacula includes commercialization of caves (e.g., tours), recreational caving, vandalism, and scientific research (USFWS 2013). Disturbance within hibernacula during the winter months causes bats to arouse from hibernation; while arousal from hibernation at regular intervals is a normal component of hibernation biology in bats, it is also the greatest source of energy depletion (Thomas et al. 1990, USFWS 2013). Different species of bats vary considerably in their degree of sensitivity to disturbance; however, it is generally understood that excessive disturbance can pose a serious threat to overwintering populations and may even result in colony level declines (Hayes et al. 2009, Kunz et al. 2009b). The extent of these threats to Eastern Small-footed Myotis or other bat species in the province has not been documented. However, threats to the bats from disturbance during hibernation is one of the most important factors taken into consideration before conducting research on overwintering populations, especially when bats are already experiencing additional stress from WNS (Thomas 1995, Warnecke et al. 2012).
The spread of WNS may also have been facilitated by human disturbance at hibernacula through the transportation of fungal spores between caves on clothing or personal gear (Coleman and Reichard 2014). As discussed, most hibernacula in Ontario are confirmed or suspected to be infected with Pd (CWHC 2016, L. Hale, pers. comm. 2015), and WNS is already present throughout the species' range (see Table 2). As a result, the threat of human-related transmission of Pd between Eastern Small-footed Myotis hibernacula has likely already passed. However, human transmission of Pd between Eastern Small-footed Myotis hibernacula may pose a risk in the recovery of the species if environmental treatment options are developed in the future that are able to eliminate the fungus from specific sites. In these cases, transmission of the fungus between hibernacula resulting from the movements of bats would likely continue to be a significant risk for transmission (Frick et al. 2010, Kunz and Reichard 2010, Blehert 2012, Lorch et al. 2011, Lorch et al. 2013, Shelley et al. 2013). However, human-related transmission still poses a threat to other bat species as it may facilitate the spread of the fungus to regions beyond the WNS-positive zone, therefore precautions should be taken to minimize spread to regions where it has not yet been observed.
As with all bat species, persecution of bats by humans is also a threat. A recent review identified that intentional killing was the third-highest source of documented accounts of multiple mortality events (i.e., mortalities of more than 10 bats per location within a maximum time frame of one year) for all bat species globally between 1975 and 2015, after wind turbine mortality and WNS (O'Shea et al. 2016). In North America, intentional killing of bats has been documented by "burning, shooting, bludgeoning, poisoning, and other activities" (O'Shea et al. 2016, pp. 6-7). This persecution is largely a result of fear or misunderstanding of bats and their behavior. There is only one documented example of Eastern Small-footed Myotis being persecuted (USFWS 2013, O'Shea et al. 2016), which may be due to its general rarity or because it uses buildings less frequently than other species such as Little Brown Myotis and Big Brown Bat, where they are more likely to come into contact with humans. Persecution of other mammals, such as rodents, may also lead to accidental mortality of bats (Jung and Slough 2005). In both intentional and accidental cases, it is likely that incidents are under-reported.
Toxins
A variety of heavy metals are of concern to bats, including arsenic, cadmium, cobalt, chromium, copper, mercury, manganese, nickel, lead, tin and thallium (Zukal et al. 2015). There are only two studies available that assess heavy metal concentrations in Eastern Small-footed Myotis (Zukal et al. 2015). In one study, mercury and zinc were recorded in Eastern Small-footed Myotis fur samples at concentrations higher than in other bat species examined in Ontario (Hickey et al. 2001). In the other study, mercury was shown to accumulate in Eastern Small-footed Myotis primarily as a result of preying on emergent aquatic insects in contaminated areas of the northeastern United States, and mercury levels were higher in Myotis species than in other genera (Yates et al. 2014). Mercury accumulation in fur and blood was higher in bats located closer to point sources than to non-point sources of contamination (Yates et al. 2014). Of concern is that mercury concentrations have been recorded at higher levels in females and may be passed on to young through breast milk. However, it is unknown to what extent this poses a threat to populations (Hickey et al. 2001, Yates et al. 2014).
Other toxins of potential concern to Eastern Small-footed Myotis that have been identified in other insectivorous bat species of eastern North America include:
- microcystin (produced by blooms of cyanobacteria in water bodies) accumulation in Little Brown Myotis and their mayfly (Hexagenia limbala) prey (Woller-Skar et al. 2015);
- polybrominated biphenyl ethers, salicylic acid, thiabendazole, and caffeine, among others, detected in Little Brown Myotis, Northern Myotis, Big Brown Bat, and Indiana Myotis (Myotis sodalis; Secord et al. 2015); and
- organochlorine pesticides and other organic contaminants in Little Brown Myotis, Northern Myotis, and Big Brown Bat, among others (Bayat et al. 2014).
Pesticides, including organochlorine pesticides and neonicotinoids, could also affect bats, including Eastern Small-footed Myotis, by reducing availability of insect prey (Pisa et al. 2015) and/or suppressing their immune systems (Gibbons et al. 2015). For example, important prey species for of Eastern Small-footed Myotis such as flies, mayflies, and caddisflies may be most sensitive to neonicotinoids (Van Dijk et al. 2013, Smit et al. 2014, Morrissey et al. 2015). Further research is needed to link effects from these pesticides and other toxins on Eastern Small-footed Myotis or availability of their prey before the extent of this potential threat can be assessed.
1.7 Knowledge Gaps
Biology
As identified in Section 1.2, the biology of Eastern Small-footed Myotis is generally not well understood. Critical knowledge gaps in the biology of the species include information on timing of life cycle stages and dispersal ability. Specifically, it will be critical to identify the maternity period in Ontario, including the dates when:
- females travel to maternity colony sites;
- females give birth and rear young; and
- adult females and juveniles depart from maternity sites.
The timing and chronology of the movements of adults and juveniles between winter and summer habitats is also unknown. For example, it is unknown if the presence of Eastern Small-footed Myotis at rocky habitats in August through October in other parts of its range indicates that the species (1) lingers at summer habitats; (2) roosts in transitional habitats on its way to hibernacula; or (3) overwinters in the same sites as summer habitats. It is also possible that the species utilizes a combination of these approaches. The extent to which males and females roost together in Ontario during the maternity period is also unknown. It will be important to identify the roosting phenology of this species in Ontario by sex and age in order to identify critical time periods when the species is most likely to be using different habitats.
More research is also needed to determine movement patterns and dispersal ability of Eastern Small-footed Myotis. General consensus among bat researchers is that longer migrations between hibernacula and maternity roost habitat do not likely occur. However, it is unknown if observations reported from sites located greater than 100 km from areas of known or possible hibernacula represent outliers or if these travel distances between hibernacula and maternity or other summer habitats may still be typical for the species. As a result, it will be important to determine overall movement patterns between hibernacula and maternity habitat to better understand dispersal ability.
In addition, the physiological factors driving the species' preference for colder and draftier sites are unknown. Information explaining this preference may help to identify the expected limit to the range of the species in Ontario and aid in addressing the extent to which the species may be affected by WNS.
Distribution
The distribution of the species in the province of Ontario is likely under-represented based on studies of bats completed to date because of:
- the species' tendency to occupy habitats not frequently surveyed for bats, and which may be difficult to access (i.e., lack of specific survey effort);
- the species' tendency to roost in crevices in both summer and winter habitats, where they are difficult to see even when conducting targeted searches;
- the species' excellent ability to detect and avoid mist-nets (Barbour and Davis 1969, Tyburec 2012); and
- the difficulty in differentiating Myotis species acoustically, based on many overlapping characteristics (Moosman et al. 2015), especially in foraging habitats where multiple Myotis species may be present.
Information on dispersal ability will help to inform the potential for distribution of the species in the province as well. There could also be as-yet unidentified hibernacula in Ontario, particularly if the species is found to hibernate in areas outside of traditional cave or abandoned mine hibernacula. If additional hibernacula are found, this could expand the known distribution, and expand the species' potential summer distribution as well.
Abundance and population viability
Population data for Eastern Small-footed Myotis in Ontario are lacking, and population viability analysis has not been completed (COSSARO 2013). This is primarily a result of the difficulty in identifying summer habitat sites and finding the species in a known bat hibernaculum even when targeted searches are conducted. Observation of the diagnostic characteristics for Eastern Small-footed Myotis usually requires handling of the bat, which limits the confidence in species identification for some survey types as well. All of the factors identified above result in a lack of population data for the species, in addition to the lack of distribution data. As a result, the species is very likely to be underrepresented through the use of traditional survey methods, making populations and trends difficult to assess. Lastly, confirmation and determination of the extent to which Eastern Small-footed Myotis leave hibernacula during warm spells in winter or use alternate hibernation sites have not yet been determined (Griffin 1945, Hitchcock 1949, Fenton 1972, USFWS 2013). This adds to the uncertainty in making population estimates.
Individual bats are most likely to congregate in hibernacula, as these habitat features are generally rarer on the landscape than summer habitats and are likely to host bats from multiple maternity sites (Kerth et al. 2003, Rivers et al. 2005, Dzal et al. 2009). As a result, counts at hibernacula present one of the easier opportunities to identify population size. However, population counts at hibernacula have fluctuated from year to year across the species' range. It is unknown if the observed fluctuations between years are a result of:
- variability in individual roost locations at the time of the count (i.e., in cracks or crevices versus walls or ceilings);
- use of different hibernacula between or within years (immigration and emigration); and/or
- variation in the number of births and deaths.
It is unknown if the species has declined in Ontario, and the global proportion of the species which occurs in the province is also unknown. A written opinion from a recognized expert on population sizes or viability for the species is also not available (COSSARO 2013). The USFWS evaluated the species and determined that Eastern Small-footed Myotis has not been affected by WNS to the same degree as other species in that country, and not at the population level (USFWS 2013, NatureServe 2015). Data is not available to perform a similar type of analysis in Ontario because WNS has not yet been confirmed in the species in the province and hibernacula counts conducted pre- and post-WNS have not identified individual species of Myotis. As a result, the protection and recovery of the species would greatly benefit from species-specific surveys in order to identify the prevalence of WNS in Eastern Small-footed Myotis in Ontario. This would be possible only if species-discriminating population counts are undertaken; however, the threat of research-related disturbance would require minimization. This could be achieved either through more invasive and thorough (but less frequent) surveys at hibernacula, e.g., visual population counts within sites, or less invasive surveys at hibernacula or in summer habitats. An example of the latter is capture surveys, which are sensitive enough to both discriminate the species effectively and produce reasonably accurate population estimates. Each method has its limitations and the best methods for determining population size in this species are still undergoing refinement (e.g. Moosman et al. 2015).
While lower female survivorship has been reported (Hitchcock et al. 1984), it is unknown how variables such as the impact of arousal from torpor as a result of human disturbance, the use of crevices and cracks during hibernation and the potential use of multiple hibernacula within and between years might impact these estimates of survival.
