Bogbean Buckmoth Recovery Strategy
This document advises the ministry on ways to ensure healthy numbers of the bogbean buckmoth, a threatened or endangered species, return to Ontario.

Recovery strategy prepared under the Endangered Species Act, 2007
About the Ontario Recovery Strategy Series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species' persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. There is a transition period of five years (until June 30, 2013) to develop recovery strategies for those species listed as endangered or threatened in the schedules of the ESA. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of Natural Resources Species at Risk webpage at: www.ontario.ca/speciesatrisk
Recommended citation
Gradish, A. and M. Tonge. 2011. Recovery Strategy for the Bogbean Buckmoth (Hemileuca sp.) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. vi + 19 pp.
Cover illustration: Michael Runtz
© Queen’s Printer for Ontario, 2011
ISBN 978–1–4435–6779–4 (PDF)
Content (excluding the cover illustration) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée Recovery strategies prepared under the Endangered Species Act, 2007, n'est disponible qu'en anglais en vertu du Règlement 411/97 qui en exempte l'application de la Loi sur les services en français. Pour obtenir de l'aide en français, veuillez communiquer avec Cathy Darevic au ministère des Richesses naturelles au 705–755–5580.
Authors
Angela Gradish, Researcher, University of Guelph.
Melissa Tonge, Wildlife Biologist Contractor for the Nature Conservancy of Canada
Acknowledgments
We would like to thank the following individuals for providing background information and technical comments: John Legge, Ted Mosquin, James Pagé, Don Sutherland, Chris Schmidt, Ross Layberry, Sandy Bonnano, Shaun Thompson, Rob Foster and Erin White. We would also like to thank Tanya Pulfer for her assistance with writing the proposal and providing copy edits. Finally, thank you to Bree Walpole who oversaw the preparation of the recovery strategy.
Declaration
This recovery strategy for the Bogbean Buckmoth has been developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). It has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all of the individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ontario Ministry of Natural Resources
Executive summary
The Bogbean Buckmoth (Hemileuca sp.) is a rare moth known to occur only in North America in New York State and near Ottawa in Ontario. In Ontario, it is classified as endangered by the Committee on the Status of Species at Risk in Ontario (COSSARO) due to its habitat specificity and extremely limited geographic range. It is currently found at two sites in southeast Ontario: Richmond Fen Wetland and White Lake Fen Wetland Complex. The actual area occupied by the species in Ontario is less than 3 square kilometers and is thought to support approximately 3,000 adult Bogbean Buckmoths.
Adults are medium to large black moths, with wide, white wing bands with wavy or scalloped outer edges, each containing a small discal (circular) spot. There is marked sexual dimorphism with females being larger than males. The species is restricted to open, calcareous, low shrub fens containing large amounts of Bogbean (Menyanthes trifoliata). This habitat requirement is unique to this species as most buckmoths are found in very dry habitats (Legge et al. 1996).
Ontario populations of Bogbean Buckmoth may be threatened by habitat changes including water level fluctuations, land development, invasive plant species, insecticide applications and long–term loss of wetland habitat from climate change. Plant species, such as European Common Reed (Phragmites australis spp. australis), Glossy Buckthorn (Frangula alnus) and Narrow–leaved Cattail (Typha angustifolia) can invade and crowd open fens and outcompete Bogbean Buckmoth host plants. Human induced water level fluctuations may threaten populations at White Lake. In addition, insecticide applications for Gypsy Moth (Lymantria dispar) are considered a potential threat depending on spray timing and concentration (NatureServe 2010).
The recovery goal is to sustain current populations and distributions of Bogbean Buckmoth at extant locations and to expand populations into suitable, but currently unoccupied habitat within its current range in Ontario. To accomplish this, several recovery objectives have been identified.
- Conduct a quantitative assessment to determine what constitutes a sustainable and secure population in Ontario.
- Fill knowledge gaps on taxonomy, ecology, distribution, behaviour, population dynamics, mortality factors and habitat use in the species' Ontario range.
- Reduce or mitigate threats on Bogbean Buckmoth populations.
- Increase public awareness and understanding of Bogbean Buckmoth populations.
- If it is determined that the species was historically widespread, then consider the introduction of Bogbean Buckmoth populations into areas of continuous, unoccupied but otherwise suitable habitat, where feasible.
Considering the Bogbean Buckmoth’s limited geographic range, it is recommended that the area prescribed as regulated habitat for this species include all occupied sites. In
Ontario, this includes the Richmond Fen Wetland and White Lake Fen Wetland Complex. Both of these sites are calcareous fens that support large populations of the Bogbean (Menyanthes trifoliata).
