Golden-eye Lichen (Great Lakes population) recovery strategy
Read the recovery strategy for the Golden-eye Lichen (Great Lakes population), a lichen species at risk in Ontario.

Cover illustration: Photo by Troy McMullin
About the Ontario recovery strategy series
This series presents the collection of recovery strategies that are prepared or adopted as advice to the Province of Ontario on the recommended approach to recover species at risk. The Province ensures the preparation of recovery strategies to meet its commitments to recover species at risk under the Endangered Species Act, 2007 (ESA) and the Accord for the Protection of Species at Risk in Canada.
What is recovery?
Recovery of species at risk is the process by which the decline of an endangered, threatened, or extirpated species is arrested or reversed, and threats are removed or reduced to improve the likelihood of a species’ persistence in the wild.
What is a recovery strategy?
Under the ESA a recovery strategy provides the best available scientific knowledge on what is required to achieve recovery of a species. A recovery strategy outlines the habitat needs and the threats to the survival and recovery of the species. It also makes recommendations on the objectives for protection and recovery, the approaches to achieve those objectives, and the area that should be considered in the development of a habitat regulation. Sections 11 to 15 of the ESA outline the required content and timelines for developing recovery strategies published in this series.
Recovery strategies are required to be prepared for endangered and threatened species within one or two years respectively of the species being added to the Species at Risk in Ontario list. Recovery strategies are required to be prepared for extirpated species only if reintroduction is considered feasible.
What’s next?
Nine months after the completion of a recovery strategy a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the strategy. The implementation of recovery strategies depends on the continued cooperation and actions of government agencies, individuals, communities, land users, and conservationists.
For more information
To learn more about species at risk recovery in Ontario, please visit the Ministry of the Environment, Conservation and Parks Species at Risk webpage.
Recommended citation
Knight, T. 2019. Recovery Strategy for the Golden-eye Lichen (Teloschistes chrysophthalmus) – Great Lakes population in Ontario. Ontario Recovery Strategy Series. Prepared for the Ministry of the Environment, Conservation and Parks, Peterborough, Ontario. v + 40 pp.
ISBN 978-1-4868-3514-0 (HTML)
ISBN 978-1-4868-3515-7 (PDF)
Content (excluding illustrations) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Recovery strategies prepared under the Endangered Species Act, 2007 », n’est disponible qu’en anglais en vertu du Règlement 671/92 qui en exempte l’application de la Loi sur les services en français. Pour obtenir de l’aide en français, veuillez communiquer avec recovery.planning@ontario.ca.
Author
Tristan Knight – Senior Ecologist/President, Terrastory Environmental Consulting Inc.
Acknowledgments
Several lichenologists and knowledgeable naturalists contributed valuable information and insights to support this recovery strategy. Sam Brinker (Natural Heritage Information Centre) offered expertise, described recent survey efforts, and assisted the author with a 2018 census of the Golden-eye Lichen colony at Sandbanks Provincial Park. Dr. Troy McMullin (Canadian Museum of Nature) and Chris Lewis (MNRF) also offered valuable expertise and insights. Roman Olszewski shed light on the circumstances surrounding the original discovery of Golden-eye Lichen at Sandbanks Provincial Park. Yvette Bree (Ontario Parks) clarified current park management priorities and recreational activities occurring near the colony at Sandbanks Provincial Park. Dr. Richard Harris (New York Botanical Garden) described historical and current records of Golden-eye Lichen from upstate New York. Finally, several iNaturalist users offered substrate and habitat details pertaining to recent records of Golden-eye Lichen from the eastern Great Lakes region.
Declaration
The recovery strategy for the Golden-eye Lichen was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This recovery strategy has been prepared as advice to the Government of Ontario, other responsible jurisdictions and the many different constituencies that may be involved in recovering the species.
The recovery strategy does not necessarily represent the views of all of the individuals who provided advice or contributed to its preparation, or the official positions of the organizations with which the individuals are associated.
The recommended goals, objectives and recovery approaches identified in the strategy are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this strategy is subject to appropriations, priorities and budgetary constraints of the participating jurisdictions and organizations.
Success in the recovery of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this strategy.
Responsible jurisdictions
Ministry of the Environment, Conservation and Parks
Environment and Climate Change Canada – Canadian Wildlife Service, Ontario
Executive summary
Golden-eye Lichen (Teloschistes chrysophthalmus) is a bright orange fruticose lichen appearing as shrubby tufts on tree bark and branches. The Great Lakes population is endangered in Ontario and represented by five historical records and one existing colony. Historical records are concentrated along the shorelines of Lake Erie (Point Pelee National Park, Port Rowan) and Lake Ontario (Presqu’ile Provincial Park, Wellington Beach), with one locality at Niagara Falls. The existing colony occurs on the bark of a mature Red Oak (Quercus rubra) near the shoreline of Lake Ontario at Sandbanks Provincial Park. Based on census counts this colony has declined from eight thalli in 2009 to two thalli in 2018. Golden-eye Lichen is also extremely rare and likely in decline within the United States (US) portion of the eastern Great Lakes region (northwestern Indiana, Michigan, northern Ohio, upstate New York).
The habitat needs of the Great Lakes population are described herein based on relatively few records from southern Ontario and the eastern Great Lakes states. Suitable substrate includes the bark and branches of deciduous and coniferous trees and shrubs, and (to a lesser extent) fence rails. The Great Lakes population is strongly associated with areas of higher humidity (e.g., Great Lakes shoreline, Niagara Falls), although several recent records are from landscaped trees at inland sites. Other habitat variables which this species appears to be associated include calcareous soil, high light penetration, and good air quality.
The recommended long-term recovery goal for the Great Lakes population of Golden-eye Lichen is to protect the known colony at Sandbanks Provincial Park and any new colonies that may be discovered in the future. The recommended objectives for this species are to:
- Maintain the known colony and any colonies that may be discovered in the future through habitat protection, management, and monitoring.
- Conduct surveys in habitats with potentially high suitability across southern Ontario.
- Provide communication and outreach materials to landowners, conservation groups, and municipalities surrounding Sandbanks Provincial Park.
- Conduct research to address knowledge gaps.
Golden-eye Lichen is an epiphyte and requires suitable microsite conditions in order to persist at an existing site and for dispersal opportunities. It is recommended that areas prescribed as habitat for this species extend to a distance of at least 100 m around each documented occurrence. A minimum 50 m radius surrounding Golden-eye Lichen will protect individual thalli by restricting human activities which may adversely affect 1) the thallus, 2) the host tree/shrub, and 3) microsite conditions (e.g., humidity, light, etc.) surrounding the host tree/shrub. A further minimum 50-100 m radius surrounding Golden-eye Lichen will protect suitable habitat for colonization and local dispersal by restricting human activities which may compromise habitat quality.
1.0 Background information
1.1 Species assessment and classification
The following list is assessment and classification information for the Golden-eye Lichen (Teloschistes chrysophthalmus). Note: The glossary provides definitions for abbreviations and technical terms in this document.
- SARO List Classification: Endangered – Great Lakes population
- SARO List History: Endangered – Great Lakes population (2018)
- COSEWIC Assessment History: Endangered – Great Lakes population (2016)
- SARA Schedule 1: No schedule, no status.
- Conservation Status Rankings: G-rank: G4, G5; N-rank: N4; S-rank: S3
1.2 Species description and biology
Species description
Golden-eye Lichen is a bright orange fruticose lichen appearing as shrubby tufts on tree bark and branches. The thallus (lichen vegetative body) colour may appear greenish or greyish on individuals growing in partial shade (Almborn 1989, Wright 2000). Individual thalli are relatively short (up to 2 cm tall) and small (up to 4 cm in diameter; Almborn 1989) but distinctive, especially if growing abundantly. The lobes (thallus branches) are typically flattened, radiate from a basal holdfast (attachment point), and may stand rigidly upright. Thalli may further affix to substrate via rhizines (Nash et al. 2004) or by entanglement. The lower lobe surface is whitish/greyish and often contains wrinkles or longitudinal ridges (Brodo et al. 2001). Apothecia (cup-shaped fruiting bodies) are typically 1-4 mm wide (Brodo et al. 2001) and terminate at the lobe ends but may occur directly on lobes or lobe margins. In its characteristic form Golden-eye Lichen apothecia are fringed with conspicuous cilia (hair-like growths) that resemble eyelashes (hence the common name). Vegetative propagules such as isidia or soredia are not produced, although lobes often terminate in cilia which may facilitate vegetative dispersal (Nyati et al. 2013).
Golden-eye Lichen exhibits considerable infraspecific variation, and populations in other parts of its range often differ somewhat morphologically. For example, some populations contain wider lobes (up to 4 mm) while others exhibit no colour variation between the upper and lower lobe surface (Almborn 1989). Thalli from the midwestern United States (US) lack or contain few apothecial cilia (Howe 1915, Almborn 1989, Nash et al. 2004) and could be mistaken for other species of Teloschistes.
Photographs of Golden-eye Lichen and its habitat from Sandbanks Provincial Park are shown in Figure 1 to Figure 4 below.

Figure 1. Golden-eye Lichen thallus on Red Oak bark at Sandbanks Provincial Park in 2009. Scale bar represents approximately 1 cm. Photo credit: C. Lewis.

Figure 2. Golden-eye Lichen thallus on Red Oak bark at Sandbanks Provincial Park in 2011. Scale bar represents approximately 1 cm. Photo credit: T. McMullin.

Figure 3. Golden-eye Lichen thallus on Red Oak bark at Sandbanks Provincial Park in 2018. Photo credit: T. Knight.

Figure 4. Habitat conditions surrounding the Golden-eye Lichen colony at Sandbanks Provincial Park in 2018. Photo credit: T. Knight.
Species biology
Lichens are composite organisms composed of an alga and/or cyanobacteria (photobiont) and a fungus (mycobiont). The photobiont is encased within fungal hyphae (filaments of fungal cells) and produces food for the lichen via photosynthesis. The mycobiont offers structure and is responsible for sexual reproduction via ascospores. Several authors report that Trebouxia (a green algae) acts as the photobiont for members of the genus Teloschistes (Murray 1960, Brodo et al. 2001, Hinds and Hinds 2007); a population of Golden-eye Lichen from the Canary Islands contained the photobiont Trebouxia gelatinosa (Nyati et al. 2014). It is unknown which species of Trebouxia is associated with the Great Lakes population.
