White-rimmed Shingle Lichen recovery strategy
Read the recovery strategy for the White-rimmed Shingle Lichen, a lichen at risk in Ontario.

About the Ontario recovery strategy series
This series presents the collection of recovery strategies prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act, 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated (lives somewhere in the world, and at one time lived in the wild in Ontario, but no longer lives in the wild in Ontario) species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species’ persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at risk in Ontario list. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published summarizing the actions the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, visit the Ministry of the Environment, Conservation and Parks Species at Risk webpage.
Recommended citation
Consiglio, J. and T. Knight. 2023. Recovery Strategy for the White-rimmed Shingle Lichen (Fuscopannaria leucosticta) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ministry of the Environment, Conservation and Parks, Peterborough, Ontario. v + 52 pp.
Cover illustration: Photo by Sam Brinker (NHIC)
© King’s Printer for Ontario, 2023
ISBN978-1-4868-6480-5 HTML
ISBN 978-1-4868-6481-2 PDF
Content (excluding illustrations) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n’est disponible qu’en anglais en vertu du Règlement 411/97 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec recovery.planning@ontario.ca.
Authors
Jessica Consiglio – Terrastory Environmental Consulting Inc.
Tristan Knight – Terrastory Environmental Consulting Inc.
Acknowledgments
Several professional lichenologists and biologists/ecologists contributed valuable information and insights to support this recovery strategy. Sam Brinker (Natural Heritage Information Centre) shared a wealth of experience and knowledge relating to White-rimmed Shingle Lichen biology, substrate/habitat associations, and threats to the species in Ontario. Dr. R. Troy McMullin (Canadian Museum of Nature) provided valuable insights into existing herbarium records of the species, as well as means of laboratory-based methods of identification. Dr. Sean Haughian (Nova Scotia Museum, St. Mary’s University) shared valuable experience surveying for the species, and provided insight into aspects of the COSEWIC report and current distribution modelling. Wasyl Bakowsky (Natural Heritage Information Centre) clarified current vegetation classification systems for northwestern Ontario. Several others shared intricate details related to the policy framework guiding Crown land forestry activities, including Dana Kinsman (MNRF), Laura Darby (MNRF) and Kaitlin Kruzick (MNRF).
Declaration
The recovery strategy for the White-rimmed Shingle Lichen (Fuscopannaria leucosticta) Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The recommended goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ministry of the Environment, Conservation and Parks
Environment and Climate Change Canada – Canadian Wildlife Service, Ontario
Executive summary
White-rimmed Shingle Lichen (Fuscopannaria leucosticta) is a lichen forming densely overlapping lobes with a grey to chestnut brown appearance and white margins. The overlapping lobes impart a “shingled” appearance (hence the common name) to the vegetative body and are usually bordered by a highly distinctive and well-developed blue-black mat of underlying fungal growth which is closely attached to the substrate. The species primarily occupies tree bark, although it is also known to occur on rocks. Records of the species throughout the Great Lakes region of Canada and the United States are sparse, and it is considered rare or extirpated in several states neighbouring Ontario. In Canada, White-rimmed Shingle Lichen has been recorded from Nova Scotia, New Brunswick and Ontario, comprising four distinct subpopulations (two of which are located in Ontario). In Ontario the species is known from two subpopulations, one in Thunder Bay District and one in Rainy River District, encompassing one historical site and seven recent extant sites. White-rimmed Shingle Lichen is listed as endangered on the Species at Risk in Ontario List (Ontario Regulation 230/08).
This species occupies highly specific habitat in Ontario, with the majority of colonies documented in undisturbed, old-growth swamps and wet forests dominated by mature Eastern White Cedar (Thuja occidentalis). The species grows exclusively on bark in Ontario and is only known to occupy mature Eastern White Cedar bark based on extant records. Occurrences are predominantly concentrated in areas protected from natural disturbances (for example, fire). Although detailed soil information within the vicinity of occurrences is not currently known, surficial soils appear to be fine mineral overlain by organics.
The most significant factor limiting recovery potential for White-rimmed Shingle Lichen is habitat availability. White-rimmed Shingle Lichen requires highly specific habitat and substrate requirements. It is known to occupy one substrate type (i.e., the bark of mature, leaning Eastern White Cedar trees), one broad ecosystem type (i.e., undisturbed, mature Eastern White Cedar swamps) and a narrow range of biophysical conditions (for example, humidity, light availability, temperature, air quality).
Direct harm to White-rimmed Shingle Lichen may result from a variety of human-mediated processes involving the removal of host trees, loss of habitat, or alterations to highly specific microclimate requirements in the surrounding biophysical environment.
The primary threats to the survival and recovery of White-rimmed Shingle Lichen (listed in order of severity) are:
- habitat loss
- habitat degradation
- alterations to the hydrologic regime
- climate change
- air pollution
The recommended recovery goal for White-rimmed Shingle Lichen is to maintain and, where possible, increase the number of thalli at all localities, and any newly-discovered occurrences, to reduce the likelihood of extirpation. Recommended protection and recovery objectives are as follows:
- Maintain or increase the long-term viability of all known occurrences
- Conduct targeted surveys in suitable habitat to determine the actual population size and distribution in Ontario
- Promote awareness of White-rimmed Shingle Lichen by collaborating with stakeholders (for example, approval authorities, landowners, industry, conservation groups and municipalities) and Indigenous organizations and communities
- Address key knowledge gaps
Like many sensitive cyanolichens, White-rimmed Shingle Lichen relies heavily upon specific microsite conditions. Maintaining existing humidity levels, light, ambient air temperature, substrate pH and presence of adjacent tree canopies is known to be critical for protecting both the host tree and thalli itself. Based on the above factors, the ecosite(s) and an area within 200 m of an ecosite(s) in which White-rimmed Shingle Lichen occurs (i.e., not an occurrence itself) is recommended for consideration in developing a habitat regulation.
1.0 Background information
1.1 Species assessment and classification
The following lists provide assessment and classification information for the White-rimmed Shingle Lichen (Fuscopannaria leucosticta). Note: The glossary provides definitions for abbreviations and technical terms in this document.
- SARO List Classification: Endangered
- SARO List History: Endangered (2022)
- COSEWIC Assessment History: Endangered (2019)
- SARA Schedule 1: No schedule, no status
- Conservation Status Rankings: G-rank: G3G5; N-rank: N3; S-rank: S2
1.2 Species description and biology
Species descriptions
White-rimmed Shingle Lichen is a grey to chestnut brown, squamulose cyanolichen in the family Pannariaceae, comprised of densely overlapping lobes with white margins. The overlapping lobes (squamules) impart a shingled
appearance (hence the common name) to the vegetative body (thallus). Individual squamules are small (2-3 mm wide) (Brodo et al. 2001) and relatively thick (0.2 mm) (Jørgensen 2000), with rounded or wavy margins which ascend from the substrate. Matted fungal filaments form a white edging (i.e., white rims
) along the squamule margins (Jørgensen 2000). Thalli are usually bordered by a highly distinctive and well-developed blue-black prothallus (mat of underlying fungal growth) which is closely attached to the substrate (Brinker 2020; Brodo et al. 2001).
Ascomycete lichens such as White-rimmed Shingle Lichen produce sexual propagules (ascospores) via microscopic organs (asci) within a fruiting body (apothecium) (Brodo et al. 2001). White-rimmed Shingle Lichen apothecia are typically 0.5 to 1.5 mm wide with a red to brown central disk and a white or grey margin (Brinker 2020; Hinds and Hinds 2007; Jørgensen 2000). Apothecial disks may be sunken and darker in appearance when dry, becoming lighter and convex when moistened (COSEWIC 2019). Ascospores are one-celled (Wedin et al. 2009), 23 to 27 × 9 to 11 µm, elliptic, colourless and surrounded by a clear layer (perispore) tapering at both ends (Jørgensen 2000). Unlike many species in the family Pannariaceae, White-rimmed Shingle Lichen does not produce vegetative propagules such as soredia or isidia (Brodo et al. 2001). Photographs of White-rimmed Shingle Lichen and surrounding substrate/habitat at known localities in northwestern Ontario are provided below in Figure 1.

Figure 1. Representative photographs of White-rimmed Shingle Lichen and substrate/habitat conditions surrounding occurrences in northwestern Ontario
Field-based separation of White-rimmed Shingle Lichen from superficially similar species (particularly other Pannariaceae species) can be reliably undertaken by experienced professionals (for example, foresters, ecologists, naturalists), but is occasionally challenging. Species with the greatest likelihood of being misidentified as White-rimmed Shingle Lichen in northwestern Ontario are listed below with a description of their distinguishing features:
- Moss Shingles Lichen (Fuscopannaria praetermissa) – broadly resembles White-rimmed Shingle Lichen but is sorediate (i.e., contains vegetative propagules), generally lacks a prothallus, is occasionally infertile (i.e., lacks apothecia), and typically occupies mossy decaying logs or bark at the base of trees (as opposed to growing on bark well-above ground level).
- Rock Shingle Lichen (Vahliella leucophaea) – upper surface is more brownish than White-rimmed Shingle Lichen, prothallus is thin or not visible, produces a darker brown to black apothecia (often lacking a thalline margin), and exhibits a crust-like growth form over rocks.
- Brown-gray Moss Shingle Lichen (Protopannaria pezizoides) – thallus with a granular-crustose appearance, apothecia are often aggregated together and share a common margin, and typically occupies soil (terricolous) but may also occur on trees or rocks.
- Black-bordered Shingle Lichen (Parmeliella triptophylla) – possesses a similar blue-black prothallus to White-rimmed Shingle Lichen but is isidiate (i.e., contains isidia) and is often infertile (i.e., lacks apothecia).
- Mealy-rimmed Shingle Lichen (Pannaria conoplea) – clearly foliose (rather than squamulose) and is sorediate.
- Coral-rimmed Shingle Lichen (Pannaria tavaresii) – foliose (rather than squamulose) with wider thallus lobes and possessing cylindrical isidia.
Laboratory-based methods may be useful for confirming small, infertile or atypical specimens. White-rimmed Shingle Lichen contains the metabolite (organic by-product) pannarin and typically expresses an orange-red colour when para-phenylenediamine is applied to the upper cortex (Hinds and Hinds 2007). The hymenium (a layer composed of sterile hyphae and asci) of White-rimmed Shingle Lichen produces a blue colour when exposed to potassium iodide, assisting in differentiation from Brown-gray Moss Shingle Lichen (Jørgensen 2000). Spore characteristics are useful and can be reviewed in thin sections of apothecia under a microscope. No cystobasidiomycete yeasts (often responsible for phenotypic variation in lichens) are known to occur in this species (Lendemer et al. 2019).
Species biology
Lichens are composite organisms composed of an alga and/or cyanobacteria (photosynthetic symbiont or photobiont) and a fungus (mycobiont). Fungal cell filaments (hyphae) comprise a significant portion of the organism, encasing the photobiont which produces food for the lichen via photosynthesis. The mycobiont provides structure and is responsible for sexual reproduction via ascospores. The mycobiont for White-rimmed Shingle Lichen is an ascomycete fungus in the family Pannariaceae (Magain and Sérusiaux 2014); all lichens are named after the mycobiont partner. Several authors report that a member of the genus Nostoc (a cyanobacteria) acts as the photobiont (Brodo et al. 2001; Magain and Sérusiaux 2014), although studies identifying the appropriate species are unknown.
