Piping Plover Recovery Strategy
This document advises the ministry on ways to ensure healthy numbers of the Piping Plover, a threatened or endangered species, return to Ontario.

Recovery strategy prepared under the Endangered Species Act, 2007
2013
About the Ontario Recovery Strategy Series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species' persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. There is a transition period of five years (until June 30, 2013) to develop recovery strategies for those species listed as endangered or threatened in the schedules of the ESA. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy.
The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of Natural Resources Species at Risk webpage .
Recommended citation
Kirk, D. A. 2013. Recovery Strategy for the Piping Plover (Charadrius melodus) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. vi + 61 pp.
Cover illustration: Photograph of Piping Plover and young at Sauble Beach by Peter Middleton.
© Queen’s Printer for Ontario, 2013
ISBN 978-1-4606-0539-4 (PDF)
Content (excluding the cover illustration) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée Recovery strategies prepared under the Endangered Species Act, 2007, n'est disponible qu'en anglais en vertu du Règlement 411/97 qui en exempte l'application de la Loi sur les services en français. Pour obtenir de l'aide en français, veuillez communiquer avec Cathy Darevic au ministère des Richesses naturelles au
Authors
David Anthony Kirk (Aquila Conservation & Environment Consulting)
Acknowledgments
The Ontario Ministry of Natural Resources funded the preparation of this recovery strategy. I thank the workshop participants who provided information on the Piping Plover, attended the workshops on 23rd March 2012 and 9th August 2012 and commented on earlier drafts (see Recovery Strategy Workshop Participants and Advisors, pg. 58). The following persons commented on earlier drafts: Amelia Argue, Andrew Chard, Leanne Jennings, Kathryn Markham, Chris Risley, Sam Short, Bree Walpole (OMNR Species at Risk Branch), Eric Cobb (OMNR Sudbury District), Sandy Dobbyn, Jessica Dunlop (Ontario Parks), Leo Heyens, John Van Den Broeck (OMNR Kenora District), Jodi Benvenuti, Suzanne Robinson (OMNR Midhurst District), Pete Ewins (World Wildlife Fund Canada), Ted Cheskey (Nature Canada), Sue Abbott (Bird Studies Canada), Peter Middleton (Bruce Stewardship Resource Network Piping Plover Monitoring Program) and the Canadian Wildlife Service of Environment Canada: Madeline Austen, Krista Holmes, Meghan Gerson, Tania Morais, Jeff Robinson, Kathy St. Laurent, Kari Van Allen (Environment Canada, Canadian Wildlife Service - Ontario Region), François Shaffer (Environment Canada, Canadian Wildlife Service - Québec Region), and Doug Bliss (Environment Canada, Canadian Wildlife Service - Atlantic Region). Francesca Cuthbert (Dept. Fisheries, Wildlife and Conservation, University of Minnesota) also reviewed the draft, and Robin Davidson-Arnott (Professor Emeritus, Department of Geography, University of Guelph) sent comments on coastal processes and beach-dune formations. Don Sutherland (Natural Heritage Information Centre, OMNR) helped compile Appendix 1 (historical records), Jon McCracken (Program Manager, Bird Studies Canada) sent relevant reports from Long Point and Val Minelga and Sarah Rupert (Park Ecologist and Park Interpreter, respectively) compiled information for Point Pelee National Park of Canada for which I am most grateful. Cheryl Widdifield helped prepare the maps.
I also thank Vince Cavalieri (Piping Plover Coordinator, United States Fish and Wildlife Service), Jonathan Cohen (Dept. Environmental and Forest Biology, SUNY College of Environmental Science and Forestry), Anne Hecht (United States Fish and Wildlife Service) and Cheri-Gratto-Trevor (Environment Canada, Canadian Wildlife Service, Saskatoon) for feedback and sending relevant reports and scientific papers. My special thanks are to: Amy Chabot for helping organize the first workshop; Warren Wilson for facilitating the second workshop; Francie Cuthbert for her tremendous support, helpful comments and sending relevant papers; Peter Middleton for his enthusiasm and love of Piping Plovers, as well as encouragement and support; Chris Risley for helping me balancing viewpoints; Don Sutherland for his great help with historical records; and last but not least to Amelia Argue, Bree Walpole and Leanne Jennings for their comments, help and guidance throughout the preparation of this strategy.
Declaration
The recovery strategy for the Piping Plover was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all of the individuals who provided advice or contributed to its preparation, nor the official positions of the organizations with which the individuals are associated.
The goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ontario Ministry of Natural Resources
Environment Canada – Canadian Wildlife Service, Ontario
Executive summary
The Piping Plover (Charadrius melodus) is a small migratory shorebird with a widespread but scattered distribution in North America. It breeds in North America but overwinters in the southern United States, Mexico and some Caribbean Islands. It is listed as endangered under Ontario’s Endangered Species Act, 2007 (ESA), Canada’s Species at Risk Act and the United States' Endangered Species Act. There are two subspecies: C. m. melodus, which occurs in the Atlantic region, and C. m. circumcinctus, which occurs in the interior of the continent. This recovery strategy focuses on the circumcinctus subspecies. Within the circumcinctus subspecies there are two populations: one in the Prairies of Canada and Northern Great Plains of the United States (hereafter 'Great Plains population') and the other in the Great Lakes of the United States and Canada (hereafter 'Great Lakes population'). In Canada, the Great Plains population occurs in Alberta, Saskatchewan, Manitoba and northwestern Ontario in the Lake of the Woods. The Great Lakes population occurs in Michigan, Wisconsin, Illinois, and Ontario.
In this recovery strategy, the populations within Ontario are referred to as subpopulations: the Ontario Lake of the Woods (subpopulation of the Great Plains population) and the Ontario Great Lakes (subpopulation of the Great Lakes population). This recovery strategy focuses on these two subpopulations.
The United States Great Lakes nesting population was almost extirpated in the mid-1980s but numbers in the Great Lakes states have since increased due to a combination of management, intensive nesting site protection (including predator control) and some captive rearing of abandoned eggs. As a direct result of these management efforts in Michigan and other states, Piping Plovers have again begun nesting in Ontario. Prior to 2007, when a pair returned to nest at Sauble Beach, Piping Plovers last nested in Ontario on the Great Lakes in 1977. Historically, they nested at 24 locations on all Great Lakes in Ontario with perhaps 70 to 90 pairs. Each year since 2007 small numbers of Piping Plovers have returned to nest in the Ontario Great Lakes; in 2012, four nests were initiated at Wasaga Beach Provincial Park, and two nests at Sauble Beach. The United States and Canadian Lake of the Woods subpopulations have declined steadily since 1991. Sporadic nesting has occurred in the Ontario Lake of the Woods since 1938; although between zero and six adult birds were present, nesting has only ever been confirmed for one or two pairs. Most recently, nesting occurred at Windy Point on the Ontario Lake of the Woods in 2007, 2009 and again in 2010.
Piping Plovers have specific habitat needs for nesting, brood rearing, foraging, staging/migration and wintering. They generally occur on beaches that are more than 10 m wide, with a shoreline of more than 400 m, with patches of gravel or sand/gravel and sandbars. Specific habitat features used by nesting Piping Plovers differ between the Great Lakes and the Great Plains populations. Great Lakes Piping Plovers nest on sand and cobble beaches with freshwater dune formations, whereas Great Plains Piping Plovers in Alberta, Saskatchewan and Manitoba are associated with reservoirs, lakes and rivers. The Ontario Lake of the Woods subpopulation nests in similar habitats to the Ontario Great Lakes subpopulation. Piping Plovers face many threats and the magnitude and relative importance of these threats vary among the Ontario subpopulations. In the Ontario Great Lakes subpopulation, the most important threats are predation, human disturbance, and habitat loss or degradation. In the Ontario Lake of the Woods subpopulation, predation and storm events that result in storm surges and flooding appear to be the main threats.
The overall recovery goal is to protect Piping Plovers at nesting locations, encourage the expansion of the current breeding population in Ontario, and ensure its persistence as part of the Great Lakes and Great Plains subpopulations. It is recommended that a population and distribution objective (including recovery targets) be set within the next three years based on: (1) the suitability of available (including historical) sites in Ontario determined from a habitat suitability model, (2) the area requirements of Piping Plovers and (3) population predictions based on a population model for the Great Lakes and Great Plains populations in the United States and Canada.
The recovery objectives identified in this recovery strategy are to:
- protect all nesting pairs and their habitat at existing sites: implement actions to address threats to territory establishment and/or nesting at occupied sites within Ontario;
- plan for the potential of greater numbers: identify potential nesting sites and establish Ontario population targets;
- promote conservation and stewardship of beach and dune ecosystems, including their overall biodiversity and associated species at risk in Ontario;
- increase knowledge of Piping Plover demography/population dynamics, habitat requirements and threats;
- foster stewardship and public outreach/education about Piping Plovers at occupied sites as well as communication within the province; and
- continue to coordinate/share information in existing databases for the Piping Plover with government and non-government conservation agencies, as required, for the Great Lakes and Lake of the Woods subpopulations.
The area to be defined in a regulation as habitat for Piping Plovers should take into account the area of beach used by Piping Plovers, the dynamic nature of beach-dune ecosystems, and the semi-colonial behaviour of nesting pairs. It is recommended that a habitat regulation include: (1) all sites occupied by nesting Piping Plovers within the last 10 years and 10 years following occupation and (2) a one-kilometre length of continuous beach habitat (generally centred around the nest site) to provide the requisites for life processes. The width of this continuous beach habitat would extend from the water’s edge to the upper or inland edge of open beach or open dune plant communities or the beginning of anthropogenic features. In instances where Piping Plovers nest in anthropogenic features, the area around the nest should be protected for one year (the season occupied), in addition to a one-kilometre strip of continuous beach habitat as defined above, where applicable.
1.0 Background information
1.1 Species assessment and classification
Common name:
Piping Plover
Scientific name:
Charadrius melodus
SARO List Classification:
Endangered
SARO List history:
Endangered (2004)
COSEWIC assessment history:
Piping Plover circumcinctus subspecies – Endangered (2001)
Piping Plover – Endangered (1985)
Piping Plover – Threatened (1978)
SARA Schedule 1:
Endangered (June 2003)
Conservation status rankings:
GRANK: G1 NRANK: N1 SRANK: S1
The glossary provides definitions for technical terms, including the abbreviations above.
1.2 Species description and biology
Species description
The Piping Plover (Charadrius melodus) is a small (18 cm long, 43-63 g mass) migratory shorebird belonging to the family Charadriidae (Elliott-Smith and Haig 2004). It has a light sand-coloured back and head, a white belly, and legs and feet that are orange in colouration. In the breeding season, adult Piping Plovers have a distinctive black breast band and a single black band between the eyes and the bill is orange with a black tip (Elliott-Smith and Haig 2004). Sexes are similar in appearance but males tend to have broader breast and head bands and a more brightly-coloured orange bill. Juveniles are also present on Ontario beaches during the breeding season and resemble adults in winter plumage (the black neck band is lost, the bill becomes mostly black and legs fade to pale yellow). Individuals of the C. m. circumcinctus subspecies tend to have intact breast bands, whereas those of the C. m. melodus subspecies are more often incomplete. Piping Plovers are distinguished from the similar Semipalmated Plover (Charadrius semipalmatus) by their sand-coloured rather than light brown backs, lores (feathers between the bill and eye) and auriculars (feathers covering ears). In comparison to the Killdeer (Charadrius vociferus), they are smaller in size and have a single rather than double black breast band. Their most common call is a clear repeated "pipe" and they also make "peep-lo" calls during the breeding season.
Species biology
There are two subspecies of Piping Plover in Canada: an Atlantic coast subspecies (C. m. melodus), which occurs in the Maritimes, Québec, Newfoundland and Saint-Pierre-et-Miquelon (the latter islands are French territories south of Newfoundland) and an inland subspecies (C. m. circumcinctus), which is found in the Prairie Provinces (Alberta, Saskatchewan, Manitoba) and in Ontario (see Figure 1).
Figure 1. Breeding (blue/grey shade) and wintering range (orange) of the Piping Plover in the Americas
(Modified from Elliot-Smith and Haig 2004, used with permission from Birds of North America online http://www.bna.birds.cornell.edu/bna [link inactive] maintained by the Cornell Lab of Ornithology; DPS is Distinct Population Segment – USFWS 2009).
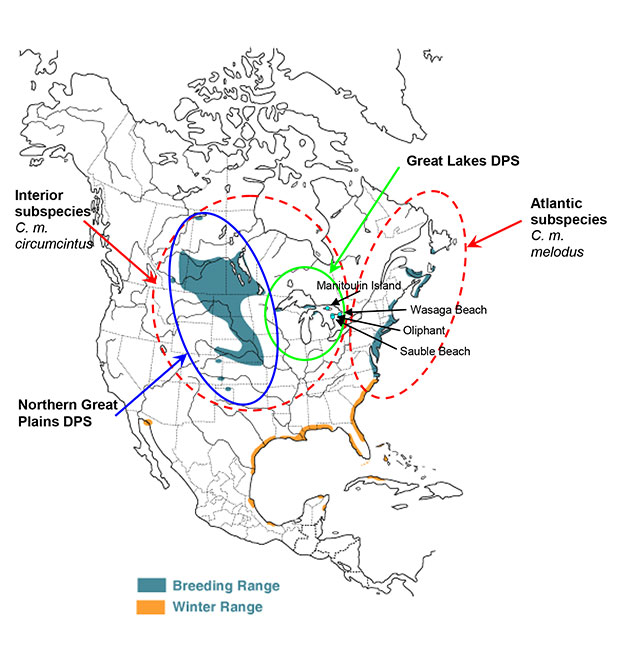
Recent mitochondrial DNA analysis has confirmed the distinction between the two subspecies (Miller et al. 2009). Within the circumcinctus subspecies, two populations exist: the Northern Great Plains (hereafter 'Great Plains') and the Great Lakes. There is some evidence for individuals mixing between these two populations. The Lake of the Woods subpopulation in Ontario and Minnesota is considered to be part of the Great Plains population but is believed by some to provide a link between the two populations (Gratto-Trevor and Abbott 2011).
It appears that exchange between the Great Plains and Great Lakes breeding populations is rare. A banded individual from the Platte River Area of Sleeping Bear Dunes National Park on Lake Michigan (Great Lakes population) was recorded at Windy Point, Lake of the Woods, Ontario in 2002 (Heyens 2002), and an adult banded in the same location defended nest scrapes (slight depressions in the ground excavated by Piping Plovers) at Windy Point in 2003 (Heyens 2003), suggesting some birds from Michigan may nest in Lake of the Woods. Evidence of interchange between the Great Plains and Lake of the Woods, Ontario comes from banding records. For example, two birds banded in 2007 in the Gavins Point reach area of the Missouri River (on the South Dakota/Nebraska border) were found breeding at Windy Point and the Sable Islands Provincial Nature Reserve. In 2011, one adult bird at Windy Point had been banded during the winter of 2010-11 near Ocean Springs, Mississippi as part of the Deepwater Horizon Oil Spill Project (L. Heyens, pers. comm. 2012). Only one recent case exists of the reverse situation where a bird from the Great Plains population in Canada bred in the United States Great Lakes. A male plover banded as a chick in Manitoba nested in Michigan in 2011 and 2012. It was first seen as a non-breeder at the Michigan site in 2010 (F. Cuthbert, pers. comm. 2012).
Piping Plovers arrive on their breeding areas generally between mid- to late April (range from early April to May). Nest initiation typically begins by late April to early May and is most closely linked to when the pair bond is formed. In some areas (though not apparently in Ontario – L. Heyens, pers. comm. 2012), it may take longer for pairs to initiate breeding at sites where few or no other breeding pairs are present (Elliott-Smith and Haig 2004). This may be because the species exhibits semi-colonial behaviour, a nesting strategy where an individual or pair is more likely to establish a nest site when conspecifics are present. Semi-colonial refers here to Piping Plovers clumping their territories together, although they still defend individual territories (Elliott-Smith and Haig 2004). It is difficult to determine why this semi-colonial nesting occurs, as attraction to conspecifics (and the reason for this) is confounded with clustering of suitable habitat and site fidelity and whether the previous breeding attempt was successful.
Piping Plovers show varying levels of territoriality throughout the year, with the most intense territorial defence being prior to the beginning of incubation. (Note that territorial behaviour here is defined by site attachment, exclusive use of an area, agonistic behaviour and attack that changes to retreat at the territory boundary; the home range is simply the area where an individual restricts itself – Huntingford and Turner 1987). Following arrival in their breeding areas, male Piping Plovers establish territories that include sections of shoreline and areas of open ground for nesting (Whyte 1985).
During this period they run up and down the length of beaches, defending their territory from conspecifics using the 'parallel-run' display (Cairns 1982) or sometimes 'horizontal threat displays' (Elliott-Smith and Haig 2009). Territory size varies both temporally (over the breeding season) and geographically. For example, in Nova Scotia average territory size was 0.4 ha (Cairns 1982), whereas in Saskatchewan it varied from 2.7 to 3.1 ha (Whyte 1985). There is also substantial variation in distances between nests: for the Atlantic subspecies it ranged from 51 to 53 m in Nova Scotia (Cairns 1982), to 85 to 99 m in New Jersey (Burger 1987) and 70 m on Long Island, New York (Wilcox 1959). For the Interior subspecies (Prairie population) inter-nest distances were 14 to 389 m in North Dakota (Murphy et al. 2001) and 14.5 to 73 m in South Dakota (Schwalbach 1988). In the Great Lakes, average home range size of Piping Plovers that fledged at least one chick was 2.9 ± 0.5 (SE) and the mean linear distance moved along beaches was 475 ± 53 m (130-1435 m; Haffner et al. 2009). There was high inter-annual variability in the area of beach used and shoreline traversed. These results from home range studies and spacing in the Great Lakes suggest that even small areas of suitable beaches may have conservation value and that individual variation in the area of habitat used must be considered when protecting and regulating habitat (Haffner et al. 2009).