Many lichens produce secondary metabolites (or “lichen substances”), some of which are a unique product of lichen symbiosis. These compounds are deposited on fungal hyphae within the thallus, sometimes as crystals. Like other members of the Teloschistaceae family (e.g., Gyalolechia, Xanthoria, etc.) Golden-eye Lichen produces parietin as a major secondary metabolite which is responsible for the orange thallus colouration (Fazio et al. 2007). Parietin affords a light screening function which protects the photobiont from excess light (Rundel 1978). This function is particularly important for Teloschistaceae members as many grow in environments with high light exposure.
Golden-eye Lichen reproduces sexually via 1-4 mm wide, cup-shaped apothecia which have been observed on thalli as small as 1 cm broad (COSEWIC 2016). The apothecia may be sessile or on short stalks (Almborn 1989) and produce 8-spored asci. The spores are hyaline (translucent) and measure 5-8 µm (Howe 1915, Murray 1960, Fletcher and Purvis 2009). The apothecial margin is thalline (contains thallus tissue and coloration) and often produces abundant cilia. These cilia (which are also produced at the lobe tips between bifurcations) are reported to contain algal cells at their base and break easily; such characteristics suggest they may be associated with vegetative propagation (Nyati et al. 2013). The apothecial cilia may also serve to condense moisture (Hannemann 1973 cited in Sanders 1993).
Many lichens reproduce vegetatively via specialized structures such as soredia and isidia which contain both the photobiont and fungal partners. Golden-eye Lichen does not produce soredia or isidia, although as described above may spread vegetatively from cilia or thallus fragments. Pycnidia (asexual fungal propagules) are frequently produced within shallow orange warts near the lobe tips (Nash et al. 2004).
Several lichenicolous fungi (parasitic fungi that grow on lichen thalli) are associated with Golden-eye Lichen. Didymocyrtis cf. infestans has been identified on Golden-eye Lichen thalli from southern Italy (von Brackel and Puntillo 2016), while Didymocyrtis karnefeltii was identified on apothecia from several locations in Australia (Kondratyuk 2008). Spaerellothecium subtile is common on Golden-eye Lichen in the Sonoran region of the southwestern United States and northwestern Mexico (Nash et al. 2004). These lichenicolous fungi form black spots that are mostly immersed in the thallus (D. cf. infestans and S. subtile) or apothecia (D. karnefeltii).
1.3 Distribution, abundance and population trends
Golden-eye Lichen has a global distribution and has been recorded from South America (Pereira et al. 2006, Fazio et al. 2007), Europe (Fletcher and Purvis 2009, Vicol 2013; Diederich et al. 2014, Sérgio et al. 2016), Africa (Elshafie and Sipman 1999, Bendaikha and Hadjadj-aoul 2016), the Middle East (Bokhary and Parvez 1993, Sipman 2002), Mexico (Nash et al. 1979), Australia (Stevens 1979), and New Zealand (Hayward and Hollis 1993). The existing US population appears to be primarily concentrated in California (along the Pacific Coast and extending somewhat inland) and the interior Midwest/southern Great Plains. There are many late 19th century and early 20th century records of Golden-eye Lichen from states bordering the Atlantic Ocean (CNALH 2018), but apparently no contemporary records from New England (Hinds and Hinds 2007) and only one recent record from North Carolina (CNALH 2018).
Two populations of Golden-eye Lichen occur in Ontario which are considered separate designatable units (COSEWIC 2016). The Prairie/Boreal population is centred around southwestern Manitoba (Prairie) and Lake of the Woods (Boreal), extending eastward to Dryden, Ontario and southward into Minnesota. The Prairie/Boreal population was assessed by COSEWIC as special concern (COSEWIC 2016). The Prairie/Boreal population and Great Lakes population were separated by COSEWIC (2016) on the basis of their apparent geographic isolation (i.e., lack of range overlap) and ecological distinctiveness (i.e., differences in substrate and habitat needs).
The Great Lakes population in Ontario is represented by five historical records and one existing colony. Four of the five historical records are collections by John Macoun who was appointed to the Geological Survey of Canada as Dominion Botanist in 1881 (Waiser 2003). Background information pertaining to these four collections (e.g., precise location, substrate, habitat, etc.) is limited and restricted to herbarium labels and a short description in Macoun’s catalogue of Canadian lichens and bryophytes (Macoun 1902) (see Figure 5). The other historical record is derived from a list of lichens observed at Queen Victoria Park in Niagara Falls (Cameron 1895). No background information is associated with this record and it is unknown if a specimen was ever collected.

Figure 5. John Macoun collection from 1892 at Point Pelee with herbarium label. Photo credit Troy McMullin 2018.
The only existing Great Lakes population colony occurs within a mature, coastal deciduous forest at Sandbanks Provincial Park and is restricted to the bark of one Red Oak (Quercus rubra) tree situated near the shoreline of Lake Ontario. This colony was first discovered on July 5, 1994 by Roman Olszewski. The exact number of individuals present when first discovered is not known but 2-3 thalli were collected at that time and “several others” were observed (R. Olszewski pers. comm. 2018). The colony was rediscovered in 2009 by Chris Lewis (Lewis 2011a) and based on a colony census later that year eight thalli were recorded from two separate Red Oak trees (COSEWIC 2016). By 2013, six thalli (four fertile) were present on the lower trunks of two Red Oak (S. Brinker pers. comm. 2018). By November 2017, the colony had been reduced to two small thalli (both fertile) on one Red Oak trunk (S. Brinker pers. comm. 2018). A November 2018 census reconfirmed the presence of two fertile thalli on one Red Oak trunk (T. Knight pers. obs. 2018, S. Brinker pers. obs. 2018). The lichen flora occupying other mature Red Oaks in the vicinity of the Golden-eye Lichen colony at Sandbanks Provincial Park is notably rich and includes several species of Ramalina (McMullin and Lewis 2014; COSEWIC 2016; T. Knight pers. obs. 2018) which are indicators of “old-growth” conditions and limited air pollution (Hinds and Hinds 2007).
Targeted surveys between 2012 and 2018 in potentially suitable habitats across southern Ontario near the Great Lakes, including at historical localities, did not yield any new records (COSEWIC 2016, S. Brinker pers. comm. 2018, C. Lewis pers. comm. 2018). Details pertaining to all known Great Lakes population records in Ontario are summarized in Table 1 and mapped on Figure 6.
Table 1. Description of historical and current records of Golden-eye Lichen (Great Lakes population) in Ontario. Adapted from (COSEWIC 2016)
| Year | Status of Colony | Recorded by | Locality | Substrate | Deposited at |
|---|---|---|---|---|---|
| 1868 | Historical | John Macoun | “Lake Ontario”; exact location unknown but possibly reflects records from Wellington Beach or Presqu’ile Point cited in Macoun (1902) | If “Lake Ontario” collection is from Wellington Beach or Presqu’ile Point, specimen grew on “trunks” (Macoun 1902) | National Herbarium of Canada lichen collection |
| 1895 or earlier | Historical | Unknown (Cameron 1895) | Queen Victoria Park, Niagara Falls | N/A | Not known to have been collected |
| 1892 | Historical | John Macoun | “Point Pelee” | “on trees” and “on trunks” (Macoun 1902 and herbarium labels) | National Herbarium of Canada lichen collection |
| 1901 | Historical | John Macoun | “Port Rowan” | “on fence-rails” (Macoun 1902) | National Herbarium of Canada lichen collection |
| 1994 | Existing | Roman Olszewski | Sandbanks Provincial Park | Bark of Red Oak | Olszewski personal herbarium |
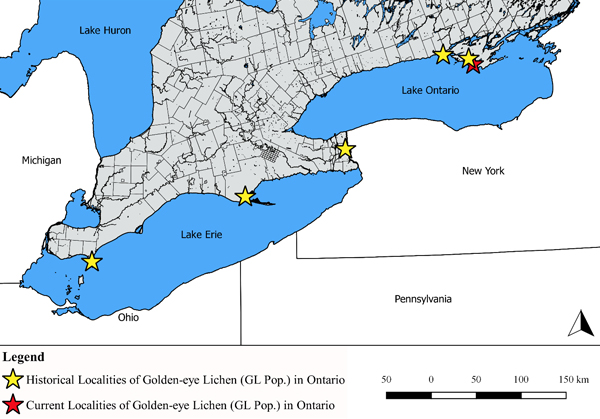
Figure 6. Historical and current distribution of Golden-eye Lichen in Ontario.
Collections from Point Pelee and Port Rowan are deposited at CANL. A third specimen at CANL is labeled “Lake Ontario” and may reflect either the Presqu’ile Point or Wellington Beach record cited by Macoun (1902). There is no known herbarium specimen associated with the Niagara Falls record.
Golden-eye Lichen is also extremely rare in the eastern Great Lakes region of the US and appears to be in decline given the dearth of recent observations. It was historically described as “so rare” in the “north” (i.e., presumably northern New York state) that “there is little likelihood of finding it at all” (Nearing and Ridgewood 1939 p. 33). Golden-eye Lichen was believed extirpated from New York (Harris 2004) and Ohio (Showman and Flenniken 2004) but was recorded recently in both states from residential areas (see Habitat needs). It is considered “critically endangered” in Michigan (Fryday and Wetmore 2002). East of the Great Lakes region, Golden-eye Lichen is described as “formerly widespread” in New England but the last known collection appears to be from Nantucket Island, Massachusetts in 1938 (Hinds and Hinds 2007 p. 469).
1.4 Habitat needs
As noted in Table 1, the known Great Lakes population is restricted to the bark of a single Red Oak tree growing in a coastal deciduous woodland at Sandbanks Provincial Park. Historical collections in southern Ontario are from trees/trunks and (in one instance) a fence rail, mostly from sites that appear to be near the Great Lakes shoreline. More detailed substrate (e.g., tree diameter, species, etc.) and habitat (e.g., vegetation community, light penetration, distance to nearest shoreline, etc.) descriptions are unfortunately lacking from herbarium labels.
Despite the paucity of southern Ontario records it is not considered appropriate to infer habitat needs of the Great Lakes population from the Prairie/Boreal population, for which current records are more voluminous. The Prairie/Boreal population was recognized as a separate designatable unit on the basis of apparent geographic isolation from the Great Lakes population and occupancy of different habitat types (COSEWIC 2016). The Prairie subpopulation primarily occupies twigs in open White Spruce (Picea glauca) dominated parklands surrounded by sandhill prairie, as well as Trembling Aspen (Populus tremuloides) dominated parkland (COSEWIC 2016). The Boreal subpopulation primarily occupies twigs in open coniferous woodlands and (to a lesser extent) mixed woodlands of Spruce (Picea spp.), Trembling Aspen, and Balsam Fir (Abies balsamea) near shorelines. Forest or woodland communities in which White Spruce was abundant were likely very rare (or virtually absent) along the shorelines of Lake Ontario and Lake Erie historically (see Puric-Mladenovic 2011 for presettlement vegetation mapping in the western Greater Toronto and Hamilton Area), although spruce plantations are widespread in this area today.