Sexual reproduction in White-rimmed Shingle Lichen occurs within the disk-shaped apothecia. Sparse, small (~0.5 mm wide) apothecia have been observed on thalli at a size of 1 to 2 cm2 wide with greater numbers of apothecia produced on thalli of 40 cm2 or larger (COSEWIC 2019). Eight ascospores are typically produced within each ascus (COSEWIC 2019). Ascospores are forcibly ejected by the asci and disperse easily by wind due to their small size (Brodo et al. 2001). Dispersal distance and survival period is not known for White-rimmed Shingle Lichen ascospores, though species which reproduce sexually tend to be more effective at dispersing widely than colonizing locally (COSEWIC 2019).
Generation time (average age of reproductively active individuals) of White-rimmed Shingle Lichen is estimated at 12 years based on secondary sources as no information is currently available for the species (S. Haughian pers. comm. 2022). The 12-year generation time is a conservative estimate derived from time to reproductive maturity and host tree longevity, as well as generation times of related species (COSEWIC 2019; S. Haughian pers. comm. 2022). The prothallus develops when spores contact a suitable substrate and encounter the appropriate photobiont, anchoring the lichen and enabling growth (COSEWIC 2019).
Many lichens reproduce vegetatively (asexually) via specialized structures such as soredia and isidia which contain both the photobiont and fungal partners. White-rimmed Shingle Lichen does not produce such structures (Brodo et al. 2001) and consequently may rely primarily on sexual reproduction for establishment and dispersal. Despite this, repeated observations of vertically oriented colonies in New Brunswick suggest that the species may also reproduce vegetatively from broken thallus tissue (COSEWIC 2019). Dispersal by fragmentation may result in colonization of additional host trees within an occupied stand (S. Haughian pers. comm. 2022); however, dispersal of vegetative fragments is probably limited by the local movements of small mammals and slugs, suggesting that colonization of new stands/habitats via fragments is unlikely (S. Haughian pers. comm. 2022).
Lichenization, the symbiosis between a fungus and photobiont, produces unique life strategies and adaptations. Cyanobacterial photobionts comprise a relatively small (8%) percentage of photobionts used by ascomycete fungi (Wedin et al. 2009). Cyanobacteria of the genus Nostoc act as the photobiont in White-rimmed Shingle Lichen (Wedin et al. 2009). Lichen fungi that employ cyanobacteria as photobionts (cyanolichens) are capable of fixing atmospheric nitrogen (Nash 2008b). Cyanolichens require moisture to sustain the process of nitrogen fixation and to photosynthesize at normal rates; thus, desiccation can halt nitrogen fixation entirely (Antoine 2004; Nash 2008b; Pearson et al. 2018). Cyanolichens also contribute to nutrient cycling through thalli decomposition and leaching a usable form of nitrogen when wetted (Nash 2008b; Richardson and Cameron 2004).
1.3 Distribution, abundance and population trends
For the purposes of this recovery strategy, the following terminology is used to describe the distribution and abundance of White-rimmed Shingle Lichen in Ontario:
Subpopulation(s)
: all White-rimmed Shingle Lichen colonies in Ontario, encompassing two of four recognized subpopulations nationally (i.e., a Rainy River District subpopulation and Thunder Bay District subpopulation).Site
orLocality
: general geographic or natural area (for example, Sleeping Giant Provincial Park) which may contain one to several geographically distinct occurrences of the species.Occurrence
orColony
: aggregation of White-rimmed Shingle Lichen thalli within a small area of contiguous habitat (often located on the same host tree).Record
: a collection or observation representing a single occurrence.
White-rimmed Shingle Lichen has a global distribution and is known from Asia (Ezhkin and Ohmura 2021; Jørgensen 2000; Jørgensen and Sipman 2007), Europe (Spribille 2009), Africa (Alstrup and Christensen 2006), Central America (Jørgensen and Sipman 2007), North America (Jørgensen 2000), and South America (Jørgensen and Palice 2010; Jørgensen and Sipman 2007). The current global distribution suggests that the species exists as a Tertiary relict; historically present across a larger continuous range and reduced by widespread extinctions to relict populations within refugia (Culberson 1972; Jørgensen 2000; Jørgensen and Sipman 2007). Records from the United States (US) are concentrated along the eastern seaboard (particularly the southeast) and Appalachia (Jørgensen 2000; Perlmutter 2005).
Within the Great Lakes region of the US, White-rimmed Shingle Lichen is considered rare in New York state (Harris 2004) and designated Special Concern in Wisconsin (Wetmore 2009; Wisconsin Natural Heritage Program 2021). Occurrences are known from Michigan although the species has not been documented there for over a decade (COSEWIC 2019). Recent occurrences are also known from Minnesota, where it is considered rare (J. Thayer pers. comm. 2022). The species is also known from Ohio, where it has not been collected since 1962 and is considered extirpated (Schumacher and Ashcraft 2021).
In Canada, White-rimmed Shingle Lichen has been recorded from Nova Scotia, New Brunswick and Ontario, comprising four distinct subpopulations (two of which are located in Ontario) (COSEWIC 2019). Specimens previously identified as White-rimmed Shingle Lichen from Newfoundland were found to be Brown-eyed Shingle Lichen (COSEWIC 2019). Similarly, a record from Alberta was examined by R. T. McMullin and determined to be Moss Shingles Lichen (R. T. McMullin pers. comm. 2022). Targeted surveys for the species in Canada are not known to have been undertaken historically; however, intensive surveys were undertaken from 2016 to 2018 in Nova Scotia, New Brunswick, Ontario and Quebec; resulting in the discovery of several new occurrences (COSEWIC 2019; S. Brinker pers. comm. 2022). One historical record of White-rimmed Shingle Lichen exists from Quebec (Lac Clair region north of Montreal). Collected in 1888 and lacking detailed location information, the occurrence is considered extirpated due to a lack of remaining suitable habitat and failure to detect during recent surveys (COSEWIC 2019). The four Canadian subpopulations are assumed isolated from one another due to the significant expanses of intervening land (COSEWIC 2019).
White-rimmed Shingle Lichen forms two distinct subpopulations in Ontario, with sites scattered across Thunder Bay District and Rainy River District. Ontario localities are represented by one historical record and seven recent sites. The historical record is a 1901 collection by B. Fink, one of North America’s foremost lichenologists of the time and Head of Botany at Miami University (Wylie 1928). Background information on this historical collection is limited to the herbarium label which describes the locality as Canada, Ontario, Rainy River, Emo
, and characterizes the substrate as on cedars in swamp
(Consortium of North American Lichen Herbaria 2022). Suitable habitat surrounding Emo was revisited in 2017 but attempts to relocate the species were unsuccessful (COSEWIC 2019).
Two additional historical records exist, one collected by R. F. Cain in 1935 near Lake Temagami (Nipissing District; CANL 62278) and a second collected by Stephen Sharnoff and Sylvia Sharnoff in 1993 within Lake Superior Provincial Park (PP) (Algoma District; CANL 116130). The 1935 record from Lake Temagami which is the basis for the species mapped North American range in Brodo et al. (2001) was examined by R. T. McMullin and determined to be Petaled Shingle Lichen (COSEWIC 2019; R. T. McMullin pers. comm. 2022). The 1993 record from Lake Superior PP is the only possible occurrence of the species currently known from Algoma District and was considered extirpated in the 2019 COSEWIC Assessment and Status Report (COSEWIC 2019). Although considered valid and supported by thin layer chromatography results, the record has been questioned on the basis of recent surveys, prevailing habitat conditions, and the fact that the specimen was collected from rock (which would represent the first and only specimen in Ontario found on a rock substrate; R.T. McMullin pers. comm. 2022; S. Brinker pers. comm. 2022).
White-rimmed Shingle Lichen is known from seven extant (existing) sites in Ontario, with all collections made from the bark of Eastern White Cedar (Thuja occidentalis). Six of the seven sites were first discovered in 2016 and 2017 by S. Brinker. The most recently discovered site (Sleeping Giant PP) was found independently by S. Brinker, R. T. McMullin, B. McCune, M. N. Singh and H. E. Schultz (S. Brinker pers. comm. 2022; R. T. McMullin pers. comm. 2022). All extant occurrences in Ontario are listed and described as follows:
- Quetico PP: The westernmost extant site in Ontario (and only occurrence in Rainy River District) is represented by Quetico PP where one colony is known. The surrounding habitat was described as coniferous swamp in a valley; associated species include Eastern White Cedar, Balsam Fir (Abies balsamea), White Spruce (Picea glauca), Speckled Alder (Alnus rugosa ssp. incana) and Yellow Clintonia (Clintonia borealis) (Brinker 2020).
- Pigeon River: Nine occurrences were recorded at a site two km north of Pigeon River in mature Eastern White Cedar dominated swamp alongside Balsam Fir, Speckled Alder, Bunchberry (Cornus canadensis), Dwarf Raspberry (Rubus pubescens) and Two-seeded Sedge (Carex disperma) (Brinker 2020).
- Dorion Cutoff: Four occurrences were found at a site north of Hick’s Lake along Dorion Cutoff Road in a mature Eastern White Cedar swamp alongside Black Ash (Fraxinus nigra), Bebb’s Willow (Salix bebbiana), Alder-leaved Buckthorn (Endotropis alnifolia), Dwarf scouring-rush (Equisetum scirpoides) and Dwarf Raspberry (Brinker 2020).
- Albert Lake: Eight occurrences were recorded at a site near Albert Lake in an old-growth Eastern White Cedar dominated forest alongside Balsam Fir, Mountain Maple (Acer spicatum), Naked Mitrewort (Mitella nuda), Common Oak Fern (Gymnocarpium dryopteris) and Dwarf Raspberry (Brinker 2020).
- Albert Lake Mesa Provincial Nature Reserve: Six occurrences were recorded at a site west of the Albert Lake Mesa Provincial Nature Reserve in a moist, old-growth mixed forest alongside Balsam Fir, Mountain Maple, Canada Yew (Taxus canadensis) and Paper Birch (Betula papyrifera) (Brinker 2020).
- Lankinen Road: Three occurrences were recorded at a site south of South Gillies near Lankinen Road, growing in an open coniferous swamp alongside Black Ash, Speckled Alder, Balsam Fir and Dwarf Raspberry (Brinker 2020).
- Sleeping Giant PP: Five extant occurrences were recorded from Sleeping Giant PP in 2019. Two occurrences were documented by S. Brinker; one found in a cedar swamp and the second from a mature mixed boreal forest alongside White Pine (Pinus strobus), Balsam Fir and Paper Birch (S. Brinker pers. comm. 2022). A third occurrence was documented by R.T. McMullin growing in a mature stand of Eastern White Cedar (R. T. McMullin pers. comm. 2022). A fourth occurrence was documented by Bruce McCune from a mixed-wood forest dominated by Balsam Fir, Alder (Alnus sp.), Birch (Betula sp.) and Eastern White Cedar (R. T. McMullin pers. comm. 2022). The fifth occurrence made at Sleeping Giant PP was documented by M. N. Singh and H. E. Schultz, from a mixed-wood forest with Eastern White Cedar stands along a creek (R. T. McMullin pers. comm. 2022).
Table 1 below provides a list of all current and historical records of White-rimmed Shingle Lichen from Ontario. Records from Lake Temagami and Lake Superior PP are omitted as the specimens were either determined to be misidentifications or are disputed, respectively. Two extant sites which occur within protected areas (Quetico PP and Sleeping Giant PP) are not known to encompass significant known threats at this time, although development activities may occur within provincial parks (S. Brinker pers. comm. 2022). One site (Lankinen Road) was considered by COSEWIC (2019) to be extirpated; however the status of this site as extirpated is in question, with flooding from beaver activity causing tree dieback and impacts to the surrounding vegetation community which are challenging to quantify (S. Brinker pers. comm. 2022). Another site (north of Pigeon River) is considered in decline due to adjacent forestry operations (COSEWIC 2019). Lastly, three sites (Dorion Cutoff Road, Albert Lake and Albert Lake Mesa Provincial Nature Reserve) are considered at risk due to potential logging activities as they occur to the west of the Albert Lake Mesa Provincial Nature Reserve (COSEWIC 2019; S. Brinker pers. comm. 2022). Additionally, the Emo site is considered historical (COSEWIC 2019).
