Recovery strategy for the Gypsy Cuckoo Bumble Bee
Read the recovery strategy for the Gypsy Cuckoo Bumble Bee (Bombus bohemicus), a species of insect at risk in Ontario.

Cover photo by Sheila Colla
About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act, 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species’ persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of Natural Resources and Forestry Species at Risk webpage.
Recommended citation
Colla, Sheila R. 2017. Recovery Strategy for the Gypsy Cuckoo Bumble Bee (Bombus bohemicus) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources and Forestry, Peterborough, Ontario. v + 23 pp.
© Queen’s Printer for Ontario, 2017
ISBN 978-1-4868-0894-6 (HTML)
ISBN 978-1-4868-0897-7 (PDF)
Content (excluding the cover illustration) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n’est disponible qu’en anglais en vertu du Règlement 411/97 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec recovery.planning@ontario.ca.
Acknowledgments
Many thanks to Leif Richardson and Paul Williams for database management and taxonomic expertise. The author would like to acknowledge the following insect collections which house Ontario specimens of this species: University of Guelph Insect Collection, Packer Collection York University, Ohio State Clement Dasch Collection, University of Kansas, Lyman Entomological Collection McGill University, the Canadian National Collection, the Royal Ontario Museum, American Museum of Natural History, University of Connecticut, Canadian Museum of Nature, Algonquin Provincial Park Collection, Peter Hallett Personal Collection, Illinois Natural History Survey, and Essig Museum of Entomology.
Declaration
The recovery strategy for the Gypsy Cuckoo Bumble Bee was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all of the individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ontario Ministry of Natural Resources and Forestry
Environment and Climate Change Canada – Canadian Wildlife Service, Ontario
Parks Canada Agency
Executive summary
The Gypsy Cuckoo Bumble Bee is a medium-sized bumble bee with a distinctive white tail and black head. Females lack pollen baskets and have a strongly curved abdomen which distinguish them from non-cuckoo bumble bees. This species likely occurred throughout much of the province of Ontario, although the central and northern portions of the province are under-surveyed. It is an obligate social parasite, usurping nests of Rusty-patched Bumble Bees and Yellow-banded Bumble Bees to rear young. This species has declined throughout its expansive North American range including in Ontario. In Ontario, Gypsy Cuckoo Bumble Bee is designated as endangered under the Endangered Species Act, 2007.
The major threat to this species’ survival is the decline of its host species, which are at-risk of extinction. In order to recover this species, host populations must reverse their declines and stabilize. Additionally, knowledge gaps with respect to ecological requirements and vulnerability to stressors require further research.
The recovery goal for the Gypsy Cuckoo Bumble Bee is to ensure the species’ long-term survival in Ontario by achieving a self-sustaining population. This will be achieved through research, protection and management of host species’ populations as well as detected extant populations of the Gypsy Cuckoo Bumble Bee throughout the province. It will be critical to mitigate threats to these species, including pathogen spillover, habitat loss, degradation and fragmentation, pesticide use and climate change.
The protection and recovery objectives are to:
- Survey, protect and monitor Gypsy Cuckoo Bumble Bees throughout their Ontario range.
- Monitor and recover host species (Rusty-patched Bumble Bee and Yellow-banded Bumble Bee).
- Monitor, create and improve habitat in or near Pinery Provincial Park and other recently occupied sites in Ontario.
- Conduct research to address knowledge gaps for the Gypsy Cuckoo Bumble Bee.
It is recommended that the area prescribed as habitat in a habitat regulation under the Endangered Species Act be based on at least one of the following criteria being met.
- Documented Gypsy Cuckoo Bumble Bee occurrence (within past 20 years).
- Documented nests of host species (within past 20 years), within 10 km of historic Gypsy Cuckoo Bumble Bee occurrence.
Recent Gypsy Cuckoo Bumble Bee sites include: Pinery Provincial Park (2008), Presqu’ile Provincial Park (2000), Dunks Bay (2000) and Oliphant Fen (2000). If this species or its hosts’ nests are located at any new sites, it is recommended that the habitat regulation be updated to include these.
At these sites, forage habitat (diverse floral resources), nesting habitat (e.g., rodent burrows containing host bumble bee species) and overwintering habitat (e.g., rotting logs and mulch) are critical to the species’ ecological requirements.
If Gypsy Cuckoo Bumble Bees are found at any site, it is recommended that habitat be prescribed as a 2 kilometre radius around the area. A radius of 2 kilometres was chosen as host bumble bees can forage up to 2.5 km from the nest. At sites where recent populations (within past 20 years) of host species occur within 10 km of historical Gypsy Cuckoo Bumble Bee occurrences, it is also recommended that habitat be prescribed as a 2 kilometre radius around the area.
