Laura’s Clubtail Recovery Strategy
This document advises the ministry on ways to ensure healthy numbers of Laura’s clubtail, a threatened or endangered species, return to Ontario.

Photo: Lois Stacey
Ontario Recovery Strategy Series
Recovery strategy prepared under the Endangered Species Act, 2007
Ministry of Natural Resources
About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species' persistence in the wild.
What is a recovery strategy?
Under the ESA, a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. There is a transition period of five years (until June 30, 2013) to develop recovery strategies for those species listed as endangered or threatened in the schedules of the ESA. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of Natural Resources Species at Risk webpage at: www.ontario.ca/speciesatrisk
Recommended citation
Pulfer, T.L., C. Bahlai and L. Mousseau. 2011. Recovery Strategy for Laura’s Clubtail (Stylurus laurae) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. v + 23 pp.
Cover illustration: Lois Stacey
© Queen’s Printer for Ontario, 2011
ISBN 978-1-4435-6783-1 (PDF)
Content (excluding the cover illustration) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée Recovery strategies prepared under the Endangered Species Act, 2007, n'est disponible qu'en anglais en vertu du Règlement 411/97 qui en exempte l'application de la Loi sur les services en français. Pour obtenir de l'aide en français, veuillez communiquer avec Cathy Darevic au ministère des Richesses naturelles au
Authors
Tanya L. Pulfer – Conservation Biologist – Nature Conservancy of Canada
Christine Bahlai – Environmental Sciences – University of Guelph
Laura Mousseau – Communications Coordinator – Nature Conservancy of Canada
Acknowledgments
The authors would like to thank Allan Edelsparre and a number of anonymous reviewers. Wendy Cridland and Mhairi MacFarlane provided expertise of the area. Cindy Chu provided aquatic species and habitat expertise and Mary Finch provided expertise on Eastern Sand Darter. Finally, Bree Walpole and Leanne Jennings from OMNR Species at Risk Branch provided invaluable guidance.
This recovery strategy was greatly improved by guidance from the core advisory group for Laura’s Clubtail, including:
- Don Sutherland (Zoologist, Natural Heritage Information Centre)
- Paul Gagnon (Lands and Waters Supervisor, Long Point Region Conservation Authority)
- Allan Harris (Biologist, Northern Bioscience)
- Dan Kraus (Conservation Science Manager, Nature Conservancy of Canada– Ontario Region)
- Alan Dextrase (Biodiversity Conservation Policy Advisor, Ontario Ministry of Natural Resources –Biodiversity Section)
- Cindy Chu (Aquatic Conservation Biologist, Nature Conservancy of Canada)
- P. Allen Woodliffe (District Ecologist, Ontario Ministry of Natural Resources – Aylmer District)
- Colin Jones (Project Zoologist, Natural Heritage Information Centre)
Declaration
The recovery strategy for Laura’s Clubtail was prepared in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all of the individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ontario Ministry of Natural Resources
Executive summary
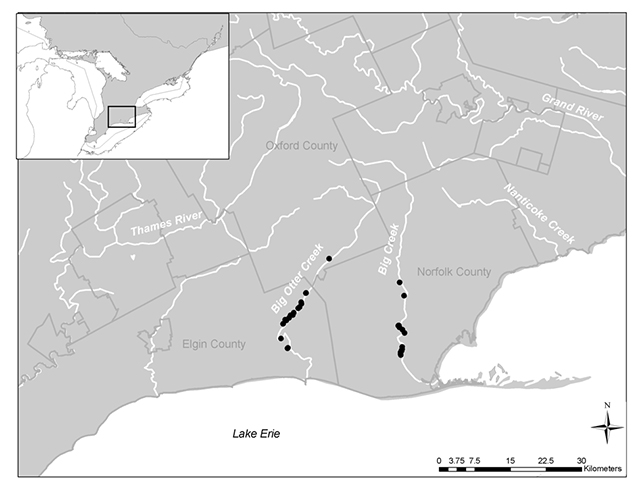
Laura’s Clubtail (Stylurus laurae) is a member of the dragonfly family Gomphidae. It is found from Texas and the Florida Panhandle up to southwest Ontario, where it is found in the Norfolk Sand Plains physiographic region. Currently there are only two known populations in Ontario – Big Creek and Big Otter Creek. Laura’s Clubtail is listed as an endangered species on the Species at Risk in Ontario (SARO) List and was assessed as endangered by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC).
Laura’s Clubtail requires a high quality aquatic environment and a vegetated riparian area, preferably consisting of mature forests. It is generally found in or near small to medium sized streams with sand or silt substrate and overhanging trees or shrubs. Adults use riffles in the stream for foraging, mating and probably to lay eggs. Eggs or recently emerged larvae are carried downstream to pools. Adults are short-lived with breeding and egg-laying occurring within weeks of adult emergence.
The main threat to the survival and recovery of Laura’s Clubtail is habitat degradation or alteration to both the aquatic and terrestrial habitat. Aquatic habitat threats include changes to water flow rate, pH, dissolved oxygen, temperature, nutrient load, pollution, dam construction and changes to water quality. Terrestrial habitat threats include shoreline alteration and loss of riparian habitat. Invasive species (especially Round Goby) and road mortalities are also thought to negatively affect Laura’s Clubtail, but the pressures these are exerting on the population are unknown and require further study. Limiting factors include a limited distribution and apparent sensitivity to specific habitat features. Knowledge gaps include an overall lack of species-specific information (including mating and foraging behaviours, physical tolerances to changes in stream condition and pesticides or herbicides), quantitative assessment of road mortalities and extent of the distribution in Ontario.