Inferring trends in the Ontario White-rimmed Shingle Lichen population is challenging given the scarcity of records, relatively recent discovery of these records, and lack of monitoring efforts. Few professionals (for example, ecologists, foresters) or naturalists are familiar with key characteristics that allow differentiation of White-rimmed Shingle Lichen from similar species from the same genus or family, particularly as some specimens may require additional lab testing to confirm the presence of fatty acids and secondary metabolites (triterpenes) in collected material (R.T. McMullin pers. comm. 2022).
Given its highly specific substrate and habitat requirements, the extent to which additional targeted searching will result in more positive identifications of White-rimmed Shingle Lichen is unknown. A 2019 estimate of the projected total number of thalli in the Ontario population was 639 (COSEWIC 2019), making the loss of a single locality detrimental to the species’ survival in Ontario.
| Date Recorded | Recorded By | Number of Thalli | Ecodistrict | Locality | Status | Source of Record & Collection Number |
|---|---|---|---|---|---|---|
| 1901 | B. Fink | N/A | 5S (5S-2) | Emo, Rainy River District | Historical | CANL 2912 |
| 2016 | S. Brinker | 1 | 4W (4W-1) | Quetico PP, Rainy River District | Extant | NHIC 13195 |
| 2017 | S. Brinker | 24 | 4W (4W-2) | North of Pigeon River, Thunder Bay District | Extant | NHIC
|
| 2017 | S. Brinker | 13 | 3W (3W-2) | Dorion Cutoff Road, Thunder Bay District | Extant | NHIC
|
| 2017 | S. Brinker | 16 | 3W (3W-3) | Albert Lake, Thunder Bay District | Extant | NHIC |
| 2016 2017 |
S. Brinker | 10 | 3W (3W-3) | Albert Lake Mesa Provincial Nature Reserve, Thunder Bay District | Extant | NHIC |
| 2017 | S. Brinker | 9 | 4W (4W-2) | Lankinen Road, Thunder Bay District | Extant | NHIC
|
| 2019 | S. Brinker | -2 | 3W (3W-3) | Sleeping Giant PP, Thunder Bay District | Extant | NHIC |
| 2019 | R. T. McMullin | N/A | 3W (3W-3) | Sleeping Giant PP, Thunder Bay District | Extant | CANL |
| 2019 | B. McCune | N/A | 3W (3W-3) | Sleeping Giant PP, Thunder Bay District | Extant | CANL |
| 2019 | M. N. Singh and H. E. Shultz | N/A | 3W (3W-3) | Sleeping Giant PP, Thunder Bay District | Extant | CANL |
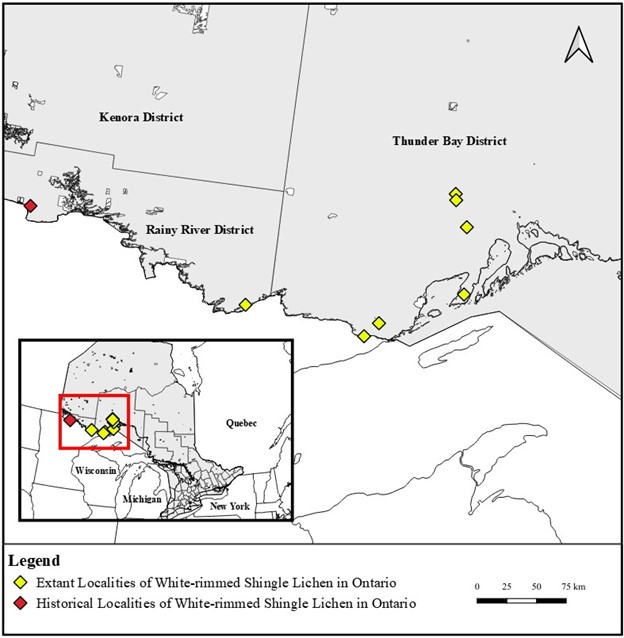
Figure 2. Extant and historical localities of White-rimmed Shingle Lichen in Ontario
1.4 Habitat needs
To date in Ontario, White-rimmed Shingle Lichen has been found exclusively in old-growth, undisturbed swamps and wet forests exhibiting structural complexity that are dominated by mature Eastern White Cedar (S. Brinker pers. comm. 2022). Colonies primarily occupy tree bark (corticolous) or occasionally rocks. Prevailing biophysical attributes that typify occupied sites in Ontario are described below.
Physiography and landscape setting
The predominant bedrock geology of occupied Ontario sites typically consists of carbonate sedimentary formations (including sandstone and shale) as well as mafic rock (Ontario Geological Survey 2021). Surficial soils appear to be loamy to fine mineral overlain by organics, though the depth of accumulated organics is unknown, and no soils investigations have occurred at extant sites to date (S. Brinker pers. comm. 2022). Occurrences are often situated in sheltered areas protected from disturbance by their physiographic positioning, such as valley slopes and bottomlands (S. Brinker pers. comm. 2022).
Ecosite description
White-rimmed Shingle Lichen is associated with Eastern White Cedar dominated swamps and wet forests. Typical woody associates in Ontario include Balsam Fir, Mountain Maple, Speckled Alder, Alder-leaved Buckthorn, Canada Yew and Dwarf Raspberry. Associated herbaceous species include Common Lady Fern (Athyrium filix-femina), Two-seeded Sedge, Sheathed Sedge (Carex vaginata), Yellow Clintonia and Bulblet Bladder Fern (Cystopteris bulbifera) (Brinker 2020; S. Brinker pers. comm. 2022; COSEWIC 2019). Reflecting the photobiont’s moisture requirements, the cool, humid environments in which White-rimmed Shingle Lichen has been documented are often associated with riparian areas, surface water flows, poor drainage or groundwater discharge (Haughian et al. 2018). Suitable Eastern White Cedar dominated swamps and wet forests typically occur in areas where wet soils reduce the frequency of fire, as well as sheltered, low-lying areas which provide protection from windthrow (Wester et al. 2015).
Extant sites in Ontario do not typically contain extensive standing water during the growing season, instead exhibiting raised hummocks and scattered pools throughout (S. Brinker pers. comm. 2022). Despite this, occupied sites have not been visited during the early spring when soil saturation typically peaks (S. Brinker pers. comm. 2022). Canopy coverage is variable but partial openings and gaps are frequent.
Substrate
White-rimmed Shingle Lichen grows primarily on tree bark throughout its range. The species is exclusively corticolous in Ontario and only known to occupy mature Eastern White Cedar bark based on extant records. Eastern White Cedar is a uniquely suitable host for the species owing to its structural attributes and habitat preferences (S. Haughian pers. comm. 2022). Structural attributes such as a twisted growth habit which often produces leaning boles, as well as the ability to continue growing after blowdown events, facilitates moisture retention (S. Haughian pers. comm. 2022). The bark of Eastern White Cedar promotes colonization by cyanolichens such as White-rimmed Shingle Lichen due to its superior water holding capacity, overall morphology (i.e., soft, spongy), and circumneutral pH (COSEWIC 2019; S. Brinker pers. comm. 2022), characteristics which are lacking in other conifers such as spruce (Picea spp.) and pine (Pinus spp.). Corticolous cyanolichens generally avoid occupying acidic substrates, preferring nutrient-rich substrates with a pH above 5.0 (Goward and Arsenault 2000). The bark of conifers is typically acidic (Goward and Arsenault 2000); however, it may become more hospitable through external nutrient enrichment. Nutrient enrichment of tree bark can occur through a drip zone effect where nutrients (notably calcium) are taken up by tree roots are later released into the environment through canopy drip (Goward and Arsenault 2000). Conversely, suitable substrates lacking sufficient buffering capacity may become inhospitable to cyanolichens over time due to acidification occurring from air pollution in the form of acid rain (Richardson and Cameron 2004).
In New Brunswick, White-rimmed Shingle Lichen has been observed on smaller trees located within mature stands, indicating that stand age may be a stronger predictor of suitable habitat than tree size (COSEWIC 2019). White-rimmed Shingle Lichen also shows a preference for the bark of medium to large Eastern White Cedars with a diameter at breast height (DBH) that averages 26.5 cm (COSEWIC 2019). Colonies have been found to predominantly grow on the upper side of Eastern White Cedar boles which exhibit a 20° lean and show a strong preference for northeastern aspects (COSEWIC 2019; S. Brinker pers. comm. 2022). Colonies in New Brunswick and Nova Scotia are most often found from 1 m to 1.8 m in height along the trunk of host trees and are rarely found below 30 cm; however, efforts to document occupation higher in the canopy (for example, via ladders) have not occurred to date (COSEWIC 2019). Additional work is needed in Ontario to address knowledge gaps by documenting the size and age of occupied trees, as well as determining the relationship between tree-lean angles and occupancy within the two Ontario subpopulations. These microhabitat characteristics provide the species with a unique light regime, allowing for adequate light exposure while reducing the likelihood of desiccation from strong southwest light exposure (COSEWIC 2019). Additionally, a sloped trunk angle allows for the thallus to receive additional exposure to rainwater (S. Brinker pers. comm. 2022) which is necessary for the photobiont to successfully fix nitrogen and photosynthesize.
Apart from Eastern White Cedar, there are other theoretically suitable substrate types that could support colonization by White-rimmed Shingle Lichen. Although the species has been recorded from Red Maple (Acer rubrum) bark in Nova Scotia, this substrate type does not typically support cyanolichens in Ontario (S. Brinker pers. comm. 2022). Like Eastern White Cedar, Balsam Fir possesses higher pH bark and routinely supports sensitive cyanolichens (particularly on twigs; S. Brinker pers. comm. 2022), though no collections of White-rimmed Shingle Lichen have been made on this species in Canada. Black Ash bark may also act as a suitable substrate for the species due to bark morphology pH buffering characteristics (S. Brinker pers. comm. 2022) and there are infrequent occurrences of White-rimmed Shingle Lichen occupying Black Ash bark in Nova Scotia; however, these are thought to be spillover effects of robust colonies on Eastern White Cedar bark to neighbouring trees (S. Haughian pers. comm. 2022). The species is also known to occasionally occupy rocks (Jørgensen 2000; Brodo et al. 2001) though this has not been documented in Ontario (with the exception of a disputed specimen) despite thorough searching in suitable habitat (S. Brinker pers. comm. 2022).
1.5 Limiting factors
Research investigating related cyanolichens (Pannariaceae) which contain the photobiont Nostoc shows that environmental and climatic requirements exert the greatest influence on cyanolichen distribution at a variety of scales, even when compared to availability of cyanobacteria associates (Lu et al. 2018). Given the highly specific habitat and substrate requirements of White-rimmed Shingle Lichen in Ontario – particularly its association with one substrate type (i.e., the bark of mature, leaning Eastern White Cedar trees), one broad ecosystem type (i.e., undisturbed, mature Eastern White Cedar swamps) and a narrow range of biophysical conditions (for example, high humidity, moderate light availability, stable temperatures, low air pollution) — it is reasonable to conclude that the species is limited by habitat availability. Where remnant cedar swamps remain, large portions of its historical range in the Great Lakes region would no longer be suitable for occupation given continent-scale declines in air quality.
As a corticolous species, White-rimmed Shingle Lichen relies on the continued health of its host tree to survive. Natural disturbance regimes occurring within the species’ habitat may also limit colony survival and longevity. Eastern White Cedar host trees which exhibit the structural characteristics that promote colonization (i.e., lean) are susceptible to blowdown and failure. Eastern White Cedar occupying mesic soils have been found to produce shallower root systems than those occupying drier, upland habitat, demonstrating reduced phenotypic plasticity and increased susceptibility to blowdown (Musselman et al. 1975). Leaning trees may also be more susceptible to blowdown or failure from snow load than those with boles in a vertical position (Coder 2013).