Alternatively, there is value in considering historical and current records from the US portion of the eastern Great Lakes region to compare with the southern Ontario records described in Table 1. Such records are summarized in Table 2 below.
Table 2. Description of historical and current records of Golden-eye Lichen from the eastern Great Lakes region of the United States
| State | Year Collected | Locality/Habitat | Substrate | Approximate Distance of Locality to Ontario (Euclidian) | Reference |
|---|---|---|---|---|---|
| Michigan | 1958 | “1 mile NE of Cross Village”, Emmet County, Michigan | “pine log in sand” | 120 km west of Cockburn Island, ON | (CNALH 2018) |
| Michigan | 1958 | “north of Cross Village”, Emmet County, Michigan | “on dead branches of Juniperus communis on bluff by beach” | 120 km west of Cockburn Island, ON | (CNALH 2018) |
| Michigan | 1961 | “bluff near Barney Lake”, Beaver Island | Spruce (Picea sp.) | 160 kilometres west of Cockburn Island, ON | (Fryday et al. 2001) |
| Michigan | 1961 | Beaver Island | Poplar (Populus sp.) | 155-165 kilometres west of Cockburn Island, ON | (Fryday et al. 2001) |
| Michigan | 2018 | “dune/swale system” approx. 200 m east of Lake Michigan, Sleeping Bear Dunes National Lakeshore | Not known with certainty but possibly Jack Pine (Pinus banksiana) | 225 kilometres west of Cockburn Island, ON | (A. Graff pers. comm. 2018) |
| New York | 1870 | Sisters Islands, Niagara Falls | “bark” | 1 km east of Queen Victoria Park, Niagara Falls, ON | (Eckel 2013, R. Harris pers. comm. 2018) |
| New York | 2016 | “Residential lawn”, southeast of village of Mexico, Oswego County | Redbud (Cercis canadensis) | 75 km southeast of Prince Edward Point, Prince Edward County, ON | (CNALH 2018) |
| Ohio | 1912 or earlier | Cedar Point, Erie County | “dead branches (Red cedar)” | 26 km south of the southern tip of Pelee Island, ON | (Claassen 1912, CNALH 2018) |
| Ohio | 1912 or earlier | Erie County | “On bark (oak)” | 26-65 km south of the southern tip of Pelee Island, ON | (Claassen 1912) |
| Ohio | 2011 | Residential area (backyard), near Plain City, Union County | On bark of a Green Ash (Fraxinus pennsylvanica) “planted at site in mid 1990s” | 215 km south of Kingsville, ON | (Riley 2011, CNALH 2018) |
| Ohio | 2017 | Residential area (front yard), west of Genoa, Ottawa County | Bark of Pin Oak (Quercus palustris) | 70 km southwest of Kingsville, ON | (S. Pogacnik pers. comm. 2018) |
| Indiana | 1986 or earlier | Indiana Dunes National Lakeshore | N/A | 330 kilometers west of Amherstburg, ON | (Wetmore 1986) |
In addition to the upstate New York records listed in Table 2 there are several historical records of Golden-eye Lichen from downstate including Putnam County, Long Island, and the Catskills (R. Harris pers. comm. 2018, CNALH 2018). These records are several hundred kilometres southeast of southern Ontario and are probably referable to a (largely historical) population stretching along the Atlantic coast from approximately North Carolina to southern Maine. A record from Hamilton County in the southwest corner of Ohio (ca. 1842) (Showman and Flenniken 2004) is also outside the Great Lakes region and is less easily placed within this species’ known distribution.
Three of the four post-2011 records listed in Table 2 are from trees situated in residential areas at inland sites. This distribution pattern may be novel as all historical collections from the eastern Great Lakes region appear to be restricted to the Great Lakes shoreline (or Niagara River). The 2011 and 2017 Ohio records are collections from trees considered (by the collector) to be planted. The 2016 upstate New York record also likely represents a collection from a planted tree as Oswego County is beyond the native range of Redbud and the habitat was described as a “residential lawn”. There is evidence that the ranges of some lichen species in North America are expanding as a result of transfers by the landscaping industry on nursery stock (Brodo et al. 2007). Whether these recent collections of Golden-eye Lichen from residential areas represent “hitchhikers” on nursery stock or natural colonization from nearby source populations is unknown but warrants further consideration.
There are also many historical and current records of Golden-eye Lichen from the western Great Lakes region in the US (Illinois, Wisconsin, and Minnesota) which are not summarized in Table 2. The western Great Lakes records are largely associated with inland sites several dozen to hundreds of kilometres from the Great Lakes shoreline. For example, apart from a historical collection at “Lake View” (Chicago) on “old oak trees near the lake shore” (Wilhelm 2018), all other Illinois records appear to be from inland sites. Records from the western Great Lakes region of the US are more appropriately referred to the population extending through the interior Midwest and southern Great Plains (i.e., Texas to Minnesota) rather than the Great Lakes population. Records from northern Minnesota are clearly associated with the Prairie/Boreal population of northwestern Ontario and southern Manitoba as defined in the COSEWIC Assessment and Status Report (COSEWIC 2016).
Several inferences can be drawn regarding the substrate and habitat needs of the Great Lakes population based on records from southern Ontario (Table 1) and the eastern Great Lakes states (Table 2) outlined above. Such habitat needs are summarized below.
Substrate
In the Great Lakes region, Golden-eye Lichen is predominantly associated with tree bark and branches/twigs. It has been recorded from deciduous trees (oak, ash, poplar), coniferous trees (spruce, Red Cedar), and shrubs (juniper). While some corticolous (bark/twig dwelling) lichen species exhibit distinct preferences for certain bark types owing to differences in bark morphology, pH, and/or nutrient content, the Great Lakes population appears to grow epiphytically on a range of tree (and shrub) genera. As a species, Golden-eye Lichen has been described as mesotrophic (COSEWIC 2016) owing to its association with circumneutral tree bark and toleration of weak eutrophication (i.e., deposition by nitrogen compounds) (Nimis and Martellos 2008).
The only record of Golden-eye Lichen in the eastern Great Lakes region from non-corticolous substrate is a collection on “fence rails” at Port Rowan (see Table 1). While records from the western Great Lakes region of the US were not reviewed in detail herein (due to apparent differences in habitat occupancy), there is also a historical collection from Illinois (Lemont, DuPage County) on “old rails in woods” (Wilhelm 2018). Outside the Great Lakes region, Golden-eye Lichen is also primarily corticolous but has been recorded to a lesser extent from rock and soil (Almborn 1989). One individual from the Prairie/Boreal population was recorded on well-lit rock in northwestern Ontario (COSEWIC 2016). Occupation of atypical substrate (fence rails, rock, soil) could in some instances be attributed to individuals being displaced from bark/twigs (by wind, etc.) which settle on and become affixed to other substrate in the local environment. Such substrate (particularly fence rails) may also be made more suitable for Golden-eye Lichen via a drip zone effect (Arsenault and Goward 2000), whereby nutrients transported into tree leaves during normal physiological processes are released back into the environment via canopy drip. While the exact mechanisms that facilitate Golden-eye Lichen occupation of non-corticolous substrate are unknown, this phenomenon appears to occur with limited frequency.
Soil nutrients
Both the Prairie/Boreal and Great Lakes populations of Golden-eye Lichen show an association with sites containing calcareous soil or underlain by base-rich bedrock (COSEWIC 2016). In fact, the Prairie/Boreal population appears to be restricted to such sites and is absent from areas containing acidic bedrock or non-calcareous soil (COSEWIC 2016). The only existing Great Lakes population colony at Sandbanks Provincial Park occurs in an area underlain by shallow limestone (which is exposed along the adjacent shoreline of Lake Ontario), and several historical sites (e.g., Presqu’ile Point, Wellington Beach) are also likely to be calcareous given the depth to bedrock and prevailing surficial geology. Still, a relationship between calcareous soil and site occupation by Golden-eye Lichen in the Great Lakes region remains speculative given the paucity of records and absence of precise locality information associated with the historical collections.
Light regime
Golden-eye Lichen has shown a preference for open or partially open canopy cover in both the Great Lakes region and across North America. Open areas are subject to greater light penetration and air circulation, conditions which may be required by this species in the Great Lakes region. Treed communities with an open canopy and uneven tree establishment (e.g., savannahs, open woodlands, treed alvars, etc.) can emerge and be maintained in a variety of ways. The existing colony at Sandbanks Provincial Park is situated in a woodland with mature Red Oak that was probably more open historically than it is today; such open conditions could have been maintained by the shallow limestone bedrock, disturbances associated with Lake Ontario (e.g., high winds, etc.), grazing, or other factors. The recently discovered colony at Sleeping Bear Dunes National Lakeshore in Michigan occurs in a dune/swale system (A. Graff pers. comm. 2018) where tree establishment is likely restricted by a combination of xeric and nutrient poor soils, shallow root systems, and aeolian processes (i.e., sand erosion by wind). Additional historical records in the eastern Great Lakes region are from beaches/dunes (see Table 2), which are typically well-lit and exposed to higher levels of humidity (see Humidity below). High light exposure is also a requirement of the Prairie/Boreal population (COSEWIC 2016).
Humidity
Most records (particularly historical) of Golden-eye Lichen in the eastern Great Lakes region are associated with areas of high humidity. The Great Lakes shoreline is known to experience a greater incidence of fog (particularly in spring/early summer) than adjacent inland sites (Visher 1943) when warm, moist air masses are cooled as they travel over the Great Lakes (Environment Canada 2014). The eastern shores of the Great Lakes often experience greater fog due to the prevailing westerly winds, and while it may be coincidental, many records of Golden-eye Lichen in the Great Lakes region are from shorelines or sand bars/spits that trend roughly north-south (i.e., have direct exposure to westerly winds). The two records of Golden-eye Lichen at Niagara Falls (both Ontario and New York) reflect a different moisture source: waterfall spray.
