Barn Swallow Recovery Strategy
This document advises the ministry on ways to ensure healthy numbers of the Barn Swallow, a threatened or endangered species, return to Ontario.
Barn Swallow (Hirundo rustica) in Ontario
Ontario Recovery Strategy Series
Recovery strategy prepared under the Endangered Species Act, 2007
Ministry of Natural Resources and Forestry
2014

About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species' persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. There was a transition period of five years (until June 30, 2013) to develop recovery strategies for those species listed as endangered or threatened in the schedules of the ESA. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of Natural Resources and Forestry Species at Risk webpage at: www.ontario.ca/speciesatrisk
Recommended citation
Heagy, A., D. Badzinski, D. Bradley, M. Falconer, J. McCracken, R.A. Reid and K. Richardson. 2014. Recovery Strategy for the Barn Swallow (Hirundo rustica) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources and Forestry, Peterborough, Ontario. vii + 64 pp.
© Queen’s Printer for Ontario, 2014
ISBN 978-1-4606-3079-2 (PDF)
Content (excluding the cover illustration) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n'est disponible qu'en Anglais en vertu du Règlement 411/97 qui en exempte l'application de la Loi sur les services en français. Pour obtenir de l'aide en français, veuillez communiquer avec Michelle Collins au ministère des Richesses naturelles et des Forêts au
Authors
Audrey Heagy Bird – Studies Canada, Port Rowan, ON
Debbie Badzinski – Bird Studies Canada, Ottawa, ON
David Bradley – Bird Studies Canada and University of Guelph, Guelph, ON
Myles Falconer – Bird Studies Canada, Port Rowan, ON
Jon McCracken – Bird Studies Canada, Port Rowan, ON
Ron Reid – Bobolink Enterprises, Washago, ON
Kristyn Richardson – Bird Studies Canada, Port Rowan, ON
Acknowledgments
Funding for the preparation of this recovery strategy was provided by the Ontario Ministry of Natural Resources and Forestry (OMNRF) through a contract to Bird Studies Canada (BSC). Many thanks to Vivian Brownell (OMNRF) for overseeing project delivery.
We would like to thank all of the people who participated in the technical workshops held at Guelph, Ontario on 10 December 2012 and 12 August 2013. Their names and affiliations are as follows:
Amelia Argue – Ontario Ministry of Natural Resources and Forestry
Natalie Boyd – Ontario Ministry of Transportation
Kyle Breault – Tallgrass Ontario
Chris Brown – Ontario Ministry of Transportation
Vivian Brownell – Ontario Ministry of Natural Resources and Forestry
Jennifer Brownlee – Ontario Ministry of Transportation
Mike Cadman – Environment Canada
Anthony Chegahno – Neyaashiinigming First Nation
Glenn Desy Ontario – Ministry of Natural Resources and Forestry
Nancy Furber Haldimand – Bird Observatory
Cathy Giesbrecht – Ontario Ministry of Transportation
Jenny den Hartog – Christian Farmers Federation of Ontario
Gail Jackson Ontario – Ministry of Natural Resources and Forestry
Peter Jeffery – Ontario Federation of Agriculture
Leanne Jennings – Ontario Ministry of Natural Resources and Forestry
Nicole Kopysh – Canadian Wind Energy Association
Rod Krick – Credit Valley Conservation
Zoé Lebrun-Southcott – Bird Studies Canada, Barn Swallow Project Biologist
Barb Macdonnell – Ontario Ministry of Transportation
Hannah MacIver – Environmental Consultant
Rob MacIver – Ontario Field Ornithologists
Megan McAndrew – Ontario Ministry of Natural Resources and Forestry
Lisa Myslicki – Infrastructure Ontario
Michael Patrikeev – Parks Canada, Bruce Peninsula National Park
Mark Peck – Royal Ontario Museum
Sarah Plant – Ontario Ministry of Agriculture, Food and Rural Affairs
Bill Read – Ontario Eastern Bluebird Society
Chris Risley – Ontario Ministry of Natural Resources and Forestry
Peter Roberts – Ontario Ministry of Agriculture, Food and Rural Affairs
Antonio Salvadori – Ontario Bird Banding Association (Wellington County Barn Swallow banding project)
Larry Sarris – Ontario Ministry of Transportation Andrew Sawyer Bruce Peninsula Bird Observatory Adrian Sgoifo Ontario Ministry of Transportation John Small Ontario Ministry of Transportation
Paul Smith – Ontario Ministry of Agriculture, Food and Rural Affairs
Sean Spisani – Environmental Consultant
Nathan Stevens – Christian Farmers Federation of Ontario
Bridget Stutchbury – York University
Sandra Turner – Ruthven Park
Mary Young – Ontario Ministry of Transportation
In addition, the authors would like to acknowledge the technical information and assistance provided by David Bell, Peter Blancher (Environment Canada, Partners in Flight), Graham Bryan (Environment Canada), Andrew Couturier (Bird Studies Canada), Marie-Anne Hudson (Environment Canada), Adam Smith (Environment Canada), Ron Tozer (Algonquin Park) and Richard A. Wolinksi (Michigan Department of Transportation).
Declaration
The recovery strategy for the Barn Swallow was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all of the individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ontario Ministry of Natural Resources and Forestry
Environment Canada – Canadian Wildlife Service
Parks Canada Agency
Executive summary
The Barn Swallow (Hirundo rustica) is a medium-sized migratory songbird found in open country habitats. The most abundant and widespread swallow in the world, this familiar species breeds in temperate regions across North America, Europe and Asia, and overwinters in Central and South America, southern Africa, and southern and southeast Asia. Throughout its range, it is found in close association with human populations. Six subspecies of Barn Swallow are recognized but only one subspecies, H.r. erythrogaster, breeds in North America. Due to population declines across the northern portion of its North American breeding range, the Barn Swallow is listed as threatened under Ontario’s Endangered Species Act, 2007 (ESA) and has been designated as threatened in Canada by COSEWIC. The Barn Swallow is one of many diverse species of common aerial-foraging insectivorous birds that are of conservation concern in Ontario, Canada and the northeastern United States due to long-term population declines for a combination of reasons that are not well understood.
This species breeds throughout Ontario but over 90 percent of the provincial population (ca. 350,000 individuals) is concentrated in southern Ontario, south of the Canadian Shield. Its distribution in northern Ontario is localized, being closely associated with roads and human settlements and largely absent in more remote areas. Since 1970 the Ontario Barn Swallow population has declined at an average annual rate of 2.56 percent, amounting to a cumulative loss of 66 percent. The rate of decline over the most recent 10-year period is similar to that since 1970.
Barn Swallow habitat needs include foraging habitat, nest sites and nests and nocturnal roost sites. Across Ontario Barn Swallows forage over a wide range of open country habitats including farmland, lakeshore and riparian habitats, road right-of-ways, clearings in wooded areas, parkland, urban and rural residential areas, wetlands and tundra. Barn Swallow nests in Ontario are commonly situated inside or outside of buildings, under bridges and wharves and in road culverts. A small portion of the population nests on cliff faces. Outside of the breeding season swallows congregate nightly in communal roosts. Roost sites in Ontario are often associated with marshes or shrub thickets in or near water.
Numerous factors have been proposed as possible explanations for the recent declines in Barn Swallows and other aerial insectivores. The information needed to critically evaluate known and potential threats to Barn Swallows in Ontario is generally lacking. Many knowledge gaps must be addressed in order to understand the most significant threats to this species' survival. It is likely that multiple direct and indirect threats at various stages (and locations) in its life cycle along with population fluctuations due to natural processes, are having an additive or synergistic impact on Barn Swallow populations.
Known and potential direct and indirect threats affecting reproduction and survival include: (1) loss of nest site habitat, (2) loss or degradation of foraging habitat, (3) environmental contaminants, pesticides and pollution, (4) reduced productivity due to predation, parasitism and persecution, (5) habitat loss, disturbance and human persecution at roost sites, and (6) climate change and severe weather.
Lack of understanding of the causes of the population decline hampers recovery planning. Key knowledge gaps that must be addressed to focus recovery efforts include: (1) vital rates and population source/sink dynamics, (2) diet and food supply, (3) habitat use, requirements and trends in Ontario, (4) wintering and migration habitat and ecology, (5) best management practices, and (6) climate change effects.
The recovery goal is to maintain a stable, self-sustaining population of Barn Swallow in Ontario by 2034 (within 20 years) and to slow the rate of decline over the next 10 years. The recovery objectives identified in this strategy are to:
- fill key knowledge gaps to increase understanding of the nature and significance of threats to Ontario Barn Swallow populations and the biological and socio-economic factors that may impede or assist recovery efforts;
- maintain or improve nesting productivity through the development of appropriate practices and policies for managing Barn Swallow nests, nest sites and associated foraging habitat in Ontario;
- promote stewardship, education and increased public awareness of the Barn Swallow in Ontario;
- identify and protect important roost sites used by Barn Swallows in Ontario; and
- inventory, monitor and report on the state of Barn Swallow breeding populations and habitats in Ontario and elsewhere to track the progress of recovery activitie
Approaches to recovery focus on research to address key knowledge gaps and also a range of short-term activities designed to maintain, and where possible enhance the reproductive output of the breeding population.
It is recommended that until key knowledge gaps are addressed, habitat for Barn Swallow in Ontario be defined narrowly as follows:
- nests (including unused nests) on natural or human-created nest sites during the current breeding season (between May 1 and August 31) plus the area within 1.5 m of the nest and the openings the birds use to access nests in enclosed situations;
- all used nests at any nest site that has been occupied by Barn Swallows within the previous three breeding seasons; and
- significant roost sites that are used regularly by at least 5,000 birds (approximately 1% of Ontario’s breeding population, adjusted for young-of-the-year) during the post-breeding season (July 1 through October 31).
1.0 Background information
1.1 Species assessment and classification
Common name (population):
Barn Swallow
Scientific name:
Hirundo rustica
SARO List Classification:
Threatened
SARO List History:
Threatened (2012)
COSEWIC Assessment History:
Threatened (2011)
SARA Schedule 1:
No Status
Conservation status rankings:
GRANK: G5
NRANK: N4N5B
SRANK: S4B
The glossary provides definitions for technical terms, including the abbreviations above.
1.2 Species description and biology
Species description
The Barn Swallow (Hirundo rustica) is a medium-sized songbird (length: 15-18 cm, mass: 17-20 g) with a long forked tail. Adults have steely-blue upperparts, cinnamon underparts, and a chestnut throat and forehead. Plumage of the sexes is similar but the elongated outer tail feathers are particularly noticeable on adult males (outer tail feather length 79-106 mm in males versus 68-84 mm in females; Pyle 1997).
This species can be distinguished readily in all plumages and ages from other North American swallows by its deeply forked tail, the white spots on the inner webs of the tail feathers and extensive cinnamon underparts (Godfrey 1986, Brown and Brown 1999a).
Six subspecies of Barn Swallow are recognized in the world and can be visually distinguished by subtle differences in plumage characteristics. Only one subspecies, H.r. erythrogaster, breeds in North America (Brown and Brown 1999a).
Species biology
Many aspects of Barn Swallow biology have been intensively studied, particularly the life history of the H.r. rustica subspecies, which breeds in Europe and winters in Africa. Caution is needed in applying the results of European studies to Barn Swallows in Ontario as there are some notable differences in the breeding biology of North American and European subspecies (Brown and Brown 1999a). For example, H.r. erythrogaster males participate in incubation whereas H.r. rustica males do not (Smith and Montgomerie 1991), and European birds can produce up to three broods per year, whereas there are no records of North American birds raising more than two broods in a year (Brown and Brown 1999a, Turner 2004). Also most breeding studies in Europe have focussed on birds nesting in dairy barns and other farm buildings where conditions may not be comparable to the range of breeding sites used in Ontario. In this recovery strategy, information for the North American subspecies is provided where available.
Food habits
The Barn Swallow is the archetypal member of the guild of aerial-foraging insectivorous birds characterized by their habit of feeding on insects while in flight. This familiar diurnal species is often observed feeding on insects flushed by grazing mammals, farm tractors, humans or flocks of other bird species (Brown and Brown 1999a). Occasionally, they land on the ground to pick up dead insects or pick insects from plants, buildings or water surfaces while in flight.
Barn Swallows forage individually or in small groups usually within 10 m of the ground in open areas (Brown and Brown 1999a). In cold weather Barn Swallows will sometimes forage in large numbers at ponds and lakes where the warmer water temperatures keep flying insects active (COSEWIC 2011).
During the breeding season most foraging takes place within 600 m of the nest site (Turner 2004). Information on foraging distances at other times of the year is currently not available.
Prey items consist almost entirely of flying insects. They also ingest grit, apparently to aid digestion of insects and possibly also for calcium (Brown and Brown 1999a). Diet reflects local insect availability with more than 80 insect families recorded as prey in European studies (Brown and Brown 1999a). Flies (Diptera) are the most common food item reported in North America along with beetles (Coleoptera), true bugs (Hemiptera), leafhoppers (Homoptera) and bees, wasps and ants (Hymenoptera; Brown and Brown 1999a). Barn Swallows often feed on single, large insects rather than on insect swarms (Brown and Brown 1999a).
Reproduction
Barn Swallows are usually socially monogamous but extra-pair paternity is common (Smith et al. 1991). Females typically first breed when one year old. Some yearling males may remain unpaired (Turner 2004). Breeding pairs are established after arrival on the breeding grounds. Pairs that nested successfully in the previous year often remain together (Shields 1984).
Barn Swallows will nest in solitary situations but are more frequently colonial with multiple pairs nesting on a single nesting structure (Brown and Brown 1999a). Barn Swallows defend a small territory (eight square metres or less in a European study) around their nest (Brown and Brown 1999a). Inter-nest distances in colonies are typically two to four metres but can be less than one metre apart if there is a visual obstruction between nests (Brown and Brown 1999a, Mercadante and Stanback 2011).
Nest record data indicate that colonies in Ontario range from 2 to 59 nests, with an average size of 10 nests (n=161) (Peck and James 1987). Colonies of up to 83 pairs have been reported elsewhere in Canada (Campbell et al. 1997).
The cup-shaped nests are made of mud pellets and lined with grasses and feathers (Brown and Brown 1999a). Nest construction starts soon after the birds return to their breeding grounds (Brown and Brown 1999a). Nest building takes 6 to 15 days (COSEWIC 2011). Old nests are often repaired and reused, which requires less time and effort than building a new nest (Brown and Brown 1999a, Turner 2006).
Egg dates for nests in Ontario range from 10 May to 21 August (Peck and James 1987). Egg-laying within a colony is largely asynchronous (Brown and Brown 1999a).
Clutch size is generally four or five eggs in Ontario ranging from one to seven (n=467) (Peck and James 1987). First clutches are larger than second clutches (Brown and Brown 1999a). Cowbird parasitism rates are negligible (4 of 3205 nests in Ontario; Peck and James 1987).
The incubation period is generally 13 to 14 days in Ontario (Peck and James 1987), and is performed mostly by the female (Smith and Montgomerie 1991). Eggs usually hatch within a 24 hour period (Brown and Brown 1999a).
Both parents feed nestlings, largely in equal proportions in North American subspecies (Brown and Brown 1999a). Young leave the nest when 19 to 24 days old (Brown and Brown 1999a) but may fledge prematurely by day 14 if handled or otherwise disturbed (Anthony and Ely 1976). Young are fed by their parents for up to a week after fledging and return to the nest at night for several days (Brown and Brown 1999a).
