Lake Whitefish (Opeongo Lake large- and small-bodied populations) recovery strategy
Read the recovery strategy for the Lake Whitefish (Opeongo Lake large- and small-bodied populations), two distinct populations of fish at risk in Ontario.

About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act, 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species’ persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of the Environment, Conservation and Parks Species at Risk webpage.
Recommended citation
Consiglio, J., T. Knight, and A. McCrum. 2024. Recovery Strategy for the Lake Whitefish (Coregonus clupeaformis) – Opeongo Lake large- and small-bodied populations in Ontario. Ontario Recovery Strategy Series. Prepared for the Ministry of the Environment, Conservation and Parks, Peterborough, Ontario. v + 54 pp.
Cover illustration: Drawing by Paul Vecsei
© King’s Printer for Ontario, 2024
ISBN 978-1-4868-7406-4 HTML
ISBN 978-1-4868-7407-1 PDF
Content (excluding illustrations) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n’est disponible qu’en anglais en vertu du Règlement 671/92 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec recovery.planning@ontario.ca .
Authors
Jessica Consiglio – Terrastory Environmental Consulting Inc.
Tristan Knight – Terrastory Environmental Consulting Inc.
April McCrum – Terrastory Environmental Consulting Inc.
Acknowledgments
Several professional biologists, park staff and researchers contributed valuable information and insights in support of this recovery strategy. This includes Trevor Middel (Ministry of Natural Resources and Forestry), Dr. Nicholas Mandrak (University of Toronto), Julia Colm (Fisheries and Oceans Canada), Dr. Andrew Drake (Fisheries and Oceans Canada), Adam Challice (Ministry of Natural Resources and Forestry), Dr. Mark Ridgway (Ministry of Natural Resources and Forestry/University of Toronto), Charlotte Ward (University of Guelph), Mary Burridge (Royal Ontario Museum), Erling Holm (Royal Ontario Museum), Dr. Erin Dunlop (Ministry of Natural Resources and Forestry/Trent University), Jennifer Hoare (Ontario Parks), Paul Gelok (Ontario Parks), Nick Lacombe (Ontario Parks) and LeSLey Baird (Ontario Parks).
Declaration
The recovery strategy for the Lake Whitefish (Coregonus clupeaformis) Opeongo Lake large- and small-bodied populations was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The recommended goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ministry of the Environment, Conservation and Parks
Fisheries and Oceans Canada
Executive summary
Lake Whitefish (Coregonus clupeaformis) is a freshwater member of the family Salmonidae (Trouts and Salmons) occupying deep, coldwater lakes. It is silvery overall in colour with a greenish-brown back, whitish underside, and overhanging snout (an adaptation to bottom-feeding). Lake Whitefish populations across North America exhibit a remarkable range and variability of physical characteristics, and uniqueness in life history, which in rare cases has given rise to physically distinct and reproductively isolated “species pairs” within the same waterbody.
The presence of separate large- and small-bodied populations of Lake Whitefish in Opeongo Lake was first reported in 1943. Attributing a particular specimen of Lake Whitefish from Opeongo Lake to either the large- or small-bodied form often requires knowledge of several traits including (i) age, (ii) reproductive status, and/or (iii) length. The large- and small-bodied populations of Lake Whitefish in Opeongo Lake are each listed as threatened on the Species at Risk in Ontario List (Ontario Regulation 230/08) and are found only in Opeongo Lake, Algonquin Provincial Park, Ontario.
Lake Whitefish has historically been captured throughout Opeongo Lake in each of its four basins. Limited records from shallower bays reflect unsuitable oxythermal (i.e., oxygen and temperature) conditions during the summer. Studies have found that the likelihood of Lake Whitefish occupancy in Opeongo Lake during summer is greatest where temperatures range between 7.7 and 13.6 °C at depths between approximately 10 and 29 m.
Opeongo Lake is situated within a protected area (Algonquin Provincial Park) managed for the purposes of maintaining natural and cultural landscapes and supporting low-intensity recreational opportunities. Maintenance of ecological integrity is the first priority for all planning and management of Ontario’s provincial parks per the Provincial Parks and Conservation Reserves Act, 2006. As a result, Lake Whitefish in Opeongo Lake are not considered vulnerable to habitat deterioration resulting from threats that emerge from human settlement and/or natural resource exploitation. The primary threats to the survival and recovery of Lake Whitefish in Opeongo Lake (listed in order of severity) include:
- accidental introduction of invasive aquatic invertebrates, particularly Spiny Water Flea (Bythotrephes longimanus)
- accidental or purposeful introduction of nonindigenous/predatory fish, particularly Rainbow Smelt (Osmerus mordax) and Northern Pike (Esox lucius)
- human-induced climate change, which may reduce habitat quantity, increase egg mortality, reduce prey availability, and/or increase the potential for harmful algal blooms
- incidental angler by-catch, the likelihood and intensity of which is low
It is generally believed that there are no confirmed limiting factors which pose a meaningful risk to the maintenance of self-sustaining populations of Lake Whitefish (both forms) in Opeongo Lake at this time. Upon further study, it may be determined that certain factors are indeed limiting for Lake Whitefish in Opeongo Lake, but only under restricted conditions.
Despite considerable historical and recent research interest, there are several gaps in current knowledge surrounding Lake Whitefish in Opeongo Lake that would benefit from further research and assessment. Most existing records represent large-bodied individuals due to biases introduced through sampling techniques (i.e., gillnet mesh size). Knowledge gaps include precise population estimates and trends, changes in habitat use across seasons and life stages, locations of spawning habitat, larval life-history, and predator-prey interactions.
The recommended long-term recovery goal for Lake Whitefish (large- and small-bodied populations) in Opeongo Lake is to maintain self-sustaining populations of both forms. Recommended protection and recovery objectives are as follows:
- Minimize risk of introducing aquatic invasive and predatory species.
- Refine population abundance estimates and project trends.
- Clarify patterns in habitat occupancy for all life stages to inform habitat protection.
- Clarify trophic niche and diet to inform recovery efforts.
- Monitor key water quality parameters to inform recovery efforts.
- Promote awareness of large- and small-bodied Lake Whitefish in Opeongo Lake and the threats facing them.
Given significant knowledge gaps in life history and habitat occupation – both for Lake Whitefish in Opeongo Lake generally and the large- and small-bodied forms individually – a habitat regulation may not be required at this time. Should a habitat regulation be developed in the future, it is recommended to include all portions of Opeongo Lake consisting of rocky shoals 10 to 50 m offshore with depths ranging from 3 to 5 m (i.e., suitable spawning and nursery habitat), and deep water areas with water depths ranging from 6 to 32 m (i.e., suitable feeding habitat for juveniles and adults). Implementation of the recovery approaches outlined herein will help to clarify the geospatial limits of Lake Whitefish habitat in Opeongo Lake and support future management, protection, and recovery of the species pair.
1.0 Background information
1.1 Species assessment and classification
The following list provides assessment and classification information for the Lake Whitefish (Coregonus clupeaformis) Opeongo Lake large- and small-bodied populations. Note: The glossary provides definitions for abbreviations and technical terms in this document.
- SARO List Classification: Threatened
- SARO List History: Threatened (2022)
- COSEWIC Assessment History: Threatened (2018)
- SARA Schedule 1: No schedule, no status
- Conservation Status Rankings: G-rank: G5TNRQ; N-rank: NU; S-rank: SU.
1.2 Species description and biology
Species description
Lake Whitefish (Coregonus clupeaformis) is a freshwater member of the family Salmonidae (Trouts and Salmons), subfamily Coregoninae (freshwater whitefishes). It was originally described by S. L. Mitchill in 1818 as Salmo clupeaformis,from a specimen originating in Lake Huron downstream of St. Marys Falls near Sault Ste. Marie (Scott and Crossman 1998). The etymology of the name Coregonus clupeaformis reflects the physical appearance of fish belonging to the population from which the specimen was taken; Coregonus derives its etymological meaning from two modern Greek words, “κόρη” (kore; pupil of the eye) and “γωνιά” (gonia; angle), referring to how the pupil tends to project forward towards the snout (Holm et al. 2021; Scott and Crossman 1998). The specific epithet clupeaformis is derived from “clupea” (herring) and “formis” (shaped), referencing its herring-like form.
Lake Whitefish is silvery overall in colour with a greenish-brown dorsal surface (back) and whitish underside (Scott and Crossman 1998). It has an elongate and somewhat laterally compressed body with large, cycloid (rounded and overlapping) scales covered by a thick layer of mucus. Its head is short with small eyes and an inferior mouth (i.e., the snout slghtly overhangs and projects forward beyond the lower jaw), an adaptation to bottom-feeding. The single dorsal fin has 11 to 13 soft rays, the anal fin has 10 to 14 rays, and the caudal fin is deeply forked. Gill rakers (bony projections on the gill arch which aid in retaining food particles) range from 19 to 33 in number and are rarely fewer than 22 (Scott and Crossman 1998). Like other Salmonidae, Lake Whitefish possess an adipose fin (a soft, fleshy fin located behind the dorsal fin) and pelvic axillary process (a small, triangular appendage at the base of the pelvic fin). Reproductive males produce nuptial tubercles (raised bumps) on their flanks along the lateral line, which are less pronounced on females. Older individuals of both sexes may develop a discrete hump behind the head (Scott and Crossman 1998).
The presence of two distinguishable morphotypes of Lake Whitefish in Opeongo Lake – referred to herein as the large- and small-bodied “forms” or “populations” – was first described by Kennedy (1943). The two forms displayed obvious differences in size and age at maturation (i.e., exhibited unique growth curves), and (when considered together) showed a bimodal length distribution of reproductively mature fish. Fewer reproductively mature individuals were found between 130 and 190 millimeters (mm) standard length (SL) compared to those less than 130 mm (attributed to the small-bodied form) and greater than 190 mm (attributed to the large-bodied form) (Kennedy 1943).
Attributing a particular specimen of Lake Whitefish from Opeongo Lake to either the large- or small-bodied form typically relies on knowledge of (i) age, (ii) reproductive status, and/or (iii) length, as further described below.
- Age: Lake Whitefish (like other fishes) are reliably aged through inspection of otoliths (ear bone inside the heads of bony fish) which requires dissection. Annuli on scales (“year marks” imprinted in response to seasonal growth patterns) were historically used for aging (e.g., Kennedy 1943) and are suitable for aging sub-adults but not reproductively mature Lake Whitefish (M. Ridgway pers. comm. 2023).
- Reproductive Status: Maturity is easily confirmed in spawning fish which are actively releasing milt or roe. Individuals which are not actively spawning typically require dissection to confirm reproductive status (i.e., to inspect gonad development) since secondary reproductive characters (e.g., nuptial tubercles) are only weakly expressed in Lake Whitefish (M. Ridgway pers. comm. 2023).
- Length: Typically expressed as fork length (FL), which is measured from the tip of the snout to the fork of the tail. Historically (e.g., Kennedy 1943) standard length (SL) was often used, which is measured from the tip of the snout to the end of the last vertebrae and does not include the caudal fin.
There is some overlap in characteristics for juveniles/young adults of the large-bodied form and most individuals of the small-bodied form, hence the need to consider multiple traits. Where all three traits (age, reproductive status, length) are known, a particular fish should be assignable to form without hesitation. Individuals displaying more distinctive or extreme characteristics may be assigned to form based on less information, as suggested by unpublished Ministry of Natural Resources and Forestry (MNRF) data from the 1980s and 2010s (referenced in Colm and Drake 2022). For example, a reproductively mature, two-year-old fish must represent the small-bodied form as the large-bodied form is not known to mature until at least age three. A reproductively mature fish that is less than or equal to 170 mm FL also represents the small-bodied form given the bimodal size distribution, wherein a gap in mature individuals has been found between 180 and 190 mm FL. In these examples, knowledge of reproductive status is compared with either age or length to assign form (i.e., two separate traits are known).
Notwithstanding the above, there is disagreement amongst the historical and recent datasets regarding the precise numerical limits of maximum age, reproductive age, and length between forms (DFO 2022). Additional sampling is planned to clarify those characteristics (and the numerical limits between them) which will facilitate differentiation of the Lake Whitefish species pair in Opeongo Lake (M. Ridgway pers. comm. 2023).
Lake Whitefish shares Opeongo Lake with two other species of coregonines, including Round Whitefish (Prosopium cylindraceum) and Cisco (C. artedi). Round Whitefish has a single flap of skin between the nostrils (i.e., nostril flap) and a notch in the rear corner of the eyelid, whereas Lake Whitefish has two nostril-flaps and no eyelid notch. Cisco has a terminal snout which does not overhang the mouth and typically possesses more gill rakers (usually more than 32) than Lake Whitefish (Scott and Crossman 1998; Holm et al. 2021). Lake Whitefish larvae have historically been visually distinguished from Cisco based on the presence and position of melanophores (specialized cells filled with the dark pigment melanin) spanning the dorsal surface (Cucin and Faber 1985). A more recent study combining visual and genetic methods suggests that visual identification using morphometric characters alone is unreliable for distinguishing larval Lake Whitefish and Cisco and will generate misidentifications (George et al. 2018). A combination of visual and genetic methods is often preferred for identifying larval coregonines depending on study purpose and scope (Overdyk et al. 2016).




Figure 1. Photographs of Lake Whitefish from Opeongo Lake in Ontario.
Species biology
Lake Whitefish populations across North America (and coregonines in general) exhibit remarkable variation of physical characteristics and uniqueness in life history (e.g., diet), which has occasionally led to unresolved taxonomic issues (Mee et al. 2015). Such morphological differentiation includes populations from hydrologically disconnected waterbodies (allopatric) and extends to intra-lake (sympatric) settings where distinguishable and reproductively isolated forms co-occur (Bernard 2006). Such intra-lake populations have been referred to as “sympatric pairs” or more commonly “species pairs” (Rogers 2008). A minimum of 19 lakes across Canada are currently known to contain Lake Whitefish species pairs, including Opeongo Lake and nearby Big Trout Lake (Mee et al. 2015; Ridgway et al. 2017). The mechanism(s) driving sympatry of these species pairs has been attributed to (1) post-glacial colonization of a waterbody by Lake Whitefish from different source populations, and (2) local (in-situ) adaptions derived from evolutionary processes including adaptive radiation and/or genetic drift (Bernard 2006; Bernatchez et al. 2010; Mee et al. 2015; Ridgway pers. comm. 2023).
