West Virginia White Management Plan
This document advises the ministry on ways to ensure healthy numbers of the West Virginia white, a species of special concern, return to Ontario.
Management plan prepared under the Endangered Species Act, 2007
June 2013

About the Ontario management plan series
This series presents the collection of management plans that are written for the Province of Ontario and contain possible approaches to manage species of special concern in Ontario. The Province ensures the preparation of the management plans meet its commitments to manage species of special concern under the Endangered Species Act, 2007 (ESA, 2007) and the Accord for the Protection of Species at Risk in Canada.
What is a species of special concern?
A species is classified as special concern if it lives in the wild in Ontario, is not endangered or threatened, but may become threatened or endangered due to a combination of biological characteristics and identified threats.
What is a management plan?
Under the ESA, 2007, a management plan identifies actions that could be taken to ensure, at a minimum, that a species of special concern does not become threatened or endangered. The plan provides detailed information about the current species population and distribution, their habitat requirements and areas of vulnerability. The plan also identifies threats to the species and sets a clear goal, possible strategies, and prioritized activities needed to address the threats.
Management plans are required to be prepared for species of special concern no later than five years of the species being added to the Species at Risk in Ontario list as a special concern species.
What’s next?
Nine months after the completion of a management plan a government response statement will be published which summarizes the actions that the Government of Ontario intends to take in response to the plan and the government priorities in taking those actions. The implementation of the management plan depends on the continued cooperation and actions of various sectors, government agencies, communities, conservation organisations, land owners, and individuals.
For more information
To learn more about species of special concern in Ontario, please visit the Ministry of Natural Resources Species at Risk webpage.
Recommended citation
Peter S. Burke. 2013. Management Plan for the West Virginia White (Pieris virginiensis) in Ontario. Ontario Management Plan Series. Prepared for the Ontario Ministry of Natural Resources, Peterborough, Ontario. v + 44 pp.
© Queen’s Printer for Ontario, 2013
ISBN 978-1-4606-2033-5 (PDF)
Content (excluding the cover illustration) may be used without permission, with appropriate credit to the source.
Cette publication hautement spécialisée « Management plans prepared under the Endangered Species Act, 2007 », n'est disponible qu'en anglais en vertu du Règlement 411/97 qui en exempte l'application de la Loi sur les services en français. Pour obtenir de l'aide en français, veuillez communiquer avec le ministère des Richesses naturelles au 1-800-667-1940.
Author
Peter S. Burke
Acknowledgments
For their contributions to the preparation of this report, thanks are given to Dawn M. Burke (OMNR), Colin D. Jones (OMNR), Donald A. Sutherland (OMNR), Alan Macnaughton (University of Waterloo) Taylor Scarr (OMNR), Michael Irvine (OMNR), Susan McGowan (OMNR), Ken Elliot (OMNR), Vivian Brownell (OMNR), Brenda Van Ryswyk (Conservation Halton), Alan Wormington, Mirek Sharp (North-South Environmental), Peter Hall, Bob Yukich, Chris Robinson (Ontario Parks), Anne White, James D. Holdsworth and Rick Cavasin. Figure 1 was provided by the Butterfly and Moth Information Network and the many participants who contribute to its Butterflies and Moths of North America (BAMONA) project.
Declaration
The management plan for the West Virginia White was developed in accordance with the requirements of the Endangered Species Act, 2007 (ESA). This management plan has been prepared for the Government of Ontario, other responsible jurisdictions, and for the many different constituencies involved in managing the species.
The management plan does not necessarily represent the views of all of the individuals who contributed to its preparation, or the official positions of the organizations with which those individuals are associated.
The goals, objectives, and management approaches identified in the plan are based on the best available knowledge and are subject to revision as new information becomes available. Implementation of this plan is subject to appropriations, priorities, and budgetary constraints of the participating jurisdictions and organizations.
Success in the management of this species depends on the commitment and cooperation of many different constituencies that will be involved in implementing the directions set out in this plan.
