Houghton’s Goldenrod Recovery Strategy
This document is the recovery strategy for a species at risk – Houghton’s Goldenrod.

Cover illustration: Houghton’s Goldenrod on the Bruce Peninsula by Michael Patrikeev.
This photo may not be reproduced separately from this document without permission of the photographer.
About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species’ persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. There was a transition period of five years (until June 30, 2013) to develop recovery strategies for those species listed as endangered or threatened in the schedules of the ESA. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of Natural Resources and Forestry Species at Risk webpage.
Recommended citation
Jones, J.A. 2015. Recovery strategy for the Houghton’s Goldenrod (Solidago houghtonii) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources and Forestry, Peterborough, Ontario. vi + 31 pp.
©Queen’s Printer for Ontario, 2015
ISBN 978-1-4606-5718-8
Authors
Judith Jones, Winter Spider Eco-Consulting, Sheguiandah, Ontario
Acknowledgments
Previous versions of some of the material in this document were prepared by Judith Jones, Jarmo Jalava, and Holly Bickerton under the direction of the Bruce-Manitoulin Alvar Recovery Team and Parks Canada Agency.
The author gratefully acknowledges the following people and agencies for sharing information: Theodore Flamand (Wikwemikong Department of Lands and Natural Resources), G’mewin Migwans (United Chiefs and Councils of M’nidoo M’nising), Will Kershaw (Ontario Parks). Thanks to Michael Patrikeev for the use of his photo for the cover.
Declaration
The recovery strategy for the Houghton’s Goldenrod was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all of the individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ontario Ministry of Natural Resources and Forestry
Executive summary
Houghton’s Goldenrod (Solidago houghtonii) is listed as threatened under Ontario’s Endangered Species Act, 2007. It is listed as special concern on Schedule 1 of the Federal Species at Risk Act. It has a global and subnational (Ontario) conservation rank of G3S2.
Houghton’s Goldenrod is a perennial plant in the Aster Family that has a cluster of narrow, strap-shaped, smooth-margined leaves growing on the ground. It produces an upright flowering stalk with leaves that become smaller toward the top. Flower heads with rough stalks are arranged in a flat-topped cluster. Flowering occurs in late August through September. Houghton’s Goldenrod is very similar to the Ohio Goldenrod (Solidago ohioensis) which may grow in the same habitat.
There are 33 populations of Houghton’s Goldenrod in Canada, all in Ontario in the Manitoulin Island region, with one occurrence on the Bruce Peninsula. Many populations are on lands belonging to or claimed by First Nations, and roughly one third of populations are on corporately-owned land. There is no information on population trends because most sites have had only one observation.
Houghton’s Goldenrod is primarily found in alvars, on bedrock shorelines, and in a few degraded alvars that resemble old fields, except on Cockburn Island where the species occurs on low sand dunes. Suitable habitat may be part of at least eight different vegetation community types. Moisture is an important feature of suitable microhabitat.
Knowledge gaps for Houghton’s Goldenrod include the status of populations, trends, population viability levels, the effects of current threats, and techniques for reintroductions. As well, genetic work is needed to know whether the Cockburn Island population may be a separate species. Other gaps pertain to habitat requirements and dynamics and potentially the effects of climate change.
Threats to Houghton’s Goldenrod include development and construction, logging and industrial activities, quarrying and aggregates extraction, off-road vehicle use, grazing and browsing, invasion by exotic species, damage to alvars as a result of a lack of public awareness and the use of herbicides and mowing.
The recovery goal for Houghton’s Goldenrod is to maintain the current abundance and distribution of all populations of Houghton’s Goldenrod in Ontario by maintaining and protecting habitat, reducing other threats, and augmenting populations if necessary.
Protection and recovery objectives include:
- assess threats and undertake actions for mitigation and reduction;
- use policy tools, where appropriate, to protect and maintain Houghton’s Goldenrod habitat and plants;
- raise awareness about Houghton’s Goldenrod and its sensitive habitats; and
- fill knowledge
A number of approaches to recovery are presented in the text.
The area recommended to be considered for a habitat regulation for Houghton’s Goldenrod includes:
- all areas where Houghton’s Goldenrod is present and any new areas that are discovered;
- on sand: the entire Ecological Land Classification (ELC) dune or beach vegetation community type polygon;
- on bedrock beach: the portion of the ELC bedrock beach vegetation community type polygon in which Houghton’s Goldenrod is present, bounded on the inland side as per the ELC, but bounded at the ends at 50 m beyond the presence of Houghton’s Goldenrod plants;
- on alvar: the entire ELC alvar vegetation community type polygon;
- in degraded, old field habitat and where the vegetation is predominantly non- native plant species: all of the continuous area around Houghton’s Goldenrod plants in which there are patches of vegetation shorter than the flowering stalks of Houghton’s Goldenrod (approximately 60 cm tall);
- in all situations above: all of the area within a minimum radial distance of 50 m from the plants, including suitable and unsuitable habitat, so that if individuals occur at the edge of a polygon, there will be sufficient distance from activities in adjacent areas to prevent the risk of impacts.
It is recommended that existing infrastructure not be prescribed as habitat.
1.0 Background information
1.1 Species assessment and classification
- Common name: Houghton’s Goldenrod
- Scientific name: Solidago houghtonii
- SARO list classification: Threatened
- SARO list history: Threatened (2008)
- COSEWIC Assessment history: Special concern (2005)
- SARA schedule 1: Special concern (2006)
- Conservation status rankings:
- GRank: G3
- NRank: N2
- SRank: S2
The glossary provides definitions for the abbreviations above and for other technical terms in this document.
1.2 Species description and biology
Species description
Houghton’s Goldenrod (Solidagohoughtonii; Figure 1) is a perennial plant in the Aster Family. In the first few years of growth it is a rosette or cluster of narrow (1-2 cm wide), strap-shaped, smooth-margined leaves usually 15 to 17 cm long. The maximum height of the rosettes is about the same as the length of these leaves. After a few years, the plant produces an upright flowering stalk 30 to 60 cm tall with leaves that become progressively smaller going up the stem. As with all members of the Aster Family, what appear to be flowers are actually heads of many tiny flowers. Houghton’s Goldenrod has the heads arranged in a more or less flat-topped capitulescence (like an inside-out umbrella), with rough stalks supporting the heads. Compared to other Goldenrods in Ontario, the flower heads are relatively large (5-8 mm tall) and generally fewer in number (usually 2-50 heads). The heads generally have 20-30 flowers (6-12 ray flowers around the outside and 8-15 disc flowers in the centre of each head) (Semple et al. 1999; Semple and Cook 2006). Both the ray flowers and the disc flowers are bright yellow. Outside of Ontario, Houghton’s Goldenrod may be more robust than the measurements given above for Ontario plants (Semple and Cook 2006). For example, in Michigan plants with 5 to 100 heads are common and plants with more than 100 heads are not unusual (Voss 1996).
Houghton’s Goldenrod is very similar to the Ohio Goldenrod (Solidagoohioensis), which often grows in similar habitats and also has a flat-topped capitulescence. These characters distinguish Houghton’s Goldenrod from the Ohio Goldenrod:
Houghton’s Goldenrod
- Narrow basal leaves
- Rough stalks under flower heads
- Heads large and few
- Blooms late August to end of September
Ohio Goldenrod
- Ovate basal leaves
- Smooth stalks under flower heads
- Heads small and numerous
- Blooms August to early September

Evolutionary origins and boundaries of the taxon
There has been a lot of speculation about the hybrid origin of Houghton’s Goldenrod due to the variations in habitat preference and morphological traits the species exhibits over its geographic range (Morton, 1979, 1981; Edwards-Wilson 1999; Semple et al. 1984, 1999). As a result, there has also been discussion about whether S. houghtonii is one species or several and which characters the taxon should include. Pringle (USFWS 1997) proposed four different entities within Houghton’s Goldenrod: (1) in Michigan on both sides of the straits of Mackinac and in Ontario on Cockburn Island; (2) in Ontario on limestone or dolostone; (3) a single disjunct octoploid population in central Michigan; and (4) a single occurrence in New York.
