Jefferson Salamander and Jefferson-dependent Unisexual Ambystoma Recovery Strategy
This document is the recovery strategy for the Jefferson salamander and the Jefferson-dependent Unisexual Ambystoma, amphibian species at risk in Ontario.

Cover photo by Jennifer McCarter (top) and Joe Crowley (bottom)
About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act, 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species’ persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of Natural Resources and Forestry Species at Risk webpage.
Recommended citation
Linton, J, J. McCarter and H. Fotherby 2018. Recovery Strategy for the Jefferson Salamander (Ambystoma jeffersonianum) and Unisexual Ambystoma (Jefferson Salamander dependent population) (Ambystoma laterale - (2) jeffersonianum) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources and Forestry, Peterborough, Ontario. vii + 58 pp.
Cover illustration: Jefferson Salamander (top) photo by Jennifer McCarter. Unisexual Ambystoma (Jefferson Salamander dependent population) (bottom) photo by Joe Crowley.
© Queen’s Printer for Ontario, 2018
ISBN 978-1-4868-2155-6 (HTML)
ISBN 978-1-4868-2156-3 (PDF)
Content (excluding the cover illustration) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n’est disponible qu’en anglais en vertu du Règlement 411/97 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec recovery.planning@ontario.ca.
Authors
- Jessica Linton, Natural Resource Solutions Inc.
- Jennifer McCarter, Natural Resource Solutions Inc.
- Heather Fotherby, Natural Resource Solutions Inc.
Acknowledgments
This document represents an update to a recovery strategy which was prepared by the Jefferson Salamander Recovery and Implementation Team (formerly called the Recovery Team) in 2010. The authors would like to acknowledge the extensive work that went into that strategy, which forms the basis of this document. In developing the original strategy the members of the Recovery and Implementation Team wished to acknowledge people who have submitted salamander eggs to the University of Guelph for identification, in particular Mary Gartshore, Bill Lamond, Al Sandilands and Craig Campbell. They also thanked David Servage, Lesley Lowcock and Alison Taylor, who made significant contributions to our understanding of the complex Ambystoma laterale (Blue-Spotted Salamander)–jeffersonianum complex during their tenures in the Master of Science program at the University of Guelph. Karine Bériault and Cadhla Ramsden’s research on habitat requirements and non-lethal sampling methods was invaluable. Leslie Rye and Wayne Weller were acknowledged for accumulating the information and producing a status report on Jefferson Salamander for the Committee on the Status of Endangered Wildlife in Canada (COSEWIC). Special mention was also extended to Brenda Van Ryswyk, John Pisapio and Albert Garofalo, who collected much of the data for radio-telemetry studies noted in this report, and to Pete Lyons, who provided property access. The Recovery and Implementation Team also thanked Fiona Reid and Don Scallen for their help with locating new populations of this species.
The authors of the recovery strategy for the Jefferson Salamander and Unisexual Ambystoma (Jefferson Salamander dependent population) would like to thank the Recovery and Implementation Team for their assistance with developing a comprehensive strategy. Dr. James Bogart at the University of Guelph provided guidance to the authors throughout the development of this strategy and is acknowledged for reviewing and providing feedback on various drafts. The following individuals are thanked for providing specific input based on their experiences in relevant jurisdictions: Sue Hayes, Heather Lynn, Anne Marie Laurence, Graham Buck, Mark Heaton, Joshua Shea, and Joe Crowley.
Declaration
The recovery strategy for the Jefferson Salamander and Unisexual Ambystoma (Jefferson Salamander dependent population) was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all of the individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ontario Ministry of Natural Resources and Forestry
Environment and Climate Change Canada – Canadian Wildlife Service, Ontario
Executive summary
The Jefferson Salamander (Ambystoma jeffersonianum) is a relatively large, uniformly grey to brownish-grey salamander with variable amounts of grey-blue speckling along the sides of the body and the long, laterally compressed tail. The snout, limbs and toes on the hind feet are relatively long compared to other Ambystoma species. The Unisexual Ambystoma (Jefferson Salamander dependent population) (Ambystoma laterale - (2) jeffersonianum) is morphologically similar to the Jefferson Salamander. Both salamanders occur sympatrically across Ontario and are only differentiated from each other through the genetic analysis of their genomotype. They generally occur in the eastern portion of the Carolinian zone and along the Niagara Escarpment in Ontario. Throughout this range, there are several geographically isolated subpopulations.
Recent estimates, based on long-term data sets for Jefferson Salamander, suggest a decline of more than 90 percent over the last three generations (33 years) of this species in Ontario. The Unisexual Ambystoma (Jefferson Salamander dependent population) have a unique reproductive strategy (kleptogenesis) where sperm from a male Jefferson Salamander is needed to initiate egg development. Their population is, therefore, dependent on the presence of Jefferson Salamander for their survival. A decline in the Jefferson Salamander would also result in a decline of unisexual Ambystoma Jefferson Salamander dependent population. The Jefferson Salamander and Unisexual Ambystoma (Jefferson Salamander dependent population) were listed on the Species at Risk in Ontario (SARO) List as endangered in 2011 and 2017, respectively, affording each of them species and habitat protection under the Endangered Species Act (ESA, 2007).
The survival and recovery of the Jefferson Salamander and Unisexual Ambystoma (Jefferson Salamander dependent population) is primarily threatened by habitat loss, degradation, and fragmentation of woodlands and breeding ponds. The vast majority of suitable habitat within the known range of these two species has been cleared, initially for agriculture and subsequently for urban development. Other major threats include road-related threats (e.g. vehicles and pollutants) and changes in pond hydrology. They are threatened to a lesser extent by forestry activities, recreational land uses, unauthorized collection, invasive and introduced species, and agricultural land uses.
The recommended recovery goal is to ensure that existing threats to populations and habitat of the Jefferson Salamander and Unisexual Ambystoma (Jefferson Salamander dependent population) are sufficiently removed to allow populations to become stable or increase in abundance and distribution throughout Ontario. The protection and recovery objectives are to:
- Identify and monitor extant populations of the Jefferson Salamander and unisexual Ambystoma Jefferson Salamander dependent populations in Ontario.
- Continue to research the species’ movements and habitat use to inform habitat protection and restoration.
- Identify historic and presently unoccupied areas with the potential for enhancement, restoration (i.e. recovery habitat) and eventual recolonization or reintroduction of the species.
- Assess and quantify threats to Jefferson Salamander and unisexual Ambystoma Jefferson Salamander dependent populations.
- Develop, test and implement threat mitigation techniques in order to reduce threats affecting Jefferson Salamander and unisexual Ambystoma Jefferson Salamander dependent populations.
- Develop a communication strategy to inform municipalities, planners, the development industry, property managers and other stakeholders of the habitat mapping and protection requirements for the Jefferson Salamander and Jefferson dependent unisexuals under the ESA 2007 and actively engage these stakeholders in effective habitat creation and restoration techniques and other recovery planning initiatives.
The short-term recovery approaches should focus on the protection of existing populations of the Jefferson Salamander and Unisexual Ambystoma (Jefferson Salamander dependent population) by minimizing further loss or degradation of known habitat or potential recovery habitat. Recovery approaches should also focus on verifying, documenting, and monitoring the distribution and habitats used by extant, historic, and potential subpopulations. Developing and evaluating mitigation and restoration techniques, actively conducting research, and developing long-term management activities should also be prioritized to ensure the recommended recovery goal will be achieved.
On February 18, 2010, a habitat regulation came into force under the ESA 2007 for the Jefferson Salamander (O. Reg. 242/08). Although the Unisexual Ambystoma (Jefferson Salamander dependent population) was not protected under the ESA at the time, the species was used as a surrogate to indicate the presence of Jefferson Salamander, since their existence is dependent on the presence of male Jefferson Salamanders. Therefore, in cases where unisexual Ambystoma Jefferson Salamander dependent populations were found, the ESA applied. Generally, the regulation includes breeding ponds for both salamanders, the 300 m area adjacent to breeding ponds which provides suitable foraging, dispersal, migration or hibernation conditions, potential breeding ponds to which juveniles could disperse to within 1 km of a known breeding pond, and suitable terrestrial dispersal habitat between these areas.
The current regulation is effective at protecting both Jefferson Salamander and the Unisexual Ambystoma (Jefferson Salamander dependent population), however some amendments should be considered. Recommended amendments include adding Unisexual Ambystoma (Jefferson Salamander dependent population) as a distinct taxon and adding additional areas (the Municipality of Chatham-Kent, Durham Region, and Oxford and Perth Counties) in which the regulation applies.
1.0 Background information
Jefferson Salamander (Ambystoma jeffersonianum) was first described by Green in 1827 (Uzzell and Goldblat 1967). Considerable confusion about the species’ taxonomy has followed because of the sympatric occurrence of polyploid, all-female unisexual populations of Ambystoma salamanders that use sperm from male Jefferson Salamanders in reproduction (COSSARO 2016). The taxonomic and nomenclature histories of the Jefferson Salamander and sympatric unisexual salamanders include taxonomic groups now considered distinct species or synonyms (Matson 2013). The debate surrounding the taxonomic status of the Unisexual Ambystoma (Jefferson Salamander dependent population) is summarized in detail in COSEWIC 2016. Briefly, they do not correspond to any species concept other than comprising a monophyletic mitochondrial lineage which makes them genetically distinct.
Populations of unisexuals which occur sympatrically with Jefferson Salamander are referred to as Jefferson dependent unisexuals (Ambystoma laterale- (2) jeffersonianum) throughout this report for simplicity. Populations of unisexual Ambystoma also occur in Canada in association with other bisexual species whose males serve as sperm donors. This includes a unisexual Ambystoma Small-mouthed Salamander dependent population (Ambystoma laterale - texanum) and a unisexual Ambystoma Blue-spotted Salamander dependent population (Ambystoma (2) laterale - jeffersonianum). The morphology and distribution of unisexual Ambystoma populations is determined by their associated sperm-donating species.
1.1 Species assessment and classification
| Assessment | Status |
|---|---|
| SARO List Classification | Endangered |
| SARO List History | Endangered (2011), Threatened (2008) |
| COSEWIC Assessment History | Endangered (2010), Threatened (2000) |
| SARA Schedule 1 | Endangered, Schedule 1 |
| Conservation Status Rankings | GRANK: G4 NRANK: N2 SRANK: S2 |
| Assessment | Status |
|---|---|
| SARO List Classification | Endangered |
| SARO List History | Endangered (2017) |
| COSEWIC Assessment History | Endangered (2016) |
| SARA Schedule 1 | No schedule, no status |
| Conservation Status Rankings | GRANK: GNR NRANK: NNR SRANK: S2 |
1.2 Species description and biology
Species description
The Jefferson Salamander is a relatively large (60-130 mm total length or 65–95 mm snout-to-vent length), uniformly grey to brownish-grey salamander with variable amounts of grey-blue speckling along the sides of the body and the long, laterally compressed tail (Figure 1) (Petranka 1998, Mills 2016). The snout, limbs and toes on the hind feet are relatively long compared to other species of Ambystoma (Mills 2016).
Jefferson dependent unisexual salamanders (Figure 2) are morphologically similar to the Jefferson Salamander; however, because they have chromosome sets from two or more species, the number and species origin of those chromosome sets dictates their morphology. All Jefferson dependent unisexuals possess at least one set of Blue-spotted Salamander (Ambystoma laterale) chromosomes which typically gives them more grey-blue-flecks or spots along the sides than Jefferson Salamanders (COSEWIC 2016).
Despite slight variations in appearance, Jefferson Salamanders and unisexuals dominated by either Jefferson or Blue-spotted Salamander genomes cannot be reliably distinguished from appearance alone; genetic analysis is required. The larvae and juveniles of both the Jefferson Salamander and Jefferson dependent unisexuals are also morphologically indistinguishable. After hatching, larvae are olive green to brown with yellow mottles on the sides. They are 8-10 mm in total length with four legs (unlike frog or toad larvae), a tall tail fin and feathery external gills behind a relatively broad head (Figure 3) (Petranka 1998, Mills 2016). Recently metamorphed individuals are 45-75 mm in total length (Petranka 1998, Matson 2013) and look like miniature adults. They are uniformly greenish or grayish-brown in colour with a dark dorsal line (Fotherby, pers. obs. 2017) and some speckling on the sides (Figure 4).

Figure 1. Adult Jefferson Salamander (Photo: Jennifer McCarter)

Figure 2. Adult unisexual Ambystoma Jefferson dependent population (Photo: Jessica Linton)

Figure 3. Young salamander larva (Photo: Jessica Linton)

Figure 4. Mature larva prior to transformation (Photo: Jessica Linton)

Figure 5. Recently metamorphed salamander (Photo: Jessica Linton)
Species biology
Jefferson Salamanders and Jefferson dependent unisexuals, like all species in the family Ambystomatidae, spend most of their lives underground (Petranka 1998, COSEWIC 2010, 2016). Their underground behaviours are not well documented, but they are thought to be ‘sit and wait’ predators, preying on earthworms and other invertebrates (Petranka 1998). Jefferson dependent unisexuals appear to exhibit the same behaviours as female Jefferson Salamanders throughout their life cycle (COSEWIC 2016). Both species are long-lived, having extremely high adult survivorship (Weller 1980) and potentially live at least 30 years (COSEWIC 2016).