Responsible jurisdictions
Ontario Ministry of Natural Resources,
Environment Canada- Canadian Wildlife Service, Ontario
Parks Canada Agency
Executive summary
The West Virginia White (Pieris virginiensis) is a medium-sized, single-brooded, all-white butterfly that doesn't stray far from the forest interior. It emerges during the mid-spring months (Apr-May) when mature hardwood forests are beginning to leaf-out. Tied to spring ephemeral host plants belonging to the Mustard (Crucifer) family, it most commonly selects Two-leaved Toothwort (Cardamine diphylla), upon which it lays single eggs on a leaf. The West Virginia White has a short flight period strongly associated with the short life span of its host plant, which wanes by mid-June. The eggs hatch shortly after being laid, and the larvae complete growth within 10-20 days. Pupation is followed by diapause (hibernation) until the following spring.
West Virginia White is a species of Special Concern in Ontario. It has a patchy distribution in southern Ontario, and sites of known occurrence are separated from each other by significant distances. It is currently found in five main areas (i) Halton region; (ii) Manitoulin Island; (iii) the Frontenac axis of eastern Ontario; (iv) central Peterborough County; and (v) southeastern shoreline of Lake Superior/St. Joseph Island. Despite the existence of suitable habitat elsewhere, it is found in very few locations outside of these sites.
Along with its small population in the province, the species faces uncertainty due to the combined threats of habitat fragmentation, invasive species, human population growth and climate change. Garlic Mustard (Alliaria petiolata), an invasive biennial plant that is difficult to control through manual and/or chemical removal, is currently spreading throughout the Ontario range of the butterfly, and has been identified as the most serious threat. Though the effects on the Ontario population have yet to be studied, Garlic Mustard is a leading cause of declines and extirpation of West Virginia White over much of its range outside of the province. Evidence shows that adults are unable to discriminate between it and their native host plant, and the larvae are unable to survive. Other potential issues relate to management for Gypsy Moth, where spraying of non-specific insecticides (Btk or Carbaryl) kill the butterflies. Also the rapid spread of Beech Bark Disease into Ontario could open up the forest leading to shorter growth cycles of toothwort. Furthermore the butterfly faces increasing pressure on its habitat from human recreational activities (off-road vehicles, collection of spring ephemeral plants for food, photography of the butterfly) and aggregate resource extraction (human population growth) and climate change which could affect the timing of butterfly and host-plant life cycles.
The goal of this management plan is to maintain or improve the health of Ontario’s West Virginia White population. To reach this goal, measurable time-defined objectives have been made that involve the support and cooperation of interested organizations, landowners, institutions and government. The key objectives are to get a better understanding of the threats across its range, in particular the effects of Garlic Mustard and human recreational activities, and to reduce habitat-related threats through Best Management Practices. Because known populations haven't been monitored regularly, the current status and range of the butterfly must be studied to help determine the risk to the provincial population. With this in mind the objectives of the management plan are:
- Determine distribution and abundance of known populations and survey for new populations.
- Initiate research and monitoring on known populations especially related to threats.
- Maintain the current distribution and abundance of West Virginia White through habitat protection/stewardship and other measures.
- Re-evaluate At Risk status both provincially and nationally.
1.0 Species assessment and classification
Common name (population): West Virginia White
Scientific name: Pieris virginiensis
SARO List Classification: Special Concern
SARO List History: Listed as Endangered in 1977; re-assessed in 1990 and status changed to Vulnerable (later changed to Special Concern on Sept. 30, 2004). [Note that these designations pre-date the establishment of COSSARO; COSSARO has never assessed the species.]
COSEWIC Assessment History: Never assessed by COSEWIC.
Species at Risk Act (SARA): Not listed on Schedule 1 (List of Wildlife Species at Risk in Canada) or Schedules 2 or 3
Conservation status rankings:
GRANK: G3 NRANK: N3 SRANK: S3
The glossary provides definitions for the abbreviations above.