Second broods are common in Ontario and usually occur in the same nest (Peck and James 1987). The average interval between initiation of the first and second clutch is poorly understood but reported as about 51 days for birds in British Columbia (Campbell et al. 1997).
Nest success in this species is generally high (approximately 70-90% of nests fledge at least one young in Ontario and elsewhere), although ectoparasites, local predation or inclement weather conditions can negatively impact nest success (Turner 2004, Turner and Kopachena 2009, Salvadori 2009).
One Ontario study involving 20 pairs found that first broods had an average of 3.1 fledglings, 30 percent of the pairs laid a second clutch and the estimated annual reproductive success was 4.2 fledglings per pair (Smith and Montgomerie 1991). In contrast a Manitoba study at a site with high insect abundance found that 90 percent of pairs produced two broods and the annual reproductive success of 21 pairs was 6.9 fledglings per pair (Barclay 1988). Nest productivity, frequency of second broods, and annual reproductive rates reported elsewhere are also quite variable between sites and between years (Turner and Kopachena 2009). The average number of fledglings per pair per year ranged from 4.2 to 7.1 in studies from across the global range (Turner 2004).
Roosts
Outside of the breeding season Barn Swallows and other North American swallows forage widely during the day and congregate nightly in dense communal roosts (Winkler 2006). In Ontario, bird numbers at swallow roosts start to build up in July, peak in early to mid-August and are negligible by September (Clark 1984, Ross et al. 1984, Long Point Bird Observatory unpub. data).
These post-breeding roosts often include a mix of swallow species and it is difficult to accurately estimate the number of each species, which vary seasonally (Ross et al. 1984). Peak counts of up to 25,000 Barn Swallows have been reported in Ontario (Godwin 1995, Weir 2008). Counts of over 100,000 birds, including a mix of Barn Swallows and other swallow species, were reported at a swallow roost on a small island at Pembroke, Ontario that was occupied annually for more than 20 years and became the focus of an annual summer festival (Clark 1984, Ross et al. 1984, Ottawa River Legacy Landmark Partners n.d.).
Information on roosting behaviour and roost locations during migration and winter for Barn Swallows in the Americas is very limited. Radar data indicate that during migration periods swallow roosts in North America tend to be spaced about 100 to 150 km apart and that there is considerable variation in how consistently these migratory roost locations are used from year to year (Winkler 2006). Though they likely exist, no major Barn Swallow roost sites have yet been documented on their wintering grounds in Central or South America.
Extensive European banding studies show that the dynamics of Barn Swallow roosts vary seasonally with high rates of turnover of individuals during migration and longer stopover times during the post-breeding and wintering periods (Pilastro and Magnani 1997, Rubolini et al. 2002, Halmos et al. 2010, Coffait et al. 2011, Neubauer et al. 2012). Individual winter roosts in excess of one million birds (H.r. rustica) that are used annually have been reported at three sites in Africa (BirdLife International 2013a, b, c).
Migrations
Barn Swallows are long-distance migrants, flying more than 8,000 km between their breeding sites and wintering grounds. Long distance movements include a marked bird that moved 12,000 km in 34 days (320 km/d average) and another that moved 3,028 km in 7 days (433 km/d; Turner 2004).
Barn Swallows migrate during the day in loose flocks, often following coastlines or river valleys (Bent 1942). During migration swallows forage as they fly and shift from one nocturnal roost site to the next (Winkler 2006). They accumulate fat reserves prior to migration and are capable of extended flights across water and other ecological barriers (Pilastro and Magnani 1997, Pilastro and Spina 1999, Rubolini et al. 2002, Halmos et al. 2010, Coffait et al. 2011).
Most Barn Swallows depart Ontario by late September with small numbers present locally through October. In spring Barn Swallows return to southern Ontario starting in April with the main passage occurring in May. The frequency with which this species is reported on eBird checklists for Ontario (1900-October 2013; N=275,541) shows a bimodal distribution with a spring migration peak on 11 May (36.2% of checklists) and a late summer peak on 7 August (29.3% of checklists) (eBird, unpub. data 2013). The mass arrival and departure dates, calculated as the earliest and latest dates with 20 percent of the peak checklist frequency (Iliff et al. 2011) for Barn Swallows in Ontario are April 15 and September 11 respectively (M.Burrell, pers. comm. 2013). The wintering grounds and migration routes of the Barn Swallows that breed in Ontario are not known (see section 1.7 for current research project).
Relatively little is known about the phenology, movements and biology of the North American Barn Swallow population outside of the breeding season. The three foreign encounters of banded birds from the Ontario population have been in the United States. A bird banded in New Jersey on spring migration was reported the following year in Ontario and two birds banded in Ontario were subsequently encountered on fall migration in Louisiana and Massachusetts, respectively (Brewer et al. 2000).
Observations and band recoveries suggest that the bulk of the North American Barn Swallow population migrates through the Central American isthmus although trans-Gulf and trans-Caribbean migration routes have also been reported (Brown and Brown 1999a, Winkler 2006). In contrast there is considerable information on the migration routes and non-breeding biology of European Barn Swallows due to intensive roost banding projects such as the collaborative European Union for Bird Ringing (EURING) Swallow Project (Spina 2001).
Demography, site fidelity, and dispersal
Limited information is available on the demography, site fidelity and dispersal patterns for the Ontario and North American population compared to European birds as there have been few long-term, intensive Barn Swallow banding projects on this continent. The largest banding project in Ontario has focussed on Barn Swallows breeding in barns and other buildings at up to 21 locations in Wellington County since 2008 (Salvadori 2009, Salvadori et al. 2011).
The Barn Swallow is a short-lived species with most individuals living for less than four years (Turner and Rose 1989, Saino et al. 2012). The longevity record for North America is eight years, one month (Clapp et al. 1983, Lutmerding and Love 2012).
The age structure of the breeding population cannot be determined except in marked populations, as Barn Swallows undergo a complete moult in their first year and yearlings cannot be distinguished from older birds by plumage or other characteristics (Pyle 1997). Return rates of birds banded as adults at breeding sites vary considerably by year and location, ranging between 12 percent and 42 percent in North American studies (Brown and Brown 1999a).
Adults show very strong fidelity to breeding sites typically with more than 99 percent of returning adults using the same site and many returning to the same nest in consecutive years (Brown and Brown 1999a, Turner 2004, Safran 2004). However, Barn Swallow yearlings seldom return to their birthplace and few settle at nest sites within 30 km of their natal site (Shields 1984, Turner and Rose 1989, Brown and Brown 1999a, Balbontín et al. 2009a). Dispersal distances and survival rates for young birds cannot be estimated from local or regional banding studies because most birds banded as nestlings that survive and return as yearlings presumably settle at sites outside of the study area (Balbontín et al. 2009a).
The Wellington County, Ontario study (Salvadori 2009) reported that at least 103 of 493 (20.8 %) banded adults returned to the same nesting site in the following years (actual return rate is higher as not all adults were captured each year; A. Salvadori, pers. comm. 2012). One bird in this study returned for seven consecutive years (Salvadori 2009). Only one re-captured adult (0.12 %) changed sites between years (Salvadori 2009). Of 4,147 birds banded as nestlings at 21 locations in Wellington County, 29 (0.7%) were subsequently recaptured as breeding birds at the same location, and 5 (0.12%) returned to a different location within the study area (Salvadori 2009). These findings are generally consistent with similar banding studies done elsewhere.
A study of the effect of nest removal (Safran 2004) found that the site fidelity of returning adults was not affected by the removal of all old nests (i.e., no adults changed sites). However, this study did find a strong relationship between the number of old nests at a site at the start of the breeding season and the number of immigrants (yearling females) that settled at those sites, indicating that used nests serve as an important cue for yearling females selecting their initial breeding location (Safran 2004, 2007). Removal of old nests reduces the number of pairs using a nest site and also results in a higher proportion of experienced females at the site (as fewer yearling females are recruited).
Mean annual adult survival probability for birds nesting at a large colony in Nebraska was 0.350 ± 0.054 SE (n=300) (Brown and Brown 1999a) and 0.38 ± 0.13 SE in a New York study (Safran 2004). Annual adult apparent survival for 21 constant effort banding stations (Monitoring Avian Productivity and Survivorship network) from across North America was estimated at 0.483 ± 0.060 SE (DeSante and Kaschube 2009). Adult survival rates in long-term studies in Europe using mark-recapture analyses include 0.343 ± 0.028 SE for males and 0.338 ± 0.047 SE for females in a declining population in Denmark (Møller and Szép 2002), 0.404 ± 0.028 (all adults) in Britain (Robinson et al. 2008) and about 0.48 in Switzerland (Schaub and Hirschheydt 2008).
1.3 Distribution, abundance and population trends
Good information regarding the distribution, abundance and populations trends of Barn Swallows breeding in Ontario is available from the Breeding Bird Survey (Environment Canada 2013) and the first and second Ontario Breeding Bird Atlas projects (Cadman et al. 1987, Cadman et al. 2007).
Distribution
The Barn Swallow is one of the most widespread swallow species, breeding in temperate regions across the Northern Hemisphere (North America, Europe and Asia) and wintering primarily in Central and South America, southern Africa and southern and southeast Asia (Turner 2004). In North America this species breeds from southern Alaska east to southern Newfoundland and south through the United States and into central Mexico (Brown and Brown 1999a).
The current Barn Swallow breeding distribution is closely tied to that of the human population. It occurs throughout southern Ontario primarily south of the Canadian Shield (Figure 1). During the first and second Ontario Breeding Bird Atlases conducted during 1981 to 1985 and 2001 to 2005 respectively, the Barn Swallow was observed in nearly 100 percent of squares south of the Canadian Shield (Clark and Clark 1987, Lepage 2007). Although the Barn Swallow breeds as far north as the coast of Hudson Bay its distribution in northern Ontario is localized. The species is largely absent from remote sections of the far north, where no roads or human settlements exist.
The non-breeding range of the North American race of Barn Swallow extends from Mexico southwards throughout Central and South America (Howell and Webb 1995). The distribution of the Ontario breeding population within this very large non-breeding range is unknown.
Historical distribution
Prior to European settlement, the North American breeding distribution of this species was presumably restricted to areas with suitable natural nest sites particularly coastal and mountainous areas (Brown and Brown 1999a). There is a record of Barn Swallow nesting on longhouses (in British Columbia) dating back to the early 1800s (Macoun and Macoun 1904), and various sources have conjectured that Barn Swallows probably also nested in First Nations settlements in eastern North America (e.g., COSEWIC 2011).
Barn Swallows readily adapted to nesting on barns, bridges and other structures built by European settlers (Bent 1942). In addition the widespread clearing of forested lands for farming created suitable foraging habitat. Consequently, Barn Swallows have expanded their range into areas of North America where they did not occur formerly (Brown and Brown 1999a). This range expansion continued throughout the 20th century, most recently in the southeast United States where the population continues to increase (Robbins et al. 1986, Brown and Brown 1999a, Sauer et al. 2012).
In Ontario, the Barn Swallow has been reported since records were first kept (COSSARO 2011). This species was well established across southern Ontario prior to 1900 and was also reported as far north as the Hudson Bay coast (McIlwraith 1886, Macoun and Macoun 1904). Its distribution continued to expand in northern Ontario in the first half of the 20th century as it was able to colonize new settlements and infrastructure (Clark and Clark 1987, Lepage 2007).
Abundance
The Barn Swallow is the most abundant swallow species worldwide with a global population of some 120 million birds including 33 million birds in North America of which about 5 million breed in Canada (Partners in Flight Science Committee 2013). The population estimates for North America are derived largely from the Breeding Bird Survey data from 1998 to 2007 (Blancher et al. 2013). The global population estimate is extrapolated from the North American estimate based on the relative proportion of the global range (Blancher et al. 2013).
The Ontario Breeding Bird Atlas estimated the Ontario Barn Swallow population at 400,000 individuals during 2001 to 2005 (Blancher and Couturier 2007). This estimate is based on more than 50,000 point counts with good spatial coverage across the province and is therefore more representative than estimates based on Breeding Bird Survey data (Blancher and Couturier 2007, P. Blancher, pers. comm. 2013).
The Ontario population represents approximately one percent of the global population, and three percent of the North American population (Partners in Flight Science Committee 2013).
Factoring in the ongoing decline since the Breeding Bird Atlas estimate, the Ontario Barn Swallow population as of 2011 was estimated as less than 350,000 individuals (COSSARO 2011). During the post-breeding period the population is augmented by young-of-the-year (estimated 50% increase to approximately 500,000 individuals).
Caution is needed in interpreting and using these population estimates as the Breeding Bird Atlas estimates were "rough ballpark figures only" and "likely to be conservative" (Blancher and Couturier 2007, p. 655). These estimates should be considered approximations within an order of magnitude (P. Blancher, pers. comm. 2013).
The atlas data showed that 91 percent of the Ontario population was concentrated south of the Canadian Shield,in the Great Lakes/St. Lawrence Plain ecoregion (Cadman et al. 2007). Within this region the areas with the highest densities of Barn Swallows are: Stormont-Dundas-Glengarry County in the east, the northeastern shore of Lake Ontario, the southern part of Kawartha Lakes District and nearly all of southwestern Ontario (Figure 2).
These high density areas all have relatively high amounts of open country habitat (Figure 3). However, there is no obvious relationship between Barn Swallow abundance patterns and the distribution of particular open country land cover types such as pasture (see Figure 11 in Neave and Baldwin 2011, S. Richmond pers comm. 2013).
