Hine’s Emerald Recovery Strategy
This document advises the ministry on ways to ensure healthy numbers of the Hine’s Emerald, a threatened or endangered species, return to Ontario.

About the Ontario Recovery Strategy Series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species' persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. There is a transition period of five years (until June 30, 2013) to develop recovery strategies for those species listed as endangered or threatened in the schedules of the ESA. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy.
The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of Natural Resources Species at Risk webpage at: www.ontario.ca/page/species-risk
Recommended citation
Pulfer, T.L., C.G. Evans, D. Featherstone, R. Post, J.I. McCarter, and J.F. Laverty. Recovery Strategy for the Hine’s Emerald (Somatochlora hineana) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. vi + 27 pp.
Content (excluding the cover illustration) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée Recovery strategies prepared under the Endangered Species Act, 2007, n'est disponible qu'en Anglais en vertu du Règlement 411/97 qui en exempte l'application de la Loi sur les services en français. Pour obtenir de l'aide en français, veuillez communiquer avec Cathy Darevic au ministère des Richesses naturelles au 705-755-5580.
Authors
Tanya L. Pulfer– Nature Conservancy of Canada
Christopher G. Evans – Brereton Field Naturalists' Club
David Featherstone– Nottawasaga Valley Conservation Authority
Ryan Post– Nottawasaga Valley Conservation Authority Jennifer I. McCarter – Nature Conservancy of Canada Jolene F. Laverty– Nature Conservancy of Canada
Acknowledgments
The authors would like to thank Dan Kraus, Allan Edelsparre, John Gerrath, Erica Thompson, Amelia Argue and Bree Walpole for their editorial comments. We would also like to thank reviewers at the Ontario Ministry of Natural Resources and Environment Canada, including: Scott Gibson, Kathryn Markham, Scott Reid, Suzanne Robinson, Krista Holmes, Meghan Gerson, Christina Rohe and Tania Morais. We also thank Andrea Hebb for creating Figure 1.
This recovery strategy was greatly improved by guidance from the core advisory group for Hine’s Emerald, including:
Bob Bowles (independent naturalist)
Tim Cashatt (Illinois State Museum)
Kim Cuddington (University of Waterloo)
Premek Hamr (Upper Canada College)
Colin Jones (Natural Heritage Information Centre)
Kristopher Law (USA Fish and Wildlife Service)
Paul M. McKenzie (USA Fish and Wildlife Service)
Meredith Mahoney (Illinois State Museum)
Suzanne Robinson (Ontario Ministry of Natural Resources – Midhurst District)
Dan Soluk (University of South Dakota)
Declaration
The Ontario Ministry of Natural Resources has led the development of this recovery strategy for the Hine’s Emerald in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all of the individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ontario Ministry of Natural Resources
Environment Canada, Canadian Wildlife Service - Ontario
Executive summary
Hine’s Emerald (Somatochlora hineana) is a globally rare dragonfly restricted to southern Ontario, Wisconsin, Michigan, Illinois and Missouri. In Ontario it is only known to occur in the Minesing Wetlands located in the County of Simcoe. It is listed as endangered under Ontario’s Endangered Species Act, 2007 due to its habitat specificity, potential threats and extremely limited geographic range. It has also been listed as endangered or extirpated throughout its known global range.
The key features used to distinguish Hine’s Emerald from other similar species are the unique conformations of its sexual appendages or genital plates on the end of the abdomen, dark metallic green thorax with two creamy yellow lateral stripes and its relatively large size (60-65 mm long and 90-95 mm wingspan).
Hine’s Emerald is restricted throughout its range to calcareous wetlands (marshes, sedge meadows and fens) dominated by graminoid vegetation and fed primarily by groundwater seeps. Adult males occur in seepage areas and fens and adjacent margins, whereas females are usually found in dry meadows, sometimes in adjacent forest openings, only coming into wetlands to lay eggs. Adults may also utilize adjacent forests, gravel roads, trails and fields for foraging before returning to the wetlands to mate and lay eggs. Hine’s Emerald deposit eggs in shallow channels or sheetflow in areas of herbaceous vegetation in marshes, meadow marshes and fens. The larvae remain in cool, shallow, slowly-moving waters of spring-fed marshes, alkaline fens, mineral-rich fens with shallow creeks, springs, small pools, marl deposits and calcareous marshy streams for three to five years before emerging as adults. In some locations, larvae use crayfish burrows, mainly of Digger Crayfish or of Devil Crawfish (also known as Meadow Crayfish), as refuge habitat in the summer and winter months. Crayfish burrows are thought to be a critical component of Hine’s Emerald habitat where seasonal drought and freezing occurs and may be a factor limiting its distribution.
The main threats to this species in Ontario are habitat loss due to changes in surface and sub-surface hydrology (including water quality), competition from invasive species (Garlic Mustard, Purple Loosestrife, Glossy Buckthorn and the non-native genotype of Common Reed) and vegetation succession from native species. The inter-species dependency of Hine’s Emerald on Digger Crayfish indicates that threats to the persistence of burrowing crayfish in Ontario would have a severe negative effect on Hine’s Emerald.
The recovery goal for Hine’s Emerald is to prevent any loss of population, genetic diversity or habitat functionality at extant sites or at any other extant locations which may be identified in the future in Ontario.
The recovery objectives outlined to achieve this goal are as follows:
- protect and maintain the quantity and quality of Hine’s Emerald habitat and habitat functionality, including the hydrological and hydrogeological function
- reduce or mitigate threats to Hine’s Emerald and its habitat
- increase knowledge of Hine’s Emerald biology in Ontario including distribution, abundance, life history and habitat needs, and
- increase public awareness and understanding of Hine’s Emerald and its habitat in Ontario
It is recommended that the area regulated as habitat include all extant locations. In Ontario, this currently includes only the Minesing Wetlands. In order to protect both the adult and larvae stages of Hine’s Emerald, it is recommended that the area prescribed as habitat include fen and wetland meadows, "(i) where Hine’s Emerald have been observed and (ii) that are connected by surface or ground water to areas where Hine’s Emerald have been observed. In addition to these areas the prescribed habitat should also include 500 metres beyond each of these habitats. For the purposes of perching, movement and roosting, all forests and dry meadows that are adjacent to the areas described above should also be prescribed as habitat.
To allow for migration and dispersal between habitat patches used by Hine’s Emerald it is recommended that corridors connecting the habitat areas described above be prescribed as habitat. Corridors are believed to be both natural (creeks, swales and other water features) and anthropogenic features (trails, utility rights-of-way and gravel roads) that have forested edges or riparian habitat.
Due to the dependence of Hine’s Emerald habitat on groundwater recharge it is recommended that prescribed habitat include the Snow Valley Uplands, where the current regional groundwater infiltration regime is maintained for the entire Minesing Wetlands.