2.0 Species information
2.1 Species description and biology
Species description
There are four stages in the life cycle of the West Virginia White – egg, larva, pupa and adult. The adult is a medium sized butterfly, very similar in appearance to the common, introduced Cabbage White (Pieris rapae) and native Mustard White (Pieris oleracea Harris) (Bess 2005). The surface of both the forewing and the hindwing are translucent, unmarked, dull white, except for the bottom third which is dusky grey to blackish. Some individuals show dusky, gray-brown margins towards the tip of the forewing. Occasionally females show two faint spots on the forewing (Hovanitz 1963). The underside of the forewing is unmarked white, while the hindwing is suffused with very pale yellow and has veins marked with indistinct grey-brown (see cover photo). At one time, the butterfly was considered a spring brood of Mustard White, due to similarity in appearance and range. However Klots (1935) and Hovanitz (1963) demonstrated that it was a separate species, due to differences in appearance and life history. The biggest difference is the brighter yellow colour and much bolder, darker grey-brown markings of the veins on the underside of the hindwing of Mustard White (see Brownell, 1980). Mustard White may also show a distinct black outer edge to the tip of the forewing and a black mark on the leading edge of the hindwing (Layberry et al. 1998). It could also be confused with the Cabbage White which flies during the same period. The main differences are that Cabbage White does not have veining on the yellower, underside surface of the hindwing, and has one (in males) or two (in females) black spots on the forewing. Some Cabbage White may have all white forewings (lacking dark markings) and must be examined carefully to confirm the species is not West Virginia White (Opler and Malikul 1992, Glassberg 1999).
The eggs of West Virginia White are 1.5 mm long, whitish-yellow (Plath 1975, Scott 1986), elongate, spindle-shaped, and marked with a latticework of tiny pits and projections that have the appearance of fine ribs. These ribs are finer than that of Mustard White or Cabbage White eggs. Eggs hatch in 5-10 days (Bess 2005) and the larvae undergo five instars (molts) within 10-20 days. The larvae become yellowish-green in color, darkening as the molts progress. The body is covered in short, dense whitish hairs, making it appear fuzzy, as well as many bristly wartlets (Plath 1975, Bess 2005). The body has yellowish markings running the entire length. The pupa is slender and angular in appearance with a longer frontal prominence than the other North American Pieridae (Klots 1954, Plath 1975). For a more complete description of the life cycle of the West Virginia White see Brownell (1980).
Some authors placed West Virginia White, Mustard White and Cabbage White in a separate genus, Artogeia Verity in the 1980s, a change proposed by mainly European taxonomists and adopted in North America for a short while. However North American taxonomy reverted back to the genus name Pieris in the 1990s.
Species biology
The West Virginia White is a univoltine (single brooded) butterfly of mesic (moist) hardwood and mixed forests. It emerges during the start of leaf out in the spring and flies for about two weeks from April until late May/early June (Hess 1975, Brownell 1980) in Ontario depending on latitude and altitude. During this period, the females lay approximately 100 eggs (Chew, pers. comm. as cited in Doak et al. 2006) singly on the leaves of forest understory ephemeral wildflowers belonging to the Mustard (Brassicaceae) family, specifically toothworts (genus Cardamine, formerly Dentaria). This includes: Two-leaved Toothwort (Cardamine diphylla), Slender Toothwort (C. angustata; = heterophylla), Cut-leaved Toothwort (C. concatenata; formerly C. lacianata) and Rock Cress (Arabis laevigata) (Klots 1935, Hovanitz 1963, Shuey and Peacock 1989, Bess 2005, NatureServe 2012). Rock Cress is used by the butterfly in several states south of the Great Lakes, though it hasn't yet been reported as a host plant in Ontario. The egg hatches within 5 to 10 days, and the larva feeds on the leaves of the host plant for 10 to 20 days before pupating on the host stem, on another nearby plant, or within the litter (Cappuccino and Kareiva 1985). Toothworts stop growing by mid June and are completely withered shortly afterwards. The butterfly has a short flight period and egg stage, quick larval growth, and long hibernation of the pupae (Shapiro 1971, Cappuccino and Kareiva 1985, Shuey and Peacock 1989, Bess 2005). Its life cycle closely matches that of the host plant.