Recent genetic studies suggest a hybrid origin for Houghton’s Goldenrod with several progenitors contributing to the genome. Laureto and Barkman (2011) analysed nuclear DNA sequence data and found that Riddell’s Goldenrod (S. riddellii), the Upland White Goldenrod (S. ptarmicoides), and the Ohio Goldenrod likely contributed to the nuclear genome of Houghton’s Goldenrod. Their analyses of chloroplast DNA suggest the Giant Goldenrod (S. gigantea) was the maternal genome donor. Their results suggest a single origin for Houghton’s Goldenrod from a complex, reticulate evolution from different types of genetic events. Interestingly, the Ontario alvar entity (#2) was the only entity of Houghton’s Goldenrod that did not contain sequences from the Upland White Goldenrod. There may be several explanations for this, ranging from to evolutionary history of the entity to aspects of the laboratory procedures. However, three parental genomes are expected for Houghton’s Goldenrod, so additional study may yet indicate the presence of the Upland White Goldenrod, which has large flower heads and may be responsible for the large size of ray flowers in Houghton’s Goldenrod (Laureto and Barkman 2011).
Today the Upland White Goldenrod frequently occurs with Houghton’s Goldenrod, but Riddell’s Goldenrod does not presently occur on the Bruce Peninsula, Manitoulin Island, or Cockburn Island in Ontario, nor anywhere in northern Michigan (Semple et al 1999; Reznicek et al. 2011). However, the events that led to the creation of Houghton’s Goldenrod as a species likely took place thousands of years ago, so the parent species and Houghton’s Goldenrod would not necessarily have remained together in the same geographic range over that time frame.
True Houghton’s Goldenrod (the plant first collected and described with that name - entity #1), is hexaploid (having six sets of chromosomes) (Semple et al. 1984, 1999). Pringle’s entity #3 is now described as a new species, Voss’s Goldenrod (S. vossii) (Laureto and Pringle 2010), because it is octoploid (having eight sets of chromosomes) and shows some evidence of separate evolutionary events. Even if the Canadian and Michigan populations (entities #1 and #2) were to be separated into two species (or designatable units by COSEWIC) at some time in the future, the change would likely not affect recovery needs for the entities that are currently called Houghton’s Goldenrod in Canada. However, it is likely the occurrence on Cockburn Island would be put into a higher risk category because it is the sole Canadian representative of the Michigan genetic entity (entity #1).
Species biology
Houghton’s Goldenrod is a perennial herb that spreads vegetatively by underground rhizomes. Plants may produce 2 to 12 ramets which can break off and become established as separate plants. Young individuals may live for one to six years as basal rosettes of leaves before flowering, and flowering plants may continue to live following successful fruit set (USFWS 1997). Jolls and Tolley (in prep.) found that flowering is size-related but that individual plants normally do not flower in consecutive years. The maximum age of individuals of Houghton’s Goldenrod is unknown but may possibly be a decade or more. Plants flower late in the season, with some flowering well into September. If successfully pollinated, each central disc flower produces a single seed- like fruit, with an attached ring of bristles (pappus) at the top which catches air movements and allows dispersal of the fruit by wind. Houghton’s Goldenrod can apparently survive transplantation, as evidenced by an inland population established by transplantation in Michigan (USFWS 1997).
Houghton’s Goldenrod flowers provide both pollen and nectar to insects, and the plants receive a range of insects that may potentially act as pollinators, including bees, moths, butterflies, flies, wasps, and beetles (USFWS 1997). Despite this, a study of the reproductive biology and environmental requirements of Houghton’s Goldenrod (Jolls and Tolley in prep.) found that successful sexual reproduction was infrequent and likely limited by pollen transfer, and self-pollination was largely unsuccessful.
In addition, fruit set for open pollination was low (17%). Growth chamber experiments showed Houghton’s Goldenrod has a low germinability (56%) which does not appear to be affected by substrate type or moisture level. Seeds require light and chilling for germination, indicating an obligate overwintering period, yet germinability declines significantly after a period of months (Jolls and Tolley in prep.). Several other species of Goldenrods also do not have long lived seeds nor maintain a seed bank. Lee (1993) found seed viability of the Seaside Goldenrod (Solidago sempervirens) declined rapidly after 1 year. Buchelé et al. (1991) found Short’s Goldenrod (Solidagoshortii) maintained no persistent seed bank and, after examining studies of other Goldenrod species, suggested that most species in the genus produce seeds that remain viable for no more than one year. Thus, a seed bank is not expected for Houghton’s Goldenrod.
The flowers of Houghton’s Goldenrod are showy and very late blooming, so the species may play some ecological role for insect populations in the late growing season. It can also be speculated that there may be some years where early frost prevents successful pollination.
1.1 Distribution, abundance and population trends
Globally, Houghton’s Goldenrod is found only on or near the northern shores of Lake Michigan, Lake Huron and Georgian Bay (Guire and Voss 1963; Morton 1979; USFWS 1997; Semple et al. 1999; COSEWIC 2005; Jones 2007, 2010, unpub. data; NatureServe 2014). Within this range, one group of populations occurs in the state of Michigan around the Straits of Mackinac and on Beaver and Drummond Islands and includes the Ontario population on Cockburn Island. Another group of populations occurs at the eastern end of the Manitoulin Island region, and a single population occurs on the Bruce Peninsula. All Canadian populations are in Ontario (Figure 2).
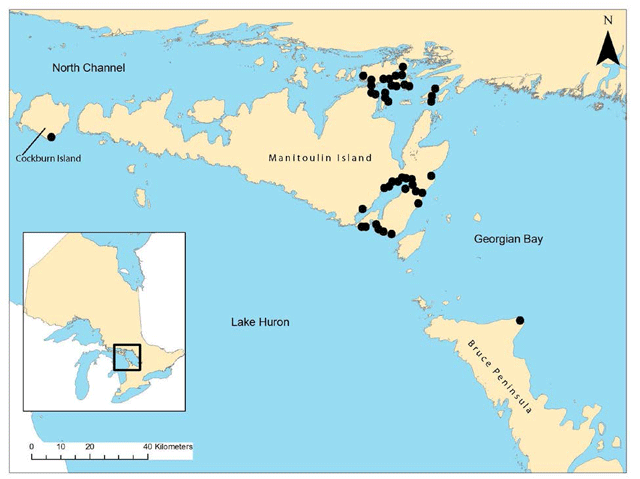
Sources: Morton and Venn 2000; Jones 2004, 2005, 2007, 2010, and pers. obs. 2013; W. Bakowsky pers. comm. 2014.
Enlarge Figure 2. Distribution of Houghton’s Goldenrod in Ontario (PDF)
There are 33 populations of Houghton’s Goldenrod in Canada (Table 1). It is currently not possible to determine population and abundance trends because most sites have had only one observation or lack abundance data. Roughly one third of Houghton’s Goldenrod populations are on land in corporate ownership. Several other populations and a large number of individuals are on lands belonging to or claimed by two First Nations communities in the Manitoulin Region: Wikwemikong Unceded Indian Reserve (WUIR) and Whitefish River First Nation. The latter is a member of the United Chiefs and Councils of M’Nidoo M’nising (UCCMM), an umbrella agency that handles most land management issues.