Kennedy (1943) performed the first morphometric analysis of the two forms of Lake Whitefish in Opeongo Lake, which revealed several key differences as summarized in Table 1 below. Until recently, evidence for reproductive isolation between the two forms was indirect (Mee et al. 2015) and inferred based on the physical differences outlined in Table 1. More recent (unpublished) genetic work has confirmed that the two forms have speciated in-situ (i.e., within Opeongo Lake) and shows evidence of limited interbreeding in the past (C. Wilson pers. comm. 2023). Therefore, occupation of Opeongo Lake by Lake Whitefish does not reflect a “double invasion” of different lineages, as is the case for nearby Big Trout Lake (M. Ridgway pers. comm. 2023). Within-population genetic diversity of the large-bodied form of Lake Whitefish in Opeongo Lake appears to be low but shows high differentiation from populations in Lake Ontario (Bay of Quinte and Chaumont Bay) and Lake Simcoe (Bernard et al. 2009).
| Morphological Attribute | Large-bodied Form | Small-bodied Form |
|---|---|---|
| Mean standard length (SL; mm) | 251 | 126 |
| Mean number of gill rakers (± SD) | 27.7 (± 1.1) | 25.4 (± 0.14) |
| Mean number of lateral line scales | 83.3 | 77.3 |
| Age of sexual maturity (years) | 4 to 7 (as early as 3) | 2 |
| Maximum age (years) | 14 | 5 |
Despite Kennedy’s study published 80 years ago, there are remaining uncertainties related to age and growth patterns of the two forms, which complicate their differentiation. There are limited comparative data for the two forms as many historical and more recent sampling efforts in Opeongo Lake did not assign the appropriate form to captured Lake Whitefish (M. Ridgway pers. comm. 2023). In some cases, age structures (i.e., otoliths) are still available from archived specimens for modern assessment (A. Challice pers. comm. 2023). Other morphological differences detected historically by Kennedy (1943) such as eye diameter, head length and caudal peduncle length were not statistically significant and have not yet been subject to modern study (M. Ridgway pers. comm. 2023).
The morphometric and age data reported by Kennedy can be compared with unpublished MNRF datasets from the 1980s and 2010s (2010, 2018, and 2019). The unpublished MNRF data revealed a maximum age of 34 years (1980s) and 24 years (2010s) for large-bodied individuals. Small-bodied individuals showed maximum ages of 26 years (1980s) and eight years (2010s). The reported maximum ages between the three datasets (i.e., Kennedy 1943, MNRF 1980s, MNRF 2010s) range between 14 and 34 (20-year difference) for the large-bodied form and between 5 and 26 (21-year difference) for the small-bodied form. The unpublished MNRF datasets also differ in mean FL, which were reported as 332.4 mm (1980s) and 301 mm (2010s) for large-bodied individuals, and 226.7 mm (1980s) and 145 mm (2010s) for small-bodied individuals (Kennedy reported SL rather than FL). Overall, Kennedy (1943) reported the lowest values for age and length, while the 1980’s MNRF data contains the greatest values. The 2010’s MNRF dataset (Table 2) represents the most recent and reliable source of information used to distinguish the two forms, though further sampling is ongoing (M. Ridgway pers. comm. 2023).
| Morphological Attribute | Large-bodied Form | Small-bodied Form |
|---|---|---|
| Mean fork length of mature individuals (FL; mm) | 301 | 145 |
| Maximum fork length of mature individuals (FL; mm) | 519 | 176 |
| Maximum age (years) | 24 | 8 |
While Kennedy (1943) likely underestimated the ages of older/reproductive individuals by using scales (as compared to otoliths recorded by MNRF; M. Ridgway pers. comm. 2023), the discrepancies in maximum reported ages within the unpublished MNRF datasets are not understood and were subject to recent scientific debate (DFO 2022). Gillnetting surveys are planned for 2024 to further clarify the morphological and physiological boundaries between the two forms and determine whether additional characteristics are useful in assigning an individual to form, such as gill raker density (i.e., number of gill rakers per length of gill arch; M. Ridgway pers. comm. 2023).
Lake Whitefish are benthivorous (i.e., feed on benthic or bottom-dwelling prey) and associated with cold, oligotrophic lakes. Given the variability in Lake Whitefish life history strategies (e.g., life cycle, diet) across its range in response to localized biophysical conditions (e.g., food availability, competition intensity, lake morphometrics), the following biological description centres primarily on what is currently known about Lake Whitefish in Opeongo Lake. Information from other populations (i.e., in Ontario or elsewhere) is drawn upon primarily to minimize knowledge gaps. Apart from the above-noted physical differences and age at maturation, limited information exists upon which to differentiate key life history attributes between the large- and small-bodied forms. As such, the description in Table 3 below and the following text largely treats both populations concurrently.
| Life Stage | Function | General Timeframe | Habitat Feature(s) |
|---|---|---|---|
| Adult spawning to hatch | Spawning | Late October to April | Nearshore areas with rocky shoals |
| Adult spawning to hatch | Egg development | Late October to April | Nearshore areas with rocky shoals |
| Adult spawning to hatch | Hatch | Late April through May (commencing the first few days following ice out) | Nearshore areas with rocky shoals |
| Larval (up to approximately 6 weeks after hatch) | Nursery; feeding | May to June | Nearshore areas with rocky shoals |
| Age 0 (approximately 50 mm, or at the onset of diet shift) | Feeding | All year | Unknown |
| Juvenile/Sub-adult (age 1 to onset of maturity; age three to five for large-bodied form and age two for small-bodied form) | Feeding | All year | Cold, deep water (hypolimnion) with access to pelagic and benthic invertebrates |
| Adult | Feeding | All year | Cold, deep water (hypolimnion) with access to pelagic and benthic invertebrates |
Lake Whitefish occupies a narrow thermal envelope and is intolerant of warmer water. The optimal thermal niche for Lake Whitefish has been reported to be between 10 and 14 °C (Christie and Regier 1988). General avoidance of temperatures greater than 10 °C during thermal stratification has been documented in northwestern Ontario (Rodrigues et al. 2022), although Lake Whitefish in Opeongo Lake were found to have a high probability of detection up to 13.6 °C (Challice et al. 2019). Cucin and Faber (1985) failed to capture larval Lake Whitefish in Opeongo Lake where surface waters exceeded 12 °C. Water temperature drives both habitat selection and diel vertical movements (Gorsky et al. 2012).
Lake Whitefish spawn in Opeongo Lake from late October to late November when water temperatures decline to 4 to 7 °C (Ihssen et al. 1981), with activity peaking between November 8 and 15 (Cucin and Faber 1985). The onset of initial and peak spawning may average later in recent years given climate change, though available data to test this assumption are lacking. Low water temperatures and extensive ice cover are considered a requirement for proper Lake Whitefish egg development (Colm and Drake 2022). Lab reared Lake Whitefish eggs have been shown to hatch successfully when developing in water temperatures ranging from 0.5 to 10 °C, with hatching unsuccessful at both higher and lower temperatures (Price 1940).
Eggs are randomly broadcast over shoals (i.e., shallow rocky areas) and rock ledges primarily consisting of cobble-sized rock with interstitial spaces (i.e., voids or crevices between the rocks), which protect the incubating eggs from displacement and/or predation (Ihssen et al. 1981; Cucin and Faber 1985; Scott and Crossman 1998). The mean diameter of Lake Whitefish eggs collected in Lake Michigan (near Elk Rapids) and Lake Ontario (Chaumont Bay) was 3.21 mm (SD=0.20, n=99), and can be reliably differentiated from Cisco (which has smaller eggs) based on the species-separating size threshold of 2.88 mm (Paufve et al. 2020). Egg hatching occurs in late April to May (Cucin and Faber 1985).
Larval Lake Whitefish in Opeongo Lake have been captured via tow netting within three to five days of ice-break (Cucin and Faber 1985). A study from Chaumont Bay (eastern Lake Ontario) from 2004 to 2006 found that larval Lake Whitefish fed overwhelmingly (81.4%) on copepods (mainly cyclopoids) – small crustaceans within the class Copepoda – and to a lesser extent water fleas within the superorder Cladocera (mainly daphnids) and chironomids (Johnson et al. 2009). Nearshore seining surveys indicated that larvae descended from the water column to the lake bottom at night (Johnson et al. 2009).
Analysis of the stomach contents of 280 adult Lake Whitefish in Opeongo Lake during the summer of 1963 (between mid-May and late-August) revealed a seasonally variable diet reliant upon benthic crustaceans, insect larvae and mollusks (Sandercock 1964). In the latter half of May, Lake Whitefish fed almost exclusively on mayfly (Ephemeroptera) nymphs, comprising 95.3 percent of stomach contents by volume. By June and July, a broader array of mostly bottom-dwelling organisms was consumed including crustaceans such as Cladocera (e.g., Sida crystallina, Ophryoxus gracilis, Eurycercus lamellatus, Latona setifera), Copepoda (Cyclops sp.) and seed shrimp (Ostracoda), along with non-biting midges (Chironomidae), freshwater molluscs (e.g., Amnicola limosa, Pisidium sp.) and water mites. By August, Cladocera (particularly S. crystallina), copepods, dipterans (particularly Chironomidae), water mites and freshwater molluscs (particularly Pisidium sp.) were taken in greatest abundance. Yellow Perch (Perca flavescens) comprised 47.4 percent of the diet by volume in early August but was not otherwise consumed during the study period. Large- and small-bodied forms were not differentiated during this study but (based on published SLs ranging between 160 to 450 mm) most were probably large-bodied (Colm and Drake 2022). Lake Whitefish in Opeongo Lake are also preyed upon by predatory fish including Burbot (Lota lota; Hackney 1973; Kennedy 1943) and Lake Trout (Salvelinus namaycush; Kennedy 1943; Martin and Fry 1973).
1.3 Distribution, abundance and population trends
The landscapes of Algonquin Provincial Park (PP) were released from glacial ice (and thus available for colonization by fish) between approximately 13,800 to 13,000 years ago, following sufficient northward retreat of the Laurentide ice sheet (Ridgway et al. 2017). Lake Whitefish is speculated to have entered watersheds emanating from the Algonquin highlands (i.e., in the area to become Opeongo Lake) soon after glacial retreat, as the distribution of Lake Whitefish spans many lakes in Algonquin PP (74 in total) which vary in elevation and watershed position (Ridgway et al. 2017). Additional colonization events by Lake Whitefish may have occurred in northern Algonquin PP between 13,000 to 12,000 years ago when proglacial Lake Algonquin discharged eastward through a series of successively lower outlets, but these watersheds are more northward and topographically below Opeongo Lake (and thus were not hydrologically connected to the Algonquin highlands). Previous genetic study suggested that all Lake Whitefish populations in Algonquin PP (and Ontario more broadly) originated from the Mississippian refuge (Bernatchez and Dodson 1991); however, more recent (unpublished) genetic evidence suggests that Lake Whitefish are represented by multiple lineages in the park which emanated from separate glacial refuges (M. Ridgway pers. comm. 2023)
The Opeongo Lake large- and small-bodied forms of Lake Whitefish are found only in Opeongo Lake, Algonquin PP. The two co-occurring forms are referred to as “populations” by COSEWIC (2018) and COSSARO (2020), and also represent separate “Designatable Units” (DUs) as defined by Fisheries and Oceans Canada (DFO) (Colm and Drake 2022). The two forms are genetically and physically distinct from each other and are evolutionarily unique (COSEWIC 2018).
Opeongo Lake (known colloquially as “Lake Opeongo”) is a coldwater, oligotrophic lake consisting of four discrete basins (South Arm, North Arm, East Arm and Annie Bay) separated by shallow narrows (Martin and Fry 1973). Opeongo is believed to derive from the Algonquian phrase “Ope au wingauk” or “sandy at the narrows”, likely reflecting the conditions separating the North and East Arms (Shaw 1998). Opeongo Lake extends approximately 14 kilometres (km) north to south and 12 km east to west, with a surface area of 5,154.2 hectares (ha), a maximum depth of 49.4 metres (m) and an average depth of 13.7 m (MNRF 2023b). Approximately 23.3 percent of Opeongo Lake exceeds 20 m in depth while 48.3 percent is less than 10 m in depth (including the entirety of Sproule Bay) (Challice et al. 2019). Water levels in Opeongo Lake are controlled by a fixed-crest weir dam (“Opeongo Lake Dam”) at the Annie Bay outlet to the Opeongo River (Colm and Drake 2022; OPG and MNR2018).
The spatial configuration and topographic relief of the Opeongo Lake watershed is illustrated below in Figure 2, highlighting the extent and character of surrounding lands which convey surface water and groundwater to the lake. Historical and current records of Lake Whitefish (not differentiated by form) in Opeongo Lake are shown below in Figure 3. Records for several years between 1936 and 1971 represent specimens deposited at the ROM (M. Burridge pers. comm. 2023) while the remaining data were provided by MNRF (T. Middel pers. comm. 2023). Records representing various years between 1981 and 1995 are also available but lack spatial attribution and are thus omitted from Figure 3.
