Riverine Clubtail Recovery Strategy
This document is the recovery strategy for a species at risk – the Riverine Clubtail.

About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species’ persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. There was a transition period of five years (until June 30, 2013) to develop recovery strategies for those species listed as endangered or threatened in the schedules of the ESA. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of Natural Resources and Forestry Species at Risk webpage.
Recommended citation
Julia J. Mlynarek. 2015. Recovery Strategy for the Riverine Clubtail (Stylurus amnicola) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources and Forestry, Peterborough, Ontario. v + 22 pp.
© Queen’s Printer for Ontario, 2015
ISBN 978-1-4606-5721-8
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n’est disponible qu’en Anglais en vertu du Règlement 411/97 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec Michelle Collins au ministère des Richesses naturelles au
Authors
Julia J. Mlynarek – University of New Brunswick
Acknowledgments
The author thanks the collection managers of the Canadian National Collection (Dr. Owen Lonsdale), the Royal Ontario Museum (Brad Hubley), the Lyman Entomological Museum and Research Laboratory (Dr. Terry Wheeler), Ouellet-Robert Collection (Louise Cloutier) and Colin Jones, Ontario Ministry of Natural Resources and Forestry, for specimen record data. I thank the reviewers for comments on the first drafts of the strategy.
Declaration
The recovery strategy for the Riverine Clubtail was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all of the individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
- Ontario Ministry of Natural Resources and Forestry
- Environment Canada – Canadian Wildlife Service
Executive summary
The Riverine Clubtail (Stylurus amnicola) is a dragonfly in the family Gomphidae, commonly referred to as the clubtails. The genus Stylurus, commonly called the hanging clubtails, differ from other clubtails in that they have relatively short hind legs and, when perched, typically “hang” vertically from vegetation with their abdomen pointing downwards. Most other clubtails typically perch horizontally on the ground or upon vegetation and have longer hind legs.
Its distribution ranges from Georgia and Louisiana to southern Manitoba and Quebec (although it is quite localized in portions of this range) and from the eastern coast of North America to eastern Nebraska and Manitoba. There are three main populations of the Riverine Clubtail in Canada: Boreal (Quebec), Great Lakes Plains (Ontario) and Prairie (Manitoba). The Great Lake Plains population, which occurs in Ontario, has been assessed as endangered by COSEWIC and COSSARO, and is currently listed as endangered under the Endangered Species Act, 2007. Within Ontario, this species has only been collected at three localities: Big Creek and Big Otter Creek, two tributaries of Lake Erie, and Aux Sables River in Chutes Provincial Park. The Riverine Clubtail was first recorded in the summer of 1999 at Big Otter Creek.
There are knowledge gaps in knowing and understanding the threats for this species. However, it is believed that the main threats to the survival of the Riverine Clubtail are habitat loss and degradation, pesticides, road mortality, invasive/introduced species and climate change.
The goals of the Recovery Strategy for the Riverine Clubtail are to ensure a viable, self- sustaining population in Ontario and maintain the Riverine Clubtail’s existing range of occurrence in Ontario. The objectives of the Recovery Strategy are to:
- protect, maintain and, where appropriate, enhance the quantity and quality of existing Riverine Clubtail habita
- increase knowledge of Riverine Clubtail biology in Ontario including distribution, abundance, life history and habitat need
- reduce and mitigate threats to the Riverine Clubtail and its habitat.
It is recommended that the streams currently occupied by the Riverine Clubtail, previously-inhabited streams with suitable habitat, and select habitat surrounding such streams extending inland 200 metres (the typical distance the dragonflies travel between reproductive and roosting habitats) be prescribed as habitat under Ontario’s Endangered Species Act, 2007.
1.0 Background information
1.1 Species assessment and classification
- Common name: Riverine Clubtail
- Scientific name: Stylurus amnicola
- SARO List Classification: Endangered
- SARO List History: Endangered (2014)
- COSEWIC Assessment History: Endangered (2012) - Great Lakes Plains (Ontario)
- SARA Schedule 1: No Schedule, No Status
- Conservation status rankings:
- GRANK: G4
NRANK: N3
SRANK: S1
- GRANK: G4
The glossary provides definitions for the abbreviations above and for other technical terms in this document.
1.2 Species description and biology
Species description
The Riverine Clubtail (Stylurus amnicola) is a dragonfly in the family Gomphidae, commonly referred to as the clubtails. Species of the family Gomphidae, including the Riverine Clubtail, can be recognized because their eyes do not meet at the top of their head (Dunkle 2000, Mead 2009) and most have a widening at the end of the abdomen known as the club (Paulson 2012). The Riverine Clubtail is one of the smallest members of its genus Stylurus measuring between 4.3 and 5.2 cm in length, from tip of head to tip of abdomen (Walker 1958). The Riverine Clubtail has turquoise-coloured eyes and a pale face with dark lines along the sutures. The male of this species is mostly black with distinctive yellow stripes on the thorax; the pattern on the back of the thorax is diagnostic of the species because of its unique three-pointed star (COSEWIC 2012). The females are very similar to the males, with the same pattern on the back but with somewhat more extensive yellow on the abdomen and paler stripes on the thorax. The club of the Riverine Clubtail is among the widest and most boldly marked of its genus (Mead 2009). The Riverine Clubtail can be confused with the Black-shouldered Spinyleg (Dromogomphus spinosus) because of the colours on the thorax, although the Riverine Clubtail is smaller with shorter legs, or the Elusive Clubtail (Stylurus notatus), but the thoracic pattern is different.