Figure 1. Breeding distribution of the Barn Swallow in Ontario in two time periods based on Breeding Bird Atlas data (see Cadman et al. 2007) Coloured squares indicate reported distribution during 2001-05. Black dots depict distributional losses, squares where Barn Swallow breeding evidence was recorded during 1981-1985, but not during 2001-2005
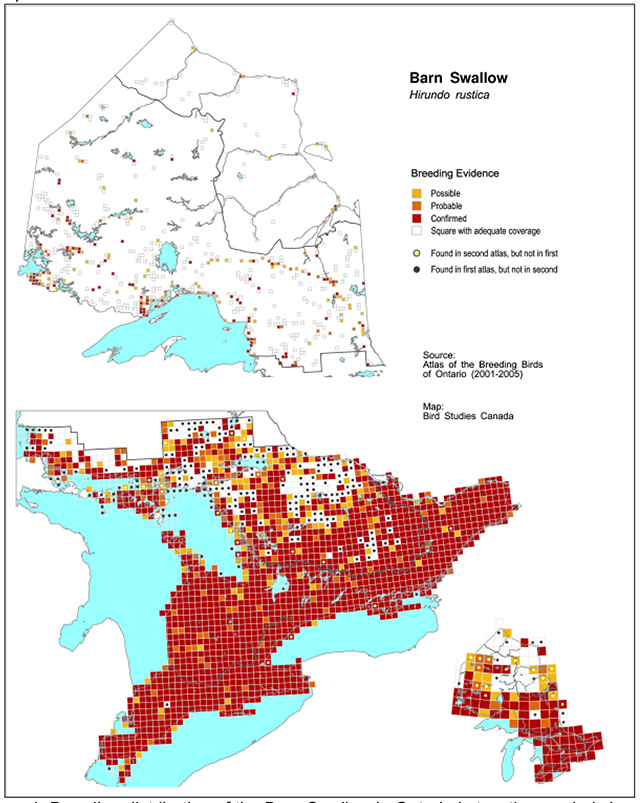
Enlarge Figure 1 - Breeding distribution of the Barn Swallow in Ontario
Figure 2. Relative abundance of the Barn Swallow in southern Ontario based on Breeding Bird Atlas point count data collected in 2001-2005 (Cadman et al. 2007). See inset map for abundance in northern Ontario. County boundaries are depicted for southern Ontario
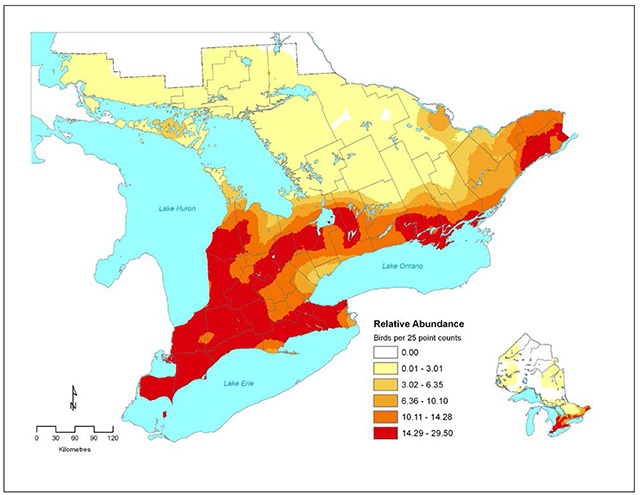
Enlarge Figure 2 - Relative abundance map
Figure 3. Total area in open country land cover classes (cropland, pasture and summer fallow) in southern Ontario during 2001 based on data from Statistics Canada (Neave and Baldwin 2011). Map courtesy of Graham Bryan, Environment Canada
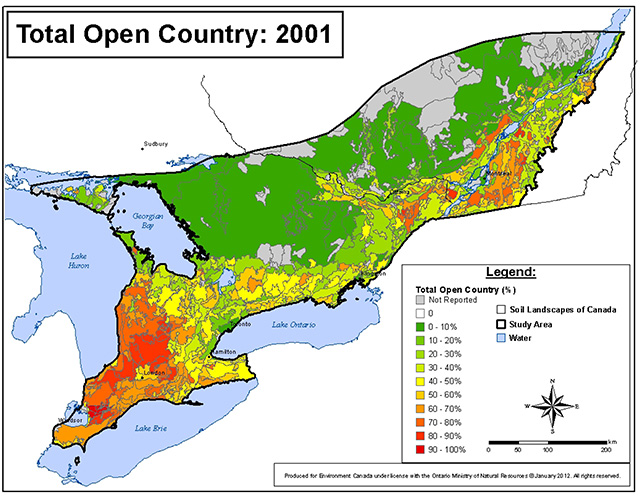
Enlarge Figure 3 - Total area map
In Europe agricultural land use, particularly livestock farming, has been shown to be a strong influence on the distribution of Barn Swallows (Møller 2001, Ambrosini et al. 2002, Evans et al. 2007, Ambrosini et al. 2011a, Ambrosini et al. 2012). There is a general spatial correlation between Barn Swallow abundance and cattle densities in southern Ontario but the pattern is not consistent (Figure 4), with some areas of high Barn Swallow abundance having few cattle (e.g., Essex County) and vice versa (e.g., Regional Municipality of Ottawa-Carleton).
Figure 4. Abundance of cattle (dairy and beef combined) in municipal boundaries of Ontario during 2006. Map is courtesy of Ontario Ministry of Agriculture and Food (P. Smith, pers. comm. 2012) based on data from Statistics Canada 2006 Census of Agriculture
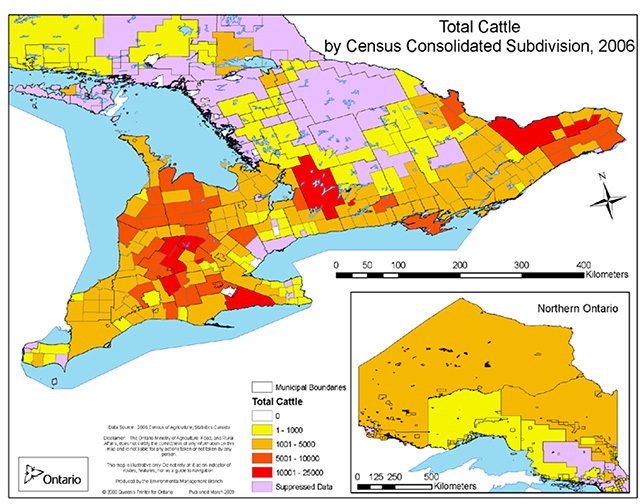
Enlarge Figure 4 -Abundance of cattle
Population trends
Historically Barn Swallow populations were likely limited by the availability of natural nest sites (Holroyd 1975, Erskine 1979, Brown and Brown 1999a). Populations in heavily forested regions were also constrained by the availability of open country habitats for foraging.
Numbers of Barn Swallows in Ontario and other parts of North America undoubtedly increased following the arrival of European settlers due to the large increase in available anthropogenic nesting sites (Brown and Brown 1999a, COSEWIC 2011). However, in many settled areas populations may have peaked a century ago as agricultural modernization has generally resulted in conditions that are less favourable for this species (Bent 1942).
Good population trend data are available for much of Ontario and North America for the past four decades through the North American Breeding Bird Survey and Ontario Breeding Bird Atlas projects (Cadman et al. 1987, Cadman et al. 2007, Environment Canada 2013).
From 1970 to 2012, the Barn Swallow population in Canada declined by 80 percent (-3.74% annually) according to Breeding Bird Survey data (Table 1; Environment Canada in prep.). In Ontario, the annual rate of decline over this period is estimated at 2.56 percent, amounting to a cumulative loss of 66 percent (see Figure 5 and Table 1; Environment Canada 2013). Barn Swallow populations declined over this period in all regions of Ontario for which data are available (Table 1), with steeper declines in the Ontario portion of Bird Conservation Region (BCR) 12 (see Figure 6, equivalent to the southern Canadian Shield region) compared to the Ontario portion of BCR 13 (Great Lakes/St. Lawrence Plain region).
The probability of observation of Barn Swallows in Ontario declined significantly between the first (1981-1985) and second (2001-2005) Breeding Bird Atlas periods (-35% overall; Lepage 2007). On a regional scale, the probability of observation declined the most in the northern and southern Canadian Shield regions by 51 percent and 32 percent respectively. The probability of observation declined by seven percent in the Lake Simcoe-Rideau region but no change was detected in the Carolinian region where Barn Swallows continued to be reported as breeding in almost every square. No significant change was detected in the Hudson Bay Lowlands region where the species is very localized.
Breeding Bird Survey data for the most recent ten-year period, 2002 to 2012, indicate that Barn Swallow populations in Canada and Ontario have continued to decline but the average annual rate of decline has lessened to 2.56 percent (statistically significant) and 1.31 percent (near significant) respectively (Table 1). While the short-term rate of decline has abated at the national and provincial scales, the pattern of population change within Ontario is not consistent. The population trend for the Great Lakes/St. Lawrence (Ontario BCR 13) region from 2002 to 2012 was -0.084 percent annually (95% credible limits of -1.94 and 1.89), suggesting that the population in this region may have stabilized over the short-term. Barn Swallows in the Southern Shield region (Ontario BCR 12) continued to experience severe declines averaging -4.68 percent annually from 2002 to 2012. While not statistically significant, the short-term population trend for the Northern Shield (Ontario BCR 8) suggests that Barn Swallows may still be declining in this region (77.6% probablity of decline, Table 1).
Breeding Bird Survey data for the North American Barn Swallow population shows a small but significant long-term decline of -1.2 percent per year (95% CI -1.4, -1.0) over the 1996-2011 period (Sauer et al. 2012). Over the past 10 years (2001-2011) the North American population has been stable (0.0, 95% CI -0.4, +0.4) with population increases in parts of the United States breeding range offsetting ongoing declines in the Canadian population (Sauer et al. 2012).
European Barn Swallow populations show a somewhat similar pattern. Moderate declines were reported for Europe from 1970-1990 and also 1990-2000 (BirdLife International 2004), but the most recent data from 21 European Union countries indicate the European population has stabilized and even recovered to a level similar to 1980 (European Bird Census Council 2012).
Table 1: Long and short-term estimates of population change for the Barn Swallow in Ontario and Canada based on Breeding Bird Survey data using Hierarchical Bayesian trend modeling (Environment Canada in prep.). Boldface denotes significant trends. Ontario trends are also sub-divided into Bird Conservation Regions (BCR) (see Figure 6).
| Geographic Area | Time Period | Start Year-End Year | Annual Trend (%) | Lower Limits (95%) | Upper Limits (95%) | n (routes during trend years) | Trend Reliability | Probability of Decrease |
|---|---|---|---|---|---|---|---|---|
| Ontario | short-term | 2002-2012 | -1.31 | -2.93 | 0.363 | 130 | Medium | 0.939 |
| Ontario | long-term | 1970-2012 | -2.56 | -3.17 | -1.97 | 152 | Medium | 1 |
| Ontario-BCR13 | short-term | 2002-2012 | -0.084 | -1.94 | 1.89 | 62 | Medium | 0.536 |
| Ontario-BCR13 | long-term | 1970-2012 | -1.2 | -1.79 | -0.577 | 68 | Medium | 1 |
| Ontario-BCR12 | short-term | 2002-2012 | -4.68 | -7.84 | -1.42 | 55 | Medium | 0.997 |
| Ontario-BCR12 | long-term | 1970-2012 | -4.73 | -5.63 | -3.79 | 66 | Medium | 1 |
| Ontario-BCR8 | short-term | 2002-2012 | -2.68 | -9.8 | 6.91 | 13 | Low | 0.776 |
| Ontario-BCR8 | long-term | 1970-2012 | -3.37 | -5.91 | -1.05 | 18 | Low | 0.998 |
| Canada | short-term | 2002-2012 | -1.85 | -2.84 | -0.747 | 631 | Medium | 0.998 |
| Canada | long-term | 1970-2012 | -3.74 | -4.15 | -3.34 | 754 | Medium | 1 |
Figure 5. Long-term population indices for Barn Swallows in Ontario during 1970-2012 based on Breeding Bird Survey data. Dotted lines depict 95 percent lower and upper credible intervals (Environment Canada in prep.).
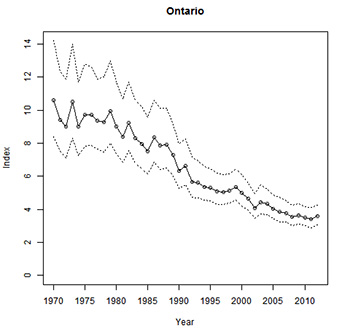
Figure 6. Bird Conservation Regions in Ontario. Map courtesy Andrew Couturier, Bird Studies Canada.
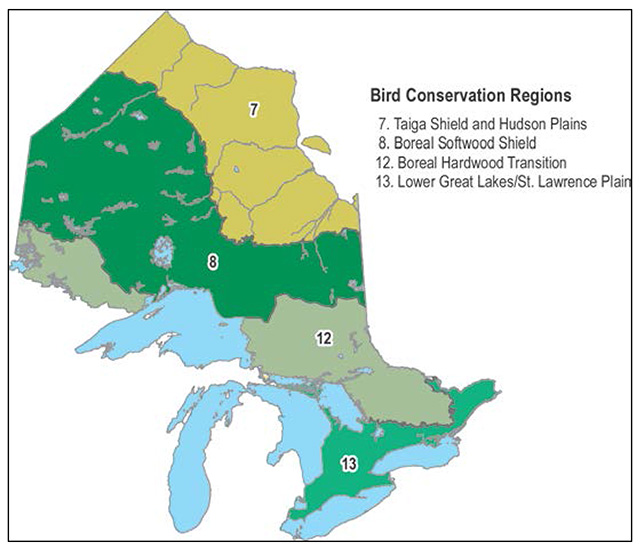
Enlarge figure 6 bird conservation regions in Ontario map
1.4 Habitat needs
Barn Swallow habitat needs include foraging habitat, nest sites and nests and nocturnal roost sites. Daily access to suitable foraging areas with a reliable supply of insect prey is necessary throughout their life cycle. For breeding they require a suitable nest site in proximity to foraging habitat. Outside of the nesting period they require suitable habitat for roosting at night.
Foraging habitat
Barn Swallows forage over a wide range of open and semi-open habitats including natural and anthropogenic grasslands, other farmland, open wetlands, open water, savannah, tundra, highways and other cleared right-of-ways, and cities and towns (Brown and Brown 1999a). They avoid forested regions and high mountains.
Foraging is concentrated in areas with high availability of flying insects close to the ground or water surface. Insect-rich habitats vary seasonally and even daily. Frequently used foraging habitats include farmyards and grazed pastures, marshes and sloughs and coastal wetlands (Samuel 1971, Turner 1980, Godfrey 1986, Brown and Brown 1999a, Petit et al. 1999, Evans et al. 2007). In Europe grazed pastures and other grasslands are the preferred foraging habitat of this species during the breeding season, as they support high insect abundances and are proximal to nest sites (Møller 2001, Ambrosini et al. 2002, Evans et al. 2007, Grüebler et al. 2010).
Foraging habitats used in Ontario include farmland, lakeshore and riparian habitats, road right-of-ways, clearings in wooded areas, parkland, urban and rural residential areas, wetlands and tundra (Peck and James 1987). This species is largely absent as a breeding species in forested and muskeg-covered areas in Ontario (Peck and James 1987).
As noted earlier, the breeding distribution of this species in Ontario is correlated with open country habitat and over 90 percent of the population breeds in southern Ontario, where agricultural lands are the predominant open country habitat (Lepage 2007, Neave and Baldwin 2011). Grazed pasture and other agricultural grasslands are likely important foraging habitat for nesting Barn Swallows in Ontario as in Europe but Barn Swallows are also frequently observed foraging over cropland in Ontario (Boutin et al. 1999).
Information on Barn Swallow foraging distances is limited, particularly for North America, as very few studies of radio-tracked birds are available. Foraging distances vary seasonally. During non-breeding periods most foraging occurs within a 50 km radius of roost sites (Turner 2006). During the breeding season, particularly during the energy-intensive nestling rearing period, most foraging activity takes place as close to the nest site as possible (Turner 2006).
Samuel (1971) reported Barn Swallows "typically" foraged within 0.75 miles (1.2 km) and "seldom" foraged more than 0.5 miles (800 m) from the nest but this information appears to be based on one marked pair observed over a one week period in West Virginia and anecdotal observations. Several intensive observational foraging studies at European Barn Swallow colonies at farms found that almost all foraging occurs within 500 m of the nest site, with most trips within a 200 m radius (Turner 1980, Bryant and Turner 1982, Møller 2001, Ambrosini et al. 2002, Turner 2006). Foraging distance of nesting Barn Swallows in non-farm settings (e.g., Zduniak et al. 2011) has not been reported. Longer foraging distances around nests have been reported for other swallow species, particularly during periods of low insect abundance (Turner 1980, Ghilain and Bélisle 2008).
Nest sites and nests
Barn Swallows throughout the world have adapted to nesting in or on human structures, including buildings, barns, bridges, culverts, wells and mine shafts (Turner 2004). Use of natural nest sites such as caves or rock cliffs with crevices or ledges protected by overhangs is rarely reported (Erskine 1979, Speich et al. 1986, Brown and Brown 1999a). Recent efforts in Canada and the United States to construct stand-alone structures specifically to provide Barn Swallow nest sites have had varying success (Bird Studies Canada 2013, van Vleck 2013).