Life cycle and reproduction
Jefferson Salamander and Jefferson dependent unisexuals are the earliest breeding Ambystoma species in Ontario. They typically migrate to breeding ponds during the first rainy nights of the spring when temperatures are above freezing, often before breeding ponds have completely thawed (COSEWIC 2016).
Breeding commences when groups of adults gather in scattered locations in a breeding pond. Male Jefferson Salamanders approach and court female salamanders, and deposit their spermatophores on pond substrates for females to pick up in their cloacae (Petranka 1998). Male Jefferson Salamanders are able to chemically distinguish between bisexual and unisexual females (Dawley and Dawley 1986) and are more likely to court and produce spermatophores for bisexual females (Uzzell and Goldblatt 1967, Uzzell 1969). Unisexual males have been documented in nature, although they are very rare. Bogart and Klemens (2008) looked at the genome composition of 1377 salamanders from 118 sites in Connecticut, Massachusetts, New Jersey, New York, Pennsylvania, and Virginia and found that the frequency of unisexual males was only 1.32 percent. Bogart (2003) suggests that such individuals are probably sterile and it is unknown if they could stimulate gynogenetic development of unisexual eggs.
One to two days after mating, females deposit small egg masses on emergent vegetation, twigs, or tree branches that have fallen into the water (Petranka 1998). Each egg mass is made up of 16 to 40 large (2.0 – 2.5 mm) eggs, which contain a black or dark brown embryo enclosed in a distinct envelope. A loose, watery layer of protective gel surrounds the eggs (Bishop 1947) (Figure 6).

Figure 6. Jefferson Salamander egg mass (Photo: Jessica Linton)
The dark melanin pigment, gel, symbiotic algae in the gel, and the dissolved organic matter in the water protect the developing embryos from damaging ultraviolet B radiation (Licht 2003). Individual females lay several egg masses, which altogether may contain more than 200 eggs, depending on the size of the female. Jefferson Salamander egg masses have lower egg mortality than Jefferson dependent unisexual egg masses. Hatching success of Jefferson Salamander eggs has been reported to be between 60 and 88 percent (Cook 1983) compared to 20 to 39 percent for Jefferson dependent unisexuals (Wilbur 1971, Bogart and Licht 1986, Bogart et al. 1987, Bogart et al. 2009).
Survival rates of larvae prior to metamorphosis are believed to be very low at 0 to 0.7 percent (Thompson et al. 1980, Mullen and Klueh 2009, Matson 2013). In Ontario, larval survival rates have been observed to be low in most breeding ponds, especially for the Jefferson dependent unisexuals, which is thought to have genetic viability issues (Bogart and Licht 1987). In addition, survival and recruitment can be highly variable year to year and can be negatively affected by ponds drying prior to larval transformation (COSEWIC 2010).
Jefferson Salamander larvae exhibit a slightly shorter larval period than Jefferson dependent unisexuals; their larval period is 94.6 days on average compared to an average of 95.8 days for unisexual larvae (Wilbur 1971). This may provide a competitive advantage to Jefferson Salamander larvae as breeding ponds begin to dry up and food supplies become depleted throughout the summer (Wilbur 1971).
Breeding success varies from year to year, depending on spring weather and water-level conditions. However, because they are long-lived, populations under normal conditions can be resilient to such variable reproductive output. Larvae hatch after two to four weeks (depending primarily on water temperature) and then spend another two to four months foraging in the pond (Petranka 1998). Jefferson Salamander larvae are known to cannibalize other salamander larvae, including conspecifics (Matson 2013). The larval stage varies in duration and can extend into early September. In Ontario, metamorphosis from the aquatic to terrestrial body form normally occurs in July and August. After transformation the salamanders move out of the pond and seek shelter in the forest litter.
Male Jefferson Salamanders in Ontario have been estimated to return to their natal ponds to breed approximately 22 months after metamorphosis (Weller 1980). Female Jefferson Salamanders and Jefferson dependent unisexuals, on the other hand, are estimated to reach sexual maturity after 34 months (Weller 1980). Jefferson dependent unisexuals breed more frequently than bisexual salamanders; Jefferson Salamanders, especially females, do not breed every year (Weller 1980).
There are no studies that examine age-specific survivorship of Jefferson Salamander, but Jefferson dependent unisexuals are thought to live longer than bisexual salamanders (COSEWIC 2010). Based on mark-recapture data, Matson (2013) found that males live for at least 9 years, bisexual females can live for more than 10 years, and Jefferson dependent unisexuals can live for more than 11 years. However, a study examining skeletochronology of another Ambystoma species, Spotted Salamanders (Ambystoma maculatum), suggests that they may live up to 32 years (Flageole and Leclair 1992). In addition, a Jefferson dependent unisexual individual first observed breeding in 1988 was still alive in 2015 meaning that it would be at least 30 years old (COSEWIC 2016).
Dispersal
Both Jefferson Salamander and Jefferson dependent unisexuals occupy terrestrial and aquatic habitats and may play an important role in channeling nutrients between the aquatic and upland wooded environments (Capps et al. 2014, Davic and Welsh 2004). Terrestrial habitat use and emigration by Jefferson Salamanders and Jefferson dependent unisexuals, especially metamorph dispersal and movements of juveniles, have not been well studied. Although radio-telemetry can provide accurate data on adult salamander movements, it has several limitations such as limited battery life and the requirement of invasive surgical procedures. In addition, the size of the transmitters only allows for implantation in adults and, therefore, no radio-telemetry studies have been conducted on juvenile salamander movements.
Weller (1980) marked metamorph Jefferson Salamanders and sympatric unisexuals using digit amputation in the Region of Peel in the late 1970s. Until recently, this was the only known mark-recapture study conducted in Ontario on dispersal of Jefferson Salamander metamorphs. Williams (1973) tracked metamorph Jefferson Salamanders using radioactive tags in Indiana and reported that individuals were found an average of 92 m from the breeding pond in 10 days, with a range of 3 to 247 m.
A multi-year metamorph and juvenile dispersal study initiated in 2015 employing pitfall trap capture-mark-recapture in the Hamilton area of Ontario has documented that the majority of metamorphs (n= 26) spent their first winter 6 to 14 m from the edge of their natal pond (Linton et al. 2016). Metamorphs travelled considerably less distance than reported in the literature (Williams 1973). It was theorized that, because the habitat is uniformly high quality around the study pond, metamorphs did not need to travel far to find suitable foraging and overwintering habitat (Williams 1973). To date, a strong correlation between time and travel distance has not been observed. Based on the distance between traps, one individual travelled 47 m in two days, while another took 10 days to travel 27 m.
Many subpopulations are separated from each other by more than one kilometre; given the maximum known movement distances, salamanders are unlikely to be able to disperse between them (COSEWIC 2016).
Intraspecific competition and/or predation
In a recent Ontario study, Jefferson Salamanders had a much higher larval transformation success rate than Jefferson dependent unisexuals despite there being a higher proportion of unisexual adults in the population (Linton et al. 2016). This suggests that, despite being in the minority of salamanders breeding in the study pond, Jefferson Salamanders were the most successful at recruiting metamorphs (Linton et al. 2016). Explanations for this observation include: Jefferson Salamanders are known to have a higher proportion of viable eggs, a shorter larval period, cannibalistic larvae, and Jefferson Salamander males prefer to breed with bisexual females. Unisexuals, in turn, may make up a higher proportion of the adult population if they live longer or return more frequently to the pond to breed.
Jefferson Salamander larvae are voracious aquatic predators that feed on moving prey such as insect larvae, small crustaceans and amphibian larvae including other Ambystoma larvae. Adults and larvae are likely prey for wetland predators, such as snakes, rodents and birds (COSEWIC 2016). At one site where pond levels receded drastically due to drought, predation of larvae by Raccoon (Procyon lotor) and Wild Turkey (Meleagris gallopavo) was observed to increase as the pond levels decreased (Linton, pers. observation 2016).
Genetics
Contrary to earlier theories, there is no evidence of past or present hybridization between Jefferson and Blue-spotted Salamanders (Bogart 2003). Mitochondrial DNA from Ambystoma unisexual individuals pre-dates that of the Jefferson Salamander (and Blue-spotted Salamander) (Bogart et al. 2007) and aligns most closely with that of a Kentucky population of the Streamside Salamander (Ambystoma barbouri) (Bogart 2003). Jefferson dependent unisexuals all share a very similar mitochondrial DNA, which arose 3 to 5 million years ago, that is distinctly different from any bisexual species, making them the oldest lineage of unisexual vertebrates known (Bi and Bogart 2010).
The nuclear genome of unisexual Ambystoma individuals is unrelated to their mitochondrial genome and is generally polyploid. Polyploid genomes contain three or more complete sets of chromosomes and Jefferson dependent unisexuals are usually triploid, however, diploid, tetraploid and pentaploid individuals have also been documented (Bogart 2003). An increase in ploidy levels in unisexual Ambystoma is a result of the incorporation of nuclear genomes from sympatric populations of bisexual species. The nuclear genome of Jefferson dependent unisexuals is predominated by chromosomes that have been incorporated from Jefferson Salamander. This is in contrast, for example, to unisexual Ambystoma Blue-spotted Salamander dependent population where chromosomes are predominated by Blue-spotted Salamander chromosomes.
The genetic mixing that occurs between unisexual Ambystoma and bisexual populations is attributed to an unusual reproductive strategy referred to as kleptogenesis (Bogart et al. 2007). Under this reproductive strategy, unisexual females lay unreduced eggs or eggs whose number of sets of chromosomes is equivalent to that of the parent’s somatic cells. Sperm from a diploid male is required to initiate development of the eggs and the male’s genome is normally not incorporated (Elinson et al. 1992). In some cases, however, the male’s genome is incorporated into the genome of the embryos (Bogart 2003). This incorporation can result in ploidy elevation (from triploid to tetraploid) or genome replacement if the male’s genome is incorporated in an egg that has possibly undergone a meiotic reduction. Ploidy elevation has been documented to occur in several populations and can be induced experimentally (Bogart et al. 1989). A possible advantage to this reproductive strategy is the incorporation of genes that are highly adapted to a particular environment as well as the ability to eliminate genomes that have deleterious alleles (Bogart et al. 2007).
All-female populations of Jefferson dependent unisexuals coexist with Jefferson Salamander populations owing to their reliance on the presence of a male Jefferson Salamander sperm donor for reproduction. In the absence of a bisexual Jefferson Salamander sperm donor, they do not appear to be able to reproduce parthenogenetically or use the sperm of other co-occurring species of Ambystoma such as Spotted Salamanders (Bogart et al. 2017). Therefore, both the Jefferson Salamander and the Jefferson dependent unisexuals are limited by their dependence on male Jefferson Salamander sperm donors for reproduction (Bogart and Licht 1987, COSEWIC 2016). Jefferson dependent unisexuals have been found in ponds without a sperm donor, although it is presumed that a sperm donor was present at one time (Bogart et al. 2017). In this regard, the presence of eggs of Jefferson dependent unisexuals necessarily and absolutely indicates the presence of a breeding bisexual Jefferson Salamander at some point in time (Rye and Weller 2000, Bogart and Klemens 1997, 2008, COSEWIC 2016).
Species and ploidy identification
At the University of Guelph, microsatellite molecular markers for the Jefferson Salamander (Julian et al. 2003) have been, and continue to be, used effectively to identify and distinguish Jefferson Salamanders from Jefferson dependent unisexuals. These markers may also help address other questions regarding population dynamics and genetics that involve the unisexual members of the complex.
With support from the Species at Risk Research Fund for Ontario, an Environmental DNA (eDNA) survey protocol was developed and tested (MNRF 2015). This method could be used to rapidly detect genetic material shed by Jefferson Salamanders into the environment and could be used to better understand the distribution and occurrence of that species across its range. The Biodiversity Institute of Ontario at the University of Guelph is currently conducting a study to assess the detection probability of eDNA for the Jefferson Salamander, quantify the distribution of eDNA across space and time in multiple vernal pools, and to determine if this type of detection is a viable means to monitor this species (S. Crooks pers. comm. 2017). Initial results have been promising for detection of Jefferson Salamander and unisexuals. Since the eDNA method currently targets mitochondrial DNA, which is virtually the same in all unisexual Ambystoma, it cannot distinguish between Jefferson dependent and Blue-spotted dependent unisexuals (J. Bogart pers. comm. 2018). Once unisexuals, which are the most common members of the complex in most ponds in southern Ontario, are confirmed in a pond through eDNA methods, more intensive sampling can be done to determine whether the sperm donor is a Jefferson Salamander (J. Bogart pers. comm. 2018).
1.3 Distribution, abundance and population trends
Global range
The global distribution of the Jefferson Salamander is restricted to eastern North America, extending from Illinois in the west, which has isolated populations in only two eastern counties (Petranka 1998), across Indiana, Kentucky and West Virginia to Virginia in the east and northeast to Vermont and New Hampshire. The Canadian range of the Jefferson Salamander is only known to occur in southern Ontario and represents the species’ northern range limit (Figure 7).