Eastern White Cedar is typically a long-lived species which tolerates shade, frost and variable moisture conditions, and may persist across multiple successional stages (Sims et al. 1990). However, this species is also susceptible to damage from a range of insects and diseases. The Boreal Carpenter Ant (Camponotus herculeanus) is known to feed on the decaying heartwood of mature trees and may further compound existing structural defects, predisposing the tree to failure or blowdown (Sims et al. 1990). Eastern White Cedars growing in wet, organic soils are also susceptible to Brown Cubical Buttress Rot (Polyporus balsameus and P. schweinitzii) which may further predispose trees to blowdown (Sims et al. 1990). Notwithstanding the above, Eastern White Cedar is generally considered at low risk of damaging agents (Carey 1993).
Naturally occurring fire regimes may play a role in limiting the distribution of White-rimmed Shingle Lichen in Ontario. Eastern White Cedar is prone to damage from fire due to its shallow root systems, thin bark, and high oil content including both leaves and twigs (Johnston 1990). This tree species often occupies wetlands and areas with a high water table which inherently exhibit lower fire risk; however, fire may spread from upland sites to wetlands if the ground layer contains a high fuel load or is composed of graminoids (Johnston 1990). All extant occurrences of White-rimmed Shingle Lichen in Ontario are from areas which appear to be protected from burning due to topographic or hydrological characteristics (S. Brinker pers. comm. 2022). Discovery of additional sites in Ontario may clarify the extent to which natural disturbance regimes may be a limiting factor for the species.
1.6 Threats to survival and recovery
Direct harm to White-rimmed Shingle Lichen may result from a variety of human-mediated processes involving the removal of host trees, loss of habitat, or alterations to highly specific microclimate requirements in the surrounding biophysical environment (for example, humidity, air temperature, light regime, ambient air quality).
The primary threats to the survival and recovery of White-rimmed Shingle Lichen in Ontario (listed in order of severity) are:
- habitat loss
- habitat degradation
- alterations to the hydrologic regime
- climate change
- air pollution
Identified threats to the species are based on direct evidence where possible, or clearly stated when inferred from evidence of impacts to related cyanolichens.
Habitat loss
Old-growth cedar swamps and wet forests represent an undisturbed, highly-sensitive ecosystem type. Based on current understandings of occupied localities and distribution, commercial forestry operations are considered the most significant threat to White-rimmed Shingle Lichen in Ontario. Although Eastern White Cedar is generally not a primary target for harvesting (D. Kinsman pers. comm. 2022), this tree species is typically managed through shelterwood or strip clearcut silviculture systems (MNRF 2021). While a variety of silvicultural treatments (for example, selection harvest, shelterwood harvest) are available which may allow for partial retention of the prevailing compositional and structural attributes of occupied sites, some degree of disturbance is inevitable when biomass is harvested and removed. Ancillary forestry operations including road and skid trail construction and small-scale aggregate extraction may also render existing habitat unsuitable for colonization. Two occupied sites are associated with protected areas (Sleeping Giant PP and Quetico PP) but most occurrences are from Crown land subject to forestry activities. The threat of habitat loss associated with forestry is evidenced by the expected decline of the species at two sites where there are active forestry operations (COSEWIC 2019).
Occurrences of White-rimmed Shingle Lichen on Crown land fall within the Ministry of Natural Resources and Forestry’s (MNRF) Northwest Administrative Region, specifically within the Black Spruce Forest (Management Unit 035) and the Lakehead Forest (Management Unit 796) (Resolute FP Canada Inc. 2021; Greenmantle Forest Inc. 2019). Sustainable Forest Licenses for both Management Units allow for harvesting of all tree species (NDMNRF 2021). Eastern White Cedar made up 5% and 2% of merchantable wood available from the Black Spruce Forest Management Unit and Lakehead Forest Management Unit respectively, based on the March 2022 Ontario Available Wood Report (NDMNRF 2022). Occupied stands within the Lakehead Forest Management Unit are not scheduled for immediate harvest based on the 2022-2023 Annual Work Schedule; however, operations are scheduled within the Black Spruce Forest Management Unit which may occur within the vicinity of the Dorion Road Cutoff site (Resolute FP Canada Inc. 2022; Greenmantle Forest Inc. 2022). If species at risk (SAR) are encountered during harvesting activities and there is no existing Area of Concern (AOC) for the species in the respective forest management plan, operations are suspended (as per the Forest Information Manual, a manual regulated under the CFSA) until the planning team develops an AOC to be amended into the plan (D. Kinsman pers. comm. 2022). If a value (such as SAR) is known to be potentially impacted by forest operations and there is no specific direction in an approved forest management guide, the planning team will develop an AOC as per the Forest Management Planning Manual (a manual regulated under the CFSA) (D. Kinsman pers. comm. 2022). Despite the foregoing, White-rimmed Shingle Lichen is highly unlikely to be field-identified by those engaged in timber harvesting layout or operations at the present time (i.e., without specialized training). The removal of suitable host trees would cause immediate (or eventual) mortality to any affixed thalli, as well as a loss of suitable substrate. The harvested area may remain unsuitable in perpetuity if other tree species (i.e., non-cedar) are planted, and (regardless of post-harvest plantings) re-establishment of cedar swamps with old-growth attributes is a process that likely takes centuries.
Activities such as trap line maintenance and the creation and maintenance of recreational trails may also occur within Crown land and have the potential to impact host trees. Other human activities such as mining claims, construction of linear infrastructure (for example, municipal roads, highways, utility corridors) and renewable energy projects may also cause habitat loss or impact host trees but are not considered to be a significant threat to the survival and recovery of White-rimmed Shingle Lichen at this time.
Habitat degradation
Certain silvicultural prescriptions and related activities (for example, road construction) may produce edge effects through the creation of an abrupt transition between harvested and non-harvested stands. Such edge effects may alter the prevailing microclimate (for example, humidity, light, wind, temperature) and could deleteriously impact nearby colonies of White-rimmed Shingle Lichen situated well beyond the harvesting limit. Cyanolichens are known to be sensitive to edge effects from timber harvesting; local extirpations in protected areas adjacent to harvesting have been reported for the related cyanolichen Boreal Felt Lichen (Erioderma pedicellatum) (Holien et al. 1995). Occurrences of White-rimmed Shingle Lichen in New Brunswick which remain in retention patches after logging were noted to have slightly necrotic thalli
(i.e., desiccating and dying) (COSEWIC 2019). Intensive forestry practices (particularly clear cutting and thinning) are known to significantly alter the habitats of cyanolichens by increasing light levels and temperature, as well as decreasing humidity and reducing beneficial nutrient enrichment provided through drip zone effects (Richardson and Cameron 2004). Significant alterations to microclimate resulting from edge effects have been found to result in loss of White-rimmed Shingle Lichen thalli in adjacent areas, even when suitable host trees are retained (COSEWIC 2019). Additional indirect impacts to habitat from timber harvesting include alterations to the water table from access road construction and increased risk of tree windthrow from the creation of canopy gaps, both of which may result in the loss of suitable host trees and a decline in habitat suitability (COSEWIC 2019). Hazard tree removal practices may also degrade habitat quality. Trees with leaning boles, such as those typically occupied by White-rimmed Shingle Lichen, are at a higher risk of failure than those with straight trunks and are more likely to be targeted during hazard tree removal work (Coder 2013; USDA 2017). Although hazard tree removals do not typically occur on Crown land, park management plans for Quetico PP and Sleeping Giant PP allow for the removal of hazard trees adjacent to trails and other infrastructure, as well as the removal of trees to enable resource management practices or the development of facilities (Ontario Parks 2007; 2018). Based on aerial imagery interpretation, all records of White-rimmed Shingle Lichen within Sleeping Giant PP appear to be located within less than 400 m of established trails. Both park management plans require the completion of an environmental assessment (Class EA-PPCR) which includes vegetation inventories and the review of potential SAR prior to the removal of trees for resource management and development, however there does not appear to be such a requirement for hazard tree removals (Ontario Parks 2007; 2018).
Alterations to the hydrologic regime
Alterations to the water balance of treed swamp communities occupied by White-rimmed Shingle Lichen could lead to flooding or drying of habitat and a resulting decline or death of host trees. Poorly planned or constructed roads may alter surficial drainage patterns; logging roads have been documented in close proximity to occupied sites (S. Brinker pers. comm. 2022).
Treed wetlands may be subject to drastic changes in water level and flooding regimes due to flooding induced by Beaver (Castor canadensis) dams. Habitat within the extirpated Lankinen Road site has declined in suitability due to tree mortality as a result of beaver-induced flooding (S. Brinker pers. comm. 2022).
Climate change
The effects of climate change on lichens primarily stem from direct changes in temperature and moisture, which also indirectly alter habitat structure and function. Cyanolichens require adequate moisture in order to photosynthesize and fix atmospheric nitrogen at regular rates, making them especially sensitive to desiccation and heat stress (Antoine 2004; Nash 2008a; Pearson et al. 2018). Modelling developed by Pearson et al. (2018) identified mean annual temperature and precipitation as the most important variables (out of the four variables included in the model) influencing White-rimmed Shingle Lichen distribution at a landscape scale.
Assessments of climate change vulnerability within Ontario’s Great Lakes Basins based on climate projections identified lichens as one of the most vulnerable taxonomic groups (Brinker et al. 2018). Climate modelling based on the Canadian Coupled Climate Global Circulation Model (Flato and Boer 2001) predicts higher summer and winter temperatures as well as decreased summer precipitation in northern Ontario by the end of the century. These outcomes may produce direct negative impacts to White-rimmed Shingle Lichen, resulting from alterations to existing moisture regimes causing an increased risk of desiccation and heat stress. Increases in temperature and decreases in precipitation may also indirectly alter habitat structure by changing the composition of vegetation communities or increasing their susceptibility to wildfire.
Climate modelling also predicts an increase in the severity and frequency of storm events (MNRF 2015). It is possible that an increase in extreme weather events may directly impact White-rimmed Shingle Lichen habitat by altering habitat structure. As the species occupies leaning Eastern White Cedar boles, it is possible that an increase in storm events may increase the risk of blowdown or tree failure. Trees with leaning boles are subject to increased risk of stem cracks and splits, and trees with progressive leans are especially susceptible to failure and blowdown (Coder 2013; USDA 2017). Similarly, trees growing in mesic habitats are often at an increased risk of blowdown due to their shallow root systems (Krause and Lemay 2022).
Air pollution
Long considered to be reliable indicators of changes in air quality (Seaward and Letrouit-Galinou 1991), lichens are known to be sensitive to air pollution. Cyanolichens are known to be sensitive to dissolved sulphur dioxide, particularly under acidic growing conditions (Richardson and Cameron 2004). Based on extensive early records and herbaria collections, cyanolichens which occur on coniferous trees have declined significantly throughout areas of eastern North America that experience acid rain (Richardson and Cameron 2004). These losses are primarily due to the low buffering capacity of conifer bark and resulting acidification of the substrate from sulphur dioxide (Richardson and Cameron 2004). As such, White-rimmed Shingle Lichen in Ontario may be sensitive to the toxic effects of sulphur dioxide given its preference for occupying the bark of conifers (Eastern White Cedar) (Goward and Arsenault 2000).