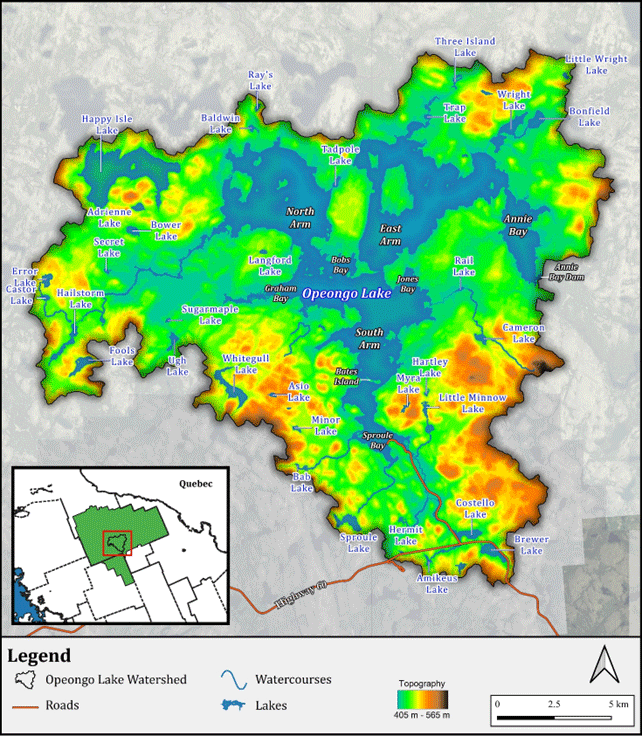
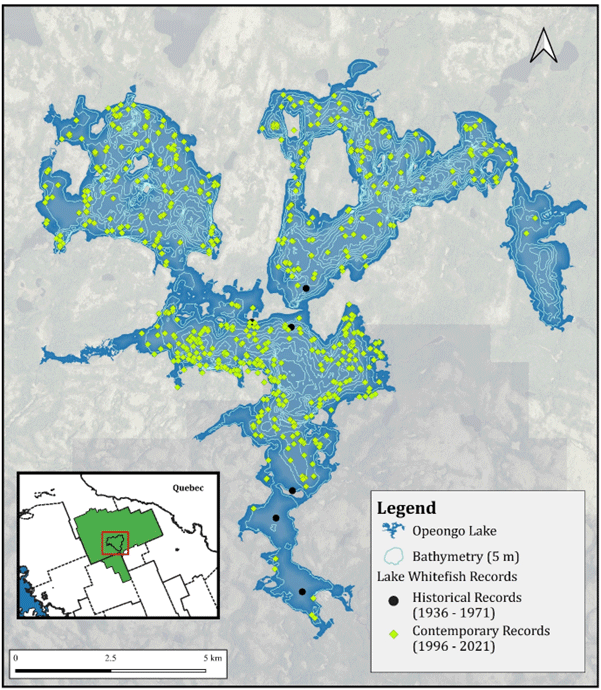
Lake Whitefish has been captured throughout Opeongo Lake in each of the four arms, though the occurrence map in Figure 3 reveals patterns reflecting summer concentration in the North, East, and South (i.e., north of Bates ISLand) arms. Limited records from shallower bays (e.g., Annie Bay, Sproule Bay) likely reflect unsuitable oxythermal habitat. Shallower areas (i.e., less than 10 m) and connected creeks (e.g., Hailstorm Creek) may be occupied by Lake Whitefish outside of thermal stratification (M. Ridgway pers. comm. 2023). The distribution of habitats occupied by Lake Whitefish in Opeongo Lake during winter (i.e., when the lake is well-mixed) is unknown (M. Ridgway pers. comm. 2023).
Based on (unpublished) MNRF datasets from 2010 and 2019, Colm and Drake (2022) report two separate lake-wide abundance estimates for the large-bodied form of Lake Whitefish in Opeongo Lake of 11,378 (95% CI, 6,509 to 18,712) and 22,792 (95% CI, 10,437 to 54,415), respectively. For both the 2010 and 2019 datasets, sampling made use of large-mesh gillnets in which most individuals captured were mature and greater than 190 mmFL (thus representing the large-bodied form). Surveys targeting the small-bodied form in 2018 using small-mesh gillnets captured 23 small-bodied individuals (i.e., mature and < 180 mmFL) and 50 large-bodied individuals (Colm and Drake 2022). The small-bodied form evades capture by large-mesh gillnets and thus has not been well-sampled historically (M. Ridgway pers. comm. 2023). Abundance estimates for the small-bodied form are not currently available.
Recent modeling suggests that the supply of available habitat in Opeongo Lake for both the large- and small-bodied populations exceeds that required by the estimated minimum viable population (MVP), and that current population estimates (assuming a low current abundance for the small-bodied population) exceed the MVP (Fung et al. 2022). Notwithstanding this, current population size, structure, and trends of Lake Whitefish in Opeongo Lake (both forms) are not known with certainty.
1.4 Habitat needs
The habitat needs of Lake Whitefish in Opeongo Lake are differentiated below based on life-stage as described in Table 3.
Adult spawning to hatch habitat
Areas selected for spawning by Lake Whitefish in Opeongo Lake are predominantly concentrated along exposed shorelines and points, including iSLands (Cucin and Faber 1985). Spawning habitat is typified by substrates consisting of gravel, rocky shoals or granite boulders and broken rocks (Cucin and Faber 1985). Spawning is assumed to occur in nearshore areas up to 50 m offshore and at depths of less than 8 m (Colm and Drake 2022), though this is based on published reports of spawning in lakes across Canada (per Scott and Crossman 1998). Historical descriptions of known Lake Whitefish spawning areas in Opeongo Lake indicate that they are found within 10 to 50 m from shore and at depths ranging between 3 to 5 m (Cucin and Faber 1985). Nearshore sampling efforts in Opeongo Lake have shown that Lake Whitefish use spawning grounds where surface water temperatures reach 4 to 7 °C during peak spawning activity (Ihssen et al. 1981).
Lake Whitefish spawning areas are assumed to overlap with those of Lake Trout, though few have been confirmed to date and Lake Whitefish may be less particular than Lake Trout (M. Ridgway pers. comm. 2023). Twenty-two Lake Trout spawning shoals are known in the North, South and East arms (T. Middel pers. comm. 2023). Suspected Lake Whitefish spawning areas occur most frequently in the East Arm and are also known from the South Arm (Martin and Fry 1973).
Whitaker and Wood (2020) describe spawning habitat for Lake Whitefish in Maine as consisting of areas with deeply lain coarse substrates of multiple size classes, which create interstitial spaces (crevices) for egg cover. Currents passing through the interstitial spaces wash them free of fine sediments; thus, aspects of shoreline morphometry such as fetch (maximum length of open water traveled by wind), aspect (orientation to the direction of prevailing winds and storms) and exposure (presence or absence of sheltering features such as iSLands) affect spawning habitat quality. Less optimal Lake Whitefish spawning habitat was found where the depth of substrate was shallower; however, these areas still contained diverse substrate particle sizes to provide egg cover (Whitaker and Wood 2020). A requirement for optimal Lake Whitefish spawning habitat is the presence of either strong currents or wave action to reduce sediment deposition on eggs (Whitaker and Wood 2020). Cucin and Faber (1985) identify the importance of rocky crevices for protecting eggs throughout the duration of development.
Key characteristics of suitable and/or optimal spawning habitat for Lake Whitefish in Opeongo Lake are unknown (M. Ridgway pers. comm. 2023; T. Middel pers. comm. 2023). Variables such as substrate type, substrate size and structure, water depths, distance from shore, and degree of wave energy likely influence spawning habitat quality in Opeongo Lake.
Larval habitat
Lake Whitefish eggs develop over winter during a four-to-six-month period in Opeongo Lake. Once hatched, larvae begin swimming immediately and move upward in the water column above spawning areas (Cucin and Faber 1985). Larvae appear to remain around their spawning grounds for approximately six weeks before dispersing to deeper waters (Ihssen et al. 1981). Triggers for dispersal may include prey availability, surface currents, avoidance of predators or innate behavioural factors (Cucin and Faber 1985). It has been speculated that larvae transition away from surface waters towards the colder lake bottom in Opeongo Lake in June (Cucin and Faber 1985).
Age 0 (approximately 50 mm) habitat
The habitat needs of age 0 (approximately 50 mm) Lake Whitefish in Opeongo Lake are largely unknown. It is possible that their tendency to specialize in a single type of prey (Pothoven et al. 2014; Pothoven and Olds 2020) may influence habitat selection.
Juvenile/Sub-adult habitat
The habitat needs of juvenile and sub-adult (i.e., age 1 to onset of maturity) Lake Whitefish in Opeongo Lake are unknown, although juveniles have been captured alongside adults during sampling, suggesting some degree of overlap in habitat use (Kennedy 1943).
Adult habitat
Available information suggests that habitat use by adults of both forms in Opeongo Lake generally overlaps throughout the summer months, with occupancy concentrated in deep water (hypolimnion). Kennedy (1943) observed that large- and small-bodied Lake Whitefish occupy similar water depths from early spring (May) through early fall (September); however, a difference in vertical distribution (depth occupancy) was detected throughout August. Sampling efforts in August revealed that large-bodied individuals typically congregated in warmer (15 °C), shallower (9.1 m, 30 ft) water, whereas small-bodied fish were found in cooler (9 °C), deeper (15.2 m, 50 ft) areas, though the results may have been influenced by two locations with exceptionally high catches (Kennedy 1943). Notwithstanding this, small-mesh gillnet surveys by MNRF in mid-August 2018 captured both forms in the same nets (Colm and Drake 2022), suggesting that each may be benthic (M. Ridgway pers. comm. 2023). Further study is needed to ascertain the extent and seasonality of niche overlap versus habitat partitioning amongst the two forms.
The summer oxythermal envelope (i.e., portion of the waterbody remaining rich in oxygen and of a suitable temperature) used by most (i.e., probability of occupancy > 50%) Lake Whitefish in Opeongo Lake (forms not differentiated) in 2003, 2009 and 2010 encompassed a temperature range of 7.6 to 20.0 °C at depths between approximately 6 and 32 m (Challice et al. 2019). Greater Lake Whitefish occupancy (i.e., probability of occupancy > 75%) was found in temperatures ranging from 7.7 to 13.6 °C at depths between approximately 10 and 29 m (Challice et al. 2019). Based on these findings and the results of acoustic substrate mapping of the lake bottom, Challice et al. (2019) found that Lake Whitefish in Opeongo Lake predominantly occupy areas where the thermocline meets the substrate during thermal stratification. Lake Whitefish in Opeongo Lake did not make significant vertical movements between water depths throughout the day but appeared to be more active during morning hours than afternoons, which may reflect foraging behaviours which optimize capture of zooplankton prey (Challice et al. 2019).
Passive acoustic telemetry of Lake Whitefish in northwestern Ontario (Lake 658 from the Experimental Lakes Area) found that individuals were mainly found in a narrow temperature band of 5.3 to 7.9 °C during stratification, and that fish avoided temperatures greater than 10 °C even where they became exposed to hypoxic conditions in the hypolimnion (DO < 2 mg/L; Rodrigues et al. 2022). The authors speculated that Lake Whitefish may be making brief foraging forays into the hypoxic hypolimnion to capture hypoxia-tolerant benthic prey including non-biting midges (Chironomidae) and phantom midges (Chaeoborus spp.).
1.5 Limiting factors
It is generally believed that there are no confirmed limiting factors which pose a meaningful risk to the maintenance of self-sustaining populations of Lake Whitefish (both forms) in Opeongo Lake at this time (M. Ridgway pers. comm. 2023; N. Mandrak pers. comm. 2023; T. Middel pers. comm. 2023). Suitable spawning habitat appears to be widespread throughout the East Arm and North Arm (and portions of the South Arm), though this requires verification. Similarly, there is no evidence of a dissolved oxygen (DO) limitation in Opeongo Lake (generally considered to be < 7 mg/L in the hypolimnion of lakes on the Precambrian Shield; MOE et al. 2010). Challice et al. (2019) found DO to be greater than 7 mg/L at all depths measured, while unpublished MNRF data (reported in Colm and Drake 2022) revealed DO levels generally above 8.5 mg/L at locations where Lake Whitefish (large-bodied form) were captured in 2010 and 2019.
The presence of nonindigenous predatory fish in Opeongo Lake including Cisco and Smallmouth Bass (Micropterus dolomieu) would constitute a limiting factor if evidence suggested they were adversely affecting the survival, growth, or recruitment of Lake Whitefish. Cisco was purposefully introduced to Opeongo Lake in 1940, then introduced again in 1948 using stock from Mary Lake in Huntsville (Cucin and Faber 1985). By the early 1950s, Cisco were documented in the stomachs of Lake Trout, confirming establishment (Martin and Fry 1973). Introducing Cisco to any waterbody containing a small-bodied form of Lake Whitefish (which occupies a similar trophic niche) is predicted to produce negative effects to Lake Whitefish due to competitive exclusion driven by an overlap in required resources (Pigeon et al. 1997; Trudel et al. 2001). It is further hypothesized that zooplankton biomass may not be sufficient to support both fishes (Trudel et al. 2001). Establishment of Cisco in Opeongo Lake preceded the intensive study of Lake Whitefish diet by Sandercock (1964) by at least ten years, so any effect of introducing Cisco on the historical diet of Lake Whitefish (if any) cannot be known. Both forms of Lake Whitefish have persisted since the establishment of Cisco approximately 70 years ago, suggesting these coregonines are using different prey items (and that a cisco-related limitation is unlikely).
Smallmouth Bass was introduced to several lakes in Algonquin PP (including Opeongo Lake in 1928) through park stocking programs beginning in 1899 and spanning well into the twentieth century (Martin and Fry 1973; Mitchell et al. 2017). Although Smallmouth Bass introductions increase recreational angling opportunities, they may result in a loss of species diversity, particularly of smaller-bodied native fish (Findlay et al. 2000). Similar to Cisco, there is a lack of baseline information to confidently assess the impacts (if any) of introducing Smallmouth Bass on Lake Whitefish, and an equal lack of evidence implying that said introduction has resulted in a biological limitation for either form. A historical analysis of Smallmouth Bass stomach contents did not reveal any Lake Whitefish eggs (Martin and Fry 1973).
Possible limiting factors for Lake Whitefish in Opeongo Lake as reported by Colm and Drake (2022; see also references therein) and synthesized herein are offered below, which are primarily based on inferences from empirical studies of Lake Whitefish in other areas. Upon further study, it may be determined that certain factors noted below are indeed limiting, but only under restricted conditions (e.g., when compounded by other factors).
- Poor recruitment due to egg predation by predatory fish could affect population viability for one or both forms (at least when other stressors are prevalent). The intensity of species-specific predation on Lake Whitefish eggs in Opeongo Lake is unknown.
- Competition with Cisco (for pelagic prey) and Round Whitefish (for benthic prey) may limit Lake Whitefish abundance. However, current evidence suggests that both Lake Whitefish forms in Opeongo Lake are benthic (M. Ridgway pers. comm. 2023) and (if so) each would have co-occurred with Round Whitefish for centuries (or perhaps much longer).
- Effects caused by genetic structure (e.g., drift, inbreeding depression, founder effects) could increase population vulnerability, particularly since the mechanisms driving reproductive isolation of the two forms are not currently understood.