Throughout northeastern North America, unisexual Ambystoma are found in association with their sperm-donating bisexual salamander species [Small-mouthed Salamander (Ambystoma texanum), Blue-spotted Salamander or Jefferson Salamander]. Unisexual Ambystoma do not extend to the northern limit of the Blue-spotted Salamander, however. Their northern limit only extends to north-central Ontario, southern Quebec, and Minnesota (COSEWIC 2016).
Jefferson dependent unisexuals are found in association with Jefferson Salamander populations throughout the Jefferson Salamander range. Genetic data describing the salamanders’ genomotypes are unavailable for much of the range, so the precise distribution of Jefferson Salamander compared to Jefferson dependent unisexuals is not known (Bogart and Klemens 1997). Jefferson dependent unisexuals have, however, been confirmed in 10 of the 14 states where the Jefferson Salamander occurs (Connecticut, Indiana, Kentucky, Massachusetts, New Hampshire, New Jersey, New York, Ohio, Pennsylvania, and Vermont) (Bogart and Klemens 2008, Bogart pers. data 2017). Jefferson dependent unisexuals have also been confirmed in southeastern Michigan, although Jefferson Salamander has not yet been documented in the state (Bogart pers. data 2017). The presence of the Jefferson dependent unisexuals in southeastern Michigan indicates that Jefferson Salamander likely were, or are still, present in the area.
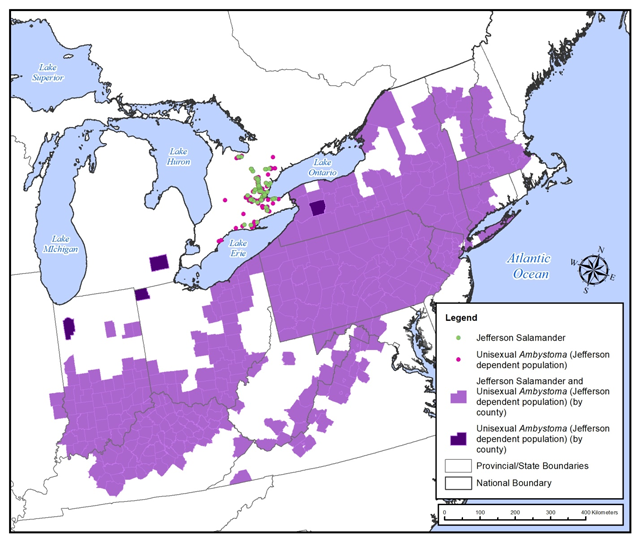
Figure 7. Global Range of Jefferson Salamander and/or unisexual Ambytoma Jefferson dependent population (Map produced by Natural Resource Solutions Inc. based on NatureServe 2016 and Bogart pers. data 2017).
The current global conservation status rank for the Jefferson Salamander is G4 or ‘Apparently Secure’, a level of ranking assigned to species with greater than 100 site occurrences and greater than 10,000 individuals (NatureServe 2016). In the United States, the Jefferson Salamander is nationally listed as ‘Apparently Secure’ (N4) as of 2001, although it is designated as ‘Imperilled’ (S2) in Illinois, Vermont and West Virginia and is considered to be ‘Apparently Secure’ (S4) in 4 of the 14 states where it is found (Table 3). In Canada, the Jefferson Salamander was assessed as ‘Imperilled’ (N2) in 2011 (NatureServe 2016) and is listed as endangered on Schedule 1 of the federal Species at Risk Act (SARA 2002). Jefferson Salamander was assessed as ‘Imperilled’ (S2) in Ontario and is listed as endangered under the Ontario provincial ESA, 2007.
Jefferson dependent unisexuals currently have no global conservation status ranking (NatureServe 2016). Several jurisdictions have provided Jefferson dependent unisexuals legal protection along with the Jefferson Salamander. In Connecticut, the Jefferson Salamander “complex”, and in Massachusetts, the Jefferson dependent unisexuals are listed as Special Concern (Connecticut Department of Energy and Environmental Protection 2015, Massachusetts Division of Fisheries and Wildlife 2016). Jefferson dependent unisexuals were assessed by the Committee on the Status of Species at Risk in Ontario (COSSARO) in December 2016 as endangered (COSSARO 2016). This resulted in the species being added to the SARO List under the provincial ESA, 2007 in June 2017 and being ranked S2 in Ontario (NHIC 2017).
| Jurisdiction | Conservation Status Rank for Jefferson Salamander | Conservation Status Rank for Jefferson dependent unisexuals |
|---|---|---|
| Global | G4 | GNR |
| Canada | N2 | NNR |
| Ontario | S2 | S2 |
| United States | N4 | SNR |
| Connecticut | S3 | SNR |
| Illinois | S2 | SNR |
| Indiana | S4 | SNR |
| Kentucky | S4 | SNR |
| Maryland | S3 | SNR |
| Massachusetts | S2S3 | SNR |
| Michigan | SNR | |
| New Hampshire | S2S3 | SNR |
| New Jersey | S3 | SNR |
| New York | S4 | SNR |
| Ohio | SNR | SNR |
| Pennsylvania | S3S4 | SNR |
| Vermont | S2 | SNR |
| Virginia | S4 | SNR |
| West Virginia | S2 | SNR |
Table 3 Legend:
- N2/S2 – Imperilled (i.e. extremely rare or especially vulnerable)
- S2S3 – The status could range from Imperilled to Vulnerable
- S3 – Vulnerable to extirpation or extinction (i.e. rare and uncommon)
- S3S4 – The status could range from Vulnerable to Apparently Secure
- G4/N4/S4 – Apparently Secure (i.e. uncommon but not rare)
- SNR/NNR/GNR –conservation status not yet assessed.
Canadian range
In Canada, Jefferson Salamander and Jefferson dependent unisexuals, generally occur in the eastern portion of the Carolinian zone and along the Niagara Escarpment in Ontario. There are also geographically isolated populations dispersed throughout the range (Figure 8).
There are records of Jefferson Salamander in Brant County, the City of Hamilton, Dufferin, Elgin, Grey and Haldimand Counties, Halton and Niagara Regions, Norfolk County, Peel and Waterloo Regions, Wellington County, and York Region (Figure 8). There are records of Jefferson dependent unisexuals, as they occur in all known Ontario Jefferson Salamander populations, in all the areas listed above as well as in the Municipality of Chatham-Kent, Durham Region, and in Oxford and Perth Counties (Figure 8).
Percentage of the global distribution in Canada
Populations of the Jefferson Salamander and Jefferson dependent unisexuals in Canada are situated at the northern limit of the species’ North American range. The Canadian populations probably represent a maximum of one to three percent of the estimated North American population, based on relative ranges (Rye and Weller 2000) (Figure 8).
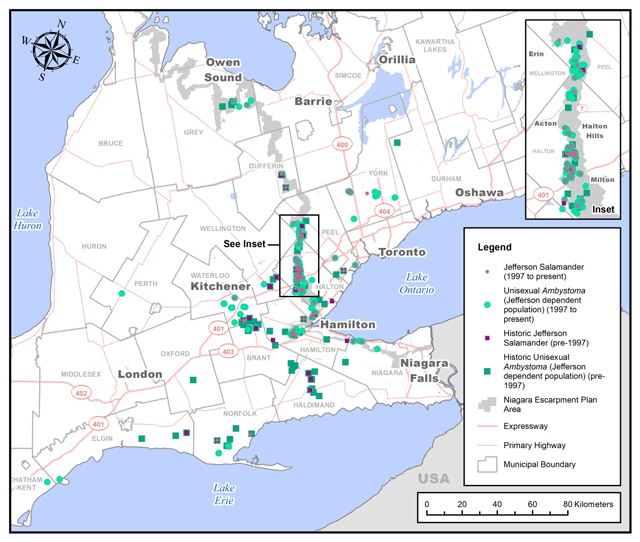
Figure 8. Historical and current distributions of the Jefferson Salamander and unisexual Ambystoma Jefferson dependent population in Ontario (based on the database of all Ontario locations that was compiled by the Recovery and Implementation Team and housed by Dr. James Bogart at the University of Guelph).
Population sizes and trends
The present knowledge of this species indicates that the current isolated sub-populations are remnants of what was once a more extensive, continuous range throughout southern Ontario. Fragmentation and loss of habitat have led to the isolation of these sub-populations. In southern Ontario, 63 percent of the original forests and over 85 percent of historic wetlands have been lost since European settlement (Butt et al. 2005, DUC 2010). Habitats have been further lost and fragmented as a result of large-scale agriculture, urbanization, road networks and resource development activities, such as aggregate extraction.
Long-term, detailed trend data are not available for Jefferson Salamander and Jefferson dependent unisexuals across the entire range in Ontario due to the difficulties differentiating these species without genetic data. Prior to 2004, the method for genetically confirming individuals required that they be sacrificed for analyses (Bogart 1982). This made it impossible to determine the absolute frequencies of bisexual and unisexual individuals in populations or to estimate trends over time (COSEWIC 2016).
Normally, estimations of the distribution of vertebrate species may be obtained from museum records and voucher specimens. Historical identifications of Jefferson Salamander and unisexual Ambystoma specimens are, however, not necessarily accurate because genetic analysis techniques were not available. Unfortunately, it is very difficult to retrieve DNA from museum specimens as they were preserved in formalin and stored in ethanol (J. Bogart pers. comm. 2017). Therefore, it is not likely possible to distinguish between individuals of the complex that are catalogued in major museum collections.
New methods for genetic testing (using microsatellite DNA loci) allow for many individuals to be genotyped in a relatively short period of time using only small tissue samples (Ramsden et al. 2006, Bogart et al. 2007). Population trends have still only been estimated for very few populations, however, using observed numbers of egg masses over time (COSEWIC 2016). To collect accurate population trend data would require intensive survey efforts over multiple years which present limitations in terms of logistics and resources. Trends in population density and inferences on presence/absence data can only be estimated through repeated annual surveys of the same ponds combined with surveying several ponds in the same year (COSEWIC 2016). To ensure the protection of the species, restrictions on sampling effort are also applied (e.g. standard protocols typically only allow for the collection of up to 20 tissue samples in a given pond per year).
Recent estimates, based on the best available long-term data sets for Jefferson Salamander, suggest a decline of more than 90 percent over the last three generations (33 years) (COSEWIC 2016). Repeat surveys over a 15-year timeframe (1990 to 2005) revealed that most subpopulations were declining and some were extirpated (COSSARO 2016). For example, surveys of 18 historically known breeding sites along the Niagara Escarpment that were documented in 1990 to 1991 revealed only three that were confirmed to still be supporting salamander populations in 2003 to 2004 (COSEWIC 2010). Overall, from 1990 to 2005, no subpopulation of Jefferson Salamanders in Ontario was estimated to be larger than when originally found (COSSARO 2011).
Based on the database that was compiled by the Recovery and Implementation Team (Figure 8) and the definition of a site as one or more breeding pond within 1 km of each other (COSEWIC 2016), a total of 40 sites in Ontario have been confirmed where Jefferson Salamander is known to occur. Twenty-eight of these sites have Jefferson Salamander observations from within the last 20 years (1997-present), while the remaining 12 sites have no recent occurrences (i.e. no documented observations since 1997). Jefferson dependent unisexuals have been confirmed in Ontario at a total of 83 sites, which includes sites where Jefferson Salamander are also known to occur. Fifty-three of these sites have observations of Jefferson dependent unisexuals from within the last 20 years (1997-present), while the other 30 sites have no recent documented occurrences.
It is difficult to determine whether or not these species still occur at sites with historic records from greater than 20 years ago. Breeding habitat can be dynamic with conditions varying from year to year, depending on precipitation and water levels. This affects levels of breeding activity and success. Due to this variability, a minimum of three consecutive years of surveys are required at historic sites to determine the species’ absence with any degree of confidence. Such monitoring effort is rare. Although data is limited, it is anticipated that some of these populations are extirpated because of habitat changes associated with anthropogenic disturbance. For example, some historically-used breeding ponds have been stocked with predatory fish, some no longer hold water for the required time for larval development, and some have been lost to development (COSEWIC 2010).
Only a few studies have genetically identified large numbers of individuals in a given subpopulation to estimate comparative abundance of bisexual and unisexual individuals (see Table 4). In six studies that involved sample sizes larger than 100 individuals, the percentage of Jefferson dependent unisexuals ranged from approximately 60 to 92 percent of sampled individuals (Table 4).
| Subpopulation | (n) | JJ | LJJ | LJJJ | Source |
|---|---|---|---|---|---|
| Kitchener (site 1) |
142 | 12 (8.45%) |
111 (78.17%) |
19 (13.38%) |
Featherstone (2007, unpubl. data) |
| Kitchener (site 1) |
190 | 15 (7.89%) |
139 (73.16%) |
36 (18.95%) |
Featherstone (2008, unpubl. data) |
| Kitchener (site 2) |
43 | 0 | 38 (88.37%) |
5 (11.63%) |
NRSI (2009, unpubl.data) |
| Kitchener (site 2) |
20 | 0 | 20 (100%) |
0 | Linton et al. (2016, unpubl. data) |
| Hilton Falls CA |
520 | 168 (32.31%) |
337 (64.81%) |
15 (2.88%) |
Ramsden (2008) |
| Waterdown |
118 | 11 (9.32%) |
103 (87.29%) |
4 (3.39%) |
Pisapio (2007, unpubl. data) |
| Erindale |
2865 | 426 (14.87%) |
2439 (85.13%) |
0 | Weller (1980) |
| Dundas |
248 | 100 (40.32%) |
140 (56.45%) |
8 (3.23%) |
Linton et al. (2017, unpubl. data) |
Note: Frequencies are provided in numbers of individuals of each genomotype. All unisexual genomotypes have at least one A. laterale (L) chromosome complement and one or more A. jeffersonianum (J) complement or genomes. Diploids have 2, triploids have 3, and tetraploids have 4 chromosome complements.