The recovery goal is to ensure a viable, self-sustaining population of Laura’s Clubtail in Ontario. The protection and recovery objectives are to:
- protect, maintain and enhance the quantity and quality of existing Laura’s Clubtail habitat;
- reduce or mitigate threats to Laura’s Clubtail and its habitat where feasible; and
- increase knowledge of Laura’s Clubtail biology in Ontario including distribution, abundance, life history and habitat needs.
It is recommended that all stream reaches (aquatic resource areas
1.0 Background information
1.1 Species assessment and classification
Common name: Laura’s Clubtail
Scientific name: Stylurus laurae
SARO List Classification: Endangered
SARO List History: Endangered (2010)
COSEWIC Assessment History: Endangered (2010)
SARA Schedule: No Schedule, No Status
Conservation status rankings:
GRANK: G4 NRANK: N1 SRANK: S1
The glossary provides definitions for the abbreviations above.
1.2 Species description and biology
Species description
Laura’s Clubtail is a dragonfly of the Family Gomphidae (Order Odonata) with several distinguishing features. Species in the gomphid family, including Laura’s Clubtail, have a widening at the end of the abdomen (segments seven through nine), that distinguishes them from other dragonfly species (Marshall 2006). Laura’s Clubtail measures approximately 60 to 64 mm in length with a hindwing length of 36 to 42 mm (Paulson 2009). It has green eyes with a pale face showing two dark cross bars (Paulson 2009). The hind wings of Ontario specimens tend to be more smoky than translucent (Catling and Catling 1999). Prominent green or yellow stripes are present on thoracic segments two and three; the thoracic stripes of males tend to be greener while those of females are more yellow. The pale thoracic stripe on the first segment (collar) is interrupted with black (Dunkle 2000, Paulson 2009). The abdomen is black fading to brown distally with pale dorsal stripes present on the abdominal segments. The legs are brown (Paulson 2009).
The larvae of the genus Stylurus (Clubtails) can be distinguished from other gomphid species by the lack of tibial burrowing hooks (COSEWIC 2010). Mature larvae of Laura’s Clubtail may be distinguished from those of other Stylurus by a slightly wider (1.3 times) than long abdominal segment nine, which is 1.3 to 1.4 times the length of abdominal segment eight and by a distinctly wider (3.5 times) than long antennal segment three (K. Tennessen unpub. in Bright and O'Brien 1999).
Species biology
Very little species-specific biology for Laura’s Clubtail is known. No systemic studies of larvae or adult behaviour have been performed for this species and all information is derived from direct observation or at the time of specimen collection. Unless otherwise noted, species biology information in this section is derived from COSEWIC (2010).
The life cycle of Laura’s Clubtail consists of egg, larval and adult states (Corbet 1962). Eggs take between 5 to 30 days to hatch. The larvae of Laura’s Clubtail are predatory. Larvae overwinter presumably within sand and mud river bottom substrates (Corbet 1962). Larvae take between two and four years to mature, depending on the quality and quantity of food resources. Larval prey ranges in size from ciliates and rotifers, to macroinvertebrates, small fish and tadpoles. The size of the prey increases as the larvae increase in size. In June, larvae emerge from the water and molt into adults, leaving behind diagnostic exuviae (Catling 2010). Adults are short-lived with breeding and egg-laying occurring within weeks of adult emergence; adults die in early autumn.
Adult Laura’s Clubtail are strong-flying, predatory and may be wide-ranging, but are typically found in the vicinity of aquatic larval habitats. Newly emerged adults, or tenerals, are more susceptible to predation and disperse into nearby forests until their exoskeletons harden. Males are the first to return to the riparian area and set up territories. Oviposition and mating behaviours are undescribed; presumably, these behaviours are similar to those of closely related species.
Adults are thought to be generalist predators, feeding on small flying insects, especially within the forest canopy (Walker 1953 as cited in COSEWIC 2010). Adults forage from leaves along forest edges. Adults perch mostly on leaves overhanging water, but also briefly on rocks and logs (MNFI 2007). Dragonflies do not typically travel more than 200 m between reproductive and roosting habitats (Corbet 1999 as cited in COSEWIC 2010).
In Ontario, as in adjacent jurisdictions, adults of Laura’s Clubtail are most frequently observed between the middle of July and the middle of August, however individuals have also been observed between the latter half of June and late September (OMNR 2010).
Predators of Laura’s Clubtail vary with life stage. Larvae are preyed upon mostly by fish, but can also be preyed upon by turtles, amphibians [e.g., frogs, Mudpuppy (Necturus maculosus)], waterbirds [e.g., Mallard (Anas platyrhynchos), American Black Duck (Anas rubripes) and Wood Duck (Aix sponsa)], as well as wading birds (especially herons). Adults are preyed upon by frogs, spiders, larger dragonflies and birds (especially blackbirds, swallows, flycatchers and small raptors).
1.3 Distribution, abundance and population trends
Laura’s Clubtail occurs throughout eastern North America, extending from eastern Texas (Figure 1) (Abbott 2001) and the Florida Panhandle (Keppner and Keppner 2007) north to central Michigan (Kudell- Ekstrum 2004) and southern Ontario (Catling and Catling 1999, OMNR 2010). A larval record from Gladwin County in central Michigan (43°58' N) represents the northernmost record of this species (Kudell-Ekstrum 2004).