The impacts of air pollution on lichens may derive from direct injury or mortality to thalli or alterations in habitat function due to acidification. The effects of air pollution on cyanolichens may be observed hundreds of kilometres away from the initial source (Richardson and Cameron 2004). The type of air pollution source also determines the nature of impact. Low elevation air pollution sources cause direct impacts to lichens by producing particulate matter which dissolves into the thallus, causing physical damage and interrupting photosynthesis (Richardson and Cameron 2004).
High elevation pollution sources produce particulate matter which remains in the atmosphere for significant periods of time, often dispersing large distances and representing a widespread threat. Particulate matter such as sulphur dioxides and nitric oxides are oxidized in the atmosphere and react with rainwater to produce sulphuric acid and nitric acid respectively, forming acid rain (Richardson and Cameron 2004). Exposure to acid rain can render habitat unsuitable for White-rimmed Shingle Lichen by leaching calcium from the host tree bark, which is necessary for maintaining a high pH and buffering capacity which supports lichen growth (Richardson and Cameron 2004). Additionally, acid rain may indirectly alter suitable habitat by leaching calcium from the soil, resulting in decreased uptake by tree roots and/or mycorrhizal fungi which may alter host tree bark characteristics and significantly alter the drip zone effect (Richardson and Cameron 2004). Within the Ontario distribution of White-rimmed Shingle Lichen, potential sources of high elevation air pollution which may contribute to acid rain include paper mills and mining operations, although the extent to which air pollution impacts the species in Ontario remains unknown (Government of Canada 2022).
1.7 Knowledge gaps
Current range
As described in Section 1.3, there are seven extant sites occupied by White-rimmed Shingle Lichen in Ontario. All extant sites were identified by a single expert (S. Brinker) with the exception of the Sleeping Giant PP site which is represented by additional collections (COSEWIC 2019; S. Brinker pers. comm. 2022; R.T McMullin pers. comm. 2022). Targeted searching and formal surveys have been extremely limited. A disputed record from Algoma District (Lake Superior PP) is the only possible record in Ontario east of Lake Superior. The current range of White-rimmed Shingle Lichen, including an understanding of available habitat, remains a significant knowledge gap.
Distribution patterns
As described in Section 1.4, White-rimmed Shingle Lichen requires highly specific conditions to persist and occurs at low densities. Based on the significant distances between known occurrences, and absences from large areas containing suitable habitat (S. Brinker pers. comm. 2022), it is possible that additional unknown habitat requirements or threats are influencing the distribution patterns of this species in Ontario. In addition to its current range, the specific factors influencing the distribution pattern of this species in Ontario are a knowledge gap.
Dispersal
As described in Section 1.2, White-rimmed Shingle Lichen predominantly reproduces sexually by ascospores which are dispersed by wind. Although the primary dispersal mechanism is known, dispersal distances and survival rates of ascospores remain unknown for this species (and most cyanolichens). Valuable comparisons may be drawn between White-rimmed Shingle Lichen dispersal and the dispersal of other macrolichen species which require old growth habitat; however, this should be done with caution, particularly as reported dispersal distances may vary significantly between studies (see: Jüriado et al. 2011). Additionally, although the species does not possess the necessary structures for vegetative propagation (such as soredia and isidia), evidence from New Brunswick suggests that vegetative reproduction from broken thallus fragments may be occurring, although dispersal distances and modes of dispersal for thallus fragments are unknown (COSEWIC 2019; S. Haughian pers. comm. 2022).
Substrate
White-rimmed Shingle Lichen is known to have specific substrate requirements (i.e., mature Eastern White Cedar bark likely enriched with nutrients through the drip zone effect) throughout its Ontario range; however, this species occupies additional substrate types in other parts of its North American range. This includes Red Maple bark in Nova Scotia and (occasionally) rocks in its range (Jørgensen 2000; COSEWIC 2019). Knowledge of substrate requirements and/or associations for this species in Ontario are based on a limited number of records and remain a knowledge gap.
Soils and Hydrologic Regime
As discussed in Section 1.4, soil type (for example, texture, depth of organic material) and hydrologic regime (for example, water transfer mechanisms, seasonal and annual variability in the water table, depth of surface water ponding) have not been investigated at occupied sites to date. Clarifying these habitat parameters, including how they may respond to anthropogenic disturbance, would refine characterizations of occupied sites and direct future survey efforts.
Viability
As discussed in Section 1.3, there are seven known sites with White-rimmed Shingle Lichen occurrences in Ontario supporting an average of 12.8 thalli per site (COSEWIC 2019; S. Brinker pers. comm. 2022). It is unknown how many of these sites (if any) contain colony densities that exceed critical population thresholds, as thresholds are not yet known. The viability of White-rimmed Shingle Lichen at all extant sites in Ontario is a knowledge gap.
Genetic distinctness
As described in Section 1.2, White-rimmed Shingle Lichen lacks specialized structures to reproduce vegetatively (soredia and isidia) suggesting that sexual reproduction is the primary mode of reproduction. The relatively large distances separating occupied sites in Ontario suggests that there may be genetic differences between them imparted by localized conditions controlling survival. Conversely, lichen ascospores are known to travel significant distances by wind (Brodo et al. 2001). The genetic distinctness of individual colonies in Ontario (and with those in eastern Canada and/or the eastern United States) is a knowledge gap.
Feasibility of propagation and transplanting
Propagation and transplantation have proven successful for some lichens, although these practices are still under development (Allen et al. 2019; Richardson and Cameron 2004). It is not known whether White-rimmed Shingle Lichen can be propagated in a controlled (ex situ) or natural (in situ) setting and/or successfully transplanted, both of which are key knowledge gaps.
Generation time
The generation time of White-rimmed Shingle Lichen is not known with certainty, although one thallus was relocated in the field after 12 years (COSEWIC 2019). An estimated generation time of 12 years is provided in the 2019 COSEWIC report, which is a conservative estimate derived from time to reproductive maturity and host tree longevity, as well as generation times of related species (COSEWIC 2019; S. Brinker pers. comm. 2022; S. Haughian pers. comm. 2022).
Browsing and grazing
The effects of browsing and grazing on White-rimmed Shingle Lichen are not known. Eastern White Cedar is an important winter browse species for White-tailed Deer (Odocoileus virginianus), and feeding damage by Porcupine (Erethizon dorsatum) has been known to injure or kill stems depending on the severity of damage (Sims et al. 1990). While grazing is a natural process mediated by wildlife, predator-prey relationships have been altered as a result of human settlement and land management regimes (for example, hunting, fire suppression). Invasive land snails (Arion spp.) are suspected in extensive grazing damage noted in Nova Scotia (COSEWIC 2019) though this has not been documented to date in Ontario (S. Brinker pers. comm. 2022).
Air pollution in Ontario
The effects of air pollution on White-rimmed Shingle Lichen in Ontario are not known, nor is the severity of the threat. As discussed in Section 1.6, cyanolichens growing on coniferous trees are known to be especially sensitive to dissolved sulphur dioxide, due to the reduced buffering capacity of conifer bark (Richardson and Cameron 2004). The extent which air quality in Ontario may directly or indirectly impact White-rimmed Shingle Lichen is a knowledge gap.
1.8 Recovery actions completed or underway
Prior to 2016, no targeted searches are known to have been conducted for White-rimmed Shingle Lichen in Ontario, although general surveys for lichens have been undertaken throughout the province. Targeted surveys were conducted in 2016 and 2017 by S. Brinker to support the 2019 COSEWIC Assessment and Status Report (COSEWIC 2019). Surveys entailed searching for species when in suitable habitat, as well as dedicated trips revisiting locations where historical occurrences were recorded (COSEWIC 2019; S. Brinker pers. comm. 2022). It is estimated that approximately 123 person-hours were spent searching for the species during these surveys (COSEWIC 2019).
2.0 Recovery
2.1 Recommended recovery goal
The recommended recovery goal for White-rimmed Shingle Lichen is to maintain and, where possible, increase the number of thalli at all localities, and any newly-discovered occurrences, to reduce the likelihood of extirpation.
2.2 Recommended protection and recovery objectives
- Maintain or increase the long-term viability of all known occurrences
- Conduct targeted surveys in suitable habitat to determine the actual population size and distribution in Ontario
- Promote awareness of White-rimmed Shingle Lichen by collaborating with stakeholders (for example, approval authorities, landowners, industry, conservation groups and municipalities) and Indigenous organizations and communities
- Address key knowledge gaps
2.3 Recommended approaches to recovery
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Management | 1.1 Develop a Habitat Regulation or General Habitat Description
|
Threats:
|
| Critical | Short-term | Protection Management |
1.2 Collaborate with species experts (for example, NHIC staff) to gather occurrence data and identify suitable habitat using a desktop approach, then work with MNRF staff to identify areas selected for activities which result in tree removals.
|
Threats:
|
| Critical | Short-term | Protection Management |
1.3 Support the protection and recovery of White-rimmed Shingle Lichen within the forest management policy framework as per the Crown Forest Sustainability Act, 1994 (CFSA), Forest Management Planning Manual (regulated under the CFSA) and forest management guides, in a manner that best support the species’ needs.
|
Threats:
|
| Critical | Short-term | Protection Monitoring and Assessment |
1.4 Complete a threats assessment and undertake mitigation for parks occurrences
|
Threats:
|
| Critical | Short-term | Inventory Monitoring and Assessment |
1.5 Conduct long-term monitoring
|
Threats:
Knowledge gaps:
|
Objective 2: Conduct targeted surveys in suitable habitat to determine the actual population size and distribution in Ontario
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Management Inventory Monitoring and Assessment |
2.1 Intensively survey suitable habitat with the intent of locating new localities
|
Threats:
Knowledge gaps:
|
Objective 3: Promote awareness of White-rimmed Shingle Lichen by collaborating with stakeholders (for example, approval authorities, landowners, industry, conservation groups and municipalities) and Indigenous organizations and communities
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Short-term | Communications Education and Outreach |
3.1 Ensure training on White-rimmed Shingle Lichen identification (including habitat identification) is available to Indigenous organizations and communities, industry, Ontario Parks staff, and local naturalists
|
Threats:
Knowledge gaps:
|
| Beneficial | Short-term | Communications Education and Outreach |
3.2 Provide training and outreach to the public
|
Threats:
Knowledge gaps:
|
Objective 4: Address key knowledge gaps
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Research | 4.1 Support Species Distribution Modeling in Ontario
|
Knowledge gaps:
|
| Beneficial | Long-term | Research | 4.2 Support Species Biology Research
|
Knowledge gaps:
|
| Beneficial | Long-term | Research | 4.3 Support Genetic Research
|
Knowledge gaps:
|
| Necessary | Long-term | Research | 4.4 Support Genetic Research
|
Knowledge gaps:
|
| Necessary | Long-term | Research Management |
4.5 Support Propagation Research
|
Threats:
Knowledge gaps:
|
| Beneficial | Long-term | Research | 4.6 Support Air Pollution Research
|
Threats:
|
Narrative to support approaches to recovery
Habitat Regulation and/or General Habitat Description
White-rimmed Shingle Lichen is a poorly known and poorly understood species which may undermine protection and recovery efforts. To date, very few professionals (<10) have observed the species in Ontario, and most occurrences are attributable to one observer (S. Brinker). These factors may result in White-rimmed Shingle Lichen being overlooked, particularly when screening areas in preparation for activities which may be harmful to the species and/or destructive to its habitat. Inclusion of a habitat regulation for White-rimmed Shingle Lichen under Ontario Regulation 832/21 or development of a General Habitat Description and associated habitat categorization scheme will inform agency staff (for example, MECP, MNRF) and proponents of this species’ level of tolerance to alterations and activities within specified distances of a known colony.
Park Management
Maintaining the longevity of the Sleeping Giant PP and Quetico PP sites is important to the continuation of the species in Ontario, particularly as certain colonies on Crown land are believed to be in decline.