1.0 Background information
1.1 Species assessment and classification
Common name: Hine’s Emerald
Scientific name: Somatochlora hineana SARO List Classification: Endangered
SARO List History: Endangered (2012)
COSEWIC Assessment History: Endangered (2011)
SARA Schedule 1: No Schedule, No Status
Conservation status rankings:
G-Rank: G2G3
N-Rank: N1
S-Rank: S1
The glossary provides definitions for technical terms, including the abbreviations above.
1.2 Species description and biology
Species description
Hine’s Emerald (Somatochlora hineana) is a dragonfly in the Family Corduliidae (Order Odonata), more commonly known as Emeralds. Emeralds are characterized by their brilliant emerald green eyes (USFWS 2001).
Identification of adults in flight is difficult between species of the Emerald family, particularly of the genus Somatochlora. These species are generally very difficult to track in flight as they are extremely swift, erratic and agile. They have excellent evasive abilities and cryptic colouration. Hine’s Emerald tends to fly high, often in dappled shade. However, in the United States they are often observed flying low over the breeding habitat during male terrestrial patrols and female oviposition flights, making identification discernible with aid of binoculars (P. McKenzie, pers. comm. 2012). In Ontario, the habitat of Hine’s Emerald is quite rare and often remote, making access very difficult. These attributes make this species very difficult to locate and even harder to capture and identify (C.G. Evans pers. obs.).
The most similar species to Hine’s Emerald are the Clamp-tipped Emerald (S. tenebrosa), Williamson’s Emerald (S. williamsoni) and the female Brush-tipped Emerald (S. walshii). The key features that distinguish Hine’s Emerald from these other species are its unique conformation of sexual appendages of the male and genital plates of the female on the end of the abdomen, either the terminal appendages (or claspers) for males or the ovipositor (or genital plates) for females. These features are readily discernible in the hand but rarely inflight (USFWS 2001). Other features used to distinguish adults from other species in the genus Somatochlora with similar sexual appendages include its dark metallic green thorax with two creamy yellow lateral stripes, and its relatively large size (60-65 mm long and 90-95 mm wingspan).
The larvae or nymphs of different species in the genus Somatochlora are extremely similar, and reliable identification can only be made in final instar larvae using characteristics observable only under a microscope (Cashatt and Vogt 2001). The only identification key available that includes Hine’s Emerald describes the final instar larvae only (Cashatt and Vogt 2001). Thus, accurate identification of earlier instars is not possible except by genetic analyses, but such analyses have not been undertaken in Ontario (P. McKenzie and T. Cashett, pers. comm. 2012).
Species biology
Similar to other dragonflies, Hine’s Emerald undergoes incomplete metamorphosis involving three stages: aquatic egg, aquatic larva (nymph) and terrestrial/aerial adult (COSEWIC 2011). The majority of the three to five year lifespan of Hine’s Emerald is spent in the aquatic larval stage (COSEWIC 2011). During this stage, they are thought to be opportunistic sit-and-wait predators that prey upon small aquatic invertebrates including mayflies (Ephemeroptera), caddisflies (Trichoptera), oligochaete worms, and also likely upon isopods, smaller dragonfly larvae, mosquito larvae, other worms, snails and even small fish (USFWS 2001, COSEWIC 2011).
As water temperatures drop in the fall, the larvae become less active and little is known about their overwintering ecology. However, in the summer and winter months they have been found inside crayfish burrows (COSEWIC 2011). During the larval period, Hine’s Emerald larvae grow and molt several times before they reach their final instar. Hine’s Emerald larvae appear to be physiologically well adapted to survive periodic conditions of drought (USFWS 2001, COSEWIC 2011).
Once in their final instar, larvae climb from the water onto emergent vegetation for their final transformation into adults. Emergence of Hine’s Emerald dragonflies continues throughout the summer and peak emergence in Ontario occurs for two to three weeks in early to mid-June (COSEWIC 2011).
Newly-emerged adults seek out protection in vegetation, where over a period of a few days, their bodies harden and their sexual organs mature (Corbet 1999). Prior to reproducing (the pre-reproductive stage), adults spend 7 to 10 days aerially foraging on gnats, mosquitoes and other small flying insects (USFWS 2001). During this pre- reproductive stage, they may forage up to three kilometres away from their larval habitats, foraging most actively in the morning but continuing throughout the day and possibly into dusk (USFWS 2001). Foraging may occur over open fields and wet or dry meadows, along hedgerows or forest margins, or along narrow roads. Foraging flight varies from fast, low (less than two metres), along roads or trails up to one kilometre long, to slow, high (treetop height), circuitous floating flights near forest/meadow margins (USFWS 2001).
During the reproductive stage, which is approximately two to four weeks long, adult males establish small territories near forests or swamps suitable for mating and near aquatic habitat suitable for ovipositing (Cashatt and Vogt 1990, Vogt and Cashatt 1994). Insufficient data on natural breeding exist to date to delineate the breeding season for the Ontario population. However, based on the breeding season for Hine’s Emerald in the climactically similar zone of Michigan, the breeding season in Ontario likely extends from late June to late July (MOS Tech. Note # 3, 2001). Adults also continue to forage, flying up to two kilometres away from their breeding sites (Cashatt and Vogt 1990, Vogt and Cashatt 1994). Males defend their territories making fast flights back and forth and occasionally hovering over territories that range in size from two to four square metres (Cashatt and Vogt 1990, Vogt and Cashatt 1994). A female entering a male’s territory is intercepted by the male and mating occurs on nearby vegetation (Cashatt and Vogt 1990, Vogt and Cashatt 1994). After fertilization, the females then repeatedly insert the tip of their abdomens into mud or shallow water, ovipositing up to 200 eggs (COSEWIC 2011).
The adult lifespan of Hine’s Emerald is typically four to six weeks (Zercher et al. 2001) but may extend up to a maximum of a few months; i.e., seven to ten days pre- reproductive, two to four weeks reproductive and up to several weeks post-reproductive (COSEWIC 2011).
Hine’s Emerald dragonflies are vulnerable to predation during all life stages. Aquatic larvae may be eaten by larger predatory insects (e.g., larger dragonfly larvae), crayfish, amphibians, fish, wading birds, shorebirds, dabbling ducks and turtles (USFWS 2001). Emerging larvae are vulnerable to predation by birds and frogs. Adults, particularly soft, poorly flying newly-emerged adults, are also susceptible to predation by birds and frogs as well as to spiders and even larger dragonflies (USFWS 2001).