1.0 Background information
1.1 Species assessment and classification
Table 1. Species assessment and classification of the Gypsy Cuckoo Bumble Bee (Bombus bohemicus)
The glossary provides definitions for the abbreviations within, and for other technical terms in this document.
| Assessment | Status |
| SARO list classification | Endangered |
| SARO list history | Endangered (2015) |
| COSEWIC assessment history | Endangered (2014) |
| SARA schedule 1 | No schedule, no status |
| Conservation status rankings | GRANK: G4 NRANK: NH SRANK: SU |
1.2 Species description and biology
Species description
The Gypsy Cuckoo Bumble Bee is a medium-sized bumble bee (11-19 mm) with a distinctive white or pale yellow ‘tail’ and almost entirely black head (Williams et al. 2014). The sides of the thorax are mostly black and the anterior of the thorax, above the wings, is primarily yellow (COSEWIC 2014). The first and second abdominal segments are black with the remaining segments having white and/or yellow hairs (COSEWIC 2014). The Gypsy Cuckoo Bumble Bee is one of six North American members of the bumble bee subgenus Psithyrus (i.e., cuckoo bumble bees) (Williams et al. 2014). Cuckoo bumble bees are obligate social parasites, where the female usurps the nest of another species and the workers of that nest rear their young. Unlike non-cuckoo bumble bees, cuckoos: a) lack pollen baskets (i.e., corbiculae) on their hind legs since they do not carry pollen to provision their young; and b) females have a strongly curved abdomen to help usurp the host colony’s queen (COSEWIC 2014). Cuckoo bumble bees do not have a worker caste; only males and queen-sized females exist.
Species biology
The Gypsy Cuckoo Bumble Bee is an obligate social parasite of species belonging to the subgenus Bombus sensu stricto. In Ontario, the host species belonging to this subgenus are the Rusty-patched Bumble Bee (Bombus affinis) and the Yellow-banded Bumble Bee (Bombus terricola). Adult females of the Gypsy Cuckoo Bumble Bee and individual queens of both of its host species emerge from their overwintering sites in late April/May (Colla and Dumesh 2010). Female cuckoos enter an established nest of their host species and either kill or subdue the queen (Suhonen et al. 2015). The female cuckoo then lays eggs which are tended by the workers of the host colony. It is unknown how the host colony is detected and whether the host colony continues to produce young after being usurped. Female Gypsy Cuckoo Bumble Bees can be detected in flight, foraging or in nests from April until September (Colla and Dumesh 2010). Male Gypsy Cuckoo Bumble Bees are reared to the adult stage in the usurped colonies, then exit to locate mates. Adult males have been detected in flight or on flowers from June to October (Colla and Dumesh 2010). They do not overwinter but instead die before the winter. Young mated females select overwintering sites and emerge in the spring. It is unknown how individuals locate mates or host colonies.
1.3 Distribution, abundance and population trends
The Gypsy Cuckoo Bumble Bee is a Holarctic species, occurring throughout Canada, the northern USA as well as much of Europe and Asia. The IUCN Red List Status for this species globally is Data Deficient (Hatfield et al. 2016). It is considered abundant/stable in Europe, where its host species are common (Hatfield et al. 2016). In North America, it has suffered drastic declines throughout its Canadian and USA range (Cameron et al. 2011; Colla et al. 2012; Hatfield et al. 2016). In Canada, it is assessed by COSEWIC as Endangered.
The Gypsy Cuckoo Bumble Bee likely occurred through much of the province of Ontario although the central and northern portions of the province have been poorly surveyed. There are historic records (dating back to the late 1800s) from a variety of ecozones including the Mixedwood Plains, Boreal Shield and Hudson Bay Lowlands. The southern Ontario portion of its range has been extensively surveyed in recent decades with few individuals detected. The most recent provincial records include Dunks Bay (2000), Oliphant Fen (2000), Presqu’ile Provincial Park (2000) and Pinery Provincial Park (2008).
The Gypsy Cuckoo Bumble Bee has exhibited declines in Ontario, where suitable data exist. Southern Ontario has been extensively searched with only a single specimen located in the past 10 years (COSEWIC 2014, S. Colla, unpublished data). The specimen was located at Pinery Provincial Park which is also the last known Canadian site for the Rusty-patched Bumble Bee (COSEWIC 2010). Historical collections, up until the 1990s, indicate 1-2% of bumble bees in Ontario were this species (i.e., 1-2% relative abundance), indicating a significant decline in recent decades (COSEWIC 2014). A survey in southern Ontario conducted between 2004 and 2006 failed to locate the Gypsy Cuckoo Bumble Bee at historical sites of occurrence where it had been found to occur at 1% relative abundance during a similar survey in 1971-1973 (Colla and Packer 2008).
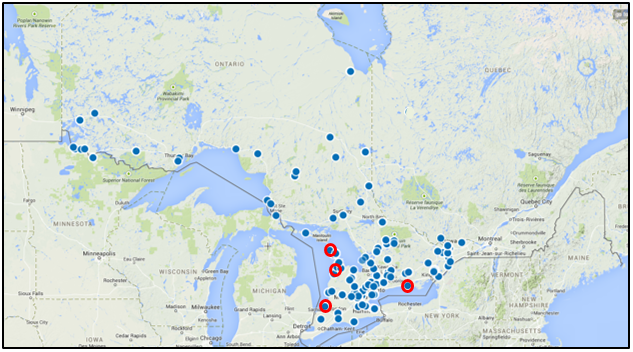
Figure 1. Historical range of the Gypsy Cuckoo Bumble Bee in Ontario(n=352)
Map created using GeoCAT and a subset of the data used in Williams et al. (2014) and COSEWIC (2014). Red circles indicate sites within last 20 years.