Population estimates of dragonflies have been attempted both on a global and regional scale, but are difficult to derive. The global population of Laura’s Clubtail is estimated at 10,000 to 1,000,000 individuals (COSEWIC 2010). In Ontario, surveys were conducted in 2008 based on searches for exuviae along the banks of Big Creek and Big Otter Creek. Based on this survey there is a minimum estimate of 580 adults between Big Creek (330) and Big Otter Creek (250). However, this is considered an incomplete population estimate because larvae were uncounted, larvae to adult ratio is unknown, and not all possible habitat was surveyed (COSEWIC 2010). The global population is thought to be stable (NatureServe 2010).
Laura’s Clubtail was first recorded in Ontario in 1999 (Catling and Catling 1999). Since 1999 it has been observed along two major tributaries of Lake Erie in the Norfolk Sand Plain area (Figure 2, OMNR 2010). Laura’s Clubtail is considered rare in areas adjacent to Ontario but is relatively widespread in the southeastern United States (Catling and Catling 1999). In Ontario, Laura’s Clubtail has only been observed in two creeks in the vicinity of Tillsonburg and Long Point near Lake Erie (OMNR 2010): Big Creek and Big Otter Creek. Laura’s Clubtail may occur undetected in adjacent areas with similar habitat (S. Marshall pers. comm. 2010) such as the Grand, Thames, Ausable and Sydenham rivers. The overlap of Eastern Sand Darter (Ammocrypta pellucida) and Laura’s Clubtail habitat in Big Creek and Big Otter Creek (Dextrase pers. comm. 2011) suggest that known Eastern Sand Darter habitat may be a good location to begin searches in the aforementioned rivers, all of which support Eastern Sand Darter. It should be noted however that dragonfly inventories along these rivers over the past 10 years have not recorded Laura’s Clubtail (COSEWIC 2010).
Figure 1. Distribution of Laura’s Clubtail in North America (COSEWIC 2010). Black dots represent areas where Laura’s Clubtail have been reported.
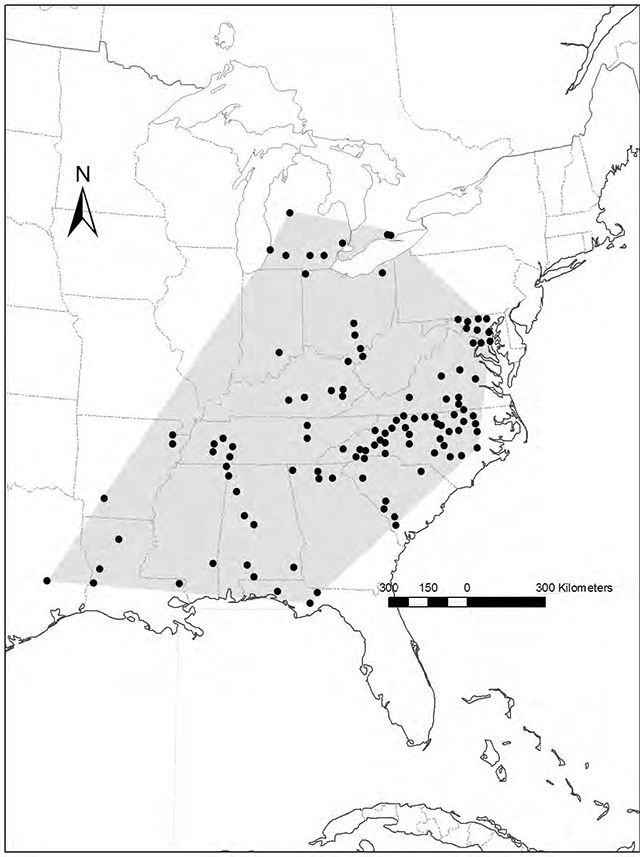
Figure 2. Known distribution of Laura’s Clubtail in Ontario. Black dots represent observations.
1.4 Habitat needs
Laura’s Clubtail requires both aquatic and terrestrial habitat for various stages of its lifecycle. During their aquatic life stage, larvae are found in shallow, sandy or sandy- muddy bottomed creeks with moderate turbidity and well-wooded banks (Williamson 1932, Catling and Catling 1999). During their adult life stage, Laura’s Clubtail requires forest cover adjacent to the aquatic habitat (COSEWIC 2010). Generally, Laura’s Clubtail populations occur in areas of creeks approximately one metre in depth or less, where riffles and pools are present and where the creek width ranges from approximately 4.5 to 6 m (Catling and Catling 1999). This is consistent with the observed habitat throughout the range of this species. In Indiana, Virginia, South Carolina and Georgia, adults have been observed near swiftly flowing, shallow and riffling creeks with rock, sand and mud bottoms, ranging from approximately three to nine metres in width, in areas well-shaded by woody vegetation (Williamson 1932). In Florida, Laura’s Clubtail adults have been collected from forested areas along a slope, directly adjacent to a sand-mud bottomed stream (Keppner and Keppner 2007).
Laura’s Clubtail larvae are generally found in small to medium sized streams. Eggs or recently emerged larvae are carried downstream to pools. Because of the dynamic nature of stream erosion and deposition, the location of these pools may change within and between years (COSEWIC 2010). Larvae are found in the water burrowed into unpolluted, well-oxygenated, loose sand or silt substrate that is interspersed by rocks (Westfall 1953, COSEWIC 2010). They spend most of their time with their abdomen raised above the sediment (COSEWIC 2010). Larvae are sensitive to water quality degradation (Jones et al. 2006, COSEWIC 2010) and are only found in unpolluted waters with moderate turbidity (Roback and Westfall 1967, COSEWIC 2010). In the southern portion of their range, larvae of Laura’s Clubtail have been found in streams with highly variable mineral content, slight to moderate acidity and with consistently moderate turbidity (Roback and Westfall 1967). However, no test has documented whether this association is obligate. Water quality requirements of more northerly populations of Laura’s Clubtail larvae are unknown. In the two rivers in Ontario where Laura’s Clubtail occur, both are streams with similar pH levels and chloride levels, but vary in turbidity and dissolved oxygen rates (COSEWIC 2010).