1.3 Distribution, abundance and population trends
Hine’s Emerald is endemic to North America and is listed as endangered or extirpated throughout its entire distribution. In the United States of America, the species is known to occur in the states of Wisconsin, Michigan, Illinois and Missouri (Figure 1). Historically, the species was known from the states of Ohio, Indiana and Alabama (Figure 1; Vogt and Cashatt 1994, COSEWIC 2011). In Canada, the species is known only from a single location in southern Ontario – the Minesing Wetlands and immediately adjacent areas (Figure 1). Based on COSEWIC convention, the extent of occurrence for this site is considered to be 28 km2(COSEWIC 2011).
The Ontario occurrence is thought to contain a single population (COSEWIC 2011), hereby referred to as the Minesing Wetlands population. Minesing Wetlands is located in the County of Simcoe, located between the towns of Barrie and Wasaga Beach. It had been suspected that Hine’s Emerald may be present in Ontario due to presence of similar habitats found at known sites in the United States. This led to targeted surveys between 1999 and 2002, all of which resulted in negative findings (COSEWIC 2011). In June 2007 a single male was observed and photographed by a local naturalist of Minesing Wetlands (report co-author, Christopher Evans) and identified by Colin Jones (Ontario Ministry of Natural Resources, OMNR). Significant survey efforts in 2007 to 2009 resulted in additional captures of adults and nymphs at Minesing Wetlands, providing a better delineation of adult and larval habitat ranges. Twenty-eight other sites that were identified as having appropriate habitat were searched (see COSEWIC 2011 for details). Although Hine’s Emerald have not been found at these sites to-date, it should be noted that negative survey results are not uncommon for this species where it is extant (COSEWIC 2011). Experts suggest there is a high likelihood of extant Hine’s Emerald populations at least at some of these twenty-eight locations. Further surveys are necessary to investigate this possibility.
Species abundance of the Ontario population is currently unknown. Mark-recapture methods have been used to estimate adult populations ranging from 1,710 (Lockport Prairie, Illinois) to 118,140 (the Ridge Sanctuary, Wisconsin) at other locations. However, these methods are not thought to be appropriate for long-term monitoring (Soluk et al. 1998 in COSEWIC 2011) and there are currently no known recommendations for population estimates that are suitable for long-term monitoring.
Figure 1. Extant and extirpated locations of Hine’s Emerald in North America (adapted from COSEWIC 2011).
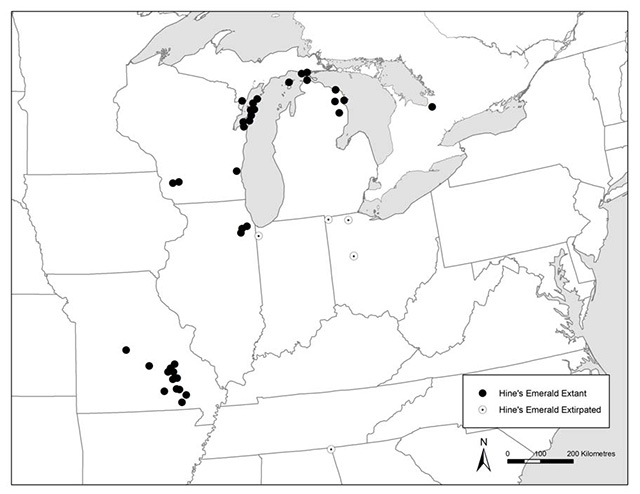
Enlarge figure 1. Extant and extirpated locations of Hine’s Emerald in North America
1.4 Habitat needs
Hine’s Emerald is a species found in calcareous wetlands including marshes, meadow marshes and fens dominated by graminoid vegetation (particularly sedges) which are fed primarily by groundwater from intermittent seeps (COSEWIC 2011). Most sites also have an underlying layer of shallow dolomitic bedrock (Cashatt and Vogt 2001).
Limestone bedrock of the Verulam Formation underlies the Minesing Wetlands at a depth of 60 to 90 metres (Hanna 1982) which is atypical for Hine’s Emerald sites, however thick glaciolacustrine silts and clays underlay the wetland complex (Post 2009). These deposits act as an impermeable layer which directs seepage flows from the adjacent bluffs laterally (northwest) through the fen thereby providing hydrogeological conditions which are similar to the shallow bedrock sites where Hine’s Emerald is found in the United States (Post 2009, COSEWIC 2011).
Hydrogeochemical analysis of Minesing Wetlands groundwater indicates that the fen is dominated by calcium and bicarbonate ions with the postulated source being from deep groundwater or mineral soils below the peat, regionally originating from the outcropping aquifers at the stranded glacial shoreline deposits and through shallow peat (Bradford 1999). Groundwater discharge along the eastern margin could account for the observed hydrogeochemical trends from the bluffs towards the fen along the principal direction of groundwater flow (Bradford 1999).
Adults use various habitats for foraging, resting, breeding and movement. As discussed in the species biology section, adults forage and breed in wet habitats such as marshy areas and open water. This includes habitats such as cattail seepage marshes, seepage sedge meadows, sedge hummocks near marshy stream edges, swales, muck, sluggish water at the edge of springs, small puddles, streamlets and in marl/muck bottomed pools (COSEWIC 2011). Adjacent forest areas provide protected, shaded areas for perching and roosting (USFWS 2001). Corridors between habitat patches are essential for movement and dispersal. Areas used as corridors include marshes, sedge meadows, dolomite prairie, fringing shrubby and forest areas bordering wetlands (Foster and Soluk 2004).
There are some observed differences in habitat use between male and female Hine’s Emerald. Generally, adult males of this species occur in seepage areas and fens, whereas the females are usually found in dry meadows, only coming into wetlands and aquatic habitat to lay eggs (Foster and Soluk 2006). Adult females deposit eggs in shallow channels or sheetflow in areas of herbaceous vegetation in marshes, meadow marshes and fens (Cashatt and Vogt 2001). Soil types range from organic muck to mineral soils such as marl (COSEWIC 2011). It should be noted that to date there have been no observations of Hine’s Emerald laying eggs in Ontario.
In Ontario, the known habitat of Hine’s Emerald is restricted to the Minesing Wetlands and immediately adjacent areas, where adults have been found in open string fen areas (elongated openings), fallow farm fields, forests, trails and roadside openings (COSEWIC 2011). The habitat in the open fens of Minesing Wetlands is dominated by herbaceous plants and include, Bog Buckbean (Menyanthes trifoliata), Twig Rush (Cladium mariscoides), Beaked Spike-rush (Eleocharis rostellata), sedges (such as Carex limosa, C. livida, C. chordorrhiza), Common Bog Arrow-grass (Triglochin maritima) and the native Common Reed (Phragmites australis ssp. americanus). "String islands" of Tamarack (Larix laricina) and Eastern White Cedar (Thuja occidentalis) swamp forest lie adjacent to these open fen habitats (COSEWIC 2011).