Habitat
Hibernacula historically used by this species in Ontario are fairly well-understood as a result of early studies on hibernating bats, many of which were conducted in Ontario (Hitchcock 1941, 1945, 1949, 1965, Fenton 1972, Hitchcock et al. 1984). However, there are many additional bat hibernacula in the province where the species has not yet been detected (Environment Canada 2015). Some of these hibernacula may provide suitable habitat for the species; in New York State, radio-tracking studies have identified many individuals hibernating in locations within hibernacula where they were not visible to the biologist (C. Herzog, pers. comm. 2015). There could also be small fissures or crevices in rocky areas that have not yet been identified as hibernacula for bats because they may be easy to overlook, difficult to survey and not able to be entered by humans to visually confirm use.
In addition, maternity habitat used by the species in Ontario is almost entirely unknown, based on the difficulties in detecting the species through traditional survey methods and the likelihood that they primarily use different habitats for maternity roosting than other species of bats. Alternative survey methods are likely to be required, e.g. mist-netting and crevice searches in talus slopes, rock barrens, and other rocky habitats (Whitby et al. 2013, Moosman et al. 2015, Bruce and Sadowski 2015). Novel methods, such as the use of trained scent dogs (Chambers et al. 2015), could also be explored.
Foraging and home range sizes
There are few studies on foraging areas and home range sizes available (Johnson et al. 2009, D. Yates and T. Divoll, in prep.). Specific foraging habitats and home range sizes in Ontario have not been identified. It will be important to identify whether foraging and home range sizes for Eastern Small-footed Myotis are similar in Ontario to elsewhere in the species' range.
Habitat management
The amount of access, and consequently human disturbance, to Eastern Small-footed Myotis hibernacula and summer habitats is unknown in the province, and there is no analysis of the number of sites already altered or destroyed. This information would help inform future management of sites.
1.8 Recovery actions completed or underway
The following sections outline recovery actions for the species which have already been completed or are currently underway. These actions are primarily related to inventory and monitoring, monitoring of threats or potential threats, threat mitigation measures, and current protection of the species or its habitat.
Inventory and monitoring
The North American Bat Monitoring Program (NABat) was established in 2015 in order to "create a continent-wide program to monitor bats at local and regional scales that will provide reliable data to promote effective conservation decision-making and the long-term viability of bat populations across the continent" (Loeb et al. 2015, p. ii). The NABat program involves the collection of data on winter hibernation counts, maternity colony counts, mobile acoustic surveys on road transects and stationary acoustic surveys. It includes detailed, standardized survey methods, a data management system and statistical approaches to analyze data. The NABat program identifies that the following survey types should be used to monitor Eastern Small-footed Myotis (Loeb et al. 2015):
Summer habitats
- Stationary acoustic surveys (primary/preferred)
- Mobile acoustic transect surveys (less preferred)
- Roost surveys (not applicable)
Winter habitats
- Hibernacula counts (primary/preferred)
- Stationary acoustic surveys (not applicable)
- Mobile acoustic transect surveys (not applicable)
Targeted inventory and monitoring efforts for Eastern Small-footed Myotis were initiated by the OMNRF in central Ontario in 2014 (Bruce and Sadowski 2015). The objective was to investigate methods for developing a standardized survey protocol to identify summer habitats. Acoustic monitoring was done, followed by ground searches (rock flipping) where the monitoring identified the presence of Myotis species. These surveys were undertaken at Kawartha Highlands and Queen Elizabeth II Wildlands Provincial Parks. Sites were identified based on land cover classes and the use of aerial imagery; factors considered included availability of loose rock and crevices for day roosting, proximity to forest cover and water, degree of solar exposure, ease of access extent of public use and access for equipment security. These preliminary efforts did not result in any confirmed observations of Eastern Small-footed Myotis, although suspected individuals may have been encountered (Section 1.4). However, the protocol is likely to benefit from further refinement and would need to be implemented to a greater extent before absence at survey sites can be confirmed (L. Bruce, pers. comm. 2015).
The OMNRF is conducting an inventory of cave sites, an important first step in identifying areas in need of further survey, as well as acoustic and harp trap capture surveys at hibernacula in caves and mine adits and tunnels). Sites were surveyed April 2016 for spring emergence (M. Browning, pers. comm. 2016).
In 2015, a set of 30 randomly-selected 10km x 10km squares were selected by the OMNRF for monitoring.efforts. Ten of the squares were surveyed following the protocol outlined for NABat in 2015. Where squares contain rock barrens or talus slope, at least one of the stationary acoustic survey points are or will be located in this habitat type, if possible, to help determine presence/absence (M. Browning pers. comm. 2016). These efforts will help to improve our understanding of the distribution of the species in the province and target areas for further study.
Neighbourhood Bat Watch is a citizen science project that encourages people across Quebec, Ontario, and Manitoba to locate, monitor and submit data on bat maternity colonies. The goals of the project are to help monitor bat population trends, raise public awareness, and encourage the building of artificial bat houses (MFFPQ et al. undated). While Eastern Small-footed Myotis maternity colonies are less frequently found in buildings than Big Brown Bat and Little Brown Myotis colonies (see Section 1.4), it is possible that previously unknown building colonies of the species may be monitored through this project.
White-nose syndrome response
The Canadian Wildlife Health Cooperative developed A National Plan to Manage White Nose Syndrome in Bats in Canada which was adopted by the Canadian Wildlife Directors Committee in 2015. This plan builds on the previous management plan (IWNSC 2012) and outlines specific goals and action items established by the following Technical Working Groups:
- Communication and Outreach;
- Data Management;
- Mitigation;
- Population Monitoring; and
- Surveillance and Diagnostics.
A number of comprehensive goals, targets, and action items are outlined within this document, which will serve to address potential recovery actions associated with the threat of WNS.
A Western Canada White Nose Syndrome Transmission Prevention communication pamphlet was also created by the Western Bat Working Group to increase awareness among recreational cavers and other users of potential bat hibernacula of the threat posed to bats from transmission of WNS from infected to uninfected sites (WBWG 2015).
In addition, the Ontario Ministry of Natural Resources and Forestry (OMNRF) developed Ontario’s White-nose Syndrome Response Plan, in collaboration with the CWHC and the Ontario Ministry of Northern Development and Mines, which identifies the risks to bat populations in the province and allows for coordination across agencies relating to prevention, surveillance and research. The plan also identifies the need to collaborate with affected stakeholders and the public to ensure that preventative steps and potential response actions are effective in supporting the conservation of Ontario’s hibernating bats. The plan will be periodically reviewed to accommodate changes in approach, organization, or emerging scientific information (OMNRF 2015a).
Monitoring impacts from wind energy projects
The Canadian Wind Energy Association, Canadian Wildlife Service (Environment Canada), Bird Studies Canada, and OMNRF have implemented an initiative to track mortalities of birds and bats, including Eastern Small-footed Myotis, in a central database called the Wind Energy Bird and Bat Monitoring Database. Wind energy project operators, or those working on their behalf, can enter data obtained from bird and bat mortality monitoring into a standardized format that allows for data to be analyzed and summaries produced of the results from monitoring. This will help increase understanding of the impacts of wind energy facilities on bats and guide future improvements to the wind energy project approvals process (Bird Studies Canada 2015).
Mitigation
One of 13 known hibernacula/swarming sites for Eastern Small-footed Myotis in Ontario has a bat-friendly gate installed at the entrance to restrict access (L. Hale, pers. comm. 2015), which will control access to the site to minimize human disturbance and prevent human-related transmission of WNS between sites.
Protection of species and habitat
Eastern Small-footed Myotis and its habitats are currently protected in the following ways:
- The species is a Specially Protected Mammal under Schedule 6 of the Fish and Wildlife Conservation Act, 1997.
- The Eastern Small-footed Myotis is listed as endangered in Ontario and therefore the species and its habitat are automatically protected under the ESA.
- Under the Provincial Policy Statement, 2014, development and site alteration are not permitted in habitat of endangered or threatened species unless in accordance with the ESA.
- Wetlands are also protected under various pieces of legislation, including the Crown Forest Sustainability Act, the Provincial Policy Statement, 2014, and the Development, Interference with Wetlands and Alterations to Shorelines and Watercourses regulations under the Conservation Authorities Act, for example.
2.0 Recovery
2.1 Recovery goal
The recovery goal for Eastern Small-footed Myotis is to maintain a self-sustaining population and to maintain sites currently and historically used for swarming, hibernation or maternity roosting in the province, unless the habitat is no longer suitable.
The number of hibernacula and maternity sites should be measured against those outlined in Section 1.3 and, in the future, should be tracked through element occurrences identified by NHIC to be swarming sites, hibernacula or maternity sites.
The following factors were considered in identifying the recovery goal for Eastern Small-footed Myotis in Ontario.
- Unlike for Little Brown Myotis, Northern Myotis, and Tri-colored Bat (Environment Canada 2015), the extent to which Eastern Small-footed Myotis may be affected by WNS is unknown.
- Estimates of Eastern Small-footed Myotis population sizes at hibernacula have traditionally fluctuated significantly, making both short- and long-term monitoring of population sizes more difficult for this species, and a benchmark for current population size is not available.
- Historical survey methods employed for bats may not have sufficiently identified the number of sites historically and currently used for swarming, hibernation, and maternity roosting by Eastern Small-footed Myotis, as the species is typically more difficult to locate than other bat species based on its roosting and hibernation preferences. Alternate survey methods may need to be implemented for monitoring for Eastern Small-footed Myotis hibernacula and maternity roosts in the future.
- Swarming, hibernation, and maternity sites are potentially limiting factors in the province, but the specific reasons for this have not yet been determined and additional research is needed.
The recovery goal outlined above was developed to acknowledge that Eastern Small-footed Myotis has historically been uncommon in the province and that under the threat of WNS and the observed mortality patterns from WNS of other bat species to date, it may not be realistic to expect population size and distribution increases in Ontario. Effective monitoring of this species including the effects of WNS and other threats will be key to ensuring the recovery goal is met.