1.4 Habitat needs
Jefferson Salamanders inhabit deciduous or mixed upland forests containing, or in close proximity to, suitable ponds for breeding (Klemens 1993). Jefferson Salamanders show fidelity to both their terrestrial and breeding habitats (Thompson et al. 1980, COSEWIC 2016, COSEWIC 2010, De Lisle and Grayson 2011). Jefferson dependent unisexuals use similar macro- and micro-habitats as bisexual Jefferson Salamanders (Bériault 2005, COSEWIC 2016, Hoffman 2017).
Terrestrial habitat
Following metamorphosis, Jefferson Salamanders and Jefferson dependent unisexuals are primarily terrestrial, using mature upland deciduous or mixed forest habitats for foraging, summer and fall movements, overwintering, and migration to and from breeding ponds (COSEWIC 2010). In general, these habitats are well-shaded, have a thick layer of leaf litter, high soil moisture, and lower substrate temperatures than random sites (Faccio 2003, Hoffman 2017). The amount of sub-canopy vegetation may or may not be an important factor for these salamanders. In Vermont, their forest habitats were characterized by a dense low shrub layer (Faccio 2003), whereas sites in Maine had lower levels of sub-canopy vegetation cover (<1 m tall) than random sites (Hoffman 2017). Various refugia are used during the active season including the underside of rocks, woody material and bark, beneath leaf litter, inside rotten logs, in rock fissures and between large rocks (Bériault 2005). The most commonly used active season refugia are small mammal burrows (Bériault 2005) which tend to be horizontal and highly branching (Faccio 2003).
Jefferson Salamanders and Jefferson dependent unisexuals may migrate through a variety of habitats during breeding migration movements including woodlands, plantations, agricultural fields, early successional areas, and across roads (COSEWIC 2010, 2016). Radio-telemetry studies have documented that post-breeding migratory movements of adults can range from hundreds of metres up to one kilometre from the breeding pond into surrounding habitat (Semlitsch 1998, Faccio 2003, Bériault 2005, COSEWIC 2016). While some individuals have been observed moving to locations outside forest habitats, under buildings, or near forest-lawn edges and roads, the vast majority of adults appear to stay within the forest habitat (Hoffman 2017). Radio-telemetry studies conducted in Ontario found that 90 percent of adults stay in the deciduous forest habitats within 300 m of their breeding pond (Bériault 2005, COSEWIC 2016).
Post-breeding observations of juveniles and adults are infrequent as they mostly remain secluded underground (Matson 2013). Very few studies have examined autumn movements of Ambystomatid salamanders. Two studies in Ontario investigated post-breeding terrestrial movements of Jefferson Salamander and/or Jefferson dependent unisexuals in Ontario through radio-telemetry (Beriault 2005, COSEWIC 2016). Neither of these studies, however, extended into the autumn to look at overwintering site selection and habitat use. For example, the Ontario Ministry of Natural Resources and Forestry (MNRF) Aurora District Office and Conservation Halton conducted radio-telemetry of post-breeding adult dispersal near Waterdown, Ontario but the radio transmitters’ batteries died by August so no insights into fall movements or specific overwintering habitats were gained (B. Van Ryswyk pers. comm. 2017).
Faccio (2003) used radio-telemetry to examine post-breeding terrestrial habitat use by Spotted Salamander (n=8) and Jefferson Salamander (n=8) between May and November in Vermont. Jefferson Salamanders moved an average of 122.6 +/- 44.4 m with a range of 11–405 m between their release point and their final overwintering site (Faccio 2003). This study concluded that overwintering occurs in deep, vertical small mammal burrows, and likely other small rock crevices or fissures, which extend below the frost line (Faccio 2003).
Breeding ponds
Mating, oviposition and larval development occurs in breeding ponds located in or near high quality forest habitats, including in limestone sinkhole ponds, kettle ponds and vernal pools (Nyman 1991) that have a sufficiently long hydro period (Matson 2013). These ponds are generally fed by groundwater, snowmelt or surface water, and dry in mid to late summer (COSEWIC 2010). Breeding ponds must be devoid of predatory fish and have sufficient egg mass attachment sites in the water, such as shrubs, twigs, fallen tree branches, submerged riparian vegetation or emergent vegetation (Thompson et al. 1980).
One study in Ontario found that breeding pond water depth, water temperature, pH, and other water-chemistry and water-quality parameters were not good predictors of the use of breeding ponds by Jefferson Salamander or Jefferson dependent unisexuals (Bériault 2005). Bériault acknowledged, however, that the sample size was small and that a narrow range of wetland types (wetlands that were confirmed breeding ponds or wetlands that looked suitable) were included in the study (2005). Jefferson Salamander larvae are not particularly susceptible to relatively low pH (COSEWIC 2010). Ample food to sustain the larvae must be present in breeding ponds. This includes small aquatic invertebrates and other amphibian larvae (COSEWIC 2010).
1.5 Limiting factors
Characteristics of Jefferson Salamander’s and Jefferson dependent unisexual’s life histories or ecology that may be limiting factors in their recovery include:
- Intermittent juvenile recruitment;
- Limited dispersal ability;
- Terrestrial and breeding site fidelity;
- Requirement of the presence of male Jefferson Salamander sperm donors; and
- For Jefferson dependent unisexuals, competition with and/or predation by Jefferson Salamander during their larval stages.
1.6 Threats to survival and recovery
The following threats to the Jefferson Salamander and Jefferson dependent unisexuals are presented in order of priority. This assessment is based on an analysis compiled by the Jefferson Salamander Recovery and Implementation Team with input from relevant land managers, a recent status assessment of Jefferson dependent unisexuals by COSSARO (2016) and an International Union for Conservation of Nature (IUCN) threats calculator to inform a COSEWIC status report on Jefferson dependent unisexuals (2016). It is inferred that threats to Jefferson Salamander and sympatrically occurring Jefferson dependent unisexuals are equal in terms of severity and scope.
High-impact threats to the survival and recovery of these salamander populations include habitat loss and degradation and fragmentation of woodlands and breeding ponds. This is attributed to a variety of activities and land uses described in more detail below. Most sub-populations are also exposed to high-medium road-related threats which include direct mortality, barriers to movement, and road-related pollutants (COSEWIC 2016). Additional low-impact threats include the introduction of carnivorous fish to breeding ponds, which can prey upon the egg, larval and adult stages of the species (MNRF 2015), agricultural land conversion, and free range livestock (COSEWIC 2016).
For consistency, the following discussion of threats is organized according to the IUCN-CMP (Conservation Measures Partnership) unified threats classification system (see Master et al. 2009 and CMP 2010 for details). Consistent with this approach, threats may be observed, inferred, or projected to occur in the near-term.
Habitat loss, fragmentation and degradation are considered the greatest threats to Jefferson Salamanders and Jefferson dependent unisexuals across their global range, including Ontario. Activities associated with urbanization, aggregate extraction and other resource development are the most significant threats to Jefferson Salamander and unisexual Ambystoma in southern Ontario.
Residential and commercial development (impact “high”)
The Carolinian forest reaches the northern limit of its distribution in southern Ontario, but the vast majority of this habitat in Ontario has been cleared, initially for agriculture and subsequently for urban development (COSSARO 2016). There is currently limited remaining habitat
Anthropogenic threats include development activities that result in the direct loss of habitat from a development footprint, cumulative loss and degradation of habitat, and fragmentation of breeding ponds and woodlands (see also Transportation and Service Corridors below). Habitat continues to be lost as a result of housing development, especially in areas experiencing rapid urban sprawl such as the Hamilton and Kitchener-Waterloo areas (COSEWIC 2016). Impacts from development include site clearing and grading that result in wetland filling, altered cover, topography and drainage patterns. Any alteration to the contributing drainage area of a wetland has the potential to negatively impact its hydrology and associated ecological function.
Development also increases impervious land cover, reduces groundwater recharge, and results in stormwater management which leads to sedimentation and altered natural hydroperiod regimes, water balance of adjacent wetlands (e.g. shorter hydroperiods), and soil moisture content. Watercourse realignments through forests and swamps also have the potential to alter wetland hydrology and salamander breeding ponds specifically. During development, silt fencing can also prevent and/or hinder migration of salamanders if it is not properly positioned or timed.
Premature drying of ponds can result from the removal of a part of the protective canopy, drawing down the water table in developed areas, or altering watercourses for snowmelt and runoff. The reduction of vernal pond “envelopes” and buffer zones also has been suggested as contributing to the reduction and possible elimination of Ambystoma species (Calhoun and Klemens 2002).
Energy production and mining (impact “high”)
The Niagara Escarpment, which represents a substantial portion of the species’ range in Ontario, is a significant aggregate extraction area. When breeding ponds are filled or drained, local extirpations are inevitable. Any resource development activity that may alter the water table or cause a disruption or modification to groundwater flow has the potential to alter wetland hydroperiods and breeding habitat, water balance, wetland function and soil moisture regimes in adjacent salamander habitat. The presence of adequate water in the breeding ponds for the duration of the larval development period is critical to population recruitment.
Transportation and service corridors (impact “high – medium”)
Some roads (and urbanization) can create barriers that limit salamander dispersal and abundance. Southern Ontario has a dense network of roads and salamanders are frequently killed on roads by vehicles while migrating to or from a breeding pond (Beebee 2013). Road-kill is expected to have severe impacts on local populations of Jefferson Salamanders and Jefferson dependent unisexuals. Using data from 500 Spotted Salamander breeding ponds in Massachusetts, Gibbs and Shriver (2005) estimated an annual risk of road mortality of more than 10 percent can lead to local population extirpation. With a mortality risk of 20 to 30 percent, the entire population would be extirpated within 25 years (Gibbs and Shriver 2005). Road-kill is substantial in some areas in southern Ontario despite mitigation attempts (e.g. road closures close to some breeding sites) (COSEWIC 2016).
Curbs can act as barriers to migratory movements and/or dispersal and catch basins can result in trapped individuals. Roads also are a source of chemicals and pollutants (e.g. salt) that degrade adjacent aquatic and terrestrial habitat. Toxic effects of road salt application can extend considerable distances into wetlands and have been observed to have detrimental effects generally on amphibians and specifically on Spotted Salamanders (Turtle 2000, Karraker et al. 2008, Collins and Russell 2009). Roads also create zones of disturbance characterized by noise and light pollution, and may contribute to the desiccation of migrating adult salamanders or increase their vulnerability to predators.
Many woodlands in the province are traversed by utility easements (e.g. pipelines and hydro corridors), which require occasional maintenance work and often removal of vegetation. This work also has the potential to negatively impact the species and its habitat if appropriate mitigation measures (e.g. avoidance of sensitive timing windows, erosion and sediment control measures) are not implemented.
Agriculture and aquaculture (impact “low”)
Current rates of agricultural land conversion in southern Ontario are low; however where it occurs, the impacts to local populations can be severe if terrestrial salamander habitats are converted and/or breeding ponds are drained (COSEWIC 2016). Ongoing and new agricultural activities have the potential to cause further habitat loss and an increase in surface runoff, which could potentially have a negative impact on adjacent wetlands, including salamander breeding ponds. The installation of tile drains also has the potential to negatively impact wetlands and their adjacent areas.
Non-vegetated open areas such as agricultural fields may be used as migratory corridors between the breeding pond and forested areas. The extent to which agricultural practices (e.g. tilling) and chemical application impact individuals travelling through these habitats is not known.
Jefferson Salamanders and Jefferson dependent unisexuals are generally associated with deciduous woodlands making them vulnerable to forestry activities such as hazard tree removal and selective harvesting. Forestry activities and the equipment used may result in the filling of vernal pools, alteration of vernal pool hydrology, sedimentation, leaf litter and soil compaction removal or alteration of associated upland habitat (removal of canopy cover, stumps, logs and leaf litter, and alteration of nutrient inputs by leaves), pollution and fragmentation or isolation of vernal pools from the terrestrial habitat. The negative effects of forestry activities are not anticipated to be frequent but when they do occur, they could be severe.
Invasive and problematic species (impact “low”)
The potential impact of invasive species on Jefferson Salamander and Jefferson dependent unisexual populations is generally understudied. Although specific data are limited, introduced zooplankton is becoming an ecosystem-level problem in southern Ontario. Native arthropods are reluctant to feed on them and as a result, the salamander’s prey base could potentially be affected (COSEWIC 2016). Invasion by aquatic plants such as Common Reed (Phragmites australis), may also degrade breeding habitat, although specific data are unavailable.
Ambystomatid salamanders do not thrive with predatory fish, and many documented Jefferson Salamander breeding sites where the species no longer exists were noted to have been stocked with fish (COSEWIC 2016). Large predatory fish will prey on all life stages of the salamanders. Goldfish in Jefferson Salamander breeding ponds and associated potential impacts is an emerging concern, particularly for the Hamilton Conservation Authority (M. Stone, pers. comm. 2017).