1.6 Threats to survival and recovery
Opeongo Lake is situated within a protected area (Algonquin PP) managed for the purposes of maintaining natural and cultural landscapes and supporting low-intensity recreational opportunities (Ontario Parks 1998). Maintenance of ecological integrity is the first priority for all planning and management of Ontario’s provincial parks per the Provincial Parks and Conservation Reserves Act, 2006. As a result, Lake Whitefish in Opeongo Lake are not considered vulnerable to habitat deterioration resulting from threats that emerge from human settlement and/or natural resource exploitation, such as riparian vegetation clearing (Martin and Fry 1973; T. Middel pers. comm. 2023). In particular, there is a minimum 120 m zone surrounding Opeongo Lake as described in the 2013 amendment to the Algonquin Park Management Plan, in which forest harvesting and intensive recreational activities are prohibited (Ontario Parks 2013, P. Gelok pers. comm. 2023).
The primary threats to the survival and recovery of Lake Whitefish in Opeongo Lake (listed in order of severity) include:
- accidental introduction of invasive aquatic invertebrates, particularly Spiny Water Flea (Bythotrephes longimanus) which is not currently present in Opeongo Lake
- accidental or purposeful introduction of nonindigenous/predatory fish, particularly Rainbow Smelt (Osmerus mordax) and Northern Pike (Esox lucius) which are not currently present in Opeongo Lake
- human-induced climate change, which may reduce habitat quantity, increase egg mortality, reduce prey availability, and increase the incidence of harmful algal blooms
- incidental angler by-catch, the likelihood and intensity of which is low
Introduction of invasive aquatic invertebrates
Invasive zooplankton
Spiny Water Flea is an invasive species of zooplankton that has spread rapidly throughout the Great Lakes basin, leading to significant reductions of pelagic zooplankton diversity in large and small waterbodies alike. Following introduction into Lake Ontario via contaminated ship ballast, Spiny Water Flea has invaded approximately 150 lakes from southcentral to northwestern Ontario (Yan et al. 2011). The recent discovery of exoskeletal remains in lake sediment cores found in two Ontario lakes (Three Mile Lake in the Township of Muskoka Lakes and Lake Nipissing in North Bay) indicates that Spiny Water Flea is at least partially native (i.e., to certain watersheds) and has occurred in the province since at least 1650, predating the earliest recorded reports in North America by nearly three centuries (DeWeese et al. 2021).
Angling is likely the primary vector of Spiny Water Flea invasion, wherein individuals and propagules are transported to new water bodies via fishing gear (e.g., fishing lines), boats, trailers and live wells (Yan et al. 2011; MAISRC 2023). The likelihood of natural dispersal of Spiny Water Flea to downstream waterbodies via river/stream connections is generally considered low and/or limited to lakes close-by (Gertzen and Leung 2011). To prevent the introduction and spread of aquatic invasive species, Ontario Regulation 354/16 under the Invasive Species Act (2015) was updated in 2022to regulate watercraft and watercraft equipment as carriers of invasive species. Boaters are now required to take mandatory precautions to remove aquatic organisms and drain water from watercraft and watercraft equipment prior to transporting overland or launching into any waterbody in Ontario.
Spiny Water Flea shows a preference for inhabiting the epilimnion of deep, cold lakes and tends to avoid the hypolimnion (Yan et al. 2001). It can reduce food supply for fish by directly impacting crustacean zooplankton diversity and abundance, or cause indirect impacts by pushing zooplankton to deeper and colder waters and/or altering zooplankton growth rates (Yan et al. 2001, 2011). There is currently no means of eradicating an established population of Spiny Water Flea, although a recent study by Martin et al. (2023) found that predation of Spiny Water Flea by Cisco in Vilas County, Wisconsin, played a direct role in Spiny Water Flea density declines.
A study in Harp Lake (northeast of Huntsville, Ontario) found that the invasion of Spiny Water Flea led to an overall decline in crustacean zooplankton richness and size structure (Yan et al. 2001). In this instance, while Cisco was present in the lake, no evidence was found to suggest that Cisco predation played any role in Spiny Water Flea declines. Instead, Spiny Water Fleas in Harp Lake adapted by seeking refuge from predation, occupying warmer, dark portions of the lake above the hypolimnion (Yan et al. 2001). These findings suggest that Spiny Water Flea responses to predation may be variable across waterbodies.
The COSEWIC Assessment and Update Status Report on Lake Whitefish in Lake Simcoe (COSEWIC 2005) indicated a lack of evidence linking Spiny Water Flea to reduced growth or survival of hatchery-reared Lake Whitefish (which prey heavily on Spiny Water Flea), but that the effect on juveniles (i.e., less than six months of age) was unknown. Notwithstanding this, Lake Simcoe presents a different context than Opeongo Lake as it does not possess a Lake Whitefish species pair. Reid et al. (2017) detailed the collapse of a Lake Whitefish species pair (i.e., “normal-bodied” and small-bodied) in Como Lake (northwest of Sudbury, Ontario) due to the introduction of Spiny Water Flea around 2011. The species pair was replaced by a single large-bodied form, which is deeper-bodied and possesses significant differences in morphology from the normal- and small-bodied forms. It was hypothesized that the introduction of Spiny Water Flea led to drastic changes in trophic niches which previouSLy maintained the species pair, causing Lake Whitefish to shift their diet from smaller prey items (such as native zooplankton) towards the larger and more abundant Spiny Water Flea (Reid et al. 2017).
Spiny Water Flea is present in many major waterbodies surrounding Algonquin PP (EDDMapS 2023). In 2022, Spiny Water Flea was first detected in the northwestern region of Algonquin PP in three lakes (North Tea Lake, Manitou Lake, Kioshkokwi Lake) forming part of the Upper Amable du Fond River watershed (J. Hoare pers. comm. 2023; P. Gelok pers. comm. 2023). It has been suggested that Spiny Water Flea poses the greatest risk to the long-term survival of the Lake Whitefish species pair in Opeongo Lake (A. Drake pers. comm. 2023; J. Colm pers. comm. 2023; N. Mandrak pers. comm. 2023; T. Middel pers. comm. 2023).
Fishhook Water Flea (Cercopagis pengoi) is another invasive zooplankton which invaded Lake Ontario in July 1998 (Jacobs and MacIsaac 2007). Unlike Spiny Water Flea, Fishhook Water Flea has not (yet) expanded into inland waterbodies in southern or central Ontario but poses similar risks to the composition, richness and abundance of native zooplankton should this species ever become established in Opeongo Lake.
Invasive bivalves
Zebra Mussels (Dreissena polymorpha)were introduced to the Great Lakes in 1988 and have rapidly colonized lake and river bottoms, rocks, and aquatic vegetation (DFO 2013; Pollux et al. 2010). Although Zebra Mussel generally occupies water depths of around four to seven metres, some populations occupy deeper waters (DFO 2013; Pollux et al. 2010). Quagga Mussel (D. begensis) was introduced to North America through contaminated ballast water and is generally limited to deep water habitats within the southern Laurentian Great Lakes (DFO 2013). Quagga Mussel also occupies a broad range of substrates in rivers and lakes, including cobble, gravel, and fine sediments (Patterson et al. 2005). Dispersal of both Zebra Mussel and Quagga Mussel larvae may occur through various pathways, including contaminated watercrafts or by passive drift (Orlova et al. 2005). In shallower waters, Quagga Mussel has replaced Zebra Mussel in many areas of the Great Lakes basin through competitive exclusion (Wilson et al. 2006). Zebra Mussel and Quagga Mussel are not known from Opeongo Lake (EDDMapS 2023).
The introduction of dreissenid mussels (i.e., mussels belonging to the family Dreissenidae) to a waterbody can significantly alter native invertebrate assemblages and nutrient dynamics. Both Quagga Mussel and Zebra Mussel are known to feed extensively on zooplankton and planktonic algae, often leading to significant changes in ecosystem structure and functions across trophic levels (see DFO 2013 and references therein). Similar to Spiny Water Flea, no evidence of direct impacts to the growth or survival of Lake Whitefish in Lake Simcoe was expected following Zebra Mussel colonization (COSEWIC 2005); however, Cunningham and Dunlop (in press) found a significant decline in Lake Whitefish larval density based on historical (1976-1986) and contemporary (2017-2019) data. Dreissenid mussel presence was associated with reduced larval densities, and dreissenid establishment was considered a potential contributing factor to SLower growth and reduced survival of Lake Whitefish as a result of changes in zooplankton biomass and composition. It is also possible that decreases in nutrient inputs to Lake Simcoe following the implementation of the Lake Simcoe Phosphorus Reduction Strategy (2010) may have influenced zooplankton biomass in conjunction with the presence of dreissenid mussels. Lake Simcoe does not contain a Lake Whitefish species pair but given these findings, the impacts to Lake Whitefish in Opeongo Lake would likely be significant should dreissenid mussels ever become established.
Low calcium availability and low pH levels in lakes on the Precambrian Shield are known to limit dreissenid establishment (Hincks and Mackie 1997; N. Mandrak pers. comm. 2023; T. Middel pers. comm. 2023). Therefore, the likelihood of dreissenid invasion in Opeongo Lake is significantly lower than for Spiny Water Flea.
Introduction of nonindigenous and predatory fish
Rainbow Smelt
Unlike Cisco and Smallmouth Bass, Rainbow Smelt is not currently established in Opeongo Lake. Introduction of Rainbow Smelt to Opeongo Lake poses a known risk to Lake Whitefish as introductions elsewhere in Ontario (e.g., Fairy Lake and Mary Lake near Huntsville) have been implicated in Lake Whitefish population declines, at least in combination with introductions of other nonindigenous game fish (MNR2009).
Rainbow Smelt larvae may compete with larval Lake Whitefish for resources, while adult Rainbow Smelt are known to feed on Lake Whitefish larvae (Evans and Loftus 1987). A study conducted in Twelve Mile Lake (north of Minden, Ontario) observed larval Lake Whitefish in the stomach contents of most (93%) captured Rainbow Smelt, with a daily average of 8.4 larvae predated per smelt (Loftus and Hulsmann 2011). The authors suggested that Lake Whitefish recruitment failure in Twelve Mile Lake was due to Rainbow Smelt predation. Another study conducted in Lake Simcoe supports these results, finding that the abundance of Lake Whitefish decreased as Rainbow Smelt numbers increased (Evans and Waring 2011). Similar Lake Whitefish population declines following the introduction of Rainbow Smelt have been documented in Maine (Wood 2016).
While Rainbow Smelt are not a permitted baitfish under the Ontario Recreational Fishing Regulations Summary (MNRF 2023a), the species is currently present in the Amable du Fond River watershed (e.g., North Tea Lake, Manitou Lake, Kioshkokwi Lake) and Petawawa watershed (e.g., Tim Lake, Rosebary Lake, Catfish Lake) in the northern and northwestern regions of Algonquin PP (Ridgway et al. 2018). Rainbow Smelt are not currently known from the Upper Madawaska drainage (EDDMapS 2023). Additional lakes in the Petawawa River watershed are accessible and predicted to be invaded by Rainbow Smelt in the future (Ridgway et al. 2018).
Northern Pike
Northern Pike is not native to Algonquin PP (Ridgway and Middel 2020) and was first discovered in the Opeongo River inside the park’s southeastern boundary in the 1980s (Strickland 2000). It was then found upstream of the Booth Lake dam (a barrier to fish passage) in 1994, suggesting that more than one individual was purposely transferred via human intervention, and by 1999 four Northern Pike were captured during sampling immediately downstream of the Opeongo Lake dam (Strickland 2000). The dam was specifically designed to prevent the passage of fish, and although fishing within 300 m downstream of the dam and transporting live sport fish overland is prohibited (MNRF 2023a), it is possible that Northern Pike will eventually gain access to Opeongo Lake, posing a significant risk to Lake Whitefish survival.
Studies examining the influence of Northern Pike introductions on the morphology of the closely related European Whitefish (C. lavaretus) in Sweden found that pike initiate a “morphological response” (i.e., altered physiology and physical attributes) in whitefish. This response is speculated to result from avoidance of predation (Enbom 2013). Trudel et al. (2011) hypothesize that predation of large-bodied Lake Whitefish by Northern Pike is likely as they tend to select larger prey items.
Human-induced climate change
The effects of human-induced climate change on coldwater species such as Lake Whitefish directly stem from (i) increasing water temperature and (ii) changes in winter ice cover, which in turn indirectly alter habitat use, habitat quality and overall survival. Clear evidence of climate change influencing the aquatic ecosystems of Algonquin PP is revealed by long-term datasets (Ridgway et al. 2018; Ridgway and Middel 2020). Ice-out dates on Opeongo Lake have been recorded since 1964 and exhibit a relatively consistent trend, averaging approximately ten days earlier today (The Friends of Algonquin Park 2022). Ice-out on Opeongo Lake in 2021 occurred on April 10 (the third earliest date recorded), while ice-out in 2022 occurred on April 25 (more consistent with the long-term trend line).
Although projected climate warming is expected to impact smaller lakes more significantly than larger lakes, Opeongo Lake exhibits a large surface area and is comprised of four smaller lake basins. Opeongo Lake may therefore respond to climate change in ways similar to a series of smaller, interconnected lakes (N. Mandrak pers. comm. 2023).
As described below, climate change threatens Lake Whitefish in Opeongo Lake via multiple pathways, although further study (and time) is required to gauge the true effect.
Reduction in suitable oxythermal habitat
Lake Whitefish require sufficient levels of DO, which can be influenced by changes in temperature (Gorsky et al. 2012). Unusually warm spring water temperatures may trigger early onset of thermal stratification, increasing the amount of time in which the hypolimnion is physically isolated from the atmosphere (which would otherwise replenish DO levels). This effect ultimately results in a decline in hypolimnetic DO and increases the likelihood (and longevity) of hypoxia and/or anoxia in a given year.
Suitable water temperature and DO collectively create an oxythermal habitat envelope for Lake Whitefish (and other coldwater fish). Projected climate warming is expected to decrease the volume and spatial extent of optimal and/or suitable oxythermal habitat conditions (Gorsky et al. 2012; Ridgway et al. 2018; Ridgway and Middel 2020), thereby reducing the quantity and/or quality of Lake Whitefish habitat in Opeongo Lake.
Increased egg mortality
Spawning and egg development in Lake Whitefish are linked to water temperature, with successful egg development occurring between 0.5 and 10 °C (Gorsky et al. 2012; Price 1940). Projected warming may delay the onset of initial and peak spawning by Lake Whitefish, decreasing the time available for egg development. Reductions in ice-cover may also expose developing eggs to greater wave intensity during storm events (particularly in late fall and/or early spring), causing damage or displacement. The incidence of egg mortality was related to the timing of ice cover during a study of Lake Whitefish in Lake Michigan (Grand Traverse Bay), with early onset of ice cover associated with the highest rates of egg survival (Freeberg et al. 1990).