The area prescribed as habitat at each of these sites should include the extent of the fen vegetation, adjoining wetland complexes and 120 metres beyond them to protect the structure and function of the fen. The 120–metre distance is a historically used set– back for resource protection in Ontario (Ontario 1992) and was chosen because developments within 120 metres of a wetland have a reasonable probability of affecting the ecological functions of the wetlands which they surround (OMNR 2010). In addition, if new locations for the Bogbean Buckmoth are discovered or the species is re– introduced, then these areas should also be prescribed as habitat in the regulation.
1. Background information
1.1 Species assessment and classification
Common name: Bogbean Buckmoth
Scientific name: Hemileuca sp.
SARO List Classification: Endangered
SARO List History: Endangered (2010)
COSEWIC Assessment History: Endangered (2009)
SARA Schedule 1: No Schedule, No Status
Conservation status rankings:
GRANK: G1Q NRANK: N1 SRANK: S1
The glossary provides definitions for the abbreviations above.
1.2 Species description and biology
Species description
In North America, there are approximately 20 species of buckmoths (Hemileuca), a well– studied group of silk moths (Saturniidae) (Tuskes et al. 1996, Rubinoff and Sperling 2004). Populations of buckmoth in the Great Lakes region vary to some extent in morphology, ecology and behaviour, and these different populations have been identified as H. maia, H. lucina, or H. nevadensis. Collectively, these species are referred to as the H. maia complex, maia being the oldest name in the group. The Great Lakes populations comprise a sub–set of this complex (Tuskes et al. 1996). Bogbean Buckmoth (also known as Cryan’s Buckmoth – see Legge et al. 1996 and Pryor 1998), is part of the H. maia species complex. Until recently, it was considered the only buckmoth in eastern Canada (COSEWIC 2009); however, in 2005, a population of the Great Plains taxon currently classified as H nevadensis latifascia was discovered west of Rainy River, Ontario (C. Schmidt pers. comm. 2010). As the species boundaries in this group are not well defined, the species–level classification of Bogbean Buckmoth remains tentative (NatureServe 2010). Because Bogbean Buckmoth differs from H. lucina and H maia (the only other northeastern North American species of the maia complex) in mating biology, morphology, primary host plant and geographic range, some experts feel that it is a valid subspecies of H nevadensis (NatureServe 2010). Genetic studies have confirmed that it belongs to the H maia complex, but molecular data could not distinguish it clearly from other named species of this group (Legge et al. 1996, Rubinoff and Sperling 2004). Similarly, Legge et al. (1996) could not find molecular evidence to delineate the species in the Great Lakes populations. However, based on ecological characteristics, they concluded that the Bogbean Buckmoth represents an evolutionary significant unit, having unique diagnostic characteristics that distinguish it from the rest of the group [e.g., Bogbean (Menyanthes trifoliate), also known as Buckbean or Common Buckbean, as the primary larval host plant], but could not assign it a specific taxonomic rank. Others speculated that the entire group may represent one widespread species with regional variability in host plant and habitat (Scholtens and Wagner 1994, Rubinoff and Sperling 2004). Scholtens and Wagner (1997) concluded that the Great Lakes populations likely represent one species showing clinal variation (i.e., gradual phenotypic or genetic variation of a species over a geographical area) in wing morphology from north to south. However, their findings cannot rule out the possibility that this morphological differentiation results from a hybrid zone between distinct species. Similarly, Rubinoff and Sperling (2004) maintain that further sampling and molecular study may reveal a monophyletic, DNA–based difference between the Bogbean Buckmoth and H maia.
Adult Bogbean Buckmoths are medium to large black moths, with wide, white wing bands, each containing a small discal (circular) spot (COSEWIC 2009, NatureServe 2010). The sexes are dimorphic, with females larger than males. Forewings measure 26 to 32 mm long for males and 32 to 36 mm for females (COSEWIC 2009). The abdomen is red–tipped in males (NatureServe 2010). Morphologically, Bogbean Buckmoths are extremely similar to other species within this group (Legge et al. 1996). Compared to other eastern buckmoths, the Ontario and New York adults are larger, very translucent, have wider wing bands with wavy or scalloped outer edges, and they demonstrate a moderate degree of maculation (i.e., spottiness) in comparison to all other species in this complex (COSEWIC 2009, NatureServe 2010).
Earlier larval instars (developmental stage between each moult until sexual maturity) of Bogbean Buckmoth larvae are similar to those of others in this complex, having a predominantly black body and spines (COSEWIC 2009). However, final instar larvae are very distinct in that the yellow spiracular (lateral) stripe characteristic of the Great Lakes Hemileuca (i.e., all maia–complex species other than Bogbean Buckmoth occurring in wetlands in the Great Lakes region, including Ohio, Indiana, Illinois, Michigan, and Minnesota) is greatly reduced or entirely absent (COSEWIC 2009, NatureServe 2010). Larval maculation also is reduced compared to others in this group (NatureServe 2010). Larvae have branched dorsal spines that most closely resemble those of H lucina larvae in colour (reddish–orange) (COSEWIC 2009). The head capsule and prolegs are reddish–brown (COSEWIC 2009). Late–instar Bogbean Buckmoth larvae measure 40 to 65 mm long and 8 mm in diameter (Pryor 1998, COSEWIC 2009).