Nest sites in Ontario are commonly inside or outside of buildings, under bridges and wharves, and in road culverts (Peck and James 1987). Only 79 of 4279 (2%) Barn Swallow nests reported for Ontario were in natural settings (Peck and James 1987), although this sample may not be representative of the actual distribution of nests.
Nests are usually two to five metres above the ground or water surface and are usually constructed on a horizontal ledge or attached to a vertical wall close to an overhang (Turner 2004, COSEWIC 2011). Installation of artificial nest supports and cups to encourage Barn Swallow nesting has been tried in different situations with varying success (Turner 2006, Mercadonte and Stanback 2011, Bird Studies Canada 2013, van Vleck 2013). Mercadonte and Stanback (2011) reported that annual occupancy of 57 nest cups placed under a pier in North Carolina ranged from 23 to 46 percent over a five-year period (natural nests had been removed). Three of 56 artificial nest cups installed by Bird Studies Canada on existing structures with nesting Barn Swallows in southern Ontario were occupied in 2013 (Richardson 2013).
Nesting Barn Swallows must have access to suitable open habitat for foraging close to the nest and generally also require access to mud for nest building, although some nests in caves contain no mud (Peck and James 1987, Brown and Brown 1999a).
Unlike most small passerine nests, Barn Swallow nests persist for many years and are frequently re-used within a breeding season and in subsequent years. Barn Swallows that reuse nests have up to 25 percent higher reproductive success compared with those that construct new nests in some locations (Safran 2004, 2006) but not in others (Barclay 1988). Used nests are also an important habitat feature as a cue for yearling females selecting an initial nest site (Safran 2004, 2007).
Roosting habitat
Nocturnal roosts are typically in reed or cane beds or other dense vegetation, usually in or near water (Turner 2004, Winkler 2006). Post-breeding and migratory roost sites in Ontario are often associated with marshes or shrub thickets in or near water [e.g., willow thicket in London, Ontario (Saunders 1898 in Bent 1942); cattail marsh at Cataraqui Creek (Weir 2008); willow thicket on a small (0.2 ha) island in Pembroke, (Ross et al. 1984); marshes at base of Long Point peninsula (D. Bell, pers. comm. 2012); marsh at Pumpkin Point near Sault Ste. Marie (D. Bell, pers. comm. 2012)].
Little information is available on winter roost habitat for the North American subspecies but it includes sugarcane fields, reed beds and marshes (Vannini 1994, Basili and Temple 1999, Brown and Brown 1999a, Petit et al. 1999). Winter roosts elsewhere are also typically in reed beds, sugarcane fields or standing corn fields in rural settings (Turner 2004). In some parts of Asia however Barn Swallows roost on overhead cables, building ledges and trees in urban settings (Ewins et al. 1991).
1.5 Limiting factors
Biological attributes that affect the feasibility of recovery approaches for this species include:
- relatively short life span (maximum of 10 years, average age in breeding population estimated at 3-4 years);
- relatively high reproductive potential (often double brooded, capable of producing 10 young per year);
- high fidelity of adults to previous breeding sites and the low fidelity of yearlings to their natal sites;
- vulnerability to extended bouts of adverse weather or other events that limit the availability of flying insects;
- vulnerability to localized hazards (e.g., severe weather events during migration, predation or persecution at roost sites, aerial spraying of pesticides on foraging flocks) due to its gregarious behaviour during the non-breeding period
1.6 Threats to survival and recovery
Numerous factors have been proposed as possible explanations of the recent declines in Barn Swallows and other aerial insectivores in Canada (Nebel et al. 2010, COSEWIC 2011, Calvert 2012). However, the information needed to critically evaluate the past, present and future impacts of these potential threats to Barn Swallows in Ontario is generally lacking (COSSARO 2011). Key knowledge gaps that must be addressed in order to assess the severity and magnitude of the many possible threats that affect the survival and recovery of this species are identified in section 1.7.
Barn Swallows breeding in Ontario are affected by the cumulative impact of stresses experienced on their breeding grounds, during the post-breeding dispersal period, during spring and fall migration and while wintering in South or Central America. Factors affecting individual fitness can result in carry-over effects from one season to the next.
This species' close association with human-modified open country habitats (most notably agricultural lands) throughout most of its life cycle makes it especially sensitive to changes in land use and other human activities. Threats affecting the Barn Swallow’s food supply (flying insects) may also be implicated in declines observed in many other species of aerial insectivore.
Given the known and potential threats to this species, it is unclear why the pattern of long-term declines is confined largely to northern Barn Swallow populations, whereas southern populations in the United States are stable or increasing.
This summary of human-caused threats mostly focuses on threats occurring in Ontario during the breeding and post-breeding periods because: (1) reproductive success is a key demographic factor for this short-lived species, (2) very little is currently known about the nature, extent and severity of threats affecting Barn Swallows during the migration and wintering periods when they are outside of Ontario, and (3) the focus of this recovery strategy is to identify key practical actions that the Ontario government could undertake to promote the recovery of Barn Swallow.
This long-distance migrant spends much of the year outside of Ontario and implementing recovery actions only in Ontario may not be sufficient to recover the population. While the recovery of Barn Swallow in Ontario will ultimately depend on reducing significant threats affecting the species' survival wherever they are occurring, recovery actions to maintain or enhance the productivity of birds in Ontario should increase the probability of success.
The following assessment of the known and potential threats to Ontario Barn Swallows is based on the best information currently available including expert opinion gathered during the development of this recovery strategy. It seems likely that multiple direct and indirect threats are having an additive or synergistic impact on Barn Swallow populations as the known threats do not appear to adequately explain the observed population decline (COSEWIC 2011, COSSARO 2011). The significance and severity of these threats should be continually reassessed as new information becomes available.
Threats are presented in order of certainty, extent and anticipated importance to the Ontario recovery strategy as their relative severity is not known at this time.
Loss of nest site habitat
Loss of Barn Swallow nest sites due to demolition, replacement or renovation of human structures is a widespread and ongoing threat. In particular, replacement of old wooden farm buildings with modern structures that do not provide accessible nest sites is often cited as a principal reason for Barn Swallow population declines in Canada (see COSEWIC 2011). The supply of nest sites on other human structures (e.g., bridges, culverts, wharves, boathouses, residential and commercial buildings) may also be reduced as old structures are replaced with modern designs or retro-fitted in a manner that birds can no longer access nest sites.
Food safety regulations require that birds be excluded from food processing facilities, including milking parlors and vegetable packing areas. In addition there are numerous bird exclusion products available on the market although the extent to which they are being installed to deter Barn Swallows from nesting is unknown.
Changes in construction materials and designs can also affect the suitability of nest sites by changing thermal conditions (e.g., increased heat-induced mortality of nestlings in metal-roofed barns, Tate 1986) or by affecting (decrease or increase) the accessibility of nests to predators and nest competitors.
There has been no net change in the availability of natural nest site habitat (COSEWIC 2011).
While there is high certainty that the availability of barns and other human structures that provide nest sites has decreased over the past several decades and will continue to decrease, the significance of this threat to the recovery of the species is less clear (COSEWIC 2011, COSSARO 2011).
Nest site availability and the severity and the magnitude (frequency and extent) of nest site loss in Ontario have not been quantified. Numerous anecdotal reports of former nest sites (including anthropogenic sites and natural cliff sites) that are currently unoccupied suggest that additional factors are contributing to the recent population decline (COSEWIC 2011, COSSARO 2011).
Given the high nest site fidelity of this species, the permanent or temporary loss of a previous nest site is likely detrimental to the annual reproductive success of returning individuals. Birds can show strong attachment to a particular nest site. Considerable effort is required to prevent Barn Swallows from nesting (e.g., where repair work is planned or in nuisance locations). The impact of the reduction in available nest sites on yearling recruitment rates is not known, and presumably varies depending on the availability of other nest sites nearby.
Loss or degradation of foraging habitat
Threats to Barn Swallow foraging habitat in Ontario include changes in land cover and land use that result in the loss or degradation of insect-rich habitats within open country habitat. The quantity and quality of foraging areas in close proximity to nest sites, near roost sites and along migratory routes are important habitat parameters. In Ontario, changes in agricultural land use are particularly important as agriculture is the dominant land cover in the open country habitats where this species is most abundant (Neave and Baldwin 2011).
The amount and nature of open country habitat in southern Ontario (including the Southern Shield ecoregion, see Figure 3) has undergone dramatic changes over the past 200 years (Neave and Baldwin 2011). Open country habitats in southern Ontario prior to European settlement included local areas of native grassland, savannah, alvar and rock barrens, and First Nations agricultural lands (Neave and Baldwin 2011). In the 19th century the amount of open country habitat in southern Ontario increased as forested lands were cleared for agriculture (Neave and Baldwin 2011). Over the past century, open country habitat in southern Ontario decreased substantially due to reforestation of marginal farmland (especially in the Southern Shield ecoregion) and urbanization (Blancher et al. 2007, Neave and Baldwin 2011). Since 1971, there has been little change in the total amount of open country habitat in southern Ontario (Neave and Baldwin 2011).
While the amount of open country habitat in southern Ontario may have stabilized in recent years, there continues to be major changes to land cover due to changes in agricultural land use that could be affecting Barn Swallows and other wildlife populations (Javorek et al. 2007, Neave and Baldwin 2011). Steady declines in the total amount of farmland in Ontario and the amount of pasture since 1921 have continued through 2011 (Javorek et al. 2007; Statistics Canada 2007, 2012). In contrast, the total amount of cropland (including tame hay) in Ontario has remained quite stable over this period (Statistics Canada 2007, 2012). Recently, there has been a shift from hay in favour of annual row crops with the proportion of cropland in hay dropping from 28 percent to 23 percent between 2006 and 2011 (P. Smith, pers. comm. 2012, Statistics Canada 2012). Changes in agricultural land use are driven by socio-economic factors. Changing dietary preferences, changing farm practices and recent high corn and soybean prices have resulted in a general shift from dairy and cattle farming to intensive annual field crop production in the Great Lakes-St. Lawrence region (Latendresse et al. 2008, Jobin et al. 2010).
In some parts of southern Ontario, rural non-farm properties with horses and pasture are increasingly common. Information on land use on these lands is not reported in the agricultural statistics as they are not considered census farms.
Changes in agricultural practices and land use that affect flying insect populations can have a major impact on this species. A study in Britain found aerial insect abundance and Barn Swallow density varied by crop type, with highest numbers over pasture and lowest numbers over row crops (Evans et al. 2007). In Europe, there is a strong link between Barn Swallow population size, distribution and productivity and the presence of livestock and pasturelands (Møller 2001, Robinson et al. 2003, Grüeebler et al. 2010, Ambrosini et al. 2011a, Ambrosini et al. 2012). A landscape-scale study in Quebec found a negative relationship between Tree Swallow breeding success and agricultural intensification (Ghilain and Bélisle 2008).
Ongoing trends that may be adversely affecting Barn Swallow foraging habitat in agricultural settings in Ontario include:
- continuing reduction in cattle herds;
- continuing reduction in pasturing of dairy cows and other livestock;
- improved sanitation practices (manure and fly management) on livestock farms;
- continuing loss of pasture;
- conversion of hayfields to annual row crops;
- increased field size and reduction in hedgerows resulting in lower insect abundance and diversity;
- changes in the types of pesticides (especially insecticides) in use; and
- increased planting of genetically-modified, insect-resistant row crop
Other insect-rich habitats that are preferred Barn Swallow foraging areas are associated with natural grasslands, wetlands, riparian habitats and water bodies. Changes in the extent and quality of these foraging habitats could be affecting food availability at various times in the Barn Swallow life cycle.
Changes in food supply are suspected as being an important factor in the decline of Barn Swallows and other aerial foraging insectivores (Nebel et al. 2010). It is uncertain as to where in the life cycle of these migratory species these changes are occurring (e.g. on wintering grounds, migration routes or breeding grounds), or whether they are related to changes in foraging habitat or other factors such as environmental pollution or climate change (see below).
Environmental contaminants, pesticides and pollution
Environmental contaminants, pesticides and pollutants that may directly or indirectly affect the survival and reproductive output of Barn Swallows (and other aerial insectivores) include:
- poisoning or sub-lethal harmful effects caused by exposure to pesticides, heavy metals, endocrine disrupters or other pollutants;
- large-scale calcium depletion (affects reproduction) due to acid precipitation;
- pesticides (particularly insecticides) impacting food supply; and
- light pollution around urban and built-up areas that may reduce local food supplie
The Barn Swallow’s close association with human-altered habitats throughout its life cycle suggests it may be at an elevated risk from pesticides and pollution. As Barn Swallows frequently forage over agricultural fields in southern Ontario they face relatively high levels of exposure to pesticides although their exposure risk is lower than ground foraging birds (Boutin et al. 1999). Although pesticide use is lower in agricultural grasslands (hay and pasture) than in other crops, a recent study found that lethal risk from insecticide exposure was the best predictor of declines in grassland birds in the United States (Mineau and Whiteside 2013). The indirect impacts of pesticides and pollution on food supply or quality is likely a more significant potential threat than direct poisoning at least on the breeding grounds in Ontario.
The quantity of agricultural pesticides applied in Ontario has declined in recent decades, with a 45 percent reduction in overall agricultural pesticide use between 1983 and 2008, and a 76 percent reduction in agricultural insecticide use in this period (McGee et al. 2010). A provincial ban on the cosmetic use of pesticides was implemented in 2009. There have however, been significant shifts in the types of pesticides being used in Ontario over time. Recently there has been considerable concern as to potential biological impacts of neonicotinoid insecticides, a new class of insecticide that was first used in Canada in the 1990s and is now widely used in Ontario and elsewhere. Neonicotinoids are systemic insecticides that act as insect neurotoxins. They have been implicated in the decline of honeybee colonies and other insect pollinators in Europe and North America and to global wildlife declines (Mason et al. 2013). There is growing evidence that these insecticides could be impacting bird populations due to direct toxicity and also as a result of the indirect impact of an overall reduction in insect biomass (Mineau and Palmer 2013).
Reduced productivity due to predation, parasitism and persecution
Predation, parasitism and persecution are known threats that can cause severe reductions in local productivity and other adverse effects. The extent and frequency of these threats has not been quantified. In general there is no indication that these threats have increased over time.
Various non-native and native predators commonly associated with human habitations may reduce productivity by predating eggs, nestlings, fledglings and/or adult Barn Swallows. Species that have been identified as particular problems include cats, rats, mice, raccoons, grackles, falcons, hawks and owls (Turner 2006, COSEWIC 2011, Salvadori et al. 2011).
Interspecific competition for nest sites with non-native House Sparrows and native Cliff Swallows can also result in reduced productivity (COSEWIC 2011, Salvadori et al. 2011). Rock Pigeons may also displace Barn Swallows at some nest sites (M.Taylor, pers.comm. 2013). House Sparrows also affect productivity directly by killing eggs and nestlings.
Barn Swallows are affected by many parasites including blood-sucking mites and lice, and internal parasites (Turner 2006). Elevated levels of ectoparasites can cause high nestling mortality and can also have carryover effects on the fitness of surviving young (COSEWIC 2011, Saino et al. 2012).
Barn Swallow nests in or on buildings are sometimes deliberately destroyed by people because the accumulation of droppings below nests is seen as a potential health hazard. Active nests may also be removed or otherwise disrupted if they are considered a nuisance because the swallows mob intruders near their nests or because of aesthetic or noise concerns. Temporary or permanent measures may be implemented to discourage birds from re-nesting at these sites (e.g., the use of predators was proposed at a marina in Toronto; Alamenciak 2012). Disturbance to active nests may severely affect local populations but the extent and severity of this threat is not known.