The association of Golden-eye Lichen with higher levels of humidity is complicated by two factors. First, recent records of Golden-eye Lichen in the eastern Great Lakes region are from inland sites away from waterbodies. Such records appear to represent transfers by the landscaping industry on nursery stock, but this is not known definitively at this time. Occupation of inland sites in the eastern Great Lakes region (either naturally or via transfers on nursery stock) suggests that Golden-eye Lichen may only require higher levels of humidity when carrying out certain life processes (e.g., sexual reproduction) and not others (e.g., thallus growth), but this remains speculative. Second, in parts of its North America range Golden-eye Lichen appears to occur naturally and abundantly at sites that lack obvious moisture sources (e.g., central Texas, Oklahoma). While this does not negate the strong historical association of Golden-eye Lichen with the Great Lakes shoreline in southern Ontario, it provides further evidence that this species exhibits somewhat different habitat requirements throughout its North American range.
Air quality
Several authorities have suggested Golden-eye Lichen may be sensitive to air pollution (Wetmore 1981; Brodo et al. 2001; Hinds and Hinds 2007; COSEWIC 2016). Certain lichen species or groups (e.g., cyanolichens) are well known to be rare or absent from areas subject to higher levels of air pollution (Jovan 2008). Wet and dry deposition of airborne pollutants such as sulfur dioxide (e.g., from fuel combustion and industrial processes, etc.) and several nitrogen compounds (e.g., from vehicle and agriculture emissions, etc.) onto lichen thalli can restrict photosynthetic activity and/or become absorbed causing mortality. Fruticose lichens (including Golden-eye Lichen) have a high surface area to volume ratio, enabling better moisture extraction from the air but greater vulnerability to air pollution. The recent return of Golden-eye Lichen to parts of southern England and Ireland has been attributed to pollution abatement and the persistence of suitable habitats (Sanderson 2012). Despite this, the relationship between Golden-eye Lichen and air quality is confounded by this species’ occurrence in several Texas metropolitan areas (e.g., Dallas, Austin, etc.) where airborne pollutant deposition on bark and branches is to be expected. The putative loss of Golden-eye Lichen at several historical localities in the Great Lakes region could be attributable to air quality in combination with habitat loss and its presumed rarity (rather than air quality alone).
1.5 Limiting factors
The most significant factor limiting the recovery potential of the Great Lakes population is its extremely small population size (i.e., two thalli on a single Red Oak tree). The formation of new thalli via sexual reproduction – which may be the primary means of Golden-eye Lichen reproduction given its frequently abundant apothecia and lack of soredia/isidia – requires the release of spores that land on appropriate substrate and encounter cells of the photobiont (Trebouxia). In other words, successful sexual reproduction requires a combination of factors that must occur in tandem and is simply less likely to occur in a population consisting of two thalli. Vegetative reproduction via fragments (either thalli or cilia) could facilitate dispersal and the generation of new thalli, but it is far more likely that any dislodged fragments (by wildlife, wind, etc.) would settle on unsuitable substrate. Long-distance dispersal opportunities (i.e., a rescue effect) from adjacent US states into southern Ontario, which is assumed to have occurred recently in southern England from populations in northern France (Sanderson 2012), are limited given the exceedingly small population size of Golden-eye Lichen in the eastern Great Lakes region.
The generation time of Golden-eye Lichen is not known with certainty but could be 10 years or less (COSEWIC 2016). Should successful reproduction by either of the two thalli occur, any new thalli must also grow to maturity in order to reproduce sexually (although vegetative dispersal via fragments could theoretically occur at any age).
Certain habitat requirements of this species, particularly its association with trees in open or partially open conditions, may limit its recovery potential in Ontario. There has been a significant loss of wooded areas (open or otherwise) within a few hundred metres of the Great Lakes shoreline since timber harvesting and settlement expanded across southern Ontario in the late 1700’s. Many of the remaining wooded areas contain closed canopies or are succeeding toward canopy closure in the absence of disturbance. It is notable that the woodland canopy at Sandbanks Provincial Park where the only existing colony occurs is rapidly closing due to woody vegetation regeneration, particularly European Buckthorn (Rhamnus cathartica).
1.6 Threats to survival and recovery
Several authorities have identified habitat loss as a significant threat to Golden-eye Lichen in North America (Brodo et al. 2001; Hinds and Hinds 2007). The removal of woody vegetation for the purposes of residential development, timber harvesting, or other activities would cause immediate (or eventual) mortality to any lichen thalli affixed epiphytically. Following woody vegetation removal such areas would undergo biophysical changes (e.g., loss of appropriate substrate, changes in microsite conditions, etc.) that may render them unsuitable for occupation by Golden-eye Lichen. While habitat loss undoubtedly threatens many existing populations of Golden-eye Lichen and may have led to localized extirpation at some historical localities in southern Ontario, the known Great Lakes population is restricted to and protected within a provincial park.
The most significant threats to the survival and recovery of the Great Lakes population of Golden-eye Lichen are described below.
Human threats
Several experts identified purposeful collecting as the most significant threat facing the Great Lakes population at this time (T. McMullin pers. comm. 2018, S. Brinker pers. comm. 2018). While documented evidence confirming this threat is lacking, the colony at Sandbanks Provincial Park has declined consistently from eight thalli in 2009 to two (thumb-sized) thalli in 2018. Prior to 2009, only one person appears to have been aware of the colony (Roman Olszewski, the original discoverer). After 2009, many individuals (e.g., naturalists, park staff, etc.) were introduced to the colony as part of naturalist field trips and following the publication of a lichen inventory at Sandbanks Provincial Park (McMullin and Lewis 2014). It is also notable that the colony had persisted between 1994 (i.e., at discovery) and 2009 despite apparently high levels of human activity in the immediate vicinity (C. Lewis pers. comm. 2018) but declined to near extirpation once its location was more widely known.
The possibility that park visitors have inadvertently damaged or dislodged Golden-eye Lichen thalli also lacks documented evidence but is plausible. Given its attachment via a basal holdfast, only a minor amount of pressure (e.g., from a human hand, thrown object, etc.) could easily damage or dislodge Golden-eye Lichen thalli affixed to the host Red Oak. An internal park access road that winds around the host Red Oak was recently closed but walking and biking on the road are still permitted and recreational activities (e.g., picnicking, etc.) occur frequently in the area (Y. Bree pers. comm. 2018).
Park management activities could also inadvertently affect the Golden-eye Lichen colony. During a November 2018 colony assessment, damage to the bark of the host Red Oak was noted and new trail signage had been stapled/nailed to the host tree’s bark (T. Knight pers. obs. 2018, S. Brinker pers. obs. 2018). Areas of damaged tree bark provide potential entry points for disease agents (e.g., bacteria, fungi, etc.) into the cambium which can compromise tree health.
Invasive species control efforts have been undertaken near the colony by park staff over the previous four years targeting Garlic Mustard (Alliaria petiolata), Dog-strangling Vine (Vincetoxicum rossicum), and European Buckthorn (Y. Bree pers. comm. 2018). The area in which the colony is situated is a priority for invasive species control given its high floristic quality (Y. Bree pers. comm. 2018). While such efforts (particularly the removal of European Buckthorn) is likely to improve habitat conditions surrounding the host Red Oak for Golden-eye Lichen, the removal of woody vegetation and use of chemical herbicides could adversely affect the colony unless implemented with care.
Biological threats
Extreme weather events also pose a major threat to the Great Lakes population, particularly given its proximity to the Lake Ontario shoreline. Strong winds, intense precipitation, hail, ice stacking, or lightening could damage/kill the host Red Oak or damage/dislodge the two thalli. Under strong winds, branch failures from adjacent trees could also damage/dislodge the two thalli. The loss of all thalli previously recorded from one of the two host Red Oak is potentially attributable to abrasion by the branches of adjacent shrubs (C. Lewis pers. comm. 2018), which is more likely to occur under strong winds. The propensity of extreme weather events is expected to increase under climate change (Hayhoe et al. 2010).
The activities of local wildlife (e.g., movement, grazing, etc.) are less manageable but equally significant threats. Small and medium-sized mammals such as Eastern Grey Squirrel (Sciurus carolinensis), Northern Flying Squirrel (Glaucomys sabrinus), and Raccoon (Procyon lotor) could easily dislodge the two thalli while climbing the host Red Oak. Birds that forage along tree trunks such as White Breasted Nuthatch (Sitta carolinensis) and woodpeckers may also inadvertently dislodge/damage thalli. While wildlife can act as dispersal agents and may actually support lichen conservation by facilitating dispersal to new areas (Heinken 1999), dislodged thalli or fragments must settle on suitable substrate and become firmly affixed. It is more likely that any Golden-eye Lichen fragments dislodged by wildlife would settle on unsuitable substrate (such as the adjacent closed internal road) where attachment and survival is unlikely.
Certain wildlife activities may target Golden-eye Lichen directly. Invertebrate grazing on lichens, particularly by gastropods, is well documented (Fröberg et al. 2006) and is a known threat to other lichens of conservation interest in Ontario (Lewis 2011b, Environment Canada 2013). While no documented evidence of invertebrate grazing on Golden-eye Lichen was identified, even minimal grazing on the remaining two thalli would be severely detrimental. Further, Golden-eye Lichen was found in the nest of a European Starling (Sturnus vulgaris) in Argentina, which the researchers attributed to mate attraction (Ibañez et al. 2018). Whether or not local breeding birds would collect Golden-eye Lichen as nest material is unknown, but such activities could swiftly result in the loss of the entire colony (and known population).
Plant pathogens also pose a threat to the host Red Oak. During the 2018 colony assessment, a decaying fungus that appeared to be Hen-of-the-woods (Grifola frondosa) was noted within approximately 1 m of the base of the host Red Oak (T. Knight pers. obs. 2018). Hen-of-the-woods is a mild parasite on the roots of oak and other hardwood trees (Baroni 2017) and may slowly weaken a tree’s structural integrity over time. Sudden Oak Death (Phytophthora ramorum) is a fungus-like pathogen known to occur in California which has been detected during annual surveys by the Canadian Food Inspection Agency in British Columbia (CFIA 2018). It infects the phloem and inner bark of susceptible species (including Red Oak) causing bleeding cankers and possible mortality by girdling the sapwood and disrupting internal water and nutrient transport (Parke and Lucas 2008). While it is not known to occur in Ontario, Sudden Oak Death has been confirmed on shipments of nursery stock to Connecticut (Marra 2012) and could conceivably be present (undetected) in northeastern North America. Oak Wilt is another pathogen that has yet to be documented in Ontario but is known from adjacent Great Lakes states including Michigan (Invasive Species Centre 2018). It is caused by an invasive fungus (Bretziella fagacearum) and may lead to rapid tree decline resulting from leaf wilting and discolouration. Other forest pests including Gypsy Moth (Lymantria dispar dispar), European Oak Borer (Agrilus sulcicollis), and Granulate Ambrosia Beetle (Xylosandrus crassiusculus) also pose a risk to oak (including Red Oak) in southern Ontario (Donley et al. 2013).