Changes in prey availability
Lake Whitefish emerge in Opeongo Lake within days of ice-out (Cucin and Faber 1985). Any changes to ice-out timing may reduce zooplankton prey availability for larval Lake Whitefish, unless prey are also able to shift life history strategies (Freeberg et al. 1990; Gorsky et al. 2012).
Increased incidence of harmful algal blooms
Increases in air and water temperature may in turn increase the likelihood of blue-green algae (i.e., cyanobacterial) blooms in Algonquin PP waterbodies, which are also known as harmful algal blooms (HABs). HABs may cause stress and/or ultimate mortality of Lake Whitefish due to a sudden decrease of oxygen (i.e., hypoxia) as excess algae die and subsequent decomposition consumes available oxygen (Ridgway and Middel 2020). Local effects of HABs may also include the creation of discrete dead zones which have low to no oxygen, a reduction in sunlight penetration below the water’s surface, or a reduction in the ability of fish to forage due to algae limiting their field of view (EPA 2023). Local effects may differ across the lake based on morphometrics within each basin (e.g., water depth, surface area, shape).
A cyanobacterial bloom in nearby (and oligotrophic) Dickson Lake has been linked to a series of conditions beginning with late ice-out and early thermal stratification (resulting in incomplete spring mixing), triggering an early onset of hypolimnetic anoxia and increased internal nutrient loading, coupled with elevated summer temperatures and low wind speeds (Favot et al. 2019). The authors of this study eliminated the possibility that increased nutrient levels from the broader watershed and/or changes in zooplankton grazing pressure drove the cyanobacterial bloom, and implicated climate change as an “ultimate driver and proximate cause”. The well-publicized cyanobacterial bloom in Dickson Lake does not appear to have adversely affected the long-term viability of either Lake Trout or Brook Trout (Salvelinus fontinalis), whose populations quickly recovered (M. Ridgway pers. comm. 2023). The potential implications for Lake Whitefish are unknown but presumed to be similar.
Incidental by-catch
Angling effort in Opeongo Lake greatly exceeds that of all other lakes in Algonquin PP, with anglers primarily targeting Lake Trout and Smallmouth Bass (Mitchell et al. 2020). MNRF creel data from Opeongo Lake indicates that an average of 8.6 Lake Whitefish were caught per year by anglers between 2005 and 2019, with an average harvest per year of 5.9 (T. Middel pers. comm. 2023). Estimated rod hours targeting Lake Whitefish within that period averaged only 30.5 hours per year, with several years (2013, 2014, 2018, 2019) representing no angling effort whatsoever. Overall angling effort targeting Lake Whitefish has been negligible historically when compared to other fishes in Opeongo Lake and throughout Algonquin PP (T. Middel pers. comm. 2023).
Angling for Lake Whitefish in Opeongo Lake was prohibited in 2022 (MNRF 2022) following provincial listing of the large- and small-bodied forms as Threatened. Given historically low angling effort and low harvest rates per year prior to prohibition, incidental by-catch likely poses a minor threat and has the greatest likelihood of occurrence when anglers target Lake Trout (which occupies similar though often deeper portions of the lake) rather than Smallmouth Bass or other littoral species (which generally feed in nearshore areas).
1.7 Knowledge gaps
Despite historical and recent research interest, there are several gaps in current knowledge that would benefit from further research and assessment to inform recovery efforts and future habitat protections. These knowledge gaps are detailed below and include:
- key physical attributes of the large- and small-bodied forms
- population abundance, structure, and trends
- genetic isolation of forms
- ontogenetic and seasonal variation in habitat use
- spawning habitat
- larval survival, diet, and dispersal
- trophic niche
Key physical attributes of the large- and small-bodied forms
From the early 1980s until about 2017, research studies focusing on Lake Whitefish in Opeongo Lake (e.g., Ihssen et al. 1981; Challice et al. 2019) along with MNRF fish monitoring programs did not often distinguish between the large- and small-bodied forms (T. Middel pers. comm. 2023). Records of Lake Whitefish from this time period generally represent large-bodied individuals due to biases introduced through sampling techniques (i.e., small-bodied forms are not typically captured in standard large-mesh gillnets; M. Ridgway pers. comm. 2023). A large historical dataset in which the forms were distinguished is available from Kennedy (1943), though certain metrics reported (e.g., maximum age) differ from more recent (unpublished) MNRF data.
While there is no scientific debate as to the presence of two physically, physiologically, and genetically distinguishable forms of Lake Whitefish in Opeongo Lake (M. Ridgway pers. comm. 2023), a modern systematic study of their physical characteristics (with a focus on key differences) is lacking. It is further unknown whether the large- and small-bodied forms can be differentiated at the larval stage, either through visual inspection or genetic methods.
Population abundance, structure, and trends
Long-term monitoring of fish populations in lakes throughout Algonquin PP is undertaken using North American standard (NA1) large-mesh gillnets (T. Middel pers. comm. 2023), which are 24.8 m long by 1.8 m high, and consist of eight panels (each 3.1 m long) with mesh sizes ranging from 38 to 127 mm (i.e., 38, 51, 64, 76, 89, 102, 114, and 127 mm; Sandstrom et al. 2013). The monitoring program in Algonquin PP involves sampling at five-year intervals and represents a modified-version of the provincial Broad-scale Monitoring (Chironomidae) program, given shorter-duration net sets (i.e., two-hour rather than overnight; T. Middel pers. comm. 2023). Sampling data from the modified-Chironomidae protocol is available from 2013 and 2019, with additional data deriving from Summer Profundal Index Netting (SPIN) sampling completed in 2009 and 2010. Population estimates have been developed for the large-bodied form (as reported in Colm and Drake 2022) based on this sampling data.
The small-bodied form of Lake Whitefish in Opeongo Lake is not typically captured by large-mesh gillnets (M. Ridgway pers. comm. 2023). An Ontario-standard (ON2) small-mesh gillnet is required to survey the small-bodied form, which is 12.5 m long by 1.8 m high, and consists of five panels (each 2.5 m long) with mesh sizes ranging from 13 to 38 mm (i.e., 13, 19, 25, 32, and 38 mm; Sandstrom et al. 2013). In addition to capturing the small-bodied form, small-mesh gillnets will also capture smaller individuals of the large-bodied form.
Modern surveys targeting Lake Whitefish specifically (rather than the pelagic and benthic fish community generally) are needed to support rigorous population abundance estimates and guide future management. Targeted surveys for the large-bodied form with standard large-mesh gillnets occurred in 2021, while small-mesh gillnet surveys targeting the small-bodied form occurred in 2018 (T. Middel pers. comm. 2023). The small-bodied form has not been afforded a population estimate due to lack of sufficient data, and population trends are not available for either form at this time (Fung et al. 2022). Current information related to population abundance, structure, and trends for both forms is limited or lacking, and thus represents a knowledge gap.
Genetic isolation of forms
Recent genetic work has shown that the large- and small-bodied forms of Lake Whitefish in Opeongo Lake are allopatric (i.e., arose in-situ, rather than arriving from separate colonization events) and show evidence of limited interbreeding in the past (C. Wilson pers. comm. 2023). Notwithstanding this, additional studies are needed to determine whether speciation of the two forms is irreversible or if coalescence between the two forms may result from future changes in habitat (or other factors).
Ontogenetic and seasonal variation in habitat use
Lake Whitefish habitat is known to vary across life stages and seasons. Spawning and larval habitat are relatively well understood; however, ontogenetic shifts in diet and prey specialization are known to occur in age 0 and juvenile Lake Whitefish (Pothoven et al. 2014; Pothoven and Olds 2020), suggesting the possibility of differences in habitat use across age classes. Additionally, the timing of certain life processes (e.g., egg development) is poorly understood given the unique challenges associated with documenting year-round habitat use (e.g., beneath ice cover). While Lake Whitefish are known to spawn in rivers (Wood 2016), occupation of creeks which are hydrologically connected to Opeongo Lake (e.g., Costello Creek, Hailstorm Creek) is unknown. Variation in habitat use for all Lake Whitefish age classes in Opeongo Lake (and connected watercourses), and the seasonality of habitat use patterns, represents a knowledge gap.
Spawning habitat
Little is known about the physical characteristics of Lake Whitefish spawning habitat in Opeongo Lake (M. Ridgway pers. comm. 2023; T. Middel pers. comm. 2023). Lake Whitefish are assumed to spawn in the same areas as Lake Trout, for which 22 spawning shoals have been identified (T. Middel pers. comm 2023). Nevertheless, the extent to which Lake Whitefish spawning habitat coincides with areas used by Lake Trout is unknown, and it is further thought that Lake Whitefish spawning habitat may be less spatially restricted (M. Ridgway pers. comm. 2023). The extent to which fine-scale physical attributes such as (among others) substrate type, substrate size and structure, water depths, distance from shore, and fetch control spawning habitat quality for Lake Whitefish (either form) in Opeongo Lake remains a key knowledge gap.
Larval survival, diet, and dispersal
Apart from previous work by Cucin and Faber (1985), limited survey effort has focused on understanding the spatial distribution and growth patterns of larval Lake Whitefish in Opeongo Lake. The diet of larval Lake Whitefish in Opeongo Lake is unknown, and there is no baseline data upon which to assess annual and long-term trends in larval survival, diet, and dispersal.
Trophic niche
Lake Whitefish species pairs in Canada have often evolved in waterbodies where Cisco are absent. In such cases, one Lake Whitefish form (the larger or “normal” form) occupies the typical benthivore (bottom-feeding) foraging niche while the other (the smaller or “dwarf” form) adopts a pelagic/limnetic (open-water) life strategy and feeds on plankton (Bernatchez 2004), effectively acting as a “Cisco mimic” (Ridgway and Middel 2020). This pattern of habitat partitioning has arisen independently in several lakes including Como Lake northwest of Sudbury (Vuorinen et al. 1993) and Big Trout Lake in Algonquin PP (Ridgway and Middel 2020). Other unusual instances of trophic specialization in Lake Whitefish have arisen elsewhere. Lake La Muir in Algonquin PP possesses only the pelagic/limnetic form of Lake Whitefish and lacks a benthic form entirely despite the availability of deep water with sufficient DO (Ridgway and Middel 2020).
At this time, evidence of habitat partitioning between the large- and small-bodied forms in Opeongo Lake is limited. Kennedy (1943) found that the large-bodied form occupied shallower water (10 m) than the small-bodied form (15 m) in August, but otherwise did not find differences in vertical distribution during the remaining survey period (May to September). It has been speculated that the large-bodied form could co-exist alongside the introduced Cisco in shallower waters (i.e., occupy a pelagic niche) given its larger size and thus greater ability to compete for plankton (J. Colm pers. comm. 2023). Notwithstanding this, gillnets set in the pelagic zone of Opeongo Lake typically only capture Cisco (M. Ridgway pers. comm. 2023). Current sampling data seems to suggest that both forms are benthic (M. Ridgway pers. comm. 2023), though the mechanisms which maintain niche partitioning are unknown. There is a need to confirm diet and overall trophic niche for both the large- and small-bodied forms individually.
1.8 Recovery actions completed or underway
Studies of Lake Whitefish in Opeongo Lake began nearly a century ago when Kennedy (1943) captured large- and small-bodied morphotypes during sampling with gillnets and fyke nets deployed in the late 1930s. Since Kennedy’s seminal study, researchers operating out of the Harkness Laboratory of Fisheries Research have made further and significant contributions to our understanding of Lake Whitefish life history (primarily the large-bodied form) in Opeongo Lake. Ihssen et al. (1981) explored variation in ecology and morphology of Lake Whitefish populations across Ontario (including Opeongo Lake), while Cucin and Faber (1985) considered reproduction and early life history. Later, Carl and McGuiness (2006) compared the Lake Whitefish community structure in Opeongo Lake to those of nine other lakes across southcentral Ontario. Most recently, Challice et al. (2019) used depth stratified gillnet sampling to reveal and model habitat associations.
Angling for Lake Whitefish (either form) in Opeongo Lake was prohibited in 2022 following provincial listing as Threatened (MNRF 2022). While angling pressure for Lake Whitefish in Opeongo Lake has been low to negligible over the previous decade (T. Middel pers. comm. 2023), the prohibition on angling for Lake Whitefish is a statutory requirement under section 9 of the ESA and provided clarity to anglers and park visitors that the species (both forms) could no longer be targeted.
Also in 2022, a pamphlet introducing anglers and park visitors to the large- and small-bodied populations of Lake Whitefish was prepared and distributed by park staff at the fish check station at the Opeongo Lake Access Point (T. Middel pers. comm. 2023). Recent articles in The Raven (LeGros 2022; published by the Friends of Algonquin Park) and the creel bulletin (N. Lacombe pers. comm. 2023) served to introduce a wide audience to the uniqueness of Lake Whitefish in Opeongo Lake.
Surveys targeting Lake Whitefish in Opeongo Lake undertaken by MNRF staff occurred in 2018 (small-bodied) and 2021 (large-bodied). A more comprehensive sampling program for the small-bodied form is planned for 2024, with preliminary surveys to occur in 2023 (M. Ridgway pers. comm. 2023).
Additionally, the Algonquin Provincial Park Management Plan (1998) guides day-to-day management and development activities within Algonquin PP as well as informing stewardship policies and wildlife management decisions. The management plan also includes direction regarding species at risk within the park. Per the management plan, provincially vulnerable, threatened, and endangered species will be prioritized in management decisions to ensure their protection, which includes encouraging further studies of species at risk within the park, such as Lake Whitefish in Opeongo Lake.
2.0 Recovery
2.1 Recommended recovery goal
The recommended recovery goal for Lake Whitefish (large- and small-bodied populations) in Opeongo Lake is to maintain self-sustaining populations of both forms.
2.2 Recommended protection and recovery objectives
The recommended protection and recovery objectives for Lake Whitefish (Opeongo Lake large- and small-bodied populations) are:
- Minimize risk of introducing aquatic invasive and predatory species.
- Refine population abundance estimates and project trends.
- Clarify patterns in habitat occupancy for all life stages to inform habitat protection.