Species biology
Bogbean Buckmoth is a day–flying moth with a one year life cycle consisting of nine stages: egg, six larval instars, pupa and adult (Pryor 1998). Adults emerge in mid– to late September, with a flight period lasting approximately three weeks (Layberry 1980, Pryor 1998, COSEWIC 2009). Bogbean Buckmoths typically fly on warm, sunny days, and they are generally inactive on cloudy, cool (less than 12°C), windy days (Pryor 1998). Adults are capable of flying for several kilometers, but rarely leave their preferred fen habitat (Pryor 1998, NatureServe 2010). Adults typically fly within a height of one metre from the ground; thus, tall shrubs and forest present potential barriers to movement (COSEWIC 2009).
Females use pheromonal cues to attract males, mate once, and deposit all of their eggs in one day (Tuskes et al. 1996). Females have short, frequent flights and males fly for longer periods and distances and mate multiple times (Pryor 1998, COSEWIC 2009). Females generally only survive for one day, whereas males live for several days (Tuskes et al. 1996, COSEWIC 2009); however, Stanton (1998) managed to recapture a female and a male after 9 and 12 days, respectively. Females lay clusters of egg rings containing 100 to 180 eggs on the stems of a variety of woody and some herbaceous plants [e.g., Red Maple (Acer rubrum), Speckled Alder (Alnus incana sp. rugosa), Virginia Chain Fern (Woodwardia virginica), sedges (Carex sp.), Leatherleaf (Chamaedaphne calyculata), Sweet Gale (Myrica gale), Bog Willow (Salix pedicellaris), Narrow–leaved Meadow–sweet (Spiraea alba) and Red Osier Dogwood (Cornus sericea)] 10 to 40 cm above the ground (Pryor 1998). Bogbean is not used as a site for egg laying because it senesces with declining light levels and water temperatures and subsequently the leafstalks die off with the frost (D. Sutherland pers. comm. 2010). Instead, eggs are laid on the stems of nearby plants and upon hatching, the larvae move to the Bogbean (Legge et al. 1996). Where a female chooses to lay her eggs appears to be dependent on structural attributes of the plant (e.g., stem diameter of typically 2.7 to 3.8 mm, but as narrow as 1.8 mm, host height of 35 to 47 cm, low foliage density), as opposed to a plant species preference (Pryor 1998). Pryor (1998) reported that egg rings increased in length as stem diameter decreased; thus, the number of eggs remains relatively constant regardless of plant type used. In Ontario at the White Lake fen, egg masses also have been observed on American Common Reed (Phragmites australis ssp. americanus), which measures at least seven millimetres in diameter and 183 to 244 cm in height (R. A. Layberry pers. comm. 2010). Adults do not feed (COSEWIC 2009).
Bogbean Buckmoth overwinters as an egg and the larvae emerge the following spring in late May or early June (Legge et al. 1996, COSEWIC 2009). In New York, newly emerged larvae feed primarily on Bog Cranberry (Vaccinium macrocarpon) for the first 12 days, at which point the Bogbean leafs out and the larvae move to this species as their primary host plant (Pryor 1998, COSEWIC 2009). In contrast, Stanton (1998) observed newly emerged larval feeding predominantly on Bogbean, with only five egg masses feeding on Bog Willow or Leatherleaf. Generally, Bogbean appears to be the preferred host plant by earlier larval instars and this host–plant association is unique to this species (Legge et al. 1996). However, populations of early instar larvae feeding on Bogbean have been observed to concurrently feed on Bog Birch (Betula pumila), Narrow–leaved Meadow–sweet, willow species (Salix sp.), and Sage–leaved Willow (Salix candida) in Ontario and/or Wisconsin. In New York, later instars have been observed to feed more heavily on other plant species including: Speckled Alder, Black Chokeberry (Aronia melanocarpa), various sedges, Leatherleaf, Common Winterberry (Ilex verticillata), oak species (Quercus sp.) and Bog Willow (Pryor 1998). Ontario populations of Bogbean Buckmoth larvae feed primarily on Bogbean until late July, but like New York populations, it is not the first host plant for newly emerged larvae (COSEWIC 2009). In Ontario, later instar larvae also have been observed feeding on Bog Birch, Narrow–leaved Meadow–sweet, Slender Willow (Salix petiolaris) and Bebb’s Willow (Salix bebbiana). It is thought that larvae may switch to these alternate host plants once Bogbean has been exhausted (COSEWIC 2009). Early instar larvae tend to feed in clusters throughout the day, whereas later instars are typically seen in isolation, feeding during the day and possibly at night (Pryor 1998, COSEWIC 2009). In late July, the larvae leave the plant and burrow into peat moss (Sphagnum sp.) to pupate (Pryor 1998, COSEWIC 2009).