Larvae are found in cool, shallow, slowly-moving waters of spring-fed marshes, alkaline fens, mineral-rich fens with shallow creeks, springs, small pools, marl deposits and calcareous marshy streams (Pintor and Soluk 2006). In the northern United States, Hine’s Emerald larvae often inhabit crayfish burrows to seek refuge, especially during drought periods (Pintor and Soluk 2006). In Missouri, Hine’s Emerald larvae have only been found in crayfish burrows (P. McKenzie, pers. comm. 2012). The burrows used have been found to belong to semi-terrestrial crayfish [i.e., Devil Crawfish (also referred to as Meadow Crayfish, Cambarus diogenes) in the United States and Digger Crayfish (Fallicambarus fodiens) in the Minesing Wetlands (COSEWIC 2011)]. The presence of crayfish burrows likely represents a critical component of Hine’s Emerald habitat, possibly limiting its distribution from otherwise suitable sites (Pinto and Soluk 2006, COSEWIC 2011).
Digger Crayfish construct fairly complex burrows mainly in wetlands but also in roadside ditches, temporary bodies of water and creek banks (Guiaşu 2007, Crandall 2010). These burrows are often made in clay or muck soils with the mud chimneys being the only portion exposed above ground (Guiaşu 2007). Despite Digger Crayfish being the most wide-spread species of the Cambaridae family, occurring in 21 states and one province (Ontario), it is not locally common and occurs in isolated patches (Guiaşu 2007).
1.5 Limiting factors
The low number of extant populations and restricted range in North America emphasizes the extremely narrow habitat preferences of Hine’s Emerald. Fen habitats like those associated with the Minesing Wetlands population comprise less than a fraction of one percent of the total wetland area south of the Canadian Shield in Ontario (Riley 1989) and suitable community types of sufficient size are found in only a portion of these habitats.
Despite significant searches for additional populations in south-central Ontario, Minesing Wetlands remains the only known population in Ontario and Canada. Though negative survey results do not necessarily suggest absence of other small populations, the negative results to date suggest that Hine’s Emerald may only occur at very few additional Canadian locations (COSEWIC 2011).
It is likely that the presence of crayfish burrows and perhaps burrows related to particular crayfish species may be a limiting factor to Hine’s Emerald distribution.
1.6 Threats to survival and recovery
Alteration of hydrology (high threat)
Reduced groundwater inputs associated with proposed development or climate change could directly impact Hine’s Emerald breeding sites by reducing the water necessary to maintain larval habitat (COSEWIC 2011). Minesing Wetlands is a provincially and internationally significant wetland, thus it is afforded protection under Ontario’s Planning Act – Provincial Policy Statement
The fen habitats in Minesing Wetlands are sustained primarily by groundwater seepage. Maintenance of current hydrogeological functions that support Hine’s Emerald habitat is dependent on effective management of recharge areas in the adjacent uplands (Post et al. 2010). Any changes to surface and sub-surface hydrology that affect the quantity of groundwater flow are likely to negatively impact larval habitat and consequently reduce populations of Hine’s Emerald (USFWS 2001).
Snow Valley Uplands
Climate change may also potentially impact hydrogeological conditions that support Hine’s Emerald habitat (Davies and Simonovic 2005). Winter and summer air temperatures are expected to increase over time (Portmann et al. 2009). Precipitation trends are less certain, although most models indicate an increase in extreme events (droughts and floods) and "flashier" storm events (Zedler 2010). Projected increases in wind speeds could increase evapotranspiration in the Lake Huron basin (Snyder et al. 2011). Any one factor or a combination of these factors could decrease the volume of precipitation and runoff recharging the Snow Valley Upland aquifer and the resulting seepage in the Minesing Wetlands.
Altered hydrology can also lead to vegetation succession. Succession is restricted based on hydrological conditions in the open fen of Minesing Wetlands. If the hydrology is altered, succession may reduce the availability of suitable breeding and larval habitat. Thus, indirectly, reduced groundwater flows across the open fen habitats could impact Hine’s Emerald breeding sites by changing the composition of the vegetation community (COSEWIC 2011). Open graminoid fen sites could shift to shrub fen or conifer swamp communities. This would reduce the amount of appropriate larvae and breeding habitat for Hine’s Emerald.
Contamination of Groundwater (low threat)
Contamination of groundwater is a potential threat to Hine’s Emerald habitat (USFWS 2001). The permeable sands and gravels associated with the Snow Valley Uplands provide a potential conduit for contaminants to enter the underlying aquifer which feeds the Minesing Wetlands. Road salt, agricultural pesticides, agricultural fertilizers, faulty septic beds and urban contaminants are potential contaminant sources that could impact groundwater quality (Post 2009). Recent investigations indicate that slightly elevated levels of nutrients (likely from agricultural activities) and sodium/chloride (from road salt) are present in groundwater at the periphery of the Minesing Wetlands (Spoelstra and Post 2012). Although current levels are considered too low to pose a risk to ecological functions and do not persist into the open fen habitats, these elevated levels strongly indicate the link between upland recharge areas and groundwater discharge to the Minesing Wetlands. Thus, this has the potential to become a high threat to Hine’s Emerald and its habitat.
Invasive Species (medium threat)
Invasive species represent another potential impact to Hine’s Emerald habitat. Approximately 14 percent of the plants documented in the Minesing Wetlands area are introduced (Bowles et al. 2007). The main species of concern include Garlic Mustard (Alliaria petiolata), Purple Loosestrife (Lythrum salicaria,), Glossy False Buckthorn (Frangula alnus) and the non-native genotype of Common Reed (Phragmites australis ssp. australis). These species have been known to act dominantly in certain ecosystems such as wetlands and forests, reduce habitat quality for sensitive native wildlife and compete with and directly exclude other native plants (Meekins and McCarthy 1999, Catling and Mitrow 2009, COSEWIC 2011). Of particular concern are the non-native Common Reed and Glossy Buckthorn, which are expanding throughout Ontario at an exponential rate. In the Minesing wetland, the non-native Common Reed has been documented around the periphery of the wetland complex, while the native Common Reed is found only in the fen portion of the wetland. While Glossy Buckthorn is not currently known within the Minesing Wetlands area, like the non-native Common Reed, it is also expanding rapidly in southern Ontario. If either of these non-native species continue to move into the open fen area of Minesing Wetland, this could significantly impact the open fen habitat and restrict or eliminate burrowing crayfish activity in current Hine’s Emerald breeding sites.