Table 1. List of populations of Houghton’s Goldenrod in Ontario
Legend:
- ANSI = area of natural and scientific interest;
- FN = First Nation;
- WUIR = Wikwemikong Unceded Indian Reserve;
- UCCMM = United Chiefs and Councils of M’Nidoo M’Nising (Manitoulin Island).
| Site Name | Region | Ownership | Abundance | Most recent observation & observer | Comments |
|---|---|---|---|---|---|
| Cabot Head Provincial Nature Reserve | Bruce Peninsula | Ontario Parks/ Bruce Peninsula National Park | 13,000 | 2003 K. Makkay | Lands are also designated as ANSI. |
| Site Name | Region | Ownership | Abundance | Most recent observation & observer | Comments |
|---|---|---|---|---|---|
| Badgely Peninsula McGregor Pt. | Manitoulin | Crown | Unknown | 2000 W. Bakowsky | Killarney Coast Proposed Provincial Park |
| Badgely Peninsula Indian Pipe Cove | Manitoulin | Crown/FN | 100 | 2009 J. Jones & WUIR staff | Killarney Coast Proposed Provincial Park/WUIR traditional territory |
| Centre Island | Manitoulin | Crown/FN | 200-300 | 2009 J. Jones & WUIR staff | Under land claim by WUIR |
| Goat Island | Manitoulin | Corporate | 100 | 2014 J. Jones | |
| Strawberry Island, northern end | Manitoulin | Ontario Parks | 1,000 | 2005 J. Jones | |
| Strawberry Island, W of Bowell Cove | Manitoulin | Ontario Parks | Frequent | 2005 J. Jones | |
| Strawberry Island, Chapleau Cove | Manitoulin | Ontario Parks | Unknown | 2005 J. Jones | |
| Gr. Cloche Island Stony Pt., English Pt. | Manitoulin | Corporate | 400 | 2003 P. Laureto | |
| Gr. Cloche Island NW, Lewis Lake | Manitoulin | Corporate | 100 | 1990’s P. Catling & V. Brownell | See Brownell & Riley 2000 |
| Gr. Cloche Island SW, W. Bell Rocks | Manitoulin | Corporate | 100 | 2003 K. Makkay | |
| Gr. Cloche Island, Little River | Manitoulin | Corporate | Unknown | 2003 K. Makkay | |
| Gr. Cloche Island, Southwest | Manitoulin | Corporate | 100s | 1996 J. Jones | |
| Little Cloche Island, Mary’s Pt. | Manitoulin | Corporate | 420 | 2003 P. Laureto | |
| Little Cloche Island, Shoal Bight | Manitoulin | Corporate | >50 | 1996 J. Jones | |
| La Cloche Peninsula #1 | Manitoulin | Whitefish River First Nation | 50 | 2010 J. Jones & UCCMM staff | |
| La Cloche Peninsula #2 | Manitoulin | Whitefish River First Nation | 1,000 | 2010 J. Jones & UCCMM staff | |
| Little Current, Harbour View Road | Manitoulin | Private/ Corp. | Unknown | 2014 J. Jones | |
| 3 km SE of Little Current | Manitoulin | Private/ Municipal | 30 | 2005 J. Jones | |
| West of Leask Point | Manitoulin | Private/ Municipal | Unknown | 2000 J. Jones | |
| South Bay, Lower Slash Rd. | Manitoulin | Private/ Municipal | >100 | 2014 J. Jones | |
| White’s Point | Manitoulin | Private/ Municipal | 10 | 2008 J. Jones | |
| Wikwemikong #1 | Manitoulin | WUIR | 50 | 2007 J. Jones & WUIR staff | |
| Wikwemikong #2 | Manitoulin | WUIR | 500 | 2007 J. Jones & WUIR staff | |
| Wikwemikong #3 | Manitoulin | WUIR | >15,000 | 2007 J. Jones & WUIR staff | |
| Wikwemikong #4 | Manitoulin | WUIR | 10,000 | 2007 J. Jones & WUIR staff | |
| Wikwemikong #5 | Manitoulin | WUIR | 30 | 2007 J. Jones & WUIR staff | |
| Wikwemikong #6 | Manitoulin | WUIR | >2,000 | 2007 J. Jones & WUIR staff | |
| Wikwemikong #7 | Manitoulin | WUIR | >1,000 | 2007 J. Jones & WUIR staff | |
| Wikwemikong #8 | Manitoulin | WUIR | 300 | 2007 J. Jones & WUIR staff | |
| Wikwemikong #9 | Manitoulin | WUIR | 6,000 | 2007 J. Jones & WUIR staff | |
| Wikwemikong #10 | Manitoulin | WUIR | 3,500 | 2007 J. Jones & WUIR staff |
| Site Name | Region | Ownership | Abundance | Most recent observation & observer | Comments |
|---|---|---|---|---|---|
| Sand Bay | Manitoulin | Private/ Municipal | 5,000 | 2005 J. Jones |
| Site Name | Region | Ownership | Abundance | Most recent observation & observer | Comments |
|---|---|---|---|---|---|
| Beauty Island | Manitoulin | Private | Presence likely | ||
| Islands off Gr. Cloche Island (Matlas, Patten, Flat, etc.) | Manitoulin | ? | Presence likely | ||
| North Channel Drive Alvars (W of Little Current) | Manitoulin | Private | Presence likely |
Totals: 33 populations; ~60,000 individuals
1.4 Habitat needs
Much of the information in this section comes from the observations of Judith Jones, unless otherwise cited. Jones visited all of the Canadian Houghton’s Goldenrod populations except the Badgely Peninsula — McGregor Point between 1996 and 2014 (Jones 2004, 2005, 2007, 2010 and unpublished data). Unpublished data collected by Jones, on sites that are not on First Nations lands, is on file with the Natural Heritage Information Centre (NHIC) of the Ontario Ministry of Natural Resources and Forestry (OMNRF). Data from First Nations lands is on file with UCCMM or WUIR.
In Canada, with the exception of the site on Cockburn Island, all Houghton’s Goldenrod populations are found in open, nearly treeless areas occurring on Ordovician limestone or Silurian dolostone. Suitable habitat is found in alvars
These communities are usually dominated by Shrubby Cinquefoil (Dasiphora fruticosa), Northern Dropseed (Sporobolus heterolepis), Little Bluestem (Schizachyrium scoparium), Tufted Hairgrass (Deschampsia cespitosa), or Creeping Juniper (Juniperus horizontalis) (Reschke et al. 1999; Brownell and Riley 2000; Jones 2004, 2005, 2010, unpub. data). In ALT1-4, the ground flora may be dominated by any of the five above- mentioned species.
On Cockburn Island, Houghton’s Goldenrod occurs on low sand dunes dominated by Little Bluestem and Long-leaved Reed Grass (Calamovilfalongifolia var. magna). The vegetation community may be classified as Little Bluestem – Long-leaved Reed Grass – Great Lakes Wheatgrass Open Dune (SDO1-2). In both alvar and sand habitats, frequent associates of Houghton’s Goldenrod include Ohio Goldenrod, Upland White Goldenrod (S.ptarmicoides), Kalm’s Lobelia (Lobeliakalmii), Shrubby Cinquefoil, Twig Rush (Cladium mariscoides), and Sticky False Asphodel (Triantha glutinosa) (Penskar et al. 2000; Jones, unpub. data).
Houghton’s Goldenrod appears to tolerate a moderate level of disturbance. The species is abundant in some weedy, degraded alvars that were used as livestock pasture in the past, for example, at parts of two Wikwemikong populations and parts of the Strawberry Island north and Great Cloche Island populations. These habitats resemble old fields and may be classified as either Bedrock Cultural Meadow (CUM2) or Common Juniper Cultural Alvar Thicket (CUT2-1).
Moisture is an important feature of suitable microhabitat. Houghton’s Goldenrod tends to grow in the moister parts of alvars and bedrock shores and at the edge of low, marshy spots and where water pools or stays for a while before evaporating. Often these areas are the result of characteristics of the underlying bedrock, such as shallow depressions and places where the rock has no fissures or fractures that would allow drainage. On sand, Houghton’s Goldenrod is most frequent in the moist flats between beach ridges and at the edges of marshy areas.
Alvars are open habitats, and similar habitats, such as prairies and oak savannas, have traditionally been maintained by periodic fire (Tester 1989; Riley 2013). Consequently, there has been much discussion on whether fire maintains alvar ecosystems (Catling and Brownell 1998; Reschke et al. 1999; Brownell and Riley 2000; Catling et al. 2001; Catling 2009). Some authors maintain that fire is harmful to alvars (Gilman 1995, 1997), while some maintain that it is beneficial or required (Catling and Brownell 1998; Catling et al. 2001; Catling 2009). Furthermore, there are different types of alvars that have different fire histories (Jones and Reschke 2005).