1.4 Habitat needs
Bumble bees require nesting habitat, forage habitat, overwintering habitat and mating habitat. Given the expansive range of this species (Williams et al. 2014), many habitat types across ecozones are suitable. Currently, nothing is known about the mating and overwintering habitat requirements for the Gypsy Cuckoo Bumble Bee. Overwintering habitat for bumble bees in Ontario may include rotting logs, leaf litter and mulch (Macfarlane 1974), burrows in soil (Macfarlane 1974), and garden compost (Goulson 2010). Forage habitat includes the plant species mentioned below as well as other flowering plants which bloom early spring (e.g. Willow) to late autumn (e.g. Goldenrod). In addition to forage plants suitable for the Gypsy Cuckoo Bumble Bee, pollen and nectar sources for the host species are also required to build resources to rear the larvae and pupae in the colony to the adult stage. These plant species can be found in Colla and Dumesh (2010). Forage habitat occurs in old fields, grasslands, dunes, alvars, woodlands (especially in the spring) and road sides. Nesting habitat requirements are those of its host species (the Rusty-patched Bumble Bee and Yellow-banded Bumble Bee). Both host species are habitat generalists which primarily use rodent burrows (e.g. Eastern Cottontail rabbit) as nest sites (Macfarlane 1974; Colla and Dumesh 2010). There has been some success rearing host species using bee boxes (e.g. Owen et al. 1980). Mating requirements are unknown. Males of some bumble bee species congregate at local hilltops (i.e., “hilltopping”), which is a common mate-finding behaviour among different insect groups (Goulson et al. 2011).
Gypsy Cuckoo Bumble Bees are not habitat specialists but have been mostly detected within or near wooded habitats (Colla and Dumesh 2010), likely because of host nest sites or early spring forage availability. The following list of native and non-native forage plants was compiled by Colla and Dumesh (2010) from museum specimens and literature: Allium, Aralia, Cephalanthus, Eupatorium, Penstemon, Rubus, Solidago, Solidago canadensis (Canada Goldenrod), Symphyotrichum novae-angliae (New England Aster), Vaccinium angustifolium (Lowbush Blueberry), Vaccinium corymbosum (Highbush Blueberry) and species non-native to Ontario including Inula helenium (Elecampane), Melilotus albus (Sweet Clover), Pilosella aurantiaca (Orange Hawkweed), Syringa vulgaris (Common Lilac), Taraxacum officinale (Dandelion), Trifolium hybridum (Alsike Clover) and Trifolium pratense (Red Clover). The non-native plants generally occur in open, disturbed habitats, not woodlands.
1.5 Limiting factors
Bumble bees (including the Gypsy Cuckoo Bumble Bee) are among the most vulnerable of our native bee species to environmental stressors related to human population density and land use (Bartomeus et al. 2013). The reason for this is not yet understood but may be related to their long flight seasons (i.e. colonies need to persist spring to fall), central place provisioning (i.e. inability to relocate), relatively large resource requirements to produce reproductive individuals at the end of the colony cycle or other shared life history traits (Colla 2016).
The sex determination system of bumble bees can be a limiting factor contributing to declines, as it can produce sterile bees when population sizes are small. Sex is determined by a single-locus complementary sex-determination system in bumble bees. Normally, fertilized (diploid) eggs become female and unfertilized (haploid) eggs become male. Ploidy level is determined based on a single locus; if more than one variant (i.e., allele) at the locus is detected, then it is considered diploid and proceeds with female development, whereas if only one variant is detected the cell proceeds with male development. When a population has little genetic variation, fertilized eggs may have two copies of the same sex-determination variant, and thus be mistaken for haploid even though they are in fact diploid. Thus in small populations, sterile diploid (Zayed and Packer 2001) and triploid (Darvill et al. 2012) males are produced through inbreeding and genetic drift. This can significantly increase extinction risk among declining populations (Zayed and Packer 2005). Due to lower dispersal ability and smaller population sizes than non-cuckoo bees, cuckoo bees may be more vulnerable to inbreeding and disease (Erler & Lattorff 2010).
Members of the subgenus Psithyrus are cuckoo bumble bees which rely on the worker caste of other bumble bee species to rear individuals from the egg to adult stage (Laverty and Harder 1988). Thus, populations of this and other cuckoo bumble bee species are limited by nest densities of their host species. As a result, cuckoo species are more vulnerable to extinction than their host species (Suhonen et al. 2015). In addition, their ability to adapt to large scale environmental stressors is likely hindered by this close reliance on their host species and the vulnerability of the host species to various threats. In Ontario, both host species have been noted to be in decline (Colla and Packer 2008; COSEWIC 2010; Colla et al. 2012; COSEWIC 2015).
1.6 Threats to survival and recovery
Decline of hosts
The primary threat to this cuckoo species is the decline of its two host species in Ontario; the Rusty-patched Bumble Bee and the Yellow-banded Bumble Bee. The declines of these host species have been documented in Ontario and surrounding jurisdictions in the USA and Canada (reviewed in COSEWIC 2010; COSEWIC 2015) deeming them to be at-risk of extinction. Despite being previously common, the Rusty-patched Bumble Bee has only been detected a few times in the last decade and the Yellow-banded Bumble Bee at relatively few sites (reviewed in COSEWIC 2010; COSEWIC 2015). Stable populations of Yellow-banded and Rusty-patched Bumble Bees are required to sustain populations of the Gypsy Cuckoo Bumble Bee (Suhonen et al. 2015).