Dragonfly larvae (also known as nymphs) are difficult to identify to species and should be verified by an expert odonatologist. The larvae of the Riverine Clubtail tend to be smaller than most species of its genus measuring between 2.8 and 2.9 cm in length (Walker 1958) but can be confused with other species of the genus. They are slender, pale brown with the head as wide as the abdomen (Walker 1958). The most diagnostic characters are the abdominal segments evenly taper from the thorax to the tip and the very hairy legs (Walker 1958).
Species biology
There is little information about the biology of the Riverine Clubtail. There are no known scientific studies on this species and all information is derived from direct observation during specimen collection or from our understanding of closely related species, like Laura’s Clubtail (Stylurus laurae), which inhabit the same types of habitats. Laura’s Clubtail is listed as endangered under Ontario’s Endangered Species Act, 2007 (Pulfer et al. 2011). Most of the following information is based on the COSEWIC (2012) report for the Riverine Clubtail and on its similarities to Laura’s Clubtail (P.M. Catling, pers. comm. 2014).
As its name suggests, the Riverine Clubtail is present in a variety of riverine habitats. The life cycle of the Riverine Clubtail consists of three stages: egg, larva and adult. It is unknown how many eggs are laid by females or the timing of egg laying. Little is also known about egg development other than females deposit them in the current of the shallow, fast-flowing areas of open streams or rivers (Corbet 1999). Larvae develop in the fine sand and silt substrates in slow to moderate flow streams and rivers (Walker 1958, Needham et al. 2000). The development time of the larva has not yet been determined but based on the biology of other Clubtail species the larval stage probably lasts two or more years (COSEWIC 2012). Larvae of certain European members of the genus Stylurus take three to four years to develop (Corbet 1999). During their larval stage in the water, the main predators of Riverine Clubtail are likely other dragonfly larvae, tadpoles, fish and waterbirds. Adults emerge in late June to early July. As they emerge in adult form, the exuviae (cast off larval skin) are left behind, attached to vegetation surrounding the stream. The young adult dragonflies can fly an unknown distance away from the stream and into the surrounding forest habitat to avoid predation until their exoskeleton hardens in about 24 hours (Corbet 1999). At this stage they are particularly vulnerable to predation. Once they are sexually mature, they return to the stream where they rest on the leaves of trees surrounding the streams or rivers, looking for flying prey or mates. Males can sometimes be seen cruising swiftly over the stream looking for females (Catling and Brownell 1999). The main predators of adult Riverine Clubtail are likely other dragonflies and birds (Corbet 1999, COSEWIC 2012). Based on Walker’s (1958) assessment of U.S. populations of Riverine Clubtail, adults of this species are generally in flight from the start of July to sometime in August. It is not known what time(s) of day adults are most active.
Riverine Clubtails are believed to be generalist predators, as are most dragonflies. As larvae, dragonflies feed on aquatic invertebrates. As adults, dragonflies are primarily predators of flying insects, with Riverine Clubtails likely hunting prey either above the water or in the surrounding forest habitat (COSEWIC 2012).
1.3 Distribution, abundance and population trends
Riverine Clubtail is one of the most northerly distributed species of the Stylurus genus. It occurs throughout eastern North America, extending from South Carolina to southern Ontario and from the eastern coast of North America (Robert 1963) to eastern Nebraska (Figure 1, Abbott 2014). In some parts of its range, as in Ontario, there are only sparse records (Figure 2) so it is unknown whether the species exists in other areas.