Batrachochytrium salamandrivorans (Bsal) is a fungal pathogen that causes chytridiomycosis in salamanders and newts (Palahnuk and Buchanan 2015). It is thought to have originated in Asia and has recently been introduced to Western Europe where it is causing rapid population declines in European Fire Salamanders (Martel et al. 2014). A Chytrid Fungus Monitoring Project began in Ontario in 2013. To date, Bsal has not been reported in Ontario or anywhere else in North America (Palahnuk and Buchanan 2015). There are several other emerging amphibian pathogens such as Severe Perkinsea Infections (SPI) that are causing significant mortality in frogs (Isidoro-Ayza et al. 2017), however at this time there is no evidence to indicate it is a threat to salamander larvae.
In at least one known site, the presence of North American Beaver (Castor canadensis) has the potential to alter breeding pond hydrology (A. Featherstone pers. comm. 2017).
Climate change and severe weather (impact unknown)
The impacts that future climate change will have on Jefferson Salamanders and sympatrically occurring unisexuals is not known. Climate change predictions for southern Ontario include warmer temperatures, more winter precipitation, less summer precipitation (McDermid et al. 2015), and more extreme weather events such as droughts or flooding (IPCC 2014). Breeding occurs in ephemeral ponds; however adequate water must remain to support larval development through to transformation. Occasional early drying of vernal pools from prolonged droughts is likely normal and not detrimental to populations because adults have several breeding seasons and are long-lived (COSEWIC 2016). Multiple years of drought, however, especially consecutive years, would likely impact populations. The balance between winter snow accumulation, summer precipitation and water losses due to increased temperatures and evaporation will ultimately determine whether drought becomes an issue for salamander breeding. Additional impacts to breeding ponds could occur due to more rapid snowmelt in the spring and intense rainfall events, which could increase runoff, erosion, sedimentation and decrease water retention, Mid-winter warm periods, which are more likely under current climate change scenario predictions (McDermid et al. 2015), can be problematic for the species, as well. In 2017, several jurisdictions reported early migrations to breeding ponds by Jefferson Salamander and Jefferson dependent unisexuals in late February. This warm period in 2017 was followed by a rapid drop in temperature and it is likely that many individuals, and potentially any egg masses laid, would have perished.
Biological resource use (impact unknown)
Collection of amphibians and reptiles for the pet trade is a growing concern and may be a threat to the Jefferson Salamander and unisexual Ambystoma. Specific location information is considered data sensitive and is not widespread in the general public.
Human intrusions and disturbance (impact unknown)
Heavy use by hikers, cyclists and all-terrain vehicle (ATV) users of recreational trails near breeding pools and in terrestrial habitats may result in salamander mortality or habitat degradation. ATVs sometimes damage breeding ponds, usually later in the summer, which may pose a threat to new metamorphs (COSEWIC 2016). At one site in Ontario, ATV-use has led to a substantial decline in high quality habitat (A. Featherstone pers. comm. 2017).
1.7 Knowledge gaps
Key knowledge gaps relating to the Jefferson Salamander and Jefferson dependent unisexuals include (but are not limited to) the following:
- Population abundance and proportion of Jefferson Salamander and Jefferson dependent unisexuals within subpopulations, as well as trends in these data over time;
- The effectiveness of mitigation efforts to address threats;
- The species’ current distribution and range, particularly in portions of the Oak Ridges Moraine Plan Area and the Greenbelt Plan Area;
- The impacts of agricultural practices on breeding ponds and migration;
- The species’ spatial ecology, including dispersal patterns, timing and distances; and
- Habitat use, particularly the location and characteristics of overwintering sites.
1.8 Recovery actions completed or underway
The Jefferson Salamander has been protected from being killed, harmed, harassed, captured or taken since the ESA, 2007 came into force in 2008. Jefferson dependent unisexuals received equal protection when they were listed as endangered in 2017. Habitat protection for Jefferson Salamander has been in place since 2010 when the Ontario government completed a habitat regulation for the species. This habitat regulation, which was developed in response to the endangered status of the Jefferson Salamander, also protected habitat for sympatrically occurring Jefferson dependent unisexuals because they cannot persist without Jefferson Salamander being present.
Protecting the Jefferson Salamander and enforcing the regulation are key components in the implementation of the ESA 2007 and continue to be government-led actions, as identified in the Government Response Statement (MNRF 2015). Through the Species at Risk Stewardship Fund, the MNRF has supported more than 40 projects designed to contribute to the protection and recovery of the Jefferson Salamander (MNRF 2015).
Work on several of the original recommended recovery objectives identified for Jefferson Salamander in the 2010 Recovery strategy for the Jefferson Salamander (Ambystoma jeffersonianum) in Ontario has begun, and a number of studies on the species have been completed. A large proportion of this work informed the revision and development of updated recovery objectives for Jefferson Salamanders and Jefferson dependent unisexuals.
Identify and monitor extant populations of the Jefferson Salamander in Ontario
(Recovery objective 1)
In 2002 and 2003, the Recovery and Implementation Team worked with the Regional Municipality of York to determine whether Jefferson Salamander and Jefferson dependent unisexual subpopulations existed in York Region. Field investigations revealed four subpopulations of Jefferson Salamander which are the only known occurrences in York Region and represent the easternmost occurrences in Ontario.
In 2003, the Recovery and Implementation Team formed a partnership with the University of Guelph to update the database of all known Jefferson Salamander and Jefferson dependent unisexuals occurrences. More than 100 wetlands with the potential to support Ambystoma species were searched to determine whether the Jefferson Salamander was present.
Also in 2003 and 2004, the Niagara Escarpment Biosphere Reserve, in partnership with Niagara Escarpment Commission’s Ontario’s Niagara Escarpment (ONE) Monitoring Program staff, and the University of Guelph, under the direction of the Recovery and Implementation Team, undertook a study to examine the location and habitat conditions of Jefferson Salamander breeding sites along the Niagara Escarpment. The study focused on historically known breeding locations that the University of Guelph had documented in 1990 and 1991.
In 2004, also under the direction of the Recovery and Implementation Team, a number of conservation authorities, including Grand River Conservation Authority, Hamilton Conservation Authority, Conservation Halton, Credit Valley Conservation, and Toronto and Region Conservation Authority, allocated staff time and resources to revisiting breeding sites previously known to support the Jefferson Salamander, and to investigating other potential habitats within their watersheds.
In 2006 and 2007, the University of Toronto Mississauga, Evergreen, EcoSource and Credit Valley Conservation, under the direction of the Recovery and Implementation Team, partnered to assess groundwater contributions and the potential impacts from recreational trails on a Jefferson Salamander breeding pond in Peel Region.
In 2013, the Recovery and Implementation Team updated a documented titled Sampling Protocol for Determining the Presence of Jefferson Salamanders (Ambystoma jeffersonianum) in Ontario. It is updated periodically to reflect the best scientific information available and feedback on the success of the methods it outlines. This document, which is equally applicable to Jefferson dependent unisexuals, includes information on salamander ecology, survey methods for determining presence/absence, recommendations on avoiding between-site contamination and permitting requirements to carry out survey work. This standardized data collection protocol has ensured consistent data collection since it was first produced in 2012. The Niagara Peninsula Conservation Authority (NPCA) completed Jefferson Salamander surveys since 2013 at Woolverton, Cave Springs, and Wainfleet Bog Conservation Areas (K. Frohlich pers. comm. 2018). Jefferson Salamander and Jefferson dependent unisexuals were documented in 2013 and 2014 at Woolverton Conservation Area. Jefferson dependent unisexuals were documented at Cave Springs Conservation Area, where NPCA completed surveys for four years (2013, 2015-2017), with a fifth year planned for 2018. Although surveys were completed at Wainfleet Bog for three consecutive years (2013-2016), no Jefferson Salamander or Jefferson dependent unisexuals were documented.
The MNRF Guelph District Office has been surveying for Jefferson Salamanders at various sites since 2009 (G. Buck pers. comm. 2018). No Jefferson Salamander or Jefferson dependent unisexuals were captured in surveys at one site in Brant County (2009) or at one site off Maltby Road south of Guelph (2011). In 2011, the MNRF Guelph District Office also partnered with the Hamilton Conservation Authority (HCA) to conduct surveys in the Dundas Valley. Jefferson Salamander and Jefferson dependent unisexuals were confirmed and re-confirmed at six new and historic ponds throughout the area. HCA has since continued surveys in this area. In 2015, surveys were completed at two ponds in the Sudden Bog Area of Natural and Scientific Interest (ANSI), and at various locations in Puslinch Township (five ponds near Crieff), Flamborough (two ponds near Troy), and just outside Paris. Of these sites, Jefferson dependent unisexuals were only captured in the Sudden Bog ANSI. Surveys conducted again in Puslinch Township in 2016 were also negative. Finally, the MNRF sampled three ponds in the Wilmot and Hoffsetter Tracts just west of Kitchener (near Petersburg) in 2017; no Jefferson Salamander or Jefferson dependent unisexuals were captured.
Species and ploidy identification
At the University of Guelph, microsatellite molecular markers for the Jefferson Salamander (Julian et al. 2003) have been, and continue to be, used effectively to identify and distinguish Jefferson Salamanders from Jefferson dependent unisexuals. These markers may also help address other questions regarding population dynamics and genetics that involve the unisexual members of the complex.
Through the Species at Risk Research Fund for Ontario, an Environmental DNA (eDNA) survey protocol was developed and tested (MNRF 2015). This method could be used to rapidly detect genetic material shed by Jefferson Salamanders into the environment and could be used to better understand the distribution and occurrence of the species across its range. The Biodiversity Institute of Ontario at the University of Guelph is currently conducting a study to assess the detection probability of eDNA for Jefferson salamander, quantify the distribution of eDNA across space and time in multiple vernal pools, and to determine if this type of detection is a viable means to monitor this species (S. Crooks pers. comm. 2017).
Apply research findings on the species’ movements and habitat use to ensure protection of habitat
(Recovery objective 2)
Post-breeding adults
In 2004, the University of Guelph initiated a radio-telemetry study focused on the movement and habitat use of 16 triploid Jefferson dependent unisexual individuals in Halton Region (Bériault 2005). The MNRF continued and expanded the study in 2005, with another 17 Jefferson dependent unisexuals from the same Halton Region location and 19 individuals at two different sites in Peel Region. In 2007 and 2008, MNRF conducted additional radio-telemetry monitoring of both unisexuals and Jefferson Salamanders Halton Region. With a total sample size of 111, these studies have generated extensive data on the movements and terrestrial habitat use of post-breeding adult Jefferson Salamander and Jefferson dependent unisexuals.
Juveniles
In 2015, a multi-year study focused on juvenile dispersal in a population of Jefferson Salamander and Jefferson dependent unisexuals was initiated by Natural Resource Solutions Inc. (NRSI) as a condition of an “overall benefit” 17(2)(c) permit under the ESA 2007. The timing and patterns of dispersal of metamorphs from the study pond were closely monitored by marking and tracking each individual encountered at pitfall trap fences and arrays. This was the first study of its kind in Ontario, and possibly throughout their range, that genetically examined metamorph Jefferson Salamander and Jefferson dependent unisexuals rather than breeding adults.
Fall movements and overwintering locations
In 2017, NRSI conducted a fall radio-telemetry study of adult Jefferson Salamander and Jefferson dependent unisexuals, which provided new insights into fall movements and the location and character of overwintering areas.
Seasonal use of habitats
Patricia Huynh, a PhD Candidate at the University of Waterloo, is exploring the limiting factors of breeding success of Jefferson Salamander by monitoring pool hydrology, water quality, food availability, and juvenile dispersal in vernal pools in the Halton and Peel Regions. This research, which is in its early stages, is in collaboration with Halton Region, Conservation Halton, Credit Valley Conservation, and MNRF.
Stephen Van Drunen, a MSc Candidate in the Norris Lab at University of Guelph, , in collaboration with Natural Resource Solutions Inc., is studying demography, survival and annual movement patterns of Jefferson Salamander and Jefferson dependent unisexuals. This study will use data collected through NRSI’s juvenile dispersal and planned radio-telemetry research.
Develop a communication strategy to inform municipalities, planners, the development industry, property managers and other stakeholders of the habitat mapping and protection requirements for the Jefferson Salamander under the ESA 2007 and other recovery planning initiatives
(Recovery objective 4)
In May 2003, MNRF ran workshops in Halton Region and Waterloo Region that provided instruction on Jefferson Salamander egg mass identification and outlined the protocol for obtaining samples for genetic analyses. Recovery and Implementation Team members, many of whom are associated with, or work for, regional conservation groups or authorities, attended these workshops.
In Niagara, Halton, and Peel Regions, viewing platforms and interpretive signs have been installed next to Jefferson Salamander breeding ponds to protect them from visitor-related impacts while providing the public with an opportunity to observe and learn about this important species and its habitat. This educational opportunity was promoted through the press for the platform and signs that were installed in the Niagara Region.