- Clarify trophic niche and diet to inform recovery efforts.
- Monitor key water quality parameters to inform recovery efforts.
- Promote awareness of large- and small-bodied Lake Whitefish in Opeongo Lake and threats facing them.
2.3 Recommended approaches to recovery
Table 4 . Recommended approaches to recovery of the Opeongo Lake large- and small-bodied populations of Lake Whitefish in Ontario.
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Management | 1.1 Install permanent watercraft and gear washing stations at Opeongo Lake access points.
| Threats:
|
| Critical | Short-term | Management | 1.2 Manage watercraft (motorized and nonmotorized) and angling activity.
| Threats:
|
| Critical | Short-term | Management | 1.3 Prepare an invasive species prevention and management response plan specific to Algonquin PP.
| Threats:
|
| Beneficial | Short-term | Management | 1.4 Install signage at the Opeongo Lake boat launch, Annie Bay dam, and other strategic locations to support aquatic invasive species prevention efforts.
| Threats:
|
| Beneficial | Short-term | Management | 1.5 Position park staff at access points (i.e., vehicular and portage), particularly during peak visitor entry/exit.
| Threats:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Ongoing | Inventory, Monitoring and Assessment | 2.1 Establish and deliver a long-term monitoring program.
| Knowledge gaps:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Short-term | Inventory, Monitoring and Assessment | 3.1 Locate, delineate and characterize spawning areas for Lake Whitefish (both forms).
| Knowledge gaps:
|
| Necessary | Short-term | Inventory, Monitoring and Assessment | 3.2 Clarify larval habitat use.
| Knowledge gaps:
|
| Necessary | Short-term | Monitoring and Assessment | 3.3 Clarify seasonal habitat use for adults.
| Knowledge gaps:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Beneficial | Short-term | Monitoring and Assessment, Research | 4.1 Clarify diet and trophic niche for adults to inform recovery efforts.
| Knowledge gaps:
|
| Beneficial | Short-term | Monitoring and Assessment, Research | 4.2 Clarify diet for larvae to inform recovery efforts.
| Knowledge gaps:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Ongoing | Monitoring and Assessment, Research | 5.1 Prepare and implement an ongoing water quality monitoring program to inform recovery efforts.
| Threats:
Knowledge gaps:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Ongoing | Education and Outreach | 6.1 Disseminate information to park staff and visitors.
| Threats:
|
Narrative to support approaches to recovery
The recommended long-term recovery goal for Lake Whitefish in Opeongo Lake emphasizes the need to maintain a “self-sustaining” population of both forms. The recovery goal does not reference increasing the size of either population (through habitat enhancement, etc.) as there is currently no evidence suggesting that this would contribute meaningfully to recovery efforts and/or be justified in terms of resource expenditure. Implementation of the monitoring program and other recovery approaches outlined herein may reveal that certain strategic enhancement efforts (e.g., improvement or creation of spawning habitat) would be beneficial or even necessary, but data which support such a conclusion is lacking at this time.
Wherever possible, all recommended survey, sampling/monitoring and research efforts should distinguish between the large- and small-bodied forms. This is not explicitly stated in the recovery objectives and approaches (for brevity) but is implied. For example, fish sampled during spawning surveys (via strategic gillnetting) should be differentiated at each discrete sampling area (if possible) to determine whether the timing and/or location of spawning habitats overlap between the two forms. This will assist with identifying reproductive barriers, which may be prezygotic (i.e., arising prior to reproduction) or postzygotic (i.e., arising after zygote formation and hampering embryo development). Additionally, research program design should strive to minimize mortality (to the extent possible). Instances of unavoidable mortality will allow for additional morphometric and genetic study of the two forms. All research activities must employ gear which has been sterilized to avoid the risk of aquatic invasive species introductions.
Minimize threats associated with the introduction of aquatic invasive and predatory species
The introduction of aquatic invasive species (AIS) and nonindigenous predatory fish poses a severe risk to both forms of Lake Whitefish in Opeongo Lake. Collapse of the Lake Whitefish species pair in Como Lake (and replacement with a single, larger species) offers an instructive and disconcerting lesson on how quickly trophic effects can reverberate through a lake ecosystem following the introduction of Spiny Water Flea (Reid et al. 2017). Predatory fishes whose historical range fell beyond the boundaries of Algonquin PP now occur in close proximity to Opeongo Lake: Northern Pike gained access to Tip Up Lake below the Opeongo Lake dam nearly three decades ago, while Rainbow Smelt occurs in at least six lakes extending across the northern region of the park.
There are several factors which increase the risk of overland transport of AIS (and eventual establishment into new waterbodies) by both motorized and nonmotorized watercraft (Johnson et al. 2001; Anderson et al. 2014; Drake 2017):
- frequency of use overall
- frequency of multi-watershed use
- overland travel distance
- cleaning (including drying) practices
Invasive zooplankton such as Spiny Water Flea, Fishhook Water Flea and others have the greatest likelihood of transfer to Opeongo Lake where visitors use watercraft or gear which was recently in contact with an invaded waterbody and had not been properly cleaned or dried before use. There are many angling restrictions in Opeongo Lake which seek to limit the risk of introduction and transfer of AIS, including prohibitions on using live bait and fishing within 300 m downstream of the Opeongo River dam (MNRF 2023a). Still, Opeongo Lake is particularly susceptible to introductions of AIS and predatory fish given vehicular access from Highway 60, intense angling and visitor interest, and permissibility of motorboats with no horsepower limits (Ridgway et al. 2018). On a busy summer day, 100 boats may be launched from the Opeongo Lake Access Point (N. Lacombe pers. comm. 2023).
AIS risk mitigation on Opeongo Lake benefits from the fact that only a single boat and parking access point is available to visitors. Installation of a mandatory boat and gear washing station(s), with an accompanying operations plan to support management by park staff, is considered a critical, short-term recovery activity. Despite this, there are significant implementation challenges associated with the management of such stations by park staff given the sheer volume of boats, timing (e.g., boats may be launched as early as 5:00 a.m.), contaminated fishing gear (which may evade cleaning), and enforcement (P. Gelok pers. comm. 2023). To partly address these issues, boat and gear wash stations could be established in multiple locations; for example, at both the Opeongo Lake Access Point and on Opeongo Road in a suitable location north of Highway 60. Effectiveness would likely be improved if the wash stations were treated as inspection stations (effectively making watercraft and gear washing mandatory), which would require additional commitment and resources from park staff.
Given the likelihood and severity of Spiny Water Flea establishment, and due to the aforementioned challenges with wash stations, there is a need to consider more effective measures to control motorized watercrafts and alter angling activity on Opeongo Lake to reduce the likelihood of Spiny Water Flea establishment. Establishing reasonable horsepower limits could be implemented swiftly to reduce motorboat traffic on the lake and would lessen the risk of invasive invertebrate introductions to some degree (Ridgway et al. 2018).
Nonmotorized watercraft such as manually-driven vessels (e.g., canoes, kayaks) and associated gear (e.g., paddles, straps, ropes) may also act as vectors of AIS transport between waterbodies, though such vessels generally offer fewer opportunities for AIS transport (given an absence of livewells, etc., see Drake 2017). AIS may adhere to either the exterior or interior hulls of canoes or kayaks or become affixed to associated straps or bungee cords. Newer canoes and kayaks may contain specialized features (e.g., foot pedals, additional compartments) and/or be packable/inflatable, which introduces additional modes of AIS transport not exhibited by more traditional nonmotorized watercraft. While the number of visitors traveling via portage routes between lakes which are known to contain Spiny Water Flea (i.e., northwestern section Algonquin PP) and Opeongo Lake is likely very low (due to distance), some visitors opt to bring their own vessels and equipment which may have been used recently within infested waterbodies. Any nonmotorized watercraft and equipment deployed by park users on Opeongo Lake should be subject to the same wash and/or inspection protocols as required for motorized vessels.
An invasive species prevention and management plan specific to Algonquin PP should be developed to prioritize actions for prevention, early detection, and rapid response efforts following detection of an introduced AIS or predatory fish. Rapid response efforts should balance the feasibility and effectiveness of various response options (e.g., eradication, containment, control). Although eradication may not be feasible, other management options – such as containment or control – may reduce impacts and spread of AIS. Similar frameworks from other jurisdictions may serve as a guide, including the Invasive Species Early Detection and Rapid Response Plan for British Columbia (IMISWG 2014).
Installation of signage at strategic locations (e.g., portage access points, Annie Bay dam) would emphasize the risks of AIS to Opeongo Lake visitors and reinforce existing rules and best management practices (e.g., cleaning and/or drying gear before use in another waterbody, watercraft cleaning and draining). Park staff should also be stationed at the Opeongo Lake Access Point and portages with cleaning supplies and educational materials outlining best management practices for reducing the spread of invasive zooplankton.
Refine population abundance estimates and project trends
Much of what is known about Lake Whitefish in Opeongo Lake has emerged from research efforts over the past several decades (e.g., Challice et al. 2019; Cucin and Faber 1985; Ihssen et al. 1981; Kennedy 1943) and sampling efforts by MNRF staff since 2009 (e.g., SPIN and modified-Chironomidae surveys). There are limitations with existing datasets, including that most do not distinguish between the large- and small-bodied forms. The small-bodied form in Opeongo Lake is not effectively captured by large-mesh gillnets (M. Ridgway pers. comm. 2023), suggesting that most published and unpublished data pertains only to the large-bodied form. Further, current population estimates of the large-bodied form are based on sampling data focused on the fish community generally (rather than Lake Whitefish specifically) and are insufficient to model population trajectories (Fung et al. 2022).
Additional sampling is needed at routine intervals to (i) verify and/or refine previous population estimates of the large-bodied form, (ii) develop a defensible population estimate of the small-bodied form, and (iii) project trends for both forms. Given the high incidence of Lake Whitefish mortality during gillnetting (i.e., approximately 95%, M. Ridgway pers. comm. 2023), the regularity of monitoring should be selected in a way that minimizes impact. Unavoidable mortality offers samples upon which modern morphometric and genetic analysis can be performed. Most individuals cannot be assigned to form without dissection (M. Ridgway pers. comm. 2023), which allows for analysis of age structures and confirmation of reproductive status. If a methodology facilitating visual identification of live specimens could be developed (i.e., supported by growth curves and/or discovery of additional and reliable physical differences) this could assist with minimizing mortality (and permit acoustic telemetry of the small-bodied form).
Clarify patterns in habitat occupancy for all life stages
Many aspects of Lake Whitefish biology and life history are understood generally across its distribution. However, the uniqueness of the large- and small-bodied forms in Opeongo Lake suggests that extrapolation from other populations (in Ontario or elsewhere) may not be appropriate. Lake Whitefish spawning locations in Opeongo Lake are assumed to overlap with those of Lake Trout, but few are known with certainty, nor have any been treated to modern study (T. Middel pers. comm. 2023; M. Ridgway pers. comm. 2023). Other key attributes of life history which are essential for informing management and conservation – such as seasonal/winter habitat use, potential occupation of connected creeks (outside of thermal stratification) and timing of larval dispersal – are based on limited empirical study dating back several decades or are inferred from Lake Whitefish populations elsewhere.
Developing a better understanding of habitat use within Opeongo Lake across forms, age classes, and seasons will inform future management and protection. Critical to this endeavour is the identification, delineation and characterization of all spawning areas, allowing for differentiation and comparisons with areas where spawning activity has not been documented. Such efforts may also clarify the functional value of different spawning areas and their relative productivity, particularly when considered in tandem with the results of offshore (ichthyoplanktontows) and nearshore (seine netting) larval sampling.
A multi-disciplinary team from DFO, MNRF, and several Ontario universities recently installed acoustic receiver arrays (InnovaSea) in Smoke Lake, Canoe Lake, and Tea Lake to assess fish movement patterns (M. Ridgway et al. 2021). These lakes are considerably smaller than Opeongo Lake, and if all 149 receivers were relocated to Opeongo Lake they would only sufficiently cover the South Arm (M. Ridgway pers. comm. 2023). Despite the obvious challenges and costs associated with installing a receiver array in Opeongo Lake (or in a particular basin), the resulting data would significantly advance knowledge of seasonal habitat use and movement patterns over a multi-year timeframe. The acoustic data would be comparable with the results of strategic gillnetting (e.g., in potential spawning habitat during November, in connected streams) to offer a more fulsome picture of Lake Whitefish habitat use (for both forms) over time and throughout the lake.
Clarify trophic niche and diet
Previous work by Sandercock (1964) and unpublished data collected by the MNRF in the 1980s revealed much of what is known about Lake Whitefish diet in Opeongo Lake through stomach contents analysis. Modern studies should combine stomach contents analysis and isotopic analysis using stable isotopes to clarify the predominant prey items for Lake Whitefish during all life stages. Further analysis of diet (coupled with the results of regular sampling and acoustic telemetry) is intended to resolve longstanding ambiguity regarding trophic niche, particularly whether the large- and small-bodied forms occupy a pelagic/limnetic and/or benthic position.
Monitor key water quality parameters
There are several pathways through which the indirect effects of climate change could adversely affect the quantity or quality of habitat for Lake Whitefish in Opeongo Lake. This includes reducing the availability of suitable oxythermal habitat (i.e., with sufficient DO and appropriate temperature), increasing egg failure (through changes in ice cover), altering prey composition and availability, and increasing the incidence of HABs. A routine monitoring program should be prepared and implemented wherein chemical parameters which are known to (or may) affect Lake Whitefish growth and survival (directly or indirectly) are analyzed, such as DO, temperature, calcium, and pH. Annual monitoring of ice-out dates should also continue.
Promote awareness
Opeongo Lake is easily accessible to park visitors from Highway 60 and offers a centralized launching point for backcountry camping throughout the park. Ease of access facilitates intense interest from anglers, campers, and other visitors, furthered by the permissibility of (and lack of horsepower restrictions on) motorboats. Awareness and outreach initiatives such as installing educational signage at the Opeongo Lake Access Point focused on the uniqueness and importance of Lake Whitefish (and the adverse effects of AIS) would be visible to many visitors. There is further opportunity to develop educational materials for dissemination at other strategic locations (e.g., permit offices), discuss threats and ongoing research in published form (e.g., additional publications in The Ravenand produce displays for exhibition at the Visitors Centre. The park reservation system could also be leveraged to disseminate information about Lake Whitefish in Opeongo Lake to visitors launching from the Opeongo Lake Access Point or camping overnight on or near Opeongo Lake. The reservation system could be further used to highlight activities which pose a greater risk to Lake Whitefish (e.g., launching unwashed watercraft) to visitors camping on or near Opeongo Lake, or to visitors more broadly.