Further to this, a threats assessment should be undertaken in areas where White-rimmed Shingle Lichen colonies occur in provincial parks by qualified staff. A threats assessment is a tool used to identify human activities and/or natural processes that may cause harm to existing White-rimmed Shingle Lichen occurrences and/or their habitat. Following completion of the threats assessment(s), implementation of mitigation measures and/or management techniques should be considered, as appropriate.
Forestry Management Planning
Forest management planning applies to forest operations conducted in accordance with an approved forest management plan, prepared under forest management framework that applies to Crown lands in the managed forest regulated by the Crown Forest Sustainability Act, 1994 (CFSA). Species at risk in these areas are addressed under the CFSA and its forest management planning policy framework and not under the ESA. Recovery approaches recommended in this recovery strategy, regarding forestry on Crown land are being offered to support the protection and recovery of White-rimmed Shingle Lichen within the forest management policy framework as per the CFSA, Forest Management Planning Manual (regulated under the CFSA) and forest management guides.
Due to the cryptic nature of the species and limited survey effort to date, a screening process should be developed in order to protect suitable habitat from areas proposed for timber harvesting and related activities. This process should be developed for all FMUs where White-rimmed Shingle Lichen is known to occur, as well as directly adjacent FMUs, and expanded to encompass the known range of the species as it changes over time. Aerial imagery interpretation (for example, Forest Resources Inventory) has been found to be an effective means of directing targeted surveys for the species (S. Brinker pers. comm. 2022) and may be used to identify areas with high potential for supporting White-rimmed Shingle Lichen occupancy. Desktop-based screening exercises should be paired with field inventories for the species conducted by specialists in suitable habitat prior to forestry operations.
Targeted surveys
Targeted inventories for White-rimmed Shingle Lichen across northwestern Ontario, particularly in areas adjacent to the northern and eastern shores of Lake Superior (where no occurrences are currently known), are critical in order to gain a better understanding of the species’ range in Ontario. In addition to identifying and protecting new colonies, results from the targeted inventories may further refine our understanding of what attributes influence habitat occupancy for White-rimmed Shingle Lichen. Additionally, the results of future targeted inventories may inform better screening practices to protect the species, as well as providing additional data to support the creation of species distribution modelling for Ontario.
Education and Outreach
Given lack of awareness of White-rimmed Shingle Lichen and few known localities documented, there is a need to circulate species identification and suitable habitat information to, for example, agencies, professional ecologists, foresters and naturalists. Although this species is sometimes challenging to field-identify, suitable habitat (and microhabitat) is distinctive enough that non-experts can readily identify suitable habitat for additional inventories by knowledgeable professionals.
Research
Currently, there is little information available on many aspects of White-rimmed Shingle Lichen biology. Supporting research to determine basic species biology, such as generation time and dispersal will fill significant gaps in the current knowledge and inform future recovery actions. Determining a species-specific generation time would also allow for more accurate predictions of future population sizes and declines in the species. Developing an understanding of species dispersal distances will support the development and refinement of species distribution modelling, helping to clarify existing knowledge gaps surrounding dispersal and current range.
Supporting research to determine the level of genetic distinctness of Ontario localities, as well as the distinctiveness of the Ontario population compared to those in eastern Canada, will also fill existing knowledge gaps as well as support feasibility assessments for transplanting options. Although restoration techniques for lichens are still being developed (Allen et al. 2019), the feasibility of propagating colonies from vegetative tissues and/or ascospores ex situ (i.e., in a laboratory setting) for eventual transplant into suitable habitat should also be explored as it offers a chance of expanding the wild population of White-rimmed Shingle Lichen in Ontario. Additionally, research exploring the potential for host tree propagation and transplantation may offer means of mitigating the impacts of host trees losses to browsing and grazing.
2.4 Performance measures
Performance measures are specific standards which permit evaluation of progress made towards achieving the recovery goals and objectives outlined in this Recovery Strategy for White-rimmed Shingle Lichen. Performance measures are offered for each recovery objective as follows:
- Increase the long-term viability of all known occurrences
- Habitat regulation under Ontario Regulation 832/21 or General Habitat Description in place (yes/no)
- Number of threats mitigated or addressed through management practices within provincial parks
- Number of sites protected through the development of approaches which direct operations away from extant occurrences
- Creation and implementation of operational approaches for the species is undertaken by all districts where the species occurs (yes/no)
- Number of projects undertaken to fill knowledge gaps and directly support the recovery of White-rimmed Shingle Lichen and its habitat (for example, through threat reduction and mitigation measures)
- The current known number of thalli at each site in Ontario, as well as any other newly identified sites has been maintained or increased (yes/no)
- Conduct targeted surveys in suitable habitat to determine the overall population size and distribution in Ontario
- Number of person hours spent surveying
- Spatial extent of suitable habitat surveyed
- Number of sites surveyed
- Number of new occurrences and thalli documented
- Promote awareness of White-rimmed Shingle Lichen by collaborating with stakeholders (for example, approval authorities, landowners, industry, conservation groups and municipalities) and Indigenous organizations and communities
- Number of workshops or training events held
- Number of attendees at workshops and training events held
- Number of new observations that can be linked back to an awareness campaign
- Number of collaborative projects to support the protection and/or recovery of White-rimmed Shingle Lichen
- Promote awareness of White-rimmed Shingle Lichen by collaborating with stakeholders (for example, approval authorities, landowners, industry, conservation groups and municipalities) and Indigenous organizations and communities
- Number of supported research projects underway
- Number of supported research projects completed
2.5 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of the Environment, Conservation and Parks on the area that should be considered if a habitat regulation is developed. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the author will be one of many sources considered by the Minister, including information that may become newly available following the completion of the recovery strategy should a habitat regulation be developed for this species.
It is recommended that a habitat regulation be prescribed for this species which encompasses the following spatial extents:
- The ecosite in which White-rimmed Shingle Lichen occurs
- Area within 200 m of an ecosite in which White-rimmed Shingle Lichen occurs, excluding the footprint of existing infrastructure (for example, roads and buildings)
These components are intended to capture the following elements:
- The species itself (i.e., occurrences, colonies).
- The host tree on which the occurrence is affixed.
- The surrounding ecosite (i.e., vegetation community) and portions of adjacent ecosites which sustain the occurrence and provide opportunities for local dispersal.
- Suitable microsite conditions (for example, high humidity, moderate light, high moisture, low wind) which sustain the occurrence and maintain habitat potential within the broader ecosite.
A rationale which supports this habitat recommendation is provided below.
Occurrence and host tree
There are a variety of human activities and processes which may adversely affect host trees (or woody vegetation generally), which include:
- Direct tree removal.
- Mechanical injury to the trunk, roots, branches, and/or foliage.
- Soil compaction and erosion within the existing or future root zone, and smothering or exposure of roots due to changes in grade resulting from soil excavation and/or placement of fill.
- Alterations to any biophysical condition (for example, light regime, soil moisture regime, etc.) which the host tree was previously accustomed.
Trees possess visible above-ground biomass (for example, leaves, needles, branches, trunks) and mostly invisible below-ground biomass (for example, roots). The maximum lateral extent of the host tree is an important consideration and is typically reflected by the canopy dripline and/or root zone. While there is an observed relationship between the maximum lateral extent of a tree’s root zone and its diameter, this relationship may be non-linear for certain species and weakens for mature trees (Day et al. 2010). Additionally, root architecture may vary significantly across species, age class and growing conditions. Guidance for establishing minimum tree protection zones with reference to trunk diameter ratios is offered in the arboricultural literature (Harris et al. 2004; Fite and Smiley 2008), but such ratios may still result in substantial loss of outer feeder roots (Fite and Smiley 2008). Similarly, the maximum extent of a dripline may vary based on species, age or competition.
The Ontario population of White-rimmed Shingle Lichen is currently known to occupy mature Eastern White Cedar trees in swamps and moist to wet forests. In contrast to Eastern White Cedars occupying upland habitat which develop relatively deep root systems, those from wetter sites tend to display shallow, flat root systems comprised of widely spreading horizontal roots (Bannan 1941a). These root systems typically occur at a soil depth of 5 cm to 7.6 cm, making Eastern White Cedar especially sensitive to changes in grade and soil compaction (Bannan 1941b).
As the broader ecosite surrounding an occurrence also forms part of this habitat recommendation, contextual variability in canopy and root dimensions of host trees will be sufficiently captured by the habitat recommendation.
Ecosite approach to habitat delineation
In Ontario, vegetation communities are typically inventoried, characterized and delineated based on Ecological Land Classification (ELC) (Lee et al. 1998; Lee 2008; Wester et al. 2015). An ecosite represents a mappable unit within a hierarchical classification system with reoccurring, relatively uniform physiography, soil conditions, hydrology and vegetation assemblages (Lee et al. 1998). Ecosites represent a classification unit which may be identified through desktop analysis of air photo imagery, often coupled with field verification and characterization. The recommended approach to regulating White-rimmed Shingle Lichen habitat includes consideration of the relevant ELC ecosite
in which thalli or colonies occur.
A variety of ecosite classification systems covering northwestern Ontario are available (Banton and Racey 2009; Racey et al. 1996; Sims et al. 1989; Wester et al. 2015). Table 3 below provides a list of ecosites which possess the greatest potential to support White-rimmed Shingle Lichen in northwestern Ontario. This list is representative but not necessarily exhaustive; it should be assumed that most moist to wet sites with mature Eastern White Cedar canopy trees in late-successional communities have some potential to support White-rimmed Shingle Lichen.
Should a thallus or colony be found overlapping with more than one ecosite (i.e., mapped polygon), all contiguous suitable ecosites should be considered habitat (provided that they are dominated by or at least contain a high proportion of Eastern White Cedar). Regulation of White-rimmed Shingle Lichen habitat based on ecosite is intended to preserve the prevailing composition, structure and function of the ecosystem surrounding the occurrence, while also supporting the preservation of required microhabitat characteristics necessary for the species’ protection and suitable host trees for local dispersal. Microhabitat characteristics required to sustain cyanolichens are known to be sensitive to alteration from anthropogenic disturbances well beyond where the impact has occurred; with several studies documenting changes in microclimate from clearcut edges from 120 m (Gauslaa et al. 2019) up to 240 m into forests (Chen et al. 1993; Ghelhausen et al. 2000).
| Document | Ecosites |
|---|---|
| Great Lakes – St. Lawrence Ecosite Fact Sheets (Wester et al. 2015) |
|
| Draft Boreal Ecosite Fact Sheets (Banton and Racey 2009) |
|
| Field Guide to the Forest Ecosystems of Northwestern Ontario (Sims et al. 1989) |
|
| Terrestrial and Wetland Ecosites of Northwestern Ontario (Racey et al. 1996) |
|
Microsite conditions
Like many sensitive cyanolichens, White-rimmed Shingle Lichen relies heavily upon specific microsite conditions. Maintaining adequate humidity levels, light, ambient air temperature, substrate pH and presence of adjacent tree canopies is known to be critical for protecting both the host tree and thallus.
Cyanolichens have been observed to experience significant direct and indirect impacts following timber harvesting activities. Studies exploring the impacts of timber harvest on cyanolichens have documented declines up to 120 m into forest interiors from cut edges (Gauslaa et al. 2019). In addition to immediate mortality, those lichens which survive initial harvesting and accompanying changes in microclimate exhibit reduced growth rates and suffer increased eventual mortality even after early tree regeneration occurs (Cameron et al. 2013; Gauslaa et al. 2019). This is due to the drastic, long-lasting shift towards warmer, drier and brighter conditions brought on by timber harvesting (Cameron et al. 2013). Microclimate influences from clearcut forest edges have been shown to extend 240 m into tall forests (Chen et al. 1993; Ghelhausen et al. 2000). Although responses to harvesting activities may vary across cyanolichen species, current research shows that species richness and total abundance decrease as dimensions of the cut area increase (Bartemucci et al. 2022). In addition to the importance of establishing buffer zones for protecting rare cyanolichens, Gauslaa et al. (2019) found that increases in size of retained forest patches also exerted a strong positive influence on cyanolichen survival.