1.3 Distribution, abundance and population trends
The rounded global status of Bogbean Buckmoth is critically imperiled (G1Q) (NatureServe 2010). Less than ten populations exist and these have been documented as occuring only in Ontario and New York (Legge et al. 1996, Tuskes 1996, Pryor 1998, NatureServe 2010, C. Schmidt pers. comm. 2010). However, Tuskes (1996), Kruse (1998), and C. Schmidt (pers. comm. 2010) also note Bogbean–feeding populations of buckmoths in Wisconsin. It is unclear whether these populations represent the same species as those found in Ontario and New York. Presumably, glacial retreat left these Great Lakes populations in disjointed habitats (Pryor 1998). Globally, the total area occupied by this species is less than 100 to 250 km2, and in Canada, it occupies less than 3 km2 (COSEWIC 2009, NatureServe 2010). It was first discovered in eastern Ontario in 1977 (Layberry 1980) and currently only occurs at four sites in this region: two fens near Richmond south of Ottawa (the Richmond and Phragmites fens), herein referred to as the Richmond Fen Wetland, and two fens 50 km west of Richmond (the White Lake and Hayes Bay fens), herein referred to as the White Lake Wetland Complex (COSEWIC 2009, Figure 1). Criteria have been developed for mapping buckmoth occurrences (NatureServe 2010): a separation distance of 2 km is required for populations with unsuitable intervening habitat and a distance of 10 km between populations with suitable intervening habitat for occurrences to be considered independent. Based on these criteria, the Ontario sites can be considered four distinct populations: the two fens at the Richmond Fen Wetland, and two fens at the White Lake Wetland Complex which are separated by 2.2 and 3.2 km of unsuitable habitat, respectively (COSEWIC 2009). However, according to COSEWIC (2009) definitions, the four sites represent two locations that may function as two metapopulations. As the two locations are 50 km apart and thus too great a distance for the Bogbean Buckmoth to be able to recolonize from one pair of fens to the other, this species is considered severely fragmented (COSEWIC 2009). Extensive searches have taken place at some other locations of suitable habitat in Ontario (Stoco Fen, Long Swamp Fen, Mer Bleue, Alfred Bog, Minesing Swamp, three fens on Lowney Lake and other road–accessible fens near White Lake), but no new populations have been found since the original discovery of the four sites (COSEWIC 2009, C. Schmidt pers. comm. 2010).
The most recent survey conducted in 2008 at the four sites in Ontario estimated the presence of approximately 6,200 larvae. Taking into account natural mortality during pupation and losses due to predation or parasitism [based on data collected from H Maia populations in Massachusetts (Selfridge et al. 2007)], approximately 3,000 of these would be expected to survive to adulthood (COSEWIC 2009). Since 1979 however, the number of Bogbean Buckmoth larvae documented at Richmond Fen alone has ranged from as low as one larva to thousands. Due to significant annual variation in population numbers and the intermittency of monitoring, determining long–term population trends in Ontario has been difficult (COSEWIC 2009). Over its entire range, Bogbean Buckmoth appears to have experienced a moderate to large long term decline of 25 to 90 percent due to habitat loss (COSEWIC 2009, NatureServe 2010). However, to date, habitat loss does not appear to have significantly impacted Ontario populations (COSEWIC 2009).
Figure 1. Ontario distribution of Bogbean Buckmoth (COSEWIC 2009)
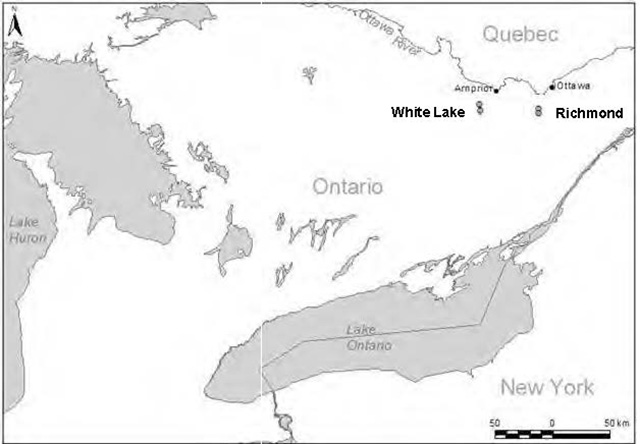
Enlarge Figure 1. Ontario distribution of Bogbean Buckmoth
1.4 Habitat needs
Bogbean Buckmoth is restricted to open, calcareous, low shrub fens containing large amounts of Bogbean (Pryor 1998, COSEWIC 2009, NatureServe 2010). This habitat requirement is unique to this species as most buckmoths are found in very dry habitats (Legge et al. 1996). The Richmond Fen Wetland site consists of palustrine fens on a limestone plain and peat landform, and the fens at the White Lake Wetland Complex site sit atop lime–rich marble (COSEWIC 2009).