2.4 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of the Environment, Conservation and Parks on the area that should be considered if a habitat regulation is developed. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the author will be one of many sources considered by the Minister, including information that may become newly available following the completion of the recovery strategy should a habitat regulation be developed for this species.
Lake Whitefish have been documented in all four basins of Opeongo Lake (see Figure 3 ), though summer habitat use (i.e., following stratification) is restricted to areas with suitable thermal characteristics (i.e., hypolimnion). Occupation of connected creek systems including Costello Creek and Hailstorm Creek is possible (particularly when the lake is well mixed) but not known. Spawning shoals have not been systematically documented but may roughly coincide with those used by Lake Trout. Considerable monitoring and research efforts are needed (and recommended herein) to clarify the spatial distribution of Lake Whitefish (both forms) in Opeongo Lake across seasons and life stages.
It is well established that upland/terrestrial riparian zones adjacent to waterbodies provide indirect (and sometimes critically important) habitat for certain species (or life stages) of freshwater fish. Alternatively, benthivores which occupy a profundal niche in lake-environments are less functionally reliant upon riparian condition or changes in riparian function (Caskenette et al. 2021; Richardson et al. 2010). As a coldwater fish that is largely restricted to deep waters (at least during summer), the persistence of both forms of Lake Whitefish in Opeongo Lake is likely insensitive to riparian conditions (T. Middel pers. comm. 2023, J. Colm pers. comm. 2023, A. Drake pers. comm. 2023, N. Mandrak pers. comm. 2023, M. Ridgway pers. comm. 2023). Thus, the riparian zone surrounding Opeongo Lake does not appear to constitute “habitat” as defined in the ESA.
Given significant knowledge gaps in life history and habitat occupation – both for Lake Whitefish generally and the large- and small bodied forms individually – and Opeongo Lake’s location within a protected area, a habitat regulation may not be required at this time. Should a habitat regulation be developed in the future, it is recommended to include all portions of Opeongo Lake consisting of rocky shoals 10 to 50 m offshore with depths ranging from 3 to 5 m (i.e., suitable spawning and nursery habitat), and deep water areas with water depths ranging from 6 to 32 m (i.e., suitable feeding habitat for juveniles and adults). Further refinement of this habitat recommendation may be possible once more information pertaining to habitat occupancy is revealed through future survey and sampling efforts.
Glossary
- Adaptive radiation
- process in which organisms diversify rapidly from an ancestral species into a multitude of new forms, particularly when a change in the environment makes new resources available, alters biotic interactions or opens new environmental niches.
- Adipose fin
- A soft, fleshy fin located behind the dorsal fin and just forward of the caudal fin, found in fish of certain families, believed to have some sensory function.
- Allopatric
- A group of organisms which are geographically isolated.
- Annulus (pl. Annuli)
- Annual markings (rings) produced on fish scales in response to seasonal growth patterns.
- Benthivore
- Fish that prey on shellfish, crustaceans and other small invertebrates that dwell on the lake bottom or seafloor.
- Caudal
- Referring to the posterior or tail.
- Caudal peduncle
- Tapered area behind the dorsal and anal fins where the caudal fin attaches to the body.
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC)
- The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
- Committee on the Status of Species at Risk in Ontario (COSSARO)
- The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
- Conservation status rank
- A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. Ranks are determined by NatureServe and, in the case of Ontario’s S-rank, by Ontario’s Natural Heritage Information Centre. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperiled
2 = imperiled
3 = vulnerable
4 = apparently secure
5 = secure
NR = not yet ranked - Cycloid
- Thin, rounded scales which overlap.
- Diel vertical movement
- Also known as diurnal vertical migration. Pattern of movement typical of certain aquatic organisms, involving changes in occupied water depth across a 24-hour period.
- Dorsal
- Referring or related to the back or upper side of an organism’s body.
- Endangered Species Act, 2007 (ESA)
- The provincial legiSLation that provides protection to species at risk in Ontario.
- Epilimnetic (Epilimnion)
- Referring to the surface layer in a body of water.
- Fork length
- A fish’s body length measured from the tip of its snout to the fork of the tail.
- Founder effects
- Reduced genetic diversity in a population, arising from descendance from a small number of colonizing ancestors.
- Genetic drift
- Changes in the gene pool of a small population owing to random chance events.
- Gill rakers
- Bony or cartilaginous projections from the gill arch which serve to sieve and retain food particles.
- Hypolimnion
- Deeper and colder layer in a thermally stratified body of water.
- Interstitial space
- Open areas or cavities between particles of substrate.
- Limnetic
- Referring to (living in) an open body of water.
- Melanophore
- Specialized cells filled with the dark pigment melanin.
- Morphotype
- Group of different types of individuals of the same species.
- Nuptial tubercles
- Raised structures made of keratin typically shed after breeding.
- Oligotrophic
- Lake or water body with relatively low productivity as a result of poor nutrient supply.
- Ontogenetic
- of or relating to the origin and development of individual organisms.
- Oxythermal
- Referring to both oxygen and temperature collectively.
- Pelagic
- Referring to open water.
- Pelvic axillary process
- A small, triangular projection at the upper end of the base of the pelvic fin.
- Postzygotic (reproductive barrier)
- Arising after zygote formation and hampering embryo development.
- Prezygotic (reproductive barrier)
- Arising prior to reproduction.
- Propagule (pl. Propagules)
- A structure which may give rise to a new individual organism.
- Rod hours
- Number of hours spent by an angler targeting a particular species.
- Species at Risk Act (SARA)
- The federal legiSLation that provides protection to species at risk in Canada. This Act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
- Species at Risk in Ontario (SARO) List
- The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008 (Ontario Regulation 230/08).
- Standard length
- A fish’s body length from the tip of its nose to the end of its last vertebrae.
- Thermocline
- Transition layer between warmer, less dense water at the surface and cooler, denser water below; a product of lake stratification in summer.
- Thermal stratification
- Settling of colder water below warmer water in a waterbody, producing layers with distinct thermal characteristics.
- Trophic Niche
- The unique position an organism occupies in a food web.
List of abbreviations
- AIS
- Aquatic Invasive Species
- CI
- Confidence Interval
- COSEWIC
- Committee on the Status of Endangered Wildlife in Canada
- COSSARO
- Committee on the Status of Species at Risk in Ontario
- CWS
- Canadian Wildlife Service
- DO
- Dissolved Oxygen
- DU
- Designatable Unit
- DFO
- Fisheries and Oceans Canada
- ESA
- Ontario’s Endangered Species Act, 2007
- FL
- Fork Length
- HAB
- Harmful Algal Bloom
- ISBN
- International Standard Book Number
- MECP
- Ministry of the Environment, Conservation and Parks
- MNRF
- Ministry of Natural Resources and Forestry
- MVP
- Minimum Viable Population
- PP
- Provincial Park
- ROM
- Royal Ontario Museum
- SARA
- Canada’s Species at Risk Act
- SAROList
- Species at Risk in Ontario List
- SD
- Standard Deviation
- SL
- Standard Length
References
- Anderson, L. G., P. C. L. White, P. D. Stebbing, G. D. Stentiford, and A. M. Dunn. 2014. “Biosecurity and Vector Behaviour: Evaluating the Potential Threat Posed by Anglers and Canoeists as Pathways for the Spread of Invasive Non-Native Species and Pathogens”. PLoS One 9 (4): e92788.
- Bernard, A. 2006. “Cryptic Structure and Diversity of Lake Whitefish (Coregonus clupeaformis) in Ontario Waters”. Master's thesis, University of Guelph.
- Bernard, A. M., M. M. Ferguson, D. L.G. Noakes, B. J. Morrison, and C. C. Wilson. 2009. “How Different Is Different? Defining Management and Conservation Units for a Problematic Exploited Species”. Canadian Journal of Fisheries and Aquatic Sciences 66 (9): 1617–30.
- Bernatchez, L. 2004. “Ecological Theory of Adaptive Radiation: An Empirical Assessment from Coregonines Fishes (Salmoniformes)”. In Evolution Illuminated: Salmon and Their Relatives, edited by A. P. Hendry and S. C. Stearns, 175–207. New York: Oxford University Press.
- Bernatchez, L., and J. J. Dodson. 1991. “Phylogeographic Structure of Mitochondrial DNA of the Lake Whitefish (Coregonus clupeaformis) and Its Relation to Pleistocene Glaciations”. Evolution 45 (4): 1016–35.
- Bernatchez, L., S. Renaut, A. R. Whiteley, N. Derome, J. Jeukens, L. Landry, G. Lu, et al. 2010. “On the Origin of Species: Insights from the Ecological Genomics of Lake Whitefish”. Philosophical Transactions of the Royal Society B: Biological Sciences 365 (1547): 1783–1800.
- Carl, L. M., and F. McGuiness. 2006. “Lake Whitefish and Lake Herring Population Structure and Niche in Ten South-Central Ontario Lakes”. Environmental Biology of Fishes 75 (3): 315–23.
- Caskenette, A., T. Durhack, S. Hnytka, C. Kovachik, and E. Enders. 2021. “Evidence of Effect of Riparian Attributes on Listed Freshwater Fishes and Mussels and Their Aquatic Critical Habitat: A Systematic Map Protocol”. Environmental Evidence 10 (18): 1–10.
- Challice, A. R., S. W. Milne, and M. S. Ridgway. 2019. “Does Habitat Occupancy by Lake Trout and Lake Whitefish in Large Lakes Match Published Thermal Habitat Envelopes?” Ecology of Freshwater Fish 28 (4): 611–23.
- Christie, G. C., and H. A. Regier. 1988. “Measures of Optimal Thermal Habitat and Their Relationship to Yields for Four Commercial Fish Species.” Canadian Journal of Fisheries and Aquatic Sciences 45 (2): 301–14.
- Colm, J., and A. R. Drake. 2022. “Information in Support of a Recovery Potential Assessment of Lake Whitefish (Coregonus Clupeaformis), Lake Opeongo Large-Bodied and Small-Bodied Designatable Units”. Ottawa.
- COSEWIC. 2005. “COSEWIC Assessment and Update Status Report on the Lake Whitefish (Lake Simcoe Population) Coregonus Clupeaformis in Canada”. Ottawa.
- ———. 2018. “Whitefish (Coregonus Spp.): COSEWIC Assessment and Status Report”.
- Cucin, D., and D. J. Faber. 1985. “Early Life Studies of Lake Whitefish (Coregonus Clupeaformis), Cisco (Coregonus Artedii), and Yellow Perch (Perca Flavescens) in Lake Opeongo, Ontario”.
- Cunningham, K. E., and E. S. Dunlop. 2023. “Declines in Lake Whitefish Larval Densities after Dreissenid Mussel Establishment in Lake Huron”. Journal of Great Lakes Research In Press.
- DeWeese, N. E., Favot, E. J., Branstrator, D. K. et al. 2021. “Early presence of Bythotrephes cederströmii (Cladocera: Cercopagidae) in lake sediments in North America: evidence or artifact?” Journal of Paleolimnology 66, 389–405.
- DFO. 2013. “Proceedings of the National Risk Assessment of Zebra Mussel, Quagga Mussel and Dark Falsemussel”.
- DFO. 2022. “Proceedings of the Regional Peer Review of the Recovery Potential Assessment for Lake Whitefish (Coregonus clupeaformis), Lake Opeongo large-bodied Designatable Unit and Lake Opeongo small-bodied Designatable Unit”.
- Drake, A. 2017. “Overland Spread of Aquatic Invasive Species among Freshwater Ecosystems due to Recreational Boating in Canada”.
- EDDMapS. 2023. “Early Detection & Distribution Mapping System”. The University of Georgia - Center for Invasive Species and Ecosystem Health. Last accessed May 2, 2023.
- Enbom, M. 2013. “Whitefish in Northern Sweden, A Study of Head Morphology between Different Morphs of Whitefish”. UMEA University.
- EPA. 2023. “Nutrient Pollution, The Effects”. Last accessed June 19, 2023.
- Evans, D., and P. Waring. 2011. “Changes in the Multispecies, Winter Angling Fishery of Lake Simcoe, Ontario, 1961–83: Invasion by Rainbow Smelt, Osmerus Mordax, and the Roles of Intra- and Interspecific Interactions”. Canadian Journal of Fisheries and Aquatic Sciences 44.
- Evans, D., and D. Loftus. 1987. “Colonization of Inland Lakes in the Great Lakes Region by Rainbow Smelt, Osmerus Mordax: Their Freshwater Niche and Effects on Indigenous Fishes”. Canadian Journal of Fisheries and Aquatic Sciences 44 (52): 249–66.
- Favot, E., J., K. M. Rühland, A. M. DeSellas, R. Ingram, A. M. Paterson, and J. P. Smol. 2019. “Climate Variability Promotes Unprecedented Cyanobacterial Blooms in a Remote, Oligotrophic Ontario Lake: Evidence from Paleolimnology”. Journal of Paleolimnology.
- Findlay, C. S., D. G. Bert, and L. Zheng. 2000. “Effect of Introduced Piscivores on Native Minnow Communities in Adirondack Lakes”. Canadian Journal of Fisheries and Aquatic Sciences 57 (3): 570–80.
- Freeberg, M. H., William W. T., and R. W. Brown. 1990. “Effect of Egg and Larval Survival on Year-Class Strength of Lake Whitefish in Grand Traverse Bay, Lake Michigan”. Transactions of the American Fisheries Society 119 (1): 92–100.
- Fung, S. R., A. S. van der Lee, and M. A. Koops. 2022. “Recovery Potential Modelling of Lake Whitefish (Coregonus Clupeaformis) in Lake Opeongo, Canada”.
- George, E. M., M. P. Hare, D. L. Crabtree, B. F. Lantry, and L. G. Rudstam. 2018. “Comparison of Genetic and Visual Identification of Cisco and Lake Whitefish Larvae from Chaumont Bay, Lake Ontario”. Canadian Journal of Fisheries and Aquatic Sciences 75 (8): 1329–36.
- Gertzen, E. L., and B. Leung. 2011. “Predicting the Spread of Invasive Species in an Uncertain World: Accommodating Multiple Vectors and Gaps in Temporal and Spatial Data for Bythotrephes Longimanus”. Biological Invasions 13 (11): 2433–44.