Usually four eggs are laid in a simple scrape, and one brood is produced each season (Elliott-Smith and Haig 2004). Replacement clutches may be laid if the first clutch is lost, especially if this happens early in the season and prior to hatching. Eggs are incubated for about 26 to 28 days (Wilcox 1959, Cairns 1977, Whyte 1985, Haig and Oring 1988) and the young are precocial, meaning they can leave the nest and walk and feed themselves on the same day that they hatch. They fledge (fly) at 21 to 35 days old (Wilcox 1959, Cairns 1982, Whyte 1985, Prindiville-Gaines and Ryan 1988). Piping Plovers will often breed during their first spring; average life span is five years with a small number of reports of birds living between 8 and 14 years (F. Cuthbert, pers. comm. 2012). Recent estimates of annual survival in the Great Lakes population have been made by LeDee et al. (2010a) and Roche et al. (2010b).
In both the Great Plains and Great Lakes populations, adult Piping Plovers show very high breeding site fidelity (70-90%: see Haig and Oring 1988, Cohen et al. 2006, Catlin 2009, Cohen and Gratto-Trevor 2011). Fidelity to breeding sites is slightly stronger for males than for females (Wemmer 2000). If pairs do not return to former breeding sites, then they may nest close by (Haig and Oring 1988). In the Great Plains site fidelity is much lower in ephemeral habitat (Knetter et al. 2002). In the Great Lakes, site fidelity is associated with previous reproductive success (Elliott-Smith and Haig 2004). Recently it has been shown that female familiarity with nest site locations significantly influences their reproductive success. Typically, females that move to a new location experience a decrease in fledging success from 2.1 to 1.5 chicks (Saunders et al. 2011). Natal site philopatry (first-year Piping Plovers returning to the sites where they hatched) is generally low and ranges from 1.6 percent in Nova Scotia to 23 percent (Haig and Oring 1988); it is much higher in the Lake of the Woods subpopulation (70%; Haig and Oring 1987). Older females and males also breed earlier, which increases their chances of nesting successfully (Saunders et al. 2011).
Piping Plovers feed on a wide variety of freshwater and marine invertebrates. In stomach and gizzard contents of chicks found dead in the Great Lakes, Cuthbert et al. (1999) and F. Cuthbert and B. Scoltens (Elliott-Smith and Haig 2004, unpub. data) found that Hymenoptera (wasps, bees and ants) dominated (32%), followed by Coleoptera (beetles, 29%), Diptera (flies, 28%), Hemiptera (true bugs) and Homoptera (which include cicadas, aphids, scales, plant-leaf and treehoppers (or hoppers), spittlebugs, whiteflies) (10%), and Ephemeroptera (mayflies), Trichoptera (caddisflies), Pseudoscorpiones (false scorpions) and Arachnida (spiders) (4%). Piping Plovers are preyed upon by a large number of predators, including mammals such as Coyotes (Canis latrans), Red Foxes (Vulpes vulpes), Raccoons (Procyon lotor), various mustelids (e.g., Striped Skunk (Mephitis mephitis), and domestic animals (dogs (Canis familiaris) and cats (Felis catus)). Avian predators include gulls such as Herring Gull (Larus argentatus) and Ring-billed Gull (Larus delawarensis), as well as Merlin (Falco columbarius), Peregrine Falcon (Falco peregrinus), Great Horned Owl (Bubo virginianus), American Crow (Corvus brachyrhynchos) and Common Raven (Corvus corax) (United States Fish and Wildlife Service 2003, 2009, Elliott-Smith and Haig 2004).
1.3 Distribution, abundance and population trends
The North American distribution of the Piping Plover is shown in Figure 1. Two subpopulations of Piping Plover circumcinctus subspecies breed in Ontario (Figure 2). One is in Lake of the Woods (Great Plains population) and the other is along Great Lakes shorelines (Great Lakes population). Currently (2012), the latter includes only Lake Huron; however, historically (prior to 1977) as many as 24 breeding sites were occupied on Lake Erie, Lake Huron and Lake Ontario and surrounding region and possibly Lake Superior (Figure 3).
This recovery strategy addresses both the Ontario Lake of the Woods (Great Plains population) and the Ontario Great Lakes (Great Lakes population) subpopulations.
Figure 2. Confirmed nesting occurrences of the Piping Plover in Ontario (2007-2011)
Modified from the Action Plan for the Piping Plover (Charadrius melodus circumcinctus) in Ontario (Environment Canada 2011 – used with permission, B. Slezak, pers. comm. 2012).
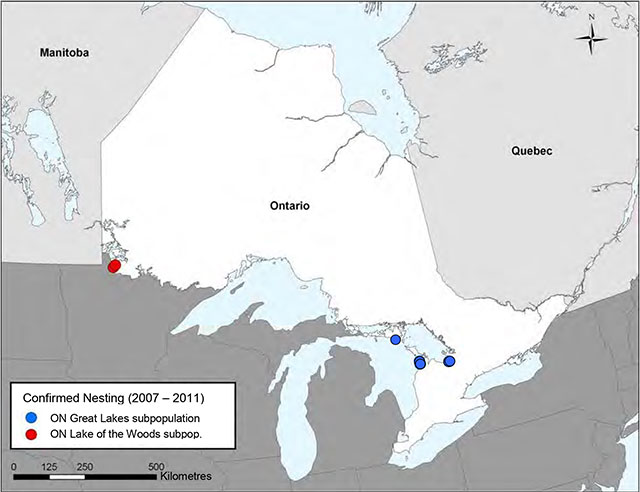
Enlarge Figure 2. Confirmed nesting occurrences of the Piping Plover in Ontario (2007-2011)
Russell (1983) estimated an 1800s total population of 492 to 682 breeding pairs of Piping Plovers in the Great Lakes region, with most located in Michigan (215), followed by Ontario (152-162), Illinois (125-130), Indiana, Ohio, Wisconsin (≤ 100 each), and Minnesota, New York, and Pennsylvania (< 30 pairs). The historical estimate for Ontario has been described as 'liberal' (Heyens 2007) and is thought to be too high (S. Matteson, Wisconsin Department of Natural Resources, pers. comm. 1988 in United States Fish and Wildlife Service 2003), because there was likely insufficient habitat available for this number of birds. Historically, Piping Plovers nested on all of the Great Lakes in Ontario, except perhaps Lake Superior (Appendix 1). Numbers declined dramatically with market hunting at the turn of the century but some recovery occurred following protection of the species (Lambert 1987, Gratto-Trevor and Abbott 2011). Following this, the Piping Plover nesting population is believed to have peaked in the 1920s but then went into a long-term decline. Russell (1983) stated that the Piping Plover was 'likely a common summer resident' in the limited habitat of the four Great Lakes in Ontario; a few scattered pairs occurred at inland lakes, though these are unlikely to have consisted of large concentrations (Quilliam 1973).
Figure 3. Historical Piping Plover locations in the Great Lakes
(data courtesy of the University of Minnesota for the United States, and the Natural Heritage Information Centre for Ontario).
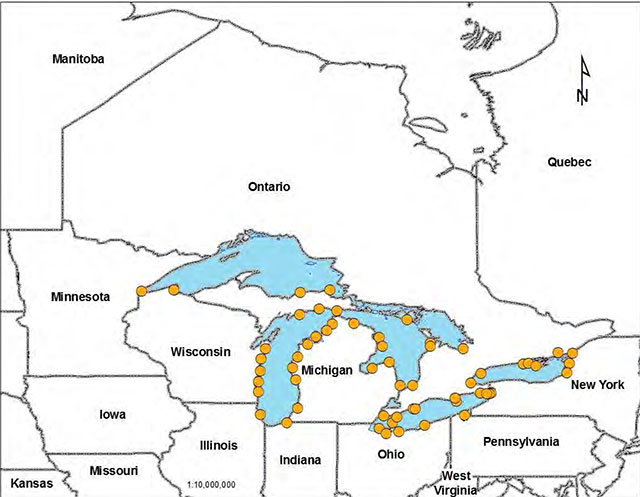
Enlarge Figure 3. Historical Piping Plover locations in the Great Lakes
On Lake Erie, there are records for 11 historical nesting locations including Point Pelee, Rondeau Provincial Park, Long Point and various beaches between Niagara Falls and Dunnville (Russell 1983; see Appendix 1). The largest number of nesting pairs is reported at Long Point on Lake Erie in 1927, where Snyder (1931) estimated 100 pairs. It is from this area that declines over time are best documented (Russell 1983; see Appendix 1 for details). Because Snyder’s estimate was based on extrapolation from only a three-mile (4.8 km) length of beach, it must be treated with some caution (Lambert 1987). In a recent study in New York, Cohen et al. (2009) reported densities of one pair every 175 m (20 pairs in a 3.5-km stretch of beach) and one pair per 93 m (64 pairs in a six-kilometre stretch of beach) on Westhampton Island, following storm damage from a hurricane. Also, Saunders (1909) estimated about six or eight pairs of Piping Plovers near the mouth of the Big Creek, Ontario, which is a density of one pair every 600 to 800 m of beach (the river mouth bar at the mouth of the Big Creek is approximately five kilometres long; D. Sutherland, pers. comm. 2012). These examples suggest that a nesting pair every 800 m was possible in the only extensive suitable nesting habitat (past and present) along the south beach at Long Point.
On July 6, 1933, Sheppard (1935) observed a large number of Piping Plovers at Long Point (50) but did not indicate whether they were adults, adults with young or if the birds were nesting (they could have been dispersing, post-breeding). Observations by Snyder (1931) and Sheppard (1935), combined with Cohen et al.'s (2009) estimates from New York State suggest that Long Point could have supported between 25 and 50 pairs of Piping Plovers during peak years in the early 1900s. Thus a realistic total for Lake Erie, including sites in other counties, is likely in the range of 50 to 75 pairs (see Appendix 1). According to Hussell and Montgomerie (1966), an average of seven pairs nested at Long Point between 1960 and 1965 but only 34 young fledged in the subsequent 10 years (Cartar 1976). By 1972, numbers at Long Point had declined to four nesting pairs, and three to five pairs by 1976; the last pair nested in 1977 and in 1978 only unmated males were observed. Gull predation on eggs is believed to be the main factor causing the decline (Miller 1977), in addition to predation of eggs and young by Raccoons and mustelids. In latter years, it became increasingly difficult for adults to recruit mates (Lambert and Nol 1978).
On Lake Huron, Piping Plovers nested at Carter Bay and Manitoulin Island (Nicholson 1981), as well as Sauble Beach and Oliphant Beach on the Bruce Peninsula and Ipperwash Beach northeast of Sarnia. Wasaga Beach is an historical site on Lake Huron not listed by Russell (1983) (there is a total of five historical nesting locations with perhaps seven pairs, which is close to Russell’s estimated historical population of 10 pairs).
In the Lake Ontario region, five nesting locations occurred historically in addition to at least one on the St. Lawrence River and one on an inland lake (Quilliam 1973; perhaps 11 pairs, again not that different from Russell’s estimate of 15-25 pairs; see Appendix 1). Russell (1983) also suggested that a few pairs of Piping Plovers nested in the Thunder Bay region of Lake Superior; although this may be possible (and is referred to by Lambert and Nol 1978), there is no strong evidence to support this (D. Sutherland, pers. comm. 2012). The Ontario Lake of the Woods nesting subpopulation has fluctuated but has remained at one or two pairs until present. Using the best available records for Piping Plovers, the Ontario nesting population in the early 1900s was likely in the range of 70 to 90 pairs, or approximately half of Russell’s (1983) estimate of 152 to 162 pairs for the Ontario Great Lakes.
Within this document, the Ontario Great Lakes and Ontario Lake of the Woods subpopulations are being examined separately because they show varying population trends, distinctive source populations, and different levels of threats. It is useful to recognize that the Ontario Great Lakes subpopulation is adjacent to the Michigan subpopulation, whereas the Ontario Lake of the Woods subpopulation is adjacent to subpopulations in Manitoba and Minnesota. However, note that some exchange occurs between birds in the Upper Peninsula of Michigan and the Ontario Lake of the Woods (see details of banding records above).
Every five years since 1991, there has been a formalized Piping Plover International Census. These censuses have provided population estimates for each subspecies and population, and at a state or provincial level (Haig et al. 2005). Results have demonstrated consistent increases in the United States Atlantic and United States Great Lakes, a slight decline in eastern Canada, and inconsistent trends in the United States Great Plains and Prairie Canada (Elliott-Smith et al. 2009). According to these surveys, the global population has increased from approximately 5,500 adults in 1991 to just over 8,000 in 2006. In the 2006 international census, detectability bias was accounted for by using the same observers for visits to a number of pre-selected sites twice during a two-week period to estimate detection error rates (the average detectability rate was 76%; United States Fish and Wildlife Service 2009).
Based on an adult annual survival estimate of 66 percent, population viability models (PVAs: models that use estimates from demographic parameters to predict future population size and growth and vulnerability to extinction) indicated that the probability of the Great Plains and Great Lakes Piping Plover populations persisting more than 80 to 100 years was low and that to stabilize these populations a 31 to 36 percent increase in reproductive output was needed (Ryan et al. 1993, Plissner and Haig 2000). In an attempt to resolve the disparity between model results and observed population growth since 1993, a more recent study (Roche 2007) conducted another PVA for the Great Lakes Piping Plover population. Although Roche’s models for the Great Lakes population also predicted decline and extirpation within 100 years, the models had low predictive accuracy. One explanation for low predictive accuracy is lower than expected juvenile survival, which may result from a small number of breeding pairs that go undetected each year at sites that are not monitored annually. The disparity between model predictions and observed population growth suggests that the Great Lakes population is larger and more widespread than documented. Thus, refined survival estimates may bring model results more in line with the observed population trends.
Great Lakes Population (United States and Ontario subpopulations)
Only 17 pairs of Piping Plovers nested in the United States' portion of the Great Lakes in 1981 (United States Fish and Wildlife Service 2003). No change occurred on the international census in 1991, with 17 pairs or 40 individual Piping Plovers counted (Haig and Plissner 1992). This number increased to approximately 125 individuals by 2005 (J. Stucker, pers. comm. to United States Fish and Wildlife Service 2009).
From 1986 to 2002, Piping Plovers nested in 12 counties in Michigan and two counties in Wisconsin. Since 2003, numbers have increased along the eastern shoreline of Lake Huron eastward into Ontario, and both west and south along the northern and eastern shorelines of Lake Michigan. This increase is attributed to nest protection, predator control, restricted access to off-road vehicles on public lands as well as to a period of lower than average water levels from 2003 to 2008 (United States Fish and Wildlife Service 2009). Important contributors to increased numbers of Piping Plovers in the United States Great Lakes population include psychological fencing and "Do Not Enter" signage at almost all nest territories as well as monitors assigned to all active nesting locations (F. Cuthbert, pers. comm. 2012). The role of water levels is equivocal. While low water levels may have created more beach habitat for Piping Plovers, this was true only in some regions of the Great Lakes (not Lake Superior), and in some areas the increase in exposed beach habitat was negated by vegetation encroachment (United States Fish and Wildlife Service 2009).
In 2008, the United States Great Lakes Piping Plover population was estimated at 59 pairs, with 53 pairs located in Michigan, and six pairs in Wisconsin. The latest estimates from field programs are 58 pairs in the Great Lakes population, of which 46 pairs are in Michigan, seven pairs in Wisconsin and five pairs in Ontario (V. Cavalieri, pers. comm. 2012, Elliott-Smith et al. in prep.). There has been sporadic breeding outside of Michigan and Wisconsin; a breeding pair nested for the first time on the Illinois side of Lake Michigan in 2009.
In the Canadian Great Lakes, the Piping Plover was officially considered extirpated as a breeding species in 1986; the last known nesting occurrence was at Long Point in 1977 (Lambert and Nol 1978, McCracken et al. 1981, Goossen et al. 2002). The decline in numbers and subsequent extirpation has been attributed to increased recreational use of beaches by humans (including disturbance, as well as direct mortality from vehicles and pedestrians) and mortality from an increasing number of predatory species (Cairns and McLaren 1980). Habitat loss and degradation were also likely factors, including residential and commercial development, as well as infrastructure such as roads, groynes, and physical removal of dune systems. Other factors that could have played a role were changes in the overall health of Great Lakes ecosystems (water levels, declines in ice-scouring and the health of the invertebrate communities on which Piping Plovers depend).
As a result of intensive management efforts by the United States Fish and Wildlife Service, organizational partners and volunteers in Michigan, Piping Plover numbers have increased substantially in that state. Without these intensive management efforts for the United States Great Lakes subpopulation (in Michigan), it is unlikely that Piping Plovers would be breeding in Ontario. Given the success of management to increase the population in Michigan, and increased sightings of non-breeding individuals in Ontario, it was expected that nesting would soon occur in Ontario (Heyens 2007). Banding information has indicated that many of the Ontario Piping Plovers originated in Michigan (United States Fish and Wildlife Service 2009). It is possible that the United States Great Lakes subpopulation had to reach a certain threshold before birds could expand into the Canadian Great Lakes.