Physicochemical threats
Over time, the loss of suitable habitat surrounding the Golden-eye Lichen colony could result from several fluctuating habitat variables. Succession towards canopy closure in the absence of disturbance is ongoing around the colony at Sandbanks Provincial Park and is problematic given the species’ need for well-lit conditions. European Buckthorn appears to be the primary understory woody species in certain areas, which not only shades adjacent tree trunks but may reduce the availability of suitable substrate for future colonization by Golden-eye Lichen.
Declines in air quality due to exogenous point sources (e.g., industry, etc.) and non-point sources (e.g., car emissions, etc.) also pose an ongoing threat. Several authorities have suggested Golden-eye Lichen may require relatively clean air (see Habitat needs). Lichen species that exhibit sensitivity to air pollution such as Tree Lungwort (Lobaria pulmonaria) (Gauslaa 1995) have largely been extirpated from southern Ontario (i.e., south/west of the Canadian Shield and northern Bruce Peninsula). Golden-eye Lichen has been described as mesotrophic (COSEWIC 2016), suggesting that it is associated with circumneutral tree bark and tolerates weak eutrophication (i.e., deposition by nitrogen compounds) (Nimis and Martellos 2008). Still, ongoing deposition of sulfur dioxide (e.g., via acid rain) and nitrogen compounds could eventually exceed the buffering capacity of tree bark rendering it unsuitable for colonization by Golden-eye Lichen (COSEWIC 2016). It is notable that while several mature Red Oak in the vicinity of the Golden-eye Lichen colony at Sandbanks Provincial Park have retained a rich lichen flora comprised of rare and sensitive species, others are dominated by nitrophytes such as Mealy Rosette Lichen (Physcia millegrana) and lack sensitive epiphytic lichen species entirely (COSEWIC 2016, T. Knight pers. obs. 2018).
1.7 Knowledge gaps
As described in Habitat needs, the Great Lakes population of Golden-eye Lichen in Ontario is represented by five historical records and one existing colony, accompanied by a few records from the eastern Great Lakes states. This dearth of records impedes our ability to define its expected range limits in the Great Lakes region with certainty. While it is plausible that Golden-eye Lichen has always been very rare in the Great Lakes region, and that existing records accurately reflect a historical distribution pattern concentrated along Lake Ontario and Lake Erie, few qualified professionals (e.g., lichenologists, naturalists, etc.) have ever actively searched for this species. While targeted survey efforts have increased since 2012, more concerted effort concentrated in habitats with high potential suitability is necessary to reduce the possibility that additional localities are simply undiscovered. The current range of the Great Lakes population of Golden-eye Lichen remains a knowledge gap.
There are several inconsistencies in the reported habitat needs of Golden-eye Lichen across its range in North America. Preferences for particular substrata, soil nutrients, light regime, humidity, and air quality were identified and reviewed in Habitat needs, yet these associations are largely based on limited records and may not hold true outside the Great Lakes region. For example, it is unknown why Golden-eye Lichen colonies in the US portion of the western Great Lakes region (e.g., Illinois, Wisconsin, Minnesota) are not associated with the Great Lakes shoreline and occur at inland sites. The presence of inland colonies, coupled with well-established populations in suburban Texas, complicate the reported association of Golden-eye Lichen with areas of high humidity and minimal air pollution. A greater understanding of the factors that affect site occupancy by Golden-eye Lichen, for both the Great Lakes population and other populations in North America, remains a knowledge gap for this species.
Three of the four recent records of Golden-eye Lichen in the Great Lakes region since 2011 are from landscaped trees in residential areas at inland sites. This distribution pattern is at odds with historical records that appear to be restricted to the Great Lakes shoreline (or Niagara River). It would be beneficial to determine with greater certainty whether the occupation of landscaped trees reflects transfer of thalli on nursery stock, or the presence of nearby inland populations that are simply undiscovered.
The known Great Lakes population of Golden-eye Lichen is represented by a single colony of two individuals. This low population size puts the Great Lakes population at an extremely high risk of extirpation. Whether or not Golden-eye Lichen can be successfully propagated in a controlled (i.e., laboratory) or natural setting, or can be transplanted from existing populations (i.e., from the Prairie/Boreal population), are also key knowledge gaps. If propagation/transplantation could be achieved cost-effectively with a reasonable likelihood of success, options for reintroducing the species to suitable sites in southern Ontario could be considered.
1.8 Recovery actions completed or underway
No specific recovery actions for Golden-eye Lichen have been completed or are underway at Sandbanks Provincial Park (Y. Bree pers. comm. 2018). Park staff have previously discussed the possibility of erecting a fence around the host Red Oak tree but were reluctant as this could draw unwanted attention to the tree or lichen (Y. Bree pers. comm. 2018). The internal access road aligned in proximity to the host Red Oak tree was recently closed to vehicles for reasons unrelated to protecting the lichen (Y. Bree pers. comm. 2018). Still, the road closure largely eliminates the potential for vehicle strikes to the host Red Oak and reduces road dust that could settle on thalli and disrupt physiological activities.
Targeted surveys for Golden-eye Lichen at historical localities and habitats with potentially high suitability were performed in 2012 to 2015 to support the COSEWIC Assessment and Status Report, and are summarized therein (COSEWIC 2016). Additional targeted surveys that have taken place since late 2015 are listed below in Table 3. No Golden-eye Lichen was found during any of the surveys listed in Table 3.
Table 3. Targeted Surveys for Golden-eye Lichen (Great Lakes Population) between 2015 and 2018
| Date | Observer | Location | Approx. effort (hours) |
|---|---|---|---|
| October 23, 2015 | C. Lewis | Municipality of Prince Edward County, Massassauga Point Conservation Area | 1 |
| October 31, 2015 | C. Lewis | City of Kingston, Lemoine Point Conservation Area | 1 |
| November 28, 2015 | C. Lewis | Township of Frontenac Islands, Wolfe Island | 1 |
| December 22, 2015 | C. Lewis | Town of Saugeen Shores | 2 |
| February 27, 2016 | C. Lewis | Presqu’ile Provincial Park | 1 |
| July 31, 2016 | C. Lewis | Town of South Bruce Peninsula, Sauble Beach | 0.5 |
| September 29, 2016 | C. Lewis | Loyalist Township, Amherst Island | 1 |
| July 7, 2017 | C. Lewis | Town of Northern Bruce Peninsula (Georgian Bay side) | 3 |
| October 23, 2017 | C. Lewis | Thousand Islands National Park (Hill Island) | 2 |
| November 24, 2017 | S. Brinker | Municipality of Prince Edward County, Wellington Beach | 4 |
| November 24, 2017 | S. Brinker | Sandbanks Provincial Park | 4 |
| April 8, 2018 | C. Lewis | Township of Frontenac Islands, Wolfe Island | 1 |
| Summer 2018 | C. Lewis | Municipality of Prince Edward County, Point Petre Wildlife Conservation Area | 2 |
| Summer 2018 | S. Brinker | Black Creek Provincial Park | 4 |
| Summer 2018 | S. Brinker | Point Pelee Provincial Park | 4 |
| Summer 2018 | S. Brinker | Wheatley Provincial Park | 4 |
| Summer 2018 | S. Brinker | Long Point Provincial Park | 1 |
2.0 Recovery
2.1 Recommended recovery goal
The recommended long-term recovery goal for the Great Lakes population of Golden-eye Lichen is to protect the known colony at Sandbanks Provincial Park and any new colonies that may be discovered in the future.
2.2 Recommended protection and recovery objectives
- Maintain the known colony and any colonies that may be discovered in the future through habitat protection, management, and monitoring.
- Conduct surveys in areas of habitat with potentially high suitability across southern Ontario.
- Provide communication and outreach materials to landowners, conservation groups, and municipalities surrounding Sandbanks Provincial Park.
- Conduct research to address knowledge gaps.
2.3 Recommended approaches to recovery
Table 4. Recommended approaches to the recovery of Golden-eye Lichen in Ontario
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Protection | 1.1 Develop a habitat regulation for Golden-eye Lichen under O. Reg. 242/08. |
|
| Critical | Short-term | Management | 1.2 Review and update (or develop an addendum to) the existing Sandbanks Provincial Park Management Plan (1993) as necessary which directs park management activities in proximity to the Golden-eye Lichen colony, and incorporates specific habitat management objectives (e.g., control European Buckthorn, etc.) that will help maintain or enhance its habitat. Should any new colonies be discovered, create and implement a site-specific management strategy which will help maintain the colony over the long-term. |
|
| Critical | Short-term | Education and Outreach, Communication, and Stewardship | 1.3 Introduce relevant Sandbanks Provincial Park staff to the Golden-eye Lichen colony and provide training that:
|
|
| Critical | Short-term | Research | 1.4 As the host Red Oak is mature and exhibits certain signs of stress, a strategy for locally translocating the Golden-eye lichen thalli should be developed for implementation in the event that the host tree declines further or suffers mortality for any reason. This would include:
|
|
| Critical | Ongoing | Monitoring and Assessment | 1.5 Develop an ongoing monitoring and assessment protocol for implementation by qualified Ontario Parks staff that involves:
|
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | AThreats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Inventory | 2.1 Intensively survey areas of habitat with potentially high suitability with the intent of locating new colonies. Survey effort should be recorded (e.g., person hours, exact sites surveyed, etc.) along with the dominant macrolichen community at each site (sites containing sensitive species are more likely to support Golden-eye Lichen). Potential survey areas (at a minimum) should include:
|
Current distribution (knowledge gap). |
| Critical | Short-term | Monitoring and Assessment | 2.2 Should any new colonies of Golden-eye Lichen be identified, the following information should be collected (with photographs) so that such colonies can be monitored and censused in the future:
|
Current distribution (knowledge gap). |
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Necessary | Short-term | Protection, Education and Outreach, Communication | 3.1 Communicate and provide outreach materials to stakeholders (e.g., landowners, conservation groups, municipalities, etc.) in the area surrounding Sandbanks Provincial Park (and any new locations, if discovered) to introduce a wider audience to Golden-eye Lichen and the threats it faces. Such information could be disseminated at (for example) workshops and may include:
|
|
| Relative priority | Relative timeframe | Recovery theme | Approach to recovery | Threats or knowledge gaps addressed |
|---|---|---|---|---|
| Critical | Short-term | Research | 4.1 Support research projects that involve propagating new Golden-eye Lichen thalli as a means to:
|
Feasibility of propagation to reintroduce new colonies (knowledge gap) |
| Critical | Short-term | Research | 4.2 Support research projects that involve transplanting existing Golden-eye Lichen thalli as a means to:
|
Feasibility of transplantation to reintroduce new colonies (knowledge gap) |
| Beneficial | Long-term | Research | 4.3 Support research projects that examine lichen communities on woody stock at nurseries in southern Ontario to better understand the likelihood that new colonies of Golden-eye Lichen could be accidentally introduced. Collected information could include:
|
Possible range expansion via the landscaping industry (knowledge gap) |
Narrative to support approaches to recovery
Despite surveys undertaken at historical localities and other areas with potentially high habitat suitability in southern Ontario since 2012 (COSEWIC 2016, S. Brinker pers. comm. 2018, C. Lewis pers. comm. 2018) only two thalli associated with the Great Lakes population of Golden-eye Lichen are known. Protection of the colony at Sandbanks Provincial Park via the approaches outlined in Table 4 above (develop a habitat regulation, direct park management activities near the colony, train relevant Ontario Parks staff, develop a translocation plan, monitor the colony) is critical and will increase the possibility that the colony will survive over the long term. Still, even the most effective park management efforts will not eliminate all threats to this colony (e.g., from wildlife activities, extreme weather, further declines in air quality, etc.); it should be accepted that the Great Lakes population of Golden-eye Lichen will be at an extreme risk of extirpation from Ontario for the foreseeable future.