The most densely populated area at the Richmond Fen Wetland is a low shrub fen dominated by Sweet Gale, Bog Birch, Bog Willow and other willow species, with patches of open fens containing sedges and Water Horsetail (Equisetum fluviatile). The other site at the Richmond Fen Wetland is an open fen surrounded by conifer swamp and dominated by sedges including Wire Sedge (Carex lasiocarpa), Twig–rush (Cladium mariscoides) and American Common Reed. Fens at White Lake have similar dominant species (COSEWIC 2009). Eastern Prairie Fringed–orchid (Platanthera leucophaea), a globally rare species, is found at three of the four fen sites (COSEWIC 2009). At all four sites, larvae are most abundant in patches of graminoid fen with shallow pools containing Bogbean and adjacent peat moss hummocks. These patches are dominated by Twig Rush or Wire Sedge, with Stunted Tamarack (Larix laricina) and Eastern White Cedar (Thuja occidentalis) in proximity (COSEWIC 2009). Bogbean Buckmoth does not appear to occur in areas lacking peat moss hummocks nearby, presumably due to the lack of suitable pupation sites (COSEWIC 2009).
1.5 Limiting factors
Bogbean Buckmoth is a specialist with relatively scarce environmental requirements (NatureServe 2010). It occurs only in fens and unlike other buckmoths, it is dependent on Bogbean for early stage larval feeding. Glacial retreat left suitable habitat for the Bogbean Buckmoth in disjointed patches. Due to its limited dispersal capacity and behaviour, it is unlikely that the Bogbean Buckmoth will move from its current fen locations to additional suitable areas naturally. Similarly, it is unlikely that adults from New York would recolonize any Ontario site extirpations (COSEWIC 2009). Adults do not migrate (Pryor 1998, NatureServe 2010). Although they are probably capable of flying several kilometers, the sites within Ontario and between Ontario and New York are approximately 50 to many times the maximum dispersal distance documented for the species. A general consequence of habitat specialization and limited dispersal capacity, either in isolation or combined with these threats, is the potential loss of genetic diversity within populations of the species.
1.6 Threats to survival and recovery
Bogbean Buckmoth populations are considered under immediate or substantial threat from a variety of factors (NatureServe 2010). The biggest threat posed to this species is habitat change. In Canada, Bogbean Buckmoth occurs in provincially recognized areas affording them some protection from on–site developments and habitat modification (i.e., Areas of Natural and Scientific Interest and Provincially Significant Wetlands) (COSEWIC 2009). However, habitat degradation can still occur through factors originating off–site such as alien invasive plants and hydrological changes. In particular, species, such as European Common Reed, Glossy Buckthorn and Narrow–leaved Cattail, can invade and crowd open fens and outcompete Bogbean Buckmoth host plants. These plant species occur in or adjacent to all Ontario sites. European Common Reed has been observed to invade and out compete native plants in a number of fens and/or prairies and wetlands in New York, along the Ontario shores of Lake Erie and Lake Hudson and in the St. Lawrence River Valley (COSEWIC 2009). Due to a lack of research, it is unclear what, if any, risk these plant species pose to Ontario populations of Bogbean Buckmoth. In New York, fen succession to denser, taller native shrubs (e.g., Sweet Gale and Leatherleaf) also is apparently degrading the habitat by shading out Bogbean. The specific reasons for this succession are unclear, but may include nitrogen enrichment due to acid precipitation and phosphorus enrichment from cottagers' septic systems (S. Bonnano pers. comm. 2010).
Human–induced water level fluctuations may threaten populations at the White Lake Wetland Complex. Large fluctuations causing excessive flooding or drying may cause mortality in any given year (COSEWIC 2009). However, this does not appear to be an issue at the northernmost White Lake fen (R. A. Layberry pers. comm. 2010).
In New York, large–scale insecticide applications for mosquito control are considered a potential threat to Bogbean Buckmoth depending on spray timing and concentration (NatureServe 2010). Similarly, it is thought that sprays for Gypsy Moth (Lymantria dispar) applied by cottagers may affect Ontario populations (COSEWIC 2009).
Finally, general long–term loss of wetland habitat from climate change poses a potential threat (COSEWIC 2009). A significant change in temperature could result in loss of host plants or directly in the mortality of larvae or adults.
1.7 Knowledge gaps
There is a general lack of scientific studies on the biology and ecology of Bogbean Buckmoth, particularly for Ontario populations. A detailed study of the life history and reproduction of the New York populations was conducted by Pryor in 1998, and remains the only published biological study on this species. Further study of the Ontario populations would provide much needed information on their specific biology, population ecology, viability and dynamics, host preferences and habitat requirements at different spatial and temporal scales.