Most alvars where Houghton’s Goldenrod is found show little or no evidence of burning (Jones and Reschke 2005). It is possible that these alvars did not originate from fire but are relics of post-glacial times that are becoming vegetated at an extremely slow rate (over centuries) (Jones and Reschke 2005). Alternatively, it may be that the drought- flood cycle and shallow soils perpetually inhibit growth of woody vegetation, keeping these alvars in a sparse, open state without fire (Rosén 1995; Reschke et al. 1999).
On the other hand, there are a few other alvars with Houghton’s Goldenrod that do have evidence of historical burning (Jones unpub. data). However, there is no evidence that these alvars are maintained by fire or in fact have ever burned more than once (Reschke et al. 1999; Jones and Reschke 2005; Jones unpub. data). Jones (2000) found that oak savannas in one township on Manitoulin Island were created in single, catastrophic fires but were not maintained by repeat, periodic fires and eventually grew into woodland. It was speculated that the oak savanna vegetation may be maintained within the landscape but that individual patches are not necessarily maintained in any one place. It is possible that alvars have a similar history but grow in extremely slowly. Therefore, it is unknown whether controlled burning on alvar would be beneficial or harmful to Houghton’s Goldenrod.
As an interesting note, Houghton’s Goldenrod occasionally occurs in the same alvars as Gattinger’s Agalinis (Agalinis gattingeri, endangered in Ontario and in Canada) and Hill’s Thistle (Cirsiumhillii, threatened in Ontario and in Canada), but these three species do not occupy the same microhabitats. Catling (1995) considered Houghton’s Goldenrod to be highly (85-100%) confined to alvar, but the study only looked at Canadian populations. In the global range of what is currently considered S. houghtonii (see genetics, above), a great many populations of this species occur on sand (Penskar et al. 2000; Reznicek et al. 2011; NatureServe 2014). However, in Canada, only the Cockburn population occurs on sand; two populations (on First Nations lands) are in savannas dominated by either Bur Oak (Quercus macrocarpa) or Jack Pine (Pinusbanksiana); and then all of the remaining 30 populations are in alvars.
The discussion is on-going as to whether alvars are restorable after disturbance, whether they can be replaced after destruction, and how restoration or replacement might be done. For example, many studies have looked at developing alvars on the floors of old limestone quarries or at creating new alvar as part of an overall plan to offset the destruction of another alvar (c.f. Larson et al. 2006; Campeau 2013). Catling (2013) reviews a number of studies on alvar restoration and points out that none provide evidence that self-sustaining and fully diverse alvars can be created or fully repaired following serious damage.
1.5 Limiting factors
Some natural limitations may affect Houghton’s Goldenrod and could potentially compound the effects of anthropogenic threats. Some of the reproductive characteristics studied by Jolls and Tolley (in prep.), such as low seed germinability, low fruit set, self-infertility, and low rates of pollen transfer, may limit Houghton’s Goldenrod. As well, the flowers of Houghton’s Goldenrod are very late blooming, so it can be speculated there may be some years when early frost prevents successful pollination.
1.6 Threats to survival and recovery
Threats to Houghton’s Goldenrod include development and construction, logging and industrial activities, quarrying and aggregates extraction, off-road vehicle use, livestock grazing, invasion by exotic species, and habitat degradation due to a lack of public awareness. The use of herbicide is a potential threat at some locations. The information in this section comes from the observations of Judith Jones unless otherwise cited. Kraus et al. (2009) conclude that the most significant threats to islands in Lake Huron, including Manitoulin, Cockburn and others that support Houghton’s Goldenrod, include incompatible development and the spread of invasive species.
Development and construction
Most Houghton’s Goldenrod habitats are in close proximity to the Lake Huron or Georgian Bay shoreline, so the locations are in demand for residential and cottage development. In one area of Manitoulin Island, industrial and commercial construction also threatens habitat. Placing buildings, yards, driveways, and roads on alvar or dunes may completely eliminate suitable habitat. Negative effects of development may result from clearing vegetation, blasting bedrock to level foundations or anchor structures, trucking in fill which introduces invasive plants and covers suitable ground, displacing shallow soil, and trampling vegetation with heavy machinery.
Logging and industrial activities
Alvar habitats are often used as staging areas for logging operations in surrounding forests and for storage of industrial materials and machinery. Driving heavy machinery and moving logs and materials across alvars tramples plants, dislodges shallow soils, and brings in exotic species that degrade habitat. These actions cause damage to the habitat as well as to the plants directly.
Quarrying and aggregates extraction
Alvars are often selected for quarry development because limestone bedrock is visible at the surface, and little clearing of forest and soil is necessary (Reschke et al. 1999). Quarrying may completely destroy alvar habitat. In the Manitoulin District, portions of several populations of Houghton’s Goldenrod have probably been destroyed by aggregate extraction. The remaining extents of these populations is not known.
Off-road vehicle use
Off-road use of vehicles is a threat to both the habitat and the plants of Houghton’s Goldenrod. Use of all-terrain vehicles (ATVs), especially, is a serious concern because ATVs are nearly unrestricted in where they can go and do not need roads or trails. Off- road vehicle use on alvar tramples plants, disturbs or destroys vegetation, displaces shallow layers of soil, and brings in weed species. Ruts from vehicle use in wet soil or sand tend to channel water and can change moisture and drainage regimes (Reschke et al. 1999). The use of ATVs causes periodic damage at many sites on Manitoulin Island, and is a potential threat at nearly all sites there.
Grazing and browsing
Livestock grazing reduces populations of plants, spreads non-native weeds, and degrades habitat (Reschke et al. 1999). Historically, some of the alvars where Houghton’s Goldenrod occurs had livestock on them, and the presence of weeds and tall Eurasian grass species have degraded habitat quality. Only one Houghton’s Goldenrod site was still being grazed in 2014 (J. Jones pers. obs.), but grazing remains a potential threat in a few places.
Some areas where Houghton’s Goldenrod is present have exceptionally large populations of White-tailed Deer (Odocoileus virginianus). Deer browsing is considered an active threat for Houghton’s Goldenrod, rather than a natural limitation, because the number of deer present is not natural where Houghton’s Goldenrod occurs. Deer densities are much higher now than they were in presettlement times, and browse may be a serious issue for herbaceous plants where deer are abundant (Waller and Alverson 1997; Rooney 2001; Côté et al. 2004). Kraus et al. (2009) note that overbrowsing by introduced deer is a threat to ecological communities on many islands in Lake Huron. Large populations of deer have caused negative effects on rare species in other parts of Ontario, such as at Pinery Provincial Park, where management to reduce deer populations became necessary (COSEWIC 2010).
Invasion by exotic species
Disturbance in alvars or on dunes may bring in seeds or other propagules of non-native species. Exotic species, especially non-native grasses, grow aggressively and take up the spaces required for the basal rosettes of Houghton’s Goldenrod. As well, many non-native grasses are taller than alvar and dune species and may shade or crowd out Houghton’s Goldenrod, may increase litter accumulation, and may change other dynamics such as moisture retention (J. Jones unpublished data). These factors cause an overall degradation of habitat for the species. Some examples of problem species in the habitat of Houghton’s Goldenrod (J. Jones, unpublished data) include European Common Reed (Phragmites australis ssp. australis), Smooth Brome (Bromus inermis), White Sweet Clover (Melilotus alba) and Canada Bluegrass (Poa compressa), which is not native to Canada (Brouillet et al. 2010+), despite its name.
Lack of public awareness
Habitat may become degraded simply due to a lack of awareness. Alvars are sometimes perceived as waste land, perhaps due to the sparse vegetation and lack of trees, and may be considered places where indiscriminate usage doesn’t matter because “there is nothing there”. As a result of a lack of awareness, alvars frequently become locations for unsanctioned activities such as illegal dumping and unmonitored camping. As well, the perception as “waste land” often leads people to select alvars preferentially for many of the activities that cause the threats listed above. In addition, a lack of knowledge about alvars sometimes leads to unintentionally damaging management, such as in 2014 on Manitoulin Island where some ditches containing Houghton’s Goldenrod were seeded with Perennial Ryegrass (Lolium perenne), presumably because the areas appeared barren. Although there has been an increase in awareness about alvars in Ontario in the last ten years, many people still do not know the word “alvar”, even on Manitoulin Island where alvars are quite common and where outreach work has been done.