2.2 Protection and recovery objectives
Table 3. Protection and recovery objectives
|
No. |
Protection or Recovery Objective |
|---|---|
|
1 |
Inventory and monitor abundance of known populations of Eastern Small-footed Myotis in Ontario. |
|
2 |
Conduct surveys in suitable habitat for the species to identify its distribution, range and abundance in Ontario. |
|
3 |
Increase understanding of Eastern Small-footed Myotis ecology in Ontario through inventory, monitoring, and research. |
|
4 |
Monitor the impacts of threats on populations and increase understanding of the effects of white-nose syndrome on the species. |
|
5 |
Protect and maintain, enhance or restore suitability of summer and winter habitats. |
|
6 |
Promote stewardship, education, and increased awareness of Eastern Small-footed Myotis, other rare and at-risk bat species, and their habitats and threats. |
2.3 Approaches to recovery
Tables 4. Approaches to recovery of the Eastern Small-footed Myotis in Ontario
|
Relative Priority |
Relative Timeframe |
Recovery Theme |
Approach to Recovery |
Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
|
Critical |
Short-term |
Inventory and Monitoring |
1.1 Identify and prioritize existing habitat for inventory and monitoring.
|
Knowledge gaps: Abundance and Population Viability |
|
Critical |
Short-term |
Inventory, Monitoring and Assessment |
1.2 Inventory and monitor species and its habitat.
|
Threats: White-nose Syndrome Knowledge gaps: Abundance and Population Viability |
|
Relative Priority |
Relative Timeframe |
Recovery Theme |
Approach to Recovery |
Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
|
Critical |
Short-term |
Inventory and Monitoring |
2.1 Identify and prioritize suitable habitat for inventory and monitoring.
|
Knowledge gaps: Distribution Habitat Population Viability |
|
Critical |
Short-term |
Inventory, Monitoring and Assessment |
2.2 Inventory and monitor habitat.
|
Threats: White-nose Syndrome Knowledge gaps: Distribution Abundance and Population Viability Habitat |
|
Necessary |
Short-term |
Protection, Inventory and Monitoring |
2.3 Develop and implement a standardized survey protocol for Eastern Small-footed Myotis.
|
Knowledge gaps: Distribution Abundance Population Viability |
|
Relative Priority |
Relative Timeframe |
Recovery Theme |
Approach to Recovery |
Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
|
Critical |
Short-term |
Inventory, Monitoring and Assessment, and Research |
3.1 Research summer roosting ecology, including:
|
Threats: Alteration of Habitat Knowledge gaps: Biology Distribution Habitat |
|
Critical |
Short-term |
Inventory, Monitoring and Assessment, and Research |
3.2 Research home range sizes and foraging ecology, including identification of:
|
Threats: White-nose Syndrome Alteration of Habitat Wind Energy Knowledge gaps: Habitat Foraging and Home Range Sizes |
|
Critical |
Short-term |
Protection, Inventory and Monitoring, Research |
3.3 Describe and define both previously known and new summer habitats (including ELC vegetation communities) used by Eastern Small-footed Myotis in Ontario for roosting and foraging.
|
Threats: Toxins Knowledge gaps: Habitat Distribution Abundance and Population Viability |
|
Necessary |
Short-term |
Protection, Inventory and Monitoring |
3.4 Describe population structure (sex, age) within known and any new winter and summer habitats used by the species. |
Knowledge gaps: Biology Distribution Abundance and Population Viability |
|
Necessary |
Short-term |
Inventory, Monitoring and Assessment, and Research |
3.5 Research movement and dispersal within summer habitats and between summer and winter habitats to determine the potential for gene flow.
|
Knowledge gaps: Biology Habitat Abundance and Population Viability |
|
Necessary |
Short-term |
Inventory, Monitoring and Assessment, and Research |
3.6 Research hibernation ecology, including:
|
Threats: White-nose Syndrome Knowledge gaps: Biology Habitat Population Viability |
|
Relative Priority |
Relative Timeframe |
Recovery Theme |
Approach to Recovery |
Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
|
Critical |
Short-term |
Monitoring and Research |
4.1 Monitor known hibernacula for signs/effects from WNS and identify opportunities to incorporate the use of Passive Integrated Transponders (PIT tags) to monitor individuals/populations, in accordance with Ontario’s White-nose Syndrome Response Plan. |
Threats: White-nose Syndrome Knowledge gaps: Biology Abundance and Population Viability |
|
Critical |
Ongoing |
Monitoring |
4.2 Carry out monitoring of populations in accordance with Ontario’s White-nose Syndrome Response Plan, with species-specific efforts whenever possible.
|
Threats: White-nose Syndrome Knowledge gaps: Abundance and Population Viability |
|
Critical |
Ongoing |
Assessment and Research |
4.3 Assess and support management approaches for WNS, accommodating scientific research and incorporating the use of emerging tools and mechanisms to reduce impacts from WNS, if appropriate, in accordance with Ontario’s White-nose Syndrome Response Plan.
|
Threats: White-nose Syndrome Knowledge gaps: Abundance and Population Viability Habitat Management |
|
Critical |
Short-term |
Monitoring and Research |
4.4 Undertake research targeting maternity colonies to assess female survivorship and reproductive success, in accordance with Ontario’s White-nose Syndrome Response Plan. |
Threats: White-nose Syndrome Knowledge gaps: Abundance and Population Viability |
|
Necessary |
On-going |
Monitoring and Assessment, Management, Stewardship |
4.5 Identify the extent to which human disturbance to populations occurs.
|
Threats: White-nose Syndrome Human Disturbance Knowledge gaps: Habitat Management |
|
Necessary |
Ongoing |
Monitoring and Assessment |
4.6 Investigate opportunities and assess the feasibility of using landscape-scale monitoring techniques to contribute species-specific data to monitor long-term population trends, in accordance with Ontario’s White-nose Syndrome Response Plan. |
Threats: White-nose Syndrome Toxins Knowledge gaps: Distribution Abundance and Population Viability |
|
Beneficial |
Ongoing |
Monitoring and Assessment |
4.7 Continue to support and improve data collection on wind turbine mortalities in the province.
|
Threats: Interactions with Wind Turbines Knowledge gaps: Distribution |
|
Beneficial |
Ongoing |
Research |
4.8 Support research on toxins that may pose a threat to bats and their prey.
|
Threats: Toxins |
|
Relative Priority |
Relative Timeframe |
Recovery Theme |
Approach to Recovery |
Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
|
Beneficial |
Ongoing |
Protection, Management, Education and Outreach, Stewardship |
5.1 Work collaboratively with stakeholders, including landowners, conservation organizations, First Nations, and recreation groups to protect known sites, and restore, where possible.
|
Threats: White-nose Syndrome Alteration of Habitat Human Disturbance Knowledge gaps: Habitat Management |
|
Beneficial |
Ongoing |
Management, Education and Outreach, Stewardship |
5.2 Explore the use of artificial roost structures as a method to supplement availability of roosting habitat for Eastern Small-footed Myotis, particularly in the vicinity of hibernacula, and to increase awareness and stewardship. |
Threats: Alteration of Habitat Human Disturbance Knowledge gaps: Habitat Management |
|
Relative Priority |
Relative Timeframe |
Recovery Theme |
Approach to Recovery |
Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
|
Beneficial |
Ongoing |
Protection, Education and Outreach |
6.1 Promote the implementation of WNS decontamination protocol amongst the caving, climbing, and research communities, along with avoidance of disturbance at hibernacula for these communities and the general public.
|
Threats: White-nose Syndrome Human Disturbance Knowledge gaps: Habitat Management |
|
Beneficial |
Ongoing |
Inventory, Education and Outreach, Communications, Stewardship |
6.2 Develop and implement education programs and information for the general public and recreational caving community on identification and reporting observations of Eastern Small-footed Myotis, in consultation with relevant stakeholders.
|
Threats: White-nose Syndrome Human Disturbance Knowledge gaps: Distribution Abundance |
|
Beneficial |
Ongoing |
Education and Outreach, Communications, Stewardship |
6.3 Assist the Communication and Outreach Technical Working Group operating under the National Plan to Manage White-nose Syndrome in Bats in Canada to establish mechanisms for the public to report observations of bats.
|
Threats: White-nose Syndrome Human Disturbance Knowledge gaps: Distribution Abundance Habitat |
|
Beneficial |
Short-term |
Education and Outreach, Communications, Stewardship |
6.4 Establish Best Management Practices (BMPs) in consultation with industry and other stakeholders for pest-control businesses and associations to address removal of at-risk bat species in buildings.
|
Threats: Alteration of Habitat Knowledge gaps: Distribution Habitat Abundance and Population Viability Habitat Management |
|
Beneficial |
Short-term |
Education and Outreach, Communications, Stewardship |
6.5 Establish Best Management Practices (BMPs) in consultation with aggregates and mining associations for addressing summer roosting or hibernation in active or abandoned sites.
|
Threats: Alteration of Habitat Knowledge gaps: Distribution Abundance and Population Viability Habitat Habitat Management |
Narrative to support approaches to recovery
Critical knowledge gaps restrict the ability to identify sites currently occupied by Eastern Small-footed Myotis in the province. Based on the species' rarity and the potential magnitude of the threats it faces, the short-term recovery approaches are to focus on:
- accurately identifying the baseline distribution, population, and habitats currently used by the species in the province (Approaches 1.1, 1.2, 2.1, 2.2, 3.1 to 3.6, 4.1 to 4.4, and 6.1 to 6.4), and
- improving knowledge of species ecology in order to effectively address threats (Approaches 3.1 to 3.6).
Knowledge and experience gained through efforts to identify habitats used by the species will help inform development of a survey protocol that can be used to improve the efficiency of efforts to identify additional sites and protect the species from threats such as alteration of habitat, human disturbance, and wind energy production (Approach 2.3).
At the same time, mitigating potential threats (Approaches 4.3, 6.3, and 6.4), actively conducting research (Approaches 4.1 to 4.8), and developing long-term management activities for habitats and threats (Approaches 4.1 to 4.8, 5.1, 5.2, 6.3 and 6.4) are needed to ensure the goal will be met.
2.4 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of Natural Resources and Forestry on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the author will be one of many sources considered by the Minister when developing the habitat regulation for this species.
Foraging habitat
Based on the lack of data available for Ontario and the broad nature of foraging habitat identified elsewhere in the range of Eastern Small-footed Myotis including forests, waterbodies and wetlands, it is not possible to identify specific areas of foraging habitat that are important to the recovery of this species. As a result, sufficient area to allow appropriate foraging resources during critical life cycle periods (swarming/hibernation and the maternity period) is included within the recommended areas for habitat regulation as outlined below.
Hibernacula and swarming sites
Suitable hibernacula are critical to ensure the overwinter survival of Eastern Small-footed Myotis during hibernation, a time when bats are solely dependent upon stored fat reserves for a period of several months. Hibernacula are known to be a limiting factor on the landscape, as temperature and humidity within these sites must meet the requirements of the species throughout the winter (see Section 1.4 for a detailed description of hibernacula). Swarming activity consists of congregations of bats flying in circles at the entrance to hibernacula, or in and out of a hibernaculum or potential hibernation feature, when mating occurs before bats prepare to hibernate. Some bat swarming sites are not sites of hibernation; however, swarming sites are usually indicative of a hibernaculum or at a minimum, are important sites for gene flow during mating. Because of the importance of both site types and their associated activities, as well as the difficulty in identifying potential crevice-based hibernacula, both swarming and hibernation sites should be prescribed as habitat in a habitat regulation.
In developing a habitat regulation for Eastern Small-footed Myotis hibernacula and swarming sites, the following should be considered.
- Bats entering into hibernation must be in prime condition with a high ratio of body fat. They are also at their weakest in the spring immediately following hibernation, when fertilization occurs and females must give birth shortly after. Suitable foraging, drinking and roosting habitats in proximity to hibernacula are depended upon in the fall in order to build up fat reserves which support individuals during a vulnerable period in the species' life cycle (hibernation), when food is not available. As a result, bats are required to forage intensively prior to hibernation.