Landowner attitudes towards Barn Swallows likely vary considerably depending on the specific situation and degree of inconvenience. Studies of landowner attitudes at farms in the Netherlands found that Barn Swallows nesting in locations where they were not wanted was not a major issue and the species was viewed as a beneficial insectivorous species rather than as a pest (Lubbe and de Snoo 2007, Kragten et al. 2009). Barn Swallows in Sweden tested negative for salmonella (Haemig et al. 2008), casting doubt on whether their faeces constitute a significant health concern.
The removal of used nests (e.g., for cosmetic reasons or for routine maintenance activities) at any time of the year may pose a threat to the recovery of the species for two reasons. In some situations there appears to be a small reproductive advantage to reusing a nest compared to building a new nest (Safran 2004, 2006 but see Barclay 1988). Second, the removal of used nests negatively affects colony size during the following breeding season as yearling females cue in on the presence of used nests when selecting a nest site (Safran 2004, 2007).
Habitat loss, disturbance and human persecution at roost sites
Habitat destruction or degradation due to activities that disturb roosting birds and land uses that attract predators are known threats at roost sites. Adjacent development as well as predation by an increasing Merlin population may have been factors in the abandonment of the summer swallow roost in downtown Pembroke, Ontario in the 1990s (Ottawa River Legacy Landmark Partners 2013). The significance of threats to roost sites is unknown as information on the locations and size of Barn Swallow roost sites in Ontario and elsewhere is very limited.
Winter roosts in Central and South America may be subject to additional specific threats including poisoning or disturbance of roost sites due to measures taken to control other avian pest species (e.g., Dickcissel) (Basilli and Temple 1999), or even direct exploitation as a food source as reported at winter roosts in parts of Asia and Africa (Ewins et al. 1991, Turner 2004).
Climate change and severe weather
Barn Swallows are vulnerable to severe weather events that exceed their temperature tolerances (e.g., cold snaps, heat-induced mortality of nestlings) or reduce the supply of flying insects (e.g., prolonged cold weather or drought) (Turner 1980, Nebel et al. 2010, Møller 2011). Severe weather events during migration can result in mass mortality of Barn Swallows (e.g., 40% reduction in Barn Swallow populations across a large area of Europe following an extreme snowfall event in the Alps in fall 1974, Møller 2011). While these events are normally infrequent (Møller 1978, Newton 2007, Brown and Brown 1999b, Møller 2011), climate change may be increasing the frequency of hurricanes and other severe weather events (Intergovernmental Panel on Climate Change 2007).
Climate change may be affecting Barn Swallows populations in various ways including changing the timing of migration, their breeding and wintering distributions, the condition of arriving birds and vital rates (Turner 2009). These effects can be positive or negative and may vary by geographic region (Balbontín et al. 2009b, Turner 2009, COSEWIC 2011, Shutler et al. 2012).
Advances in European Barn Swallow migration dates and a northward shift in their wintering distribution can extend the breeding season and increase seasonal productivity (Møller 2008, Balbontín et al. 2009b, Ambrosini et al. 2011b). However,
hotter, drier weather can reduce insect populations and adversely affect productivity and survival (Balbontín et al. 2009b, Saino et al. 2004, Saino et al. 2011). A rapidly changing climate can also lead to a temporal mismatch between food availability and energy requirements for Barn Swallows and other aerial insectivores (Ambrosini et al. 2011b, Saino et al. 2011, Calvert 2012). Various European studies have found that survival and productivity rates are correlated with climatic factors that affect insect populations on the breeding and wintering grounds (Møller 1989, Møller and Szép 2002, Saino et al. 2004, Robinson et al. 2008, COSEWIC 2011).
Climate change effects on North American Barn Swallow populations remain largely speculative but are likely to be mixed as has been reported for Tree Swallows in North America (Dunn and Winkler 1999, Hussell 2003, Shutler et al. 2012).
1.7 Knowledge gaps
The Barn Swallow has been the focus of extensive studies particularly in Europe and Africa but much less is known about the ecology and conservation needs of the North American subspecies. The few published studies carried out at breeding colonies in Ontario have been limited in focus, geographic scope and duration (Holroyd 1975, Smith et al. 1991, Smith and Montgomerie 1991, Smith and Montgomerie 1992, Boutin et al. 1999, Salvadori 2009, Salvadori et al. 2011).
Fundamental uncertainties that are pertinent to the recovery of this species include:
- the demographic processes and proximate and ultimate factors driving recent declines in Barn Swallow and other aerial insectivores; and
- the extent and severity of current and potential threats in Ontario and elsewhere.
Uncertainty as to the threats and environmental factors limits our ability to determine what constitutes an achievable long-term recovery goal, prioritize recovery objectives, and predict the efficacy of various recovery approaches.
Several specific information deficiencies regarding Ontario Barn Swallow biology, habitat needs and threats were identified at the expert consultation workshop held in December 2012. These knowledge gaps were grouped into six themes: (1) vital rates, (2) diet and food supply, (3) habitat requirements and trends on the breeding grounds, (4) habitat and ecology during winter and on migration, (5) best management practices (BMPs), and (6) climate change effects. Research is currently underway to address some of these knowledge gaps (see section 1.7).
Vital rates and population source/sink dynamics
Information on the vital rates of the Ontario Barn Swallow population is needed to understand the demographic processes underlying the population decline and to identify where in the life cycle recovery action will be most effective. Comparable demographic data are also needed from other parts of the North American range including areas with population increases.
Specifically, systematic sampling is needed across the Ontario breeding range to assess:
- productivity;
- nestling growth rates under various conditions;
- adult return rates to previous nest sites and nests;
- recruitment of yearling birds to breeding locations; and
- survival rates of adults and young at each stage of the annual life cycle (breeding, post-breeding, and overwintering periods).
This information is needed to address key questions regarding population source/sink dynamics such as:
- demographic parameters correlated with nest substrate or colony size (e.g., is nest success affected by structure type); and
- what impact food shortages, nest competitors, predators, parasites and loss of used nests have on productivity.
Diet and food supply
Widespread declines in aerial insectivore populations has raised concern as to whether there have been large-scale changes in insect populations due to insecticides, environmental contaminants, habitat degradation, climate change or other factors.
Specific information needs are:
- Barn Swallow diet in Ontario and elsewhere in the life cycle;
- patterns of insect abundance and diversity among various habitat types;
- contaminant loads (e.g., neonicotinoids) in Barn Swallows and their food supply; and
- trends in insect abundance in Ontario and elsewhere that may be causing a bottleneck in the Barn Swallow’s life cycle.
Habitat use, habitat requirements and habitat trends in ontario
Specific information that is needed to evaluate the significance of habitat loss and degradation in Ontario as a factor in past declines and a threat to recovery are as follows.
- Nest sites and used nests
- What proportion of the Ontario Barn Swallow population uses various nest sites (barns, bridges/culverts, boathouses, natural cliffs e)?
- How is the availability of various types of nest site changing over time?
- What are the physical characteristics of nest sites?
- How severe an impact do nest competitors have on nest site availability?
- What is the extent and impact of deliberate and inadvertent nest site exclusion and nest destruction (of both active and used nests) in various settings?
- How important are used nests as a habitat feature in various settings in Ontario?
- Foraging habitat
- foraging distances around nest sites and roost sites;
- size, habitat type and quality (food availability) of foraging habitat close to nest sites;
- amount of foraging habitat required to sustain different colony sizes;
- relationships among foraging habitat, insect availability and Barn Swallow productivity;
- spatial correlations between Barn Swallow abundance and habitat features (including presence of livestock) at various scales (nest-to landscape-scale); and
- spatial and temporal correlations between Barn Swallow populations and changes in land use, agricultural practices, and pesticide us
- Roosting habitat
- Location and habitat features of major post-breeding roost sites in Ontari
Wintering and migration habitat and ecology
Basic information on migration connectivity is generally lacking and this limits our understanding of the threats to the species' recovery. Studies on the European population highlight the importance of understanding the full life cycle of this long-distance migrant to determine whether, where and what conservation action is needed to address population declines. Key information gaps include:
- general location(s) in Central and/or South America where birds from Ontario spend the winter (see section 1.7);
- specific habitats and locations used by Barn Swallows for foraging and roosting during winter;
- routes and stopover locations used during spring and fall migration; and
- nature, severity and significance of threats to Barn Swallows during wintering and migration periods.
Best management practices (BMPs)
Due to their close association with humans and their propensity for nesting on human structures, Barn Swallow management issues are a fairly common occurrence. Information is needed to develop BMPs for specific situations including:
- the design, placement and management of artificial nest structures to replace or enhance existing nest sites;
- management of nest sites to maintain and protect used nests;
- effective methods for temporarily or permanently deterring nesting in inappropriate locations while minimizing any adverse impact on productivity;
- methods for enhancing productivity and encouraging nesting in appropriate locations; and
- approaches to addressing sanitation and health concerns at nest site
Climate change effects
Two aspects of climate change have been identified as particularly relevant to understanding the decline of Barn Swallows, as well as other aerial insectivores (Nebel et al. 2010, Calvert 2012).
- Has there been a shift in phenology of birds or insects related to climate change?
- Has there been increased mortality and/or reduced nesting success due to increased weather variability?
1.8 Recovery actions completed or underway
The decline in Barn Swallow populations and its designation as a threatened species has prompted several agencies, organizations and individuals to initiate activities relevant to this recovery strategy. These activities are grouped by recovery theme, with some activities fitting into more than one theme (e.g., several monitoring projects have a research component).
Inventory, monitoring and assessment of species, habitats or threats
- General bird surveys and monitoring programs that collect information on Barn Swallow populations include the following.
- North American Breeding Bird Survey: annual bird population survey since 1966 (Environment Canada 2013).
- Breeding Bird Atlas: bi-decadal survey of bird distribution and abundance across Ontario, last cycle completed 2001-05 (Cadman et a 1987, Cadman et al. 2007).
- Ontario Nest Records Scheme/Project Nestwatch (ONRS 2012): ongoing project to compile nest monitoring information in a computer databas
- As of October 2012 this database contained information on 4520 Barn Swallow nests in Ontario spanning the period from 1875 through 2012 (Lebrun-Southcott, pers. comm. 2013). Environment Canada has used these data to determine seasonal nest phenology at various temporal and spatial scales (B. Drolet, pers. comm. 2012). Bird Studies Canada has used the nest site and habitat variables contained in this database to compile a broad-scale description of Barn Swallow habitat in Ontario and will be analysing productivity and investigating other aspects of the dataset in 2013 (Z. Lebrun-Southcott, pers. comm. 2013).
- Local intensive Barn Swallow nest monitoring and banding projects include:
- annual nest census and monitoring along the Highway 60 corridor in Algonquin Park since 2007 (Tozer 2012);
- Wellington County banding and nest monitoring study by A. Salvardori since 2008 with additional monitoring by M. Cadman, Environment Canada since 2010 (A. Salvadori, per. comm. 2012, M. Cadman, pers. comm. 2012);
- 2011 follow up survey of a Killarney study site where Barn Swallow nests were monitored during the 1980s (P. Blancher, per. comm. 2012); and nest monitoring and nestling banding at Ruthven Historic Site in 2012 and 2013 (N. Furber, pers. comm. 2012).
- Local targeted bird surveys (e.g., roadside point counts focussed on grassland birds and Species at Risk) by various organizations have collected Barn Swallow abundance data in various regions in recent years including:
- grassland bird surveys coordinated by Wildlife Preservation Canada in the Carden Plain, Napanee Plain and other priority areas from 2010 to 2012;
- grassland bird surveys by Bird Studies Canada in Norfolk County region from 2011 to 2013; and
- Species at Risk bird surveys at the Alderville First Nations, in Prince Edward County by the Prince Edward Point Bird Observatory in 2011 and on the Bruce Peninsula by the Bruce Peninsula Bird Observatory in 2012 and 201
- Regional field surveys by Bird Studies Canada in 2013 to determine nest site availability and the relative importance of various types of nest substrates and habitats to Barn Swallows in Ontario.
Research
- Statistical analyses of Breeding Bird Survey data are underway by A. Smith (Environment Canada) to determine the timing of Barn Swallow declines in Canada.
- A project is underway using geolocators and stable isotope analyses of feathers to identify the wintering locations, migration routes and migration timing of Barn Swallows breeding in Ontario and other locations in Canada and the United States (coordinated by K. Hobson, Environment Canada). Preliminary results suggest that birds breeding in southwestern Ontario winter in southern Brazil, Paraguay and/or Bolivia, and stopover at various locations during fall migration (Hobson and Van Wilgenburg 2013).
- Additional relevant research is underway on other aerial insectivores in Ontario, especially Tree Swallows, Purple Martins, Bank Swallows and Chimney Swif
Communications, education and outreach
- A Barn Swallow workshop for the public was held at the Ruthven Park Historic Site in 2012 (S. Turner, per. comm. 2012).
- Bird Studies Canada will be conducting stakeholder interviews in 2013 to gauge landowner attitudes towards Barn Swallow
- New resources and web site materials have been developed to promote Barn Swallow NestWatch (within the existing Project NestWatch program). The goal is to engage and educate citizens and increase the collection of data on nesting Barn Swallows in Ontario and across the country.
Stewardship
- Bird Studies Canada initiated a multi-year Barn Swallow stewardship project in 2012 that includes:
- a summary of available information on alternate nesting structures and their success in attracting Barn Swallows (Bird Studies Canada 2013);
- installation of artificial nest cups and alternate nesting structures to investigate nest site and broader scale preferences;
- roadside surveys to determine Barn Swallow occupancy of potential nest sites in southern Ontario; and
- an analysis of available Barn Swallow nest record data from the Ontario Nest Record Schem
- Various individuals and groups are experimenting with the use of artificial nest structures to encourage Barn Swallows to nest in specific location
Management of species, habitat or threats
- The Ministry of Transportation (MTO) has used timing restrictions to avoid disturbing birds during the nesting season (Boyd, pers. comm. 2012).
- MTO has been using various methods to prevent or discourage Barn Swallows from nesting prior to scheduled maintenance work on MTO bridges and structures (Boyd, pers. comm. 2012).
- MTO has been deploying artificial nest structures to replace nest sites that are being temporarily disturbed (e.g., bridge repairs) or permanently altered (culvert replacement, bridge replacement) (Boyd, pers. comm. 2012).
- Bird Studies Canada will be developing BMPs guidelines using new and historic monitoring data as well as information gathered through stakeholder consultation
Protection of species and habitat
- OMNRF has prepared a general habitat description for Barn Swallow to provide greater clarity on the area of habitat protected under the general habitat definition in the ESA (OMNR 2013b).
- OMNRF has developed a streamlined regulatory process that sets out rules for approval and registration of activities that alter a building or structure (e.g., barn or bridge) that is habitat for Barn Swallow (OMNR 2013a)
2.0 Recovery
2.1 Recovery goal
The recovery goal is to maintain a stable, self-sustaining population of Barn Swallow in Ontario by 2034 (within 20 years) and to slow the rate of decline over the next 10 years.
Narrative to support recovery goal
The Barn Swallow is a native species to Ontario and should be maintained in keeping with Ontario’s Biodiversity Strategy (OBC 2011). Despite past declines the species is presently common and widespread. Achieving a stable population appears to be a realistic long-term target.