Eggs are laid on the underside of the leaf, up to 10 per plant (Plath 1974). In Ontario, Two-leaved Toothwort is the primary host of West Virginia White; although larvae have been found on Cut-leaved Toothwort (Plath 1979 as cited in Brownell 1980) which is widespread in southern Ontario. In Indiana, Massachusetts and Pennsylvania, West Virginia White does use this host, albeit sparingly (Wagner 1978, Cappuccino and Kareiva 1985, Shuey and Peacock 1989) along with two other native mustards. The earlier growing period (in May) of Cut-leaved Toothwort in Ontario (Plath 1975), when the larvae are still in need of food, limits its use by West Virginia White (Cappuccino and Kareiva 1985). The later growth period of Two-leaved Toothwort, by as much as two weeks, normally allows the larvae to fully develop and pupate (Plath 1974). Recently larvae and eggs have been recorded on Garlic Mustard, (Courant et al. 1994, Porter 1994) an invasive plant which has rapidly expanded in northeastern North America.
The first instars of the larva (instars 1-3) remain on the undersides of the host leaf to hide and feed (Cappuccino and Kareiva 1985, Bess 2005). During later stages (instars 4-5) they move to the top of the leaves and fruit as the plant develops (see Brownell 1980). Larvae will search for another host plant if differences in the timing of host plant and butterfly development occur (Cappuccino and Kareiva 1985).
Pupation typically occurs from late May into June (Brownell 1980) on stems of understory plants or in the leaf litter. The pupa becomes dormant for summer, fall and winter (Klots 1935, Shapiro 1971). It remains unknown whether West Virginia White pupae are capable of overwintering for more than one season (i.e. double diapause).
Observations that populations are scarce in some years and then recover quickly suggest the butterfly does use this survival strategy (NatureServe 2012).
The adult flight period in Ontario has been recorded from 4 April to 13 June (Ontario Butterfly Atlas Database 2012). In Halton Regional Forest flight times were observed to begin April 25, peaking about May 10 depending on weather conditions, and ending around May 20 to 25 (Plath 1975, Mainguy 1991). Records into June are typical of the most northerly sites in the province (viz. Jones et. al. 2012). Further south in the US, they may begin flying as early as late March and finish by mid-May (Bess 2005). In Indiana, individuals typically live 5 to 10 days (Bess 2005) and flight activity is associated with temperatures above 19°C and sunny conditions (Plath 1975, Cappuccino and Kareiva 1985). Prolonged periods of inactivity are observed when conditions are cloudy and cool, which are not uncommon during the flight period, especially in the first 2 weeks. Therefore, the exact timing of the flight season depends on the availability of sunshine, nectar, and larval host plants (Cappuccino and Kareiva 1985, Brownell 1980, Mainguy 1991). Males are often associated with forest streams and damp areas near woodland roads and trails (Cappuccino and Karieva 1985) and quickly seek out females by patrolling through the forest, stopping rarely, or briefly to rest or feed. The females appear to require sunny glades on the forest floor, often perching on the host plant or other spring ephemeral wildflowers. Both sexes nectar on various flowers of their hardwood/mixed forest habitat including Large-flowered Trillium (Trillium grandiflorum), Yellow Dog’s-Tooth Violet (Erythronium americanum), Canada Violet (Viola canadensis), Spring Beauty (Claytonia virginica) (Hess 1974, Stewart 1988) and Garlic Mustard (Alliaria petiolata) (Yahner 1998). It has been suggested that abundant nectar is necessary to increase populations in otherwise good years, offsetting mortality in years with cold springs (Mainguy 1991). The flight is usually slow, low and direct, within 1 m of the forest floor, however it will occasionally fly quickly at a greater height (Cooper 1980), even into the canopy if disturbed (Bess 2004). West Virginia White is strictly confined to mature forested habitats and has been demonstrated to avoid forest edges, unshaded stream crossings, utility right-of-ways, and open field habitats (Cappuccino and Kareiva 1985). It will cross smaller roads within the canopy of the forest, even traversing the roadways and landing on them (Stewart 1988, NatureServe 2012). As such it is considered to be a forest-interior species and sensitive to hard edges. In light of this and its poor dispersal ability, isolated populations that become extirpated are unlikely to be recolonized (Bess 2005).