- Only two studies are available that identify home range sizes. One study (D. Yates and T. Divoll, in prep.), from Maine, assesses the home ranges of juveniles and adult males during the period of July to October, which significantly overlaps with the fall time period when bats must forage for adequate resources to survive the winter. Home ranges were up to 1,489 ha in size (96 to 1,489 ha, mean 652 ha ±488 ha ), with juveniles having both the smallest and largest home ranges. Core use areas (50 % isopleth) for all bats tracked were 8 ha to 293 ha (average size of 117 ha ±92 ha). This suggests that large areas are relied upon by bats prior to entering hibernation, when it is critical for their survival to develop sufficient fat reserves. The other study, from Maryland, (Johnson et al. 2009) identifies the home range size for females emerging from hibernation in the spring. In this study, open sunny rock roosting habitat, abundantly available less than 100 m from the hibernaculum, was used by females for roosting. Home ranges where foraging primarily occurred were less than 100 ha in size, significantly smaller than the home ranges identified in Maine. This is likely a result of high site fidelity by females associated with the upcoming maternity period, or may be related to weaker body condition in spring.
- Hibernacula support individuals from multiple maternity colonies and are likely to support more individuals than any one maternity site. Also, bats of multiple species congregate at hibernacula and swarming sites. As a result, they are likely to compete for foraging resources and may be forced to travel further in order to forage adequately. It is therefore recommended that a larger area be considered when identifying habitat associated with a hibernaculum or swarming site in order to provide greater available foraging resources needed by individuals during the fall, as well as by the collective number of bats using the site.
- The effects of the removal of foraging or roosting resources surrounding hibernacula are largely unknown. The removal of foraging resources may impact insect availability (USFWS 2013), which could add stress to bats during the swarming season by forcing them to travel greater distances in order to build up sufficient fat reserves. In addition, industrial activities such as forestry conducted near, even if not overlapping, hibernacula may alter microclimate, air flow or hydrology within them, which could make them unsuitable for use by bats (Environment Canada 2015). Currently, all known hibernacula/swarming sites are situated in, or in close proximity to, large forests.
- Based on the roosting habits of Eastern Small-footed Myotis across its range, this species could likely use a variety of natural and anthropogenic roosting resources in proximity to hibernacula during the fall and spring or even during warm spells in winter; however, these potential roosting sites and their prevalence of use are unknown.
- Hibernacula and swarming sites are very limited in Ontario. There are only 13 sites known for this species in the province, one of which is no longer suitable. Data also indicate that each of these sites is relied upon by only a small number of individuals (Section 1.3) because of the general rarity of the species. This means each site is likely to be extremely important for the survival and recovery of Eastern Small-footed Myotis in the province.
- Because of the limited availability of hibernacula on the landscape and the consistent provision of the stable microclimates required for hibernation, individual hibernacula may be used by Eastern Small-footed Myotis for decades or for long as conditions remain suitable and the site is not altered by human disturbance or structural collapse. However, the number of individuals using a site in any given year may fluctuate.
- Identifying a larger area to support foraging and roosting resources in proximity to hibernacula and swarming sites follows a precautionary approach for a species that is poorly understood. Research is lacking on: (1) identification of home range sizes during specific time periods in the species' life cycle; and (2) effects that removal of foraging or roosting resources near hibernacula may have on the species and/or its use of a particular hibernaculum. Given these unknowns and the overall rarity of Eastern Small-footed Myotis, a precautionary approach is warranted.
It is recommended that, until knowledge gaps are addressed, the following criteria be used to identify regulated habitat for hibernation and swarming.
- All known hibernacula and swarming sites for Eastern Small-footed Myotis (historical and current) are recommended to be prescribed as habitat in a habitat regulation, unless a site is no longer suitable.
- It is recommended that the area within 610 m of a hibernaculum and/or swarming site used by Eastern Small-footed Myotis be identified as habitat in the habitat regulation. This distance is based on the average core use area of 117 ha identified between July and October in Maine (described in Section 1.4) as it may represent the area most likely to be relied upon by individuals prior to hibernation (D. Yates and T. Divoll, in prep). The area within 610 m of a hibernaculum or swarming site is equivalent to a 117-ha area based on a point centroid with a circular buffer.
- The 610 m distance should extend from all known or suspected entrances of a hibernaculum or the total underground extent of a hibernaculum, if known, and/or the concentrated area of swarming activity. If one entrance is known, the entrance would represent a point in the center of a circle with a radius of 610 m. If multiple entrances are known, but the full underground extent of the site is not known, a polygon should be drawn between the entrance locations, using straight lines. For sites with multiple entrances, or where the underground extent of a hibernaculum is known, an area extending 610 m from the boundary of the known hibernaculum polygon should be included in the site area. This method is recommended in order to protect the hibernaculum as the core feature of the habitat, supported by adequate foraging areas and alternate fall and spring roosting sites in proximity to the hibernaculum. It is also recognized that the extent of caves or abandoned mines is generally poorly understood and also, how conditions within them (e.g., temperature, humidity and air flow) may be affected by nearby surface or ground activities.
- Within this area, potential foraging and roosting resources should be protected if they are used by foraging or roosting Eastern Small-footed Myotis between August 1 and May 15. Foraging resources include forests, wetlands, and waterbodies. Roosting resources include rocky habitats, both natural and anthropogenic, and structures unoccupied by humans such as bridges, barns, sheds and empty houses.
- The presence of one or more Eastern Small-footed Myotis within or at the entrance to a cave or abandoned mine (as described in Section 1.4) or any other site reasonably believed to contain hibernating individuals between August 1 and May 15 should be the basis for identifying a hibernaculum or swarming site. This recognizes that population sizes at hibernacula may fluctuate within and between years, and may be as low as zero individuals detected in a given year.
Maternity sites
Maternity sites are relied upon by female Eastern Small-footed Myotis in the spring and early summer, not long after emerging from hibernation, when they are recuperating from poor body condition. Females, and typically at least some males, are likely to congregate in suitable sites containing similar characteristics, such as sufficient protection from predators, abundance of roosting locations, and adequate solar exposure to achieve appropriate body temperatures.
In developing a habitat regulation for Eastern Small-footed Myotis maternity sites, the following should be considered.
- Eastern Small-footed Myotis change roost sites frequently, and are likely to use a variety of roosting locations within a given maternity site rather than one particular crevice.
- Where studies have identified sex and age of individuals roosting in a site during the maternity period, males have been observed to roost in the same areas as females (O'Keefe and Lavoie 2010, Johnson et al. 2011, Whitby et al. 2013, Moosman et al. 2015).
- Maternity roosting habitat, typically crevices associated with rocky areas or buildings/other structures, is different from foraging habitat which is typically forests, waterbodies and wetlands, and water is a critical resource to lactating female bats; therefore, areas surrounding maternity sites should provide adequate foraging and drinking resources to support the associated population using a maternity site.
- There are no data available on home range sizes for females and insufficient data for juveniles during the maternity period. However, data are available for adult females emerging from hibernation (prior to the maternity period) and for juveniles during the latter portion of the maternity period and continuing into the summer and fall. In Maryland, home range sizes of adult females after hibernation were a maximum of 100 ha (Johnson et al. 2009). In Maine, home range sizes of juveniles in the summer and fall (between July and October) were a minimum of 96 ha (D. Yates and T. Divoll in prep.). The Maine home range sizes could represent: (1) the area of habitat during the latter portion of the maternity period, (2) dispersal; and/or (3) fall swarming, and therefore may represent substantially larger areas than typically used by adult females and juveniles during the maternity period. As a result of these studies, it is appropriate to use an estimated home range of 100 ha to identify the area of foraging resources needed to support a maternity site, and until additional data specific to the maternity period is available.
It is recommended that, until knowledge gaps are addressed, the following criteria be used to identify regulated habitat for the maternity period.
- Maternity sites should be identified based on any location where an observation of roosting Eastern Small-footed Myotis has been made between May 15 and July 31 and the observation is linked to an identifiable roosting feature, e.g., a rock feature or in a building, unless the feature is confirmed not to support pregnant or lactating females or juveniles. This precautionary approach is warranted since only one confirmed maternity site is currently known in the province. Future identification of maternity sites should focus on the identification of adult females or juveniles day-roosting in the area between May 15 and July 31.
- It is recommended that maternity sites in natural communities (e.g., cliff, talus, rock barren, alvar, or other rocky areas occurring within meadow, shrub, or forest habitat) be described based on the Ecological Land Classification (ELC) methods for southern Ontario (Lee et al. 1998) or ELC methods for the Far North, the Boreal, the Great Lakes-St. Lawrence, or the South (Operational Drafts or their successors, as applicable and as they may become available) (Banton et al. 2009), and identified to at least the ecosite level. The portion of contiguous ecosite containing roosting Eastern Small-footed Myotis, to a maximum of 100 ha, should be included as the site. The 100-ha size is based on the species' tendency to be highly mobile, changing roost sites frequently within an area, and also recognizing that the likelihood of detecting all specific roosts being used is low. The boundaries of the roosting area may be further refined if sufficient information is available to clearly refine the core roosting area used (i.e., through a radio-telemetry study).
- For anthropogenic sites such as buildings, bridges, rip-rap, and quarries, the contiguous area providing the same type of roosting habitat used by the species should be identified. Examples are: a contiguous extent of rip rap, a cut rock face or rock pile; an entire bridge; or a building.
- For maternity sites where the ecosite is less than 100 ha in size, it is recommended that the area no more than 565 m beyond the boundary of a maternity site be identified as habitat in the habitat regulation. The area should be no greater than 100 ha , including the ecosite plus the surrounding area. This is recommended to protect foraging habitat used during the maternity period. It is based on the maximum home range size identified for adult females emerging from hibernation between March and April in Maryland (100 ha , Johnson et al. 2009) and the minimum home range sized used by juveniles during the summer and fall in Maine (96 ha , D. Yates and T. Divoll in prep). The 565 m distance from the maternity site represents a 100-ha area based on a point centroid with a circular buffer.
- Within this 100-ha area, potential foraging resources should be protected if used by foraging Eastern Small-footed Myotis between May 15 and July 31. Foraging resources include forests, wetlands, and waterbodies.
- Caves and abandoned mines do not provide maternity habitat in Ontario, and the observation of Eastern Small-footed Myotis within caves or abandoned mines between May 15 and July 31 should not constitute identification of a maternity site. Also, anthropogenic objects such as vehicles or mobile homes that could be moved should not be considered maternity habitat.
Future research on the roosting and foraging habitats used by the species (particularly during the maternity period), dispersal distances, home range sizes and roosting ecology, as recommended in this recovery strategy, may inform a larger or smaller extent of area that should be included in the habitat regulation.
Glossary
Adit: A horizontal (or near-horizontal) mine entrance or passage.
Aerial insectivore: A species that primarily forages for aerial insects, and does so in flight.