Adults use riffles in the stream for foraging and presumably, oviposition although no direct observations of oviposition by this species have been made to date. Adults require vegetation (from 0.5 to 6 m above the water) along the creek to perch between flights toward riffles (Williamson 1932, Corbet 1962, COSEWIC 2010).
Exuviae have been collected on sand banks and exposed root mats up to one metre from the river bed (COSEWIC 2010). Adults are most frequently collected from vegetation directly overhanging riffles in the stream (Williamson 1932, Price 1950, Catling and Catling 1999). Males are very rarely collected, suggesting that either they are most likely found in the upper reaches of the canopy (COSEWIC 2010) or there is a strong sex-ration bias in the population towards females.
1.5 Limiting factors
Laura’s Clubtail require a very specific combination of habitat attributes which may not always co-occur at a given location and does not typically disperse great distances. Though adults are strong fliers, they may not move between habitat patches: they are not typically observed outside wooded river habitats (Williamson 1932, Catling and Catling 1999). If habitats become fragmented, local populations may be prone to extirpation, should conditions become even temporarily sub-optimal within a habitat patch, as it is unlikely local populations will be supplemented by immigration. Temporary or permanent alterations to habitat could include both human-caused and natural events, such as river damming, pollution, extreme weather events or a combination of these factors.
Combined, the need for a combination of specific habitat attributes and low dispersal rates may confound the species' ability to cope with habitat loss/alteration, contributing to its limited distribution in Ontario.
1.6 Threats to survival and recovery
Due to a lack of knowledge about Laura’s Clubtail, there are currently no known direct threats to the Ontario populations. Potential threats are discussed below.
Habitat loss or degradation
Habitat loss and degradation pose a significant threat to Laura’s Clubtail given the species' sensitivity to pollution and specific habitat requirements (COSEWIC 2010, NatureServe 2010). Any changes to water flow rate, pH, dissolved oxygen, temperature and nutrients could lead to extirpation (COSEWIC 2010). Use of pesticides and herbicides on agricultural lands, golf courses and for the control of Sea Lamprey (Petromyzon marinus) have potential to impact Laura’s Clubtail, its prey or its habitat (COSEWIC 2010). Road maintenance through salting, sanding and construction activities can alter the water quality, water temperature, flow rates and increase sedimentation (Williams et al. 1999, Helmreich et al. 2010). Because Laura’s Clubtail are thought to be sensitive to changes in water quality, these alterations may reduce the amount of suitable habitat.
Because Laura’s Clubtail do not disperse great distances beyond forested habitat (Corbet 1999 as cited in COSEWIC 2010) it is important that forest cover is present adjacent to aquatic habitat. The loss of riparian vegetation along streams supporting larval habitat areas could negatively impact Laura’s Clubtail populations. Presently, the shorelines of Big Creek and Big Otter Creek are mainly forested (COSEWIC 2010). Intense logging adjacent to these creeks could lead to increased sedimentation and would remove species-required shade cover. An increase in sedimentation would further result in a decrease of dissolved oxygen and an increase in water temperature, making the habitat unsuitable for Laura’s Clubtail (COSEWIC 2010).
Hardening of landscapes, including urbanization and shoreline hardening, can result in reduced infiltration and increased runoff, changing water conditions in Laura’s Clubtail’s habitat. Areas with hardened shorelines generally experience surges in water flow during storm events, and associated changes to normal patterns of erosion and sedimentation. Hardening of shorelines upstream of Big and Big Otter creeks, as well as within them, could lead to a change in water quality, increased water flow rates and increased sedimentation during construction (Gore and Shields 1995). Although shoreline hardening, as is occurring along shores of the Great Lakes, could reduce erosion and agricultural run-off, it would also require removal of necessary creek-side vegetation and would thus negatively impact Laura’s Clubtail populations (Gore and Shields 1995, COSEWIC 2010). It may also alter natural erosion and deposition processes that are important for maintaining habitat.
Laura’s Clubtail occurs in rural areas. Agricultural runoff can degrade water quality and removal of water for agriculture and other uses impacts flow-rates (COSEWIC 2010). Runoff can lead to an increase in phosphorus and nitrate levels, which decreases the dissolved oxygen content in the system (COSEWIC 2010). Water removal for irrigation, especially in years of low precipitation, may reduce Laura’s Clubtail habitat and lead to decline in habitat quality through increased concentration of pollutants (COSEWIC 2010). Tobacco farming was a major crop in southwestern Ontario that required large amounts of water irrigation. While tobacco farming has been reduced in recent years, it has been replaced with other high value crops (e.g., sweet potato, carrot and Chinese vegetables) (Wales 2004) that also require water irrigation. Several tributaries of Big Creek and Big Otter Creek are sources for irrigation water, and because the habitat requirements and threats of Laura’s Clubtail are poorly understood, it is possible that regulation of water use is negatively influencing the habitat of this species (COSEWIC 2010).