Studies in Nova Scotia on Boreal Felt Lichen (Erioderma pedicellatum), a related foliose cyanolichen in the family Pannariaceae, found significant mortality of thalli on trees adjacent to timber harvesting operations (Cameron et al. 2013). Of 41 thalli documented between 2004-2005 and monitored until 2009, 22 died during the monitoring period, with the mean distance of all monitored Boreal Felt Lichen thalli from harvest being 259 m. While some loss was attributable to non-human factors (for example, grazing), forest harvesting was believed to be primarily responsible for mortality. The authors also reported the mean distance of harvest from thalli which did not survive (159 m) and mean distance of harvest from surviving thalli (320 m); recommending that a minimum 100 m area of uncut buffer be applied to thalli (Cameron et al. 2013). In recognition of these studies, the Nova Scotia Department of Natural Resources (NSDNR) has established Special Management Practices that constrain forestry activities in areas known to support at-risk lichens, applying a 200 m buffer protection zone (i.e., no disturbance) around occurrences of Boreal Felt Lichen and a 200-500 m restricted zone where harvesting and related operations must meet specific guidelines (NSDNR 2018). Other sensitive and at-risk lichens (including several cyanolichens) are afforded either a 200 m or 100 m protected buffer around occurrences. Boreal Felt Lichen shares similar requirements to White-rimmed Shingle Lichen, including a need for moist microhabitats and old-growth conifer dominated forest stands, providing a suitable model for protection and recovery efforts (Maass and Yetman 2002).
Based on the above discussion, the ecosite(s) and an area within 200 m of the ecosite(s) in which White-rimmed Shingle Lichen occurs (i.e., not an occurrence itself) is recommended for consideration as habitat (Figure 3).
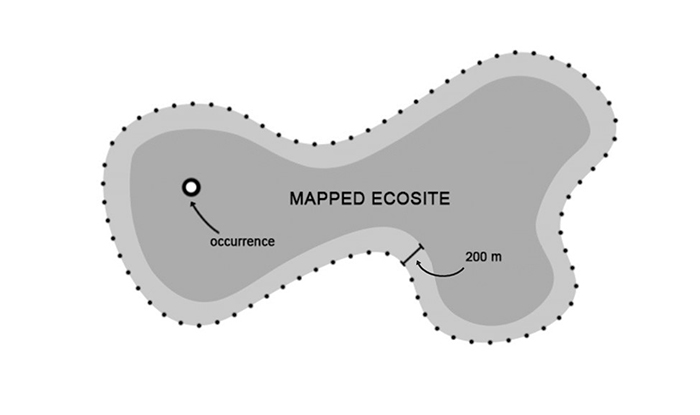
Figure 3. Habitat recommendation for White-rimmed Shingle Lichen established by applying a 200 m area surrounding the ecosite in which an occurrence is present
The 200 m area contributes to the maintenance of suitable microsite conditions and provides opportunities for local dispersal. This recommendation is based on the best available information (reviewed above) which overall is scant; long-term monitoring and additional research will assist with verifying the appropriateness of this recommendation.
Geographic scope
It is recommended that the geographic scope of the habitat regulation cover the province of Ontario in full (without geographic limitation). While currently restricted to northwest Ontario, there is the potential for this lichen to occur in other parts of the province where habitat is suitable. Although extant occurrences of White-rimmed Shingle Lichen are restricted to sites within Rainy River District and Thunder Bay District, additional colonies may be discovered in neighbouring or nearby municipalities. We further recommend that the habitat regulation described herein also be applied to any new White-rimmed Shingle Lichen occurrences discovered in the future.
Glossary
- Apothecium (pl. Apothecia)
- Disk- or cup-shaped fruiting bodies
- Ascomycete (pl. Ascomycetes)
- Fungi (including lichens) which produce spores in an ascus, now forming part of the phylum Ascomycota
- Ascus (pl. Asci)
- A sac-like structure in which ascospores are formed
- Ascospore
- A spore produced within an ascus by species in the phylum Ascomycota
- Bole
- Main stem or trunk of a tree
- Circumneutral
- Having a pH near neutral
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC)
- The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
- Committee on the Status of Species at Risk in Ontario (COSSARO)
- The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
- Confamilial
- An organism belonging to the same taxonomic family as another
- Congener
- An organism belonging to the same genus as another
- Conservation status rank
- A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. Ranks are determined by NatureServe and, in the case of Ontario’s S-rank, by Ontario’s Natural Heritage Information Centre. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
- 1 = critically imperiled
- 2 = imperiled
- 3 = vulnerable
- 4 = apparently secure
- 5 = secure
- NR = not yet ranked
- Cortex
- Outer layer of the lichen thallus
- Corticolous
- Growing on tree bark
- Crown Forest Sustainability Act, 1994 (CFSA)
- The provincial legislation that provides for the sustainability of Crown forests and, in accordance with that objective, to manage Crown forests to meet social, economic and environmental needs of present and future generations.
- Crustose
- Lichen growth habitat forming a crust on the substrate
- Cyanolichen
- Lichens which contain cyanobacteria (blue-green algae) as the photobiont
- Cystobasidiomycete
- Class of fungi in the subdivision Pucciniomycotina of the Basidiomycota
- Endangered Species Act, 2007 (ESA)
- The provincial legislation that provides protection to species at risk in Ontario
- Epiphyte (adj. Epiphytic)
- An organism that grows on the surface of a plant and predominantly derives its moisture and nutrients from the air and precipitation.
- Ex situ
- Activities occurring off-site or away from the field (for example, in a lab.)
- Foliose
- Lichen growth habit displaying a distinct upper and lower side
- Fruticose
- A type of lichen form characterized by a coral-like shrubby or bushy structure, attached only at the base, with little difference between the upper and lower branch/lobe surface
- Fungal
- Pertaining to fungi
- Host
- An animal or plant on or in which a parasite or commensal organism lives
- Hypha (pl. Hyphae)
- A microscopic filament of fungal cells
- Hymenium
- Structure within apothecia containing asci (spore producing structure) and sterile fungal hyphae to maintain form
- In situ
- Activities occurring on-site or in the field
- In vitro
- performed outside of an organism’s normal biological context
- Isidia
- Small vegetative propagules on the upper surface of a lichen covered with cortex and assisting with vegetative reproduction
- Lobe
- A branch or division in the lichen thallus
- Mafic
- Silicate dominated rock formed through the cooling of lava
- Mesic
- Habitat containing a moderate amount of water
- Micrometre (μm)
- Unit of length equaling one millionth of a metre
- Mycobiont
- A fungal partner in a lichen symbiosis
- Mycorrhizal
- Fungi growing in symbiotic association with plant roots
- Pannarin
- Lichen metabolite isolated from several species
- Perispore
- Gelatinous layer surrounding the spore
- Photobiont
- The photosynthetic partner in a lichen, either a green alga or a cyanobacterium
- Propagation
- Reproduction by any number of natural or artificial means
- Propagule
- A structure for reproductive dispersal, either sexual (for example, ascospore) or asexual/vegetative (for example, soredia, isidia)
- Prothallus
- Weft of dense fungal hyphae lacking photobiont projecting beyond the thallus margin onto the substrate, typically different in colour from the thallus
- Soredium (pl. Soredia)
- Small vegetative propagules on the upper surface of a lichen that contain fungal hyphae and alga but are not covered by cortex
- Species at Risk Act (SARA)
- The federal legislation that provides protection to species at risk in Canada. This Act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
- Squamulose
- Small, scale-like thalli, appearing intermediate between foliose and crustose growth forms
- Species at Risk in Ontario (SARO) List
- The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
- Terricolous
- Growing on soil
- Thalline margin
- The margin around an apothecium containing algae or cyanobacteria which is coloured like the thallus
- Thallus (pl. Thalli)
- The vegetative body of a lichen consisting of a fungus and alga and/or cyanobacteria
- Triterpenes
- Secondary metabolites synthesized through chemical transformations within lichens
List of abbreviations
- AOC
- Area of Concern
- CANL
- National Herbarium of Canada Lichen Collection
- CFSA
- Ontario’s Crown Forest Sustainability Act, 1994
- CNALH
- Consortium of North American Lichen Herbaria
- COSEWIC
- Committee on the Status of Endangered Wildlife in Canada
- COSSARO
- Committee on the Status of Species at Risk in Ontario
- CRO
- Conditions on Regular Operations
- CWS
- Canadian Wildlife Service
- ELC
- Ecological Land Classification
- ESA
- Ontario’s Endangered Species Act, 2007
- FMU
- Forest Management Units
- ISBN
- International Standard Book Number
- MECP
- Ministry of the Environment, Conservation and Parks
- NDMNRF
- Ministry of Northern Development, Mines, Natural Resources and Forestry
- MNRF
- Ministry of Natural Resources and Forestry
- NHIC
- Natural Heritage Information Centre
- PP
- Provincial Park
- SARA
- Canada’s Species at Risk Act
- SARO List
- Species at Risk in Ontario List
- US
- United States (of America)
References
Allen, J. L., R. T. McMullin, E. A. Tripp, and J. C. Lendemer. 2019. Lichen Conservation in North America: A Review of Current Practices and Research in Canada and the United States.
Biodiversity and Conservation.
Alstrup, V., and S. N. Christensen. 2006. New Records of Lichens with Cyanobacteria from Tanzania and Kenya.
Cryptogamie, Mycologie 27 (1): 57–68.
Antoine, M. E. 2004. An Ecophysiological Approach to Quantifying Nitrogen Fixation by Lobaria Oregana.
The Bryologist 107 (1): 82–87.
Bannan, M. W. 1941a. Variability in Wood Structure in Roots of Native Ontario Conifers.
Bulletin of the Torrey Botanical Club 68 (3): 173–94.
Bannan, M. W. 1941b. Wood Structure of Thuja Occidentalis.
Gazette 103 (2): 295–309.
Banton, E., and G. Racey. 2009. Draft Boreal Ecosite Factsheets.
Thunder Bay, ON.
Bartemucci, P., Lilles, E., and Y. Gauslaa. 2022. Silvicultural strategies for lichen conservation: Smaller gaps and shorter distances to edges promote recolonization.
Ecosphere 13(1).
Brinker, S. R., M. Garvey and C. D. Jones. 2018. Climate change vulnerability assessment of species in the Ontario Great Lakes Basin. Ontario Ministry of Natural Resources and Forestry, Science and Research Branch, Peterborough, ON. Climate Change Research Report CCRR-48. 85 p. + append.Brinker, S. R. 2020. Contributions to the Ontario Flora of Lichens and Allied Fungi, with Emphasis on the Great Lakes Basin.
Opuscula Philolichenum 19: 58–157.
Brodo, I., S. D. Sharnoff, and S. Sharnoff. 2001. The Lichens of North America. Edited by Yale University Press.
Cameron, R., T. Neily, and H. Class. 2013. Forest Harvesting Impacts on Mortality of an Endangered Lichen at the Landscape and Stand Scale.
Canadian Journal of Forest Research 43: 507–11.
Carey, J. H. 1993. Thuja Occidentalis.
Fire Effects Information System. 1993.
Coder, K. 2013. Tree Risk and Hazard Assessment Concepts.
Chen, J., Franklin, J. F., and T. A. Spies. 1995. Growing-Season Microclimatic Gradients from Clearcut Edges into Old-Growth Douglas-Fir Forests.
Ecological Applications 5(1), 74–86.