A public tour program ran in the Oak Ridges Moraine from 2009 to 2011 to educate the public about the Jefferson Salamander (in addition to other species at risk) and its habitat. This program reached a total of 257 students and members (MNRF 2015). Over 55 “protection and recovery” 17(2)b permits under the ESA 2007 have been issued by the MNRF pertaining to the Jefferson Salamander (MNRF 2015). These types of permits are issued if the activity of the permit holder would assist in the protection or recovery of the species. These permits enabled a variety of organizations to undertake activities such as conducting surveys to verify and document the locations of Jefferson Salamander populations, restoring habitat, managing invasive species, and installing a boardwalk and fencing to minimize human impacts (MNRF 2015).
Develop and evaluate mitigation and restoration techniques employed to address threats
(Recovery objective 5)
Road closures
Annual road closures during the spring migration period have been implemented in the City of Burlington and the City of Kitchener to reduce mortalities associated with vehicle traffic. King Road in the City of Burlington has been closed every spring for a period of three weeks since 2012, and was determined to be effective if physical barriers (i.e. cement barriers) were put in place (B. Van Ryswyk pers. comm. 2017).
Stauffer Drive in the community of Doon South in the City of Kitchener has been closed for spring salamander movement formally since 2012 and was informally closed in several springs prior to 2012 (B. Steiner pers. comm. 2018). Road mortality of Ambystomatid salamanders was monitored on Stauffer Drive for five nights each year during their spring migration to/from suitable breeding habitat starting in 2008. This monitoring was originally required and undertaken as a condition of approval of Draft Plans for a Subdivision on the adjacent lands (B. Steiner pers. comm. 2018). From 2012 to 2016, as a condition of an ESA permit for the development north of Stauffer Drive, monitoring was increased to every night of the road closure, which varied year to year depending on weather conditions but generally ran from mid-March through to the first of May. The City anticipates permanently closing this road in 2018 (B. Steiner pers. comm. 2018).
These sorts of measures can be logistically challenging, particularly in years where the conditions suitable for migration occur at atypical times, however the approach and timing of the road closures are adequate to mitigate mortality during peak movements.
Eco-passages
MNRF Aurora District Office has five eco-passages being implemented, under ESA permits to reduce road mortality of Jefferson Salamanders in Peel Region (M. Heaton pers. comm. 2018).
Managing hydroperiod in breeding ponds
Dufferin Aggregates (a division of CRH Canada Group Inc.) has implemented a method to protect and enhance the hydroperiod in Jefferson Salamander breeding pools located near their Milton Quarry Extension. A Water Management System (WMS) was established around the perimeter of the quarry cells, in order to protect offsite water-dependent features (e.g. creeks, wetlands, Jefferson Salamander breeding pools, etc.) from the dewatering effects of quarrying dolostone from below the water table. The system includes a reservoir that holds groundwater and a system of pump stations, watermains and recharge wells that maintain offsite groundwater levels at seasonal targets and discharges water to nearby wetlands.
This method has proven to be very effective. One pond that was monitored annually from 2003 to 2008 had a suitable hydroperiod for salamander recruitment in only 1 of the 6 years. Since the commencement of WMS in 2009, this pond now has a suitable hydroperiod every year regardless of local climatic conditions and successful salamander breeding has occurred every year which has been confirmed through juvenile recruitment. Artificially maintaining the hydroperiod of salamander breeding ponds with similar WMS may be an important recovery strategy as climate change progressively renders the hydroperiod of more ponds unsuitable for salamander recruitment.
Address emerging pathogens
The Government of Canada has implemented a one-year import restriction on salamanders. The restriction, which is implemented through an amendment to the Wild Animal and Plant Trade Regulations (WAPTR) was approved by the Governor in Council and was published in the Canada Gazette, Part II, on May 31, 2017.
The purpose of the amendment is to prevent the introduction of Batrachochytrium salamandrivorans (Bsal), a pathogenic chytrid fungus that infects salamanders and newts (Palahnuk and Buchanan 2015), into Canadian ecosystems by temporarily prohibiting the import of all species of the order Caudata (such as salamanders, newts and mudpuppies) unless authorized by a permit issued by Environment and Climate Change Canada, for a period of one-year, until May 11, 2018.
During this time, the Government of Canada will explore longer-term measures to protect Canadian salamanders. Prohibiting the import of all salamander species is consistent with the precautionary principle, and takes into consideration the limited and evolving understanding of the disease, as well as the enforcement challenges associated with identifying different salamander species at Canada’s numerous ports of entry.
2.0 Recovery
2.1 Recommended recovery goal
The recommended recovery goal is to ensure that existing threats to populations and habitat of the Jefferson Salamander and Unisexual Ambystoma (Jefferson Salamander dependent population) are sufficiently removed to allow populations to become stable or increase in abundance and distribution throughout Ontario.
2.2 Recommended protection and recovery objectives
The focus of the short-term recovery objectives, and the recommended overall recovery goal, is the protection of existing populations of the Jefferson Salamander and Jefferson dependent unisexuals by ensuring that no further loss or degradation of known habitat or potentially suitable habitat (recovery habitat) occur. Habitat protection is critical to the survival of these species. Protection of existing habitat and enforcement of the habitat regulation should have priority over compensation for lost habitat (i.e. the creation of habitat). Consistent with general principles of conservation biology for species at risk, the avoidance of negative impacts should be the first approach and compensatory measures such as habitat creation and species relocation efforts should be undertaken only as a last resort and when other measures (e.g. mitigation) have proven unsuccessful.
Protection, restoration and enhancement of existing Jefferson Salamander and Jefferson dependent unisexual habitat are the priority recovery planning recommendations. Habitat alterations that would adversely affect these species should be discouraged.
Although habitat created through the permitting process is protected as part of the overall benefit for the species, at present, there is no basis for protecting other newly created features (e.g. breeding ponds) because colonization and use of such features has not been sufficiently documented. Created habitat cannot immediately replace existing habitat that Jefferson Salamanders use. In addition, restoration of forests and wetlands over the long-term (i.e. 50+ years) intended to compensate for habitat loss are not in keeping with recovery planning for Jefferson Salamander, Jefferson dependent unisexuals and other species at risk.
| Number | Protection or recovery objective |
|---|---|
| 1 | Identify and monitor extant populations of the Jefferson Salamander and Jefferson dependent unisexuals in Ontario. |
| 2 | Continue to research the species’ movements and habitat use to inform habitat protection and restoration. |
| 3 | Identify historic and presently unoccupied areas with the potential for enhancement, restoration (i.e. recovery habitat) and eventual recolonization or reintroduction of the species. |
| 4 | Assess and quantify threats to Jefferson Salamander and Jefferson dependent unisexuals. |
| 5 | Develop, test and implement threat mitigation techniques in order to reduce threats affecting Jefferson Salamander and Jefferson dependent unisexuals. |
| 6 | Develop a communication strategy to inform municipalities, planners, the development industry, property managers and other stakeholders of the habitat mapping and protection requirements for the Jefferson Salamander and Jefferson dependent unisexuals under the ESA 2007 and actively engage these stakeholders in effective habitat creation and restoration techniques and other recovery planning initiatives.” |
2.3 Recommended approaches to recovery
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Ongoing | Protection, Management, Inventory, Monitoring and Assessment. |
|
Threats:
Knowledge gaps:
|
| Critical | Ongoing | Protection, Management, Monitoring and Assessment, Research. |
|
Threats:
Knowledge gaps:
|
| Necessary | Long-term | Protection, Management, Monitoring and Assessment, Research |
|
Threats:
Knowledge gaps:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Long-term | Protection, Management, Monitoring and Assessment, Research |
|
Threats:
Knowledge gaps:
|
| Necessary | Ongoing | Protection, Management, Monitoring and Assessment, Research |
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Beneficial | Long-term | Protection, Management, Inventory, Monitoring and Assessment, Stewardship |
|
Threats:
|
| Beneficial | Long-term | Protection, Management, Inventory, Monitoring and Assessment, Stewardship |
|
Threats:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Ongoing | Protection, Management, Monitoring and Assessment, Research |
|
Threats:
Knowledge gaps:
|
| Critical | Long-term | Protection, Management, Monitoring and Assessment, Research |
|
Threats:
Knowledge gaps:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Ongoing | Protection, Management, Monitoring and Assessment, Research |
|
Threats:
Knowledge gaps:
|
| Necessary | Long-term | Protection, Management, Monitoring and Assessment, Research |
|
Threats:
Knowledge gaps:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Ongoing | Protection, Management, Education and Outreach, Communication |
|
Threats:
Knowledge gaps:
|
| Beneficial | Ongoing | Protection, Management, Education and Outreach, Communication |
|
Threats:
Knowledge gaps: ·
|
2.4 Area for consideration in the development of the habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of Natural Resources and Forestry on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the author will be one of many sources considered by the Minister when developing the habitat regulation for this species.
On February 18, 2010, the following habitat regulation came into force under the ESA 2007 for the Jefferson Salamander (O. Reg. 242/08):
Jefferson salamander habitat
- For the purpose of clause (a) of the definition of “habitat” in subsection 2 (1) of the Act, the following areas are prescribed as the habitat of the Jefferson salamander:
- In the City of Hamilton, the counties of Brant, Dufferin, Elgin, Grey, Haldimand, Norfolk and Wellington and the regional municipalities of Halton, Niagara, Peel, Waterloo and York,
- a wetland, pond or vernal or other temporary pool that is being used by a Jefferson salamander or Jefferson dominated polyploid or was used by a Jefferson salamander or Jefferson dominated polyploid at any time during the previous five years,
- an area that is within 300 metres of a wetland, pond or vernal or other temporary pool described in subparagraph i and that provides suitable foraging, dispersal, migration or hibernation conditions for Jefferson salamanders or Jefferson dominated polyploids,
- a wetland, pond or vernal or other temporary pool that,
- would provide suitable breeding conditions for Jefferson salamanders or Jefferson dominated polyploids,
- is within one kilometre of an area described in subparagraph i, and
- is connected to the area described in subparagraph i by an area described in subparagraph iv, and
- an area that provides suitable conditions for Jefferson salamanders or Jefferson dominated polyploids to disperse and is within one kilometre of an area described in subparagraph i.
- In the City of Hamilton, the counties of Brant, Dufferin, Elgin, Grey, Haldimand, Norfolk and Wellington and the regional municipalities of Halton, Niagara, Peel, Waterloo and York,
Although the Jefferson dependent unisexuals were not protected under the ESA 2007 at the time the regulation came into force, their presence triggered the application of the habitat regulation. This is because the presence of Jefferson dependent unisexuals indicates the presence of Jefferson Salamander at some point in time (Bogart et al. 2017).
Considerations for the refinement of the habitat regulation:
The current regulation is effective at protecting both Jefferson Salamander and Jefferson dependent unisexuals, however the following amendments should be considered:
- Jefferson dependent unisexuals should be added as a distinct taxon to which the regulation applies.
- The terminology in the regulation should be updated to replace “Jefferson dominated polyploids” with Unisexual Ambystoma (Jefferson Salamander dependent population) for consistency.
- The Municipality of Chatham-Kent, Durham Region, and Oxford and Perth Counties should be added to the areas in which the regulation applies.
Exclusions
The following features should not be included within the habitat regulation:
- Existing houses, buildings, and structures that are within 300 m of a breeding pond;
- Open areas such as agricultural fields that are within 1 km of a breeding pond that do not directly separate the pond from forested areas or other breeding ponds and therefore do not serve as corridors between habitats and/or breeding areas.
Naturalized anthropogenic features
Jefferson Salamanders and Jefferson dependent unisexuals occasionally breed in old farm ponds and human-created depressions that have reached a substantial state of wetland succession (probably after decades) and that occur within, or close to, existing forested or other naturally vegetated areas. Most of these ponds/depressions occur in locations where wetlands had originally existed or where portions of wetlands have been deepened. The vast majority of wetlands on the landscape that existed before agricultural conversion have been eliminated and at higher rates in areas where drainage and filling has been feasible and practicable. Therefore, these salamanders may use some naturalized human-created depressions as breeding habitat. Naturalized anthropogenic features such as old farm ponds and human-created depressions where Jefferson Salamanders and Jefferson dependent unisexuals are confirmed to breed should be included in the habitat regulation.
Glossary
- Bisexual species
- The condition of an organism capable of producing both male and female gametes (sex cells).
- Extant population
- A population that has been confirmed in the last 20 years.
- Chromosome
- A threadlike structure of nucleic acids and protein found in the nucleus of most living cells, carrying genetic information in the form of genes.
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC)
- The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
- Committee on the Status of Species at Risk in Ontario (COSSARO)
- The committee established under section 3 of the ESA, 2007 that is responsible for assessing and classifying species at risk in Ontario.
- Conservation status rank
- A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. Ranks are determined by NatureServe and, in the case of Ontario’s S-rank, by Ontario’s Natural Heritage Information Centre. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperilled
2 = imperilled
3 = vulnerable
4 = apparently secure
5 = secure
NR = not yet ranked - Conspecific
- A member of the same species.
- Control site
- A study site against which all other study sites will be compared. In the case of the Jefferson Salamander, a control site is one where conditions are known to be typical for the species and where there is a lack of disturbance.
- Demography
- Demography is the study of the size, structure, and distribution of populations, and population changes in space and time in response to birth, death, aging, and migration.
- Diploid
- When an organism has two complete sets of chromosomes, one from each parent.