The future rate of development in towns near Big Creek and Big Otter Creek is uncertain. There has been an increase in the population of Tillsonburg which is expected to add pressure to the Big Otter Creek subwatershed (COSEWIC 2010). Development such as golf course construction adjacent to habitats of Laura’s Clubtail can remove natural creek-side vegetation. Golf courses adjacent to water sources have been shown to impair water quality through fertilizer and pesticide run-off (Odanka et al. 1994).
Dams can alter Laura’s Clubtail habitat by removing riffles, raising surface water temperatures and increasing the accumulation of sediment, thus potentially reducing the flushing effect from spring freshets (COSEWIC 2010). Water level draw-downs from dams can also influence the natural rates and patterns of sediment accumulation and the natural temperature regimes of a river. Conversely, the removal of a dam may change current water flow rates although it reduces suspended sediment delivery downstream it increases sediment retention upstream. In 2009 the mean annual discharge rates in Big Creek and Big Otter Creek were 6.63 m3/s and 8.24 m3/s respectively (COSEWIC 2010). The dam on Big Otter Creek above Tilllsonburg was removed in September 2010 (Cridland pers. comm. 2010). The flow rates in Big Otter Creek did not change following dam removal since this reservoir was strictly an "in and out" dam with no capability for level manipulation or storage. As a result of the removal of the dam, it is anticipated that water temperatures in Big Otter Creek will decrease, along with an overall improvement to the water quality (Gagnon pers. comm. 2010).
Invasive and introduced species
Invasive and introduced species with the potential to alter aquatic habitat [e.g., Round Goby (Neogobius melanostomus), Zebra Mussel (Dreissena polymorpha), Common Carp (Cyprinus carpio), Curly Pondweed (Potamogeton crispus) and Rainbow Trout (Oncorhynchus mykiss)] pose a threat to Laura’s Clubtail, although many of these effects have not yet been studied for this system or specific species (COSEWIC 2010). Round Goby is of the greatest concern to Laura’s Clubtail and has been detected in Big Creek and its tributaries (COSEWIC 2010; Dextrase pers. comm. 2011). A scientific fish collection in Little Otter Creek, just east of Big Otter Creek, in August 2010 recorded 133 Round Goby catches from a total of 385 fish (34 %) suggesting that Round Goby is a significant threat in the area (Gagnon pers. comm. 2011). Round Goby is a prolific breeder and voracious feeder, the combination of which could potentially upset a small and isolated population of Laura’s Clubtail (Corkum et al. 2004). Round Goby has the ability to alter food web dynamics and has been linked to enhanced algal biomass, both impacts that could affect Laura’s Clubtail (Corkum et al. 2004). There is speculation that Round Goby may predate Laura’s Clubtail larvae, especially at the time of emergence, but this has yet to be studied (COSEWIC 2010). Another invasive species with the potential to alter Laura’s Clubtail habitat is the Zebra Mussel. Zebra Mussels can cause increased light penetration in water bodies and may increase occurrences of blue-green algal blooms, either of which changes could upset sensitive species like Laura’s Clubtail (MacIsaac 1996). Zebra Mussels also change food web dynamics by removing phytoplankton biomass through ingestion or trapping in pseudofeces (MacIsaac 1996); this reduces food available to other consumers. These impacts are increased in shallow or well-mixed water systems (MacIsaac 1996). Zebra Mussel abundance in known locations of Laura’s Clubtail is thought to be low (Dextrase pers. comm. 2011; Gagnon pers. comm. 2011) but should be studied further in order to dismiss the threat. Common Carp increase turbidity and destroy aquatic vegetation needed by Laura’s Clubtail for both travel and reproduction (COSEWIC 2010). Carp are known to enter Big Creek and Big Otter Creek in summer months. The recent removal of a dam on Big Otter Creek has increased presence of carp downstream into at least one known location of Laura’s Clubtail but the removal of the dam also removed carp breeding habitat (the reservoir) so the threat of carp is expected to decrease (Gagnon pers. comm. 2011). A second dam at Tillsonberg lies upstream of the majority of known location of Laura’s Clubtail on Big Otter Creek and should prevent carp from travelling into the downstream area (Gagnon pers. comm. 2011). Despite only seasonal abundance of carp, they could pose a greater threat than more abundant fish (e.g., Rainbow Trout) because of their behaviour (Gagnon pers. comm. 2011). Curly Pondweed can alter aquatic habitat and water quality by creating dense mats and lowering oxygen conditions (Global Invasive Species Database 2006). Curly Pondweed can currently be found downstream of known locations of Laura’s Clubtail on both Big Creek and Big Otter Creek (Dextrase pers. comm. 2011; Gagnon pers. comm. 2011). Historic presence of Curly Pondweed further upstream in Big Otter Creek (this species was removed) (Gagnon pers. comm. 2011) suggests that it could reoccur and impact Laura’s Clubtail. Finally, Rainbow Trout can alter food web dynamics (Buria et al. 2010). Rainbow Trout are more abundant than Common Carp in the Big and Big Otter Creek systems, but likely pose less threat due to their habit of feeding on large aquatic hatches such as those of mayflies (Gagnon pers. comm. 2011). Invasive and introduced species that have the potential to threaten Laura’s Clubtail should be assessed regularly as they become established in areas identified as habitat for Laura’s Clubtail.
Road mortality
Dragonflies are also susceptible to road mortalities (Rao and Girish 2007). Dragonfly road deaths generally happen in two stages: (i) dragonflies that are hit by cars exceeding 50 to 60 km/h experience severe shock and fall to the ground; (ii) dragonflies either recover from the shock and fly away or, as is usually the case, they are run over by a second vehicle (Rao and Girish 2007).