Based on historical and current records of Golden-eye Lichen from across the eastern Great Lakes region, this species was likely historically rare in southern Ontario and restricted to specific habitat types (i.e., partially open woodlands with good air quality and high humidity along the Great Lakes shoreline) that are now limited in areal extent. Should any new Great Lakes population colonies be discovered, several of the recovery approaches listed for objective 1 in Table 4 remain largely applicable. A specific management strategy should be developed by relevant authorities for any new colonies discovered on public land (e.g., other provincial parks, conservation areas, County/municipal forests, etc.) supported by a monitoring and assessment protocol. Any colonies discovered on private land would likely require a management strategy prepared by the local MNRF district (or area) office with the support of the landowner.
The recent discovery of Golden-eye Lichen at Sleeping Bear Dunes National Lakeshore in Michigan in 2018 offers hope that concerted survey efforts will yield new localities in southern Ontario. While several habitats with potentially high suitability have been surveyed in the last few years (S. Brinker pers. comm. 2018, C. Lewis pers. comm. 2018), survey effort has been relatively limited (often an hour or two) at many sites. Due to the small size of Golden-eye Lichen thalli (<4 cm broad, often smaller than 1 cm), suitable habitats must be slowly and methodically surveyed by qualified experts. Such techniques often result in only portions of a particular area or site being surveyed, and several days may be required to reasonably conclude that Golden-eye Lichen is likely absent from a given site.
There is further value in communicating with and providing outreach materials regarding Golden-eye Lichen to stakeholders near Sandbanks Provincial Park. Such stakeholders could include conservation groups (e.g., Nature Conservancy of Canada, Prince Edward County Field Naturalists, etc.), local landowners, and the Municipality of Prince Edward County. Disseminating information about Golden-eye Lichen to stakeholders could increase the likelihood of incidental discovery (since it is relatively easy to field identify) and will introduce the importance of protecting this species to the local community. A workshop (or series of workshops) is one option for disseminating such information. Should any additional colonies be discovered in other parts of southern Ontario, an outreach strategy with the local community could also be developed consistent with the recovery actions outlined objective 3.
Finally, research projects that involve propagating or transplanting Golden-eye Lichen could be supported as a means to assess the feasibility of reintroduction to suitable sites in southern Ontario. There are several ways in which lichens can be cultured in vitro (i.e., grown in a laboratory) or in natural settings. Some techniques involve propagating the mycobiont (fungal partner) from spores or thallus fragments, while others involve recombining the mycobiont and photobiont under controlled conditions (see Stocker-Worgotter 2001 for several examples of lichen culturing). Vegetative propagation of two lichen species common in southern Ontario – Hammered Shield Lichen (Parmelia sulcata) and Hooded Rosette Lichen (Physcia adscendens) – was successfully undertaken via soredia transferred onto plastic cover slips placed outdoors (Anstett et al. 2014). Harvesting thallus or cilial fragments from the two remaining thalli at Sandbanks Provincial Park would be very risky; fragments suitable for propagation likely would need to be sourced from other populations. The possibility of propagating (in laboratory or natural settings) or transplanting (from the Prairie/Boreal population or other populations) Golden-eye Lichen successfully and cost-effectively offers perhaps the best hope of securing the population and minimizing the risk of extirpation over the long term.
Other research projects could focus on studying lichen communities on nursery stock as a means to better understand this potential dispersal vector. As noted in Habitat needs, there is evidence (though not definitive) that Golden-eye Lichen is being accidentally transported to new areas in the eastern Great Lakes region by the landscaping industry on nursery stock.
2.4 Area for consideration in developing a habitat regulation
Under the ESA, a recovery strategy must include a recommendation to the Minister of the Environment, Conservation and Parks on the area that should be considered in developing a habitat regulation. A habitat regulation is a legal instrument that prescribes an area that will be protected as the habitat of the species. The recommendation provided below by the author will be one of many sources considered by the Minister when developing the habitat regulation for this species.
It is recommended that a habitat regulation be prescribed for this species which encompasses the following areal extents:
- A minimum 50 m radius surrounding Golden-eye Lichen to protect individual thalli and the host tree/shrub in which it is affixed.
- An additional minimum 50 m radius (i.e., between 50 m and 100 m) surrounding Golden-eye Lichen to protect suitable habitat for local dispersal.
A rationale which supports this approach is provided below.
Protection of individual thalli and the host tree/shrub
In order to protect Golden-eye Lichen individuals, any tree/shrub in which it is growing epiphytically must also be protected from adverse effects stemming from human activities, which may include:
- direct tree/shrub removal
- mechanical injury to the trunk, roots, branches, and/or foliage
- soil compaction within the existing or future rooting zone
- smothering or exposure of roots due to changes in grade
- alterations to any biophysical condition (e.g., light regime, soil moisture regime, etc.) in which the host tree/shrub was previously accustomed
In order to protect a host tree/shrub on which Golden-eye Lichen exists from adverse human activities, the maximum lateral extent of the host tree/shrub should be considered first. This is usually reflected by its root zone (which is not visible) and/or dripline. While there is an empirical relationship between the maximum lateral extent of a tree’s root zone and its diameter, this relationship may be non-linear and weakens for larger diameter trees (Day et al. 2010). Further, the maximum root zone extent depends on a wide array of factors such as species, age, slope, soil type, soil moisture, soil depth, obstructions, among others. Guidance for establishing minimum tree protection zones with reference to trunk diameter ratios (e.g., 6:1, 12:1,18:1, etc.) is offered in the arboricultural literature (R. Harris et al. 2004, Fite and Smiley 2008), but such ratios may still result in substantial loss of outer feeder roots (Fite and Smiley 2008). Similarly, the maximum extent of a dripline varies based on species, age, competition, canopy coverage, etc.
The only existing Great Lakes population colony grows on a mature Red Oak. Larger (i.e., 75 cm diameter), open-grown Red Oak frequently have driplines extending within the 10-15 m range (T. Knight pers. obs.). While empirical data are sparse, one major root lateral of a 60 year-old 30 cm diameter Red Oak at Harvard Forest was measured to be 15 m long (Lyford 1980). As 30 cm represents a medium sized trunk diameter for Red Oak, which may occasionally grow to 120 cm in diameter (Farrar 1995), a larger tree (such as the host Red Oak at Sandbanks Provincial Park) can be expected to exhibit lateral root growth in excess of 15 m. Shallow soils are present in the vicinity of the Golden-eye Lichen colony at Sandbanks Provincial Park, and may also promote greater lateral tree root extension.
Consideration for the maximum lateral extension of a host/tree shrub is a useful starting point but is insufficient to protect it from direct impacts resulting from many adjacent human activities. For example, many tree species in southern Ontario can grow to heights of 25-30 m or more (Farrar 1995), and any Golden-eye Lichen host tree/shrub within striking distance (i.e., target zone) could be severely damaged during tree removal (felling) activities. Further, maintaining the existing microsite conditions surrounding the host tree/shrub (e.g., canopy cover, wind, humidity, etc.) is critical not only to protect the health and structural integrity of the host tree/shrub but also any Golden-eye Lichen thalli affixed epiphytically. The literature on edge effects suggests that altered microsite conditions (e.g., light, temperature, humidity, etc.) often extend from 50 m (Matlack 1993) to more than 200 m (Chen et al. 1995) into forests from adjacent open/semi-open habitats, depending on the microsite variable under consideration and other site-specific factors.
Based on the above discussion, a minimum 50 m radius surrounding Golden-eye Lichen thalli is considered necessary to protect it from human activities that may adversely affect 1) the thallus, 2) the host tree/shrub, and 3) microclimate conditions surrounding the host tree/shrub. This minimum 50 m radius should include adjacent waterbodies (e.g., Great Lakes, etc.) as such features influence microsite conditions surrounding the Golden-eye Lichen thalli. A 50 m radius for protecting Golden-eye Lichen individuals is also consistent with the current habitat regulation for Pale-bellied Frost Lichen (Physconia subpallida) per paragraph 28.2(2)1 of O. Reg. 242/08.
Protection of suitable habitat for local dispersal
Habitat protection for Golden-eye Lichen involves not only protecting suitable substrate (i.e., trees/shrubs) that can be colonized through local dispersal but also maintaining suitable microsite characteristics in such areas. While no studies assessing dispersal distances by Golden-eye Lichen could be found, Tree Lungwort (Lobaria pulmonaria) has been shown to disperse under natural conditions at mean distances of 37 metres (Ockinger et al. 2005) to 97 metres (Belinchon et al. 2017). The results of lichen dispersal studies may not be directly applicable out of context, since dispersal distances vary widely by species (due to different reproduction strategies, etc.), study design (e.g., studies of a longer duration may capture greater maximum dispersal distances), and habitat suitability in the surrounding environment (Werth et al. 2006).
An additional minimum 50 m (i.e., 50-100 m) radius surrounding all Golden-eye Lichen thalli will allow for the restriction of human activities which may compromise the suitability of surrounding habitat for dispersal and colonization. This minimum 50-100 m radius should include adjacent waterbodies (e.g., Great Lakes, etc.) as such features influence microsite conditions surrounding potential colonization sites and contribute to habitat suitability. This 50-100 m radius to protect Golden-eye Lichen habitat is also consistent with the current habitat regulation for Pale-bellied Frost Lichen (Physconia subpallida) per paragraph 28.2(2)2 of O. Reg. 242/08.