Road Mortality (low threat)
Dragonflies are susceptible to road mortality (Rao and Girish 2007, Soluk et al. 2011), however the extent to which Hine’s Emerald is impacted by road mortality in Ontario is unknown. Hine’s Emerald does not seem to avoid roads, and may in fact use them as corridors (Soluk et al. 2011, C.G. Post pers. obs.). Dragonfly road mortalities generally happen in two stages: (i) dragonflies that are hit by cars exceeding 50 to 60 km/h experience severe shock and fall to the ground; (ii) dragonflies either recover from the shock and fly away or, as is usually the case, they are run over by a second vehicle (Rao and Girish 2007). This coupled with the distribution of carcasses (usually in vehicle grills), make quantifying this threat difficult.
Soluk and Moss (2003) conducted a road mortality study of a Hine’s Emerald population in Wisconsin. They estimated that dragonfly road mortality rates were roughly 16 per day, and a minimum estimate of 699 per year.
Several roads surrounding Minesing Wetlands have been observed as active foraging habitat for both male and female Hine’s Emerald and Harlequin Darners (Gomphaeschna furcillata; C.G. Evans pers. obs.). Male Hine’s Emerald commonly patrol these routes in what are thought to be territorial patrols at heights of between one and two metres, which may make them vulnerable to road mortality (C.G. Evans pers. obs.). Adult females appear to forage mainly at heights greater than three metres in these areas and thus are not particularly susceptible to significant road mortality. However, one injured teneral female Hine’s Emerald was removed from the surface of Portage Trail, apparently a victim of a collision with a vehicle (C.G. Evans pers. obs.). Little research has been conducted on the effects of road mortality on dragonflies and so the exact impact of this threat on the Canadian Hine’s Emerald population is unknown.
Anthropogenic disturbance to habitat (low threat)
Fen habitats are sensitive to human disturbance, such as trampling. Unrestricted access to these habitats could impact the open fen habitats that support Hine’s Emerald in the Minesing Wetlands. Disturbance of the fen habitat by all terrain vehicles (ATVs) is currently not an issue due to restricted vehicle access in the fen as well as the difficulty of the terrain. All terrain vehicles could be of potential threat to adult Hine’s Emerald along the trail networks in Minesing Wetlands; the trails have restricted access but this is not easily enforced as many land owners use the trails to access their properties in Minesing Wetlands.
Direct human-caused mortality (unknown)
The exact threat from direct mortality, such as collections, is unknown. Dragonflies have longer breeding periods and generally smaller population sizes than many other insects (Soluk et al. 2011). Therefore direct mortality of an individual will likely have a larger impact on the overall population compared to species with short breeding periods and larger population sizes.
1.7 Knowledge gaps
Population size and range
Hine’s Emerald abundance in Ontario is unknown. Further, other population sizes in North America are relatively unknown. A standardized, repeatable population estimate technique is required to understand baseline populations and future trends. Adults are generally encountered during focused surveys during their summer flight period (COSEWIC 2011). Despite efforts being made to mark and recapture individuals, this technique is not favoured (COSEWIC 2011). Removal method and use of exuviae have been suggested as other potential population estimate techniques (Foster and Soluk 2004, COSEWIC 2011). However, this has proved to be unsuccessful in the Midwest United States, where there are too few excuviae or lavae to use such a method (P. McKenzie, pers. comm. 2012).
In Ontario, focused surveys from 2007 to 2009 have provided further insight into the range of Hine’s Emerald in the vicinity of the Minesing Wetlands but the full extent of the population range in Minesing has likely not been fully determined to date.
Other extant populations of Hine’s Emerald may be present in southern Ontario. Although focused survey efforts have failed to find any other populations, Hine’s Emerald can be very difficult to find, especially in areas with small populations (COSEWIC 2011). New populations in Missouri were found only after two to three years of surveys by experts (P. McKenzie, pers. comm. 2012). Additional surveys in suitable habitats elsewhere in southern Ontario may be warranted to search for additional populations.
Dispersal
Based on current knowledge, it is unknown if any dispersal is happening between Minesing Wetlands and other Hine’s Emerald populations. It is unknown how far Hine’s Emerald can travel. Currently, the maximum recorded dispersal distance of an individual is 5.4 km (COSEWIC 2011). However, there are genetic studies that link populations together over larger distances. The evidence so far suggests that these dispersal events were relatively recent (approximately 4000 years ago during the Pleistocene age) leading experts to suggest that dispersal distances would be larger than the current observed maximum distance (5.4 km).
Habitat requirements
Hine’s Emerald habitat in Ontario differs from that of the populations in the United States. Similarities between sites include the importance of groundwater to Hine’s Emerald habitat and the use of crayfish burrows by larvae. The populations in the United States are found in areas of shallow soil over the bedrock, whereas Minesing Wetlands has a deep layer of soil over bedrock. Thus, questions arise as to what habitat features Hine’s Emerald are selecting for.
There is uncertainty about how the hydrogeological processes provide critical support for Hine’s Emerald habitat in the Minesing Wetlands. Although recent hydrogeological studies (e.g., Post 2009, Post et al. 2010, Spoelstra and Post 2012) have provided important baseline information on wetland hydrogeology (groundwater levels and chemistry), a regular, dedicated monitoring program is required to fully understand seasonal habitats and to identify trends over time. Hydrogeological conditions associated with the recharge area that feeds the groundwater system which, in turn, support Hine’s Emerald habitat are not well known.
Inter-species interactions
It appears that Hine’s Emerald use crayfish burrows during the larval phase of their lifecycle (USFWS 2001, COSEWIC 2011), but the nature and extent of this use is not yet understood. Studies in the United States have determined that Hine’s Emerald use Devil Crawfish burrows (USFWS 2001), whereas investigations in Ontario have found Hine’s Emerald in Digger Crayfish burrows (COSEWIC 2011). It is unknown if these are the only two crayfish species' burrows that Hine’s Emerald use for larval habitat. It is unknown whether or not this use of burrows is obligatory, opportunistic or incidental in Minesing Wetlands, however, it has been shown to be beneficial and likely obligatory at one site in the United States (Pintor and Soluk 2006). Further study of the interaction between Hine’s Emerald and burrowing crayfish in Minesing Wetlands habitats would potentially be valuable in locating other extant populations of Hine’s Emerald in Ontario and Canada and in identifying and restoring historical habitats.
Identified threats
Most of threats listed above are not fully understood. While most experts agree that the listed threats are valid, the degree of impact (current and/or future) is uncertain. Of note are the exact threats from direct human-related mortalities and road mortalities to this population.
1.8 Recovery actions completed or underway
Population size and range
In 2007 to 2009, relatively intensive Hine’s Emerald (adult) surveys were undertaken in fen and boreal swamp habitats as well as upland habitats along the eastern periphery of the Minesing Wetlands. Larval habitats (crayfish burrows) were sampled and confirmed in 2008 (COSEWIC 2011). These surveys have assisted with identification of the range of Hine’s Emerald habitat in Minesing Wetlands, although additional surveys may be required to document the full extent of the population range.