Use of herbicides and mowing
Houghton’s Goldenrod sometimes occurs in roadside ditches or in utility corridors in a few places where roads and power lines have been put across alvar habitat. These types of areas are sometimes mowed or sprayed with herbicide to reduce unwanted vegetation, and this may be a potential threat to Houghton’s Goldenrod. Approximately eight sites have some part of the population in a ditch or utility corridor (J. Jones pers. obs. 1996-2014). Presumably, areas where Houghton’s Goldenrod occurs have not been sprayed in the past. However, with lack of awareness (above), this could inadvertently occur.
1.7 Knowledge gaps
There are knowledge gaps about the status of some populations of Houghton’s Goldenrod and the effects of current threats. Most populations have had only one observation, so little is known about trends or viability for most of the Canadian population. The continued presence of the species at some sites not visited in decades or where threats are on-going remains a question. In addition, at least three sites with potential populations (listed at the bottom of Table 1) have never been surveyed.
Information on population viability is needed as well in order to know whether additional recovery actions, such as reintroductions or augmentation by seeding or transplantation may be required in smaller populations. Filling these knowledge gaps would allow recovery to be targeted where it is most urgently needed.
Filling genetic knowledge gaps may be necessary to clarify taxonomic questions, such as whether all genetic entities belong in a single species. Addressing this taxonomic question may be quite important because if Houghton’s Goldenrod is divided into two species, the new taxa may have additional habitat protection or recovery needs (although this recovery strategy attempts to address all such needs for all populations of Houghton’s Goldenrod for the time being).
There are also knowledge gaps pertaining to habitat requirements and dynamics. It would be useful to know more about the factors that create or maintain habitat in a suitable state in order to better protect and manage habitat. For example, the importance of fire in the maintenance of alvars suitable for Houghton’s Goldenrod is still unknown, and the impact of the drought and flooding cycle on habitat suitability, population size and other factors is also a knowledge gap. A better understanding of the moisture regime required by the species would also allow a better delineation of suitable habitat.
Penskar and Derosier (2012) ranked Houghton’s Goldenrod as highly vulnerable to the effects of climate change (on a scale of extremely vulnerable, highly vulnerable, moderately vulnerable, or presumed stable). The vulnerability of many species, including Houghton’s Goldenrod, is presumed from projected changes in the levels of the Great Lakes and surrounding moisture regimes. Whether climate change is in fact affecting Houghton’s Goldenrod and by what mechanisms is a knowledge gap.
1.8 Recovery actions completed or underway
Major alvar studies
The International Alvar Conservation Initiative (IACI) (Reschke et al. 1999) conducted surveys and research across the entire Great Lakes basin and provided a great deal of data on alvar locations, vegetation communities, and ecological dynamics. A number of alvars with Houghton’s Goldenrod were surveyed as part of the IACI. As well, outreach to alvar landowners and the aggregates industry was conducted, and the ecological significance of alvars also became more widely known through exposure in magazines and other media.
The Ontario Alvar Theme Study (Brownell and Riley 2000) collected information on all Ontario alvars and ranked the alvars according to provincial and regional significance. As a result of this study, many alvars were recommended for designation as Areas of Natural and Scientific Interest (ANSI) including several that support Houghton’s Goldenrod. However, no alvar ANSIs in the Manitoulin District have been confirmed (Manitoulin Planning Board 2013).
Field work
Field surveys of Houghton’s Goldenrod and its habitat were done at several locations as part of different projects (Jones 2004, 2005; Jalava 2008). Both WUIR and UCCMM have completed surveys for this species and have baseline data on where it occurs.
The communities are working on protection and management of habitat for Houghton’s Goldenrod (T. Flamand pers. comm. 2014; G. Migwans pers. comm. 2014).
Outreach
Educational booklets about species at risk including Houghton’s Goldenrod have been prepared by WUIR (Wikwemikong Department of Lands and Natural Resources 2012). The booklets are very popular and have quickly become out of print. Presentations on Species at Risk (SAR) have been made to elders’ groups, school classes, community college programs, Anishnaabe language conferences, and local naturalist clubs (Madahbee et al. 2010; Flamand pers. comm. 2014).
Stewardship and acquisitions
Strawberry Island, which supports three populations of Houghton’s Goldenrod, has become an Ontario provincial nature reserve.
Policy and planning
WUIR is in the process of preparing a land use plan that will guide future development of its lands. Alvars and lands with SAR, including Houghton’s Goldenrod, are already designated as areas of concern and will have some protection during planning (J. Manitowabi pers. comm. 2014). In addition, the community is working on a process where an assessment of SAR will be done before new projects get approved (T. Flamand pers. comm. 2014).
In the Manitoulin Region, a new official plan that will guide land use and development is in the process of being approved (Manitoulin Planning Board 2013). The new official plan restricts site alteration in alvar habitats unless an environmental study shows there will be no impacts from the proposed project. Local municipalities still have to develop by-laws to implement the new plan, but this is expected in the next two years.
2.0 Recovery
2.1 Recovery goal
The recovery goal for Houghton’s Goldenrod is to maintain the current abundance and distribution of all populations of Houghton’s Goldenrod in Ontario by maintaining and protecting habitat, reducing other threats, and augmenting populations if necessary.
2.2 Protection and recovery objectives
The protection and recovery objectives (Table 2) and the approaches to recovery (Table 3) are intended to assist all jurisdictions, whether they be governments, First Nations, private or corporate landowners, or non-governmental organizations, with guidance on recovery.
Table 2. Protection and recovery objectives for Houghton’s Goldenrod. (Converted to list for accessibility)
Protection or recovery objectives
- Assess threats and undertake actions for mitigation and
- Use policy tools, where appropriate, to protect and maintain Houghton’s Goldenrod habitat and
- Raise awareness about Houghton’s Goldenrod and its sensitive
- Fill knowledge
2.3 Approaches to recovery
Table 3. Approaches to recovery of Houghton’s Goldenrod in Ontario
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term |
|
1.1 Liaise with and support UCCMM and WUIR in recovery actions developed by the communities. Actions may include but are not limited to the following.
|
|
| Critical | Long-term |
|
1.2 Ensure appropriate zoning and protection within parks and protected areas.
|
Invasion by Exotic Species Off-road Vehicle Use Lack of Public Awareness Grazing and Browsing |
| Critical | Long-term |
|
1.3 After 3.3 and 3.4, liaise with and support corporate and private owners in recovery actions. Some actions may include the following.
|
Any or all threats |
| Necessary | On-going |
|
1.4 Provide enhanced enforcement of ESA 2007 and SARA if stewardship and other actions are not effective. | Any or all threats |
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Protection |
2.1 Encourage municipalities to consider alvars and SAR in development of new by-laws.
|
|
| Critical | Long-term | Protection |
2.2 Recognize alvar ANSIs in the Manitoulin Official Plan.
|
|
| Necessary | Long-term | Protection |
2.3 Design community-based policies to protect alvars and Houghton’s Goldenrod on First Nations lands.
|
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Protection, Communications, Education and Outreach |
3.1 Discuss Houghton’s Goldenrod with municipal planners.
|
|
| Critical | Short-term | Protection Communications, Education and Outreach |
3.2 Discuss Houghton’s Goldenrod with enforcement officials.
|
Any or all threats |
| Necessary | On-going | Protection, Communications, Education and Outreach |
3.3 Discuss Houghton’s Goldenrod and alvars with corporate landowners and aggregate operators.
|
Quarrying & Aggregates Extraction Logging and Industrial Activities Grazing and Browsing Invasion by Exotic Species Development/Construction Lack of Public Awareness Use of Herbicide |
| Necessary | On-going | Protection, Communications, Education and Outreach Stewardship |
3.4 Discuss Houghton’s Goldenrod and alvars with private landowners.
|
Any or all threats |
| Beneficial | Short-term | Education and Outreach, Communications Stewardship |
3.5 Assist with reprinting and updating WUIR educational materials and assist with preparations of materials for UCCMM communities if requested.