In 2005, a pair of Piping Plovers returned to Wasaga Beach Provincial Park on Georgian Bay in Ontario (but failed to breed) and in 2006 a single bird was verified. This was followed in 2007 by a confirmed nesting (with three young) of a pair at Sauble Beach on the Bruce Peninsula in Ontario (Cartwright 2007, Toews et al. 2008). In 2008 Piping Plovers nested at three sites, including Sauble Beach (one pair), Wasaga Beach (two pairs) and another location – Oliphant Beach (one pair): three chicks fledged in total. Nesting occurred at another new location (Carter Bay on Manitoulin Island) in 2009, and Piping Plovers again nested at Sauble Beach and Wasaga Beach (total of seven pairs and 15 chicks hatched, 11 chicks fledged). In 2010, nesting occurred at both Sauble Beach (two pairs) and Wasaga Beach (four pairs, including a male that attempted to nest with two different females; two chicks fledged). Both Sauble and Wasaga Beach were occupied again in 2011 (five pairs in total; nine chicks fledged). For the 2012 breeding season, two pairs nested at Sauble Beach but nesting success was lower overall due to predation. Although each pair incubated and hatched four chicks each, six birds (two adults and their four chicks) disappeared from the north end of the beach (S. Jefferis, pers. comm. 2012). At the nest at the south end, three chicks were fledged and one chick was predated. The male bird also disappeared and may either have been predated or migrated. In addition, two transient males were observed during the season; one of these birds was from Carter Bay and had successfully nested there in 2009 (S. Jefferis, pers. comm. 2012).
Four nest sites were occupied at Wasaga Beach Provincial Park in 2012. There were three nests in Beach Area 1: the first nest produced four fledged chicks; the second nest produced two fledged chicks (the adult male died halfway through incubation, the female continued to incubate and three of the four eggs hatched. One of the chicks was predated three weeks later); and the third nest was unsuccessful (this was a re-nesting attempt by the first pair, but the male disappeared halfway through incubation and the female abandoned the nest). Piping Plovers also occupied a new nest site within the Wasaga Beach location (New Wasaga Beach east of the river mouth). However, the male disappeared one day prior to eggs hatching (cause unknown). Two of the four eggs hatched, but one chick was predated within 24 hours. The female and her chick were predated three weeks later by a Merlin (P. Davidson, pers. comm. 2012).
Individual birds were seen on Manitoulin Island but no known breeding took place in 2012.
Lake of the Woods subpopulation
The Lake of the Woods Piping Plover subpopulation in Minnesota and Ontario has been considered a remnant part of the Great Plains population of circumcinctus (Elliott-Smith and Haig 2004, Gratto-Trevor and Abbott 2011) and is isolated from the closest nesting Piping Plovers in Manitoba by 300 km. Although the relationship with the Great Lakes population is unknown (few Great Lakes birds have been recorded in Lake of the Woods), the relationship with the Great Plains population is under review (V. Cavalieri, pers. comm. 2012). In the Ontario Lake of the Woods subpopulation, the number of breeding pairs has been sporadic. At Windy Point, no birds were confirmed nesting from 1987 to 1991, or in 2001. However, single pairs nested (nests with more than one egg) in 1979, 1995 to 1997, 1999, 2000, 2009 and 2010. Two pairs nested in 1998, 2002, and 2007. It is not known whether Piping Plovers nested in 1938, 1974, 1978 or 1986. Note that these are conservative estimates of the numbers of breeding pairs. In many cases, between one and six adult Piping Plovers may have been observed on a single day either showing territorial behaviour or creating nest scrapes; however, no nest was found (L. Heyens, pers. comm. 2012).
The last successful nesting at Windy Point was in 2009 when one chick was reported to have fledged. While nesting was initiated in 2010 and again in 2011, these nests were not successful. No successful nesting was recorded in 2012 on the Ontario side of Lake of the Woods (L. Heyens, pers. comm. 2012).
At Sable Islands Provincial Nature Reserve, no confirmed nesting took place between 1992 and 2006. Single pairs nested in 1979, 1986 to 1988, 1990, 1991 and 2007, and two pairs in 1989. No birds were confirmed nesting between 1992 and 2006. It is unknown whether birds nested at this location in 1938, 1974 and 1978. The last (unsuccessful) nesting attempt at Sable Islands Provincial Nature Reserve was in 2007.
Numbers in the United States' side of Lake of the Woods in Minnesota reached a peak of 47 to 50 adults in 1984 and have declined steadily to only two adults in 2010 (Figure 4; Elliott-Smith et al. in prep.). In 2012, two chicks were reported to have fledged from the Minnesota side of the lake on Pine and Curry Islands (L. Heyens, pers. comm. 2012).
Figure 4. Number of Piping Plovers in Lake of the Woods from 1974-2011
Upper line (magenta) represents the Minnesota Lake of the Woods subpopulation and lower line (dark blue) indicates the Ontario Lake of the Woods subpopulation; based on data from L. Heyens, pers. comm. 2012.
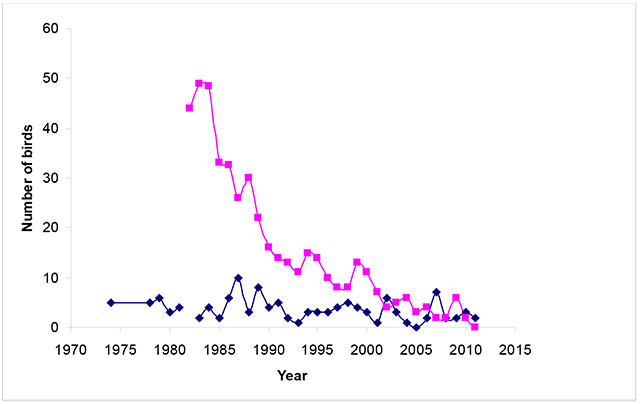
Enlarge Figure 4. Number of Piping Plovers in Lake of the Woods from 1974-2011
Winter distribution
Piping Plovers winter along the South Atlantic, Gulf Coast, and Caribbean beaches and barrier islands, mainly on intertidal beaches with sand and/or mud flats with no or very sparse vegetation (Elliott-Smith and Haig 2004, United States Fish and Wildlife Service 2009).
1.4 Habitat needs
In Ontario, Piping Plover nesting habitat occurs in complex and dynamic beach-dune ecosystems that are maintained by coastal, climate-related, processes such as storm events, water and wave action, ice-scouring and wind. Freshwater dune complexes are unique to the Great Lakes and harbour many endemic rare and provincially tracked species, such as the Beach Dune Tiger Beetle (Cicindela hirticollis - Wasaga Beach), Great Lakes Wheatgrass (Elymus lanceolatus var. psammophilus – western Bruce Peninsula and southern Manitoulin Island), Pitcher’s Thistle (Cirsium pitcheri – southern Bruce Peninsula and Manitoulin Island), Gillman’s Goldenrod (Solidago simplex var. gillmanii - Carter Bay) and Eastern Prickly Pear Cactus (Opuntia humifusa - Lake Erie dune spits; Jalava 2004, Dougan and Associates and McKay 2006).
Despite large inland lakes, rivers and reservoirs being available in the Great Lakes, Piping Plovers do not currently breed in these habitats nor did they historically, though they do so in the Great Plains. It has been suggested that this may be because more complex dune systems provide cover from human disturbance (Flemming et al. 1988, Burger 1994, Loegering and Fraser 1995). However, Piping Plovers probably nested in dune ecosystems prior to human disturbance and so their use of beach-dune systems may be more related to food availability, cover and microhabitat for camouflage from predators. Moreover, outside of the Great Lakes, few or no suitable natural beaches (e.g., one kilometre or more in length) exist inland because of reduced wave action (fetch) and lack of sediment supply in some areas (mostly on the Canadian Shield), and are dominated by glacial erosion rather than deposition (R. Davidson-Arnott, pers. comm. 2012).
Dune ecosystems are dynamic and depend on beach erosion for their sand supply (Saunders and Davidson-Arnott 1990, Reed et al. 2009, Davidson-Arnott 2010). They grow towards the waterline in low water years, with the reverse trend in high water years. Storms and ice-scouring determine how close dunes occur to the shoreline (see later section on threats for more discussion of storms and ice-scouring). In some areas, the lack of major storm events, especially at times of high seasonal water levels, combined with low lake levels over the previous few years have led to vegetation encroachment (R. Davidson-Arnott, pers. comm. 2012), which has reduced available nesting habitat for Piping Plovers (United States Fish and Wildlife Service 2009).
Specific natural features are required by Piping Plovers for nesting, brood-rearing, foraging, staging, migrating and wintering. Even though there is considerable geographical variation in the specific habitat types occupied, there are some similarities in the general habitat features preferred by the species. Typically, Piping Plovers occur on beaches more than 10 m wide, with a shoreline of more than 0.4 km, and patches of gravel or sand/gravel and sandbars. It has been suggested that 400 m to one kilometre of shoreline may be used for nesting, brood-rearing and foraging depending on beach size (Lambert and Risley 1989, J. Robinson, pers. comm. to Environment Canada 2011). In the northern lower peninsula of Michigan, Piping Plovers used an average linear beach distance of 475 ± 53 m (± standard error; range 130-1,435 m; Haffner 2005, Haffner et al. 2009).
No information is available on home range size in the Northern Great Plains population. However, in the Great Lakes population on the northern lower peninsula of Michigan, Piping Plovers that fledged at least one chick had home ranges of 0.4 to 11.2 ha (mean 2.9 ± SE 0.5 ha; Haffner et al. 2009).
Nesting habitat
Piping Plovers prefer to nest on flat, wide stretches of sand, cobble or alkaline substrate with sparse vegetation (Elliot-Smith and Haig 2004). They occur on beaches separated by at least 50 m between the high water mark and the tree line (Environment Canada 2006; see also Cohen et al. 2008). Sections of shoreline that have the widest strip of beach below the high watermark are often chosen for nesting – usually this area is between the shore and crest or peak of the vegetated dune (Environment Canada 2011). The distance to the tree line is related to the risk of predation since woody cover provides concealment for predators.
Preferred nesting substrates are sand, gravel and pebble because these provide camouflage for incubating adults, nests and young and conceal them from predators (Whyte 1985, Boyne 2001, United States Fish and Wildlife Service 2003). Nests have been found in open areas or near grass, stones, cottonwoods or willow saplings (Peck and James 1983); sparsely vegetated areas and cobble are important features. Other features, such as driftwood and other woody material, also provide concealment and shelter. Piping Plovers avoid areas of dense vegetation such as shrub or tree cover and thus prefer dunes in early stages of succession maintained by disturbance (in New York state, nest sites had less than 10% vegetation and nesting beaches less than 47% vegetation; Cohen et al. 2008).
Brood-rearing habitat
Habitat used by broods is similar to that used for nesting and foraging and includes sparsely vegetated areas that provide cover to shelter from predators or inclement weather. Piping Plovers often feed on aquatic, benthic and terrestrial invertebrates (Cuthbert et al. 1999), within five metres of the lakeshore edge and including the high watermark. However, adults not caring for young or have young that can fly will forage outside the territory/nesting area. Most nesting areas tend to be close to a seep or river, and in some cases marshes (McCracken et al. 1981, Elliott-Smith and Haig 2004), features that are also important for foraging. There was a positive relationship between home range size and human disturbance; adult Piping Plovers showed greater movement, and thus had larger home ranges where there was increased human disturbance (Haffner 2005, Haffner et al. 2009).
Great Lakes subpopulation
In the Great Lakes, nesting occurs along sandy shorelines separated from adjacent forest by wide expanses of dune habitat (Wemmer 2000, Price 2002). Sauble Beach is a 10-kilometre long, wide sandy beach that occurs between two headlands, Chief’s Point to the north, and Frenchman’s Point to the south (Peach 2004). Dunes at the north end of the beach at Sauble Beach are substantially wider, have higher floristic diversity and lower human impact than those to the south, which are narrow, have lower plant diversity and high anthropogenic influence (Peach 2004). Some dunes at Sauble Beach may be relic (Peach 2004), but there is extensive Aeolian (wind) transport of sand as well as large waves from the northwest through to the southwest, which wash sand onshore and could create new dunes (R. Davidson-Arnott pers. comm. 2012). There is a relic dune field inland (especially further north towards Oliphant and Red Bay); a relative lake level fall is occurring in this area due to the ongoing isostatic shift (part of the earth’s crust emerging or submerging). Further to the north this had led to reduced sand availability and thus narrow and shorter beaches and smaller dunes (R. Davidson-Arnott, pers. comm. 2012).
At Wasaga Beach Provincial Park, the 14 km of shoreline include diverse and dynamic habitats, such as wet-sedge meadows, wide-open sand beach and dune complexes. Over the past four years (2008-2012), Piping Plovers have mainly used the area in Beach Section 1 (two nests), which has the highest level of human disturbance within the park (P. Davidson, pers. comm. 2012). The widest of eight beaches within the park (83 m from the boardwalk to the water’s edge), this beach is flat with sparse vegetation, a section of cobble-stratified sand, and a high drift line. A third nesting area is at the point where the beach is approximately 18 m wide, flanked by heavily vegetated dunes (P. Davidson, pers. comm. 2012).
The coastline at Oliphant is comprised of a headland-bay complex as well as the Fishing Islands offshore. These islands, as well as rock reefs, protect rocky bays from wave action and have allowed the development of coastal meadow marshes and narrow sandy gravel beaches. Dune grassland ridges occur along the shoreline and are only replenished during low lake levels when the sand substrate of lakes is exposed and wind erosion transports sand inland. The beaches are gently sloping (gradient <0.5%), which means they are vulnerable to changing lake levels (Peach and Donnelly 2010).
Carter Bay, located at the south end of Manitoulin Island, is a long, sandy beach with fine sand and limestone outcropping and dune systems. Extensive development is slated to occur in this area (see Threats - Habitat loss and degradation).
Lake of the Woods subpopulation
In the interior Great Plains population, Piping Plovers nest on sand/gravel beaches of permanent to semi-permanent alkaline lakes and wetlands, freshwater lakes, reservoirs and sometimes river shorelines and sandbars (Boyne 2001). However, in the Lake of Woods subpopulation in Ontario nesting habitat is somewhat different and more similar to that used by the Great Lakes subpopulation. In the past, Piping Plovers nested in the Sable Islands Provincial Nature Reserve in Lake of the Woods and breeding was attempted there again in 2007. These islands consist of a six-kilometre-long narrow barrier island; at high watermark, parts of the island are submerged, forming two or three islands. Vegetation is composed of a large sand dune with scattered trees and shrubs in the elevated sites and some marshes on the shoreward side. Wide, sandy beaches surround the entire island. Windy Point was previously a narrow peninsula extending two kilometres from the mainland, but was breached by storms in the mid- 1990s and is now an island three kilometres northeast of the Sable Islands Provincial Nature Reserve. The maximum width of the point is 200 m, tree and shrub growth is sparse and limited to elevated sites, and wide sandy shores surround the peninsula. There is a marsh at the base of the peninsula. Available nesting areas for Piping Plovers in Lake of the Woods are dependent on suitable low water levels that fluctuate from year to year (L. Heyens, pers. comm. 2012).
Winter habitat
Piping Plovers stage on natal lakes and use stopover sites during migration between the Great Lakes and their major wintering areas in the southeastern United States and Gulf of Mexico (F. Cuthbert, pers. comm. 2012). During winter various habitats are used, including beaches, dunes, mudflats, sand flats and algal flats (United States Fish and Wildlife Service 2003, Elliott-Smith and Haig 2004).
1.5 Limiting factors
Limiting factors are intrinsic biological attributes that limit species' populations. For Piping Plovers, the main factor limiting the population is the availability of suitable beach breeding habitat. However, in the Great Lakes numbers of Piping Plovers may be currently below the threshold at which beach habitat is limiting. Moreover, small population size, relatively low reproductive output, and high first-year mortality make Piping Plovers in the Great Lakes and Lake of the Woods subpopulations vulnerable to stochastic events that affect overall population size and trends.
1.6 Threats to survival and recovery
The main threats to the Piping Plover are predation, human disturbance and habitat degradation and loss (Environment Canada 2006). The relative importance of these threats varies by subpopulation. For example, for Great Lakes breeding pairs recently re-established in Ontario, newly identified threats relate to predation, specific human disturbance factors and certain natural weather events. In the Ontario Lake of the Woods subpopulation, high water levels and storm events seem to be the most important factors, and predation may be a secondary threat. Most major threats (habitat degradation, predation, and human disturbance) are persistent and pervasive within the Great Lakes basin and present many challenges (United States Fish and Wildlife Service 2009, LeDee et al. 2010b).
Predation
Many avian and mammalian predator populations have expanded as a result of human alteration of landscapes. While predation is a normal ecological process, predator populations that are augmented by humans can represent a threat to species at risk. These 'subsidized' predators are often attracted to recreational areas, such as beaches, because of the direct and indirect sources of food found there. Among these food sources are direct feeding of wildlife (e.g., gulls) by people as well as indirect sources such as garbage or discarded food. Predator attractants are usually higher at sites with high human use. As a result of being attracted to beaches, these predators may also prey upon Piping Plover eggs, nestlings or adults.