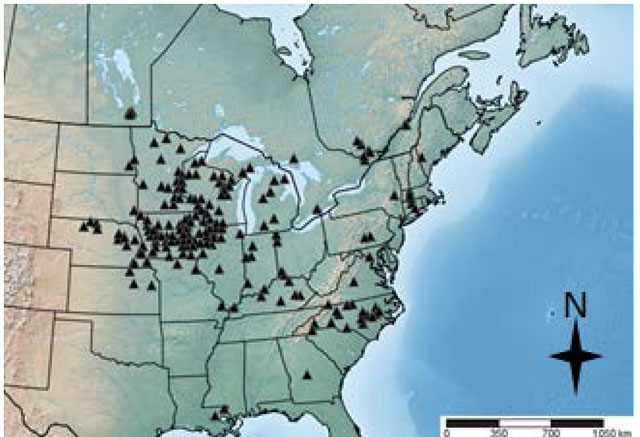
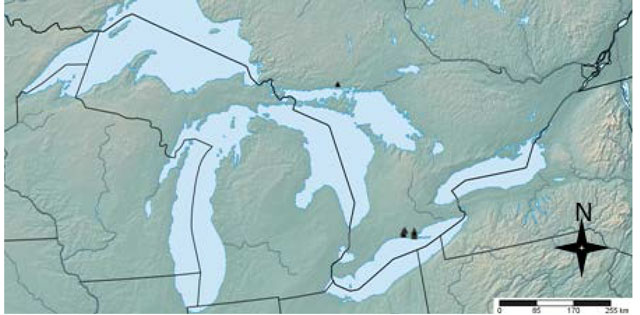
The global population size of the Riverine Clubtail is estimated at 2,500 to 10,000 individuals (NatureServe 2014). The global population trend is believed to be stable at this time (Paulson 2009). The Riverine Clubtail is considered rare in the northern states of the United States adjacent to Ontario (Paulson and Dunkle 1999, Paulson and Dunkle 2009).
There are three populations of the Riverine Clubtail in Canada (COSEWIC 2012). The population size and trend for the Canadian populations is unknown. Additionally, too little is known about the other two Canadian populations, the Prairie population in Manitoba and the Boreal population in Quebec, to allow conservation assessment, thus they are currently considered data deficient (COSEWIC 2012). The Ontario population is known as the Great Lakes Plains population.
The Riverine Clubtail from the Great Lakes Plains population was first recorded in Ontario at Big Creek in 1999 (Catling and Brownell 1999, Pratt 1999). Since that time it has been recorded fewer than one hundred times along two major tributaries of Lake Erie (Figure 2) in Elgin and Norfolk Counties: Big Otter Creek and Big Creek (Catling and Brownell 1999, COSEWIC 2012, Natural Heritage Information Centre 2014) and a single specimen was collected in Chutes Provincial Park, North of Lake Huron, in July 2014, but more were observed (B. Korol pers. comm.). The population trends in Ontario are currently unknown (COSEWIC 2012).
One of the challenges of observing the Riverine Clubtail is its habit of perching on the leaves of high branches of trees surrounding the flowing water body from which it has emerged (Mead 2009). The height of its perch can result in the species being rarely observed or documented.
1.4 Habitat needs
The Riverine Clubtail, like other dragonflies, requires an aquatic environment for its larval stage and a terrestrial environment for its adult stage. Overall this species requires sandy- or silty-bottomed streams with continuous vegetation along the river bank (Walker 1958). The first time Riverine Clubtails were documented in Ontario at Big Otter Creek, they were flying 30 cm above the water surface and flew into the surrounding vegetation (Catling and Brownell 1999). The creek at that location was sandy-bottomed, shallow (0.5 - 1 m) and clear with a fairly rapid flow (Catling and Brownell 1999). The habitat where the Riverine Clubtail was collected from the Chutes Provincial Park was very similar to those of Big Otter Creek and Big Creek: vegetated shoreline and sandy bottom stream. The characteristics of these sites are representative of the preferred habitat of the Riverine Clubtail throughout its global distribution (K. Mead, pers. comm.).
As a larva, the Riverine Clubtail requires streams or rivers with sandy bottoms in which to burrow for protection against predators. Clubtail larvae tend to move away from very shallow water and into deeper pools for protection from predators (Corbet 1999).
Freshly emerged adults (i.e., tenerals) require trees and shrubs as perching locations within 200 m of the stream for about 24 hours, the time it takes for their exoskeletons to harden (Corbet 1999), during which time adults are poor fliers and are vulnerable to predation (Paulson 2012). Sexually mature male and female Riverine Clubtails perch high in the tree canopy on broad leaves along the shore of the stream to bask and to find prey. Males additionally find mates by patrolling the tree canopies. The Riverine Clubtail requires large-leaved vegetation and would not use anthropogenic areas such as croplands or pastures. They can also be seen coasting above riffles in the water catching insect prey (Catling and Brownell 1999).
In general, Riverine Clubtails require slow to fast flowing streams or rivers that are wide enough so that the canopy does not completely cover the width of the stream (Catling et al. 1999). The streams generally have fast flowing areas in which adults lay their eggs and wider stretches with slower moving water, such as pools in the streams, where larvae can develop (Catling et al. 1999). The Riverine Clubtails prefer stream habitats with a mix of slow and fast (e.g. riffle forming) moving water. Information on home range size, foraging distances, and several other aspects of movement behaviour are not yet known for this species.