Genetic studies
The Natural Heritage Information Centre (NHIC) and the Illinois State Museum (under the direction of Drs Cashett and Mahoney) are currently working together to determine the genetic diversity of the Minesing Wetlands population of Hine’s Emerald. Work is being conducted on both mitochrondial and nuclear DNA sequencing from populations across the United States and Ontario. When completed we should understand the variation within the Ontario population compared with the entire Hine’s Emerald geographic range (Mahoney pers. comm. 2012). This work will play an integral part of guiding future conservation efforts for this population.
Further work has been conducted in the United States which used microsatellite markers of both adult and larval Hine’s Emerald adults to examine relatedness between populations in Illinois and Wisconsin (Monroe et al. 2010). An exciting part of this study was using non-lethal methods to collect genetic material (including the use of excuvae, fecal pellets and wing-clippings). Using the 10 microsatellites markers from this study will aid in understanding the population across the entire Hine’s Emerald range. This work will also contribute to a greater understanding of population structure that may aid in any attempts at re-establishment or augmentation for Hine’s Emerald in the United States populations.
Habitat requirements
Recent hydrogeological studies in Minesing Wetlands (Post 2009, Post et al. 2010, Spoelstra and Post 2012) have assisted in documenting hydrogeological conditions (groundwater levels and groundwater chemistry) in ecotone gradients from the east periphery of the wetland through the boreal swamps to the open fen habitats along two transects of shallow nested monitoring wells. The 2010 study provided a useful comparison between 1999 hydrogeological and ecological conditions (Bradford 1999) and current (Post 2009) conditions.
Intensive groundwater monitoring was conducted (Bradford 1999, Post et al. 2010) at twelve locations within the wetland. Collectively, these groundwater-focused studies provide important baseline information regarding hydrogeological conditions that provide critical support for Hine’s Emerald habitat in Minesing Wetlands.
Anticipated future hydrogeochemical characterization in the wetland includes:
- establishing a series of monitoring wells up-gradient of the wetland for collecting water samples and monitoring water levels in the groundwater source
- investigating the intra-annual variability of groundwater chemistry in the wetland and in nearby upland groundwater; and
- establishing a regular monitoring program of water levels and groundwater chemistry for the Minesing Wetlands for the purpose of detecting and quantifying long-term changes (Spoelstra and Post 2012)
Habitat protection and stewardship
The Nature Conservancy of Canada and the Nottawasaga Valley Conservation Authority’s Minesing Wetlands Natural Area Conservation Plan (2011) identifies conservation actions within Hine’s Emerald habitat. Actions include working with landowners to conserve areas identified as Hine’s Emerald breeding
2.0 Recovery
2.1 Recovery goal
The recovery goal for Hine’s Emerald is to prevent any loss of population, genetic diversity, or habitat functionality at extant sites or at any other extant locations which may be identified in the future in Ontario.
2.2 Protection and recovery objectives
Table 1. Protection and recovery objectives
| No. | Protection or Recovery Objective |
|---|---|
| 1 | Protect and maintain the quantity and quality of Hine’s Emerald habitat and habitat functionality, including the hydrological and hydrogeological function |
| 2 | Reduce or mitigate threats to Hine’s Emerald and its habitat |
| 3 | Increase knowledge of Hine’s Emerald biology in Ontario including distribution, abundance, life history and habitat needs |
| 4 | Increase public awareness and understanding of Hine’s Emerald and its habitat in Ontario |
2.3 Approaches to recovery
Table 2. Approaches to recovery of Hine’s Emerald in Ontario
Objective 1: Protect and maintain the quantity and quality of Hine’s Emerald habitat and habitat functionality, including the hydrological and hydrogeological function
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Critical | Ongoing | Habitat protection |
1.1 Work with municipalities and other planning agencies to protect habitat and populations through municipal land use planning processes
|
|
| Critical | Short-term | Habitat protection | 1.2 Develop a habitat regulation to provide enhanced protection and clarity on the area defined as habitat for Hine’s Emerald in Ontario. |
|
| Necessary | Ongoing | Habitat protection | 1.3 Identify high-priority private lands within Hine’s Emerald distribution and promote securement through conservation easement, stewardship agreement or land acquisition when there are willing sellers.
|
|
| Necessary | Ongoing | Habitat protection | 1.4 Identify areas of probable habitat for Hine’s Emerald.
|
|
| Beneficial | Ongoing | Stewardship | 1.5 Where feasible, restore altered hydrological and/or hydrogeological regimes.
|
|
| Beneficial | Long-term | Stewardship | 1.6 Explore and encourage land owner incentive programs for Hine’s Emerald habitat protection and restoration (e.g., Conservation Land Tax Incentive Program) |
|
Objective 2: Reduce or mitigate threats to Hine’s Emerald and its habitat
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Critical | Ongoing | Management | 2.1 Develop and implement management actions to maintain or increase known population
|
|
| Critical | Ongoing | Habitat protection | 2.2 Protect burrowing crayfish and their habitat within known and suspected Hine’s Emerald locations in Ontario.
|
|
| Necessary | Ongoing | Habitat protection | 2.3 Ensure protection and recovery approaches are identified in management plans for all applicable levels of government (including federal, provincial, municipal and conservation authorities)
|
|
| Necessary | Ongoing | Management | 2.4 Monitor and mitigate invasive species in and around Hine’s Emerald habitat
|
|
Objective 3: Increase knowledge of Hine’s Emerald biology in Ontario including distribution, abundance, life history and habitat needs
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Critical | Ongoing | Inventory, Monitoring and Assessment | 3.1 Develop and implement a monitoring program to assess changes in populations and habitats over time
|
|
| Critical | Ongoing | Inventory, Monitoring and Assessment | 3.2 Conduct surveys to locate new populations
|
|
| Critical | Short-term | Research | 3.3 Investigate the sensitivity of Hine’s Emerald to various Factors that may influence habitat constraints (e.g., water quality/quantity)
|
|
| Necessary | Short-term | Research | 3.4 Investigate the relationship between Hine’s Emerald and crayfish species
|
|
| Necessary | Ongoing | Research | 3.5 Investigate interspecies relationships
|
|
| Necessary | Ongoing | Monitoring | 3.6 Monitor hydrogeological function within Minesing Wetlands
|
|
| Beneficial | Ongoing | Communication | 3.7 Participate in inter-jurisdictional recovery and research teams, including the United States Fish and Wildlife Service Hine’s Emerald recovery team |
|
Objective 4: Increase public awareness and understanding of Hine’s Emerald and its habitat in Ontario
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Necessary | Short-term | Communication | 4.1 Provide information to stakeholders and interested parties to increase their awareness of this species
|
|
2.4 Area for Consideration in Developing a Habitat Regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of Natural Resources on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the authors will be one of many sources considered by the Minister when developing the habitat regulation for this species.