Other predator species not subsidized include the Merlin – a small bird-eating falcon that is increasing in the shoreline regions of the Great Lakes – and in some areas, Peregrine Falcons (Elliott-Smith and Haig 2004). According to the North American Breeding Bird Survey (NABBS), Merlin populations increased significantly in the Lower Great Lakes/St. Lawrence region at a rate of 2.5 percent per annum between 1966 and 2010 (Sauer et al. 2011). Furthermore, Breeding Bird Atlases showed that in Ontario Merlin populations doubled in the 20-year period between the first and second atlas (Gahbauer 2007); similar increases and range expansion have occurred in Michigan where the species increased by 220 percent between the first and second atlas (Haas 2011).
In some areas (e.g., inner cities), Merlins have adapted to urban environments, partly as a result of the passerine populations found there, which are often augmented by humans. Their adaptability to urban habitats has contributed directly to expanding populations in some areas (Warkentin et al. 2005). However, there is no direct evidence that numbers of Merlins in the vicinity of beaches frequented by Piping Plovers have been influenced by humans, and their behaviour may be seen as opportunistic. Merlins are an important predator of Piping Plovers in Michigan and in Ontario (Roche et al. 2010a). Individual Merlins can specialize on certain prey species or groups of species (e.g., shorebirds), although often prey are taken in proportion to their abundance (Warkentin et al. 2005).
The extent of predation on Piping Plovers by different species may vary depending on the stage of Piping Plover breeding. For example, late-nesting birds may be more vulnerable to nest predation by flocking gulls, the numbers of which build up in late July or August. Gulls are a major subsidized predator and are a concern for Piping Plover conservation. Predation of eggs by most mammal species can be prevented by the use of exclosures. However, except when Piping Plovers are incubating, exclosures are not effective in preventing gull predation, which is mostly on chicks outside the exclosures (L. Heyens, pers. comm. 2012, F. Cuthbert, pers. comm. 2012). It is also important to point out that although certain types of exclosures have increased reproductive success in some jurisdictions, they may also increase adult Piping Plover mortality and lead to nest abandonment (Murphy et al. 2003, Neuman et al. 2004, Barber et al. 2010). Managers using exclosures should be knowledgeable about the literature on exclosures and alert to possible problems.
Predator species may differentially prey on eggs, nestlings and adults. For example, most mammalian predators are likely to take eggs and chicks and less likely to prey on adults unless they are on the nest. Crows and gulls are most likely to prey on eggs and chicks. Small raptors such as Merlin prey on chicks and adults. Predator species also vary geographically or even on a site-by-site basis, depending on local predator populations and the predator community.
Great Lakes subpopulation
At beaches in the Ontario Great Lakes, confirmed predators of Piping Plover include Red Foxes, Raccoons, American Crows, Merlin, and gulls – both Ring-billed and Herring Gulls. Other possible predators include Striped Skunk, Coyote and Great Horned Owls (Powell and Cuthbert 1992). In addition, domestic pets (dogs and cats) often disturb birds and nests and have killed adult and juvenile Piping Plovers in Michigan (F. Cuthbert, pers. comm. 2012).
Lake of the Woods subpopulation
In the Lake of the Woods, the primary predation threats come from loafing gulls (L. Heyens, pers. comm. 2012). These include Ring-billed Gulls and Herring Gulls but also Franklin’s Gull (Leucophaeus pipixcan), which has a more westerly distribution and does not breed in the eastern Great Lakes. While evidence of predation on chicks by gulls is circumstantial, it is convincing (L. Heyens, pers. comm. 2012). After gulls, the American Crow is thought to be the most important predator. Some predation by the Merlin may also occur. Dogs may also be a threat to Piping Plovers at Lake of the Woods though probably less so than in the Great Lakes subpopulation.
Human use of beaches in this area is much less than in the eastern Great Lakes, so fewer subsidized predators are attracted to the beaches. Moreover, because Windy Point and Sable Islands Provincial Nature Reserve are islands, mammalian predators, such as Raccoon and Red Fox, are much less of a threat (L. Heyens, pers. comm. 2012).
Human disturbance
Great Lakes subpopulation
The primary form of human disturbance is recreational use of beaches. Many human activities on beaches can directly impact nesting Piping Plovers (Burger 1994). It is important to point out that Piping Plovers arrive on their breeding areas early in April and begin nest initiation in mid-May, which is prior to large crowds gathering on beaches. Nevertheless, even subtle forms of human disturbance (e.g., recreational users walking or sitting on a beach) can impact Piping Plover courtship, territory selection and nest initiation or foraging in a given area. The result may be that Piping Plovers leave the area or abandon a nest attempt (S. Robinson, pers. comm. 2012).
Later in the summer large crowds of people gather on beaches, and this crowding, combined with various activities, can disrupt nesting and foraging of Piping Plovers at a time when they are already heavily invested in reproductive activities at a specific site. Crowds of people can force Piping Plovers to shift foraging areas or alter their brood- rearing habitat use. In a comparison of adult home range size on beaches with different intensities of human use in Michigan, Haffner et al. (2009) found that on beaches with low human use, Piping Plovers had the smallest home ranges and suggested that larger protected areas may be required on beaches with high levels of disturbance.
Conversely, in some situations it is possible that chicks could be forced to forage in a very small area (e.g., a promontory of land extending into the lake), resulting in their being malnourished and subsequently reducing their chances of survival.
A plethora of human recreational activities are focused on beaches used by nesting Piping Plovers. Pedestrians walking on the beach can disturb adult Piping Plovers and their young. People often walk with dogs on beaches and let their dogs off the leash. Dogs will often intentionally chase adult Piping Plovers and can cause mortality to chicks. Dogs can also unintentionally trample on the nests or chicks. Alternatively, chicks run from their parents and/or siblings and, isolated from shelter, are exposed to predation or become vulnerable to storm events or collisions with off-road vehicles. The latter include off-road motorized and non-motorized vehicles such as trucks, all terrain vehicles (ATVs), dirt bikes and mountain bikes, all of which also disturb adult Piping Plovers and their chicks. Direct, inadvertent damage is done when Piping Plover eggs and nests (which are highly cryptic) are destroyed (Environment Canada 2006). Setting off fireworks in the vicinity of nests is also highly detrimental to the birds and can disrupt their nesting activities (J. Benvenuti, pers. comm. 2012).
It has been suggested that flying kites or kite-boarding can cause disturbance to Piping Plovers and frighten them off nests or interfere with foraging; kites can mimic avian predators, and Piping Plovers respond by freezing, thus reducing the amount of time they spend feeding (Environment Canada 2006). However, compared to other threats this threat may be of less significance (J. Benvenuti, pers. comm. 2012).
Lake of the Woods subpopulation
Human disturbance probably has much less of an effect on Piping Plovers in the Lake of the Woods subpopulation than in the Great Lakes subpopulation. This is partly related to access; some of the nesting sites are now located on islands (such as Windy Point) and so are largely inaccessible. Human densities and use of beaches are also much lower in Lake of the Woods and so crowds of people are less of a threat to Piping Plovers. However, human disturbance can be subtle and even individuals walking their dogs may have an impact on nesting Piping Plovers (see above for Great Lakes subpopulation). The ESA Shorebird Nesting Area signs appear to be effective at discouraging people and their dogs from entering nesting or brood-rearing locations (L. Heyens, pers. comm. 2012).
Habitat loss and degradation
Local habitat change – Great Lakes subpopulation
Many human activities contribute directly to loss and degradation of habitat for Piping Plovers. Increased access to transportation and an expanding tourism industry have fueled coastal development; according to Alig et al. (2004), p. 230, coastlines are predicted to accommodate 'ever-increasing residential, commercial and industrial use". Increasingly larger and heavier machinery have made large-scale construction activities much more feasible than they were earlier in this century (Nordstrom 2000). These activities include the construction of jetties or armoured features (piers and rip rap) to stabilize shorelines (Melvin et al. 1991, United States Fish and Wildlife Service 1996, 2003, Environment Canada 2006). For example, during high water levels in 1985 to 1986, millions of dollars were spent protecting coastal waterfronts and municipal properties along the Lake Huron shoreline (Peach 2004). Such activities interfere with natural ecological processes such as the interchange of sand between open beach and beach-dune communities.
Other forms of coastal development include construction of cottages and other types of residential or commercial buildings. For example, land sales and the potential for future waterfront development exist in the general area of Carter Bay (McCutcheon 2012), which could (indirectly) impact Piping Plover nesting habitat.
Food sources and shelter for the Piping Plover are affected by activities that remove vegetation and natural materials. Vegetation in Great Lakes dune ecosystems (e.g., American Beachgrass (Ammophila breviligulata)) plays a critical role in stabilization of sand and is thus a component of the dynamic interchange of sand between dunes and open-beach (Nickling and Davidson-Arnott 1990, Peach et al. 2007, Reed et al. 2009). Such vegetation may also include provincially-tracked species like Long-leaved Reed Grass (Calamovilfa longifolia), which plays an important role in dune stabilization at Sauble Beach (Peach et al. 2007). Trampling of dunes causes erosion and dune blow- outs and leads to loss of sand from beach-dune systems. Furthermore, any activities that compact sand or change its moisture content can make sand more vulnerable to translocation by wind (Peach et al. 2007).
Many recreational beaches are raked and groomed for human recreational use. On many coastlines, grooming has decreased wrack cover and native plant species abundance richness, and increased the unvegetated dry sand zone (Dugan and Hubbard 2010). Beach grooming can also reduce the numbers of birds using beaches (Defeo et al. 2009). Raking and grooming can interfere with nesting or foraging Piping Plovers in at least four ways. First, raking is done specifically to remove wrack (strand lines) from beaches. Wrack supports invertebrate communities and the biomass of these communities has been shown to be positively related to shorebird numbers (Tarr and Tarr 1987, Dugan et al. 2000, Hubbard and Dugan 2003, Dugan et al. 2003). It also provides nutrients for plant species such as the Long-leaved Reed Grass (Peach 2004). Wrack and other natural materials are used by Piping Plovers for foraging, as well as refuge from predators and storms and for resting and brooding chicks. Second, raking activities can directly affect reproductive success of Piping Plovers, since heavy machinery is often used to rake the beach. For example, nests or eggs could be directly covered over or destroyed and adult birds could be disturbed during this process. In New Jersey, raking has caused mortality of Piping Plover chicks (C. Davis, pers. comm. 2012). Third, natural debris/material contributes to beach stabilization and removing it could lead to sand blow-outs and destabilization of beaches and dune systems (Reed et al. 2009). Raking allows moist sand to be loosened and more easily dislodged by wind (Peach 2004). Fourth, natural debris/material provides cover for Piping Plover chicks and removing it could expose them to predation or weather events such as storms. Finally, raking interferes with the relationship between lake levels and dune development; during low lake levels pioneer plants (e.g., American Beachgrass) colonize the dune margin and upper beach through underground rhizomes, but raking destroys this dune-building process (Saunders and Davidson-Arnott 1990, Peach 2006).
Another activity that could disturb Piping Plovers is beach nourishment (United States Fish and Wildlife Service 2009). On some beaches sand is shipped in and used to augment or improve the beach aesthetically for human use. As with raking, deposition of sand beach nourishment could (at certain times of the year) cover or destroy nests, or change site characteristics by burying debris or transporting invasive species.
Use of off-road motorized and non-motorized vehicles such as trucks, all-terrain vehicles (ATVs), dirt bikes and mountain bikes damages vegetation and compacts and disturbs natural materials, which affects Piping Plovers indirectly. Similarly, though less invasive, horse-back riding can cause similar effects through trampling.
Although Piping Plovers are negatively affected by the anthropogenic disturbance factors listed previously, they depend on natural disturbances to create and maintain beaches in early stage of succession. Piping Plovers tend to select areas with sparse vegetation but with natural materials and they avoid dense woody plant growth (see Habitat Needs). Normally, natural processes (ice-scouring, wind erosion, storm water erosion) would ensure that beaches remain relatively free of vegetation. However, in some cases, declines in the incidence of natural events (such as storms or ice-scouring; see Wang et al. 2012), combined with increased human use of beaches have reduced the habitat available for nesting Piping Plovers. This has also increased the prevalence of invasive plant species (including some woody species).
Local habitat change - Lake of the Woods subpopulation
There are fewer impacts of coastal development for this subpopulation than the Great Lakes subpopulation. However, there is a trend along Canadian Lake of the Woods shorelines for seasonal cottages to be replaced by permanent homes, presenting more challenges and opportunities for Piping Plover conservation (L. Heyens, Ontario Ministry of Natural Resources, pers. comm. 2012). Establishment of permanent homes increases human impact, including year-round presence, and increased inputs of pollutants from septic systems into water sources. On the other hand, long-term stability of ownership may facilitate the efficacy of communication and education about protection and conservation of Piping Plovers and their habitat in the long-term. In the United States, turnover in summer rentals has increased the challenge of communicating Piping Plover conservation issues (F. Cuthbert, pers. comm. 2012).
Watershed level habitat change
At the landscape level, a wide variety of human activities can affect Piping Plover habitat. Because coastal ecosystems of the Great Lakes occur at the interface of land and water, they are influenced by both terrestrial (landward) and offshore processes (Morrice et al. 2008). Changes in watershed management can result in habitat loss and degradation in coastal ecosystems. For example, loss of permanent cover higher in watersheds can lead to sedimentation in coastal systems, as well as to transport of pollutants. Several studies demonstrate a link between agricultural, urban land and atmospheric pollutant loads and water quality in the Great Lakes (Crosbie and Chow- Fraser 1999, Uzarski et al. 2004, Morrice et al. 2009, Allan et al. 2013). These pollutants could enter the food chain in beaches adjacent to lake water, as well as coastal seeps and watercourses and thus expose Piping Plovers as they forage in these habitats. It may not be fortuitous that indicators of coastal Great Lakes ecosystem health (invertebrate communities in coastal wetlands) are most healthy (the highest index of biotic integrity) in northern Lake Michigan and northern Lake Huron (Uzarski et al. 2004), where Piping Plovers are nesting. However, note that according to Allan et al. (2013) cumulative stressors are still high in some of these areas. Piping Plovers have not yet returned to nest on Lake Erie and Lake Ontario, where the ecosystem health index is lower. Invertebrate communities along these Great Lakes shorelines have been detrimentally impacted by physical alteration of shorelines and eutrophication, as well as resulting changes in plant communities, including expansion of invasive plant species.
More broadly, water levels in the Great Lakes are impacted by a wide variety of factors and, historically, had high variability due to variation in precipitation, evaporation from surface water, inflow from upstream and outflow to downstream lakes. Moreover, the magnitude of seasonal and long-term fluctuations vary by lake and there is differential isostatic uplift on different Great Lakes, meaning that some areas are emerging while others are submerging with subsequent variation in the characteristics of beach-dune systems (R. Davidson-Arnott, pers. comm. 2012). Generally there has been a decline in the variability of Great Lakes water levels over the period 1919 to 2007 (Environment Canada and United States Environmental Protection Agency 2009). Water levels are regulated directly in both Lake Superior (since 1918) and Lake Ontario (since c.1960) and ultimately variability in water levels has declined. Other factors also affect water levels, including control structures, dredging, dams, canals and diversions (Wilcox et al. 2007).
Since about 1999, low water levels have occurred in the Great Lakes (Wilcox et al. 2007); these have had both positive and negative effects on habitat for Piping Plovers. While more open beach habitat has been created, and even new dune systems in some areas, there has also been substantial vegetation encroachment, both by woody and invasive species.
Water levels in the Lake of the Woods are regulated by the Lake of the Woods Control Board, which influences habitat availability for Piping Plovers.
Ice-scouring, storms and other natural events
Because Piping Plovers nest in open, exposed and unstable habitats at the juncture of land and water, they are vulnerable to both natural and unnatural events that lead to changes in hydrology. At the same time, coastal (geomorphic) processes (e.g., fluctuating water levels, stormwater events or ice-scouring) are essential for maintaining Piping Plover habitat.
Breeding success has been negatively affected in Ontario by natural events such as storms, flooding and heavy wind, which can cover nests with snow, water or sand. Nest failure has been documented in both the Great Lakes and Lake of the Woods subpopulations following such events. Storms and weather events are believed to pose the greatest threat to the Lake of the Woods subpopulation. For example, during the 1980s, high water levels in the Lake of the Woods flooded many nests, and led to widespread breeding failure (Wiens 1986, Haig and Oring 1987, Wiens and Cuthbert 1988, Maxson and Haws 2000).
Habitat loss in wintering areas
During winter, Piping Plovers show high site fidelity to specific stretches of beach. Their relatively small home ranges increase their susceptibility to activities such as dredging, stabilization and alteration of shorelines and beaches (Stucker and Cuthbert 2006). Because survival of first-year birds is a critical factor limiting population growth, winter habitat quality is believed to have major implications for the recovery of the listed populations.
Climate change
The role of global climate change and its effects on lake levels in the Great Lakes is presently unknown. Although marine water levels are predicted to rise with increasing temperatures and cause widescale losses of shorebird habitat (Galbraith et al. 2002), including habitat for Piping Plovers (United States Fish and Wildlife Service 2003), in the Great Lakes it is possible that global warming will cause lake levels to further recede (Lofgren et al. 2002, 2011). Climate change has been implicated as a factor in the decline of ice cover in the Great Lakes, but the main controlling factors appear to be the North Atlantic Oscillation and El Niño Oscillation (Wang et al. 2012). Another effect of climate change on Piping Plovers in the Great Lakes comes from changes in the severity and frequency of summer storms. While storms are natural processes, their frequency and magnitude could be influenced by anthropogenically-influenced climate change; moreover, when populations are reduced to small numbers as a result of human actions, natural events can pose threats to their continued existence. In contrast, storms can also create the beach habitat used by Piping Plovers.