Areas where road crossings fragment stream habitat have a high potential for Laura’s Clubtail adult mortality (COSEWIC 2010). This is especially true of most stream crossings within the relevant counties (Norfolk and Elgin) where paved roads have a posted speed limit of at least 60 km/h (Rao and Girish 2007, COSEWIC 2010) and where most of the crossings in question have a posted speed limit of 80 km/h. Within Laura’s Clubtail habitat, there are 19 bridges which fit these criteria (12 bridges on Big Otter Creek and 7 on Big Creek) (COSEWIC 2010).
1.7 Knowledge gaps
Knowledge gaps around Laura’s Clubtail are extensive. The factors affecting the distribution of this species are very poorly understood for Ontario populations, which may hinder the efficacy of protection strategies until the biology of Laura’s Clubtail is better understood. Research on the following knowledge gaps would contribute to a more complete understanding for the protection and recovery of the species and its habitat:
- species-specific biology/behaviour data for both adults and larvae including territoriality, foraging, mating and oviposition;
- extent within Ontario (may occur in locales other than along Big Creek and Big Otter Creek);
- physiological tolerance to changes in stream condition (temperature, pH, mineral content, pollution);
- physiological tolerance of Laura’s Clubtail and their prey to pesticides and herbicides; and
- extent of road mortality at river crossings.
1.8 Recovery actions completed or underway
- Surveys for Laura’s Clubtail within known areas and areas with suitable habitat (NHIC – Colin Jones, Rob Foster, Allan Harris, Paul Catling, Peter Burke – roughly 40 hours of search time)
- Water quality monitoring through the Provincial Water Quality Monitoring Program at Big Otter Creek (Calton and Maple Dell) and two locations on Big Creek (Concession 7 north of Walsingham and Concession 2 south of Kelvin) (Long Point Region Conservation Authority)
- Protection and enhancement of habitat through acquisition and restoration efforts in Big Creek area (Nature Conservancy of Canada)
2.0 Recovery
2.1 Recovery goal
The recovery goal is to ensure a viable, self-sustaining population of Laura’s Clubtail in Ontario.
2.2 Protection and recovery objectives
Table 1. Protection and recovery objectives
| No. | Protection or Recovery Objective |
|---|---|
| 1 | Protect, maintain and enhance the quantity and quality of existing Laura’s Clubtail habitat. |
| 2 | Reduce or mitigate threats to Laura’s Clubtail and its habitat where feasible. |
| 3 | Increase knowledge of Laura’s Clubtail biology in Ontario including distribution, abundance, life history and habitat needs. |
2.3 Approaches to recovery
It is recommended that recovery efforts for Laura’s Clubtail be coordinated with recovery efforts for the Eastern Sand Darter (Ammocrypta pellucida) where the occurrences overlap since they share similar habitat and threats (Finch pers. comm. 2011; Dextrase pers. comm. 2011).
Table 2. Approaches to recovery of Laura’s Clubtail in Ontario
1. Protect, maintain and enhance the quantity and quality of Laura’s Clubtail habitat
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Critical | Short-term | Protection | 1.1 Develop a habitat regulation to protect the habitat at known locations of Laura’s Clubtail. |
|
| Necessary | Short-term | Assessment, Stewardship, Education and Outreach |
1.2 For lands surrounding the known sites develop, implement and support education, awareness and stewardship programs:
|
All |
| Beneficial | Long-term | Stewardship, Protection |
1.3 Restore potential Laura’s Clubtail habitat within dispersal distance to facilitate natural expansion of current sites:
|
|
| Necessary | Ongoing | Protection, Stewardship | 1.4 Protect habitat through land acquisition, stewardship agreements, conservation easements, and pertinent legislation, policies and guidelines. |
|
2. Reduce or mitigate threats to Laura’s Clubtail and its habitat where feasible
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed | |
|---|---|---|---|---|---|
| Critical | Ongoing | Research | 2.1 Determine the extent to which invasive and introduced species such as Round Goby and Rainbow Trout may be feeding on Laura’s Clubtail larvae. – investigate impacts on extant populations. |
|
|
| Necessary | Ongoing | Monitoring and Assessment | 2.2 Conduct surveys for aquatic invasive species such as Round Goby, Zebra Mussel and Common Carp within and adjacent to Laura’s Clubtail habitat:
|
|
|
| Critical | Ongoing | Protection | 2.3 Work with local partners such as municipalities and conservation authorities to mitigate negative impacts at known locations:
|
|
|
| Necessary | Ongoing | Stewardship, Protection | 2.4 Work with conservation authorities and Ministry of Environment to monitor water quality of Laura’s Clubtail habitat:
|
|
|
| Necessary | Ongoing | Education and Outreach | 2.5 Work with partners to develop an outreach strategy to mitigate and prevent the spread of invasive species:
|
|
|
| Necessary | Short-term | Research | 2.6 Quantify threat of road mortality to Laura’s Clubtail and explore tactics for mitigation.