Geographic scope
Although the entire existing Great Lakes population of Golden-eye Lichen occurs within Sandbanks Provincial Park, restricting its habitat regulation to a single locality (i.e., Municipality of Prince Edward County) is not recommend at this time given the possibility that additional colonies will be discovered during implementation of this recovery strategy. We further recommend that the habitat regulation described herein also be applied to any newly discovered Great Lakes population colonies in the future.
A schematic of the recommended habitat regulation is provided below in Figure 7.
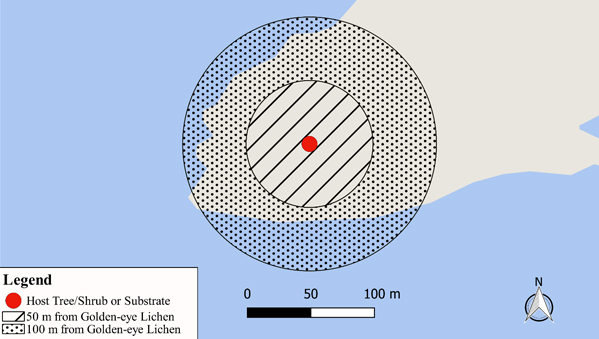
Figure 7. Habitat regulation recommendation for Golden-eye Lichen (Great Lakes population)
Glossary
- Apothecium (pl. Apothecia)
- Disk- or cup-shaped fruiting bodies.
- Ascus (pl. Asci)
- A sac-like structure in which ascospores are formed.
- Ascospore
- A spore produced within an ascus by species in the phylum Ascomycota.
- Bryophyte
- An informal group consisting of mosses, liverworts, and hornworts.
- Cilium (pl. Cilia)
- A slender, hair-like outgrowth usually along lobe margins, not used for attachment.
- Committee on the Status of Endangered Wildlife in Canada (COSEWIC)
- The committee established under section 14 of the Species at Risk Act that is responsible for assessing and classifying species at risk in Canada.
- Committee on the Status of Species at Risk in Ontario (COSSARO)
- The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
- Conservation status rank
- A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. Ranks are determined by NatureServe and, in the case of Ontario’s S-rank, by Ontario’s Natural Heritage Information Centre. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperiled
2 = imperiled
3 = vulnerable
4 = apparently secure
5 = secure
NR = not yet ranked - Corticolous
- Growing on tree bark.
- Endangered Species Act, 2007 (ESA)
- The provincial legislation that provides protection to species at risk in Ontario.
- Epiphyte
- An organism that grows on the surface of a plant and predominantly derives its moisture and nutrients from the air and precipitation.
- Fruticose
- A type of lichen form characterized by a coral-like shrubby or bushy structure, attached only at the base, with little difference between the upper and lower branch/lobe surface.
- Fungal
- Pertaining to fungi.
- Holdfast
- Modified tissue specialized for attachment to substrate.
- Host
- An animal or plant on or in which a parasite or commensal organism lives.
- Hyaline
- Having a glassy, translucent appearance.
- Hypha (pl. Hyphae)
- A microscopic filament of fungal cells.
- Infraspecific
- Occurring within a species.
- In vitro
- Performed outside of an organism’s normal biological context.
- Isidia
- Small vegetative propagules on the upper surface of a lichen covered with cortex and assisting with vegetative reproduction.
- Lichenicolous fungi
- Non-lichenized fungi growing on lichens.
- Lignicolous
- Growing on lignan (i.e., growing on wood which lacks bark).
- Lobe
- A branch or division in the lichen thallus.
- Macrolichen
- A lichen with a large thallus that is not considered crustose.
- Mycobiont
- A fungal partner in a lichen symbiosis.
- Nitrophyte
- A plant that tolerates or prefers nitrogen rich substrate.
- Parietin
- An orange pigment produced in the cortex of several lichen species, including members of the family Teloschistaceae.
- Photobiont
- The photosynthetic partner in a lichen, either a green alga or a cyanobacterium.
- Pycnidium (pl. Pycnidia)
- A small, immersed, flask-shaped structure in which special spores (conidia) are produced, which may function either in sexual reproduction or vegetative dispersal.
- Propagation
- Reproduction by any number of natural or artificial means.
- Propagule
- A structure for reproductive dispersal, either sexual (e.g., ascospore) or asexual/vegetative (e.g., soredia, isidia).
- Rhizine
- A strand of hyphae that arises from the lower surface of many lichens and attaches them to substrate.
- Secondary Metabolite
- An organic compound produced by bacteria, fungi, or plants which is not directly involved in the normal growth, development, or reproduction of the organism.
- Soredium (pl. Soredia)
- Small vegetative propagules on the upper surface of a lichen that contain fungal hyphae and alga but are not covered by cortex.
- Species at Risk Act (SARA)
- The federal legislation that provides protection to species at risk in Canada. This act establishes Schedule 1 as the legal list of wildlife species at risk. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
- Species at Risk in Ontario (SARO) List
- The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
- Thalline Margin
- The margin around an apothecium containing algae or cyanobacteria which is coloured like the thallus.
- Thallus (pl. Thalli)
- The vegetative body of a lichen consisting of a fungus and alga and/or cyanobacteria.
List of abbreviations
- CANL
- National Herbarium of Canada Lichen Collection
- CNALH
- Consortium of North American Lichen Herbaria
- COSEWIC
- Committee on the Status of Endangered Wildlife in Canada
- COSSARO
- Committee on the Status of Species at Risk in Ontario
- ESA
- Ontario’s Endangered Species Act, 2007
- ISBN
- International Standard Book Number
- MECP
- Ministry of the Environment, Conservation and Parks
- MNRF
- Ministry of Natural Resources and Forestry
- SARA
- Canada’s Species at Risk Act
- SARO List
- Species at Risk in Ontario List
- US
- United States (of America)
References
Almborn, O. 1989. Revision of the lichen genus Teloschistes in central and southern Africa. Nordic Journal of Botany 8(5):521–38.
Anstett, D.N., A. Salcedo, and E.W. Larsen. 2014. Growing foliose lichens on cover slips: A method for asexual propagation and observing development. The Bryologist 11(2):179–86.
Arsenault, A., and T. Goward. 2000. The drip zone effect: New insights into the distribution of rare lichens. Proceedings of a Conference on the Biology and Management of Species and Habitats at Risk (Vol. 2), Kamloops,
British Columbia. B.C. Ministry of Environment, Lands and Parks, Victoria, B.C.
Baroni, T.J. 2017. Mushrooms of the northeastern United States and eastern Canada. Portland, Oregon: Timber Press Field Guide. 599 pp.
Belinchon, R., P.J. Harrison, L. Mair, G. Varkonyi, and T. Snall. 2017. Local epiphyte establishment and future metapopulation dynamics in landscapes with different spatiotemporal properties. Ecology 98(3):741-50.
Bendaikha, Y., and S. Hadjadj-aoul. 2016. Diversity of lichens flora in Oran area (north-western Algeria). Advances in Environmental Biology 10:180–91.
Bokhary, H.A., and S. Parvez. 1993. Lichen flora from high altitude areas of Saudi Arabia. Nova Hedwigia 56(3–4):491–96.
Brodo, I.M., C. Lewis, and B. Craig. 2007. Xanthoria parietina, a coastal lichen, rediscovered in Ontario. Northeastern Naturalist 14(2):300–306.
Brodo, I., S.D. Sharnoff, and S. Sharnoff. 2001. The lichens of North America. Yale University Press, New Haven, Connecticut. 828 pp.
Cameron, R. 1895. Queen Victoria Niagara Falls park: Catalogue of plants which have been found growing without cultivation in the park and tts outlying territories / collected, mounted and catalogued for the park herbarium in the superintendent’s office, by Roderick Cameron; Appendix to the report of the superintendent of the park for the year 1894. Warwick Bros. & Rutter, Toronto, Ontario.
CFIA. 2018. Sudden oak death – Phytophthora ramorum. 2018.[accessed November 2018].
Chen, J., J.F. Franklin, and T.A. Spies. 1995. Growing-season microclimatic gradients from clearcut edges into old-growth Douglas-Fir forests. Ecological Applications 5(1):74–86.
Claassen, E. 1912. Alphabetical list of lichens collected in several counties of northern Ohio. The Ohio Naturalist XII(8):543–48.
CNALH 2018. Consortium of North American Lichen Herbaria. [accessed November 2018].
COSEWIC. 2016. COSEWIC assessment and status report on the Golden-Eye Lichen Teloschistes chrysophthalmus, Prairie / Boreal population and Great Lakes population, in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xv + 50 pp.
Day, S.D., P.E. Wiseman, S.B. Dickinson, and J.R. Harris. 2010. Contemporary concepts of root system architecture of urban trees. Arboriculture and Urban Forestry 36(4):149–59.
Diederich, P., D. Ertz, M. Eichler, R. Cezanne, P. van den Boom, D. Van den Broeck, and E. Serusiaux. 2014. New or interesting lichens and lichenicolous fungi from Belgium, Luxembourg and Northern France. Bulletin de La Société Des Naturalistes Luxembourgeois 115:157–65.
Donley, R.N., J. Jalava, and J. van Overbeeke. 2013. Management plan for the Shumard Oak (Quercus shumardii) in Ontario. Ontario Management Plan Series. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. v + 59 pp.
Eckel, P.M. 2013. Botanical heritage of islands at the brink of Niagara Falls. 374 pp.
Elshafie, A.E., and H.J.M. Sipman. 1999. Mediterranean lichens in the tropics: Lichens of the mist oasis of Erkwit, Sudan. Tropical Bryology 16:103–8.
Environment Canada. 2013. Recovery strategy for the flooded jellyskin lichen (Leptogium rivulare) in Canada. Species at Risk Act Recovery Strategy Series. Environment Canada, Ottawa. Iv + 23 pp.
Environment Canada. 2014. National marine weather guide: Ontario regional guide.
Farrar, J.L. 1995. Trees in Canada. Fitzhenry & Whiteside Limited, Markham, Ontario. x + 501.
Fazio, A.T., M.T. Adler, M.D. Bertoni, C.S. Sepúlveda, E.B. Damonte, and M.S. Maier. 2007. Lichen secondary metabolites from the cultured lichen mycobionts of Teloschistes chrysophthalmus and Ramalina celastri and their antiviral activities. Zeitschrift Fur Naturforschung - Section C Journal of Biosciences 62(7–8):543–49.