Consortium of North American Lichen Herbaria. 2022. Site Records for Fuscopannaria leucosticta.
2022.
COSEWIC. 2019. COSEWIC Assessment and Status Report on the White-rimmed Shingle Lichen (Fuscopannaria leucosticta) in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 85 pp.
Culberson, W. L. 1972. Disjunctive Distributions in the Lichen-Forming Fungi.
Annals of the Missouri Botanical Garden 59 (2): 165–73.
Day, S. D., P. E. Wiseman, S. B. Dickinson, and J. R. Harris. 2010. Contemporary Concepts of Root System Architecture of Urban Trees.
Arboriculture and Urban Forestry 36 (4): 149–59.
Ezhkin, A. K., and Yoshihito Ohmura. 2021. Notes to Pannariaceae Species in Taiwan.
Taiwania 66 (4): 575–79.
Fite, K., and E. T. Smiley. 2008. Managing Trees during Construction. Champaign, Illinois: International Society of Arboriculture.
Flato, G., and G. J. Boer. 2001. Warming asymmetry in climate change simulations.
Geophysical Research Letters 28: 195-198.
Gauslaa, Y., Bartemucci, P., and K. A., Solhaug. 2019. Forest edge-induced damage of cephalon- and cyanolichens in northern temperate rainforests of British Columbia.
Canadian journal of forest research 49(5), 434–439.
Gehlhausen, S. M., Schwartz, M. W., and C. K. Augspurger. 2000. Vegetation and Microclimatic Edge Effects in Two Mixed-Mesophytic Forest Fragments.
Plant Ecology 147(1), 21–35.
Government of Canada. 2022. National Pollutant Release Inventory: sulphur dioxide.
Goward, T., and A. Arsenault. 2000. Cyanolichen Distribution in Young Unmanaged Forests: A Dripzone Effect?
Bryologist 103 (1): 28–37.
Greenmantle Forest Inc. 2019. Summary of the Long-Term Management Direction for the 2020-2030 Lakehead Forest Management Plan.
Greenmantle Forest Inc. 2022. Lakehead Forest 2022/2023 Annual Work Schedule.
Harris, R., J. Clark, and N. Matheny. 2004. Arboriculture: Integrated Management of Landscape Trees, Shrubs, and Vines. 4th ed. Upper Saddle River, New Jersey: Prentice Hall.
Harris, R. C. 2004. A Preliminary List of the Lichens of New York.
Opuscula Philolichenum 1: 55–74.
Haughian, S. R., S. R. Clayden, and Robert Cameron. 2018. On the Distribution and Habitat of Fuscopannaria Leucosticta in New Brunswick, Canada.
Ecoscience 26 (2): 99–112.
Hinds, J. W., and P. L. Hinds. 2007. The Macrolichens of New England. New York, USA: The New York Botanical Garden Press.
Holien, H., G. Gaarder, and A. Håpnes. 1995. Erioderma Pedicellatum Still Present, but Highly Endangered in Europe.
Graphis Scripta 7: 79–84.
Johnston, W. F. 1990. Thuja Occidentalis L. (Northern White Cedar).
In Silvics of North America, edited by R. M. Burns and B. H. Honkala, 1:580–89. Washington: U.S. Department of Agriculture, Forest Service.
Jørgensen, P. M. 2000. Survey of the Lichen Family Pannariaceae on the American Continent, North of Mexico.
The Bryologist 103 (4): 670–704.
Jørgensen, P. M., and Z. Palice. 2010. Additions to the Lichen Family Pannariaceae in Ecuador.
Nordic Journal of Botany 28 (5): 623–28.
Jørgensen, P. M., and H. J. M. Sipman. 2007. The Lichen Fuscopannaria Leucosticta (Tuck.) P. M. Jørg. Found in the Tropics.
Lichenologist 39 (3): 305–7.
Jüriado, I., Liira, J. and D. Csencsics. 2011. Dispersal ecology of the endangered woodland lichen Lobaria pulmonaria in managed hemiboreal forest landscape. Biodivers Conserv 20, 1803–1819.
Knight, T. 2019. Recovery Strategy for the Golden-Eye Lichen (Teloschistes chrysophthalmus) - Great Lakes Population in Ontario. Peterborough, Ontario: Ministry of the Environment, Conservation and Parks.
Krause, C., and Lemay, A. 2022. Root adaptations of black spruce growing in water-saturated soil.
Canadian journal of forest research 52(5), 653–661.
Lee, H. T. 2008. Southern Ontario Ecological Land Classification: Vegetation Type List.
Lee, H. T., W. D. Bakowsky, J. Riley, J. Bowles, M. Puddister, P. Uhlig, and S. McMurray. 1998. Ecological Land Classification for Southern Ontario: First Approximation and its Application.
Lendemer, J. C., Keepers, K. G., Tripp, E. A., Pogoda, C. S., McCain, C. M., and Kane, N. C.. 2019. A taxonomically broad metagenomic survey of 339 species spanning 57 families suggests cystobasidiomycete yeasts are not ubiquitous across all lichens.
American Journal of Botany 106( 8): 1090– 1095.
Lu, J., N. Magain, J. Miadlikowska, J. R. Coyle, C. Truong, and F. Lutzoni. 2018. Bioclimatic Factors at an Intrabiome Scale Are More Limiting than Cyanobiont Availability for the Lichen-Forming Genus Peltigera.
American Journal of Botany 105 (7): 1198–1211.
Maass, W., and D. Yetman. 2002. COSEWIC Assessment and Status Report on the Boreal Felt Lichen Erioderma pedicellatum in Canada. Committee on the Status of Endangered Wildlife in Canada.
Ottawa.
Magain, N., and E. Sérusiaux. 2014. Do Photobiont Switch and Cephalodia Emancipation Act as Evolutionary Drivers in the Lichen Symbiosis? A Case Study in the Pannariaceae (Peltigerales).
PLoS ONE 9 (2).
Musselman, R. C., D. T. Lester, and M. S. Adams. 1975. Localized Ecotypes of Thuja Occidentalis L. in Wisconsin.
Ecology 56 (3): 647–55.
Nash, T. H. 2008a. Lichen Biology. Edited by T.H. Nash. Cambridge, U.K.: Cambridge University Press.
Nash, T. H. 2008b. Lichen Biology - Nitrogen, Its Metabolism and Potential Contribution to Ecosystems.
In Lichen Biology, 216–33.
NSDNR. 2018. At-Risk Lichens – Special Management Practices.
Ontario Geological Survey. 2021. Bedrock Geology.
Ontario Ministry of Natural Resources and Forestry (OMNR). 2010. Forest Management Guide for Conserving Biodiversity at the Stand and Site Scales.
Toronto: Queen’s Printer for Ontario.
Ontario Ministry of Northern Development, Mines, Natural Resources, and Forestry (NDMNRF). 2019. Forest Management: Great Lakes and St. Lawrence Landscapes.
Ontario Ministry of Natural Resources and Forestry (MNRF). 2021. Ontario Forest Management Guide.
Ontario.
Ontario Ministry of Northern Development, Mines, Natural Resources, and Forestry (NDMNRF). 2021. Sustainable Forestry Licences.
Ontario Ministry of Northern Development, Mines, Natural Resources, and Forestry (NDMNRF). 2022. Available wood report.
Ontario Parks. 2007. Sleeping Giant Provincial Park Management Plan.
Ontario Parks. 2018. Quetico Provincial Park Management Plan.
Pearson, K., R. Cameron, and R. T. McMullin. 2018. Habitat Associations and Distribution Model for Fuscopannaria Leucosticta in Nova Scotia, Canada.
Lichenologist 50 (4): 487–97.
Perlmutter, G. 2005. Lichen Checklist for North Carolina, USA.
Evansia 22.
Racey, G. D., A. G. Harris, J. K. Jeglum, R. F. Foster, and G. M. Wickware. 1996. Terrestrial and Wetland Ecosites of Northwestern Ontario.
Vol. FG-02.
Resolute FP Canada Inc. 2021. Forest Management Plan for the Black Spruce Forest.
Resolute FP Canada Inc. 2022. Black Spruce Forest Management Plan AWS 2022-2023 Harvest Index Map.
Richardson, D. H. S., and R. P. Cameron. 2004. Cyanolichens: Their Response to Pollution and Possible Management Strategies for Their Conservation in Northeastern North America.
Northeastern Naturalist 11 (1): 1–22.
Schumacher, C., and B. Ashcraft. 2021. Ohio Bryology et Lichenology, Identification, Species, Knowledge Newsletter of the Ohio Moss and Lichen Association
18 (1).
Seaward, M., and M. A. Letrouit-Galinou. 1991. Lichen Recolonization of Trees in the Jardin Du Luxembourg, Paris.
Lichenologist 23: 181–86.
Sims, R. A., H. M. Kershaw, and G. M. Wickware. 1990. The Autecology of Major Tree Species in the North Central Region of Ontario. Ontario Ministry of Natural Resources.
Sims, R. A., W. D. Towill, K. A. Baldwin, and G. M. Wickware. 1989. Field Guide to the Forest Ecosystem Classification for Northwestern Ontario.
Thunder Bay, ON: Forestry Canada and Ontario Ministry of Natural Resources.
Spribille, T. 2009. Fuscopannaria leucosticta (Tuck.) P.M. Jørg. Rediscovered in Europe.
The Lichenologist 41 (2): 209–10.
United States Department of Agriculture. 2017. A Guide to Identifying, Assessing, and Managing Hazard Trees in Developed Recreational Sites of the Northern Rocky Mountains and the Intermountain West.
Wedin, M., E. Wiklund, P. M. Jørgensen, and S. Ekman. 2009. Slippery When Wet: Phylogeny and Character Evolution in the Gelatinous Cyanobacterial Lichens (Peltigerales, Ascomycetes).
Molecular Phylogenetics and Evolution 53 (3): 862–71.
Wester, M., P. Uhlig, W. Bakowsky, and E. Banton. 2015. Great Lakes-St. Lawrence Ecosite Fact Sheets.
Sault Ste. Marie,ON.
Wetmore, C. M. 2009. “Lichens Collected in the Brule River State Forest, Wisconsin.”
Wisconsin Natural Heritage Program. 2021. Wisconsin Natural Heritage Working List.
Wylie, R. 1928. Bruce Fink Lichenologist.
Mycologia 20 (1): 1–2.
Personal communications
Bakowsky, W. 2022. Email correspondence with J. Consiglio and T. Knight. January 2022. Provincial Botanist, Natural Heritage Information Centre.
Brinker, S. 2022. Telephone and email correspondence with J. Consiglio and T. Knight. January 2022. Provincial Botanist, Natural Heritage Information Centre.
Darby, L. 2022. Telephone correspondence with J. Consiglio and T. Knight. January 2022. Regional Planning Ecologist, Ministry of Northern Development, Mines, Natural Resources and Forestry.
Haughian, S. 2022. Telephone correspondence with J. Consiglio. January 2022. Botany Curator and Postdoctoral Fellow, Nova Scotia Museum and St. Mary’s University.
Kinsman, D. 2022. Telephone correspondence and correspondence through second draft comments with J. Consiglio and T. Knight. January 2022. Forest Management Species at Risk Biologist, Ministry of Northern Development, Mines, Natural Resources and Forestry.
Kruzick, K. 2022. Telephone correspondence with J. Consiglio and T. Knight. January 2022. Regional Planning Ecologist, Ministry of Northern Development, Mines, Natural Resources and Forestry.
McMullin, R.T. 2022. Telephone and email correspondence with J. Consiglio. January 2022. Research Scientist, Canadian Museum of Nature.
Thayer, J. 2022. Email correspondence with J. Consiglio. May 2022. Minnesota Lichen Survey.