Long–term population trend data also are absent for Ontario populations because the four sites have only been monitored intermittently. Ongoing monitoring of these areas should occur to determine potential causes for yearly variation in the number of larvae and adults.
Due to a lack of surveys for Bogbean Buckmoth eggs, larvae and/or adults in areas of suitable habitat, many questions remain unanswered with respect to the species' distribution in Ontario. Furthermore, it is unclear why it is currently only found in the Richmond Fen Wetland and White Lake Fen Complex, despite the presence of suitablehabitat in other areas. It is thought that plants such as Eastern Prairie Fringe–orchid may serve as an indicator species for Bogbean Buckmoth (COSEWIC 2009). However, this needs to be confirmed.
The taxonomy of the Bogbean Buckmoth remains unresolved. Although it exhibits unique morphological and ecological characteristics, there remains a lack of evidence and agreement regarding its taxonomic position and repeated molecular analyses have yielded inconclusive results. Additionally, of the molecular studies conducted on the H maia complex, only Legge et al. (1996) included Canadian specimens. Clarifying the taxonomy would give a better understanding of the populations in a biogeographical and evolutionary context, and may help to determine the global gene pool of the species as a whole, and provide information on this species' evolutionary history, which can be used to further define its conservation status.
1.8 Recovery actions completed or underway
- A Draft Recovery Plan for New York Bogbean Buckmoth populations is targeted for publication in March, 2011.
- The Richmond Fen Wetland is prescribed as habitat for Eastern Prairie Fringed– orchid in a regulation under the Endangered Species Act, 2007 and as such indirectly supports the habitat protection of the Bogbean Buckmoth. Richmond Fen has been classified as a Provincially Significant Wetland (PSW) as well as an Area of Natural and Scientific Interest (ANSI).
- The White Lake Fen Wetland Complex has also been classified as a PSW and an ANSI. The Crown land portion is a conservation reserve and is protected through Ontario’s Living Legacy – a land use strategy developed to ensure long– term health and protection of Ontario’s natural resources (OMNR 2001).
- A survey for larvae was conducted at the Ontario sites in 2008 (COSEWIC 2009).
- The Central and Western New York Chapter of the Nature Conservancy has published: Development of a Population Monitoring Program for the Bog Buckmoth (Saturniidae: Hemileuca sp.) (2003).
2. Recovery
2.1 Recovery goal
The recovery goal is to sustain current populations and distributions of Bogbean Buckmoth at extant locations, and to expand populations into suitable, but currently unoccupied habitat within its current range in Ontario.
2.2 Protection and recovery objectives
Table 1. Protection and recovery objectives
| No. | Protection or Recovery Objective |
|---|---|
| 1 | Conduct a quantitative assessment to determine what constitutes a sustainable and secure population in Ontario. |
| 2 | Fill knowledge gaps on taxonomy, ecology, distribution, behaviour, population dynamics, mortality factors and habitat use in the species' Ontario range. |
| 3 | Reduce or mitigate threats on Bogbean Buckmoth populations. |
| 4 | Increase public awareness and understanding of Bogbean Buckmoth populations. |
| 5 | If it is determined that the species was historically widespread, then consider the introduction of Bogbean Buckmoth populations into areas of continuous unoccupied but otherwise suitable habitat, where feasible. |
2.3 Approaches to recovery
Table 2. Approaches to recovery of the Bogbean Buckmoth in Ontario
1. Conduct a quantitative assessment to determine what constitutes a sustainable and secure population in Ontario
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Necessary | Long–term | Research | 1.1 Compile and map entomological records for Bogbean Buckmoth in Ontario.
|
|
| Critical | Short–term | Inventory and Monitoring | 1.2 Determine current population numbers, trends and fluctuation in populations based on standardized inventory and monitoring protocols.
|
|
| Necessary | Short–term | Research | 1.3 Based on current population numbers, conduct population modeling and population viability analysis to determine what constitutes a sustainable population of Bogbean Buckmoth in Ontario. |
|
2. Fill knowledge gaps on taxonomy, ecology, distribution, behaviour, population dynamics, mortality factors and habitat use in the species' Ontario range
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Necessary | Short–term | Research | 2.1 Develop a habitat suitability model for Ontario populations of Bogbean Buckmoth.
|
|
| Necessary | Short–term | Research | 2.2 Based on suitability model results, estimate potential densities of Bogbean Buckmoth in suitable, but currently unoccupied habitat.
|
|
| Necessary | On–going | Research | 2.3 Determine potential biological and/or ecological differences between the Ontario and New York populations.
|
|
| Necessary | Short–term | Research | 2.4 Determine taxonomic position and rank of Bogbean Buckmoth. |
|
3. Reduce or mitigate threats on Bogbean Buckmoth populations
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Critical | On–going | Research | 3.1 Understand the specific hydrology of Richmond Fen Wetlands and the White Lake Fen Complex.