1.5 Limiting factors
The Riverine Clubtail requires a specific combination of habitat characteristics, wide stream with thicket or wooded riparian vegetation, which is uncommon in southern Ontario. Dragonflies will, depending on their size, disperse on average 200 m from where they emerged to sexually mature (Corbet 1999, Rouquette and Thompson 2007, Keller et al. 2010). Smaller dragonflies, those with smaller wings, tend to disperse shorter distances than larger dragonflies because the wing aspect ratio is greater in smaller dragonflies, which means that they have to expend more energy to disperse greater distances (McCauley 2013). This distance is also needed for sexually mature adults of this genus to move between breeding grounds and roosting grounds (Pulfer et al. 2011). It is unlikely that local populations could be supplemented by immigration if the Riverine Clubtail were extirpated from these two sites. Dispersal between local populations of Riverine Clubtail may be restricted due to the limited availability of continuous suitable aquatic and terrestrial habitat. The northern range limit of the Riverine Clubtail may be restricted by water temperature, although this is uncertain.
1.6 Threats to survival and recovery
We do not know with certainty what threatens the Riverine Clubtail in Ontario, or the impact of those threats. Therefore, potential threats based on expert opinion and other closely related species with similar life histories and overlapping ranges (e.g. Laura’s Clubtail) are presented (P.M. Catling pers. comm., Pulfer et al. 2011).
Habitat loss and degradation
Dragonflies can be good indicators of environmental health in aquatic habitats e.g. whether the water is clean for their survival (Corbet 1999). Fluctuations in pH, dissolved oxygen, temperature and nutrients could result in habitat being uninhabitable by the Riverine Clubtail if the dragonflies or their prey cannot survive the new concentrations or their rate of change (COSEWIC 2012). Dragonflies, such as the Riverine Clubtail, are believed to be sensitive to changes in water quality, habitat loss and degradation through excessive human alterations that reduce the suitability of habitat (Samways and Steytler 1996).
The Riverine Clubtail is currently reported from areas where its habitat is threatened by various types of development (e.g. road maintenance, wood cutting, and damming activities around streams). These activities can alter water quality, temperature, flow rate, depth, and increase sedimentation (i.e. more particles falling to the stream bottom; Williams et al. 1999, Helmreich et al. 2010), which could hinder the development of the immature stages of the Riverine Clubtail (COSEWIC 2012).
Damming of streams is a potential threat to the Riverine Clubtail. Damming and surrounding agricultural use can change flow rate, water depth and sedimentation, resulting in alteration of the preferred shallow, medium to fast flowing water needed for egg laying by Riverine Clubtail (Catling and Brownell 1999). Upstream of a dam, water flow is slowed and depth is increased, resulting in conditions that might not be suitable for females to lay their eggs in, and silt accumulation could result in a lack of oxygen to developing larvae. Downstream of a dam, flow is controlled which may be detrimental to Riverine Clubtail larvae if they are not able to adjust to frequently changing water speed and depth. Riverine Clubtails inhabit areas downstream of the dam on Big Creek and downstream of the three dams on Big Otter Creek. There are no dams at the location of the Chutes population. Drawdown of water levels from agricultural uses may also pose a threat in a similar way to dams.
Pesticides and other toxins
This species seems to be quite tolerant to pollution as the agricultural runoff levels exceed the Canadian guidelines in Big Creek and Big Otter Creek (COSEWIC 2012). Even though the tolerance level for pH, dissolved oxygen and temperature are unknown for the Riverine Clubtail, pollution from runoff and other sources could threaten the larval stage of the Riverine Clubtail by promoting eutrophication, which could exceed their tolerance levels, and decreasing dissolved oxygen. Use of pesticides and especially insecticides on surrounding agricultural lands and golf courses can have an effect on the reproductive success of the Riverine Clubtail. Neonicotinoids, such as imidacloprid, are of increasing concern to the conservation of insects in North America (Pisa et al. 2014). Neonicotinoid pesticides are widely used in Ontario agriculture, and can leach into local water. Neonicotinoids can alter water chemistry in a way that makes it less habitable by some aquatic invertebrates (Morrissey et al. 2015). Neonicotinoids can reduce dragonfly larval survival, emergence into adults, and abundance of prey insects (Jinguji et al. 2013, Van Dijk et al. 2013).
Various other pollutants negatively affect dragonflies (Johnson 1991, Campero et al 2007, Van Gossum et al. 2009). Dragonflies are sensitive to copper exposure and they bioaccumulate cadmium, lead, copper and other heavy metals (Tollett et al. 2009). Dragonflies can also bioaccumulate pharmaceuticals including antihistamines (De Lange et al. 2006, Jonsson et al. 2014). Although insecticides, such as organochlorines, may not have as strong an effect on dragonflies as they do on other insects, the abundance of prey may be reduced so dragonflies will not have enough food (Brewer and Atchison 1999). Phosphorous is also of concern in many Ontario streams, where levels often exceed provincial objectives (Ontario Ministry of the Environment 2013). Phosphorous can reduce the diversity of benthic invertebrates (Ontario Ministry of the Environment 2013). The levels of pesticides are continuously changing as their popularity changes with time (Stone et al. 2014), and their full effects on the Riverine Clubtail remain unknown.