2.2 Population and distribution
The West Virginia White is believed to be extirpated from a portion of its range (eastern NY south through NJ), creating a gap between populations (NatureServe 2012). Overall, it is found from southern and western Vermont south to south central Connecticut, west through northern Pennsylvania and New York to northern Ohio, across southern Ontario to Michigan and northern Wisconsin, and down the Appalachians from Pennsylvania to northern Georgia and northwestern Alabama (Figure 1). Isolated populations also occur in a limited area near the Ohio River in Indiana and Kentucky (Opler and Malikul 1992). It has most likely been extirpated from southwestern Connecticut and southeastern New York (Cech and Tudor 2005), New Jersey (Gochfeld and Burger 1997) and eastern Pennsylvania (NatureServe 2012).
The butterfly is in "alarming decline, in the northern and central Appalachians, possibly as far south as northern North Carolina," (LeGrand and Howard 2010). Although the species may still be found in these regions, it is likely that the range has become fragmented and is shrinking (NatureServe 2012).
Figure 1. Range of West Virginia White (Pieris virginiensis) in the United States. Taken from Butterflies and Moths of North America (BAMONA) (Opler et al., 2012). Yellow dots represent all records in the database (may be a general as a county) while orange dots represent the 400 most recent records with complete latitude and longitude information in the BAMONA database.
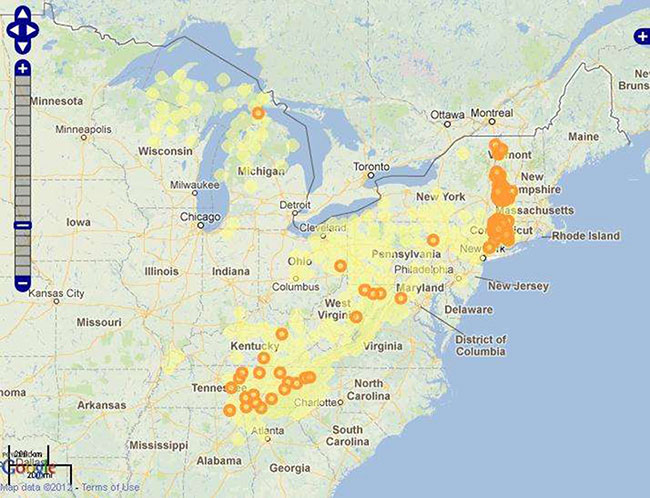
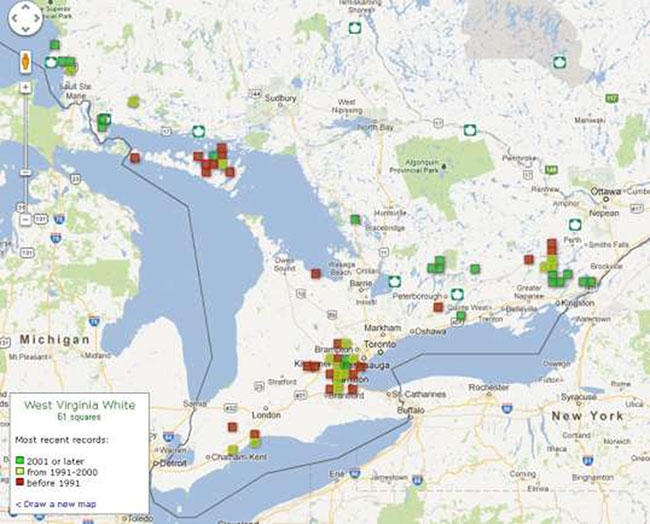
In Ontario, the West Virginia White has been recorded in 17 counties/regional municipalities (RM): ten of which have no records since 2000 (Brant, Chatham-Kent, Elgin, Grey, Hamilton, Lanark, Middlesex, Peel, Waterloo and Wellington counties) (Jones et al. 2012). Search efforts appear to have declined, as many known experts reported little recent activity updating known locations. As such, the status of West Virginia White within many of these sites remains unknown. There are exceptions to this. Current observations (post 2002) have been clustered within five main areas of Ontario: Halton RM in the vicinity of the Halton Regional Forest; Manitoulin Island; Algoma District near the southeastern shore of Lake Superior and adjacent St. Joseph Island; central Peterborough County; and the Frontenac Axis in Leeds & Grenvillle and Frontenac counties. Other recent records are from Northumberland and Parry Sound District (see Fig. 2).