Pathogens and parasites
Pathogen spillover from managed bees is cited to explain declines of the Rusty-patched and Yellow-banded Bumble Bees (e.g. Cameron et al. 2011; Szabo et al. 2012; Graystock et al. 2016). Spillover occurs when managed populations introduce new pathogens to wild populations or amplify pathogens (spillback) which may have been naturally in lower abundances (Graystock et al. 2016). The mechanism for disease transfer is the use of shared floral resources (Durrer & Schmid-Hempel 1994). Pathogen spillover has been documented among bumble bees in Ontario (Colla et al. 2006). Laboratory studies provide increasing evidence that multiple honey bee pathogens are transferable to bumble bees (e.g., Meeus et al. 2011; Peng et al. 2011; Plischuk et al. 2009). Inbreeding depression and low genetic diversity due to small population size can also result in increased disease levels among declining species (Cameron et al. 2011). Pathogen spillover may impact the Gypsy Cuckoo Bumble Bee directly or by causing the decline of its host species (e.g. Cameron et al. 2011; Szabo et al. 2012).
Habitat loss, fragmentation and degradation
There are numerous types of habitat alteration which can have a negative impact on the Gypsy Cuckoo Bumble Bee and its hosts. Bumble bees require forage, nesting and overwintering habitat within accessible distances. Loss of any of these habitat components within a colonies range would negatively impact colony fitness. Fragmentation of these habitats, such as with roads, has been noted to negatively impact bumble bees (e.g., Bhattacharya et al. 2003).
Forage habitat consists of a diversity of flowering plants with bloom times which range from early spring (often spring ephemerals or shrubs) to late autumn (e.g., old fields, roadsides, grasslands). The loss of forage habitat due to urbanization and agricultural conversion has been shown to decrease bumble bee diversity and abundance (e.g., Hines and Hendrix 2005). The quality of forage can also be impacted by the use of pesticides. Herbicides can remove good quality forage plant species (e.g., Nicholls and Altieri 2013). Pollen and nectar resources can also be removed from forage habitat by managed bees (e.g., Colla & MacIvor, in press). In Ontario this includes colonies of non-native honey bees and managed colonies of native bumble bees (most commonly the Common Eastern Bumble Bee, Bombus impatiens) used for agricultural purposes. Honey bees can extract floral resources up to 10 km from their hives (Beekman and Ratnieks 2000).
The nesting habitat of the Gypsy Cuckoo Bumble Bee is that of the Rusty-patched and Yellow-banded Bumble Bee. These host species rely on rodent burrows for nest sites (Colla & Dumesh 2010). Thus, threats to local rodent populations would result in the loss of nesting habitat.
The mating habitat for Gypsy Cuckoo Bumble Bee is unknown. Given the low density of cuckoo bee populations, finding a mate may pose a challenge.
Overwintering habitat is not well understood for bumble bees in Ontario but may include rotting logs, leaf litter and mulch (Macfarlane 1974), burrows in soil (Macfarlane 1974), and garden compost (Goulson 2010). Removing these components at a site may result in the loss of overwintering habitat.
Pesticide use
Wild bumble bees can be exposed to pesticides while foraging on treated plants or if their nests are contaminated (by workers bringing in contaminated resources). In agricultural systems, insecticides (e.g. neonicotinoids) can negatively impact non-target organisms, like bees, while they forage on the crop or adjacent flowering plants or nests can be sprayed directly. Flowering plants within the watershed can also be contaminated as many insecticides are water soluble (David et al. 2016). In urban settings, exposure may occur on treated garden plants or adjacent to treated grass seed. Systemic insecticides (i.e. soluble chemicals targeting pests which travel through plant tissues and are present in tissues and fluids) present in pollen and/or nectar can have lethal or sub-lethal affects at the individual or colony levels in bumble bees (Rundlof et al. 2015; Morandin et al. 2005; Franklin et al. 2004). Fungicides may also impact populations as studies show sub-lethal impacts on bee health and behaviour though studies have been mostly in the laboratory thus far (e.g., Elston et al. 2013; Sprayberry et al. 2013). Given the growing number of studies on other bee species, it is likely extant populations of the Gypsy Cuckoo Bumble Bee and its hosts can be negatively impacted by exposure to a variety of pesticides and combinations of pesticides.
Climate change
There is some indication that with climate change, many bumble bee species have declined more in southern portions of their ranges (Bartomeus et al. 2013; Kerr et al. 2015) while not expanding northward (Kerr et al. 2015). Climate change has been noted to alter the emergence date of wild bees as well as forage plant species (Bartomeus et al. 2011; Thomson 2010). Husband et al. (1980) suggests spring storms (i.e. snow, rain or hail) could be particularly dangerous for early-emerging bumble bees (such as the Gypsy Cuckoo Bumble Bee and its hosts). Spring storms could damage early food sources or kill newly emerged queens. This is concerning given the increase in storms predicted by future climate change scenarios in Ontario (Wang et al. 2014). Whether these contribute to the decline of the Gypsy Cuckoo Bumble Bee or its host species remains to be determined but evidence from other species suggest is it possible.
1.7 Knowledge gaps
There are many knowledge gaps which exist for this and other native bumble bee species.
- The current distribution and abundance of this species is uncertain in central and northern Ontario where there have been few recent surveys.
- Ecological and habitat requirements for overwintering and mating are unknown for the Gypsy Cuckoo Bumble Bee and its host species.