|
Any or all threats |
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed | |
|---|---|---|---|---|---|
| Urgent | Short-term | Inventory, Monitoring and Assessment, Research |
4.1 Develop and undertake a monitoring program to assess population levels and to monitor threats.
|
|
|
| Necessary | Long-term | Research | 4.2 Determine if Cockburn Island genetic entity may be a separate species from alvar entity. | Whether additional recovery efforts may be required. | |
| Necessary | Long-term | Inventory, Monitoring and Assessment |
4.3 Determine population viability levels;
|
Whether reintroductions or augmentations are warranted. | |
| Beneficial | Long-term |
Inventory, Monitoring and Assessment, Research |
4.4 Study factors that create or maintain suitable habitat to guide potential habitat management.
|
Habitat requirements and dynamics; improved success of recovery actions. Threats: Invasion by Exotic Species Grazing and Browsing |
|
| Beneficial | Long-term | Research | 4.5 Study moisture regime in Houghton’s Goldenrod habitat for a better understanding of the parameters of suitable habitat. | Habitat requirements and dynamics | |
| Beneficial | Long-term | Research, Stewardship | 4.6 Study reintroduction and augmentation techniques to be used as necessary to maintain abundance levels. | Any or all threats | |
Narrative to support approaches to recovery
Houghton’s Goldenrod has narrow habitat requirements that occur in a very restricted geographic range in Ontario. The distribution of the species is unlikely to expand much, even with recovery efforts, because suitable alvar habitat is limited. Furthermore, the main threats to Houghton’s Goldenrod are threats to its habitat. Therefore, the recovery goal for this species focuses on maintaining the existing distribution and abundance by protecting habitat. However, reintroductions or augmentation may be needed to achieve the recovery goal of maintaining the current distribution and abundance of Houghton’s Goldenrod. In addition, population viability is a knowledge gap, so reintroductions or augmentation may also be needed if it becomes determined that some populations are not viable.
Development of range-wide monitoring protocol will allow comparisons of trends among populations. This could potentially allow the effects of threats at individual populations to be separated natural cycles possibly to track the effects of climate, which would probably affect all populations.
It is recognized that First Nations will have a key role to play in recovery for Houghton’s Goldenrod. First Nations community members are in contact with many of the sites where this species is found, so it is likely that community members will need to be involved on many levels in order for recovery to be successful.
Corporate owners will also have a key role to play as nearly one third of populations are on land in corporate ownership. Improved data on populations and locations may be useful to corporate owners in planning business activities since some areas may potentially need to be excluded while others could be freed up for use. Municipal planners may also require similar information to ensure alvars are protected when new development is proposed.
Some Houghton’s Goldenrod populations are on lands where there is little presence of any type of ownership or jurisdictional authority, be it First Nations, Crown, a corporation, or a municipality. A person entering these lands would have no way of knowing whose land it is because there is no building, sign, or evidence of regular human usage. Furthermore, some of these lands are under land claim by First Nations or are included in lands proposed for provincial park status, and ownership may be in dispute. During the time that may elapse until legal ownership is clarified and resolved, recovery actions may still be undertaken through many different means. It is recommended that the various jurisdictions contact each other and work together for the protection and recovery of Houghton’s Goldenrod.
2.4 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of Natural Resources and Forestry on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the author will be one of many sources considered by the Minister when developing the habitat regulation for this species.
Considerations
Occupancy
Houghton’s Goldenrod is a long-lived, rhizomatous, perennial plant that requires a specific moist microhabitat on alvars, dunes, or bedrock shores. During the growing season, if Houghton’s Goldenrod plants are visible, a site is occupied. However, this species (and most Goldenrods) require considerable expertise to identify correctly and even then can only be conclusively identified (without examining DNA) when the plants are in flower or fruit. Therefore, it is recommended that occupancy or a lack of occupancy should only be determined from flowering or fruiting plants. As plants in these stages are usually only present from late-August through September, it is recommended that occupancy can only be determined during this period.
There may be some situations in which flowering and fruiting plants are not present, but the habitat is still occupied by sterile rosettes of Houghton’s Goldenrod. This could be the case if browsing removes flowering stalks or if a population consists only of young individuals. Houghton’s Goldenrod is not known to maintain a seed bank, and seeds are known to be viable only for one year. Therefore, as a precautionary measure, it is recommended that occupancy be presumed even if no flowering plants are seen, for a period of six years, which is the time in which rosettes normally achieve mature size.
Dynamics and maintenance of suitable microhabitat
It is recognized that Houghton’s Goldenrod requires specific patches within a vegetation community type (listed in Section 1.4) and may not occur throughout the entire polygon. However, in dune and alvar situations, it is suggested that the entire ELC vegetation type polygon is required to maintain suitable habitat conditions. Suitable microhabitat patches occur as a result of the dynamics of the surrounding area and may depend on precipitation levels, Great Lakes water levels, ice scour, flooding, accumulation of organic materials, frost heaving of shallow soil, fire (at some sites), and many other factors. In addition, the position of suitable microhabitat patches may shift somewhat over a number of years depending on the dynamics listed, so the entire surrounding ELC vegetation type polygon is needed to allow space for such changes to occur.
Furthermore, at least in alvar situations, the same dynamic processes involved in maintenance of suitable microhabitat may be involved in the maintenance of the alvar community itself (Reschke et al. 1999), which in turn supports the necessary microhabitat. Thus, more than just the moist patches themselves are required.
On the other hand, on bedrock beaches, it is suggested that the entire polygon may not be required. Bedrock beach vegetation communities may be many kilometres in length, and not all of this area will be occupied by Houghton’s Goldenrod. In degraded, old field situations, most of the natural alvar vegetation is no longer present and much of the field will be unsuitable, although open, moist microhabitat conditions still remain to support the species.
Suitable microhabitat for Houghton’s Goldenrod occurs within at least eight different ELC vegetation types, on both sand and bedrock substrates, as well as in degraded situations. These vegetation types may include:
- Little Bluestem – Long-leaved Reed Grass – Great Lakes Wheatgrass Open Dune (SDO1-2)
- Shrubby Cinquefoil Carbonate Open Bedrock Beach (BBO2-1)
- Dry-Fresh Little Bluestem Open Alvar Meadow (ALO1-3)
- Fresh-Moist Tufted Hairgrass Open Alvar Meadow (ALO1-5)
- Creeping Juniper-Shrubby Cinquefoil Dwarf Shrub Alvar (ALS1-2)
- Jack Pine – White Cedar – White Spruce Treed Alvar (ALT1-4)
- Bedrock Cultural Meadow (CUM2)
- Common Juniper Cultural Alvar Thicket (CUT2-1)
or any other vegetation type in which Houghton’s Goldenrod is found.
It is suggested that different habitat prescriptions are needed for the different types of situations in which Houghton’s Goldenrod occurs. Therefore, it is recommended that the habitat to be considered for regulation for Houghton’s Goldenrod be prescribed as follows.
- All areas where Houghton’s Goldenrod is present and any new areas that are discovered.
- On sand:
The entire ELC dune or beach vegetation community type polygon in which Houghton’s Goldenrod is present, with boundaries defined as per the ELC for the appropriate vegetation community type. The beach polygon where Houghton’s Goldenrod occurs is small (less than 10 ha), so it is recommended that all of the area be prescribed to maintain suitable habitat parameters.
- On bedrock beach:
The portion of the ELC bedrock beach vegetation community polygon in which Houghton’s Goldenrod is present, bounded on the inland side as per the ELC for the appropriate vegetation community type, but bounded at the ends (the short sides of a semi-rectangular shape running parallel to the shore) at 50 m beyond the presence of Houghton’s Goldenrod plants. A distance of 50 m beyond the plants is recommended to protect the plants from activities which may cause impacts. In many places, bedrock beach polygons transition on the inland side to alvar vegetation communities which may also be occupied by Houghton’s Goldenrod. In these cases, it is recommended that the bedrock beach habitat to be prescribed would continue into the occupied alvar which would be prescribed according to (4), next.
- In alvar vegetation communities:
The entire alvar vegetation community polygon in which Houghton’s Goldenrod is present, with boundaries as per the ELC for the appropriate vegetation community type.