Ice-scouring is an important ecological process that creates habitat for Piping Plovers by disturbing shorelines. Over the period of 1973 to 2010 there were significant declines in lake ice cover, which are attributed to the Arctic Oscillation (also known as the North Atlantic Oscillation, NAO) and the El Niño Oscillation (La Niña, ENSO), as well as to global climate change (Wang et al. 2012). Ice cover has declined by 71 percent overall with the greatest declines being recorded in Lake Ontario, and the least in Lake Superior. The lack of ice-scouring in recent years, combined with low water levels in the Great Lakes, has exacerbated vegetation encroachment along shorelines and reduced Piping Plover habitat (F. Cuthbert, pers. comm. 2012).
Disease and pollution
Other potential threats are Type-E Botulism, West Nile Virus and pollution (such as oil, or chemicals like PCBs (polychlorinated biphenyls) in the Great Lakes; United States Fish and Wildlife Service 2009). Piping Plovers can contract botulism by consuming maggots or beetles that are feeding on carcasses of infected waterfowl or other bird species (United States Fish and Wildlife Service 2009). However, whether Piping Plovers are directly affected is questionable and is an area for further research.
Energy development
In some areas and at certain times of year there is potential for wind turbines to have a negative effect on Piping Plovers (United States Fish and Wildlife Service 2009, United States Fish and Wildlife Service 2012). Wind turbines have caused mortality in some other shorebird species in Ontario such as the Killdeer, Upland Sandpiper (Bartramia longicauda) and Wilson’s Snipe (Gallinago delicata) between May and October (Environment Canada et al. 2011). To assess the magnitude of the risk posed by wind turbines more information is needed on Piping Plover movements and behavior as well as potential effects of wind turbine location, height and density. In the United States, some wind projects are located on barrier beaches, bay shorelines or in bays and thus Piping Plovers may be susceptible during the breeding season (depending on their flight routes and flight altitude to and from foraging and roosting areas, as well as behavioral avoidance under different conditions; United States Fish and Wildlife Service 2012). Rising sea levels may cause some inland wind farms to become closer to intertidal habitats used by Piping Plovers. Collisions with wind turbines could also occur during spring or fall migration (Burger et al. 2011). Windfarms may also be located offshore.
In the Atlantic region, Piping Plovers migrate mainly along the coast, so they would be at low risk from turbines located far from land (Burger et al. 2011). Windfarm projects occur in several areas of the Great Lakes and along known major migration routes, such as Ontario’s Essex County in the vicinity of Point Pelee National Park of Canada (Kirk 2007).
1.7 Knowledge gaps
There are numerous knowledge gaps that need to be filled to ensure that recovery objectives for the Piping Plover are met continent-wide. Some of these knowledge gaps can only be answered at the international level (including the breeding, migration and wintering areas) or population level (Great Lakes or Great Plains) rather than just within Ontario. Broad-scale questions that need to be addressed include the following:
- the impact of long-term and short-term habitat management on reproductive success of Piping Plovers in Ontario;
- the effect of habitat quality in relation to reproductive success – local and landscape level habitat analysis (including the role of beach dynamics in providing habitat) and its relationship to Piping Plover productivity;
- the role of various predator species as potential threats to Piping Plovers;
- the structure of invertebrate communities at nesting beaches and how this influences foraging and reproductive success;
- the effects of human recreation or other factors on behaviour, foraging development of chicks and juvenile survival;
- the relationship between climatic variability in recent decades (and influence on water levels) and effects on reproductive success and a related factor – the effect of changes in lake water levels on the distribution of Piping Plovers and influence on census results;
- movements of adults and young among breeding sites and breeding and wintering areas (information-sharing among jurisdictions);
- survival and mortality of dispersing young and adults dispersing from nest sites; and
- wintering ground locations, detectability, and threats for Ontario Piping Plovers.
1.8 Recovery actions completed or underway
Management
The success of Piping Plover recovery in the United States Great Lakes subpopulation is largely attributable to the implementation of intensive management. This management has been implemented by a network of dedicated government officials, and conservation partners (e.g., university contributors and private partners), including many volunteers.
In Ontario, management efforts have included:
- beach monitoring to reduce impacts on nesting Piping Plovers from recreational human disturbance through education;
- nest protection devices and symbolic fencing to protect nests and chicks from predation and human disturbance (i.e., recreational activities and domestic animals); this includes signage advising human beach users to be cautious as they move through an occupied site, when young Piping Plovers have fledged and are using the open beach. These signs are moved in response to fledgling movements;
- nest translocation
footnote 1 as a last resort in response to an immediate, immitigable threat of storm surges and flooding, either natural or from human-made water control structures; and - water management (including controlling spring inflows on managed rivers to reduce egg, chick and habitat losses).
Education and communication
Working together with the local community in the vicinity of nesting Piping Plovers is critical. As part of volunteer monitoring programs, extensive outreach and communication with the public has occurred, both informally during beach monitoring but also through an active outreach campaign with municipalities and businesses. While nesting of Piping Plovers in busy recreational beaches such as Wasaga Beach and Sauble Beach presents many challenges and risks to nesting Piping Plovers, it also provides huge opportunities for conservation education with a broad spectrum of the public. This outreach and awareness has been achieved through:
- signage, leaflets and verbal communication, media events, web sites, appreciation events and open houses; and
- engagement of business and the community in Piping Plover conservation. For example, placing posters about Piping Plover conservation at local businesses (e.g., Home Hardware) at Wasaga Beach has instilled pride and interest in the local community about the day-to-day activities of nesting plovers.
2.0 Recovery
2.1 Recovery goal
In this strategy, the overall recovery goal is to protect Piping Plovers at nesting locations, encourage the expansion of the current breeding population in Ontario, and ensure its persistence as part of the Great Lakes and Great Plains subpopulations. Quantitative targets cannot be set at this time because they could only be based on historical estimates that are approximate.
A population and distribution objective (including recovery targets) should be set within the next three years based on: (1) the suitability of available (including historical) sites in Ontario determined from a habitat-suitability model developed by the United States Fish and Wildlife Service, (2) the area requirements of Piping Plovers and (3) additional information on populations based on habitat modeling for the Great Lakes population in the United States and Canada.
Based on estimates from the International Piping Plover Census and other estimates, recovery targets have been set for the different subspecies and populations/ subpopulations by other jurisdictions (United States Fish and Wildlife Service 2003, 2009, Environment Canada 2006, 2011).
2.2 Protection and recovery objectives
Table 1. Protection and recovery objectives
| No. | Protection or Recovery Objective |
|---|---|
| 1 | Protect all nesting pairs and their habitat at existing sites: implement actions to address threats to territory establishment and/or nesting at occupied sites within Ontario. |
| 2 | Plan for the potential of greater numbers: identify potential nesting sites and establish Ontario population targets. |
| 3 | Promote conservation and stewardship of beach and dune ecosystems, including their overall biodiversity and associated species at risk in Ontario. |
| 4 | Increase knowledge of Piping Plover demography/population dynamics, habitat requirements and threats. |
| 5 | Foster stewardship and public outreach/education about Piping Plovers at occupied sites as well as communication within the province. |
| 6 | Continue to coordinate/share information in existing databases for the Piping Plover with government and non-government conservation agencies, as required, for the Great Lakes and Lake of the Woods subpopulations. |
2.3 Approaches to recovery
Table 2. Approaches to recovery of the Piping Plover in Ontario
1. Protect all nesting pairs and their habitat at existing sites: implement actions to address threats to territory establishment and/or nesting at occupied sites within Ontario
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Long-term | Protection, Management |
1.1 Conduct activities to protect breeding birds, territorial individuals and habitat
|
Predation, human disturbance, habitat loss and degradation |
| Critical | Short-term | Research |
1.2 Prioritize threats at existing nest sites
|
All threats |
| Critical | Long-term | Management |
1.3 Adjust management activities to optimize recovery efforts; identify and implement best management practices for predator management, water and terrestrial habitat
|
Predation, human disturbance, habitat loss and degradation |
2. Plan for the potential of greater numbers: identify potential nesting sites and establish Ontario population targets
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Long-term | Protection, Management, Monitoring and Assessment |
2.1 Survey potential breeding sites in the Great Lakes and Lake of the Woods subpopulations, including historical sites and follow up on observations
|
Predation, human disturbance, habitat loss and degradation |
| Critical | Ongoing | Monitoring and Assessment |
2.2 Establish Ontario population targets based on habitat, area requirements and population analysis
|
Predation, human disturbance, habitat loss and degradation |
3. Promote conservation and stewardship of beach and dune ecosystems, including their overall biodiversity and associated species at risk in Ontario
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed | |
|---|---|---|---|---|---|
| Critical | Long-term | Protection, Management |
3.1 Align actions for Piping Plover recovery with beach management plans and beach-dune ecosystem plans and dynamic beach regulations
|
All threats | |
| Critical | Ongoing | Management |
3.2 Evaluate habitat for potential reestablishment
|
Habitat loss and degradation | |
| Critical | Ongoing | Management | 3.3 Develop standardized criteria for identifying habitat to be regulated | Habitat loss and degradation | |
| Critical | Ongoing | Management |
3.4 Negotiate with agencies (e.g., Lake of the Woods Water Control Board), municipalities, organizations (e.g., Friends of Sauble Beach, Friends of Wasaga Beach, The Lake Huron Centre for Coastal Conservation) and First Nations (e.g., Chippewas of Saugeen First Nation) with land, water and recreational responsibilities over developments and management
|
Habitat loss and degradation |
4. Increase knowledge of demography/population dynamics, habitat requirements and threats
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed | |
|---|---|---|---|---|---|
| Beneficial | Ongoing | Monitoring and Research, Management | 4.1 Investigate survival, recruitment and movement patterns Band chicks and record banded adults to monitor movements, dispersal and lifetime reproductive success Contribute data to the United States Fish and Wildlife Service for analyses at regional level on survival and dispersal | All threats, population/ demography knowledge gaps | |
| Critical | Ongoing | Monitoring and Research, Management |
4.2 Monitor reproductive success at all sites in relation to predator abundance and distribution
|
Predation threat, lack of knowledge of specific predators and impacts | |
| Critical | Ongoing | Research, Monitoring |
4.3 Investigate invertebrate communities (food source for Piping Plovers) as indicator of shoreline health
|
Habitat loss and degradation | |
| Critical | Ongoing | Management |
4.4 Investigate effects of beach maintenance (including raking) and dune erosion on Piping Plovers and their invertebrate food supply
|
Human disturbance, all threats |
5. Foster stewardship and public outreach/education about Piping Plovers at occupied sites as well as communication within the province
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Beneficial | Ongoing | Outreach |
5.1 Develop and implement habitat conservation activities Use signage, education and protected areas to protect birds and habitats
|
Human disturbance |
| Beneficial | Ongoing | Outreach |
5.2 Expand stewardship and outreach program
|
All threats |
6. Continue to coordinate/share information in existing databases for the Piping Plover with government and non-government conservation agencies, as required, for the Great Lakes and Lake of the Woods subpopulations
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Long-term | Monitoring, Management, Research |
6.1 Continue to manage, maintain and improve, as required, existing databases (dates/times nest checks, visitation rates, etc.)
|
All threats |
| Beneficial | Ongoing | Management, Research |
6.2 Continue ongoing liaison with United States Great Lakes Piping Plover Recovery Implementation Group, Prairie Piping Plover Recovery Team, Universities, stewardship councils, municipalities
|
All threats |
| Beneficial | Ongoing | Outreach | 6.3 Expand on multi-partner recovery initiatives | All threats |
Supporting narrative
Approach 1.1
In Michigan, enhancing reproductive output and subsequent population increase was achieved through intensive management. Recovery actions in Ontario should focus on maintaining and enhancing the productivity of Piping Plovers in Ontario to contribute to the population expansion overall in the Great Lakes, and perhaps stem the declines in the Lake of the Woods. Numbers may fluctuate from year to year, but it is important to ensure that pairs have a minimal fledging success rate. Fledging success is one population parameter that is more amenable to management, in contrast to adult survival (United States Fish and Wildlife Service 2009).
The Environment Canada recovery strategy suggested that the productivity objective be 1.25 chicks fledged per pair (Environment Canada 2006). Based on modeling efforts, the United States Fish and Wildlife Service seeks to maintain 1.5 to 2.0 chicks fledged per nest per year for the Great Lakes population (Plissner 2000) but this estimate was based on apparent survival rather than true survival (Cohen and Gratto-Trevor 2011). Moreover, to maintain the Great Plains Piping Plover population, Prindiville-Gaines and Ryan (1988) estimated a fledging rate of at least 1.15 chicks per pair was needed based on an adult survival rate of 0.66. However, a more recent modeling effort (Cohen and Gratto-Trevor 2011) of Great Plains Piping Plovers suggested that an objective of 0.75 chicks per pair would be adequate to stabilize the population. The reproductive success objective (or outcome) required for fledging success of the Ontario Piping Plover population requires further discussion.
It is critical that activities are coordinated to protect nesting Piping Plovers and that there is excellent communication among all partners. Indirect effects of human disturbance, which includes people (individuals to large crowds) and disturbance by dogs or other pets can have very detrimental effects on Piping Plover breeding success. This is because, as mentioned in the threats section, this limits their foraging ability or affects the survival of their chicks. Mitigating these impacts through education and outreach – and in some cases enforcement – is critical.
Active predator control measures have been implemented in other jurisdictions such as in the United States Great Lakes with varying degrees of success. The main method for reducing effects of predators on Piping Plover nesting success in Ontario is through the use of exclosures and appropriate waste management. Direct predator control has been a low priority. However, it is important to recognize that in some jurisdictions, the success of exclosures and waste management programs have been limited since predators have been found to readily adapt to new situations. Provided that specific types and sizes of exclosures and protocols are followed, exclosures are very successful in preventing predation at Ontario Piping Plover nests (L. Heyens, pers. comm. 2012). Some exceptions occur such as when a fox entered an exclosure at Sauble Beach in 2010 (S. Robinson, pers. comm. 2012). The Ministry of Natural Resources and the Canadian Wildlife Service have since developed a modified predator exclosure that incorporates an apron (a buried flap around the perimeter of the exclosure) to be used at locations where it is deemed necessary. In the United States it has been found that some management activities, such as tall nest exclosures with perch sites, actually attract certain raptors and can increase predation on Piping Plovers (Murphy et al. 2003, Neuman et al. 2004, Isaksson et al. 2007). When exclosures are used to manage egg predation managers should monitor for the presence of avian predators such as Merlins, which can threaten the survival of adult Piping Plovers or indicate that nest abandonment may be about to occur. Another approach that can be used to reduce predation is education of the public to reduce the amount of garbage and other food sources on nesting beaches.
Translocation of nests to avoid flooding would only be carried out in specific and extenuating circumstances and only by qualified government biologists under permit using approved protocols.
Approach 1.2
Prioritizing threats and evaluating their significance to reproductive success of Piping Plovers are critical. In ideal situations and with sufficient sample sizes, Population Viability Analysis (PVA) would determine which specific threats contribute most at different life stages (e.g., egg predation, predation of chicks or adults) and have the greatest impact on the population. Although PVAs have been conducted for the Great Lakes (United States) and Great Plains populations, sample sizes are much too small to conduct this analysis for Ontario, but may be sufficient in the future.
Approach 1.3
It is important that management activities be adapted quickly in response to changing conditions. Examples include accurate timing for the erection of nest exclosures to coincide with the period when pairs show strong site fidelity and their use does not disturb nesting birds and cause abandonment.
Identifying and implementing best management practices could mean the difference between success and failure for Piping Plover nests. Investigations should be carried out into the optimal beach management or maintenance plans for Piping Plover habitat. In some cases this may involve beach restoration or rehabilitation. Best management practices for predators were discussed in 1.1 (above).
Approaches 2.1 and 2.2
While surveys are already conducted every five years for the International Piping Plover Census, more frequent surveys may be required to confirm presence or absence of Piping Plovers in suitable habitat in intervening years.
Scoping out potential habitat for Piping Plovers is part of this approach and overlaps with approaches 1.1 and 1.2. Scoping out potential suitable habitat for Piping Plovers at historical or other sites is an important component of recovery. This could involve identifying sites that meet suitable criteria, and perhaps in some (already protected areas), restoration and/or stewardship. A response team could be set up using birder’s networks to identify where Piping Plovers are prospecting and could potentially breed.
If new nesting pairs are discovered then the approaches in objective 1 should be implemented. Because beach habitat for Piping Plovers is continually changing in response to lake levels and vegetation encroachment it would be informative to use Geographic Information Systems (GIS) to monitor these changes.
Use of natural features mapping from the OMNR GIS land cover (OMNR 1998) would also enable suitable habitat to be identified throughout the Great Lakes shorelines of Ontario by using the habitat suitability model developed by the United States Fish and Wildlife Service (Wemmer et al. 2001, and more recent updates). By incorporating the area requirements (territory size) of Piping Plovers into this GIS map, the number of breeding pairs that could be currently supported in Ontario could be calculated. This would provide an empirical basis to set a range of population objectives.
Approaches 3.1 and 3.2
Recovery efforts for the Piping Plover should be fully integrated with actions being carried out for ecosystem recovery strategies developed for multiple species in freshwater dune systems, and more generally with management plans and regulations developed for beaches (see Peach 2004, Peach et al. 2006, Peach and Donnelly 2010). These include linking Piping Plover conservation efforts to dynamic beach regulations (planning for natural hazards – see OMNR 2001).