Barotrauma: Trauma to the lungs of a mammal associated with a sudden change in air pressure, such as the low-pressure zone found at the tips of moving wind turbine blades.
Committee on the Status of Endangered Wildlife in Canada (COSEWIC): The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
Committee on the Status of Species at Risk in Ontario (COSSARO): The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
Conservation status rank: A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperilled
2 = imperilled
3 = vulnerable
4 = apparently secure
5 = secure
Element occurrence: An area of land or water where a species is or was present; represents an area important to the conservation of the species. For Eastern Small-footed Myotis, includes areas thought to represent a bachelor colony, breeding area, maternity colony, hibernaculum, or recurring/migrating non-breeding population (NatureServe 2015).
Endangered Species Act, 2007 (ESA): The provincial legislation that provides protection to species at risk in Ontario.
Extant (element occurrence): An area of land or water where a species is still in existence.
Frequency of maximum energy: The sound frequency measured in kilohertz (kHz)) of an individual bat vocalization where the greatest energy occurs. It is typically measured on a search-phase call to aid in identification of a bat call sequence to species level.
Hibernaculum (pl. hibernacula): A location with specific and relatively stable atmospheric conditions (temperature and humidity) where animals overwinter. These are often sites where a species will congregate.
Hibernation: A state of inactivity and greatly reduced metabolism which occurs seasonally for a species that regulates its body temperature primarily through its own bodily functions, rather than by ambient temperature. Hibernation is characterized by low body temperature, slower breathing, reduced heart rate, and low metabolic activity.
Historical (element occurrence): An area of land or water where a species was present historically (not observed in the last 20 to 40 years, NatureServe 2015). In this Recovery Strategy, Historical refers to dates prior to 1995.
Karst: A landscape formed in areas of soluble rock such as limestone, dolostone or gypsum. It is characterized by underground drainage that results in features such as sinkholes, caves, and disappearing streams.
Low Frequency: The lowest sound frequency, of an individual bat vocalization, measured in kilohertz (kHz). It is typically measured on a search-phase call to aid in identification of a bat call to species level.
Phenology: The study of life cycle events of a species and how they are influenced by periodic variations in climate and habitat, such as those brought on by seasonality.
Pd (Psaccesseseudogymnoascus destructans): A fungus that causes white-nose syndrome and results in lesions in the skin and wing membranes of bats.
Rip rap: Rock or other material used to armour shorelines or structures to prevent scour erosion from water or ice.
Species at Risk Act (SARA): The federal legislation that provides protection to species at risk in Canada. This act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
Species at Risk in Ontario (SARO) List: The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
Swarming: The activity in which male and female bats gather to mate and prepare for hibernation. Swarming usually occurs at the entrance to a hibernaculum.
White-nose Syndrome (WNS): A disease of hibernating bats caused by the fungus Pseudogymnoascus destructans (Pd) that has caused widespread mortality of cave-hibernating bats throughout eastern North America, where it was first discovered in New York State in 2006. Signs of white-nose syndrome include a white substance on the muzzle, ears, and wings of an infected bat during hibernation. Mortality from white-nose syndrome is thought to be a result of wing lesions leading to dehydration that causes excessive arousal and depleted fat reserves, which can subsequently cause emaciation and death.
References
Adams, R.A. and M.A. Hayes. 2008. Water availability and successful lactation by bats as related to climate change in arid regions of western North America. Journal of Animal Ecology 77:1115-1121.
Amelon, S. and D. Burhans. 2006. Conservation Assessment: Myotis leibii (Eastern Small-footed Myotis) in the Eastern United States. Pp. 57-68 in F.R. Thompson, III (ed.). Conservation Assessments for Five Forest Bat Species in the Eastern United States. United States Department of Agriculture, Forest Service. General Technical Report NC-260.
Arnett, E.B., W.K. Brown, W.P. Erickson, J.K. Fiedler, B.L. Hamilton, T.H. Henry, A. Jain, G.D. Johnson, J. Kern, R.R. Koford, C.P. Nicholson, T.J. O'Connell, M.D.Piorkowski, and R.D. Tankersley Jr. 2008. Patterns of bat fatalities at wind energy facilities in North America. Journal of Wildlife Management 72(1):61-78.
Arnett, E.B. and E.F. Baerwald. 2013. Impacts of wind energy development on bats: implications for conservation. Pp. 435-456 in R.A. Adams and S.C. Pedersen (eds.). Bat Evolution, Ecology, and Conservation, Springer Science+Business Media, New York.
Arnett, E.B., E.F. Baerwald, F. Mathews, L. Rodrigues, A. Rodríguez-Durán, J. Rydell, Villegas-Patraca, and C.C. Voigt. 2016. Impacts of wind energy development on bats: a global perspective. Pp. 295-323 in Voigt, C.C. and T. Kingston (eds.). Bats in the Anthropocene: Conservation of Bats in a Changing World. Springer International, DOI 10.1007/978-3-319-25220-9.
Ballman, A., pers. comm. 2015. Email correspondence to J. Segers and C. Humphrey. February 2016. Wildlife Disease Specialist, United States Geological Survey National Wildlife Health Centre. Madison, Wisconsin.
Banton, E., J. Johnson, H. Lee, G. Racey, P. Uhlig, and M. Wester. 2009. Ecosites of Ontario, Operational Draft. Ecological Land Classification Working Group. v + 91pp.
Barclay, R.M.R. 1999. Bats are not birds: a cautionary note on using echolocation calls to identify bats: a comment. Journal of Mammalogy 80(1):290-296.
Baerwald, E.F., G.H. D'Amours, B.J. Klug, and R.M.R. Barclay. 2008. Barotrauma is a significant cause of bat fatalities at wind turbines. Current Biology 18(16):R695-R696.
Baerwald, E.F. and R.M.R. Barclay. 2009. Geographic variation in activity and fatality of migratory bats at wind energy facililties. Journal of Mammalogy 90:1341-1349.
Banfield, A.W.F.1974. The Mammals of Canada. University of Toronto Press, Toronto, Ontario. xxv + 438 pp.
Barbour, R.W. and W.H. Davis. 1969. Bats of America. The University Press of Kentucky, Lexington, Kentucky. 286 pp.
Bat Conservation and Management, Inc. 2003. Bat inventory for the project lands of the Upper Connecticut River basin. Prepared for the U.S. Army Corps of Engineers New England District. iv +103 pp.
Bayat, S., F. Geiser, P. Kristiansen, and S.C. Wilson. 2014. Organic contaminants in bats: trends and new issues. Environment International 63:40-52.
Best, T.L., and J.B. Jennings. 1997. Myotis leibii. Mammalian Species 547:1-6. Bird Studies Canada, Canadian Wind Energy Association, Environment Canada, and Ontario Ministry of Natural Resources. 2014. Wind energy bird and bat monitoring database: summary of the findings from post-construction monitoring reports. July 2014. 50 pp.
Bird Studies Canada. 2015. Wind energy bird and bat monitoring database. Web site: http://www.bsc-eoc.org/birdmon/wind/main.jsp [accessed November 19, 2015].
Blehert, D.S., A.C. Hicks, M. Behr, C.U. Meteyer, B.M. Berlowski-Zier, E.L. Buckles, J.T.H. Coleman, S.R. Darling, A. Gargas, R. Niver, J.C. Okoniewski, R.J. Rudd, W.B. Stone. 2009. Bat white-nose syndrome: an emerging fungal pathogen? Science 323:10.1126/science.1163874.
Blehert, D.S. 2012. Fungal disease and the developing story of bat white-nose syndrome. PLoS Pathogens 8(7): e1002779. DOI: 10.1371/journal.ppat.1002779.
Boratyński, J.S., C.K.R. Willis, M. Jefimow, and M.S. Wojciechowski. 2014. Huddling enhances survival of hibernating bats by reducing evaporative water loss. Comparative Biochemical Physiology 179:125-132.
Boyles, J.G., J.J. Storm, and V. Brack Jr. 2008. Thermal benefits of clustering during hibernation: a field test of competing hypotheses on Myotis sodalis. Functional Ecology 22(4):632-636.
Brooks, R.T. 2011. Declines in summer bat activity in central New England 4 years following the initial detection of white-nose syndrome. Biodiversity Conservation 20:2537–2541.
Browning, M., pers. comm. 2016. Email correspondence to C. Humphrey. February, April, and July 2016. Restoration and Rehabilitation Research Biologist, Ontario Ministry of Natural Resources and Forestry. Peterborough, Ontario.
Bruce, L., pers. comm. 2015. Email correspondence to C. Humphrey. November 2015 and February 2016. Wildlife Research Technician, Ontario Ministry of Natural Resources and Forestry. Peterborough, Ontario.
Bruce, L. and C. Sadowski. 2015. A preliminary survey of the Eastern Small-footed Bat (Myotis leibii) in central Ontario. Wildlife Research and Monitoring Section, Ontario Ministry of Natural Resources and Forestry. Peterborough, Ontario. 21 pp.
Brunton, F.R. and J.E.P. Dodge. 2008. Karst of Southern Ontario and Manitoulin Island. Ontario Geological Survey, Groundwater Resources Study 5. ISBN 978-1-4249-8376-6.
Butler, B., pers. comm. 2015. Email correspondence to C. Humphrey. November 2015. Bat Conservation and Management Inc., Carlisle, Pennsylvania.
Caldwell, K., pers. comm. 2015. Email correspondence to C. Humphrey. September Mammalogist, Associate Wildlife Biologist, North Carolina Wildlife Resources Commission, Asheville, North Carolina.
Carr, J.A., R.F. Bernard, and W.H. Stiver. 2014. Unusual bat behaviour during winter in Great Smoky Mountains National Park. Southeastern Naturalist 13:N1-N4.
Chambers, C.L., C.D. Vojta, E.D. Mering, and B. Davenport. 2015. Efficacy of scent-detection dogs for locating bat roosts in trees and snags. Wildlife Society Bulletin 39(4):780-787.
Chenger, J. 2008. Summer woodland bat survey: Shaffer Mountain Wind Project. Bat Conservation and Management, Inc. Carlisle, Pennsylvania. 71 pp. In USFWS 2011. 90-day finding on a petition to list the Eastern Small-footed Bat and the Northern Long- eared Bat as Threatened or Endangered. United States Fish and Wildlife Service.
Federal Register 76(125):38095-38106. Chenger, J. 2012. Very quick MYOLEI roosts. Presentation at the Northeast Bat Working Group Annual Meeting, January 11-13, 2013. Carlisle, Pennsylvania.
Chenger, J., pers. comm. 2015. Email correspondence to C. Humphrey. November President, Bat Conservation and Management Inc., Carlisle, Pennsylvania.
CWHC (Canadian Wildlife Health Cooperative). Undated. White Nose Syndrome Specimen Submission Protocol. Canadian Wildlife Health Cooperative. Web site: http://www.cwhc-rcsf.ca/docs/WNS_Specimen_Submission_Protocol.pdf [accessed February 26, 2016].