- It is unknown how Gypsy Cuckoo Bumble Bees find: a) each other for mating, and b) host nests to usurp. If olfactory (i.e., smell) cues are used, such chemicals might be used to attract Gypsy Cuckoo Bumble Bees for monitoring and reintroduction work, if deemed appropriate.
- The minimum required host population size (Rusty-patched Bumble Bee and Yellow-banded Bumble Bee) to maintain a sustainable Gypsy Cuckoo Bumble Bee population is unknown. The minimum viable population size for the Gypsy Cuckoo Bumble Bee has not been determined.
- The extent of environmental stressors (e.g., pesticide use, forage loss, habitat fragmentation, disease and parasite dynamics, climate change and competition with invasive species), and possible synergies between these stressors, requires additional research.
- This species’ dispersal ability and forage range size are unknown, and are needed to inform land use planning and conservation efforts. Similar information is unknown for its host species.
- While there is evidence cuckoo bumble bees can be reared in captivity (Lhomme et al. 2013), the feasibility of conservation management tools such as translocation, captive breeding and co-reintroduction with hosts requires further research.
1.8 Recovery actions completed or underway
Recovery actions for one host species, the Rusty-patched Bumble Bee, are currently underway as described in its Ontario recovery strategy (Colla and Taylor-Pindar 2011), draft Canadian recovery strategy ( ECCC 2016), and Ontario government response statement (OMNR 2012). This species is currently listed as Endangered federally (under SARA) and provincially (under Ontario’s ESA). The Yellow-banded Bumble Bee was recently assessed as Special Concern federally by COSEWIC, and has been listed as special concern under Ontario’s ESA, though it has not yet been listed under SARA. Recovery actions for this host species will be undertaken in the future.
The citizen science program Bumble Bee Watch aids monitoring of bumble bees across North America, including the Gypsy Cuckoo Bumble Bee and its host species. It is a citizen science program that collects data and photos of bumble bees from volunteers across North America, in an effort to track and conserve bumble bees. All species are identified or verified by regional experts, making it an increasingly valuable data source for current and future analyses. There is currently a website (Bumble Bee Watch) and an iOS app was launched in 2017. While it welcomes and is primarily for incidental ad-hoc observations, there are already areas using it for formal monitoring of bumble bees (e.g. Pinery Provincial Park). The collaborative program is run by several partners, including The Xerces Society for Invertebrate Conservation, the Faculty of Environmental Studies - York University, Wildlife Preservation Canada, University of Ottawa, Montreal Insectarium, Natural History Museum (London), and BeeSpotter.
Ontario released its Pollinator Health Action Plan in 2017. This includes objectives for wild pollinator monitoring and habitat availability. As part of Ontario’s Pollinator Health Strategy, the coating of corn and soybean seeds with neonicotinoid insecticides is being regulated. This should reduce the amount of neonicotinoids taken up by flowering plants in agricultural areas and their watersheds in future years.
2.0 Recovery
2.1 Recovery goal
The recovery goal for the Gypsy Cuckoo Bumble Bee is to ensure the species’ long-term survival in Ontario by achieving a self-sustaining population. This should be achieved by protecting and managing host species’ populations and by detecting extant populations of Gypsy Cuckoo Bumble Bee throughout the province. While the Rusty-patched Bumble Bee is increasingly rare and has not been detected at its last known Canadian site (Pinery Provincial Park), there are still numerous small populations of Yellow-banded Bumble Bees which make this goal feasible. This should be accomplished by mitigating threats to these species, including pathogen spillover, habitat loss, fragmentation and degradation, pesticide use and climate change.
2.2 Protection and recovery objectives
Table 2. Protection and recovery objectives
| Number | Protection or recovery objective |
|---|---|
| 1 | Survey, protect and monitor Gypsy Cuckoo Bumble Bees throughout their Ontario range. |
| 2 | Monitor and recover host species (Rusty-patched Bumble Bee and Yellow-banded Bumble Bee). |
| 3 | Monitor, create and improve habitat in or near Pinery Provincial Park and other recently occupied sites in Ontario. |
| 4 | Conduct research to address knowledge gaps for the Gypsy Cuckoo Bumble Bee. |
2.3 Approaches to recovery
Table 3. Approaches to recovery of the Gypsy Cuckoo Bumble Bee in Ontario
Objective 1: Survey, protect and monitor Gypsy Cuckoo Bumble Bees throughout their Ontario range
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Ongoing | Monitoring and Assessment Education and Outreach Stewardship |
|
Knowledge gaps:
|
| Critical | Ongoing | Inventory, Monitoring and Assessment Research |
|
Knowledge gaps:
|
| Beneficial | Ongoing | Inventory, Monitoring and Assessment Education and Outreach Communication, Stewardship |
|
Knowledge gaps:
|
Objective 2: Monitor and recover host species (Rusty-patched Bumble Bee and Yellow-banded Bumble Bee)
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Ongoing | Protection |
|
Threats:
|
| Beneficial | Ongoing | Monitoring and Assessment Education and Outreach Stewardship |
|
Knowledge gaps:
|
| Beneficial | Long-term | Protection Management |
|
Threats:
Knowledge gaps:
|
Objective 3: Monitor, create and improve habitat in or near Pinery Provincial Park and other recently occupied sites in Ontario
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Ongoing | Management |
|
Threats:
|
| Necessary | Ongoing | Protection Management Education and Outreach Communication,Stewardship |
|
Threats:
|
| Necessary | Short term | Stewardship, management, education and outreach |
|
Threat:
|
Objective 4: Conduct research to address knowledge gaps for the Gypsy Cuckoo Bumble Bee
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Ongoing | Research |
|
Knowledge gaps:
|
| Critical | Ongoing | Research Management |
|
Threats:
Knowledge gaps:
|
| Critical | Ongoing | Research |
|
Knowledge gaps:
|
| Necessary | Ongoing | Research |
|
Threats:
Knowledge gaps:
|
| Necessary | Ongoing | Research |
|
Knowledge gaps:
|
2.4 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of Natural Resources and Forestry on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the author will be one of many sources considered by the Minister when developing the habitat regulation for this species.