The timeline for slowing the current population decline and achieving a stable population is unknown due to uncertainty as to causal factors and the magnitude of current threats. Twenty years is considered a realistic estimate of the time required to complete the extensive multi-year research studies identified in this recovery strategy, and to implement priority recovery actions.
Given the high reproductive potential of this species, it is anticipated that implementation of recovery actions in Ontario over the short-term to maintain or enhance the productivity of the population and improve habitat conditions to enhance survival rates could show measurable results (i.e., a slower rate of population decline) within 10 years.
It is acknowledged that achieving the goal of stabilizing the population by 2034 would likely nonetheless result in an approximately 50 percent further reduction in the current size of the Ontario Barn Swallow breeding population.
Short-and long-term population abundance, distribution and/or trend targets should be established once the factors driving the recent decline are better understood.
2.2 Protection and recovery objectives
Table 2. Protection and recovery objectives
| No. | Protection or Recovery Objective |
|---|---|
| 1 | Fill key knowledge gaps to increase understanding of the nature and significance of threats to Ontario Barn Swallow populations and the biological and socio-economic factors that may impede or assist recovery efforts. |
| 2 | Maintain or improve nesting productivity through the development of appropriate practices and policies for managing Barn Swallow nests, nest sites and associated foraging habitat in Ontario. |
| 3 | Promote stewardship, education and increased public awareness of the Barn Swallow in Ontario. |
| 4 | Identify and protect important roost sites used by Barn Swallows in Ontario. |
| 5 | Inventory, monitor and report on the state of Barn Swallow populations and habitats in Ontario and elsewhere to track the progress of recovery activities. |
2.3 Approaches to recovery
Table 3. Approaches to recovery of the Barn Swallow in Ontario
- Fill key knowledge gaps to increase understanding of the nature and significance of threats to Ontario Barn Swallow populations and the biological and socio-economic factors that may impede or assist recovery efforts.
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Critical | Long-term | Research Inventory and Monitoring Communications Education and Outreach |
1.1 Investigate the vital rates of Barn Swallows in Ontario in various nest types (new, used and artificial) across a range of nest sites, landscapes, regions and years, using well-designed multi-year collaborative studies that engage volunteer Citizen Scientists where appropriate.
|
|
| Critical | Short-term | Research |
1.2 Investigate whether environmental contaminants are directly affecting productivity and/or survival rates.
|
|
| Critical | Short-term (yielding results over longer-term) | Communications Research Education and outreach |
1.3 Promote and coordinate applied research targeting key knowledge gaps.
|
|
| Critical | Long-term | Research Communications | 1.4 Encourage coordinated research into the link between food supply and population declines in Barn Swallow and other aerial insectivores breeding in Ontario and northeastern North America. |
|
| Critical | Short-term | Research |
1.5 Investigate possible links between Barn Swallow foraging habitat quantity and/or quality and population declines.
|
|
| Critical | Short-term | Research Management |
1.6 Identify and describe the key characteristics of the nest site and foraging habitats used by Barn Swallows in Ontario at various scales (nest-scale to landscape-scale).
|
|
| Critical | Short-term | Inventory and Monitoring Research |
1.7 Investigate historical changes in nest site availability and occupancy in Ontario.
|
|
| Necessary | Short-term | Research |
1.8 Identify the wintering grounds and migration routes used by Ontario Barn Swallows and investigate migratory connectivity.
|
|
| Necessary | Short-term | Research Communications |
1.9 Identify and collaborate with other relevant jurisdictions and organizations to research the ecology of Ontario Barn Swallows in their wintering areas in South America and along their migration routes.
|
|
| Necessary | Long-term | Research |
1.10 Analyse existing data sets to investigate possible direct or indirect links between climate change and/or severe weather events and changes in Barn Swallow populations.
|
|
| Necessary | Short-term | Research |
1.11 Investigate Barn Swallow diet and food supply in Ontario and elsewhere.
|
Knowledge gap: diet and food supply
|
| Necessary | Short-term | Management Protection |
1.12 Investigate how public policies and socio-economic trends are affecting Barn Swallow habitats, populations and recovery efforts.
|
|
| Beneficial | Long-term | Communications Research | 1.13 Develop a broad-scale collaborative study of Barn Swallow vital rates across North America to identify ecological correlates of regional population trends. |
|
- Maintain or improve nesting productivity through the development of appropriate practices and policies for managing Barn Swallow nests, nest sites and associated foraging habitat in Ontario.
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Critical | Short-term and Ongoing (evaluation) | Research Management Education and Outreach |
2.1 Work with relevant agencies and organizations to develop, promote and evaluate BMPs for the management of nests on various human structures (barns and other agricultural buildings, bridges and other transportation infrastructure, boathouses and wharves etc.).
|
|
| Critical | Ongoing | Research Management Stewardship Communications |
2.2 Develop and evaluate guidelines for the design, placement and management of artificial Barn Swallow nest structures (ledges, nest cups, kiosks or other free-standing structures).
|
|
| Critical | Short-term | Research Management |
2.3 Assess the effectiveness of measures to mitigate the impact that the loss of traditional nest sites and/or loss of used nests has on nesting productivity.
|
|
| Critical | Ongoing | Communications Research |
2.4 Coordinate the sharing of information on BMPs and the effectiveness of mitigation measures.
|
|
- Promote stewardship, education and increased public awareness of the Barn Swallow in Ontario.
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Critical | Ongoing | Protection Management Stewardship | 3.1 Encourage regulatory agencies including the Ontario Ministry of Natural Resources and Forestry and Environment Canada, to recognize and promote stewardship activities, safe harbour agreements, BMPs and incentive programs as an effective approach to the protection of Barn Swallow nests and habitats. |
|
| Critical | Short-term | Communications Education and Outreach Protection |
3.2 Develop a communications and outreach strategy focused on education of key audiences to promote the protection and enhancement of Barn Swallow nests and nest sites through engagement and stewardship.
|
|
| Necessary | Short-term | Education and Outreach | 3.3 Develop and distribute outreach materials and tools (e.g., fact sheets, presentations, mobile displays, town hall meetings and booths at farm shows) on various topics (e.g., species at risk status, nuisance nest management options, stewardship success stories and BMPs) for various target audiences (public, farmers, Ministry of Transportation, municipalities, marina operators etc.) |
|
| Beneficial | Short-term | Education and Outreach Stewardship Management |
3.4 Develop programs to facilitate and encourage good stewardship and implementation of BMPs.
|
|
| Beneficial | Ongoing | Communications Research Education and Outreach |
3.5 Develop and maintain a central repository for Barn Swallow information.
|
|
- Identify and protect important roost sites used by Barn Swallows in Ontario.
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Critical | Short-term | Inventory and Monitoring Research |
4.1 Inventory and assess roost sites used by Barn Swallows (and other swallow species) during the post-breeding period.
|
|
| Necessary | Ongoing | Protection Inventory and Monitoring |
4.2 Identify, protect and monitor significant roost sites used by Barn Swallows (and other related species) in Ontario.
|
|
- Inventory, monitor and report on the state of Barn Swallow populations and habitats in Ontario and elsewhere to track the progress of recovery activities.
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Necessary | Short-term | Inventory and Monitoring |
5.1 Complete a baseline assessment of the current state of Barn Swallow breeding habitats across Ontario.
|
|
| Necessary | Ongoing | Inventory and Monitoring |
5.2 Continue to monitor and report on Barn Swallow population trends in Ontario to determine effectiveness of recovery actions.
|
|
| Beneficial | Long-term | Inventory and Monitoring Research |
5.3 Design and implement a long-term intensive Barn Swallow demographic monitoring program across the Ontario breeding range.
|
|
2.4 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of Natural Resources and Forestry on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the authors will be one of many sources considered by the Minister when developing the habitat regulation for this species.
A habitat regulation may be developed to safeguard those areas on which the Barn Swallow depends directly or indirectly to carry out its life processes including feeding, reproduction and migration. Barn Swallow habitat use in Ontario varies seasonally and includes breeding habitat and non-breeding habitat. Habitat used during the breeding season (i.e., between about May 1 and August 31) includes the nest (nest material and support), the nest site (human structure or natural substrate to which the nest is attached) and the associated foraging habitat. Non-breeding habitat includes post-breeding roost sites where Barn Swallows congregate at night which may be used between about July 1 and October 31.
In developing a habitat regulation for Barn Swallow breeding habitat the following should be considered.
- Despite severe population declines the Barn Swallow is still common and widespread in much of Ontario and there are likely well in excess of 15,000 sites with one or more nests (based on a current population estimate of ~350,000 adults and reported average colony size of ~10 pairs).
- The overwhelming majority of Barn Swallow nests in Ontario are situated on human structures in anthropogenic open country habitats.
- Barn Swallows show very strong fidelity to their previous nest site and temporary or permanent loss of nest sites may be detrimental to reproductive output.
- Barn Swallow nests are persistent structures that are often re-used and the loss of old nests may have detrimental impacts on reproductive output and local recruitment.
- Active and used Barn Swallow nests are currently afforded protection under the federal Migratory Bird Convention Act.
- It is not known if nest site availability is limiting the recovery of this species.
- Barn Swallows defend a small territory around their nest (8 m2 or less in a European study by Møller in 1990, equivalent to circle of 1.5 m radius).
- Barn Swallows require a small opening (e.g., can use a gap between boards that is just a few centimetres wide) to access nests located within enclosed nest sites (Brown and Brown 1999a).
- Nest sites often support multiple nests (i.e., a colony). The average distance between active nests within a colony is approximately two to four metres although nests can be as close as 60 cm if visual barriers are present (Brown and Brown 1999a, Mercadante and Stanback 2011).
- Very little is known about the foraging habitat used by North American Barn Swallows.
- Efforts to further regulate the protection of Barn Swallow nests and nest sites associated with human structures may impede stewardship efforts and could be counterproductive to species recovery.
In developing a habitat regulation for Barn Swallow roosting habitat the following should be considered.
- For bird conservation purposes a one percent threshold is often used to define "significant" concentrations (e.g., sites that regularly hold one percent or more of the biogeographic population of a congregatory species for any length of time are considered to be globally significant Important Bird Areas, see http://www.birdlife.org/datazone/info/ibacritglob.
- The location, size, seasonality, duration and significance of Barn Swallow roosts in Ontario are not known at present.
It is recommended that until key knowledge gaps are addressed, habitat for Barn Swallow in Ontario be defined as follows.
- Nests (including unused nests) that are located on natural or human-created nest sites during the current breeding season (between May 1 and August 31) plus the area within 1.5 m of the nest and the openings the birds use to access nests in enclosed situation
- All used nests at any nest site that has been occupied by Barn Swallows within the previous three breeding seasons.
- Significant roost sites that are used regularly by at least 5,000 birds (ca. 1% of Ontario’s breeding population, adjusted for young-of-the-year) during the post-breeding season (July 1 through October 31). Regular use would be defined as roosting on more than one night per year in at least two years within the past four year This habitat should continue to be protected for a further three years (average age of adults) after the last record of occupancy of significant numbers of birds. The extent of habitat at a significant roost site will need to be defined on a case-by-case basis but should encompass all vegetation that is directly used (e.g., as perches or cover) by roosting birds plus the air space they use to approach the site. Use of ecosite polygons as defined by the most current Ecological Land Classification scheme for Ontario, would be an appropriate tool for delineating the boundaries of significant roost sites.
Foraging habitat is also very important particularly in the vicinity of nest sites, but suitable information for defining the extent and delineating the type of habitat required for foraging is not presently available. Furthermore foraging habitat is not static and likely changes throughout the breeding season. Protection and enhancement of foraging habitat through stewardship and best management practices should be encouraged.
Glossary
Aerial insectivore: This term refers to the suite of bird species that primarily feed on flying insects especially birds that catch insects while in flight.
Asynchronous: Not occurring at the same time. With respect to egg-laying, pairs in a colony often start egg-laying on different dates and therefore nests in a colony are at different stages at any given time during the breeding season.
Committee on the Status of Endangered Wildlife in Canada (COSEWIC): The committee responsible for assessing and classifying species at risk in Canada.
Committee on the Status of Species at Risk in Ontario (COSSARO): The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
Conservation status rank: A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperilled
2 = imperilled
3 = vulnerable
4 = apparently secure
5 = secure
Credible Intervals (lower and upper limits): The credible intervals are a statistical measure of the precision of the population trend. In the case of the Breeding Bird Survey trends provided in this report given the data and the accuracy of the model, there is a 95 percent probability that the average annual trend in the population lies somewhere between the lower and upper credible intervals (i.e., for Barn Swallow in Ontario the BBS data for 2002-2012 indicate there is a 95% probability that the average annual population change is somewhere between -2.93% and 0.363%).
Diurnal: active during the daytime (rather than at night).
Endangered Species Act, 2007 (ESA): The provincial legislation that provides protection to species at risk in Ontario.
Ecological Land Classification scheme: The Ontario Ecological Land Classification (ELC) scheme is a hierarchical system for consistently defining ecological units on the basis of bedrock, climate, physiography and vegetation that is widely used for land use and conservation planning in Ontario.
Extra-pair paternity: Offspring resulting from female copulations with a male other than her social mate (as identified by genetic tests).
Hierarchical Bayesian model: Breeding Bird Survey (BBS) data are analyzed using a Hierarchical Bayesian model that accounts for the effects of variation among observers and routes, first-year observer effects, variations in trend and abundance among strata and annual variation around a long-term trend. More information about BBS analytical methods is available on the BBS web site, http://www.ec.gc.ca/ron-bbs/P006/A001/?lang=e.
Population source-sink dynamics: The concept of how spatial variation in habitat quality affects vital rates and the growth or decline of a population at various scales. Population sources are occupied habitats that produce surplus young whereas population sinks are occupied habitats where productivity is insufficient to offset annual mortality. Persistence of sink populations is dependent on immigration.
Plumage: The pattern, colour and arrangement of feathers covering a bird. Probability of decrease: Indicates the statistical probability that the population has decreased during the specified time period. A value of 1 indicates a 100% probability that the population has declined; whereas a value of 0.939 indicates a 93.9% probability that a population decline has occurred over the time period.
Safe Harbour Agreements: Agreements that enable landowners to assist in the protection or recovery of species at risk or create or enhance their habitat in exchange for legal assurance that doing so will not result in additional restrictions should they wish to modify their land at a later date.
Socially monogamous: Common avian mating system where individuals form pair bond with single member of opposite sex and jointly raise young (include extra-pair young sired by other males).
Species at Risk Act (SARA): The federal legislation that provides protection to species at risk in Canada. This act establishes Schedule 1 as the legal list of wildlife species at risk to which the SARA provisions apply. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
Species at Risk in Ontario (SARO) List: The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
Trend reliability: The overall reliability of the Breeding Bird Survey (BBS) trend information is categorized as low, medium or high based on three measures: the proportion of the species' regional breeding population within the area covered by BBS, the degree of fit of the Hierarchical Bayesian model to the observed data and the width of the 95% credible intervals. Detailed explanations of how each measure is assessed are available on the Definitions section of the Canadian BBS web site, http://www.ec.gc.ca/ron-bbs/P006/A001/?lang=e.
Vital rates: Demographic statistics that determine population growth including productivity (e.g., number of young produced per female per year or lifetime), survivorship (e.g., proportion of adults and young that survive from one year to the next), immigration (e.g., number of yearlings recruited to a breeding population) and emigration (e.g., number of yearlings that disperse to breed elsewhere).
References
Alamenciak, T. 2012. Toronto police pursue fowl foe. Toronto Star article, 12 November 2012. Web site: http://www.thestar.com/news/gta/2012/11/12/toronto_police_pursue_fowl_foe.html [accessed November 2012].