Fite, K., and E.T. Smiley. 2008. Managing trees during construction. International Society of Arboriculture, Champaign, Illinois. 35 pp.
Fletcher, A., and O.W. Purvis. 2009. Teloschistes Norman (1853). Pp. 874. In C. Smith, A. Aptroot, B. Coppins, A. Fletcher, O. Gilbert, P. James and P. Wolseley (eds.). The Lichens of Great Britain and Ireland, The British
Lichen Society, London, England.
Fröberg, L., A. Baur, and B. Baur. 2006. Field study on the regenerative capacity of three calcicolous lichen species damaged by snail grazing. The Lichenologist 38(5):491–93.
Fryday, A.M., J.B. Fair, M.S. Googe, and A.J. Johnson. 2001. Checklist of lichens and allied fungi of Michigan. Contributions from the University of Michigan Herbarium 23:145–223.
Fryday, A.M., and C.M. Wetmore. 2002. Proposed list of rare and/or endangered lichens in Michigan. The Michigan Botanist 41(2001):89–93.
Gauslaa, Y. 1995. The lobarion, an epiphytic community of ancient forests threatened by acid rain. The Lichenologist 27(1):59–76.
Hannemann, B. 1973. Anhangsorgane Der Flechten, Ihre Strukturen Und Ihre Systematische Verteilung. Bibliotheca Lichenologica 1:1–192.
Harris, R.C. 2004. A preliminary list of the lichens of New York. Opuscula Philolichenum 1:55–74.
Harris, R., J. Clark, and N. Matheny. 2004. Arboriculture: Integrated management of landscape trees, shrubs, and vines. Prentice Hall, Upper Saddle River, New Jersey. 592 pp.
Hayhoe, K., J. VanDorn, T. Croley, N. Schlegal, and D. Wuebbles. 2010. Regional climate change projections for Chicago and the US Great Lakes. Journal of Great Lakes Research 36(SUPPL. 2):7–21.
Hayward, B.W., and C.J. Hollis. 1993. Ecology of Waimamaku River estuary, north of Kawerua, North Aukland. Tane 34:69–78.
Heinken, T. 1999. Dispersal patterns of terricolous lichens by thallus fragments. Lichenologist 31(6):603–12.
Hinds, J.W., and P.L. Hinds. 2007. The macrolichens of New England. The New York Botanical Garden Press, New York, New York. xx + 584.
Howe, R.H., Jr. 1915. The genus Teloschistes in North America. Bulletin of the Torrey Botanical Club 42(10):579–83.
Ibañez, L.M., R.A. García, V.D. Fiorini, and D. Montalti. 2018. Lichens in the nests of European Starling Sturnus vulgaris serve a mate attraction rather than insecticidal function. Turkish Journal of Zoology 42(3):316–22.
Invasive Species Centre. 2018. “Oak Wilt.” 2018.
Jovan, S. 2008. Lichen bioindication of biodiversity, air quality, and climate: Baseline results from monitoring in Washington, Oregon, and California. United States Department of Agriculture, Forest Service, General Technical Report PNW-GTR-737.
Kondratyuk, S.Y. 2008. Polycoccum kaenefeltii Sp. Nova (Dothideales), a new lichenicolous fungus on Teloschistes chrysophthalmus (L.) Th. Fr. Ukrayins’kyi Botanichnyi Zhurnal 65(4):565–71.
Lewis, C. 2011a. Lichens of Sandbanks Provincial Park. Prepared for Ontario Parks, Peterborough, Ontario. 1-13 pp.
Lewis, C. 2011b. Recovery strategy for the Pale-Bellied Frost Lichen (Physconia subpallida) in Ontario. Ontario Recovery Strategy Series. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. vi + 24 pp.
Lyford, W.H. 1980. Development of the root system of northern Red Oak (Quercus rubra L.). Harvard Forest Paper No. 21. 30 pp.
Macoun, J. 1902. Catalogue of Canadian plants. Part VII, Lichenes and hapaticae; Geological Survey of Canada, Government Printer, Ottawa.
Marra, R. 2012. Ramorum blight (Sudden Oak Death) (Phytophthora ramorum) [accessed November 2018].
Matlack, G.R. 1993. Microenvironment variation within and among forest edge sites in the eastern United States. Biological Conservation 66(1993):185–94.
McMullin, R.T., and C.J. Lewis. 2014. The unusual lichens and allied fungi of Sandbanks Provincial Park, Ontario. Botany 92:85–92.
Murray, J. 1960. Studies of New Zealand II – The Teloschistaceae. Transactions of the Royal Society of New Zealand 88(2):197–210.
Nash, T.H., III, G.T. Nebeker, T.J. Moser, and T. Reeves. 1979. Lichen vegetational gradients in relation to the Pacific coast of Baja California: The maritime influence. Madroño 26(4):149–63.
Nash, T.H., B.D. Ryan, C. Gries, and F. Bungartz. 2004. Lichen flora of the Greater Sonoran Desert Region. Vol 2. Arizona State University. Web site: http://lichenportal.org/portal/taxa/index.php?taxon=56375 [accessed November 2018].
Nearing, G.G., and N.J. Ridgewood. 1939. Guide to the lichens of the New York Area. Torreya 39(2):29–37.
Nimis, P.L., and S. Martellos. 2008. Teloschistes chrysophthalmos (L.) Th. Fr. 2008 [accessed November 2018].
Nyati, S., S. Scherrer, S. Werth, and R. Honegger. 2014. Green-algal photobiont diversity (Trebouxia spp.) in representatives of Teloschistaceae (Lecanoromycetes, lichen-forming ascomycetes). The Lichenologist 46(2):189–212.
Nyati, S., S. Werth, and R. Honegger. 2013. Genetic diversity of sterile cultured Trebouxia photobionts associated with the lichen-forming fungus Xanthoria parietina visualized with RAPD-PCR fingerprinting techniques. The Lichenologist. Vol. 45.
Ockinger, E., M. Niklasson, and S. Nilsson. 2005. Is local distribution of the epiphytic lichen Lobaria pulmonaria limited by dispersal capacity or habitat quality? Biodiversity and Conservation 14:759-73.
Parke, J.L., and S. Lucas. 2008. Sudden oak death and ramorum blight. 2008. [accessed November 2018].
Pereira, I., F. Müller, and A. Valderrama. 2006. Diversity and distribution of bryophytes and lichens of El Colorado, Central Chile. Nova Hedwigia 83(1–2):117–27.
Puric-Mladenovic, D. 2011. Pre-settlement Vegetation Mapping for the Greater Toronto Area, including the Regions of Hamilton, Halton, Peel and York and the Credit Valley Watershed. Faculty of Forestry, University of Toronto.
Riley, B. 2011. Found alive! OBELISK Newsletter of the Ohio Moss and Lichen Association 8(1):2-3.
Rundel, P.W. 1978. The ecological role of secondary lichen substances. Biochemical Systematics and Ecology 6(3):157–70.
Sanders, W.B. 1993. Apical formation of cilia and associated branching of the axis in the lichen Teloschistes flavicans. International Journal of Plant Sciences 154(1):75–79.
Sanderson, N.A. 2012. History & ecology of Goldeneyes Teloschistes chrysophthalmus in England. 2012 [accessed November 2018].
Sérgio, C., P. Carvalho, C.A. Garcia, E. Almeida, V. Novais, M. Sim-Sim, H. Jordão, and A.J. Sousa. 2016. Floristic changes of epiphytic flora in the metropolitan Lisbon area between 1980-1981 and 2010-2011 related to urban air quality. Ecological Indicators 67:839–52.
Showman, R.E., and D.G. Flenniken. 2004. The macrolichens of Ohio. Ohio Biological Survey, Columbus, Ohio. Iv + 277 pp.
Sipman, H.J.M. 2002. Lichens of Mainland Yemen. Willdenowia 32(1):127–35.
Stevens, G.N. 1979. Distribution and related ecology of macrolichens on mangroves on the East Australian Coast. Lichenologist 11(3):293–305.
Stocker-Worgotter, E. 2001. Experimental Lichenology and Microbiology of Lichens: Culture Experiments, Secondary Chemistry of Cultured Mycobionts, Resynthesis, and Thallus Morphogenesis. The Bryologist 104(4):576–581.
Vicol, I. 2013. Distribution of the Teloschistes chrysophthalmus (L.) Th. Fr. in Romania. Romanian Journal of Biology - Plant Biology 58(2):105–8.
Visher, S.S. 1943. Some climatic influences of the Great Lakes, latitude and mountains: An analysis of climatic charts in Climate and Man, 1941 (II). Bulletin American Meteorological Society 24:205–10.
von Brackel, W., and D. Puntillo. 2016. New records of lichenicolous fungi from Calabria (Southern Italy), including a first checklist. Herzogia 29(2):277–306.
Waiser, W.A. 2003. MACOUN, JOHN. Dictionary of Canadian Biography, vol. 14 [accessed December 2018].
Werth, S., H.H. Wagner, F. Gugerli, R. Holderegger, D. Csencsics, J.M. Kalwij, and C. Scheidegger. 2006. Quantifying dispersal and establishment limitation in a population of an epiphytic lichen. Ecology 87(8):2037-46.
Wetmore, C.M. 1981. Lichens and air quality in Big Bend National Park, Texas. 24 pp.
Wetmore, C.M. 1986. Lichens and air quality in Indiana Dunes National Lakeshore. 34 pp.
Wilhelm, G. 2018. Working draft of the lichens of the Southern Lake Michigan Region. 115 pp.
Wright, D.M. 2000. Guide to the macrolichens of California: Part 1, the orange pigmented species. Bulletin of the California Lichen Society 7(1):7–16.
Personal communications
Bree, Y. 2018. Telephone correspondence with T. Knight. October 2018. Natural Heritage Education Coordinator, Ontario Parks.
Brinker, S. 2018. Telephone correspondence with T. Knight. October 2018. Project Botanist, Natural Heritage Information Centre.
Graff, A. 2018. Electronic correspondence with T. Knight via iNaturalist. October 2018. Naturalist.
Harris, R.C. 2018. Email correspondence with T. Knight. October 2018. Research Associate, New York Botanical Garden.
Lewis, C. 2018. Telephone correspondence with T. Knight. October 2018. Resources Management Technician, Ministry of Natural Resources and Forestry.
McMullin, R.T. 2018. Telephone correspondence with T. Knight. October 2018. Research Scientist, Canadian Museum of Nature.
Olszewski, R. 2018. Telephone correspondence with T. Knight. October 2018. Naturalist.
Pogacnik, S. 2018. Electronic correspondence with T. Knight via iNaturalist. October 2018. Naturalist.