Ecosystem recovery plans have been developed for Lake Huron Dune Grasslands (Jalava 2004) and Lake Erie Sand Spit Savannas (Dougan and Associates and McKay 2006). It is important to identify where there are commonalties and differences in threats, habitat or management requirements for species at risk.
Approach 3.4
This recovery strategy for the Piping Plover presents many opportunities for partnerships and stewardship, since Piping Plovers are a flagship species and have a high public profile. It is important that recovery be achieved through liaison and partnerships with the many groups and agencies concerned with the conservation of freshwater beach and dune systems (e.g., Friends of Sauble Beach, Friends of Wasaga Beach, The Lake Huron Centre for Coastal Education) as well as government agencies and non-government organizations (e.g., Bird Studies Canada) and universities. It is also critical that liaison occurs over developments that may impact Piping Plover habitat, and that they are considered in environmental and ecological site assessments. Piping Plovers are highly vulnerable to changes in water levels, which can cause flooding, so agencies in control of water levels (e.g., dams in Lake of the Woods) need to be advised about protecting nesting areas and potential impacts on nesting birds.
Approach 4.1
Investigations of survival, recruitment and movement patterns of Piping Plovers are beneficial to recovery. Banding is an integral and critical part of this process and does not influence chick survival (Roche et al. 2010c); chicks are routinely banded in Ontario. The majority of known breeding adults in Ontario are already colour-banded through United States Fish and Wildlife Service programs; banding of nesting adults in Ontario has not been carried out to minimize disturbance to nesting birds. If each chick is banded with a unique plastic band (e.g., a colour band with unique readable number), then it does not need to be recaptured to read the United States Geological Survey band. These bands are now used in the United States Great Lakes and could be used in the future in Ontario. If and when the population in Ontario becomes larger then banding of adults should be reconsidered and potentially included in the recovery program in Ontario. It is important to note that the majority of breeding adult Piping Plovers in Ontario already carry United States Geological Survey bands and colour- band combinations so that their origin can be traced. Also, when Piping Plovers that hatched in Ontario are found nesting in the United States, they are captured and unique bands are added to aid in individual identification.
Approach 4.2
Detailed studies are essential to determine the effects of predators on Piping Plover nesting success (both indirect and direct) and the effectiveness of different predator management strategies. Predation is currently an important issue in the recovery of Piping Plover populations in Ontario, and is likely to become more so as breeding adults and chicks continue to be predated.
Approach 4.3
The relationship between the health of invertebrate communities and Piping Plover distribution, abundance and reproductive success has not been fully explored, either in natural systems (unmanaged) or in managed (i.e., raked or other management) systems. Invertebrate communities are one of the indicator groups used to assess the state of the Great Lakes coastal wetlands. While large amounts of site specific data have already been collected on plants, diatoms, invertebrates and birds for coastal wetlands as part of ongoing assessments of the state of the Great Lakes (State of the Lakes Ecosystem Conferences, e.g., Albert and Minc 2001, Uzarski et al. 2004, Niemi et al. 2009) and for some nearshore benthic communities, this type of information is also needed for open beach-dune ecosystems. This should involve sampling of beach invertebrates and their relationship with management regimes, substrates, stormwaters (pollutant loadings) and other disturbances, as well as analysis of fecal material from Piping Plovers to determine dietary composition.
Approach 4.4
The threats posed by raking were discussed earlier; the evidence suggests that raking is detrimental to nesting Piping Plovers. More research is needed on the timing, intensity and frequency of beach raking and grooming to determine if this activity is acceptable and, if so, what the optimal timing of this activity from the perspective of breeding Piping Plovers might be, especially in relation to impacts at different stages of the reproductive cycle.
Approaches 5.1 and 5.2
For recovery of the Piping Plover to be effective and for the population to meet target levels, it is critical that there be extensive liaison and negotiation over developments and management with agencies and organizations that have responsibility for land water and recreation. This collaborative integration is essential to be able to address and adequately respond to development activities that have the potential to impact Piping Plover habitat.
Communication with the public and fostering stewardship among landowners and agencies are key recovery actions since so much depends on the goodwill and cooperation of people. Signs and leaflets can be distributed by volunteers to provide information about Piping Plovers and their habitat.
Continuing to give presentations at local schools and for local residents and engaging local businesses in recovery efforts are also highly beneficial.
Approach 6.1
It is important that standard reporting is implemented so that this is consistent with other jurisdictions. The continued regular updating and quality control, maintenance and enhancement of a Canadian Wildlife Service database, which has been designed to track crucial Piping Plover information (i.e., nesting dates) and statistics (i.e., fledgling rates) (in 2011), are important components of this approach.
Approach 6.2
A key component of recovery is to continue liaison and cooperation with government agencies (e.g., the United States Great Lakes Piping Plover Implementation Group and Prairie Piping Plover Recovery Team). This includes the sharing of techniques and data to address threats and challenges to ensure enhanced integration and management options.
2.4 Area for consideration in developing a habitat regulation
Although important habitat for the Piping Plover has been identified elsewhere (Environment Canada 2007, 2011), it is not recommended that this definition be adopted for the provincial habitat regulation as some sites recently used for nesting would be excluded. In developing a habitat regulation, the following should be considered:
- the area of beach used by nesting Piping Plovers; 400 m to 1 km of shoreline may be used for nesting, brood-rearing and foraging depending on beach size (Lambert and Risley 1989, J. Robinson, pers. comm. to Environment Canada 2011);
- the dynamic nature of beach habitat. The area to be defined in a regulation as habitat for Piping Plovers should take into account the dynamic nature of beach and dune ecosystems, and this means that nest site locations may vary from year to year; and
- the fact that Piping Plovers are semi-colonial so that the habitat regulation applies to single nests, or in the case of multiple nests, from the outer nest locations.
It is recommended that habitat for Ontario be defined as follows.
- All sites occupied by nesting Piping Plovers within the last 10 years and 10 years following occupation (the maximum lifespan of Piping Plover is 8-14 years). An occupied site is defined as a site where one breeding pair occurs
footnote 3 . The designation of Piping Plover habitat would persist for 10 years following the last year of occupation. For example, if Piping Plovers nested at a site in 2007 and 2008, the habitat would remain regulated until 2018, even if Piping Plovers do not use the nesting site for nesting after 2008. If they reoccupied that site for nesting in 2015, then the habitat would be regulated as Piping Plover habitat until 2025. - A one-kilometre length of continuous beach habitat (generally centred around the nest site) is required to provide the requisites for life processes. This one kilometre of beach around the nest pertains to the length of shoreline and not one kilometre in all directions. The width of this continuous beach habitat would extend from the water’s edge to the upper or inland edge of open beach or open dune plant communities or the start of any anthropogenic features such as parking lots, manicured lawns, roads or boardwalks (but not a bench on the beach). In some situations the nest may not be centered within the one-kilometre length as a different configuration is required to ensure adequate access for birds to important habitat features. For example, in situations where suitable shoreline habitat is limited in one direction from the nest (e.g., 200 m), this would be compensated for by a larger distance (in this case 800 m) in the opposite direction.
In instances where Piping Plovers nest in anthropogenic features (such as a parking lot), then the immediate area around the nest should be protected for one year (the season occupied), in addition to a one-kilometre strip of continuous beach habitat as defined above.
Piping Plovers usually occur individually and do not concentrate in large numbers at inland stopover sites (shorelines of reservoirs, industrial ponds, natural lakes, and rivers; Pompei 2005). The use of these sites is influenced by local water levels and water management policy, and is therefore highly variable and difficult to include in a habitat regulation.
Glossary
Aeolian transport: the process of sand being transported by wind.
Auriculars: A circle of feathers surrounding the opening of the ear of birds.
Benthic: Occurring on the bottom of a body of water.
Breeding pairs: 'Breeding' can include a confirmed nest, a confirmed breeding pair or a probable breeding observation. A probable breeding observation, in suitable nesting habitat during the breeding season, includes a male and female pair, a courtship or display between a male and a female or two males (including courtship feeding or copulation), an adult visiting a probable nest location or building a nest, agitated behaviour or anxiety calls of an adult, or breeding evidence such as a brood patch or cloacal protuberance (Environment Canada 2011).
Committee on the Status of Endangered Wildlife in Canada (COSEWIC): The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
Committee on the Status of Species at Risk in Ontario (COSSARO): The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
Conservation status rank: A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperiled
2 = imperiled
3 = vulnerable
4 = apparently secure
5 = secure.
Dynamic beach and hazard regulations: The dynamic beach hazard limit is the combined flooding hazard limit, (the 100-year flood level plus an allowance for wave uprush and other water-related hazards), plus the dynamic beach allowance of 30 m on the Great Lakes-St. Lawrence River system (or 15 m on large inland lakes). If the dynamic beach is subject to erosion or is receding, the flooding hazard limit is added to the horizontal distance representing 100 times the average annual recession rate, plus dynamic beach allowance of 30 m on the Great Lakes-St. Lawrence River system or 15 m on large inland lakes (see OMNR 2001).
Endangered Species Act, 2007 (ESA): The provincial legislation that provides protection to species at risk in Ontario.
Endemic: Restricted or peculiar to a locality or region.
Lore: The region between the eye and beak.
Species at Risk Act (SARA): The federal legislation that provides protection to species at risk in Canada. This Act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedules 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
Species at Risk in Ontario (SARO) List: The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
Translocation: The transport and release of plant, animal or habitat from one location to another. In this document it refers to translocation of eggs.
References
Albert, D. A., and L. D. Minc. 2001. Abiotic and floristic characterization of Laurentian Great Lakes Coastal Wetlands. Verhandlungen des Internationalen Verein Limnologie 27(6):3,413-3,419.
Alig, R., J. Kline, and M. Lichtenstein. 2004. Urbanization on the U.S. landscape: looking ahead in the 21st century. Landscape and Urban Planning 69:219–234.
Allan, D. J., P. B. McIntyre, S. D. P. Smith, B. S. Halpern, G. L. Boyer, A. Buchsbaum, G. A. Burton, Jr., L. M. Campbell, W. L. Chadderton, J. J. H. Ciborowski, P. J. Doran, T. Eder, D. M. Infante, L. B. Johnson, C. A. Joseph, A. L. Marino, A. Prusevich, J. G. Read, J. B. Rose, E. S. Rutherford, S. P. Sowa, and A. D. Steinman. 2013. Joint analysis of stressors and ecosystem services to enhance restoration effectiveness. Proceedings of the National Academy of Sciences (PNAS) 110: 372-377.
Barber, C., A. Nowak, K. Tulk, and L. Thomas 2010. Predator exclosures enhance reproductive success but increase adult mortality of Piping Plovers (Charadrius melodus). Avian Conservation and Ecology 5(2): 6. [online] URL: DOI 10.5751
Boyne, A. 2001. Updated COSEWIC status report — Piping Plover Charadrius melodus. Committee on the Status of Endangered Wildlife in Canada. Sackville, New Brunswick. 46 pp.
Burger, J. 1987. Physical and social determinants of nest site selection in Piping Plover in New Jersey. Condor 89:881-918.
Burger, J. 1994. The effect of human disturbance on foraging behavior and habitat use in Piping Plover (Charadrius melodus). Estuaries 17:695-701.
Burger, J., C. Gorden, J. Lawrence, J. Newman, G. Forcey, and L. Vlietstra. 2011. Risk evaluation for federally listed (roseate tern, piping plover) or candidate (red knot) bird species in offshore waters: A first step for managing the potential impacts of wind facility development on the Atlantic Outer Continental Shelf. Renewable Energy 36:338-351.
Cairns, W.E. 1977. Breeding biology of the Piping Plover in southern Nova Scotia. Master’s Thesis. Dalhousie University, Halifax, Nova Scotia. 115 pp.
Cairns, W.E. 1982. Biology and behavior of breeding Piping Plovers. Wilson Bulletin 94:531-545.
Cairns, W. E. and I. A. McLaren. 1980. Status of the Piping Plover on the east coast of North America. American Birds 34:206-208.
Cartar, R. 1976. The status of the Piping Plover at Long Point, 1966-1975. Ontario Field Biologist 30(2):42-45.
Cartwright, C. 2007. Piping Plovers nest at Sauble Beach. OFO News 25(2):15.
Catlin, D.H. 2009. Population dynamics of Piping Plovers (Charadrius melodus) on the Missouri River. Ph.D. dissertation, Fisheries and Wildlife Sciences, Virginia Polytechnic Institute and State University, Blacksburg, Virginia, United States.
Cohen, J. B., and C. Gratto-Trevor. 2011. Survival, site fidelity, the population dynamics of Piping Plovers in Saskatchewan. Journal of Field Ornithology 82(4):379-394.
Cohen, J. B., J. D. Fraser, and D. H. Catlin. 2006. Survival and site fidelity of Piping Plovers on Long Island, New York. Journal of Field Ornithology 77(4): 379-394.
Cohen, J. B., E.H. Wunker, and J.D. Fraser. 2008. Substrate and vegetation selection by nesting Piping Plovers (Charadrius melodus) in New York. Wilson Journal of Ornithology 120:404-407.
Cohen, J. B., L.M. Houghton, and J.D. Fraser. 2009. Nesting Density and Reproductive Success of Piping Plovers in Response to Storm- and Human-Created Habitat Changes. Wildlife Monographs 173:1-24.
Crosbie, B., and P. Chow-Fraser. 1999. Percentage land use in the watershed determines the water and sediment quality of 22 marshes in the Great Lakes basin. Canadian Journal of Fisheries and Aquatic Sciences 56:1781–1791.
Roche, E.A. 2007. Great Lakes Piping Plover Persistence Despite Projected Declines: Can Predictive Inaccuracy Inform Management? M.S. Thesis, University of Minnesota-Twin Cities, Minnesota, United States.
Cuthbert, F.J., B. Scholtens, L.C. Wemmer, and R. McLain. 1999. Gizzard Contents of Piping Plover Chicks in Northern Michigan. Wilson Bulletin 111:121-123.
Davidson-Arnott, R.G.D. 2010. Introduction to Coastal Processes and Geomorphology. Cambridge University Press, Cambridge, England. 442 pp.
Defeo, O., A. McLachlan, D.S. Schoeman, T.A. Schlacher, J. Dugan, A. Jones, M. Lastra, and F. Scapini. 2009. Threats to sandy beach ecosystems: a review. Estuarine, Coastal and Shelf Science 81:1-12.
Dougan and Associates, and V.L. McKay. 2006. An Ecosystem-Based Recovery Strategy for the Eastern Prickly Pear Cactus (Opuntia humifusa) – Lake Erie Sand Spit Savannas in Canada. Parks Canada Agency. 89 pp. + 1 Appendix
Dugan, J.E., and D.M. Hubbard. 2010. Loss of coastal strand habitat in southern California: the role of beach grooming. Estuaries and Coasts 33:67–77.
Dugan, J.E., D.M. Hubbard, J.M. Engle, D.L. Martin, D.M. Richards, G.E. Davis, K.D.
Lafferty, and R.F. Ambrose. 2000. Macrofauna communities of exposed sandy beaches on the southern California mainland and Channel Islands. Pp. 339-346, in D. Browne (ed.). Fifth California Islands Symposium, Outer Continental Shelf Study, Camarillo, California.
Dugan, J.E., D.M. Hubbard, M.D. McCrary, and M.O. Pierson. 2003. The response of macrofauna communities and shorebirds to macrophyte wrack subsidies on exposed sandy beaches of southern California. Estuarine, Coastal and Shelf Science 58S:133–148.
Elliott-Smith, E. and S.M. Haig. 2004. Piping Plover (Charadrius melodus). The Birds of North America Online (A. Poole, ed.). Ithaca: Cornell Lab of Ornithology.
Elliott-Smith, E., S.M. Haig, and B.M. Powers. 2009. Data from the 2006 International Piping Plover Census: U.S. Geological Survey Data Series 426. 332 pp.
Environment Canada. 2006. Recovery strategy for the Piping Plover (Charadrius melodus circumcinctus) in Canada. Species at Risk Act Recovery Strategy Series. Environment Canada, Ottawa, Ontario. 30 pp.
Environment Canada. 2007. Addendum to the final recovery strategy for the Piping Plover (Charadrius melodus circumcinctus) in Canada. Re: Identification of Critical Habitat. Environment Canada, Ottawa, Ontario.
Environment Canada, and the United States Environmental Protection Agency. 2009. State of the Great Lakes, 2009.
Environment Canada. 2011. Action Plan for the Piping Plover (Charadrius melodus circumcinctus) in Ontario [Proposed]. Species at Risk Act Action Plan Series. Environment Canada, Ottawa. iii + 19 pp.
Environment Canada, the Canadian Wind Energy Association, Bird Studies Canada, and the Ontario Ministry of Natural Resources. 2011. Wind Energy Bird and Bat Monitoring Database: Summary of the findings from post-construction monitoring reports.
Flemming, S.P., R.D. Chiasson, P.C. Smith, P.J. Austin-Smith, and R.P. Bancroft. 1988. Piping Plover Status in Nova Scotia related to its reproductive and behavioural responses to human disturbance. Journal of Field Ornithology 59(4):321-330.
Gahbauer, M. 2007. Merlin (Falco columbaris). Pp. 192-193, in M.D. Cadman, D.A. Sutherland, G.G. Beck, D. Lepage, and A.R. Couturier (eds.). Atlas of the Breeding Birds of Ontario, 2001-2005. Bird Studies Canada, Environment Canada, Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature. Toronto, Ontario, Canada.