Ambrosini, R., A.M. Bolzern, L. Canova, S. Arieni, A.P. Møller and N. Saino. 2002. The distribution and colony size of barn swallows in relation to agricultural land use. Journal of Applied Ecology 39: 524-534.
Ambrosini, R., L. Bani, D. Massimino, L. Fornasari and N. Saino. 2011a. Large-scale spatial distribution of breeding Barn Swallows Hirundo rustica in relation to cattle farming. Bird Study 58: 495-505.
Ambrosini, R., D. Rubolini, A.P. Møller, L. Bani, J. Clark, Z. Karcza, D. Vangeluwe, C. de Feu, F. Spina, and N. Saino. 2011b. Climate change and the long-term northward shift in the African wintering range of the barn swallow Hirundo rustica Climate Research 49(2): 131-14.
Ambrosini, R., D. Rubolini, P. Trovo, G. Liberini, M. Bandini, A. Romano, C. Sicurella, C. Scandolara, M. Romano, and N. Saino. 2012. Maintainance of livestock farming may buffer population decline of the Barn Swallow Hirundo rustica. Bird Conservation International 22: 411-428.
Anthony, L.W. and C.A. Ely. 1976. Breeding biology of Barn Swallows in west-central Kansas. Kansas Ornithological Society Bulletin 27(4): 37-44.
Balbontín, J., A.P. Møller, I.G. Hermosell, A. Marzal, M. Reviriego, and F. de Lope. 2009a. Geographic patterns of natal dispersal in barn swallows Hirundo rustica from Denmark and Spain. Behavioral Ecology and Sociobiology 63:1197–1205.
Balbontín, J., A.P. Møller, I.G. Hermosell, A. Marzal, M. Reviriego, and F. de Lope. 2009b. Divergent patterns of impact of environmental conditions on life history traits in two populations of a long-distance migratory bird. Oecologia 159: 859-872.
Barclay, R.M.R. 1988. Variation in the costs, benefits and frequency of nest reuse by Barn Swallows (Hirundo rustica). Auk 105: 53-60.
Basili, G.D. and S.A. Temple. 1999. Winter ecology, behavior, and conservation needs of Dickcissels in Venezuela. Studies in Avian Biology 19:289-299.
Bent, A.C. 1942. Barn Swallow Hirundo rustica. In Newforth, P.Q. (ed.). Life Histories of Familiar North American Birds [electronic book]. Web site: www.birdsbybent.com/ch21-30/bswallow.html [accessed March 2013].
Bell, D., pers.comm. 2012. Email correspondence with A. Heagy. Several dates between November 2012 and January 2013. Bank Swallow Project SAR Intern (Summer 2012), Bird Studies Canada.
BirdLife International. 2004. Birds in the European Union: a status assessment. Wageningen, The Netherlands: BirdLife International.
BirdLife International. 2013a. Important Bird Areas factsheet: Ebok-Kabaken, Nigeria. Web site: http://www.birdlife.org/datazone/sitefactsheet.php?id=6760 [accessed June 2013].
BirdLife International. 2013b. Important Bird Areas factsheet: La Mercy/Mount Moreland South Africa. Web site: http://www.birdlife.org/datazone/sitefactsheet.php?id=20353 [accessed June 2013].
BirdLife International. 2013c. Important Bird Areas factsheet: Okavango Delta, Botswana. Web site: http://www.birdlife.org/datazone/sitefactsheet.php?id=6047 [accessed June 2013].
Bird Studies Canada. 2013. Creating nesting sites for Barn Swallows: summary report. Unpublished report prepared for Ontario Ministry of Natural Resource Species at Risk Fund. February 2013. 15 pp.
Blancher, P.J., and A.R. Couturier. 2007. Population Size Estimates for Ontario Birds, based on point counts. Pp. 655-657 in M.D. Cadman, D.A. Sutherland, G.G. Beck, D. Lepage, and A.R. Couturier (eds.). 2007. Atlas of the Breeding Birds of Ontario, 2001-2005. Bird Studies Canada, Environment Canada, Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature, Toronto, xxii + 706 pp.
Blancher, P.J., M.D. Cadman, B.A. Pond, A.R. Couturier, E.H. Dunn, C.M. Francis and R.S. Rempel. 2007. Changes in Bird Distributions between Atlases. Pp. 32-48 in M.D. Cadman, D.A. Sutherland, G.G. Beck, D. Lepage, and A.R. Couturier (eds.). 2007. Atlas of the Breeding Birds of Ontario, 2001-2005. Bird Studies Canada, Environment Canada, Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature, Toronto, xxii + 706 pp.
Blancher, P.J., K.V. Rosenberg, A.O. Panjabi, B. Altman, A.R. Couturier, W.E Thogmartin and the Partners in Flight Science Committee. 2013. Handbook to the Partners in Flight Population Estimates Database, Version 2.0. PIF Technical Series No 6. Web site: http://www.partnersinflight.org/pubs/ts/ [accessed July 2013] [link inactive].
Blancher, P., pers.comm. 2012. Communications with A. Heagy et al. at Aerial Forager Workshop at Bird Studies Canada March 2012 and related email correspondence with A. Heagy, November 2012. Research Scientist Emeritus, Canadian Wildlife Service, Environment Canada, Ottawa.
Blancher, P., pers.comm. 2013. Email communications with A. Heagy. 24 April 2013. Research Scientist Emeritus, Canadian Wildlife Service, Environment Canada, Ottawa.
Boutin, C., K.E. Freemark, and D.A. Kirk. 1999. Farmland bird in southern Ontario: field use, activity patterns and vulnerability to pesticide use. Agriculture, Ecosystems and Environment 72: 239-254.
Boyd, N., pers. comm. 2012. pers. comm. 2012. Communication with A. Heagy et al. at the Barn Swallow workshop, Guelph Arboretum, Guelph, Ontario. December 2012. Team Leader, Environmental Policy Branch, Ontario Ministry of Transportation, St. Catherines.
Brewer, D., A. Diamond, E.J. Woodsworth, B.T. Collins, and E.H. Dunn. 2000. Canadian Atlas of Bird Banding, Volume 1: Doves, Cuckoos, and Hummingbirds through Passerines, 1921-1995. Special Publication, Canadian Wildlife Service, Canada. 395 pp.
Brown, C.R. and M.B. Brown. 1999a. Barn Swallow (Hirundo rustica), The Birds of North America Online (A. Poole, Ed.). Ithaca: Cornell Lab of Ornithology. Web site: http://bna.birds.cornell.edu/bna/species/452 [accessed October 2012].
Brown, C.R. and M.B. Brown. 1999b. Natural selection on tail and bill morphology in Barn Swallows Hirundo rustica during severe weather. Ibis 141:652-659.
Bryant, D.M. and A.K. Turner. 1982. Central place foraging by swallows (Hirundinidae) -the question of load size. Animal Behaviour 30: 845-856.
Burrell, M., pers. comm. 2013. Email communications with A. Heagy. October 2013. eBird Ontario editor, Bird Studies Canada.
Cadman, M., pers. comm. 2012. Verbal communications with A. Heagy. November and December 2012. Also, Communication with A. Heagy et al. at Aerial Forager Workshop at Bird Studies Canada (March 2012) and at the Barn Swallow workshop, Guelph Arboretum, Guelph, Ontario (December 2012). Landbird Biologist, Canadian Wildlife Service – Ontario Region, Environment Canada.
Cadman, M.D., P.F.J. Eagles, and F.M. Helleiner (eds.). 1987. Atlas of the Breeding Birds of Ontario. University of Waterloo Press, Waterloo, ON. 617 pp.
Cadman, M.D., D.A. Sutherland, G.G. Beck, D. Lepage, and A.R. Couturier (eds.). 2007. Atlas of the Breeding Birds of Ontario, 2001-2005. Bird Studies Canada, Environment Canada, Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature, Toronto, xxii + 706 pp.
Calvert, A.M. 2012. Research priorities to support the conservation of aerial insectivores in Canada. Unpublished report. Environment Canada and Bird Studies Canada. May 2012. 18 pp.
Campbell, W., G.E.J. Smith, M.C.E. McNall, G.W. Kaiser, J.M. Cooper. I. McTaggart-Cowan, N.K. Dawe. 1997. Birds of British Columbia, Volume 3: Passerines – Flycatchers through Vireos. University of British Columbia Press, Vancouver. 696 pp.
Clapp, R.B., M.K. Klimkiewicz, and A.G. Futcher. 1983. Longevity records of North American Birds: Columbidae through Paridae. Journal of Field Ornithology. 54(2): 123-137.
Clark, W.R. 1984. Canada’s Capistrano. Nature Canada 13(1): 14-15.
Clark, W.R. and C.A. Clark. 1987. Barn Swallow in M.D. Cadman, P.F.J. Eagles, and F.M. Helleiner (eds.): Atlas of the breeding birds of Ontario. University of Waterloo Press, Waterloo, ON.
Coffait, L., R.A. Robinson, J.A. Clark and B.M Griffin. 2011. Fattening strategies of British and Irish Barn Swallows Hirundo rustica prior to autumn migration. Ringing & Migration 26: 15–23.
COSEWIC. 2011. COSEWIC assessment and status report on the Barn Swallow Hirundo rustica in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. ix +37 pp. Web site: www.sararegistry.gc.ca/status/status_e.cfm [accessed October 2012].
COSSARO. 2011. COSSARO candidate species at risk evaluation form for Barn Swallow (Hirundo rustica). Committee on the Status of Species at Risk in Ontario.14 pp.
DeSante, D.F. and D.R. Kaschube. 2009. The Monitoring Avian Productivity and Survivorship (MAPS) Program 2004, 2005, and 2006 report. Bird Populations 9: 86-169.
Drolet, B., pers. comm. 2012. Email correspondence with Z. Lebrun-Southcott. November to December 2012. Biologist, Environmental Stewardship Branch, Canadian Wildlife Service – Quebec Region, Environment Canada.
Dunn, P.O. and D. W. Winkler. 1999. Climate change has affected breeding date of Tree Swallows throughout North America. Proceedings of the Royal Society of London, Series B 266:2487–2490. Web site: http://dx.doi.org/10.1098/rspb.1999.0950 [accessed February 2013].
Ebird Canada. 2013. Ebird Canada (electronic database). Bird Studies Canada. Web site: http://ebird.org/content/canada/ [accessed 21 October 2013].
Environment Canada. 2013. North American Breeding Bird Survey -Canadian Trends Web site, Data-version 2011. Environment Canada, Gatineau, Quebec. Web site: http://www.ec.gc.ca/ron-bbs/ [accessed September 2013].
Environment Canada. in prep. North American Breeding Bird Survey -Canadian
Trends, Data-version 2012. Environment Canada, Gatineau, Quebec.
Erskine, A.J. 1979. Man’s influence on potential nesting sites and populations of swallows in Canada. Canadian Field-Naturalist 93: 371-377.
European Bird Census Council. 2012. Trends of common birds in Europe, 2012 update. Pan-European Common Bird Monitoring Scheme web site: http://www.ebcc.info/index.php?ID=500 [accessed March 2013].
Evans, K.L., J.D. Wilson and R.B. Banbury. 2007. Effects of crop type and aerial invertebrate abundance on foraging barn swallows Hirundo rustica. Agriculture, Ecosystems and Environment 122:267-273.
Ewins, P.J., P.D. Round, and D.R. Bazely. 1991. Urban roosting of Barn Swallows Hirundo rustica wintering in Thailand. Forktail 6: 68-70.
Furber, N., pers. comm. 2012. Communication with A. Heagy et al. at the Barn Swallow workshop, Guelph Arboretum, Guelph, Ontario. December 2012. Bander, Ruthven Park station, Haldimand Bird Observatory.
Ghilain, A. and M. Bélisle. 2008. Breeding success of Tree Swallows along a gradient of agricultural intensification. Ecological Applications 18(5): 1140-1154.
Godfrey, W.E. 1986. The Birds of Canada. Revised edition. National Museum of Canada, Ottawa, ON. 595 pp.
Godwin, C.E. 1995. A bird-finding guide to Ontario, revised edition. University of Toronto Press, Toronto. 477 pp.
Grüeebler, M.U., F. Korner-Nievergelt, J. von Hirschheydt, 2010. The reproductive benefits of livestock farming in barn swallows Hirundo rustica: qualify of nest site or foraging habitat? Journal of Applied Ecology 47(6): 1340-1347.
Haemig, P.D., H. Hernandez, J. Waldenstrom, J. Bonnedahl, B. Olsen. 2008. Barn swallows (Hirundo rustica) test negative for Salmonella. Vector-Borne and Zoonotic Diseases 8(4): 451-453.
Halmos, G., Z.S. Karcza, A. Némethod, and T. Csörgő. 2010. The migratory fattening of the Barn Swallow Hirundo rustica in Hungary. Acta Zoologica Academiae Scientiarum Hungaricae 56 (1): 73–87.
Hobson, K.A. and S. van Wilgenburg. 2013. Update on connectivity research: inferring connectivity using geolocators. Presentation by Environment Canada at Barn Swallow workshop held at Holiday Inn, Guelph, Ontario. August 2013.
Holroyd, G.L. 1975. Nest site availability as a factor limiting population size of swallows. Canadian Field-Naturalist 89: 60-64.
Howell, S.N.G. and S. Webb. 1995. A guide to the birds of Mexico and northern Central America. Oxford University Press. 1010 pp.
Hussell, D.J.T. 2003. Climate change, spring temperatures, and timing of breeding of Tree Swallows (Tachycineta bicolor) in southern Ontario. Auk 120:607-618.
Illif, M.J., B.L. Sullivan, and C.L. Wood. 2011. The Changing Seasons: the eBird era. North American Birds 65: 394-405.
Intergovernmental Panel on Climate Change. 2007. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Solomon S., D. Qin, M. Manning, Z. Chen, M. Marquis et al. (eds). Cambridge, UK and New York, USA: Cambridge University Press.
Javorek, S.K., R. Antonowitsch, C.Callaghan, M.Grant, and T. Weins. 2007. Changes to wildlife habitat on agricultural land in Canada, 1981–2001. Canadian Journal of Soil Science 87: 225–233.
Jobin B., C. Latendresse, M. Grenier, C. Maisonneuve, and D. Sebbane. 2010. Recent landscape change at the ecoregion scale in Southern Quebec (Canada): 1993-2001. Environmental Monitoring and Assessment 164: 631-647.
Kragten, S, E. Reinstra, and E. Gertenaar. 2009. Breeding Barn Swallows Hirundo rustica on organic and conventional arable farms in the Netherlands. Journal of Ornithology 150 :515-518.
Latendresse, C., B. Jobin, A. Baril, C. Maisonneuve, C. Boutin and D. Côté. 2008. Dynamique spatiotemporelle des habitats fauniques dans l'écorégion des Basses terres du fleuve Saint-Laurent, 1950-1997. Série de rapports techniques no 494, Environnement Canada, Service canadien de la faune, région du Québec, Québec, 83 p. et annexes.
Lebrun-Southcott, Z., pers. comm. 2013. Email and verbal communications with A. Heagy. Multiple dates between October 2012 and March 2013. Also, Communication with A. Heagy et al. at the Barn Swallow workshop, Guelph Arboretum, Guelph, Ontario. December 2012. Barn Swallow Project Biologist, Bird Studies Canada.