|
|
| Critical | On–going | Research | 3.2 Determine long–term impacts of invasive species (e.g., Narrow–leaved Cattail, European Common Reed) on the sustainability of occupied fen ecosystems and Bogbean Buckmoth host plant species.
|
|
| Critical | On–going | Research | 3.3 Understand the thresholds for Bogbean Buckmoth with respect to fluctuations in water level, especially at White Lake |
|
| Necessary | On–going | Research | 3.4 Determine what/if any insecticide applications are affecting Ontario Bogbean Buckmoth populations.
|
|
| Necessary | Long–term | Research | 3.5 Monitor long–term impacts of climate change on occupied fen ecosystems.
|
|
4. Increase public awareness and understanding of Bogbean Buckmoth populations
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Necessary | Long–term | Stewardship, Education and Outreach | 4.1 Work with local partners, such as municipalities, field naturalists, provincial governments, wetland conservation groups (e.g., Ducks Unlimited, Wetland Habitat Canada), cottage associations and private landowners to mitigate negative impacts at known locations.
|
|
| Necessary | Long–term | Protection, Stewardship | 4.2 Protect habitat through land acquisition, stewardship agreements, conservation easements, and pertinent legislation, policies and guidelines. – Develop habitat regulation for Bogbean Buckmoth under the ESA. |
|
5. If it is determined that the species was historically widespread, then consider the introduction of Bogbean Buckmoth populations into areas of suitable habitat, where feasible
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Necessary | Long–term | Research | 5.1 Explore the feasibility of introducing larvae and adult Bogbean Buckmoths into identified areas of suitable habitat. |
|
2.4 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of Natural Resources on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the authors will be one of many sources considered by the Minister when developing the habitat regulation for this species.
It is recommended that the area prescribed as habitat for this species include all occupied sites. In Ontario, this includes the Richmond Fen Wetland and White Lake Fen Complex. Both sites are calcareous fens supporting large populations of Bogbean. If it is found that Bogbean Buckmoth was more widespread than its current distribution in Ontario, then areas of continuous unoccupied but otherwise suitable habitat should be prescribed as recovery habitat. Recovery habitat for this species should be calcareous fens where Bogbean is present.
The Richmond Fen Wetland hosts palustrine fens on a limestone plain and organic (peat) landform (Chapman and Putnam 1984, NHIC 2008a as cited in COSEWIC). The fens at Richmond are characterized as low shrub fens dominated by Sweet Gale, Bog Birch, Bog Willow and other willows, with open areas dominated by Water Horsetail and various graminoids, including Wire Sedge, Twig–rush and the native grass, American Common Reed. The White Lake Fen Complex overlays lime–rich marble and has similar dominant plant species to Richmond Fen Wetland (NHIC 2008b–taken from COSEWIC 2009). Area boundaries of both fens should be described using the Ontario Wetland Evaluation System (OMNR 1993).
At each of the known locations, the area prescribed as habitat should include the extent of the fen vegetation
Glossary
Calcareous soils: soils containing a lot of calcium carbonate from underlying chalk or limestone rock.
Committee on the Status of Endangered Wildlife in Canada (COSEWIC): The committee responsible for assessing and classifying species at risk in Canada.
Committee on the Status of Species at Risk in Ontario (COSSARO): The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
Conservation status rank: A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G–rank, N–rank and S–rank, are not legal designations. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
- = critically imperilled
- = imperilled
- = vulnerable
- = apparently secure
- = secure
Q indicates questionable taxonomy where taxonomic distinctiveness of the entity is questionable.
Endangered Species Act, 2007 (ESA): The provincial legislation that provides protection to species at risk in Ontario.
Fen: Wetlands with unique hydrology that provides mineralized water to the soil’s surface.
Larva (pl: larvae): The immature, free–living form of any animal that develops into a structurally dissimilar adult through the process of metamorphosis
Palustrine: Non–tidal wetlands that are substantially covered with emergent vegetation such as trees, shrubs, moss, etc.
Senesce: To reach maturity.
Species at Risk Act (SARA): The federal legislation that provides protection to species at risk in Canada. This act establishes Schedule 1 as the legal list of wildlife species at risk to which the SARA provisions apply. Schedules 2 and 3 contain lists of species that at the time the act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
Species at Risk in Ontario (SARO) List: The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
References
Bonnano, S. pers. comm. 2010. Email correspondence to M. Tonge. December 2010. Consulting Ecologist, Fulton, New York.
Chapman, L.J. and D.F. Putnam. 1984. The Physiography of southern Ontario; Ontario Geological Survey, Special Volume 2. Government of Ontario, Ontario. 270p.