- In degraded, old field habitat and where the vegetation is predominantly non- native plant species:
All of the continuous area around Houghton’s Goldenrod plants that contains patches of vegetation shorter than the flowering stalks of the species (approximately 60 cm tall). It is recommended that the boundary of this area be the presence of a continuous cover of trees, or tall shrub or herbaceous vegetation, all of which are unsuitable for Houghton’s Goldenrod.
- In all situations, all of the area within a minimum radial distance of 50 m from the plants, including both suitable and unsuitable habitat, so that if individuals occur at the edge of any prescribed area, there will be sufficient distance from activities in adjacent areas to prevent negative It is recognized that, in some cases, a 50 m radial distance may include a small amount of unsuitable habitat. It is recommended that all of the area in a 50 m radial distance be prescribed, whether suitable or unsuitable as habitat, as a protective measure.
A distance of 50 m has been shown to provide a minimum critical function zone to ensure microhabitat properties for rare plants. A study on micro-environmental gradients at habitat edges (Matlack 1993) and a study of forest edge effects (Fraver 1994) found that effects such as changes in light, moisture, shrub cover, humidity, species richness, sapling density, etc., could be detected as far as 50 m into habitat fragments. Forman and Alexander (1998) and Forman et al. (2003) found that most roadside edge effects on plants resulting from construction and repeated traffic have their greatest impact within the first 30 to 50 m.
Reschke et al. (1999) studied the hydrology of alvars. In their study, they found that most water on alvars derived from surface rainwater rather than from ground water, and surface waters outside the alvars were not the source of water flooding grasslands. Therefore, for alvars, 50 m may be a sufficient distance to protect the hydrological regime. On dunes, it is recognized that additional distance may be warranted to protect the moisture regime. However, no research was available to suggest a particular protective distance. Therefore, for dunes, it is recommended that the 50 m distance be used as a guideline until additional information becomes available.
It is recommended that any existing infrastructure present in the habitat of Houghton’s Goldenrod, such as existing buildings, roads, parking lots, campgrounds, industrial facilities, active agricultural areas, and other man-made structures not be prescribed as habitat.
Glossary
- Alvar:
- Alvars are open, usually treeless or sparsely treed ecosystems, that occur on level limestone or dolostone bedrock with very shallow soils. Alvar vegetation is usually dominated by grasses and sedges or low, creeping shrubs. There are different types of alvars just as there are different types of forests.
- Capitulescence:
- An arrangement of heads of flowers, especially in the Aster Family.
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC):
- The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
- Committee on the Status of Species at Risk in Ontario (COSSARO):
- The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
- Conservation status rank:
- A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
- 1 = critically imperilled: At very high risk of extinction due to extreme rarity (often 5 or fewer populations), very steep declines, or other factors.
- 2 = imperilled: At high risk of extinction or elimination due to very restricted range, very few populations, steep declines, or other factors.
- 3 = vulnerable: At moderate risk of extinction or elimination due to a restricted range, relatively few populations, recent and widespread declines, or other factors.
- 4 = apparently secure: Uncommon but not rare; some cause for long-term concern due to declines or other factors.
- 5 = secure: Common; widespread and abundant.
- DNA:
- Deoxyribonucleic acid. The genetic material of almost all living organisms.
Strands of molecules that make up DNA contain chemical sequences that are called genes. - Endangered Species Act, 2007 (ESA):
- The provincial legislation that provides protection to species at risk in Ontario.
- Genome:
- The total of all the genetic material in a cell or organism. Hexaploid: Having six copies of each chromosome in the nucleus.
- Octoploid:
- Having eight copies of each chromosome in the nucleus.
- Open pollination:
- Pollen transfer by any vector that normally occurs, such as by wind or bees; the opposite of self-pollination.
- Ordovician limestone:
- Sedimentary rock formed mainly of calcium and magnesium carbonates, deposited during the Ordovician period, which lasted from about 510 to 439 million years ago.
- Reticulate evolution:
- (Reticulate: like a net; Evolution: in living things, the change in inherited traits over successive generations). The development of several closely related species especially by doubling or further replication of chromosomes. A map of the history of the development of these species appears net-like, rather than as a linear time line.
- Rhizome:
- A horizontal stem from which roots or shoots may grow. A rhizome may grow along the top of or under the ground
- Silurian dolostone:
- A type of limestone containing the mineral dolomite, deposited during the Silurian period, which lasted from about 439 to 409 million years ago.
- Species at Risk Act (SARA):
- The federal legislation that provides protection to species at risk in Canada. This act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
- Species at Risk in Ontario (SARO) List:
- The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
- Taxon (plural taxa):
- A taxonomic group of any level, such as a species, family or class.
References
Bakowsky, Wasyl. pers. comm. 2014. Email correspondence to J. Jones. January 15, 2014. Community Ecologist, Natural Heritage Information Centre, Ontario Ministry of Natural Resources, Peterborough, ON.
Brouillet, L., F. Coursol, S.J. Meades, M. Favreau, M. Anions, P. Bélisle and P. Desmet 2010+. Poa compressa L. in VASCAN, the Database of Vascular Plants of Canada. [accessed September 17, 2014].
Brownell, V.R. and J.L. Riley. 2000. The Alvars of Ontario: Significant Natural Areas in the Ontario Great Lakes Region. Federation of Ontario Naturalists, Don Mills, Ontario. 269 pp.
Buchelé, D.E., J.M. Baskin, and C.C. Baskin. 1991. Ecology of the endangered species <>Solidago shortii. II. Ecological life cycle. Journal of the Torrey Botanical Club 118(3):281-287.
Campeau, S. 2013. Establishing Alvars Mosses on Quarry Floors: A Necessary Step in the Restoration of Quarries to Alvars. Final Report to the Ontario Aggregate Resources Corporation (TOARC). Bryophyta Technologies Inc., Lambton, Québec. 65 pp. [accessed September 19, 2014]
Catling, P. 1995. The extent of confinement of vascular plants to alvars in southern Ontario. Canadian Field-Naturalist 109(2):172-181.
Catling, P. 2009. Vascular plant diversity in burned and unburned alvar woodland: more evidence of the importance of disturbance to biodiversity and conservation. Canadian Field-Naturalist 123(3):240-245.
Catling, P.M. 2013. Can we create alvars or fully restore those damaged? Canadian Field-Naturalist 127(1): 97–101.
Catling, P.M. and V.R. Brownell. 1998. Importance of fire in alvar ecosystems-evidence from the Burnt Lands, eastern Ontario. Canadian Field-Naturalist 112(4):661- 667.
Catling, P.M., A. Sinclair, and D. Cuddy. 2001. Vascular plants of a successional alvar burn 100 days after a severe fire and their mechanisms of re-establishment. Canadian Field-Naturalist 115(2):214-222.
COSEWIC. 2005. COSEWIC assessment and status report on the Houghton’s Goldenrod Solidago houghtonii in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 17 pp.
COSEWIC. 2010. COSEWIC assessment and status report on the Pitcher’s Thistle Cirsium pitcheri in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 32 pp.
Côté, S.D., T.P. Rooney, J.P. Tremblay, C. Dussault and D.M. Waller. 2004. Ecological impacts of deer overabundance. Annual Review of Ecology, Evolution, and Systematics 35:113-147.
Edwards-Wilson, M.L. 1999. A taxonomic study of Solidago houghtonii: an attempt to discover its hybrid origins. M.S. thesis, Central Michigan University, Mount Pleasant, MI.
Flamand, Theodore. pers. comm. 2014. In person correspondence to J. Jones. January 15, 2014. Species at Risk program coordinator, Wikwemikong Department of Lands and Natural Resources, Wikwemikong, ON.
Forman, R.T.T. and L.E. Alexander. 1998. Roads and their major ecological effects. Annual Review of Ecology and Systematics 29:207-231.
Forman, R.T.T., D. Sperling, J.A. Bissonette, A.P. Clevenger, C.D. Cutshall, V.H. Dale,L. Fahrig, R. France, C.R. Goldman, K. Heanue, J.A. Jones, F.J. Swanson, T. Turrentine, and T.C. Winter. 2003. Road ecology: Science and solutions. Island Press. Covelo, CA.