- Element occurrence
- As used by NatureServe conservation data centers, an occurrence of an element of biodiversity (e.g. species or ecological community) on the landscape, an area of land and/or water on/in which an element is or was present. The NHIC uses a 1 kilometre radius to define element occurrences of the Jefferson Salamander in Ontario.
- Endangered Species Act, 2007 (ESA)
- The provincial legislation that provides protection to species at risk in Ontario.
- Extant
- Still in existence; not extirpated.
- Extirpated
- Species, community or population is believed to be lost from the nation, state/province or site.
- Genomotype
- The genetic constitution of an individual organism.
- Gynogenetic development
- Development in which the embryo contains only maternal chromosomes due to activation of an egg by a sperm that degenerates without fusing with the egg nucleus.
- Historic population
- A population that has not been confirmed in the last 20 years but is not yet confirmed as extirpated.
- Hydroperiod
- The duration, depth and extent of saturation of water in a vernal pool or other wetland.
- Isozymes
- Each of two or more enzymes with identical function but different structure, from different genes such that they can be used for identification of species.
- Juvenile
- An individual in its second, or subsequent years, which has not yet reached sexually maturity.
- Kleptogenesis
- Reproduction by a unisexual species using sperm 'stolen' by mating with members of a related bisexual species.
- Metamorph
- An individual that has recently transformed or completed metamorphosis.
- Metamorphosis
- The process of transformation from an immature form to an adult form in two or more distinct stages. In salamanders, metamorphosis is the process where an individual changes from a gilled larva into a juvenile that lacks gills.
- Microsatellite DNA
- A section of repetitive DNA widely used for DNA profiling in population genetics, in which certain DNA motifs (ranging in length from 2–5 base pairs) are repeated, typically 5–50 times.
- Mitochondrial lineage
- Mitochondrial DNA is the DNA located in mitochondria, cellular organelles within cells that convert chemical energy from food into a form that cells can use. In most species, mitochondrial DNA is inherited solely from the mother, leading to distinguishable lineages of related organisms.
- Monophyletic
- A group of organisms descended from a common evolutionary ancestor or ancestral group.
- Morphology
- They physical form of living things.
- Natal
- Relating to the place or time of an organism’s birth.
- Pentaploid
- When an organism has five complete sets of chromosomes.
- Ploidy
- The number of sets of chromosomes in a cell, or in the cells of an organism.
- Polyploid
- [Of] An organism that contains more than two sets of chromosomes (e.g. triploid – three sets of chromosomes, tetraploid – four sets of chromosomes). Examples within the Ambystoma laterale–jeffersonianum complex include LJJ, LLJ, LJJJ, and so on.
- Population
- For the purposes of this report, a population is defined as a group of salamanders that use one or many breeding ponds in a contiguous area of suitable habitat.
- Recruitment
- When juvenile organisms survive to be added to a population, by birth or immigration.
- Refugia
- Areas in which organisms can survive through a period of unfavorable conditions.
- Site
- For the purposes of this report, a site is defined as a single salamander breeding pond or group of breeding ponds that function to support a subpopulation.
- Skeletochronology
- Used to determine the chronological age of an organism by counting the concentric growth rings found in a cross section of bone.
- Species at Risk Act (SARA)
- The federal legislation that provides protection to species at risk in Canada. This act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
- Species at Risk in Ontario (SARO) List
- The regulation made under section 7 of the ESA, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
- Spermatophore
- A capsule of sperm on a gelatinous base that male salamanders deposit on the substrate of a breeding pond for females to take up.
- Snout to vent length (SVL)
- A standard measurement of an animal’s body length. The measurement is from the tip of the nose (snout) to the cloaca (vent), and excludes the tail.
- Subpopulation
- For the purposes of this report, a subpopulation is the group of salamanders that use a particular breeding pond or set of breeding ponds within 1km of each other.
- Sympatric
- Occurring within the same geographical area, overlapping in distribution.
- Triploid
- When an organism has three complete sets of chromosomes.
- Tetraploid
- When an organism has four complete sets of chromosomes.
- Unisexual Ambystoma
- A female member of the Ambystoma laterale–jeffersonianum complex that uses a form of reproduction whereby sperm is required to stimulate egg development but the male’s genes are normally not incorporated. The offspring are genetically identical to their mothers.
- Vernal pool
- Also known as an “ephemeral wetland,” a landform depression that temporarily fills with water following snowmelt in the spring and heavy rainfall or as a result of a high water table. Vernal pools vary in their size, shape, depth, timing and duration of flooding, and the types of species that are able to use them. A defining feature of vernal pools is that they usually dry by the middle of the summer, some vernal pools, however, may dry only every couple of years.
- Water balance
- In hydrology, the term water balance refers to the flow of water into and out of a system. Water sources include groundwater, surface water and precipitation while water losses can occur through evaporation, plant transpiration from plants, runoff, or drainage.
References
Beebee, T.J.C. 2013. Effects of road mortality and mitigation measures on amphibian populations. Conservation Biology 27:657-668.
Bériault, K.R.D. 2005. Critical habitat of Jefferson Salamanders in Ontario: an examination through radiotelemetry and ecological surveys. M.Sc. thesis, University of Guelph, Ontario, Canada. 69 pp.
Bi, K., and J. P. Bogart. 2010. Time and time again: unisexual salamanders (genus Ambystoma) are the oldest unisexual vertebrates. BMC Evolutionary Biology 10: 238.
Bishop, S.C. 1947. Handbook of salamanders. Comstock Publishing Company, Ithaca, New York. 555 pp.
Bogart, J.P. 1982. Ploidy and genetic diversity in Ontario salamanders of the Ambystoma jeffersonianum complex revealed through an electrophoretic examination of larvae. Canadian Journal of Zoology 60:848-855.
Bogart, J.P. 2003. Genetics and polyploidy of hybrid species. Pp 109-134. in D.M. Sever, and B.G.M. Jamieson (eds.). Reproductive Biology and Phylogeny of Urodela, vol. 1. CRC Press, Enfield, New Hampshire.
Bogart, J.P. 2017. Professor Emeritus, University of Guelph. Personal Database.
Bogart, J.P. 2017. Phone correspondence with J. Linton. July 20, 2017. Professor Emeritus, University of Guelph. Guelph, Ontario.
Bogart, J.P. 2018. Phone correspondence with J. McCarter. March 20, 2018. Professor Emeritus, University of Guelph. Guelph, Ontario.
Bogart, J. P., J. Bartoszek, D.W.A. Noble, and K. Bi. 2009. Sex in unisexual salamanders: discovery of a new sperm donor with ancient affinities. Heredity 103:483-493.
Bogart, J.P., K. Bi, J. Fu, D.W.A. Noble, and J. Niedzwieki. 2007. Unisexual salamanders (genus Ambystoma) present a new reproductive mode for eukaryotes. Genome 50:119-136.
Bogart, J.P., R.P. Elinson, and L.E. Licht. 1989. Temperature and sperm incorporation in polyploid salamanders. Science 246:1032-1034.
Bogart, J.P., and M.W. Klemens. 1997. Hybrids and genetic interactions of mole salamanders (Ambystoma jeffersonianum and A. laterale) (Amphibia: Caudata) in New York and New England. American Museum Novitates No. 3218: 78 pp.
Bogart, J.P., and M.W. Klemens. 2008. Additional distributional records of Ambystoma laterale, A. jeffersonianum (Amphibia: Caudata) and their unisexual kleptogens in northeastern North America. American Museum Novitates No. 3627: 58 pp.
Bogart, J.P., and L.E. Licht. 1986. Reproductive biology and the origin of polyploids in hybrid salamanders of the genus Ambystoma. Canadian Journal of Genetics and Cytology 28:605-617.
Bogart, J.P., and L.E. Licht. 1987. Evidence for the requirement of sperm in unisexual salamander hybrids (genus Ambystoma). Canadian Field Naturalist 101:434-436.
Bogart, J.P., J.E. Linton, and A. Sandilands. 2017. A population in limbo: unisexual salamanders (genus Ambystoma) decline without sperm-donating species. Herpetological Conservation and Biology 12:41–55.
Bogart, J.P., L.A. Lowcock, C.W. Zeyl, and B.K. Mable. 1987. Genome constitution and reproductive biology of hybrid salamanders, genus Ambystoma, on Kelleys Island in Lake Erie. Canadian Journal of Zoology 65:2188-2201.
Buck, G. 2018. Email correspondence to J. McCarter. March 20, 2018. Management Biologist. Ministry of Natural Resources and Forestry, Guelph District Office, Guelph, Ontario.
Butt, S., Ramprasad, P., and Fenech, A. 2005. Changes in the landscape of southern Ontario Canada since 1750: impacts of European colonization (PDF). In A. Fenech, D. MacIver, & H. Auld (Eds.), Integrated Mapping Assessment (pp. 83-92). Toronto, Ontario: Meteorological Service of Canada, Environment Canada.
Calhoun, A.J.K., and M.W. Klemens. 2002. Best development practices: conserving pool-breeding amphibians in residential and commercial developments in the northeastern United States. MCA Technical Paper No. 5. Metropolitan Conservation Alliance, Wildlife Conservation Society, Bronx, NY. vi + 57 pp.
Canadian Endangered Species Conservation Council. 2016. Wild Species 2015: The General Status of Species in Canada. National General Status Working Group: 128 pp.
Capps, K.A., Berven, K, and S.D. Tiegs. 2014. Modeling nutrient transport and transformation by pool-breeding amphibians in forested landscapes using a 21 year dataset. Freshwater Biology 60:500-511
Collins, S.J., and R.W. Russell. 2009. Toxicity of road salt to Nova Scotia amphibians. Environmental Pollution 157:320-324.
Connecticut Department of Energy and Environmental Protection. 2015. Connecticut’s Endangered, Threatened and Special Concern Species: 2015. State of Connecticut Department of Energy and Environmental Protection Bureau of Natural Resources. Hartford Connecticut. 1-20 pp.
CMP (Conservation Measures Partnership). 2016. Classification of Conservation Actions and Threats. Version 2.0. [accessed November 17, 2017]
Cook, R.P. 1983. Effects of acid precipitation on embryonic mortality of Ambystoma salamanders in the Connecticut Valley of Massachusetts. Biological Conservation 27:77-88.
COSSARO. 2011. COSSARO Candidate Species at Risk Evaluation Form for Jefferson Salamander (Ambystoma jeffersonianum). Committee on the Status of Species at Risk in Ontario. 1-9 pp.
COSSARO. 2016. Ontario species at risk evaluation report for unisexual Ambystoma (Ambystoma laterale). Committee on the Status of Species at Risk in Ontario. 1-29 pp.
COSEWIC. 2010. COSEWIC assessment and status report on the Jefferson Salamander Ambystoma jeffersonianum in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 38 pp.
COSEWIC. 2016. COSEWIC assessment and status report on the unisexual Ambystoma, Ambystoma laterale, Small-mouthed Salamander–dependent population, Jefferson Salamander–dependent population and the Blue-spotted Salamander–dependent population, in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xxii + 61 pp.
Crooks, S. 2017. Email correspondence to J. Linton. July 13, 2017. Postdoctoral Research Fellow Biodiversity Institute of Ontario University of Guelph. Guelph, Ontario.
County of Brant. 2012. The County of Brant Official Plan. Revised 2016. Web site: http://www.brant.ca/en/open-for-business/resources/Text---Official-Plan-final-2016.pdf [inactive link]
County of Elgin. 2015. Consolidated Official Plan of the County of Elgin. Web site: http://www.elgincounty.ca//files.ontario.ca/FINAL%20CONSOLIDATED%20OFFICIAL%20PLAN%20OF%20THE%20COUNTY%20OF%20ELGIN.pdf [inactive link]
Davic, R.D., and H.H. Welsh. 2004. On the ecological roles of salamanders. Annual Review of Ecology, Evolution, and Systematics 35:405-34.
Dawley, E.M., and R.M. Dawley. 1986. Species discrimination by chemical cues in a unisexual-bisexual complex of salamanders. Journal of Herpetology 20(1):114-116.
De Lisle, S.P., and K.L. Grayson. 2011. Survival, breeding frequency, and migratory orientation in the Jefferson Salamander, Ambystoma jeffersonianum. Herpetological Conservation and Biology 6(2):215–227.
Ducks Unlimited Canada (DUC). 2010. Southern Ontario wetland conversion analysis: final report (PDF). Ducks Unlimited Canada, Barrie, ON.
Elinson, R.P., J.P. Bogart, L.E. Licht, and L.A. Lowcock. 1992. Gynogenetic mechanisms in polyploid hybrid salamanders. Journal of Experimental Zoology 264:93-99.
Faccio, S.D. 2003. Post breeding emigration and habitat use by Jefferson and Spotted salamanders in Vermont. Journal of Herpetology 37:479-489.
Featherstone, A. 2017. Email correspondence to J.Linton. July 2017. Senior Planning Ecologist, Manager Cambridge Office LGL Limited environmental research associates, Cambridge, Ontario.
Featherstone, A. 2007. Email correspondence to J.P. Bogart. April 2007. Senior Planning Ecologist, Manager Cambridge Office LGL Limited environmental research associates, Cambridge, Ontario.
Featherstone, A. 2008. Email correspondence to J.P. Bogart. November 2008. Senior Planning Ecologist, Manager Cambridge Office LGL Limited environmental research associates, Cambridge, Ontario.