CWHC (Canadian Wildlife Health Cooperative). 2014. Canadian Bat White-nose Syndrome Necropsy Protocol. Canadian Wildlife Health Cooperative. Web site: http://www.cwhc-rcsf.ca/docs/Canadian%20Bat%20WNS%20Necropsy%20Protocol.pdf [accessed August 17, 2016].
CWHC (Canadian Wildlife Health Cooperative). 2015. A national plan to manage white nose syndrome in bats in Canada. Canada’s Inter-agency White Nose Syndrome Committee, reporting to the Canadian Wildlife Directors Committee. 13 pp.
CWHC (Canadian Wildlife Health Cooperative). 2016. Surveillance Data – White Nose Syndrome. Canadian Wildlife Health Cooperative. Web site: http://www.cwhc-rcsf.ca/data_products_wns.php [accessed February 24, 2016].
Clare, E.L. 2011. Eating local: influences of habitat on the diet of little brown bats(Myotis lucifugus). Molecular Ecology 20:1772-1780.
Coleman, J.T.H. and J.D. Reichard. 2014. Bat White-nose syndrome in 2014: a brief assessment seven years after discovery of a virulent fungal pathogen in North America. Outlooks on Pest Management 25:374-377.
COSEWIC (Committee on the Status of Endangered Wildlife in Canada). 2013.COSEWIC assessment and status report on the Little Brown Myotis Myotis lucifugus, Northern Myotis Myotis septentrionalis and Tri-coloured Bat Perimyotis subflavus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xxiv + 93 pp.
COSSARO (Committee on the Status of Species at Risk in Ontario). 2013. COSSARO Candidate Species at Risk evaluation for Eastern Small-footed Myotis (formerly Eastern Small-footed Bat) (Myotis leibii). Committee on the Status of Species at Risk in Ontario. 12 pp.
Cryan, P., C.U. Meteyer, J. Boyles, and D. Blehert. 2010. Wing pathology of white-nose syndrome in bats suggests life-threatening disruption of pathophysiology. BioMed Central Biology 8:135.
Davies, W. E. 1970. Karstlands and Cavern Areas [map]. P. 77 in United States Department of the Interior, Geological Survey. The National Atlas of the United States of America.
Davis, E. 1931. First Canadian record of Least Brown Bat. The Canadian Field- Naturalist 45(5):118.
Davis, E. 1955. Myotis subulatus leibii found in unusual situations. Journal of Mammalogy 36(1):130.
Dewar, H.J., C.L. Furlonger, and M.B. Fenton. 1985. A survey of the bats of the Carolinian Zone of Ontario. World Wildlife Fund Canada. Toronto. 38 pp.
Divoll, T.J. 2013. Mist-netting, passive ultrasonic detection, and stable isotopes determine community structure and temporal variation in bats (Chiroptera) at Acadia
National Park, Maine. Master of Science Thesis, University of Southern Maine, Portland, Maine, United States of America. 75 pp.
Dobbyn, J.S. 1994. Atlas of the Mammals of Ontario. Federation of Ontario Naturalists, Don Mills, Ontario. viii + 120 pp.
Dodd, L.E., M.J. Lacki, D.R. Cox, and L.K. Rieske. 2014. Prey consumed by bats across central Appalachia prior to detection of white-nose syndrome. Journal of the Kentucky Academy of Science 75(1):85-93.
Dzal, Y., L.A. Hooton, E.L. Clare, and M.B. Fenton. 2009. Bat activity and genetic diversity at Long Point, Ontario, an important bird stopover site. Acta Chiropterologica 11(2):307-315.
Environment Canada. 2015. Recovery Strategy for Little Brown Myotis (Myotis lucifugus), Northern Myotis (Myotis septentrionalis), and Tricolored Bat (Perimyotis subflavus) in Canada [Proposed]. Species at Risk Act Recovery Strategy Series. Environment Canada, Ottawa. ix + 110 pp.
Fagan, K.E., E.V. Willcox, R.F. Bernard, and W.H. Stiver. 2016. Myotis leibii (Eastern Small-footed Myotis) roosting in buildings of Great Smoky Mountains National Park, Tennessee. Southeastern Naturalist 15(2): N23-N27.
Fenton, M.B. 1969. Summer activity of Myotis lucifugus (Chiroptera: Vespertilionidae) at hibernacula in Ontario and Quebec. Canadian Journal of Zoology 47: 597-602.
Fenton, M.B. 1970. Population studies of Myotis lucifugus (Chiroptera: Vespertilionidae) in Ontario. Life Science Contributions of the Royal Ontario Museum 77:1-34.
Fenton, M.B. 1972. Distribution and overwintering of Myotis leibii and Eptesicus fuscus (Chiroptera: Vespertilionidae) in Ontario. Royal Ontario Museum Life Sciences Occasional Papers. 21 8 pp.
Fenton, M.B. and R.M.R. Barclay. 1980. Myotis lucifugus. Mammalian Species 142: 1-8.
Fenton, M.B. and G.P. Bell. 1981. Recognition of species of insectivorous bats by their echolocation calls. Journal of Mammalogy 62(2): 233-243.
Fenton, M.B. and W. Bogdanowicz. 2002. Relationships between external morphology and foraging behaviour: bats in the genus Myotis. Canadian Journal of Zoology 80(6):1004-1013.
Ford, W.M., E.R. Britzke, C.A. Dobony, J.L. Rodrigue, and J.B. Johnson. 2011. Patterns of acoustical activity on bats prior to and following white-nose syndrome occurrence. Journal of Fish and Wildlife Management 2:125-134.
Fraser, E. and C. Davy. 2004. The Bats of Algonquin Park. University of Western Ontario, London, Ontario. 11 pp.
Fraser, E., A. MacKenzie, and C. Davy. 2007. Photo Field Guide to the Bats of Ontario. St. Thomas Field Naturalist Club Inc; St. Thomas, Canada. 40 pp.
Frick, W.F., J.F. Pollock, A.C. Hicks, K.E. Langwig, D.S. Reynolds, G.G. Turner, C.M. Butchkoski, and T.H. Kunz. 2010. An emerging disease causes regional population collapse of a common North American bat species. Science 329(5992):679-682.
Furey, N.M. and P.A. Racey. 2016. Conservation ecology of cave bats. Pp. 463-500 in C.C. Voigt and T. Kingston (eds.). Bats in the Anthropocene: Conservation of Bats in a Changing World. Springer International, DOI 10.1007/978-3-319-25220-9.
Furlonger, C.L., H.J. Dewar, and M.B. Fenton. 1987. Habitat use by foraging insectivorous bats. Canadian Journal of Zoology 65(2):284-288.
Gannon, M.R., C.A. ludica, S. Mistry. 2012. Bats of Pennsylvania. Indiana State University Center for North American Bat Research and Conservation, Publication Number 6. 64 pp.
Griffin, D.R. 1945. Travels of banded cave bats. Journal of Mammalogy 26(1):15-23.
Griffin, D.R. 1971. The importance of atmospheric attenuation for the echolocation of bats (Chiroptera). Animal Behaviour 19:55-61.
Grodsky, S.M., M.J. Behr, A. Gendler, D. Drake, B.D. Dieterle, R.J. Rudd, and N.L. Walrath. 2011. Investigating the causes of death for wind turbine-associated bat fatalities. Journal of Mammalogy 92(5):917-925.
Hallam, T.G. and P. Federico. 2009. Application of dynamic population models to bats. Pp. 177-194 in T.H. Kunz and S. Parsons (eds.). Ecological and Behavioral Methods for the Study of Bats, 2nd ed. Johns Hopkins University Press, Baltimore, Maryland.
Hale, L., pers. comm. 2015. Email correspondence to C. Humphrey. September 2015. Protected Areas Species at Risk Biologist (Acting), Protected Areas Section, Ontario Ministry of Natural Resources and Forestry, Peterborough, Ontario.
Hall, E.R. 1981. The Mammals of North America. 2nd edition, Volume 1. John Wiley & Sons, Toronto, Ontario. 600 pp.
Hayes, J.P and S.C. Loeb. 2007. The influences of forest management on bats in North America. Pp. 207-235 in M.J. Lacki, J. P. Hayes, and A. Kurta (eds.). Bats in Forests: Conservation and Management. Johns Hopkins University Press, Baltimore, Maryland.
Hayes, J.P., H.K. Ober, and R.E. Sherwin. 2009. Survey and monitoring of bats. Pp. 112-129 in T.H. Kunz and S. Parsons (eds.). Ecological and Behavioral Methods for the Study of Bats, 2nd ed. Johns Hopkins University Press, Baltimore, Maryland.
Herzog, C., pers. comm. 2015. Eastern Small-footed Myotis Recovery Strategy Workshop, Email correspondence to C. Humphrey. October and November 2015. Wildlife Biologist, Wildlife Diversity Unit, New York State Department of Environmental Conservation, Albany, New York.
Hickey, M.B.C., M.B. Fenton, K.C. MacDonald, and C. Souliere. 2001. Trace elements in the fur of bats (Chiroptera: Vespertilionidae) from Ontario and Quebec, Canada. Bulletin of Environmental Contamination and Toxicology 66(6):699-706.
Hitchcock, H.B. 1941. Myotis subulatus leibii and other bats hibernating in Ontario and Quebec. The Canadian Field-Naturalist 55(3):46.
Hitchcock, H.B. 1945. Myotis subulatus leibii in Ontario. Journal of Mammalogy 26(4):433.
Hitchcock, H.B. 1949. Hibernation of bats in southeastern Ontario and adjacent Quebec. The Canadian Field-Naturalist 63:47-59.
Hitchcock, H.B. 1955. A summer colony of the least bat, Myotis subulatus leibii (Audubon and Bachman). The Canadian Field-Naturalist 69(2):31.
Hitchcock, H.B. 1965. Twenty-three years of bat banding in Ontario and Quebec. The Canadian Field-Naturalist 79:4-14.
Hitchcock, H.B., R. Keen, and A. Kurta. 1984. Survival rates of Myotis leibii and Eptesicus fuscus in southeastern Ontario. Journal of Mammalogy 65(1):126-130.
Hoyt, J.R., K.E. Langwig, J. Okoniewski, W.F. Frick, W.B. Stone, and A.M. Kilpatrick. 2014. Long-term persistence of Pseudogymnoascus destructans, the causative agent of white-nose syndrome, in the absence of bats. EcoHealth 12(2):330-333.
IWNSC (Inter-agency White Nose Syndrome Committee). 2012. A National Plan to Manage White Nose Syndrome in Bats in Canada. Management Plan Drafting Subcommittee of Canada’s Inter-agency White Nose Syndrome Committee. 15 pp.
Johnson, J.B., and J.E. Gates. 2007. Food habits of Myotis leibii during fall swarming in West Virginia. Northeastern Naturalist 14(3):317-322.
Johnson, J.B., and J.E. Gates. 2008. Spring migration and roost selection of female Myotis leibii in Maryland. Northeastern Naturalist 15(3):453-460.