The potential habitat for the Gypsy Cuckoo Bumble Bee covers much of the province. It is recommended that the area prescribed as habitat in the habitat regulation be based on at least one of the following criteria being met.
- Documented Gypsy Cuckoo Bumble Bee occurrence (within past 20 years).
- Documented nests of host species (within past 20 years), within 10 km (estimated bumblebee dispersal distance, e.g. Kraus et al. 2009 (gene flow)) of historic Gypsy Cuckoo Bumble Bee occurrence.
Recent COSEWIC reports provide records of occurrence for the Rusty-patched Bumble Bee (COSEWIC 2010) and Yellow-banded Bumble Bee (COSEWIC 2015) within past 20 years. In addition, the following sites are considered recent for the Gypsy Cuckoo Bumble Bee: Pinery Provincial Park (2008), Presqu’ile Provincial Park (2000), Dunks Bay (2000) and Oliphant Fen (2000). If this species or its hosts’ nests are located at any new sites, the habitat regulation should be updated to include these.
If individual Gypsy Cuckoo Bumble Bees or host species’ nests are found at any site (as per criteria above), it is recommended that habitat be prescribed as a two kilometre radius around the site where the individual or host species’ nest was seen. The distance selected for the habitat regulation is based on studies completed for Buff-tailed Bumble Bee (Bombus terrestris), a known host to the Gypsy Cuckoo Bumble Bee in the Old World. The foraging distances of host species found in Ontario (Yellow-banded Bumble Bee and Rusty-patched Bumble Bee) have not been studied, so information from the closely-related and well-studied Buff-tailed Bumble Bee is used instead. The selected distance is based on the host species foraging range, indicating where nests are most likely to be found when an individual bee is sampled while foraging (i.e. Gypsy Cuckoo Bumble Bee habitat). A radius of two kilometres is recommended as it is known that the Buff-tailed Bumble Bee forages from 625 up to 2500 m, though the further distance is less likely due to energetic costs (Walther-Hellwig and Frankl 2000; Darvill et al. 2004; Wolf & Moritz 2008; Hagan et al. 2011).
Habitat can be, but is not limited to, natural or anthropogenic structures (e.g. buildings with nests) or landscapes, including farms, forests, grasslands, and urban gardens. At these sites, forage habitat (diverse floral resources), nesting habitat (e.g., rodent burrows containing host bumble bee species) and overwintering habitat (e.g., rotting logs and mulch) are critical to the species’ ecological requirements. Habitat which would not be considered suitable within the radius includes open water, rocky cliffs and other types of habitat which would not provide forage, nesting, mating or overwintering habitat.
Glossary
Central place provisioning: Where foraging individuals travel from a home base (i.e. nest) to foraging location rather than passing through an area or travelling at random.
Committee on the Status of Endangered Wildlife in Canada (COSEWIC): The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
Committee on the Status of Species at Risk in Ontario (COSSARO): The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
Conservation status rank: A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. Ranks are determined by NatureServe and, in the case of Ontario’s S-rank, by Ontario’s Natural Heritage Information Centre. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperilled
2 = imperilled
3 = vulnerable
4 = apparently secure
5 = secure
NR = not yet ranked
NH = Possibly extirpated
SU = Unrankable
Endangered Species Act, 2007 (ESA): The provincial legislation that provides protection to species at risk in Ontario.
Obligate social parasite: A species which cannot complete its life cycle without laying eggs in a host colony, which are then tended by the host species.
Species at Risk Act (SARA): The federal legislation that provides protection to species at risk in Canada. This act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
Species at Risk in Ontario (SARO) List: The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
Worker Caste: Specialized individuals within Eusocial insects which tend to the nest and in general, do not reproduce.
References
Bartomeus, I., J.S. Ascher, J. Gibbs, B.N. Danforth, D.L. Wagner, S.M. Hedtke and R. Winfree. 2013. Historical changes in northeastern US bee pollinators related to shared ecological traits. Proceedings of the National Academy of Sciences 110:4656-4660.
Bartomeus, I., J.S. Ascher, D. Wagner, B.N. Danforth, S.R. Colla, S. Kornbluth and R. Winfree. 2011. Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proceedings of the National Academy of Sciences 108: 20645-20649.
Beekman, M and F.L. Ratnieks. 2000. Long range foraging by the honey-bee, Apis mellifera. Functional Ecology 14:490-496.
Bhattacharya, M., R.B. Primack, and J. Gerwein. 2003. Are roads and railroads barriers to bumble bee movement in a temperate suburban conservation area? Biological Conservation 109:37-45.