|
|
3. Increase knowledge of Laura’s Clubtail biology in Ontario including distribution, abundance, life history and habitat needs
| Relative Priority | Relative Timeframe | Recovery Theme | Approach to Recovery | Threats or Knowledge Gaps Addressed |
|---|---|---|---|---|
| Critical | Ongoing | Inventory and Monitoring | 3.1 Report observations of Ontario Odonata for inclusion in the Ontario Odonata Atlas Database and the Natural Heritage Information Centre (NHIC). |
|
| Critical | Ongoing | Inventory and Monitoring | 3.2 Survey and identify potential habitat for Laura’s Clubtail:
|
|
| Necessary | Short-term | Research | 3.3 Investigate the sensitivity of Laura’s Clubtail to various factors that may influence water quality:
|
|
| Necessary | Long-term | Research | 3.4 Carry out research on the basic biology of Laura’s Clubtail to address knowledge gaps:
|
|
| Necessary | Ongoing | Inventory and Monitoring | 3.5 Include information on Laura’s Clubtail in ongoing inventory programs in streams across the province:
|
|
| Beneficial | Ongoing | Education and Outreach | 3.6 Provide information to the public and land managers on Laura’s Clubtail, its habitat and how to report sightings, especially within the Norfolk Sand Plain area. |
|
| Beneficial | Short-term | Research | 3.7 Determine why Laura’s Clubtail appears to occur naturally in only a few of the apparently suitable rivers in Ontario. |
|
2.4 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of Natural Resources on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the authors will be one of many sources considered by the Minister when developing the habitat regulation for this species.
Laura’s Clubtail requires both aquatic and terrestrial habitats to complete its life cycle. Further research is required to understand the distribution, dispersal and life history of this species. It is recommended that the area prescribed as habitat in a habitat regulation include only locations where the species has been found (currently Big Creek and Big Otter Creek).
It is recommended that all stream reaches (aquatic resource areas
Glossary
Ciliates: Small to microscopic single-celled organism found in the water. Committee on the Status of Endangered Wildlife in Canada (COSEWIC): The committee responsible for assessing and classifying species at risk in Canada.
Committee on the Status of Species at Risk in Ontario (COSSARO): The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
Conservation status rank: A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperiled
2 = imperiled
3 = vulnerable
4 = apparently secure
5 = secure
Endangered Species Act, 2007 (ESA): The provincial legislation that provides protection to species at risk in Ontario.
Exuviae: The cast-off skins or coverings. In this case, the cast off shell or covering of the dragonfly larvae, shed after the larva emerges from the water to moult to the adult life stage.
Gomphidae: A family of dragonflies commonly referred to as "Clubtails". This family contains approximately 90 genera and 900 species
Larva (pl: larvae): An immature stage of any invertebrate that differs from the adult stage.
Macroinvertebrates: Animals that have no backbone and are visible to the naked eye (i.e. not microscopic in size). Some examples of aquatic macroinvertebrates include crayfish, mussels, aquatic snails, aquatic worms, and aquatic insects and their larvae.
Odonata: The taxonomic order comprising of dragonflies and damselflies.
Oviposition: To lay eggs, especially by means of an ovipositor.
Ovipositor: An organ found in some species of insects at the end of the female abdomen. This organ is used to deposit eggs.
Riffles: A stretch of turbulent water flow caused by such a shoal or sandbar; a rapid where the water’s surface is typically broken and has an obvious slope.
Rotifers: Microscopic animals found in the water.
Shoreline Hardening: The placement of an anthropogenic structure, such as concrete or rock, along the shoreline. Usually done as an effort to reduce shoreline erosion or as a measure for flood control.
Species at Risk Act (SARA): The federal legislation that provides protection to species at risk in Canada. This act establishes Schedule 1 as the legal list of wildlife species at risk to which the SARA provisions apply. Schedules 2 and 3 contain lists of species that at the time the act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
Species at Risk in Ontario (SARO) List: The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
Thoracic: Arising from the thorax, the portion of the body between the head and the abdomen.
Tibial (Tibia): The fourth segment of the insect leg, between the femur and tarsi.
Turbidity: A measure of water clearness. Turbid water is unclear, often cloudy.
References
Abbott, J.C. 2001. Distribution of dragonflies and damselflies (Odonata) in Texas. Transactions of the American Entomological Society 127: 199-228.
Bright, E. and M.F. O'Brien. 1999. Odonata Larvae of Michigan: Keys for, and notes on, the dragon- and damselfly larvae found in the State of Michigan [web application]. University of Michigan Museum of Zoology, Insect Division [Last Updated: 07
January 1999]. Available online: http://insects.ummz.lsa.umich.edu/michodo/mol/Home.htm[Accessed December 20, 2010.]
Buria, L., R. Albarino, V. Diaz Villanueva, B. Modenutti and E. Balseiro. 2010. Does predation by the introduced rainbow trout cascade down to detritus and algae in a forested small stream in Patagonia. Hydrobiologia 651: 161-172.
Catling, P.M. and C.H. Catling. 1999. Laura’s Clubtail (Stylurus laurae) new to Canada. Argia 11: 10-11.
Catling, P.M. 2000. An illustrated key to the mature nymphs and exuviae of eastern Canadian Hanging Clubtails (Stylurus). Ontario Odonata 1: 52-54.
Corkum, L.D., M.R. Sapota and K.E. Skora. 2004. The round goby, Neogobius melanostomus, a fish invader on both sides of the Atlantic Ocean. Biological Invasions 6: 173-181.
COSEWIC. 2010. COSWEIC assessment and status report on the Laura’s Clubtail Stylurus laurae, in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. VI + 34 pp.
Corbet, P.S. 1962. A Biology of Dragonflies. H.F.&G. Witherby Ltd. London. Corbet, P.S. 1999. Dragonflies: Behavior and Ecology of Odonata. Cornstock
Publishing Associates.