Johnson, J.B., J.E. Gates, and W.M. Ford. 2009. Notes on Foraging Activity of Female Myotis leibii in Maryland. United States Department of Agriculture, Forest Service, Northern Research Station. Research Paper NRS-8. U.S. Forest Service, Delaware, Ohio, United States of America. 8 pp.
Johnson, J.S., J.D. Kiser, K.S. Watrous, and T.S. Peterson. 2011. Day-roosts of Myotis leibii in the Appalachian Ridge and Valley of West Virginia. Northeastern Naturalist 18(1):95-106.
Johnson, J.S., L.E. Dodd, J.D. Kiser, T.S. Peterson, and K.S. Watrous. 2012. Food habits of Myotis leibii along a forested ridgetop in West Virginia. Northeastern Naturalist 19(4):665-672.
Jonasson, K., pers. comm. 2015. Email correspondence to C. Humphrey. October and November 2015. Ph.D. Candidate, Advanced Facility for Avian Research, Department of Biology, University of Western Ontario, London, Ontario.
Jung, T.S. and B.G. Slough. 2005. Mortality of Little Brown Bats, Myotis lucifugus, in a rodent trap in the boreal forest. The Canadian Field-Naturalist 119(4):589-590.
Kerth, G., A. Kiefer, C. Trappmann, and M. Weishaar. 2003. High gene diversity at swarming sites suggest hot spots for gene flow in the endangered Bechstein’s bat. Conservation Genetics 4:491-499.
Kunz, T.H., R.A. Adams, and W.R. Hood. 2009a. Methods for assessing size at birth and postnatal growth and development in bats. Pp. 273-314 in T.H. Kunz and S. Parsons (eds.). Ecological and Behavioral Methods for the Study of Bats, 2nd ed. Johns Hopkins University Press, Baltimore, Maryland.
Kunz, T.H., R. Hodgkison, and C.D. Weise. 2009b. Methods of capturing and handling bats. Pp. 4-35 in T.H. Kunz and S. Parsons (eds.). Ecological and Behavioral Methods for the Study of Bats, 2nd ed. Johns Hopkins University Press, Baltimore, Maryland.
Kunz, T.H. and J.D. Reichard. 2010. Status review of the Little Brown Myotis (Myotis lucifugus) and determination that immediate listing under the Endangered Species Act is scientifically and legally warranted. Center for Ecology and Conservation Biology, Boston University. Boston, Massachusetts. 30 pp.
Langwig, K.E., W.F. Frick, J.T. Bried, A.C. Hicks, T.H.Kunz and A.M.Kilpatrick. 2012. Sociality, density-dependence and microclimates determine the persistence of populations suffering from a novel fungal disease, white-nose syndrome. Ecology Letters 15(9):1-8.
Langwig, K.E., W.F. Frick, R. Reynolds, K.L. Parise, K.P. Drees, J.R. Hoyt, T.L. Cheng, T.H. Kunz, J.T. Foster, and A.M. Kilpatrick. 2015. Host and pathogen ecology drive the seasonal dynamics of a fungal disease, white-nose syndrome. Proceedings of the Royal Society of London B: Biological Sciences 282(1799):20142335.
Lausen, C.L. and R.M.R. Barclay. 2002. Roosting behaviour and roost selection of female Big Brown Bats (Eptesicus fuscus) roosting in rock crevices in southern Alberta. Canadian Journal of Zoology 80:1069-1076.
Lee, H.T., W.D. Bakowsky, J. Riley, J. Bowles, M. Puddister, P. Uhlig and S. McMurray. 1998. Ecological Land Classification for Southern Ontario: First Approximation and its Application. Ontario Ministry of Natural Resources, Southcentral Science Section, Science Development and Transfer Branch. SCSS Field Guide FG-02.
Leopardi, S., D. Blake, and S.J. Puechmaille. 2015. White-nose syndrome fungus introduced from Europe to North America. Current Biology 25(6):R217-R219.
Lewis, S. 1995. Roost fidelity of bats: a review. Journal of Mammalogy 76:481-496.
Lindner, D.L., A. Gargas, J.M. Lorch, M.T. Banik, J. Glaeser, T.H. Kunz, and D.S. Blehert. 2011. DNA-based detection of the fungal pathogen Geomyces destructans in soils from bat hibernacula. Mycologia 103(2): 241-246.
Loeb, S.C., T.J. Rodhouse, L.E. Ellison, C.L. Lausen, J.D. Reichard, K.M. Irvine, T.E. Ingersoll, J.T.H. Coleman, W.E. Thogmartin, J.R. Sauer, C.M. Francis, M.L. Bayless, T.R. Stanley, and D.H. Johnson. 2015. A plan for the North American Bat Monitoring Program (NABat). United States Department of Agriculture, Forest Service, Research and Development, Southern Research Station. General Technical Report SRS-208. June 2015.
Lorch, J.M., C.U. Meteyer, M.J. Behr, J.G. Boyles, P.M. Cryan, A.C. Hicks, A.E. Ballmann, J.T.H. Coleman, D.N. Redell, D.M. Reeder, and D.S. Blehert. 2011. Experimental infection of bats with Geomyces destructans causes white-nose syndrome. Nature 480:376-378.
Lorch, J.M., L.K. Muller, R.E. Russell, M. O'Connor, D.L. Lindner, and D.S. Blehert. 2013. Distribution and environmental persistence of the causative agent of white-nose syndrome, Geomyces destructans, in bat hibernacula of the eastern United States. Applied and Environmental Microbiology 79(4):1293-1301.
MacPherson, M. 2016. Email correspondence to D. Morningstar. February 2016. Undergraduate student, University of Guelph. Guelph, Ontario.
Martin, R.L., J.T. Pawluk, and T.B. Clancy. 1966. Observations on hibernation of Myotis subulatus. Journal of Mammalogy 47(2):348-349.
McGuire, L.P., C.G. Guglielmo, S.A. Mackenzie, and P.D. Taylor. 2012. Migratory stopover in the long-distance migrant Silver-haired Bat, Lasionycteris noctivagans. Journal of Animal Ecology 81:377-385.
McGuire, L., pers. comm. 2015. Email correspondence to C. Humphrey. October 2015. Assistant Professor, Department of Biological Sciences, Texas Tech University, Lubbock, Texas.
Mering, E.D. and C.L. Chambers. 2014. Thinking outside the box: a review of artificial roosts for bats. Wildlife Society Bulletin 38(4):741-751.
Meteyer, C.U., E.L. Buckles, D.S. Blehert, A.C. Hicks, D.E. Green, V. Shearn-Bochsler N.J. Thomas, A. Gargas, and M.J. Behr. 2009. Histopathologic criteria to confirm white-nose syndrome in bats. Journal of Veterinary Diagnostic Investigation 21:411-414.
MFFPQ (Ministère des Forêts, de la Faune et des Parcs du Québec), Quebec Centre for Biodiversity Science, and the University of Winnipeg. Undated. Neighbourhood Bat Watch. Principal Partners: MFFPQ, QCBS, and the University of Winnipeg. Additional Partners: Zoo de Granby, Biodôme de Montréal, and Groupe Chiroptères du Québec. Available at http://www.batwatch.ca [accessed August 19, 2016].
Morningstar, D., pers. comm. 2016. Email correspondence to C. Humphrey and M. Browning. February 2016. Terrestrial Ecologist, Golder Associates Ltd., Cambridge, Ontario.
Morrissey, C.A., P. Mineau, J.H. Devries, F. Sanchez-Bayo, M. Liess, M.C. Cavallaro, and K. Liber. 2015. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: a review. Environment International 74:291-303.
Moosman, P.R. Jr., pers. comm. 2015. Email correspondence to C. Humphrey. October 2015. Associate Professor of Biology, Virginia Military Institute, Lexington, Virginia.
Moosman, P.R. Jr., H.H. Thomas, and J.P. Veilleux. 2007. Food habits of eastern small-footed bats (Myotis leibii) in New Hampshire. American Midland Naturalist 158(2):354-360.
Moosman, P.R. Jr., J.P. Veilleux, G.W. Pelton, and H.H. Thomas. 2013. Changes in capture rates in a community of bats in New Hampshire during the progression of white-nose syndrome. Northeastern Naturalist 20(4):552-558.
Moosman, P.R. Jr., D.P. Warner, R.H. Hendren, and M.J. Hosler. 2015. Potential for monitoring Eastern Small-footed Bats on talus slopes. Northeastern Naturalist 22(1):1-13.
Mukhida, M., J. Orprecio, and M.B. Fenton. 2004. Echolocation calls of Myotis lucifugus and Myotis leibii (Vespertilionidae) flying inside a room and outside. Acta Chiropterologica 6(1):91-97.
Murray, K.L., E.R. Britzke, and L.W. Robbins. 2001. Variation in search-phase calls of bats. Journal of Mammalogy 82(3):728-737.
NatureServe. 2015. NatureServe Explorer: An online encyclopedia of life (web application). Version 7.1. NatureServe, Arlington, Virgina. Available at http://explorer.natureserve.org [accessed August 25, 2015].
Obrist, M.K. 1995. Flexible bat echolocation: the influence of individual, habitat, and conspecifics on sonar signal design. Behavioural Ecology and Sociobiology 36(3):207-219.
OMECC (Ontario Ministry of the Environment and Climate Change). 2015. Renewable energy projects listing. Ontario Ministry of Natural Resources and Forestry. Web site: /environment-and-energy/renewable-energy-projects-listing [accessed November 18, 2015].
OMNDM (Ontario Ministry of Northern Development and Mines). 2016. OGSEarth, Ontario Ministry of Northern Development and Mines. Web site:
http://www.mndm.gov.on.ca/en/mines-and-minerals/applications/ogsearth [accessed November 6, 2015 and August 8, 2016).
OMNR (Ontario Ministry of Natural Resources). 2000. Significant wildlife habitat technical guide. Fish and Wildlife Branch, Wildlife Section and Science Development and Transfer Section, Southcentral Sciences Section. October 2000. 139 pp. plus appendices.
OMNR (Ontario Ministry of Natural Resources). 2010. Forest management guide for conserving biodiversity at the stand and site scales. Ontario Ministry of Natural Resources. 212 pp.
OMNRF (Ontario Ministry of Natural Resources and Forestry). 2015a. Ontario’s white-nose syndrome response plan. Wildlife Section, Species Conservation and Policy Branch, Ontario Ministry of Natural Resources and Forestry. 22 pp.
OMNRF (Ontario Ministry of Natural Resources and Forestry). 2015b. How species at risk are protected. Ontario Ministry of Natural Resources and Forestry. Web site: /page/how-species-risk-are-protected [accessed November 19, 2015].
O'Keefe, J.M. and M. LaVoie. 2010. Maternity colony of Eastern Small-footed Myotis (Myotis leibii) in a historic building. Southeastern Naturalist 10(2):381-383.