Fraver, S. 1994. Vegetation responses along edge-to-interior gradients in the mixed hardwood forests of the Roanoke River Basin, North Carolina. Conservation Biology 8(3):822-832.
Gilman, B. 1995. Vegetation of Limerick cedars: pattern and process in alvar communities. Doctoral dissertation, State University of New York. College of Environmental Science and Forestry, Syracuse, NY.
Gilman, B. 1997. Recent Fire History Data for the Perch River Barrens Alvar Site.Unpublished report for the International Alvar Conservation Initiative, The Nature Conservancy, Chicago, IL.
Guire, K.E. and E.G. Voss 1963. Distributions of distinctive shoreline plants in the Great Lakes Region. The Michigan Botanist 2:99-114.
Jalava, J.V. 2008. Alvars of the Bruce Peninsula: A Consolidated Summary of Ecological Surveys. Prepared for Parks Canada, Tobermory, Ontario. iv + 350 pp + appendices.
Jolls, C.L. and P.M. Tolley. in preparation, 2014. The reproductive ecology of a Great Lakes endemic, Houghton’s Goldenrod (Solidago houghtonii T. & G.). C.L. Jolls, Associate Professor, Dept. of Biology, East Carolina University, Greenville, NC.
Jones, J.A. 2000. Fire history of the bur oak savannas of Sheguiandah Township, Manitoulin Island. The Michigan Botanist 39(1):3-15.
Jones, J.A. 2004. Alvars of the North Channel Islands. Unpublished report prepared for NatureServe Canada, Ottawa, Ontario. 10 pp.
Jones, J.A. 2005. More alvars of the North Channel Islands and the Manitoulin Region: Unpublished report prepared for Ontario Ministry of Natural Resources, Espanola Office. 15 pp.
Jones, J.A. 2007. Report from the species at risk surveys on the Wikwemikong Reserve. Unpublished report to the Wikwemikong Department of Lands and Natural Resources, Wikwemikong, Ontario. 18 pp.
Jones, J.A. 2010. Interim report to UCCMM on species at risk survey work. Unpublished report to the United Chiefs and Councils of M’nidoo M’nising, M’chigeeng, Ontario. 3 pp.
Jones, J.A. and C. Reschke. 2005. The role of fire in Great Lakes alvar landscapes. The Michigan Botanist 44(1):13-27.
Kraus, D., B. Henson and D. Ewert 2009. Biodiversity and conservation of Lake Huron’s islands. Aquatic Ecosystem Health and Management 12(1):90-100.
Larson, D.W., U. Matthes, P. Richardson and S. Tomlinson. 2006. The Quarry to Alvar Initiative. Final report to the Ontario Aggregate Resources Corporation (TOARC). University of Guelph, Guelph, Ontario.
Laureto, P. J. and J.S. Pringle. 2010. Solidago vossii (Asteraceae), a new species of Goldenrod from northern Michigan. The Michigan Botanist 49:105-117.
Laureto, P.J. and T.J. Barkman. 2011. Nuclear and chloroplast DNA suggest a complex single origin for the threatened allopolyploid Solidago houghtonii (Asteraceae) involving reticulate evolution and introgression. Systematic Botany 36(1):209- 226.
Lee, H.T., W.D. Bakowsky, J. Riley, J. Bowles, M. Puddister, P. Uhlig and S. McMurray. 1998. Ecological Land Classification for Southern Ontario: First Approximation and Its Application. OMNR, Southcentral Science Section, Science Development and Transfer Branch. SCSS Field Guide FG-02. 225 pp.
Lee, P.C. 1993. Seed dispersal limitations on the spatial distribution of a gap species, seaside Goldenrod (Solidago sempervirens). Canadian Journal of Botany 71:978- 984.
Madahbee, S., M. Cooper, and J.A. Jones. 2010. Species at risk around us. Powerpoint presentation created for United Chiefs and Councils of M’nidoo M’nising, and presented at community schools, January 2011. UCCMM, M’chigeeng, ON.
Manitoulin Planning Board. 2013. District of Manitoulin Official Plan, final draft December 2013. [Accessed January 21, 2014].
Manitowabi, John. pers. comm. 2014. In person correspondence to J. Jones, January 17, 2014. Planner, Wikwemikong Department of Lands and Natural Resources.
Matlack, G.R. 1993. Microenvironment variation within and among forest edge sites in the eastern United States. Biological Conservation 66(3):185-194.
Migwans, G’mewin. pers. comm. 2014. In person correspondence to J. Jones, January 17, 2104. Community Engagement Coordinator, United Chiefs and Councils of M’nidoo M’nising, M’chigeeng, Ontario.
Morton, J.K. 1979. Observations on Houghton’s Goldenrod (Solidago houghtonii). The Michigan Botanist 18:31-36.
Morton, J.K. 1981. Chromosome numbers in Compositae from Canada and the USA. Botanical Journal of the Linnean Society 82(4):357-368.
Morton, J.K. and J.M. Venn. 2000. The Flora of Manitoulin Island, 3rd ed. University of Waterloo Biology Series Number 40. Waterloo, Ontario.
NatureServe. 2014. Solidago houghtonii in NatureServe Explorer: an online encyclopedia of life. Version 7.1. NatureServe, Arlington, Virginia. [accessed January 22, 2014]
Penskar, M.R., P.J. Higman and S.R. Crispin. 2000. Special plant abstract for Solidago houghtonii (Houghton’s Goldenrod). Michigan Natural Features Inventory, Lansing, MI. 3 pp. [Accessed January 22, 2014]
Penskar, M.R. and A.L. Derosier. 2012. Assisting the Michigan Wildlife Action Plan: Tools and Information for Incorporating Plants. Final Report to NatureServe. Michigan Natural Features Inventory Report No. 2012-, Lansing, MI. 26 pp. + appendices.
Reschke, C., R. Reid, J. Jones, T. Feeney and H. Potter. 1999. Conserving Great Lakes Alvars: Final Technical Report of the International Alvar Conservation Initiative. The Nature Conservancy, Chicago, Illinois. 230 pp.
Reznicek, A.A., E.G. Voss and B.S. Walters. 2011.Michigan Flora Online. University of Michigan Herbarium. [Accessed January 28, 2014]
Riley, J.L. 2013. The Once and Future Great Lakes Country. McGill Queens University Press, Montreal. 488 pp.
Rooney, T.P. 2001. Deer impacts of forest ecosystems: North American perspective. Forestry 74(3):201-208.
Rosén, E. 1995. Periodic droughts and long-term dynamics of alvar grassland vegetation on Öland, Sweden. Folia Geobotanica et Phytotoxonomica 30(2):131- 140.
Semple, J.C., G.S. Ringius, C. Leeder and G. Morton. 1984. Chromosome numbers of Goldenrods, Euthamia and Solidago (Compositae: Astereae). II. Additional counts with comments on cytogeography. Brittonia 36:280–292.
Semple, J.C., G.S. Ringius and J.J. Zhang. 1999. Goldenrods of Ontario, 3rd ed. University of Waterloo Biology Series, No. 39.
Semple, J.C. and R.E. Cook. 2006. Solidago. In Flora of North America vol. 20. Oxford University Press, New York, NY. pp. 107-166.
Tester, J.R. 1989. Effects of fire frequency on oak savanna in east-central Minnesota. Bulletin of the Torrey Botanical Club 116(2):134-144.
United States Fish and Wildlife Service (USFWS). 1997. Recovery Plan for Houghton’s Goldenrod (Solidago houghtonii A. Gray). Region 3, U.S. Fish and Wildlife Service, Fort Snelling, Minnesota. 58 pp.
Waller, D.M. and W.S. Alverson. 1997. The white-tailed deer: a keystone herbivore. Wildlife Society Bulletin 25(2):217-226.
Wikwemikong Department of Lands and Natural Resources. 2012. Interesting plants and shrubs of Wikwemikong. Wikwemikong Unceded Indian Reserve, Wikwemikong, Ontario. 48 pp.
Voss, E.G. 1996. Michigan Flora, vol. 3. Cranbrook Institute of Science and Regents of the University of Michigan, Ann Arbor, Michigan. 622 pp.