Flageole, S., and R. Leclair, Jr. 1992. Étude démographique d’une population de salamandres (Ambystoma maculatum) à l’aide de la méthode squelettochronologique. Canadian Journal of Zoology 70:740-749.
Fotherby, H. 2017. Personal Observation. Natural Resource Solutions Inc., Waterloo, Ontario.
Frohlich, K. 2018. Email correspondence to J. McCarter. March 20, 2018. Ecologist, Niagara Peninsula Conservation Authority. Welland, Ontario.
Gartner Lee Limited. 2002. Final Report: Rationale and Methodology for Determining Significant Woodlands in the Regional Municipality of Halton. Prepared for the Regional Municipality of Halton. Web site: Regional Municipality of Halton Significant Woodlands Report Online (PDF)
Gibbs, J.P., and W.G. Shriver. 2005. Can road mortality limit populations of pool breeding amphibians? Wetlands Ecology and Management 13:281-289.
Hoffmann, K.E. 2017. Breeding ecology and habitat use of unisexual salamanders and their sperm-hosts, Blue-Spotted Salamanders (Ambystoma Laterale). Ph.D. dissertation, University of Maine, Orono, Maine. 127 pp.
Intergovernmental Panel on Climate Change (IPCC). 2014. Climate Change 2014: Synthesis Report Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, R.K. Pachauri and L.A. Meyer (eds.)]. IPCC, Geneva, Switzerland, 151 pp.
Isidoro-Ayza, M., J.M. Lorch, D.A. Grear. M. Winzeler, D.L. Calhoun, and W.J. Barichivich. 2017. Pathogenic lineage of Perkinsea associated with mass mortality of frogs across the United States. Scientific Reports 7: 1-10.
Jefferson Salamander Recovery Team. 2012, Sampling Protocol for Determining the Presence of Jefferson Salamanders (Ambystoma jeffersonianum) in Ontario. 9 pp.
Julian, S.E., T.L. King, and W.K. Savage. 2003. Novel Jefferson Salamander, Ambystoma jeffersonianum, microsatellite DNA markers detect population structure and hybrid complexes. Molecular Ecology Notes 3:95-97.
Karraker, N.E., J.P. Gibbs, and J.R. Vonesh. 2008. Impacts of road deicing salt on the demography of vernal pool-breeding amphibians. Ecological Applications 18:724-734.
Klemens, M.W. 1993. Amphibians and reptiles of Connecticut and adjacent regions. State Geological and Natural History Survey of Connecticut. Bulletin 112: 1-318.
Licht, L.E. 2003. Shedding light on ultraviolet radiation and amphibian embryos. BioScience 53:551-561.
Licht, L.E. 2015. Email correspondence to J.P. Bogart. January 2015. Professor of Zoology (retired), York University, Toronto, Ontario.
Linton, J. 2016. Personal Observation. Natural Resource Solutions Inc., Waterloo, Ontario.
Linton, J., J.P. Bogart, and A. Sandilands. 2016. Research on the Jefferson Salamander complex: Year 1. Natural Resource Solutions Inc. Waterloo, Ontario. 1-56 pp.
Linton, J., J.P. Bogart, and A. Sandilands. 2017. Research on the Jefferson Salamander complex in the Dundas Valley: Year 2. Natural Resource Solutions Inc. Waterloo, Ontario. 1-69 pp.
Martel, A., M. Blooi, C. Adriaensen, P. Van Rooij, W. Beukema, M. C. Fisher, et al. 2014. Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science 346:630–631.
Massachusetts Division of Fisheries and Wildlife. 2016. Jefferson Salamander fact sheet. A Species of Greatest Conservation Need in the Massachusetts State Wildlife Action Plan. Westborough, Massachusetts. 1-7 pp.
Master, L.L., D. Faber-Langendoen, R. Bittman, G.A. Hammerson, B. Heidel, L. Ramsay, and A. Tomaino. 2009. NatureServe Conservation Status Factors. NatureServe, Arlington, VA.
Matson, T.O. 2013. Jefferson Salamander (Ambystoma jeffersonianum). Pp. 83-100. in Amphibians of Ohio. Pfingsten, R.A, J.G. Davis, T.O. Matson, G.J. Lipps, Jr., D.E. Wynn, and B.J. Armitage (eds.). Ohio Biological Survey Bulletin New Series 17(1). Ohio Biological Survey, Columbus, Ohio.
McDermid, J. S. Fera, and A. Hogg. 2015. Climate change projections for Ontario: An Updated Synthesis for Policymakers and Planners. Ontario Ministry of Natural Resources and Forestry, Science and Research Branch, Peterborough, Ontario. Climate Change Research Report CCRR-44. vi + 27 pp.
McNamara, M., and T. Sadonoja. 2012. Emerald Ash Borer (EAB) Action Plan (PW10088a) – (City Wide). Prepared for the City of Hamilton Public Works Department. Web site: http://www2.hamilton.ca/NR/rdonlyres/E213E655-CA4C-4DA7-B6F1-63D7DC0BF49/0/Sep06EDRMS_n348943_v1_7_1__PW10088a.pdf [inactive link]
Mills, P.B. 2016. Metamorphosis: Ontario’s amphibians at all stages of development. SLG Group, Brampton, Ontario. 1-102 pp.
Mullen, S. J., and S. Klueh. 2009. Demographics of a geographically isolated population of a threatened salamander (Caudata: Ambystomatidae) in central Illinois. Herpetolocial Conservation and Biology 4:261- 269.
NatureServe. 2016. NatureServe Explorer: An online encyclopedia of life. Version 7.1
NatureServe, Arlington, Virginia. Web site: NatureServe Explorer Online [accessed July 2017].
Natural Heritage Information Centre (NHIC). 2017. Get natural heritage information: species lists: species of conservation concern. Web site: Natural Heritage Information Centre Online [accessed July 2017].
Natural Resource Solutions Inc. (NRSI). 2009. Summary of 2009 Salamander Surveys. Waterloo, Ontario. Submitted Graham Buck, Ontario Ministry of Natural Resources, Guelph District. 5 pp.
Natural Resource Solutions Inc. (NRSI). 2017. Grey County Natural Heritage system Study “Green in Grey”. Prepared for Grey County.
Norfolk County. 2017. Forestry. Web site: Norfolk County Forestry Website
North-South Envronmental Inc., Dougan & Associates, and Sorensen Gravely Lowes Planning Associates Inc. 2009. Peel-Caledon Significant Woodlands and Significant Wildlife Habitat Study. Prepared for the Region of Peel and the Town of Caledon, Ontario. Web site: Peel Region Significant Woodland and Significant Wildlife Habitat Study (PDF)
Nyman, S. 1991. Ecological aspects of syntopic larvae of Ambystoma maculatum and the A. laterale-jeffersonianum complex in two New Jersey ponds. Journal of Herpetology 25:505-509.
Ontario Ministry of Natural Resources and Forestry (MNRF). 2015. Five-Year Review of Progress Towards the Protection and Recovery of Species at Risk. Species Conservation Policy Branch, Peterborough, Ontario. 215 pp.
Ontario Woodlot Association. 2015. Niagara Chapter. Web site: Niagara Chapter Ontario Woodlot Association Website
Palahnuk, A., T. Buchanan. 2015. Ontario chytrid fungus surveillance project 2015. Ministry of Natural Resources and Forestry. Wildlife Research and Monitoring Section, Peterborough, Ontario. 1-7 pp.
Petranka, J.W. 1998. Salamanders of the United States and Canada. Smithsonian Institution Press. Washington, DC. 587 pp.
Pisapio, J. 2007. Email correspondenceto J.P. Bogart. June 2007. Wildlife Biologist, Ontario Ministry of Natural Resources, Aurora District, Aurora, Ontario.
Ramsden, C., K. Bériault, and J.P. Bogart. 2006. A non-lethal method of identification for Ambystoma laterale, A. jeffersonianum and sympatric unisexuals. Molecular Ecology Notes 6:261-264.
Ramsden, C. 2008. Population Genetics of Ambystoma jeffersonianum and sympatric unisexuals reveal signatures of both gynogenetic and sexual reproduction. Copeia 3: 586-594.
Region of Waterloo. 2006. Planning, Housing and Community Services 2006 Census Bulletin #12. Web site: http://www.regionofwaterloo.ca/en/doingBusiness/resources/2013_Census_Bulletin_Agriculture_06.pdf [inactive link]
Rye, L., and W.F. Weller. 2000. COSEWIC status report on Jefferson salamander, Ambystoma jeffersonianum, in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. ii + 19 pp.
Semlitsch, R.D. 1998. Biological delineation of terrestrial buffer zones for pond-breeding salamanders. Conservation Biology 12(5):1113-1119.
Steiner, B. 2018. Email correspondence to J. McCarter. March 21, 2018. Senior Environmental Planner, City of Kitchener, Kitchener, Ontario.
Stone, M. 2017. Email correspondence to J. Linton. November 2017. Manager, Watershed Planning Services, Hamilton Conservation Authority, Ontario.
Thompson, E.L., J.E. Gates, and G.S. Taylor. 1980. Distribution and breeding habitat selection of the Jefferson salamander, Ambystoma jeffersonianum, in Maryland. Journal of Herpetology 14:113-120.
Tremblay, B. 2013, April 18. County votes to cut down tree bylaw. The Orangeville Banner. Web site: Orangeville Banner News Story Online
Turtle, S.L. 2000. Embryonic survivorship of the spotted salamander (Ambystoma maculatum) in roadside and woodland vernal pools in southeastern New Hampshire. Journal of Herpetology 34:60-67.
Urban Forest Innovations Inc. and Dougan & Associates. 2007. Framework for the Strategic Urban forest Management Plan. Prepared for the Operations Department of the City of Guelph. Web site: City of Guelph Strategic Urban Forest Management Plan Online (PDF)
Uzzell, T.M., S.M. Goldblatt. 1967. Serum proteins of salamanders of the Ambystoma jeffersonianum complex, and the origin of the triploid species of this group. Evolution 21(2): 345-354.
Uzzell, T.M. 1969. Notes on spermatophore production by salamanders in the Ambystoma jeffersonianum complex. Copeia 1969:602-612.
Uzzell, T.M. 1964. Relations of the diploid and triploid species of the Ambystoma jeffersonianum complex (Amphibia, Caudata). Copeia 1964:257-300.
Van Ryswyk, P. 2017. Email correspondence to J. Linton. July 2017. Terrestrial Ecologist, Conservation Halton, Ontario.
Weller, W. F. 1980. Migration of the salamanders Ambystoma jeffersonianum (Green) and A. platineum (Cope) to and from a spring breeding pond, and the growth, development and metamorphosis of their young. M.Sc. thesis, University of Toronto, Ontario. 248 pp.
Wilbur, H.M. 1971. The ecological relationship of the salamander Ambystoma lateraleto its all-female, gynogenetic associate. Evolution 25:168-179.
Williams & Associates Forestry Consultants Ltd. 2016. Haldimand County Forest Strategy. Prepared for Haldimand County. Web site: Haldimand County Forest Strategy Online (PDF)
Williams, P.K. 1973. Seasonal Movements and Population Dynamics of Four Sympatric Mole Salamander, genus Ambystoma. Ph.D. dissertation. Indiana University, Bloomington.
York Region. 2016. York Region Forest Management Plan. Web site: York Region Forest Management Plan Online (PDF)
List of abbreviations
- COSEWIC: Committee on the Status of Endangered Wildlife in Canada
- COSSARO: Committee on the Status of Species at Risk in Ontario
- CWS: Canadian Wildlife Service
- ESA: Ontario Endangered Species Act, 2007
- ISBN: International Standard Book Number
- IUCN: International Union for Conservation of Nature and Natural Resources
- MNRF: Ontario Ministry of Natural Resources and Forestry
- NHIC: Natural Heritage Information Centre
- SARA: Canadian Species at Risk Act
- SARO: Species at Risk in Ontario
Footnotes
- footnote[1] Back to paragraph Salamanders were collected using minnow traps placed in the breeding pond as well as drift fences with pit-fall traps that surrounded the breeding pond.
- footnote[2] Back to paragraph Salamanders were collected using drift fences around the breeding pond. This study only distinguished males and females. The number of Jefferson Salamanders (JJ) were derived by doubling the number of males and assumed a 1:1 sex ratio.
- footnote[3] Back to paragraph Includes adults collected using drift fences with pit-fall traps that surrounded a breeding pond and were strategically placed throughout the surrounding terrestrial habitat.
- footnote[4] Back to paragraph Approximate remaining total forest cover in counties in which these species occurs are: Brant County - 13%, City of Hamilton - 18%, Dufferin County - 24%, Elgin County - <20%, Grey County - 39%, Haldimand County - 14%, Halton - 22%, Niagara County - 12%, Norfolk County - 25%, County of Peel - 20%, Waterloo Region - 14.4%, Wellington County - 17%, and York - 31% (Gartner Lee Limited 2002, Region of Waterloo 2006, Urban Forest Innovations Inc. and Dougan & Associates 2007, North-South Environmental et al. 2009, County of Brant 2012, McNamara and Sadonoja 2012, Tremblay 2013, County of Elgin 2015, Ontario Woodlot Association 2015, Williams & Associates Forestry Consultants Ltd. 2016, York Region 2016, Norfolk County 2017, NRSI 2017).