Cameron, S.A., J.D. Lozier, J.P. Strange, J.B. Koch, N. Cordes, L.F. Solter and T.L. Griswold. 2011. Patterns of widespread decline in North American bumble bees. Proceedings of the National Academy of Sciences 108:662-667.
Colla, S.R. 2016. Status, threats and conservation recommendations for wild bumble bees (Bombus spp.) in Ontario, Canada: a review for policymakers and practitioners. Natural Areas Journal 36:412-426.
Colla, S.R., F. Gadallah, L. Richardson, D. Wagner and L. Gall. 2012. Assessing declines of North American bumble bees using museum specimens. Biodiversity and Conservation 21:3585-3595.
Colla, S.R. and S. Dumesh. 2010. The bumble bees of southern Ontario: notes on natural history and distribution. Journal of the Entomological Society of Ontario 141:38-67.
Colla, S.R. and J.S. MacIvor. In press. Questioning public perception, conservation policy, and recovery actions for honey bees in North America. Conservation Biology.
Colla, S.R., M.C Otterstatter, R.J. Gegear and J.D. Thomson. 2006. Plight of the bumble bee: pathogen spillover from commercial to wild populations. Biological Conservation 129:461-467.
Colla, S.R. and L. Packer. 2008. Evidence for decline in eastern North American bumble bees (Hymenoptera: Apidae), with special focus on Bombus affinis Cresson. Biodiversity and Conservation 17:1379-1391.
Colla, S.R. and A. Taylor-Pindar. 2011. Recovery Strategy for the Rusty-patched Bumble Bee (Bombus affinis) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. vi + 21 pp.
COSEWIC. 2010. COSEWIC assessment and status report on the Rusty–patched Bumble Bee Bombus affinis in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. vi + 34 pp.
COSEWIC. 2014. COSEWIC assessment and status report on the Gypsy Cuckoo Bumble Bee Bombus bohemicus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. ix + 56 pp.
COSEWIC. 2015. COSEWIC assessment and status report on the Yellow-banded Bumble Bee Bombus terricola in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. ix + 60 pp.
Darvill, Ben, Mairi E. Knight, and Dave Goulson. 2004. Use of genetic markers to quantify bumblebee foraging range and nest density. Oikos 107: 471-478.
Darvill, B., O. LePais, L.C. Woodall and D. Goulson. 2012. Triploid bumble bees indicate a direct cost of inbreeding in fragmented populations. Molecular Ecology 21:3988-3995.
David, A., Botías, C., Abdul-Sada, A., Nicholls, E., Rotheray, E. L., Hill, E. M., and Goulson, D. 2016. Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environment international, 88: 169-178.
Durrer, S and P. Schmid-Hempel. 1994. Shared use of flowers leads to horizontal transmission Proceedings of the Royal Society B-Biological Sciences 258: 299-302.
ECCC. 2016. Recovery Strategy for the Rusty-patched Bumble Bee (Bombus affinis) in Canada [Proposed]. Species at Risk Act Recovery Strategy Series. Environment and Climate Change Canada. Ottawa, ON. vii + 56 p.
Elston, C., H.M. Thompson and K.F.A. Walter. 2013. Sub-lethal effects of thiamethoxam, a neonicotinoid pesticide, and propiconazole, a DMI fungicide, on colony initiation in bumblebee (Bombus terrestris) micro-colonies. Apidologie 44:563-574.
Erler, S. and H.M.G. Lattorff. 2010. The degree of parasitism of the bumblebee (Bombus terrestris) by cuckoo bumblebees (Bombus (Psithyrus) vertalis). Insectes Sociaux 57:371-377.
Franklin, M.T., M.L. Winston and L.A. Morandin. 2004. Effects of clothianidin on Bombus impatiens (Hymenoptera: Apidae) colony health and foraging ability. Journal of Economic Entomology 97:369-373.
Goulson, D., Sangster, E.L. and Young, J.C. 2011. Evidence for hilltopping in bumblebees? Ecological Entomology (2011), 36: 560-563.
Goulson, D. 2010. Bumblebees: behaviour, ecology, and conservation. Oxford University Press on Demand. 317 pp.
Goulson, D., & Hughes, W. O. 2015. Mitigating the anthropogenic spread of bee parasites to protect wild pollinators. Biological Conservation, 191: 10-19
Graystock, P., Blane, E. J., McFrederick, Q. S., Goulson, D., & Hughes, W. O. 2016. Do managed bees drive parasite spread and emergence in wild bees?. International Journal for Parasitology: Parasites and Wildlife, 5(1), 64-75.
Hagen M, Wikelski M, Kissling WD 2011. Space Use of Bumblebees (Bombus spp.) Revealed by Radio-Tracking. PLoS ONE 6(5): e19997. doi:10.1371/journal.pone.0019997
Hatfield, R., S. Jepsen, R. Thorp, L. Richardson and S. Colla. 2016. Bombus bohemicus. The IUCN Red List of Threatened Species 2016. [website accessed January 2017].
Hines, H.M. and S.D. Hendrix. 2005. Bumble bee (Hymenoptera: Apidae) diversity and abundance in tallgrass prairie patches: effects of local and landscape floral resources. Environmental Entomology 34:1477-1484.
Husband R.W., R. Fischer and T.W. Porter. 1980. Description and biology of bumble bees (Hymenoptera: Apidae) in Michigan. Great Lakes Entomologist. 13:225-239.