Road mortality
Road mortality affects dragonflies much more than other insects because of their daily movements and low flight behavior (Riffel 1999, Soluk et al. 2011). Young adult dragonflies, in general, move away from the water during their pre-reproductive stage and return to the water later once they are ready to mate (Corbet 1999). If roads run parallel to streams at a distance of less than one kilometer in some places, as they do near Big Creek and Big Otter Creek, the Riverine Clubtail will have to frequently cross the road to go between the stream and forest. This frequent movement increases the risk of road mortality if there are many roads in the surrounding areas. While flying low above the road, dragonflies are at an increased risk of getting hit by passing vehicles although it is currently unknown how high this species flies above the road. If they do not die right away, they may be disoriented and get hit by a subsequent vehicle (Riffel 1999). As urban and agricultural development increase in the areas surrounding current Riverine Clubtail habitat, so will the number of roads, which may increase rates of collision.
Invasive and introduced species
Invasive and introduced species can either alter the habitat [e.g. Zebra Mussel (Dreissena polymorpha) and Kudzu (Pueraria lobata)] or be novel predators [e.g. Round Goby (Neogobius melanostomus), Rusty Crayfish (Orconectes rusticus)] to the Riverine Clubtail. The effects of these invasive and introduced species on the Riverine Clubtail are unknown (COSEWIC 2012) because there have been no known studies to directly compare the effects of invasive and introduced species on the Riverine Clubtail.
Zebra Mussels can alter habitat by changing water chemistry, water clarity, and species composition (Bulté et al. 2012). Kudzu is an invasive vine spreading northward from the United States and present on the shore of Lake Erie (Waldron and Larson 2012), which can cover and choke shorelines and riparian vegetation (OFAH/OMNR 2012). Kudzu could be an issue if its leaves are too dense for the Riverine Clubtails to perch on and because dragonfly prey are often less abundant in habitats dominated by invasive plants (Litt et al. 2014). If these invasive species are present in Riverine Clubtail habitat, they may indirectly affect Riverine Clubtail survival.
Other predatory invasive/introduced species (Round Goby, Rusty Crayfish) may be present in the streams surrounding the Great Lakes and can potentially impact egg or larval stages of the Riverine Clubtail through predation (Jude 2001, Cox and Lima 2006, Gunderson 2008). Dragonfly larvae bury themselves in the sandy bottom to hide from predators, but if they do not recognize an invasive species as a predator they will not hide and will be prone to higher predation pressure (Polis et al. 1989). It may require the consumption of naïve individuals for a species to evolve the ability to recognize and cue in on a novel predator such as Round Goby or Rusty Crayfish (Wisenden et al. 1997).
Invasive and introduced species that have the potential to threaten the Riverine Clubtail should be assessed regularly as they become established in areas identified as habitat for the Riverine Clubtail, especially the streams where the Riverine Clubtail is currently known to occur.
1.7 Knowledge gaps
There are gaps in our knowledge of the Riverine Clubtail. The factors affecting the distribution of this species are very poorly understood for Ontario populations. Knowledge gaps for this species may hinder efforts to protect it. Research on the following knowledge gaps would contribute to a more complete understanding of the threats to the Riverine Clubtail and the effectiveness of protection and recovery of the species and its habitat.
- General natural history and ecology information for the Riverine Clubtail.
- Tolerance of each life stage to environmental changes (e.g. sensitivity to pesticides and flow changes).
- Population sizes of known Riverine Clubtail population
- Distribution within Ontario.
- Extent of road mortality.
- Effect of invasive and introduced species on the Riverine Clubtail.
2.0 Recovery
2.1 Recovery goal
The recovery goal for the Riverine Clubtail is to ensure viable, self-sustaining populations in Ontario by increasing our knowledge of the Riverine Clubtail and maintaining and, where appropriate, enhancing the quality of existing Riverine Clubtail habitat.
2.2 Protection and recovery objectives
Table 1. Protection and recovery objectives
[Table 1 has been converted into a list]
- Protect, maintain and, where appropriate, enhance the quantity and quality of existing Riverine Clubtail habitat.
- Increase knowledge of Riverine Clubtail biology in Ontario including distribution, abundance, life history and habitat needs.
- Reduce threats to Riverine Clubtail and its habitat.
2.3 Approaches to recovery
Table 2. Approaches to recovery of the Riverine Clubtail in Ontario
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Ongoing | Protection and Stewardship |
1.1 Protect known terrestrial and aquatic Riverine Clubtail habitats through:
|
Threats:
|
| Beneficial | Long-term | Stewardship, Education and Outreach | 1.2 Develop, implement and support education and stewardship programs at known sites of Riverine Clubtail. |
Threats:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Ongoing | Monitoring, Assessment and Research | 2.1 Report observations of Ontario dragonflies for inclusion in the Ontario Odonata Atlas Database and the Natural Heritage Information Centre (NHIC). |
Knowledge gaps:
|
| Critical | Ongoing | Monitoring, Assessment and Research |
2.2 Monitor the Riverine Clubtail.
|
Knowledge gaps:
|
| Critical | Short-term | Research |
2.3 Carry out research on the biology of the Riverine Clubtail to determine aspects of its natural history and ecology including:
|
Knowledge gaps:
|
| Beneficial | Short-term | Monitoring and Research | 2.4 Sequence Riverine Clubtail genes to allow identification of the species in DNA-based monitoring programs. |
Knowledge gaps:
|
| Beneficial | Short-term | Research |
2.5 Investigate the sensitivity of Riverine Clubtail to anthropogenic factors.
|
Threats:
Knowledge gaps:
|
| Beneficial | Short-term | Research | 2.6 Investigate the sensitivity of Riverine Clubtail to introduced and invasive species. |
Knowledge gaps:
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Long-term | Stewardship and Protection |
3.1 Work with local partners (municipalities and conservation authorities) and the Ministry of Environment and Climate Change to monitor water quality of Riverine Clubtail habitat.
|
Threats:
|
| Beneficial | Ongoing | Education and outreach |
3.2 Work with partners to develop an outreach strategy to mitigate and prevent the spread of invasive species:
|
Threats:
|
| Beneficial | Short-term | Research | 3.3 Quantify the threat of road mortality to the Riverine Clubtail and, if appropriate, explore tactics for mitigation. |
Threats:
|
Supporting narrative
It is recommended that recovery efforts for the Riverine Clubtail be coordinated with the recovery efforts for Laura’s Clubtail (Stylurus laurae) where occurrences overlap since they share similar habitats and threats (Pulfer et al. 2011).
2.4 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of Natural Resources and Forestry on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the author will be one of many sources considered by the Minister when developing the habitat regulation for this species.
The Riverine Clubtail requires both freshwater and terrestrial habitats to complete its life cycle. Even though further research is required to document its distribution, dispersal, population size, and life history, it is recommended that the area prescribed as habitat in a habitat regulation include the locations occupied by the species or those that were occupied by the species but still might be re-colonized because of suitable habitat.
Aquatic habitat suitability should be assessed according to the following guidelines, and extend up to the high water mark.
- Riffles (important for adult egg-laying and male mate-finding flights).
- Pools below riffles (important for egg and larval growth) with a depth of 60 cm for larval growth (P.M. Catling pers. comm.). It should be noted that areas deeper than 60 cm can be used as movement habitat for eggs or larvae just passing through.
It is recommended that regulated habitat under the ESA include the following terrestrial features.
- Up to 30 m of natural vegetation inland to: 1) maintain river quality (Sweeney and Newbold 2014); and 2) allow teneral dragonflies to find refuge near emergence sites (c.f. Eastern Sand Darter; Fisheries and Oceans Canada 2012).
- Broad-leaved vegetation such as trees, shrubs and thickets extending inland 200 m (the typical distance dragonflies travel between reproductive and roosting habitats; Corbet 1999) used for: 1) foraging; 2) roosting; and 3) reproduction habita Coniferous vegetation should be excluded from habitat protection since Riverine Clubtails are not known to perch on conifers.
Should additional occupied areas be found in the future, habitat should automatically be prescribed under the ESA. It is also recommended that the area prescribed as habitat for the species is re-evaluated as new information is gathered, given that there are extensive knowledge gaps.
Additionally, it is important to acknowledge that the health of the entire watershed, especially upstream, could have an effect on river life downstream (Sweeney and Newbold 2014). An activity that occurs beyond the area of regulated habitat, and has the potential to adversely affect the regulated habitat, may require authorization under the ESA.
Glossary
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC)
- The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
- Committee on the Status of Species at Risk in Ontario (COSSARO)
- The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
- Conservation status rank
-
A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperilled
2 = imperilled
3 = vulnerable
4 = apparently secure
5 = secure - Endangered Species Act, 2007 (ESA)
- The provincial legislation that provides protection to species at risk in Ontario.
- Exuviae
- Cast-off skins or coverings. For the Riverine Clubtail, refers to the cast off covering of the dragonfly larva, shed after the larva emerges from the water to molt into the adult life stage.
- Larva (pl. larvae)
- The immature form of an insect that is active and differs greatly from the adult form.
- Odonata
- The taxonomic order comprising dragonflies and damselflies.
- Nymph
- A larva of an insect that resembles the adult somewhat but needs to transform into the adult form to breed.
- Riffles
- Areas of relatively fast, turbulent flow, where the water’s surface is typically broken.
- Riparian
- Terrestrial area directly adjacent to a water body.
- Species at Risk Act (SARA)
- The federal legislation that provides protection to species at risk in Canada. This act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
- Species at Risk in Ontario (SARO) List
- The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
- Thorax
- Division of an animal’s body that lies between the head and the abdomen.
- Teneral
- The period when the adult insect is newly emerged from its larval skin. During the teneral period, the insect’s exoskeleton has not hardened or darkened, leaving it vulnerable to predators.
References
Abbott, J.C. 2014. OdonataCentral: An online resource for the distribution and identification of Odonata. Web site: http://www.odonatacentral.org. [accessed July 21, 2014].