Galbraith, H., R. Jones, R. Park, J. Clough, S. Herrod-Julius, B. Harrington, and G. Page. 2002. Global climate changes and sea level rise: Potential loss of intertidal habitat for shorebirds. Waterbirds 25:173-183.
Goossen, J.P., D.L. Amirault, J. Arndt, R. Bjorge, S. Boates, J. Brazil, S. Brechtel, R. Chiasson, G.N. Corbett, R. Curley, M. Elderkin, S.P. Flemming, W. Harris, L. Heyens, D. Hjertaas, M. Huot, B. Johnson, R. Jones, W. Koonz, P. Laporte, D. McAskill, R.I.G. Morrison, S. Richard, F. Shaffer, C. Stewart, L. Swanson, and E. Wiltse. 2002. National Recovery Plan for the Piping Plover (Charadrius melodus). National Recovery Plan No. 22. Recovery of Nationally Endangered Wildlife, Ottawa, Ontario. 47 pp.
Gratto-Trevor, C.L. and S. Abbott. 2011. Conservation of Piping Plover (Charadrius melodus) in North America: science, successes, and challenges. Canadian Journal of Zoology 89:5, 401-418.
Haas, S.C.G. 2011. Merlin (Falco columbarius) in A.T. Chartier, J.J. Baldy, and J.M. Brenneman (eds.). The Second Michigan Breeding Bird Atlas. Kalamazoo Nature Center. Kalamazoo, Michigan.
Haffner, C.D. 2005. Breeding season spatial requirements of Great Lakes Piping Plovers in Northern Lower Michigan. M.S. Thesis, University of Minnesota, St. Paul, Minnesota, USA.
Haffner, C.D., F.J. Cuthbert, and T.W. Arnold. 2009. Space use by Great Lakes Piping Plovers during the breeding season. Journal of Field Ornithology 80:270-279.
Haig, S.M., and L.W. Oring. 1987. Population studies of Piping Plovers at Lake of the Woods, Minnesota, 1982-1987. Loon 59:113-117.
Haig, S.M. and L.W. Oring. 1988. Mate, site, and territory fidelity in Piping Plovers. The Auk 105:268-277.
Haig, S.M. and J.H. Plissner. 1992. 1991 International Piping Plover Census. Report to United States Fish and Wildlife Service Region 3, Division of Endangered Species, Ft. Snelling, Minnesota. 148 pp.
Haig, S., C. Ferland, F. Cuthbert, J. Dingledine, J. Goosen, A. Hect, and N. McPhillips. 2005. A complete species census and evidence of regional declines in Piping Plovers. Journal of Wildlife Management 69:160–173.
Heyens, L. 2002. Ontario 2002 report: Prairie Piping Plover Recovery Team. Unpublished report.
Heyens, L. 2003. Ontario 2003 report: Prairie Piping Plover Recovery Team. Unpublished report.
Heyens, L. 2007. Piping Plover Charadrius melodus. Pp. 214-215, in M. Cadman, D.A. Sutherland, G.G. Beck, D. Lepage, and A.R. Couturier (eds.). Atlas of the Breeding Birds of Ontario. Bird Studies Canada, Environment Canada, Ontario Field Ornithologists, Ontario Ministry of Natural Resources, and Ontario Nature.
Hubbard, D.M. and J.E. Dugan. 2003. Shorebird use of an exposed sandy beach in southern California. Estuarine Coastal Shelf Science 58:41-54.
Huntingford, F. A., and A. K. Turner. 1987. Animal Conflict. Chapman & Hall, London, United Kingdom.
Hussell, D.J.T. and R.D. Montgomerie. 1966. The status of the Piping Plover at Long Point, 1960-1965. Ontario Field Biologist 20:14-16.
Isaksson, D., J. Wallander, and M. Larsson. 2007. Managing predation on ground- nesting birds: The effectiveness of nest exclosures. Biological Conservation 136:136-142.
Jalava, J.V. 2004. Pitcher’s Thistle – Lake Huron Dune Grasslands Recovery Strategy, May 2004. Pitcher’s Thistle – Lake Huron Dune Grasslands Recovery Team. Parks Canada and Ontario Ministry of Natural Resources. ii + 32 pp. + appendices.
Kirk, D.A. 2007. Literature review and search questions for assessing impacts of wind turbines on avian and bat mortality at Point Pelee National Park of Canada. Report to Parks Canada Agency headquarters, Gatineau, Québec.
Knetter, J.M., R.S. Lutz, J.R. Cary, and R.K. Murphy. 2002. A multi-scale investigation of Piping Plover productivity on Great Plains alkali lakes, 1994-2000. Wildlife Society Bulletin 30:683-694.
Lambert, A.B. 1987. Piping Plover Pluvier siffleur Charadrius melodus. Pp. 162–163, in M.D. Cadman, P.F.J. Eagles, and F.M. Helleiner (compilers). Atlas of the breeding birds of Ontario. University of Waterloo Press, Waterloo, Ontario. 617 pp.
Lambert, A.B. and E. Nol. 1978. Status of the Piping Plover at Long Point. Unpublished report prepared by Long Point Bird Observatory for Wildlife Branch, Ontario Ministry of Natural Resources. 40 pp.
Lambert, A.B. and C.J. Risley. 1989. An investigation of the status of the Piping Plover at Lake of the Woods, Ontario. Unpublished report to Endangered Species Recovery Fund and Ontario Ministry of Natural Resources. 49 pp.
LeDee, O., T.W. Arnold, E.A. Roche, F.J. Cuthbert. 2010a. The use of breeding and nonbreeding encounters to estimate survival and fidelity in the Great Lakes Piping Plover. The Condor 112(4):637-643.
LeDee, O., F.J. Cuthbert, K.C. Nelson. 2010b. The challenge of threatened and endangered species management in coastal areas. Coastal Management Journal 38(4):337-353.
Loegering, J.P. and J.D. Fraser. 1995. Factors affecting Piping Plover chick survival in different brood-rearing habitats. Journal of Wildlife Management 59:646-655.
Lofgren, B.M., F.H. Quinn, A.H. Clites, R.A. Assel, A.J. Eberhardt, C.L. Luukkonen. 2002. Evaluation of potential impacts on Great Lakes water resources based on climate scenarios of two GCMs. Journal of Great Lakes Research 28: 537–554.
Lofgren, B.M., T.S. Hunter, and J. Wilbarger. 2011. Effects of using air temperature as a proxy for potential evapotranspiration in climate change scenarios of Great Lakes basin hydrology. Journal of Great Lakes Research 37:744-752.
McCracken, J.D., M.S.W. Bradstreet, and G.L. Holroyd. 1981. Breeding birds of Long Point, Lake Erie: a study in community succession. Canadian Wildlife Service Report Series No. 44.
McCutcheon, A. 2012. Carter Bay listed for $3.75 million. The Manitoulin Expositor. Web site: [accessed August 15, 2012].
McRae, R.D. 1982. Birds of Presqu'ile, Ontario. Ontario Ministry of Natural Resources. 74 pp.
Maxson, S.J. and K.V. Haws. 2000. Population studies of Piping Plovers at Lake of the Woods, Minnesota: 19 year history of a declining population. Waterbirds 23:475–481.
Melvin, S.M., C.R. Griffin, and L.H. MacIvor. 1991. Recovery strategies for Piping Plovers in managed coastal landscapes. Coastal Management 19:21-34.
Miller, G.W. 1977. The current status and breeding performance of the Long Point Piping Plovers – a survey of an endangered species population. Long Point Bird Observatory. 28 pp.
Miller, M.P., S.M. Haig, C.L. Gratto-Trevor, and T.D. Mullins. 2009. Molecular population genetic structure in the Piping Plover: U.S. Geological Survey Open-File Report 2009-1032. 30 pp.
Morrice, J.A., N.P. Danz, R.R. Regal, J.R. Kelly, G.J. Niemi, E.D. Reavie, T. Hollenhorst, R.P. Axler, A.S. Trebitz, A.M. Cotter, G.S. Peterson. 2008. Human influences on water quality in Great Lakes coastal wetlands. Environmental Management 41:347-357.
Morrice, J.A., A.S. Trebitz, J.R. Kelly, A.M. Cotter, and M.L. Knuth. 2009. Nutrient variability in Lake Superior coastal wetlands: the role of land use and hydrology. Pp. 217-238, in M. Munawar and I.F. Munawar (eds.). State of Lake Superior, Ecovision World Monograph Series, Aquatic Ecosystem Health and Management Society, Burlington, Ontario, Canada.
Munro, J.A. 1911. The spring migration of birds at Fisherman’s Island, Toronto, 1910. Ottawa Naturalist 25:27-48.
Murphy, R. K., K. A. Smith, B. R. Casler, and D. P. Althoff. 2001. Reproduction and spacing of Piping Plovers breeding at high density Appam Lake, North Dakota. Wilson Bull. 113:331-335.
Murphy, R.K., I.M.G. Michaud, D.R.C. Prescott, J.S. Ivan, B.J. Anderson, and M.L. French-Pombier. 2003. Predation on adult Piping Plovers at predator exclosure cages. Waterbirds 26:150-155.
Neuman, K.K., G.W. Page, L.E. Stenzel, J.C. Warriner, and J.S. Warriner. 2004. Effect of mammalian predator management on Snowy Plover breeding success. Waterbirds 27:257-263.
Nickling, W.G. and R.G.D. Davidson-Arnott. 1990. Aeolian sediment transport on beaches and coastal dunes. Pp. 1-35, in R.G.D. Davidson-Arnott (ed.). Proceedings of the Symposium on Coastal Sand Dunes. National Research Council of Canada.
Nicholson, J.C. 1981. Birds of Manitoulin Island and adjacent islands within Manitoulin district. Sudbury, Ontario.
Niemi, G.J., V.J. Brady, T.N. Brown, J.J.H. Ciborowski, N.P. Danz, D.M. Ghioca, J.M. Hanowski, T.P. Hollenhorst, R.W. Howe, L.B. Johnson, C.A. Johnston, E.D. Reavie. 2009. Development of ecological indicators for the U.S. Great Lakes coastal region – a summary of applications in Lake Huron. Aquatic Ecosystem Health & Management 12(1):77-89.
Nordstrom, K.F. 2000. Beaches and Dunes of Developed Coasts. Cambridge University Press. 338 pp.
OMNR. 1998. Ontario 28-Class Land Cover Database. Ontario Ministry of Natural Resources, Peterborough, Ontario.
OMNR 2001. Understanding Natural Hazards. Ontario Ministry of Natural Resources, Peterborough, Ontario.
Ontario Nest Records Scheme. 2008. Ontario Nest Records Scheme Database. Web site: [accessed in 2008].
Peach, G.H. 2004. Sauble Beach Management Plan: Conserving a finite resource. Prepared by the Lake Huron Centre for Coastal Conservation.
Peach, G. 2006. Management of Lake Huron’s beach and dune ecosystems: Building up from the grassroots. The Great Lakes Geographer 13(1):39-49.
Peach, G. and P. Donnelly. 2010. Oliphant Coastal Stewardship Plan. Prepared by the Lake Huron Centre for Coastal Conservation. 62 pp. + appendices
Peach, G. H., J. Bowles, and L. Porter. 2007. Conserving a delicate balance: Management plan for North Sauble Beach, Ontario, Canada. Prepared by the Lake Huron Centre for Coastal Conservation.
Peck, G.K. and R.D. James. 1983. Breeding Birds of Ontario: Nidiology and Distribution. Volume 1: Nonpasserines. Life Sciences Miscellaneous Publications, Royal Ontario Museum, Toronto.
Plissner, J.H. and S.M. Haig. 2000. Viability of Piping Plover Charadrius melodus metapopulations. Biological Conservation 92:163-173.
Pompei, V. D. 2005. Spring and fall distribution of Piping Plovers in North America: implications for conservation of migration stopover sites, MS Thesis. University of Minnesota-Twin Cities, Minnesota, USA.
Powell, A.N. and F.J. Cuthbert. 1992. Habitat and reproductive success of Piping Plovers nesting on Great Lakes islands. Wilson Bulletin 104: 155–161.
Price, E.W. 2002. Piping Plover (Charadrius melodus) recolonization potential in the Great Lakes: Assessment of the historic habitat and dispersal events. M.S. Thesis, University of Minnesota, St. Paul, Minnesota, USA.
Prindiville Gaines, E.M. and M.R. Ryan. 1988. Piping Plover habitat use and reproductive success in North Dakota. Journal of Wildlife Management 52:266- 273.
Quilliam, H.R. 1973. History of the Birds of Kingston, Ontario. Kingston Field Naturalists, Kingston, Ontario. 209 pp.
Reed, D.J., R. Davidson-Arnott, and G.M.E. Perillo. 2009. Estuaries, coastal marshes, tidal flats and coastal dunes. Pp. 130-157, in O. Slaymaker, T. Spencer, and C. Embleton-Hamann (eds.). Geomorphology and Global Environmental Change, Cambridge. Cambridge University Press.
Roche, E.A., T.W. Arnold, and F.J. Cuthbert. 2010a. Apparent nest abandonment as evidence for breeding season mortality in Great Lakes Piping Plovers. The Auk 127(2):402-410.
Roche, E.A., J.B. Cohen, D.H. Catlin, D.L. Amirault, F.J. Cuthbert, C. Gratto-Trevor, J. Felio, and J.D. Fraser. 2010b. Range-wide estimation of apparent survival for the Piping Plover. Journal of Wildlife Management 74(8):1,784-1,791.
Roche, E.A., T.W. Arnold, J.H. Stucker, and F.J. Cuthbert. 2010c. Colored plastic and metal leg bands do not affect survival of Piping Plover chicks. Journal of Field Ornithology 81:317-324.
Russell Jr., R.P. 1983. The Piping Plover in the Great Lakes region. American Birds 37:951-955.
Ryan, M.R., B.G. Root, and P.M. Mayer. 1993. Status of Piping Plover in the Great Plains of North America: A demographic simulation model. Conservation Biology 7:581-585.
Sauer, J.R., J.E. Hines, J.E. Fallon, K.L. Pardieck, D.J. Ziolkowski, Jr., and W.A. Link. 2011. The North American Breeding Bird Survey, Results and Analysis 1966 - 2010. Version 12.07.2011. USGS Patuxent Wildlife Research Center, Laurel, Maryland.
Saunders, K.E. and R.G.D. Davidson-Arnott. 1990. Coastal dune response to natural disturbances. Pp. 321-346, in R.G.D. Davidson-Arnott (ed.). Proceedings of the Symposium on Coastal Sand Dunes. National Research Council of Canada.
Saunders, S.P., E.A. Roche, T.W. Arnold and F.J. Cuthbert. 2011. Female site familiarity increases fledging success in Piping Plovers (Charadrius melodus). The Auk 129:329-337.
Saunders, W.E. 1909. Summer birds of the southern edge of western Ontario. Wilson Bulletin 21:152-155.
Schwalbach, M. J. 1988. Conservation of Least Terns and Piping Plovers along the Missouri River and its major tributaries in South Dakota. Master’s Thesis. South Dakota State University, Brookings, South Dakota, United States.
Sheppard, R.W. 1935. Mid-summer bird notes from Long Point, Norfolk County, Ontario. The Auk 52:196-197.
Snyder, L. L. 1931. A faunal investigation of Long Point and vicinity, Norfolk County, Ontario. Transactions of the Royal Canadian Institute 18:139-227.
Snyder, L.L. 1941. The birds of Prince Edward County, Ontario. University of Toronto Studies, Biological Series 48:25-92.
Stucker, J.H. and F.J. Cuthbert. 2006. Distribution of nonbreeding Great Lakes Piping Plovers along Atlantic and Gulf coastlines: 10 years of band resightings. Report to the United States Fish and Wildlife Service, East Lansing, Michigan and Panama City, Florida Field Offices.
Tarr, J.G. and P.W. Tarr. 1987. Seasonal abundance and the distribution of coastal birds on the northern Skeleton Coast, South West Africa/Nimibia. Madoqua 15:63-72.
Toews, B.A., K.J. Toews, and C.E.J. Cartwright. 2008. The successful nesting of the Piping Plover at Sauble Beach marks a return to the Canadian Great Lakes after 30 years. Ontario Birds 26:16-49.
United States Fish and Wildlife Service (USFWS). 1996. Piping Plover (Charadrius melodus), Atlantic Coast population. Revised recovery plan. Hadley, Massachusetts.
United States Fish and Wildlife Service (USFWS). 2003. Recovery plan for the Great Lakes Piping Plover (Charadrius melodus). Ft. Snelling, Minnesota. 141 pp.
United States Fish and Wildlife Service (USFWS). 2009. Piping Plover (Charadrius melodus): 5-year review: summary and evaluation. Hadley, Massachusetts. vi + 206 pp.
United States Fish and Wildlife Service (USFWS). 2012. Draft Comprehensive Conservation Strategy for the Piping Plover (Charadrius melodus) in its Coastal Migration and Wintering Range in the Continental United States. East Lansing, Michigan, United States.
Uzarski, D.G., T.M. Burton, and J.A. Genet. 2004. Validation and performance of an invertebrate index of biotic integrity for Lakes Huron and Michigan fringing wetlands during a period of lake level decline. Aquatic Ecosystem Health & Management 7:269–288.