It is recommended that the area regulated as habitat for Hine’s Emerald include all extant locations. In Ontario, the extant sites currently include only the Minesing Wetlands and possibly some adjacent lands where adults have been observed in potential larval habitat. This recommendation is specific to Minesing Wetland, given our current knowledge of Hine’s Emerald distribution in Ontario. If other extant locations are found in the future, additional habitat should be prescribed under the ESA on a site- specific basis. Furthermore, due to the lack of understanding of Hine’s Emerald biology and knowledge of the Ontario population, serious knowledge gaps exist on the habitat for Hine’s Emerald. Given this, it is recommended that the area prescribed as habitat for Hine’s Emerald be re-evaluated as new information is gathered.
Minesing Wetlands and the surrounding area are found over limestone bedrock, with rich and deep, mineral soils. Groundwater discharge plays an essential role in the fen areas that relate to Hine’s Emerald habitat. The principal source of groundwater to the fen areas correspond to the Snow Valley Upland area, east of the Minesing Wetlands (Bradford 1999, Beckers and Frind 2001, Post et al. 2010).
Hine’s Emerald has two different life stages (larval and adult) that depend on different habitat. First, the larvae require areas that support their foraging, overwintering and refuge habitats. These areas are characterized as string fen and wet meadow areas. There is strong evidence of Hine’s Emerald larvae being dependent on Digger Crayfish and Devil Crawfish burrows as refuges (Pintor and Soluk 2006). Second, the adults require areas that support breeding, foraging and roosting habitats. These habitat requirements differ between female and male adult Hine’s Emerald during the breeding season (Foster and Soluk 2006). Males have been found to occupy string fens and wet intermediate meadow areas, whereas females were found to occupy dry meadow areas (Foster and Soluk 2006, COSEWIC 2011). Adjacent forest areas provide protected, shaded areas for perching and roosting by both females and males (USFWS 2001).
Dispersal patterns of Hine’s Emerald in Minesing Wetlands have not been studied. However, experts suggest that corridors that connect prescribed habitat are essential to the recovery of Hine’s Emerald, particularly to enable their movement and dispersal (C.G. Evans pers. obs).
In order to protect both the adult and larvae stages of Hine’s Emerald, it is recommended that the area prescribed as habitat include all fen, wetland meadows, and hydrologically connected fen and wet meadow habitat where Hine’s Emerald have been observed (Foster and Soluk 2006). The groundwater area that feeds the fen must be considered in order to protect the current hydrological regime. It is thought that 500 meters surrounding this area be considered the minimum local recharge area (R. Post, pers. obs.). Thus, in addition to the hydrologically connected fen and wet meadow habitat, the prescribed habitat should also include 500 metres beyond each of these habitats. Additionally, for the purposes of perching, movement and roosting, all forests and dry meadows that are adjacent to the areas described above should also be prescribed as habitat.
Corridors are believed to be both natural (creeks, swales and other water features) and anthropogenic features (trails, utility rights-of-way and gravel roads) that have forested edges or riparian habitat (C.G. Evans and D. Featherstone, pers. obs.). To allow for migration and dispersal between habitat patches used by Hine’s Emerald it is recommended that corridors connecting these areas be prescribed as habitat.
Due to the dependence of Hine’s Emerald habitat on groundwater recharge it is recommended that prescribed habitat include the Snow Valley Uplands areas as defined by Becker and Frind (2001), where the current regional groundwater infiltration regime is maintained for the entire Minesing Wetlands (i.e., maintaining the current water balance and water quality).
3.0 Glossary
Calcareous: A type of sediment, sedimentary rock, or soil type formed from, or contains a high proportion of calcium carbonate.
Committee on the Status of Endangered Wildlife in Canada (COSEWIC): The committee responsible for assessing and classifying species at risk in Canada.
Committee on the Status of Species at Risk in Ontario (COSSARO): The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
Conservation status rank: A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperiled
2 = imperilled
3 = vulnerable
4 = apparently secure
5 = secure
Conspecific: From the same species
Discharge Area: An area in which there are upward components of hydraulic head in the aquifer. Ground water is flowing toward the surface in a discharge area and may escape as a spring, seep or baseflow or by evaporation and transpiration.
Endangered Species Act, 2007 (ESA): The provincial legislation that provides protection to species at risk in Ontario.
Exuviae: The cast-off skins or coverings. In this case, the cast off shell or covering of the dragonfly larvae, shed after the larva emerges from the water to molt to the adult life stage.
Fen: Wetlands with unique hydrology that provides mineralized water to the soil’s surface.
Haplotype: The genetic makeup of an individual with respect to a specific pair of alleles or genes.
Hydrogeology: The study of the interrelationships of geologic materials and processes with water, especially ground water.
Hydrology: The study of the occurrence, distribution, and chemistry of all waters of the earth.
Instar: An insect or other arthropod between molts
Larva (pl: larvae): An immature stage of any invertebrate that differs from the adult stage.
Metapopulation: A population belonging to a group of populations of the same species that exchange individuals through migration and recolonize sites in which other metapopulations have become extinct.
Molt: Shed of old shell to make way for new growth
Nymph: A larva of an insect with incomplete metamorphosis
Odonata: The taxonomic order comprising dragonflies and damselflies.
Oviposition: To lay eggs, especially by means of an ovipositor.
Ovipositor: An organ found in some species of insects at the end of the female abdomen. This organ is used to deposit eggs.
Recharge area: An area in which there are downward components of hydraulic head in the aquifer. Infiltration moves downward into the deeper parts of an aquifer in a recharge area.
Species at Risk Act (SARA): The federal legislation that provides protection to species at risk in Canada. This act establishes Schedule 1 as the legal list of wildlife species at risk to which the SARA provisions apply. Schedules 2 and 3 contain lists of species that at the time the act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
Species at Risk in Ontario (SARO) List: The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
Succession: The progressive replacement of one dominant type of species or community by another in an ecosystem.
Teneral: The period when the adult insect is newly emerged from the pupal case or nymphal skin. During the teneral period, the insect’s exoskeleton has not hardened or darkened, leaving it vulnerable.
Thoracic: Arising from the thorax, the portion of the body between the head and the abdomen.
Tibial (Tibia): The fourth segment of the insect leg, between the femur and tarsi.
Water balance: An equation that can be used to describe the flow of water in and out of a system.
Water budget: An evaluation of all the sources of supply and the corresponding discharges with respect to an aquifer or a drainage basin.
Water table: The surface in an unconfined aquifer or confining bed at which the pore water pressure is atmospheric. It can be measured by installing shallow wells extending a few feet into the zone of saturation and then measuring the water level in those wells.