Kerr, J.T., A. Pindar, P. Galpern, L. Packer, S.G. Potts, S.M. Roberts, P. Rasmont, O. Schweiger, S.R. Colla, L.L. Richardson, D.L. Wagner, L.F. Gall, D.S. Sikes and A. Pantoja. 2015. Climate change impacts on bumblebees converge across continents. Science 349:177-180.
Kraus, F. B., Wolf, S., & Moritz, R. F. A. 2009. Male flight distance and population substructure in the bumblebee Bombus terrestris. Journal of Animal Ecology, 78: 247-252.
Laverty, T.M. and L.D. Harder. 1988. The bumble bees of eastern Canada. Canadian Entomologist 120:965-987.
L'homme, P., A. Sramkova, K. Kreuter, T. Lecocq, P. Rasmont and M. Ayasse. 2013. A method for year-round rearing of cuckoo bumblebees (Hymenoptera: Apoidea: Bombus subgenus Psithyrus). Annales de la Société Entomologique de France 49:117-125.
Macfarlane, R.P. 1974. Ecology of Bombinae (Hymenoptera: Apidae) of Southern Ontario, with emphasis on their natural enemies and relationships with flowers. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada.
Meeus, I., M.J.F. Brown, D.C. De Graaf and G. Smagghe. 2011. Effects of invasive parasites on bumble bee declines. Conservation Biology 25:662-671.
Morandin, L.A., M.L. Winston, M.T. Franklin and V.A. Abbott. 2005. Lethal and sub-lethal effects of spinosad on bumble bees (Bombus impatiens Cresson). Pest Management Science 61:619-626.
Nicholls, C.I. and M.A. Altieri. 2013. Plant biodiversity enhances bees and other insect pollinators in agroecosystems. A review. Agronomy for Sustainable Development 33:257-274.
OMNR. 2012. Rusty-patched Bumble Bee: Ontario Government Response Statement. Ontario Ministry of Natural Resources. Peterborough, ON. 4 pp.
Owen, R. H. Rodd, R.C. Plowright. 1980. Sex ratios in bumble bee colonies: Complications due to orphaning? Behavioural Ecology and Sociobiology 7: 287-291.
Peng, W.J., J.L. Li, H. Boncristiani, J.P. Strange, M. Hamilton and Y.P. Chen. 2011. Host range expansion of honey bee Black Queen Cell Virus in the bumble bee, Bombus huntii. Apidologie 42:650-658.
Plischuk, S., R. Martin-Hernandez, L. Prieto, M. Lucia, C. Botias, A. Meana, A.H. Abrahamovich, C. Lange and M. Higes. 2009. South American native bumble bees (Hymenoptera: Apidae) infected by Nosema ceranae (Microsporidia), an emerging pathogen of honey bees (Apis mellifera). Environmental Microbiology Reports 1:131-135.
Rundlöf, M., G.K.S. Andersson, R. Bommarco, I. Fries, V. Hederström, L. Herbertsson, O. Jonsson, B.K. Klatt, T.R. Pedersen, J. Yourstone and H.G. Smith. 2015. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521:77-80.
Spencer, H.G. and M. Zuk. 2016. For host’s sake: The pluses of parasite preservation. Trends in Ecology and Evolution 31:341-343.
Sprayberry, J.D.H., K.A. Ritter, J.A.Riffell. 2013. The Effect of Olfactory Exposure to Non-Insecticidal Agrochemicals on Bumblebee Foraging Behavior PLoS One 8: e76273.
Suhonen, J., J. Rannikko and J. Sorvari. 2015. The rarity of host species affects the co-extinction risk in socially parasitic bumblebee Bombus (Psithyrus) species. Annales Zoologici Fennici 52:236-242.
Szabo, N.D., S.R. Colla, D.L. Wagner, L.F. Gall, and J.T. Kerr. 2012. Do pathogen spillover, pesticide use, or habitat loss explain recent North American bumblebee declines? Conservation Letters 5:232-239.
Thomson, J.D. 2010. Flowering phenology, fruiting success, and progressive deterioration of pollination in a nearly flowering geophytes. Philosophical Transactions of the Royal Society-Biological Sciences 365:3187-3199.
Walther-Hellwig, K. and R. Frankl. 2000. Foraging habitats and foraging distances of bumblebees, Bombus spp. (Hym., Apidae), in an agriculture landscape. Journal of Applied Entomology 124:299-306.
Wang, X.Q, G.H. Huang and J.L. Liu. 2014. Projected increases in intensity and frequency of rainfall extremes through a regional climate modeling approach. Journal of Geophysical Research-Atmospheres 119:13271-13286.
Williams, P.H., R.W. Thorp, L.L. Richardson and S.R. Colla. 2014. Bumble Bees of North America: An Identification Guide. Princeton University Press, USA. 208 pp.
Wolf, S., & Moritz, R. F. 2008. Foraging distance in Bombus terrestris L.(Hymenoptera: Apidae). Apidologie, 39: 419-427.
Zayed A. and L. Packer. 2001. High levels of diploid male production in a primitively eusocial bee (Hymenoptera: Halictidae). Heredity 87:631-636.
Zayed A. and L. Packer. 2005. Complementary sex determination substantially increases extinction proneness of haplodiploid populations. Proceedings of the National Academy of Sciences 102:10742-10746.