Wang, J., X. Bai, H. Hu, A. Clites, M. Colton, and B. Lofgren. 2012. Temporal and Spatial Variability of Great Lakes Ice Cover, 1973–2010. Journal of Climate 25:1,318–1,329.
Warkentin, I.G., N.S. Sodhi, R.H.M. Espie, A.F. Poole, L.W. Oliphant, and P.C. James. 2005. Merlin (Falco columbarius), The Birds of North America Online (A. Poole, ed.). Ithaca: Cornell Lab of Ornithology.
Wemmer, L.C. 2000. Conservation of the Piping Plover (Charadrius melodus) in the Great Lakes Region: A Landscape–Ecosystem Approach. Ph.D. dissertation, University of Minnesota, St. Paul, Minnesota, USA.
Wemmer, L.C., U. Ozesmi, and F.J. Cuthbert. 2001. A habitat-based population model for the Great Lakes population of the Piping Plover (Charadrius melodus). Biological Conservation 99:169-181.
Whyte, A.J. 1985. Breeding ecology of the Piping Plover in central Saskatchewan. M.S. Thesis, University of Saskatchewan, Saskatoon, Canada. 153 pp.
Wiens, T.P. 1986. Nest-site tenacity and mate retention in the Piping Plover (Charadrius melodus). M.S. Thesis, University of Minnesota at Duluth.
Wiens, T.P. and F.J. Cuthbert. 1988. Nest-site tenacity and mate retention of the Piping Plover. Wilson Bulletin 100:545-553.
Wilcox, D.A., T.A. Thompson, R.K. Booth, and J.R. Nicholas. 2007. Lake-level variability and water availability in the Great Lakes. U.S. Geological Survey Circular No. 1311. 25 pp.
Wilcox, L. 1959. A twenty year banding study of the Piping Plover. The Auk 76:129-152.
Personal communications
Cavalieri, V., pers. comm. 2012. Communication with D.A. Kirk at the Piping Plover workshop, Simcoe County Museum, Barrie, Ontario. August 2012. United States Fish and Wildlife Service, East Lansing, Michigan.
Benvenuti, J., pers. comm. 2012. Email correspondence with D.A. Kirk. Several dates August 2012. Species at Risk Biologist, Ontario Ministry of Natural Resources, Midhurst District.
Cuthbert, F., pers. comm. 2012. Email correspondence with D.A. Kirk. Several dates March to September 2012. University of Minnesota, St. Paul’s.
Davidson, P., pers. comm. 2012. Email correspondence with D.A. Kirk. Several dates August 2012. Piping Plover Coordinator, Wasaga Beach Provincial Park.
Davidson-Arnott, R.G.D., pers. comm. 2012. Email correspondence with D.A. Kirk. Several dates September 2012. Professor Emeritus, University of Guelph.
Davis, C., pers. comm. 2012. Email correspondence with D.A. Kirk. September 2012. Endangered and Nongame Species Program, New Jersey Division of Fish and Wildlife.
Fisher, J., pers. comm. 2012. Email correspondence with D.A. Kirk. Several dates March 2012. Superintendant, Wasaga Beach Provincial Park.
Heyens, L., pers. comm. 2012. Email correspondence with D.A. Kirk. Numerous dates March to August 2012. Communication with D.A. Kirk at the Piping Plover workshop, Simcoe County Museum, Barrie, Ontario. March 23, 2012. Ontario Ministry of Natural Resources, Kenora District.
Jefferis, S., pers. comm. 2012. Communication with D.A. Kirk at the Piping Plover workshop, Simcoe County Museum, Barrie, Ontario. August 9, 2012. Email correspondence with D.A. Kirk. August 2012. Piping Plover Coordinator, Ontario Ministry of Natural Resources, Sauble Beach.
Robinson, S., pers. comm. 2012. Email correspondence with D.A. Kirk. Numerous dates March to August 2012. Communication with D.A. Kirk at the Piping Plover workshops, Simcoe County Museum, Barrie, Ontario. March 23, 2012 and August 9, 2012. Telephone communication with D.A. Kirk. August 2012. Ontario Ministry of Natural Resources, Midhurst District.
Rupert, S., pers. comm. 2012. Email correspondence with D.A. Kirk. September 2012. Park Interpreter, Point Pelee National Park of Canada.
Recovery strategy workshop participants and advisors
Workshop participants and Piping Plover Recovery Implementation Group
| Name | Affiliation and office |
|---|---|
| Sue Abbott | Bird Studies Canada |
| Amelia Argue | Ontario Ministry of Natural Resources, Peterborough |
| Debbie Badzinski | Bird Studies Canada |
| Jodi Benvenuti | Ontario Ministry of Natural Resources, Midhurst |
| Amy Chabot | Consulting Species at Risk Biologist |
| Andrew Chard | Ontario Ministry of Natural Resources, Peterborough |
| Sandy Dobbyn | Ontario Parks |
| Patricia Davidson | Ontario Ministry of Natural Resources, Wasaga Beach |
| Jessica Dunlop | Ontario Parks |
| Laurel Finney | Ontario Ministry of Natural Resources, Wasaga Beach |
| John Fisher | Ontario Ministry of Natural Resources, Wasaga Beach |
| Leo Heyens | Ontario Ministry of Natural Resources, Kenora District |
| Samantha Jefferis | Piping Plover Coordinator, Ontario Ministry of Natural Resources, Sauble Beach |
| Leanne Jennings | Ontario Ministry of Natural Resources, Peterborough |
| David Anthony Kirk | Aquila Conservation & Environment Consulting, Ottawa |
| Greg Lucking | Ontario Ministry of Natural Resources, Peterborough |
| Peter Middleton | Huron Fringe Field Naturalists |
| Erica Nol | Trent University, Peterborough |
| Megan Rasmussen | Ontario Ministry of Natural Resources, Sudbury |
| Chris Risley | Ontario Ministry of Natural Resources, Peterborough |
| Jeff Robinson | Environment Canada, Canadian Wildlife Service, London |
| Suzanne Robinson | Ontario Ministry of Natural Resources, Midhurst |
| Kathy St. Laurent | Environment Canada, Canadian Wildlife Service, Ontario Region, Downsview |
| Don Sutherland | Ontario Ministry of Natural Resources, Peterborough |
| John Van Den Broeck | Ontario Ministry of Natural Resources, Fort Frances |
| Bree Walpole | Ontario Ministry of Natural Resources, Peterborough |
| Warren Wilson | Facilitator, Intersol Group Ltd., Ottawa |
Advisors
| Vince Cavalieri | Piping Plover Coordinator, US Fish and Wildlife Service, East Lansing, Michigan |
| Francie Cuthbert | Piping Plover Researcher, University of Minnesota |
| Anne Hecht | Piping Plover Coordinator, United States Fish and Wildlife Service |
| Cheri Gratto-Trevor | Recovery Team Chair for Piping Plover, Federal Recovery Strategy |
Appendix 1
Historical nesting sites for the Piping Plover in the Ontario Great Lakes (information courtesy of the Natural Heritage Information Centre, Ontario Ministry of Natural Resources, D.A. Sutherland, pers. comm.).
Location: Lake Erie
| Number | County | Location | Date last nested | Estimated number of nesting pairs | Comments |
|---|---|---|---|---|---|
| 1 | Chatham-Kent | Erieau | 1926 | 1 | Ontario Nest Record Scheme (ONRS) 2008 |
| 2 | Chatham-Kent | Rondeau | 1936 | 1 | Last nest record 1947 (ONRS 2008) |
| 3 | Essex | Big Creek Marsh [Holiday Beach] | 1909 | 6-8 | "About six or eight pairs of these birds were scattered along the lake shore beside the marsh near the mouth of the big creek a few miles from the Detroit River. One nest was found with four nearly hatched eggs and the other birds were manifestly concerned at our presence." (Saunders 1909) |
| 4 | Essex | Pelee Island | 1933 | 1 | ONRS 2008 |
| 5 | Essex | Point Pelee | 1938 | 5-10 | "It is a common summer resident and regular breeder on the east beach" (Taverner and Swales 1907). No indication of what was meant by 'common'. J.H. Fleming (Fleming Book 1, p. 7 in Stirret files) said in May 1913: 'Saw a number of pairs. Mr. Young found 4 nests, saw 2 of these on June 2, 1913." (S. Rupert, pers. comm. 2012) |
| 6 | Niagara | Crescent Beach | 1936 | 1 | Last report of nesting was in 1936 (Beardslee and Mitchel 1965) though presumably nesting occurred in the years prior to 1936. |
| 7 | Niagara | Crystal Beach | 1934 | 1 | Last nest record in 1934 (ONRS 2008) ONRS 2008 |
| 8 | Niagara | Long Beach | 1938 | 1 | ONRS 2008 |
| 9 | Niagara | Sherkston Beach | 1944 | 1 | Last nest record in 1944 (ONRS 2008) |
| 10 | Norfolk | Long Point | 1976 | 35 (25-50) |
Chronology of events: Between turn of century and 1940s, Piping Plovers believed to be 'remarkably common.' (McCracken et al. 1981) |
| 11 | Norfolk | Turkey Point | 1924 | 1 | Baillie and Harrington (1936) Estimated total for Lake Erie: 75 pairs (Russell’s estimate was 125 pairs). |
Location: Lake Huron
| Number | County | Location | Date last nested | Estimated number of nesting pairs | Comments |
|---|---|---|---|---|---|
| 12 | Bruce | Oliphant Beach | 1 | ||
| 13 | Bruce | Sauble Beach | 1 | 13 | |
| 14 | Lambton | Ipperwash Beach | 1953 | 1 | Last documentation of breeding: pair with downy young (Kelley 1978). |
| 15 | Manitoulin | Carter Bay | 1 | 15 | |
| 16 | Simcoe | Wasaga Beach | 2-3 | No indication of historical abundance (cf. Baillie and Harrington 1936; Devitt 1943, 1967); presumably several pairs bred in the 1920s and 30s. Estimated total for Lake Huron: 7 pairs (Russell’s estimate was 10 pairs). |
Location: Lake Ontario
| Number | County | Location | Date last nested | Estimated number of nesting pairs | Comments |
|---|---|---|---|---|---|
| 17 | Hamilton | Van Wagner’s Beach | 1934 | 1 | "Noted at Hamilton Beach on only two occasions by McIlwraith, but beginning in 1930 and ending in 1947, George North encountered a pair of these plovers almost every year along the Lake Ontario Beaches between Burlington and Stoney Creek. The birds probably nested successfully on more than one occasion; the observation dates suggest they attempted to nest during at least five summers." (Curry 2006). Only documented nest was in 1934 [Van Wagner’s Beach], but indications are that individuals and perhaps a pair summered until 1947. |
| 18 | Metro Toronto | Toronto Island | 1934 | 3 | Presumably nested on the Toronto peninsula in late 19th century, but the earliest chroniclers of the area’s birds (e.g., Fleming, 1906) make no mention of nesting; Fleming states only 'regular migrant, not very common." Munro (1911) mentions 'three pairs arrived on May 10th [1910] and began nesting on Fisherman’s Island, where they nested at least until 1928 (Baillie and Harrington 1936), so the population was minimally 3 pairs and possibly somewhat larger. The last confirmation of breeding seems to have been in 1934 at Hanlan’s Point. |
| 19 | Northumberland | Presqu'ile Provincial Park | [last documented nest in 1916, but convincing reports of nesting into the 1950s] | 1 | At least one pair present annually between 1914 and 1918 with actual nesting documented only in 1915 and 1916 (McRae 1982). Birds present periodically into the 1950s and additional nesting likely occurred. |
| 20 | Prince Eward | Consecon [Bald Head] | 1926 | 5 | E. Beaupre collected a clutch of partially incubated eggs at "Consecon" in 1926 and Beaupre and C.J. Young had found five breeding pairs there in 1924 (Snyder 1941). Presumably "Consecon" refers to the baymouth bars (Bald Head Beach, Bald Head Island, and Barcovan Spit) spanning the mouth of Weller Bay. |
| 21 | Prince Edward | Sandbanks | [1930; species observed in suitable habitat in 1952] | 1 | Pair on beach on baymouth bar at West Lake [Sandbanks Provincial Park] and one bird flushed from nest scrape in 1930 (Snyder 1941) is the only actual evidence of breeding. Individuals observed periodically in suitable habitat until 1952 (Sprague and Weir 1984). |
Location: St Lawrence
| Number | County | Location | Date last nested | Estimated number of nesting pairs | Comments |
|---|---|---|---|---|---|
| 22 | Leeds & Grenville | Rockport | 1894 | 1 | Baillie and Harrington (1936). This is only report of nesting in the Thousand Islands and, given that the eggs are in CMN and presumably correctly identified, must be acceptable. Most likely location for this nest record is Tar Island, a long sandy island within a kilometre to the east of Rockport (D. Sutherland, pers. comm. 2012). Toner et al. (1942) state that 'this bird is found breeding along the St. Lawrence river but is more often noted as a spring and fall migrant", but just cited the C.J. Young 1894 nest record from Rockport. |
| 23 | Frontenac | Collins Lake | 1903 | 1 | Only record of nesting and presumably an anomaly (Quilliam 1973). Estimated total for Lake Ontario region: 11 pairs (Russell estimated 15-25 pairs). |
Location: Lake Superior
| Number | County | Location | Date last nested | Estimated number of nesting pairs | Comments |
|---|---|---|---|---|---|
| 24 | Thunder Bay District | Russell (1983) mentions Lake Superior as a nesting area, but there have been very few records of Piping Plover on the Canadian shore of Lake Superior (D. Sutherland, pers. comm.). Lambert and Nol (1978) stated 'Birds no longer nest in the Thunder Bay area". Thus there may have been a few pairs historically. Estimated total for Lake Superior: 2-3 pairs (Russell estimated a few pairs). |
References cited
Baillie, J.L. and P. Harrington. 1936. The Distribution of Breeding Birds in Ontario, Part 1. Transactions of the Royal Canadian Institute 21:1-50.
Beardslee, C.S. and H.D.l. Mitchell. 1965. Birds of the Niagara Frontier Region. Bulletin of the Buffalo Society of Natural Sciences, Buffalo, New York. Vol. 22. Xix + 478 pp.
Curry, R. 2006. Birds of Hamilton and Surrounding Areas. Hamilton Naturalists' Club. 647 pp.
Devitt, O.E. 1943. The Birds of Simcoe County, Ontario. Transactions of the Royal Canadian Institute 24(2):241-313.
Devitt, O.E. 1967. The Birds of Simcoe County, Ontario. The Brereton Field Naturalists' Club, Barrie. 192 pp.
Fleming, J.H. 1906. Birds of Toronto, Ontario. Part 1, Water Birds. The Auk 23:437-453.
Hussell, D. J. T. and R. D. Montgomerie. 1966. The status of the Piping Plover at Long Point, 1960-1965. Ontario Field Biologist 20: 14-16.
Kelley, A.H. 1978. Birds of Southeastern Michigan and Southwestern Ontario. Cranbrook Institute of Science, Broomfield Hills, Michigan. 99 pp.
Lambert, A. and E. Nol. 1978. Status of the Piping Plover at Long Point. Long Point Bird Observatory, Ontario. 40 pp.
McRae, R.D. 1982. Birds of Presqu'ile, Ontario. Ontario Ministry of Natural Resources. 74 pp.
Munro, J.A. 1911. The spring migration of birds at Fisherman’s Island, Toronto, 1910. Ottawa Naturalist 25:27-48.
ONRS. 2008. Ontario Nest Records Scheme Database. Web site: [accessed in 2008].
Quilliam, H.R. 1973. History of the Birds of Kingston, Ontario. Kingston Field Naturalists Club, Kingston. 209 pp.
Rupert, S., pers. comm. 2012. Email correspondence with D.A. Kirk. September 2012. Park Interpreter, Point Pelee National Park of Canada. Russell Jr., R.P. 1983. The Piping Plover in the Great Lakes region. American Birds 37:951-955.
Saunders, W.E. 1909. Summer birds of the southern edge of western Ontario. Wilson Bulletin 21:152-155.
Sheppard, R.W. 1935. Mid-summer bird notes from Long Point, Norfolk County, Ontario. The Auk 52:196-197.
Snyder, L.L. 1941. The birds of Prince Edward County, Ontario. University of Toronto Studies, Biological Series 48:25-92.
Sprague, R.T. and R.D. Weir. 1984. The Birds of Prince Edward County. 2nd edition. Kingston Field Naturalists, Kingston. 206 pp.
Taverner, P.A. and B.H. Swales. 1907. The birds of Point Pelee. Wilson Bulletin 19:82-99.
Toner, G.C., W.E. Edwards, and M.W. Curtis. 1942. Birds of Leeds County. Canadian Field-Naturalist 56:1-12; 21-24; 34-44; 50-55.
Footnotes
- footnote[1] Back to paragraph Implementation of nest protection and translocation is conducted using specific existing protocols and is carried out by agency staff under appropriate government permits.
- footnote[2] Back to paragraph Jalava 2004, Peach 2004, Dougan and Associates and McKay 2006, Peach and Donnelly 2010
- footnote[3] Back to paragraph "The definition of one breeding pair can include a confirmed nest, a confirmed breeding pair or a probable breeding observation. A probable breeding observation, in suitable nesting habitat during the breeding season, includes a male and female pair, a courtship or display between a male and a female or 2 males (including courtship feeding or copulation), an adult visiting a probable nest location or building a nest, agitated behaviour or anxiety calls of an adult, or breeding evidence such as a brood patch or cloacal protuberance" (taken from Environment Canada 2011).