Brewer, S.K. and G.J. Atchison. 1999. The effects of chlorpyrifos on cholinesterase activity and foraging behaviour in the dragonfly, Anax junius (Odonata). Hydrobiologia 394:201-208.
Bulté G., S.A. Robinson, D.J. Marcogliese and M.R. Forbes. 2012. Is there such things as a parasite free lunch? Ecohealth 9:6-16.
Campero, M., S. Slos, F. Ollevier and R. Stoks. 2007. Sublethal pesticide concentrations and predation jointly shape life history: behavioral and physiological mechanisms. Ecological Applications 17:2111–2122.
Catling, Paul M. pers. comm. 2014. Email personal communication to J. Mlynarek. July 2014.
Catling, P.M. and V.R. Brownell. 1999. Riverine Clubtail (Stylurus amnicola) new to Ontario. Argia 11:9-10.
Corbet, P.S. 1999. Dragonflies: Behavior and ecology of Odonata. Cornell University Press. NY. 829 pp.
COSEWIC. 2012. COSEWIC assessment and status report on the Riverine Clubtail Stylurus amnicola in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xiv + 60 pp.
Cox, J.G. and S.L. Lima. 2006. Naiveté and an aquatic-terrestrial dichotomy in the effects of introduced predators. Trends in Ecology and Evolution 21:674-680.
Crespo, J.G. 2010. A review of chemosensation and related behavior in aquatic insects. Journal of Insect Science 11:1-39.
De Lange, H.J., W. Noordoven, A.J. Murk, M. Lürling and E.T.H.M. Peeters. 2006. Behavioural responses of Gammarus pulex (Crustacea, Amphipoda) to low concentrations of pharmaceuticals. Aquatic toxicology 78:209-216.
Dunkle, S.W. 2000. Dragonflies Through Binoculars. Oxford University Press, Oxford. 206 pp.
Fisheries and Oceans Canada. 2012. Recovery strategy for the Eastern Sand Darter (Ammocrypta pellucida) in Canada: Ontario populations. Species at Risk Act Recovery Strategy Series, Fisheries and Oceans Canada, Ottawa. vii + 58 pp.
Gunderson, J. 2008. Rusty Crayfish: A Nasty Invader. Minnesota Sea Grant. http://www.seagrant.umn.edu/ais/rustycrayfish_invader
Helmreich, B., R. Hilliges, A. Schriewer and H. Horn. 2010. Runoff pollutants of a highly trafficked urban road – correlation analysis and seasonal influences. Chemosphere 80:991-997.
Jinguji, H., D.Q. Thuyet, T. Uéda and H. Watanabe. 2013. Effect of imidacloprid and fibronil pesticide application on Sympetrum infuscatum (Libellulidae: Odonata) larvae and adults. Paddy and Water Environment 11:277-284.
Johnson, D.M. 1991. Behavioral ecology of larval dragonflies and damselflies. Trends in Ecology and Evolution 6:8-13.
Jonsson, M., J. Fick, J. Klaminder and T. Brodin. 2014. Antihistamines and aquatic insects: bioconcentration and impacts on behavior in damselfly larvae (Zygoptera). Science of the Total Environment 472:108-111.
Jude, D.J. 2001. Round and Tubenose Gobies: 10 Years with the Latest Great Lakes Phantom Menace. Dreissena 11(4):1-14.
Keller, D., S. Brodbeck, I. Flöss, G. Vonwil and R. Holderegger. 2010. Ecological and genetic measuremements of dispersal in a threatened dragonfly. Biological Conservation 143:2658-2663.
Litt, A.R., E.E. Cord, T.E. Fulbright and G.L. Schuster. 2014. Effects of invasive plants on arthropods. Conservation Biology 28(6):1532-1549.
McCauley, S.J. 2013. Relationship between morphology, dispersal, and habitat distribution in three species of Libellula (Odonata: Anisoptera). Aquatic Insects 34:195-204.
Mead, Kurt. pers. comm. 2014. Email personal communication to J. Mlynarek. December 2014.
Mead, K. 2009. Dragonflies of the North Woods. Kollath and Stensaas Publishing, Duluth, Minnesota. 193 pp.
Morrissey, C.A., P. Mineau, J.H. Devries, F. Sanchez-Bayo, M. Liess, M.C. Cavallaro and K. Liber. 2015. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environment International 74:291-303.
NatureServe. 2014. NatureServe Explorer: An online encyclopedia of life [web application]. Version 7.1. NatureServe, Arlington, Virginia. Website: http://explorer.natureserve.org. [accessed July 16, 2014].
Needham, J.G., M.J. Westfall Jr. and M.L. May. 2000. Dragonflies of North America. Scientific Publishers, Gainesville, Florida. 939 pp.
OFAH/OMNR Invading species awareness program. 2012. Kudzu. Retrieved from http://www.invadingspecies.com. [accessed October 25, 2014].
Ontario Ministry of the Environment. 2013. Water Quality in Ontario 2012 Report. 92 pp. Website: /environment-and-energy/water-quality-ontario-report-2012. [accessed 12 December, 2014].