- Gorsky, D., J. Zydlewski, and D. BaSLey. 2012. “Characterizing Seasonal Habitat Use and Diel Vertical Activity of Lake Whitefish in Clear Lake, Maine, as Determined with Acoustic Telemetry”. Transactions of the American Fisheries Society 141 (3): 761–71.
- Hackney, P. A. 1973. “Ecology of the Burbot (Lota Lota) with Special Reference to Its Role in the Lake Opeongo Fish Community”. Toronto: University of Toronto.
- Hincks, S. S., and G. L. Mackie. 1997. “Effects of PH, Calcium, Alkalinity, Hardness, and Chlorophyll on the Survival, Growth, and Reproductive Success of Zebra Mussel (Dreissena Polymorpha) in Ontario Lakes”. Canadian Journal of Fisheries and Aquatic Sciences 54 (9): 2049–57.
- Holm, E., N. E. Mandrak, and M., E. Burridge. 2021. A Field Guide to Freshwater Fishes of Ontario. Second. Toronto: Royal Ontario Museum.
- Ihssen, P. E., D. O. Evans, W. J. Christie, J. A. Reckhan, and R. L. DesJardine. 1981. “Life History, Morphology, and Electrophoretic Characteristics of Five Allopatric Stocks of Lake Whitefish (Coregonus clupeaformis) in the Great Lakes Region”. Canadian Journal of Fisheries and Aquatic Sciences 38: 1790–1807.
- IMISWG. 2014. “Invasive Species Early Detection and Raid Response Plan for British Columbia”.
- Jacobs, M. J., and H. J. MacIsaac. 2007. “Fouling of Fishing Line by the Waterflea Cercopagis Pengoi: A Mechanism of Human-Mediated Dispersal of Zooplankton?” Hydrobiologia 583 (1): 119–26.
- Johnson, L. E., A. Ricciardi, and J. T. Carlton. 2001. “Overland Dispersal of Aquatic Invasive Species: A Risk Assessment of Transient Recreational Boating”. Ecological Applications 11 (6): 1789–99.
- Johnson, J. H., J. E. McKenna, M. A. Chalupnicki, T. Wallbridge, and R. Chiavelli. 2009. “Feeding Ecology of Lake Whitefish Larvae in Eastern Lake Ontario”. Journal of Great Lakes Research 35 (4): 603–7.
- Kennedy, W. A. 1943. “The Whitefish, Coregonus Clupeaformis (Mitchill), of Lake Opeongo, Algonquin Park, Ontario”. Pub. Onto Fish. Res. Lab. Vol. 46. BioI. 47. Pub.
- LeGros, D. 2022. “Another Fish Story”. The Raven, May 1, 2022, 1–2.
- Loftus, D., and P. Hulsmann. 2011. “Predation on Larval Lake Whitefish (Coregonus Clupeaformis) and Lake Herring (C. artedii) by Adult Rainbow Smelt (Osmerus Mordax)”. Canadian Journal of Fisheries and Aquatic Sciences 43: 812–18.
- Martin, N. V., and F. E. J. Fry. 1973. “Lake Opeongo: The Ecology of the Fish Community and of Man’s Effects on It”. Technical Report No. 24. Ann Arbor, MI: Great Lakes Fishery Commission.
- Mee, J. A., L. Bernatchez, J. D. Reist, S. M. Rogers, and E. B. Taylor. 2015. “Identifying Designatable Units for Intraspecific Conservation Prioritization: A Hierarchical Approach Applied to the Lake Whitefish Species Complex (Coregonus Spp.)”. Evolutionary Applications 8 (5): 423–41.
- MAISRC. 2023. “Determining highest-risk vectors of spiny water flea spread.”
- Martin, B. E., Mrnak, J. T., and M. J. Vander Zanden. 2023. Evaluating the potential role of predation by native fish regulating the abundance of invasive spiny water flea, Journal of Freshwater Ecology, 38:1.
- Mitchell, K. A., A. Challice, N. Lacombe, C. Taylor, G. Forward, T. Middel, and M. Ridgway. 2020. “Science and Research Assessing Recreational Angling in Algonquin Provincial Park”. Peterborough.
- Mitchell, K., S. Luke, A. Lake, N. Lacombe, and M. Ridgway. 2017. “Science and Research Branch Information Report: A History of Fish Stocking in Algonquin Provincial Park”. Ontario Ministry of Natural Resources and Forestry, Science and Research Branch, Peterborough, ON. Science and Research Technical Report TR-35. 28 p. + appendices.
- MNR. 2009. “A Fisheries Management Review for Fairy, Mary, Peninsula and Vernon Lakes”.
- MNRF. 2022. “2022 Fishing Ontario: Recreational Fishing Regulations Summary”.
- ———. 2023a. “2023 Fishing Ontario: Recreational Fishing Regulations Summary”.
- ———. 2023b. “Fish ON-Line”.
- MOE, MNR, and MMAH. 2010. “Lakeshore Capacity Assessment Handbook: Protecting Water Quality in Inland Lakes”.
- Ontario Parks. 1998. “Algonquin Provincial Park Management Plan”.
- ———. 2013. “Algonquin Park Management Plan Amendment”.
- OPG and MNR. 2018. “Madawaska River Water Management Plan”.
- Orlova, M. I., T. W. Therriault, P. I. Antonov, and G. K. Shcherbina. 2005. “Invasion Ecology of Quagga Mussels (Dreissena rostriformis bugensis): A Review of Evolutionary and Phylogenetic Impacts”. Aquatic Ecology 39 (4): 401–18.
- Overdyk, L., E. Holm, S. Crawford, and R. Hanner. 2016. “Increased Taxonomic Resolution of Laurentian Great Lakes Ichthyoplankton through DNA Barcoding: A Case Study Comparison against Visual Identification of Larval Fishes from Stokes Bay, Lake Huron”. Journal of Great Lakes Research 42 (4): 812–18.
- Patterson, M., J. J.H. Ciborowski, and D. R. Barton. 2005. “The Distribution and Abundance of Dreissena Species (Dreissenidae) in Lake Erie, 2002.” Journal of Great Lakes Research 31 (Suppl. 2): 223-237.
- Paufve, M., S. A. Sethi, L. G. Rudstam, B. C. Weidel, B. F. Lantry, M. A. Chalupnicki, K. Dey, and M. E. Herbert. 2020. “Differentiation between Lake Whitefish and Cisco Eggs Based on Diameter”. Journal of Great Lakes Research 46 (4): 1058–62.
- Pigeon, D., A. Chouinard, and L. Bernatchez. 1997. “Multiple Modes of Speciation Involved in the Parallel Evolution of Sympatric Morphotypes of Lake Whitefish (Coregonus Clupeaformis, Salmonidae)”. Evolution 51 (1): 196–205.
- Pollux, B. J. A., G. van der Velde, and A. bij de Vaate. 2010. “A Perspective on Global Spread of Dreissena polymorpha: A Review on Possibilities and Limitations”. In The Zebra Mussel in Europe, 45–58. Backhuys Publishers.
- Pothoven, S. A., T. O. Hook, and C. R. Roswell. 2014. “Feeding Ecology of Age-0 Lake Whitefish in Saginaw Bay, Lake Huron”. Journal of Great Lakes Research 40 (1): 148–55.
- Pothoven, S., and C. Olds. 2020. “Spatial Variation in Feeding Ecology of Age-0 Lake Whitefish Coregonus Clupeaformis in Lake Huron”. Journal of Freshwater Ecology 35 (1): 349–66.
- Price, J. W. 1940. “Time-Temperature Relations in the Incubation of the Whitefish, Coregonus Clupeaformis (Mitchell)”. The Journal of General Physiology, 449–68.
- Reid, S. M., M. Parna, and J. D. Reist. 2017. “Collapse of Lake Whitefish Coregonus Clupeaformis (Mitchill, 1818) Species Pair in Como Lake, Ontario”. Journal of Applied Ichthyology 33 (5): 933–39.
- Richardson, J. S., E. Taylor, D. Schluter, M. Pearson, and T. Hatfield. 2010. “Do Riparian Zones Qualify as Critical Habitat for Endangered Freshwater Fishes?” Canadian Journal of Fisheries and Aquatic Sciences 67 (7): 1197–1204.
- Ridgway, M., B. McMeans, and M. Wells. 2021. “The Smoke, Canoe, and Tea Lakes Fish Movement Project in Algonquin Provincial Park”. Peterborough, ON.
- Ridgway, M., and T. Middel. 2020. “From Aquatic Connectivity to Aquatic Conservation in Algonquin Provincial Park”. Ontario Ministry of Natural Resources and Forestry, Science and Research Branch, Peterborough, ON. Science and Research Information Report IR-20. 41 p.
- Ridgway, M., T. Middel, and A. Bell. 2017. “Aquatic Ecology, History, and Diversity of Algonquin Provincial Park”. Ontario Ministry of Natural Resources and Forestry, Science and Research Branch, Peterborough, ON. Science and Research Information Report IR-10. 203 p.
- Ridgway, M., T. Middel, and L. Wensink. 2018. “Aquatic Connectivity, Fish Introductions, and Risk Assessment for Lakes in Algonquin Provincial Park”. Ontario Ministry of Natural Resources and Forestry, Science and Research Branch, Peterborough, ON. Science and Research Information Report IR-13. 64 p. + append.
- Rodrigues, T. H., A. J. Chapelsky, L. E. Hrenchuk, G. R. Mushet, L.J. Chapman, and P. J. Blanchfield. 2022. “Behavioural Responses of a Cold-Water Benthivore to Loss of Oxythermal Habitat”. Environmental Biology of Fishes 105 (10): 1489–1507.
- Rogers, S. M. 2008. “COSEWIC Special Report: Designatable Units at an Appropriate Scale for the Lake Whitefish (Coregonus clupeaformis) in Canada”.
- Sandercock, K. F. 1964. “Contribution to the Ecology of the Whitefishes Prosopium cylindraceum and Coregonus clupeaformis of Algonquin Park, Ontario”. Master's thesis, University of Toronto.
- Sandstrom, S., M. Rawson, and N. Lester. 2013. “Manual of Instructions for Broad-Scale Fish Community Monitoring Using North American (NA1) and Ontario Small Mesh (ON2) Gillnets”.
- Scott, W. B., and E. J. Crossman. 1998. Freshwater Fishes of Canada. Oakville, ON: Galt House Publications.
- Shaw, S. Bernard. 1998. Lake Opeongo: Untold Stories of Algonquin Park’s Largest Lake. Burnstown, ON: General Store Publishing House.
- Strickland, D. 2000. “The Sad Story of Algonquin’s Very Own Lake Mess Monster”. In The Raven, edited by D. Strickland. Vol. 41. The Friends of Algonquin Park.
- The Friends of Algonquin Park. 2022. “Algonquin Park Ice Conditions”. 2022.
- Trudel, M., A. Tremblay, R. Schetagne, and J. B. Rasmussen. 2001. “Why Are Dwarf Fish so Small? An Energetic Analysis of Polymorphism in Lake Whitefish (Coregonus Clupeaformis)”. Canadian Journal of Fisheries and Aquatic Sciences 58 (2): 394–405.
- Vuorinen, J. A., R. A. Bodaly, J. D. Reist, L. Bernatchez, and J. J. Dodson. 1993. “Genetic and Morphological Differentiation between Dwarf and Normal Size Forms of Lake Whitefish (Coregonus clupeaformis) in Como Lake, Ontario”. Canadian Journal of Fisheries and Aquatic Sciences 50 (1): 210–16.
- Whitaker, D. S., and J. Wood. 2020. “An Investigation of Lake Whitefish Spawning and Early Life History in the Allagash Watershed: Preliminary Results”.
- Wilson, K. A., E. T. Howell, and D. A. Jackson. 2006. “Replacement of Zebra Mussels by Quagga Mussels in the Canadian Nearshore of Lake Ontario: The Importance of Substrate, Round Goby Abundance, and Upwelling Frequency”. Journal of Great Lakes Research 32 (1): 11–28.
- Wood, J. 2016. “Current Status of Lake Whitefish in Maine; an Update to MDIFW’s 2001 Whitefish Assessment”.
- Yan, N. D., A. Blukacz, W. G. Sprules, P. K. Kindy, D. Hackett, R. E. Girard, and B. J. Clark. 2001. “Changes in Zooplankton and the Phenology of the Spiny Water Flea, Bythotrephes, Following Its Invasion of Harp Lake, Ontario, Canada”. Canadian Journal of Fisheries and Aquatic Sciences 58 (12): 2341–50.
- Yan, N. D., B. Leung, M. A. Lewis, and S. D. Peacor. 2011. “The Spread, Establishment and Impacts of the Spiny Water Flea, Bythotrephes longimanus, in Temperate North America: A Synopsis of the Special Issue”. Biological Invasions 13 (11): 2423–32.
Personal communications
- Challice, A. 2023. Email correspondence. January 17, 2023. Regional Aquatic Ecosystem Science Specialist, MNRF.
- Colm, J. 2023. Verbal correspondence. January 27, 2023. Aquatic Biologist, Great Lakes Laboratory for Fisheries and Aquatic Sciences, DFO.
- Drake, A. 2023. Verbal correspondence. January 27, 2023. Research Scientist, Great Lakes Laboratory for Fisheries and Aquatic Sciences, DFO.
- Gelok, P. 2023. Verbal correspondence. February 15, 2023. Ecologist, Indigenous Relations Unit, Ontario Parks.
- Hoare, J. 2023. Verbal correspondence. February 15, 2023. Acting Zone Ecologist, Algonquin Zone, Ontario Parks.
- Lacombe, N. 2023. Verbal correspondence. February 15, 2023. Acting Fisheries Biologist, Algonquin Zone, Ontario Parks.
- Mandrak, N. 2023. Verbal correspondence. January 27, 2023. Professor, Department of Biological Sciences, University of Toronto (Scarborough).
- Middel, T. 2023. Verbal and email correspondence. January and February 2023 (various dates). Aquatic Analyst, MNRF.
- Ridgway, M. 2023. Verbal correspondence. February 8, 2023. Research Scientist & Director, Harkness Laboratory of Fisheries Research, MNRF.
- Wilson, C. 2023. Email correspondence. May 19, 2023. Research Scientist, MNRF