O'Shea, T.J., P.M. Cryan, D.T.S. Hayman, R.K. Plowright, and D.G. Streiker. 2016. Multiple mortality events in bats: a global review. Mammal Review. DOI: 10.1111/mam.12064.
Paradiso, J.L. and A.M. Greenhall. 1967. Longevity records for American bats. American Midland Naturalist 78(1):251-252.
Parsons, S. and J.M. Szewczak. 2009. Detecting, recording, and analyzing the vocalizations of bats. Pp. 91-111 in T.H. Kunz and S. Parsons (eds.). Ecological and Behavioral Methods for the Study of Bats, 2nd ed. Johns Hopkins University Press, Baltimore, Maryland.
Pennsylvania Game Commission. 2014. Eastern Small-footed Bat Roost Structures and Examples. Pennsylvania Game Commission, Harrisburg, Pennsylvania. 6 pp.
Pikula, J., H. Bandouchova, L. Novotný, C.U. Meteyer, J. Zukal, N.R. Irwin, J. Zima, and N. Martínková. 2012. Histopathology confirms white-nose syndrome in bats in Europe. Journal of Wildlife Diseases 48(1):207-211.
Pisa, L.W., V. Amaral-Rogers, L.P. Belzunces, J.M. Bonmatin, C.A. Downs, D. Goulson, D.P. Kreutzweiser, C. Krupke, M. Leiss, M. McField, C.A. Morrissey, D.A. Noome, J. Settele, N. Simon-Delso, J.D. Stark, J.P. Van der Sluijs, H. Van Dyck, and M. Weimers. 2015. Effects of neonicotinoids and fipronil on non-target invertebrates. Environmental Science and Pollution Research 22(1): 68-102.
Reichard, J.D. and T.H. Kunz. 2009. White-nose syndrome inflicts lasting injuries to the wings of little brown myotis (Myotis lucifugus). Acta Chiropterologica 11(2):457-464.
Ren, P., K.H. Haman, L.A. Last, S.S. Rajkumar, M.K. Keel, and V. Chaturvedi. 2012. Clonal spread of Geomyces destructans among bats, Midwestern and Southern United States. Emergent Infectious Disease 18(5):883-885.
Rivers, N.M., R.K. Butlin, and J.D. Altringham. 2005. Genetic population structure of Natterer’s bats explained by mating at swarming sites and philopatry. Molecular Ecology 14:4299-4312.
Roble, S.M. 2004. Notes on an autumn roost of Eastern Small-footed Bat (Myotis leibii). Banisteria 23:42-44.
Rollins, K.E., D.K. Meyerholz, G.D. Johnson, A.P. Capparella, and S.S. Loew. 2012. A forensic investigation into the etiology of bat mortality at a wind farm: barotrauma or traumatic injury? Veterinary Pathology 49(2):362-371.
Ryckman, A., pers. comm. 2016. Communication with C. Humphrey. January 2016. Senior Terrestrial and Wetland Biologist, Natural Resource Solutions Inc., Ontario.
Rydell, J. L. Bach, M. Dubourg-Savage, M. Green, L. Rodrigues, and A. Hedenstrom. 2010. Bat mortality at wind turbines in northwestern Europe. Acta Chiropterologica 12(2):261-274.
Secord, A.L., K.A. Patnode, C. Carter, E. Redman, D.J. Gefell, A.R. Major, and D.W. Sparks. 2015. Contaminants of emerging concern in bats from the northeastern United States. Archives of Environmental Contamination and Toxicology 69(4):411-421.
Segers, J., pers. comm. 2015. Email correspondence to C. Humphrey. October 2015. National White-nose Syndrome Coordinator, Canadian Wildlife Health Cooperative, Prince Edward Island.
Segers, J., pers. comm. 2016a. Email correspondence to C. Humphrey. January 2016. National White-nose Syndrome Coordinator, Canadian Wildlife Health Cooperative, Prince Edward Island.
Segers, J., pers. comm. 2016b. Email correspondence to C. Humphrey. July 2016. National White-nose Syndrome Coordinator, Canadian Wildlife Health Cooperative, Prince Edward Island.
Shelley, V., S. Kaiser, E. Shelley, T. Williams, M. Kramer, K. Haman, K. Keel, and H.A. Barton. 2013. Evaluation of strategies for the decontamination of equipment for Geomyces destructans, the causative agent of white-nose syndrome (WNS). Journal of Cave and Karst Studies 75(1):1-10. DOI:10.4311/2011LSC0249.
Simmons, J.A. and R.A. Stein. 1980. Acoustic imaging in bat sonar: echolocation signals and the evolution of echolocation. Journal of Comparative Physiology 135(1):61-84.
Smit, C.E., C.J.A.M. Posthuma-Doodeman, P.L.A. van Vlaardingen, and F.M.W. de Jong. 2014. Ecotoxicity of imidacloprid to aquatic organisms: derivation of water quality standards for peak and long-term exposure. Human and Ecological Risk Assessment 00:1-23, DOI:10.1080/10807039.2014.964071
Steffen, B.J., T.L. Osborne, T.C. Carter, and G.A. Feldhamer. 2006. The first record of Eastern Small-footed Myotis (Myotis leibii) in Illinois. Transactions of the Illinois State Academy of Science 99(1&2):87-89.
Thomas, D.W., M. Dorais, and J.M. Bergeron. 1990. Winter energy budgets and cost of arousals for hibernating little brown bats, Myotis lucifugus. Journal of Mammalogy 71(3):475-479.
Thomas, D.W. 1995. Hibernating bats are sensitive to non-tactile human disturbance. Journal of Mammalogy 76(3):940-946.
Thomas, H.H., P.R. Moosman Jr., J.P. Veilleux, and J. Holt. 2012. Food of bats (family Vespertilionidae) at five locations in New Hampshire and Massachusetts. The Canadian Field-Naturalist 126:117-124.
Thompson, T. 2013. Roost ecology of Eastern Small-footed Bats (Myotis leibii) in the southern Appalachian Mountains. Master of Science Thesis, Indiana State University, Terre Haute, Indiana, United States of America. 75 pp.
Turner, G.G., D.M. Reeder, and J.T.H. Coleman. 2011. A five-year assessment of mortality and geographic spread of white-nose syndrome in North American bats and a look to the future. Bat Research News 52(2):13-27.
Tuttle, M.D. 1964. Myotis subulatus in Tennessee. Journal of Mammalogy.
Tyburec, J. 2012. The effectiveness of harp traps for the capture of the Eastern Small-footed Myotis (Myotis leibii) during summer surveys in Pennsylvania (pre-WNS). Presentation at the Northeast Bat Working Group Annual Meeting, January 11-13, 2013. Carlisle, Pennsylvania.
USFWS (U.S. Fish and Wildlife Service). 2012. North American bat death toll exceeds 5.5 million from white-nose syndrome. United States Fish and Wildlife Service. News Release, January 17, 2012. 2pp.
USFWS (U.S. Fish and Wildlife Service). 2013. 12-month finding on a petition to list the Eastern Small-footed Bat and the Northern Long-eared Bat as endangered or threatened species; listing the Northern Long-eared Bat as an endangered species. United States Fish and Wildlife Service. Federal Register 78(191):61046-61080.
USFWS (U.S. Fish and Wildlife Service). 2016. whitenosesyndrome.org: A Coordinated Response to the Devastating Bat Disease. United States Fish and Wildlife Service. Web site: https://www.whitenosesyndrome.org/ [accessed August 19, 2016].
Van Dijk, T.C., M.A. van Staalduinen, and J.P. Van Der Sluijs. 2013. Invertebrate decline in surface water polluted with imidacloprid. PLoS ONE 8(5): e62374. DOO:10.1371/journal.pone.0062374
van Zyll de Jong, C.G. 1985. Handbook of Canadian Mammals. Volume 2: Bats. National Museum of Natural Sciences, Ottawa. 212 pp.
Veilleux, J.P. 2007. A noteworthy hibernation record of Myotis leibii (Eastern Small-footed Bat) in Massachusetts. Northeastern Naturalist 14(3):501-502.
Veilleux, J.P. and S. Reynolds. 2005. Eastern Small-footed Bat, Myotis leibii. Pp. A-328-A-334 in New Hampshire Wildlife Action Plan, Appendix A: Species Profiles – Mammals. New Hampshire Fish and Game Department, Concord, New Hampshire.
Verant, M.L., M.U. Carol, J.R. Speakman, P.M. Cryan, J.M. Lorch, and D.S. Blehert. 2014. White-nose syndrome initiates a cascade of physiologic disturbances in the hibernating bat host. BMC Physiology 14(1):10.
Warnecke, L., J.M. Turner, T.K. Bollinger, J.M. Lorch, V. Misra, P.M. Cryan, G. Wibbelt, D.S. Blehert, and C.K.R. Willis. 2012. Inoculation of bats with European Geomyces destructans supports the novel pathogen hypothesis for the origin of white-nose syndrome. Proceedings of the National Academy of Sciences of the United States of America 109(18): 6999-7003.
WBWG (Western Bat Working Group). 2015. Western Canada white-nose syndrome transmission prevention. Available on the Canadian Wildlife Health Cooperative Web site: http://www.cwhc-rcsf.ca/docs/WNS_Western_Transmission_Prevention.pdf [accessed November 19, 2015].
Whitby, M., S. Bergeson, T. Carter, S. Rutan, and R. McClanahan. 2013. The discovery of a reproductive population of Eastern Small-footed Bat, Myotis leibii, in Southern Illinois using a novel survey method. American Midland Naturalist 169:229-233.
Wilcox, A., L. Warnecke, J.M. Turner, L.P. McGuire, J.W. Jameson, V. Misra, T.C. Bollinger, and C.K.R. Willis. 2014. Behaviour of hibernating Little Brown Bats experimentally inoculated with the pathogen that causes white-nose syndrome. Animal Behaviour 88:157-164.
Woller-Skar, M.M., D.N. Jones, M.R. Luttenton, and A.L. Russell. 2015. Microcystin detected in Little Brown Bats (Myotis lucifugus). The American Midland Naturalist 174(2):331-334.
Worton, B.J. 1989. Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70(1):164-168.
Wright, A.H. and S.E.R. Simpson. 1920. The vertebrates of the Otter Lake region, Dorset, Ontario. The Canadian Field-Naturalist 34(8):141-168.
Yates, D.E., E.M. Adams, S.E. Angelo, D.C. Evers, J. Schmerfeld, M.S. Moore, T.H. Kunz, T. Divoll, S.T. Edmunds, C. Perkins, R. Taylor, and N.J. O'Driscoll. 2014. Mercury in bats from the northeastern United States. Ecotoxicology 23:45-55.
Yates, D. and T. Divoll. in prep. 2015. Draft report on capture and radio-telemetry surveys conducted in Acadia National Park (Maine) in 2014. BioDiversity Research Institute, Portland, Maine.
Zukal, J., J. Pikula, and H. Bandouchova. 2015. Bats as bioindicators of heavy metal pollution: history and prospect. Mammalian Biology 80(3):220-227.