References
Beckers, J. and E.O. Frind. 2001. Simulating groundwater flow and runoff for the Oro Moraine aquifer system. Part II. Automated calibration and mass balance calculations. Journal of Hydrology, 243: 73-90
Bowles, R.L., J. Laverty and D. Featherstone. 2007. Minesing Wetlands Biological Inventory. Utopia, Ontario: Nottawasaga Valley Conservation Authority.
Bradford, A. 1999. A hydrobiological study of Minesing Swamp, Ontario. PhD thesis. Queen’s University, Kingston, ON, Canada. 392 pages.
Cashatt, T., 2012. pers. comm. Email correspondence to T.L. Pulfer. October 2012. (Curator of Zoology, Illinois State Museum)
Cashatt, E.D. and T.E. Vogt. 2001. Description of the larva of Somatochlora hineana with a key to the larvae of the North American species of Somatochlora (Odonata: Corduliidae). International Journal of Odonatology 4:93.
Corbet, P.S. 1999. Dragonflies: Behaviour and Ecology of Odonata. Cornell University Press, Ithaca, New York.
COSEWIC. 2011. COSEWIC assessment and status report on the Hine’s Emerald Somatochlora hineana in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. Ix + 41 pp.(www.sararegistry.gc.ca/status/status_e.cfm) [link no longer active].
Crandall, K.A. 2010. Fallicambarus fodiens. In: IUCN 2011. IUCN Red List of Threatened Species. Version 2011.2. <www.iucnredlist.org>. Downloaded on 05 March 2012.
Cuthrell, D.L. 1999. Special animal abstract for Somatochlora hineana (Hine’s Emerald dragonfly). Michigan Natural Features Inventory, Lansing, MI. 3 pp.
Davies, E.G.R. and S.P. Simonovic. 2005. Climate change and the hydrological cycle. 17th Canadian Hydrotechnical Conference Proceedings: 47-58.
Foster, S.E. and D.A. Soluk, 2004. Evaluating exuvia collection as a management tool for the federally endangered Hine’s emerald dragonfly, Somatochlora hineana Williamson (Odonata: Cordulidae). Biological conservation 118: 15.
Foster, S.E. and D.A. Soluk 2006. Protecting more than the wetland: The importance of biased sex ratios and habitat segregation for conservation of the Hine’s emerald dragonfly, Somatochlora hineana Williamson. Biological Conservation 127: 158-166.
Guiaşu, R.C. 2007. Conservation and diversity of the crayfishes of the genus Fallicambarus Hobbs, 1969 (Decapoda, Cambaridae), with an emphasis on the status of Fallicambarus fodiens (Cottle, 1863) in Canada. Crustaceana 80: 207-223.
Hamr, P. 2005. The distribution and conservation status of burrowing crayfishes Fallicamabarus fodiens and Cambarus diogenes in Canada. Freshwater Crayfish 15: 271-282.
Hanna, R. 1982. A Detailed Life Science Inventory Check-sheet for Minesing Swamp.
Unpublished
McKenzie, P. PhD. pers. comm. 2012. Email correspondence T.L. Pulfer October 2012. (Endanagered Species Coordinator, USA Fish and Wildlife Service, Columbia, MO).
Meekins, J.F. and B.C. McCarthy. 1999. Competitive ability of Alliaria petiolata (Garlic Mustard, Brissicaceae), an invasive, nonindiguous forest herb. International Journal of Plant Sciences 160: 743-752.
Monroe, E.M., C. Lynch, D.A. Soluk and H.B. Britten. 2010. Nonlethal tissue sampling techniques and microsatellite markers used for first report of genetic diversity in two populations of the endangered Somotochlora hineana (Odonata: Corduliidae). Annuals of the Entomological Society of America 103: 1012-1017.
Pintor, L.M. and D.A. Soluk. 2006. Evaluating the non-consumptive, positive effects of a predator in the persistence of an endangered species. Biological Conservation 130: 584-591.
Portmann, R.W., S. Soloman and G.C. Hegerl. 2009. Spatial and seasonal patterns in climate change, temperatures, and precipitation across the United States. Proceedings of the National Academy of Sciences of the United States of America 106: 7324-7329.
Post, R. 2009. Desktop Hydrogeological Review of the Minesing Wetlands. Report submitted to the Nature Conservancy of Canada. Nottawasaga Valley Conservation Authority, Utopia, Ontario. 33 pp.
Post, R., D. Featherstone, I. Ockenden and A. Rodie. 2010. Integrated Minesing Wetlands Monitoring Program – 2009 Results. Nottawasaga Valley Conservation Authority, Utopia, Ontario. 59 pp.
Pulfer, T.L., C. Bahlai and L. Mousseau. 2011. Recovery Strategy for Laura’s Clubtail (Stylurus laurae) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario.v + 23 pp.
Rao, R.S.P. and M.K.S. Girish. 2007. Road kills: assessing insect causalities using flagship taxa. Current Science 92: 830-837.
Riley, J.L. 1989. Laboratory methods for testing peat: Ontario peatland inventory project. Ontario Ministry of Northern Development and Mines. 51 pp.
Snyder, R.L., R. Moratiel, Z. Song, A, Swelam, I. Jomaa and T. Shapland. 2011. Evapotranspiration response to climate change. Acta Horticulturae 922:91-98. Soluk, D.A., D.S. Zercher and A.M. Worthington. 2011. Influence of roadways on patterns of mortality and flight behavior of adult dragonflies near wetland areas. Biological Conservation 144: 1638-1643.
Soluk, D.A. and K. Moss. 2003. Roadway and exuvial surveys of the Hines Emerald dragonfly (Somatochlora hineana) in Door County, Wisconsin: Part II Roadway fatalities. Final Report to the United States Fish and Wildlife Service. 12 pp.
Soluk, D.A. D.S. Zercher, A.M. Worthington. 2011. Influence of roadways on patterns of mortality and flight behavior of adult dragonflies near wetland areas.
Spoelstra, J. and Post, R. 2012. Hydrogeochemical characterization of the eastern Minesing Wetlands. National Water Research Institute, Report Number 12‐001. Environment Canada. 19 pp.
United States Fish and Wildlife Service (USFWS). 2001. Hine’s Emerald Dragonfly (Somatochlora hineana) Recovery Plan. Fort Snelling, MN. 120 pp.
Zedler, J.B. 2010. How frequent storms affect wetland vegetation: a preview of climate-change impacts. Frontiers in Ecology and the Environment 8: 540-547.
Footnotes
- footnote[*] Back to paragraph No longer with NCC
- footnote[1] Back to paragraph The Planning Act – Provincial Policy Statement protects the area deemed provincially significant and up to 120 meters surrounding this area
- footnote[2] Back to paragraph See Figure 1.1 in Spoelstra and Post 2012 for a description of the Snow Valley Uplands.