COSEWIC. 2009. COSEWIC assessment and status report on the Bogbean Buckmoth Hemileuca sp. in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vii + 29 pp. (www.sararegistry.gc.ca/status/status_e.cfm).[link inactive]
Endangered Species Act. 2007. Statutes of Ontario 2007, Chapter 6. Available at http://www.e–laws.gov.on.ca/html/statutes/english/
Kruse, J.J. 1998. New Wisconsin records for a Hemileuca (Lepidoptera: Saturniidae) using Menyanthes trifoliata (Solanales: Menyanthaceae) and Betula pumila (Betulaceae). Great Lakes Entomologist 31:109–112.
Layberry, R. A. pers. comm. 2010. Email correspondence to M. Tonge. December 2010. Entomologist, Canadian National Collection of Insects and Arthropods, Ottawa, Ontario (retired).
Layberry, R. A. 1980. Hemileuca sp. near lucina Henry Edwards. Pp 9. in Q. F. Hess (ed.). Butterflies of Ontario and Summaries of Lepidoptera Encountered in Ontario in 1979, Toronto Entomologists Association, Toronto, ON.
Legge, J. T., R. Roush, R. Desalle, A. P. Volger, B. May. 1996. Genetic criteria for establishing evolutionary significant units in Cryan’s buckmoth. Conservation Biology 10: 85–98.
NatureServe. 2010. NatureServe Explorer: An online encyclopaedia of life [web application]. NatureServe, Arlington, Virginia. Available http://www.natureserve.org/explorer. Accessed November 2010.
OMNR. 2001. Ontario’s Living Legacy – Ontario’s Crown Land Use Policy Atlas. Peteroborogh, Ontario. 51 pp. (https://ozone.scholarsportal.info/bitstream/1873/6997/1/10281337.pdf)[link inactive].
OMNR. 1992. Province of Ontario’s Wetland Policy Statement. Toronto. 42 pp.
OMNR. 1993. Ontario Wetland Evaluation System. Northern manual covering hill’s site regions 2,3,4 and 5. 1st ed. Toronto. 181 pp.
OMNR. 2010. Natural Heritage Reference Manual for Natural Heritage Policies of the Provincial Policy Statement, 2005. 2nd ed. Toronto. xi + 233 pp.
Pryor, G. S. 1998. Life history of the bog buck moth (Saturniidae: Hemileuca) in New York state. Journal of the Lepidopterists' Society 52: 125–138.
Rubinoff, D. and F. A. H. Sperling. 2004. Mitochondrial DNA sequence, morphology and ecology yield contrasting conservation implications for two threatened buckmoths (Hemileuca: Saturniidae). Biological Conservation 118: 341–351.
Schmidt, C. pers. comm. 2010. Email correspondence to A. Gradish. December 2010. Entomologist, Canadian Food Inspection Agency, Ottawa, Ontario.
Scholtens, B. G. and W. H. Wagner. 1994. Biology of the genus Hemileuca (Lepidoptera: Saturniidae) in Michigan. The Great Lakes Entomologist 27: 197–207.
Selfridge, J.A., D. Parry, and G.H. Boettner. 2007. Parasitism of barrens buck moth Hemileuca maia Drury in early and late successional pine barrens habitats. Journal of the Lepidopterists' Society 61: 213–221.
Sutherland, D. pers. comm. 2010. Email correspondence to M. Tonge. December 2010. Zoologist, Ontario Ministry of Natural Resources. Peterborough, Ontario.
Tuskes, P. M., Tuttle, J. P., Collins, M. M. 1996. The Wild Silk Moths of North America. Cornell University Press, Ithaca, New York. 264 pp.
Recovery strategy advisors
Advisors
| Name | Affiliation and office |
|---|---|
| Sandy Bonnano | Consulting Ecologist, Fulton, NY |
| Dan Kraus | Nature Conservancy of Canada, Guelph, ON |
| Ross Layberry | Entomologist, Canadian National Collection of Insects and Arthropods, Ottawa, ON |
| John Legge | West Michigan Conservation Director, The Nature Conservancy, MI |
| Ted Mosquin | Naturalist, Lanark County, ON |
| James Page | Natural Heritage Biologist, OMNR, Kemptville, ON |
| Christian Schmidt | Canadian Food Inspection Agency, Ottawa, ON |
| Don Sutherland | OMNR, Peterborough, ON |
| Erin White | Zoologist, New York Natural Heritage Program, NY |
Footnotes
- footnote[1] Back to paragraph Eastern Prairie Fringed Orchids may serve as an indicator species for the presence of Bogbean Buckmoth (COSEWIC 2009).
- footnote[2] Back to paragraph Effects on highly restricted fen species such as Eastern Prairie Fringed–orchid, Spotted Turtle (Clemmys guttata) and the Jumping Spider(Paradamoetas fontanus) should be considered before the removal of invasive species takes place.
- footnote[3] Back to paragraph Thresholds for restricted fen species (listed above) should also be considered with respect to water–level fluctuations.
- footnote[4] Back to paragraph Extent of fen vegetation will be determined using appropriate Ecological Land Classification (ELC) classes.