Lepage, D. 2007. Barn Swallow. Pp. 398-399 in M.D. Cadman, P.F.J. Eagles, and F.M. Helleiner (eds.): Atlas of the breeding birds of Ontario. Bird Studies Canada, Environment Canada, Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature, Toronto, xxii + 706 pp.
Lubbe, S.K. and G.R. de Snoo. 2007. Effect of dairy farm management on Swallow Hirundo rustica abundance in the Netherlands. Bird Study 54: 176-181.
Lutmerding, J.A. and A.S. Love. 2012. Longevity Records of North American Birds. Version 2012.2. Patuxent Wildlife Research Center. Bird Banding Laboratory. Laurel MD.
Macoun, J. and J.M. Macoun. 1904. Catalogue of Canadian birds, Part III. Geological Survey of Canada, Ottawa.
Mason,R., H. Tennekes, F. Sánchez-Bayo, and P.U. Jepsen. 2013. Immune suppression of neonicotinoid insecticides at the root of global wildlife declines. Journal of Environmental Immunology and Toxicology 1:3-12.
McGee, B., H. Berges and D. Beaton. 2010. Survey of pesticide use in Ontario, 2008.
Ontario Ministry of Agriculture, Food and Rural Affairs. Toronto, ON. McIlwraith, T. 1886. Birds of Ontario. Hamilton Association. Hamilton, ON.
Mercadante, A.N. and M.T. Stanback. 2011. Out of sight, out of mind? Visual obstructions affect settlement patterns in Barn Swallows (Hirundo rustica). Auk 128: 230-236.
Mineau, P. and C. Palmer. 2013. The impact of the Nation’s most widely used insecticides on birds. Report prepared for the American Bird Conservancy, March 2013.
Mineau, P. and M. Whiteside. 2013. Pesticide acute toxicity is better correlate of U.S. grassland bird declines than agricultural intensification. PLoS One: 8(2): e57457.
Møller, A.P. 1978. Migration of Danish swallows Hirundo rustica in connection with the weather induced disaster in Central Europe in autumn 1974. Dansk Ornitologisk Forenings Tidsskrift 72: 59-60.
Møller, A.P. 1989. Population dynamics of a declining Swallow Hirundo rustica population. Journal of Animal Ecology 58: 1051-1063.
Møller, A. P.1990. Changes in the size of avian breeding territories in relation to the nesting cycle. Animal Behaviour 40: 1070-1079.
Møller, A.P. 2001. The effect of dairy farming on barn swallow, Hirundo rustica abundance, distribution and reproduction. Journal of Applied Ecology 38: 378-389.
Møller, A.P. 2008. The distribution of arrival dates in a migratory bird in relation to environmental conditions, natural selection and sexual selection. Ethology, Ecology & Evolution 20: 193-210.
Møller, A.P. 2011. Behavioral and life history responses to extreme climatic conditions: Studies on a migratory songbird. Current Zoology 57(3): 351-362.
Møller, A.P. and T. Szép. 2002. Survival rate of adult Barn Swallows Hirundo rustica in relation to sexual selection and reproduction. Ecology 83(8): 2220-2228.
Neave, E. and D. Baldwin. 2011. Mixedwoods Plain and Southern Boreal Shield Open Country Birds Habitat Assessment: History and Trends. Unpublished report to Environment Canada, Canadian Wildlife Service – Ontario Region. Downsview, Ontario. 75 pp.
Nebel, S., A. Mills, J.D. McCracken, and P.D. Taylor. 2010. Declines of aerial insectivores in North America follow a geographic gradient. Avian Conservation and Ecology -Écologie et conservation des oiseaux 5(2): 1. Web site: http://www.ace-eco.org/vol5/iss2/art1/ [accessed October 2012].
Newton, I. 2007. Weather-related mass-mortality events in migrants. Ibis 149: 453–467.
Neubauer, G., P. Zielinski, Z. Wojciechowski, E. Buszkiewicz, J. Siekiera, and A. Sierkiera. 2012. Leaving on migration: estimating departure dates of Barn Swallows Hirundo rustica from summer roost using a capture-mark-recapture approach. Bird Study 59(2): 144-154.
OMNR. 2013a. Alter a structure (habitat for Barn Swallow) webpage. Effective July 1, 2013 Web site: /environment-and-energy/alter-structure-habitat-barn-swallow [accessed September 2013].
OMNR. 2013b. General Habitat Description for the Barn Swallow (Hirundo rustica) webpage. Posted February 7, 2013. Web site: /page/barn-swallow-general-habitat-description [accessed September 2013].
Ontario Biodiversity Council (OBC). 2011. Ontario’s Biodiversity Strategy, 2011: Renewing our Commitment to What Sustains Us. Ontario Biodiversity Council, Peterborough.
ONRS 2012. Ontario Nest Record Scheme (electronic database). Royal Ontario Museum and Bird Studies Canada. Web site: http://www.birdsontario.org/onrs/onrsmain.html [accessed October 2012].
Ottawa River Legacy Landmark Partners. n.d. Pembroke: Canada’s Capistrano. Canada Heritage River -Ottawa River. Web site: http://www.ottawariver.org/html/landmarks/pembroke_e.html [accessed 6 March 2013].
Partners in Flight Science Committee. 2013. Population Estimates Database, version 2013. Web site : http://rmbo.org/pifpopestimates [accessed 22 July 2013].
Peck, G.K. and R.D. James. 1987. Breeding Birds of Ontario: Nidiology and Distribution. Vol. 2. Royal Ontario Museum, Toronto.
Petit, L.J., D.R. Petit, D.G. Christian and H.D.W. Powell. 1999. Bird communities of natural and modified habitats in Panama. Ecography 22(3): 292-304.
Pilastro, A. and A. Magnani. 1997. Weather conditions and fat accumulation dynamics in pre-migratory roosting Barn Swallows Hirundo rustica. Journal of Avian Biology 28: 338-344.
Pilastro, A. and F. Spina. 1999. Fat accumulation in pre-migratory roosting Barn Swallows in Europe. Proceedings of the 22nd International Ornithological Congress, Durban 22: 219-228.
Pyle, P. 1997. Identification Guide to North American Birds, Part 1: Columbidae to Ploceidae. Slate Creek Press, Bolinas, CA. 732 pp.
Richardson, K. 2013. 2013 Barn Swallow nesting structure study – Results. Unpublished report prepared for Ontario Ministry of Natural Resource Species at Risk Fund. Bird Studies Canada, October 21, 2013. 3 pp.
Richmond, S., pers.comm. 2013. Personal conversation with A. Heagy regarding preliminary results of a comparison of Ontario Breeding Bird Atlas Barn Swallow abundance and frequency data with Ontario land cover data. August 2013. Post-doctoral researcher, Bird Studies Canada.
Robbins, C.S., D. Bystrak, and P.H. Geissler. 1986. The breeding bird survey: Its first fifteen years 1965–1979. U.S. Fish and Wildlife Service Res. Publ. No. 157. 196 pp.
Robinson, R.A., H.Q.P. Crick, and W.J. Peach. 2003. Population trends of Swallows Hirundo rustica breeding in Britain. Bird Study 50: 1-7.
Robinson, R.A., D.E. Balmer and J.H. Marchant. 2008. Survival rates of hirundines in relation to British and African rainfall. Ringing & Migration 24:1-6.
Ross, R.K., W.R. Clark and J.M. Bouvier. 1984. Survey of a major swallow roost in Pembroke. Ontario Birds 2(1): 34-37.
Rubolini, D., A. Gardiazabal Pastor, A. Pilastro and F. Spina. 2002. Ecological barriers shaping fuel stores in barn swallows Hirundo rustica following the central and western Mediterranean flyways. Journal of Avian Biology 33: 15–22.
Safran, R.J. 2004. Adaptive site selection rules and variation in group size of Barn Swallows: individual decisions predict population patterns. American Naturalist 164(2): 121-131.
Safran, R.J. 2006. Nest site selection in the barn swallow Hirundo rustica: What predicts seasonal reproductive success? Canadian Journal of Zoology 84: 1533-1539.
Safran, R.J. 2007. Settlement patterns of female barn swallows Hirundo rustica across different group sizes: access to colorful males or favored nests? Behavioral Ecology and Sociobiology 61: 1359-1368.
Saino, N., T. Szep, R. Ambrosini, M. Romano and A.P. Møller. 2004. Ecological conditions during winter affect sexual selection and breeding in a migratory bird. Proceedings of the Royal Society Biological Sciences B 271: 681-686.
Saino, N., R. Ambrosini, D. Rubolini, J. von Hardenburg, A. Provenzales, K. Hü, O. Hü, A. Lehikoinens, E. Lehikoinens, K. Rainio, M. Romano, and L. Sokolov. 2011. Climate warming, ecological mismatch at arrival and population decline in migratory birds. Proceedings of the Royal Society B 278: 835-842.
Saino, N., M. Romano, R. Ambrosini, D. Rubolini, G. Boncoraglio, M. Caprioli and A. Romano. 2012. Longevity and lifetime reproductive success of barn swallow offspring are predicted by their hatching date and phenotypic quality. Journal of Animal Ecology 81:1004-1012.
Salvadori, A. 2009. A study of Barn Swallow nestings during the summer of 2008 in Ontario. North American Bird Bander 34(4): 160-164.
Salvadori, A., pers. comm. 2012. Email and verbal communications with A. Heagy. Multiple dates between November and December 2012. Also, Communication with A. Heagy et al. at the Barn Swallow workshop, Guelph Arboretum, Guelph, Ontario. December 2012. Barn Swallow Project Biologist, Bird Studies Canada.
Salvadori, A., M. Cadman, K. Horner and L. Rae. 2011. Barn Swallow populations in Wellington County, 2008-2010. Ontario Birds 29(1): 2-12.
Samuel, D.E. 1971. The breeding biology of Barn and Cliff Swallows in West Virginia. Wilson Bulletin 83(3): 284-301.
Sauer, J.R., J E. Hines, J.E. Fallon, K.L. Pardieck, D.J. Ziolkowski, Jr., and W.A. Link. 2012. The North American Breeding Bird Survey, Results and Analysis 1966 -2011. Version 12.13.2011. USGS Patuxent Wildlife Research Center, Laurel, MD. 22 February 2013 update. Web site: http://www.mbr-pwrc.usgs.gov/bbs/ [accessed March 2013].
Schaub, M. and J. von Hirschheydt. 2008. Effect of current reproduction on apparent survival, breeding dispersal, and future reproduction in barn swallows assessed by multistate capture–recapture models. Journal of Animal Ecology 78: 625-635.
Shields, W.M. 1984. Factors affecting nest and site fidelity in Adirondack Barn Swallows (Hirundo rustica). Auk 101(4): 780-789.
Shutler, D., D.J.T. Hussell, D.R. Norris, D.W. Winkler, R.J. Robertson, F. Bonier, W.B. Rendell, M. Bélisle, R.G. Clark, R.D. Dawson, N.T. Wheelwright, M.P. Lombardo, P.A. Thorpe, M.A. Truan, R. Walsh, M.L. Leonard, A.G. Horn, C.M. Vleck, D. Vleck, A.P. Rose, L.A. Whittingham, P.O. Dunn, K.A. Hobson and M.T. Stanback. 2012. Spatiotemporal patterns in nest box occupancy by Tree Swallows across North America. Avian Conservation and Ecology 7(1): 3. Web site: http://dx.doi.org.proxy1.lib.uwo.ca/10.5751/ACE-00517-070103.
Smith, H.G. and R. Montgomerie. 1991. Sexual selection and the tail ornaments of North American barn swallows. Behavioral Ecology and Sociobiology 28: 195-201.
Smith, H.G. and R. Montgomerie. 1992. Male incubation in Barn Swallows: the influence of nest temperature and sexual selection. The Condor 94:750-759.
Smith, H.G., R. Montgomerie, T.Pðldmaa, B.N.White, and P.T. Boag. 1991. DNA fingerprinting reveals relation between tail ornaments and cuckoldry in barn swallows, Hirundo rustica. Behavioral Ecology 2(1): 90-98.
Smith, P., pers. comm. 2012. Communication with A. Heagy et al. at the Barn Swallow workshop, Guelph Arboretum, Guelph, Ontario. December 2012. Senior Policy Advisor, Ontario Ministry of Agriculture and Food.
Speich, S.M., H.L. Jones, and E.M. Benedict. 1986. Review of the natural nesting of the Barn Swallow in North America. American Midland Naturalist 115(2): 248-254.
Spina, F. 2001. EURING swallow project third newsletter years 1999–2000. EURING Newsletter 3. Web site: http://www.euring.org/about_euring/newsletter3/index.html [accessed October 2012].
Statistics Canada. 2007. Selected Historical Data from the Agricultural Census. Electronic resource, release date December 11, 2007. Web site: http://www.statcan.gc.ca/pub/95-632-x/2007000/t/4185570-eng.htm#35 [accessed July 2013].
Statistics Canada. 2012. 2011 Census of Agriculture. Electronic resource, release date December 10, 2012. Web site: http://www.statcan.gc.ca/ca-ra2011/ [accessed July 2013].
Tate, J. 1986. The blue list for 1986. American Birds 40: 227-235.
Taylor, M., pers.comm. 2013. Communication with A. Heagy et al. at the Barn Swallow workshop, Holiday Inn, Guelph, Ontario. August 2013. Environmental planner, Northeast Region, Ontario Ministry of Transportation.
Tozer, R. 2012. Birds of Algonquin Park. Friends of Algonquin Park, Whitney, ON. 474 pp.
Turner, A. 1980. The use of time and energy by aerial feeding birds. Ph.D. Thesis, Department of Biology, University of Stirling, UK. 353 pp.
Turner, A.K. 2004. Family Hirundinidae (Swallows and Martins). Pp. 602-685 in Del Hoyo, J., A. Elliott, and D.A. Christie (eds). Handbook of the Birds of the World. Volume 9. Cotingas to pipits and wagtails. Lynx Edicions, Barcelona.
Turner, A. 2006. The Barn Swallow. T & AD Poyser, London.
Turner, A. 2009. Climate change: a Swallow’s eye view. British Birds 102: 3-16. Turner, A. and C. Rose. 1989. Swallows and martins: an identification guide and handbook. Houghton-Mifflin.
Turner, K. T. and J.G. Kopachena. 2009. Breeding biology of the Barn Swallow (Hirundo rustica) in northeast Texas with temporal and geographic comparisons to other North American studies. Texas Journal of Science 61(2): 131-146.
Turner, S., pers.comm. 2012. Communication with A. Heagy et al. at the Barn Swallow workshop, Guelph Arboretum, Guelph, Ontario. December 2012. SAR Coordinator, Ruthven Park Historic Site, Lower Grand River Land Trust.
Vannini, J.P. 1994. Nearctic avian migrants in coffee plantations and forest fragments of Southwestern Guatemala. Bird Conservation International 4(2-3): 209-232.
Van Vleck, R. 2013. Artificial Barn Swallow nests. http://www.americanartifacts.com/smma/per/b4info.htm [accessed 22 July 2013].
Weir, R.D. 2008. Birds of the Kingston region, 2nd edition. Kingston Field Naturalists, Kingston, Ontario. 611 pp.
Winkler, D.W. 2006. Roosts and migrations of swallows. Hornero 21(2): 85-97. Zduniak, P., P. Czechowski, and G. Jedro. 2011. The effect of nesting habitat on reproductive output of the Barn Swallow (Hirundo rustica): a comparative study of populations from atypical and typical nesting habitats in western Poland. Belgium Journal of Zoology 141(1): 38-43.