Paulson, D.R. 2009. Stylurus amnicola. The IUCN Red List of Threatened Species. Version 2014.1. Website: www.iucnredlist.org. [accessed on 08 July 2014].
Paulson, D.R. 2012. Dragonflies and Damselflies of the East. Princeton University Press, Princeton. 544 pp.
Paulson, D.R. and S.W. Dunkle. 1999. A Checklist of North American Odonata. Slater Museum of Natural History, University of Puget Sound Occasional Paper, 56: 86 pp. Website: http://www.ups.edu/x7015.xml [link inactive] [accessed July 8, 2014].
Paulson, D.R. and S.W. Dunkle. 2009. A checklist of North American Odonata including English name, etymology, type locality, and distribution. Originally published as Occasional Paper No. 56, Slater Museum of Natural History, University of Puget Sound, June 1999; completely revised March 2009. Online. Website: http://www.odonatacentral.org/docs/NA_Odonata_Checklist_2009.pdf. [accessed July 9, 2014].
Pisa, L.W. et al. 2015. Effects of neonicotinoids and fipronil on non-target invertebrates. Environmental Science and Pollution Research 22(1):68-102.
Polis, G.A., C.A. Myers and R.D. Holt. 1989. The ecology and evolution of intraguild predation: potential competitors that eat each other. Annual Review of Ecology and Systematics 20:297-330.
Pratt, P.D. 1999. Regional lists of Ontario Odonata. Website: www.netcore.ca/prairie/odonata.html. [accessed July 16, 2014]. [link inactive]
Pulfer, T.L., C. Bahlai and L. Mousseau. 2011. Recovery Strategy for Laura’s Clubtail (Stylurus laurae) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. v + 23 pp.
Riffel, S.K. 1999. Road mortality of dragonflies (Odonata) in a Great Lakes wetland. The Great Lakes Entomologist 32:63-74.
Robert, A. 1963. Les libellules du Québec. Ministère du transport, de la chasse et de la pêche, Province du Québec. 223 pp.
Rouquette, J.R. and D.J. Thompson. 2007. Patterns of movement and dispersal in an endangered damselfly and the consequences for its management. Journal of Applied Ecology 44:692–701.
Samways, M.J. and N.S. Steytler. 1996. Dragonfly (Odonata) distribution patterns in urban and forest lanscapes, and recommendations for riparian management. Biological Conservation 78:279-288.
Shorthouse, D.P. 2010. SimpleMappr, an online tool to produce publication-quality point maps. Website: http://www.simplemappr.net. [accessed July 29, 2014].
Soluk, D.A., D.S. Zercher and A.M. Worthington. 2011. Influence of roadways on patterns of mortality and flight behavior of adult dragonflies near wetland areas. Biological Conservation 144:1638-1643.
Stone, W.W., R.J. Gilliom, and J.D. Martin. 2014. An overview comparing results from two decades of monitoring for pesticides in the Nation’s streams and rivers, 1992–2001 and 2002–2011: U.S. Geological Survey Scientific Investigations Report 2014–5154, 23 pp., http://dx.doi.org/10.3133/sir20145154.
Sweeney, B.W., and J.D. Newbold. 2014. Streamside forest buffer width needed to protect stream water quality, habitat, and organisms: a literature review. Journal of the American Water Resource Association 50:560-584.
Tollett, V.D., E.L. Benvenutti, L.A. Deer and T.M. Rice. 2009. Differential toxicity toCd, Pb, and Cu in dragonfly larvae (Insecta: Odonata). Archives of Environmental Contamination and Toxicology 56:77-84.
Van Dijk, T.C., M.A. Van Staalduinen and J.P. Van der Sluijs. 2013. Macro-invertebrate decline in surface water polluted with imidacloprid. PLOS One 8:e62374.
Van Gossum, H., J. Bots, T. Snijkers, J. Meyer, S. Van Wassenbergh, W. De Coen and L. De Bruyn. 2009. Behaviour of damselfly larvae (Enallagma cyathigerum) (Insecta, Odonata) after long-term exposure to PFOS. Environmental Pollution157(4):1332-1336. doi:10.1016/j.envpol.2008.11.031.
Waldron, G.E. and B.M.H. Larson. 2012. Kudzu Vine, Pueraria montana, adventive in southern Ontario. Canadian Field-Naturalist 126(1):31–33.
Walker, E.M. 1958. The Odonata of Canada and Alaska. Volume II, Part III, The Anisoptera of Canada and Alaska. University of Toronto Press. 318 pp.
Williams, D.D., N.E. Williams and Y. Cao. 1999. Road salt contamination of groundwater in a major metropolitan area and development of a biological index to monitor its impact. Water Resources 34:127-138.
Wisenden, B.D., D.P. Chivers and R.J.F. Smith. 1997. Learned recognition of predation risk by Enallagma damselfly larvae (Odonata, Zygoptera) on the basis of chemical cues. Journal of Chemical Ecology 23(1):137-151.