Dextrase, A. pers. comm. 2011. Personal communication to T.L. Pulfer. March 2011. Dunkle, S.W. 2000. Dragonflies through binoculars. Oxford University Press. Oxford, NY. 266 pp.
Englund, R.A. and D.A. Polhemus. 2010. Evaluating the effects of introduced rainbow trout (Oncorhynchus mykiss) on native stream insects on Kauai Island, Hawaii. Journal of Insect Conservation 5: 365-281.
Finch, M. pers. comm. 2011. Personal communication to L. Mousseau. February 2011.
Gagnon, P. pers. comm. 2011. Personal communication to L. Mousseau. March 2011. Global Invasive Species Database. 31 March 2006. Potamogeton crispus. www.issg.org/database/species/ecology.asp?si=447&fr=1&sts. [Accessed December 28, 2010.]
Gore, J.A. and F.D. Shields, Jr. 1995. Can large rivers be restored? BioScience. 45(3):134-141.
Helmreich, B., R. Hilliges, A. Schriewer, and H. Horn. 2010. Runoff pollutants of a highly trafficked urban road – Correlation analysis and seasonal influences. Chemosphere 80: 991-997.
Jones, F.C., D. Baird, M. Bowman, G. Cameron, B. Craig, B. Cutler, J. Diamond, N. Dmytrow, M. Nicol, J. Parker, T. Pascoe, H. Vaughan, and G. Whitelaw. 2006. Performance of Ontario’s Benthos Biomonitoring Network: Impacts on Particpants' Social Capital Environmental Action, and Problem-solving Ability. Environments Journal Volume 34. 18 pp.
Keppner, E.J. and L.A. Keppner. 2007. Dragonflies and Damselflies (Odonata) of the St. Andrew Bay Ecosystem and Bay County, Florida. St. Andrew Bay Environmental Study Team, Inc. Available online: http://www.friendsofstandrewbay.org/publications/odonates_sabe.pdf[Accessed December 28, 2010.]
Kudell-Ekstrum, J. 2004. Hiawatha National Forest ode surveys, 2003. Williamsonia: a publication of the Michigan Odonata Survey. 8(4): 2,8.
MacIsaac, H.J. 1996. Potential Abiotic and Biotic Impacts of Zebra Mussels on the Inland Waters of North America. American Zoology 36: 287-299.
Marshall, S. pers. comm. 2010. Personal communication to J. Gerrath. November 2010. Marshall, S.A. 2006. Insects: Their Natural History and Diversity, With a Photographic Guide to Insects of Eastern North America. Firefly Books Ltd. Richmond Hill. 720 pp.
Michigan Natural Features Inventory. 2007. Rare Species Explorer (Web Application). Available online at http://web4.msue.msu.edu/mnfi/explorer [Accessed Dec. 20, 2010]
NatureServe. 2010. NatureServe Explorer: An online encyclopedia of life [web application]. Version 6.1. NatureServe, Arlington, Virginia. Available online: http://www.natureserve.org/explorer[Accessed December 28, 2010.]
OMNR (Ontario Ministry of Natural Resources). 2010. Element Occurrence data from Biotics database. Natural Heritage Information Centre. December 2010.
Odanaka, Y., T. Taniguchi, Y. Shimamura, K. Iijima, Y. Koma, T. Takechi, and O. Matano. 1994. Runoff and leaching of pesticides in golf courses. Journal of Pesticide Science 19:1–10.
Paulson, D.R. 2009. Dragonflies and damselflies of the West. Princeton University Press. Princeton, NY. 535 pp.
Price, H.J. 1950. Notes on the Dragonflies of Northwestern Ohio. Ohio Journal of Science L: 71-78.
Rao, R.S.P. and Girish, M.K.S. 2007. Road kills: assessing insect casualties using flagship taxa, Current Science 92: 830–837.
Roback, S.S. and M.J. Westfall Jr. 1967. New records of Odonata nymphs from the United States and Canada with water quality data. Transactions of the American Entomological Society 93: 101-124.
Sutherland, D.A. pers. comm. 2010. Personal communication to T.L. Pulfer. December 2010.
Vidrine, M.F., C.M. Allen, B. Borsari and L. Allain. 2001. Lepidopteran and Odonate Communities in the Cajun Prairie Ecosystem in Southwestern Louisiana. Proc. 17th N.A. Prairie Conference: 206-214
Wales, M. 2004. Searching for alternative crops to replace or enhance tobacco in Canada. 2004 Neufield Scholar Final Report. 36 pp. Available online: http://nuffield.ca/wp-content/uploads/2009/09/MarkWalesReport.pdf[Accessed December 29, 2010.]
Walker, E.M. 1953. The Odonata of Canada and Alaska. Vol 1. Part 1: General University Toronto Press, Toronto, Canada. 292 pp.
Westfall, M.J. Jr. 1953. Notes on Florida Odonata, including additions to the state list. The Florida Entomologist 36: 165-173.
Williams, D.D., N.E. Williams and Y. Cao. 1999. Road salt contamination of groundwater in a major metropolitan area and development of a biological index to monitor its impact. Water Resources 34: 127-138.
Williamson, E.B. 1932. Two new species of Stylurus (Odonata Gomphinae). Occasional Papers of the Museum of Zoology, University of Michigan. 247: 1-18.
Footnotes
- footnote[1] Back to paragraph Aquatic resource areas are aggregations of stream segments with similar physical and biological characteristics.
- footnote[2] Back to paragraph Aquatic resource areas are aggregations of stream segments with similar physical and biological characteristics.