Only rough population data are available for Ontario (Brownell 1980). A study by Cooper (1980) in eastern Ontario, within one of the butterfly’s core areas, revealed a density of two to three adult butterflies per 90m2 of suitable habitat. Within this same study area, sampling also revealed a maximum density of 2 eggs per 100 Two-leaved Toothwort leaves examined. During good weather conditions, as many 15 to 25 individuals were encountered at any one time along a 152m transect (frequency of 10-16/100m) within the core area (Cooper, 1980). In other Frontenac County populations, Cooper (1980) reported much lower densities of one to five individuals per site. In comparison, Martin (1980) reported an average of 4.8 butterflies per hour in good weather conditions, with a maximum of eight per hour, within the Halton Regional Forest.
Figure 2. Historical and current distribution of West Virginia White (Pieris virginiensis) in Ontario. Map courtesy of Ontario Butterfly Atlas Database online, 2012. Accessed 5 November, 2012. Toronto Entomologists'.
2.3 Habitat requirements
The West Virginia White is found exclusively in mesic hardwood and mixed forests (Hovanitz 1963, Cappuccino and Kareiva 1985, Opler 2004) with rich soils and hardwood swamps (NatureServe 2012). Although it will fly across shaded roadways in continuous forest, it avoids edges and open fields in fragmented landscapes (Wagner 1978, NatureServe 2012). Host plant populations are typically abundant (Catling et al. 1974, Cappuccino and Kareiva 1985, Benson et al. 2003) and distributed across the sites in which the butterfly occurs. Although its presence is not guaranteed on all sites with Two-leaved Toothwort (Brownell 1980), the likelihood of occurrence increases in large tracts of unbroken forest where many patches of the host plant exist.
In Ontario, West Virginia White is associated mainly with forests growing on calcareous bedrock and/or calcareous till (D. Sutherland, pers. comm. 2012). These forests are usually mature to semi-mature, deciduous or mixed forests, with a layer of dark, mesic soil over the bedrock/till. In eastern Ontario it is also found where Precambrian rock is overlaid with thin soils on the Frontenac Axis. Sites are typically dominated by Sugar Maple (Acer saccharum) and have a closed canopy and an abundant herb layer. Within Ontario, other tree species associated with its habitat include Black Maple (A. nigrum), American Beech (Fagus grandifolia), Basswood (Tilia americana), American Elm (Ulmus americana), Eastern White Pine (Pinus strobus), Eastern Hemlock (Tsuga canadensis), White Ash (Fraxinus americana), Bitternut Hickory (Carya cordiformis), Shagbark Hickory (Carya ovata), Ironwood (Ostrya virginiana), Black Cherry (Prunus serotina), Blue Beech (Carpinus caroliniana), Red Oak (Quercus rubra) and Bur Oak (Q. macrocarpa). The shrub layer tends to be poorly developed, creating an open lower understory, with species such as Leatherwood (Dirca palustris) and Choke Cherry (Prunus virginiana) usually prevalent. Overall, the herb layer tends to be remarkably diverse and abundant (Catling et al. 1974), with dense patches and/or extensive coverage of the host plant Two-leaved Toothwort (Catling et al. 1974). For a complete list of the vascular plant associates in the Halton County Forest, see Catling et al. (1974) and McKay (1976). Elsewhere, Morton and Tasker (1980) reported that on Manitoulin Island the butterfly was more closely associated with portions of a site dominated by Cut-leaved Toothwort rather than Two-leaved Toothwort. In central and eastern Ontario, sites have been shown to be more characteristic of earlier successional stages due to thinner soils, past forestry practices resulting in younger classes of trees, and less dense Two-leaved Toothwort cover (C.D. Jones, D. Sutherland, pers. comm. 2012).
West Virginia White prefers continuous forest cover. In a 1990 study it was most commonly found in Ontario in forests with areas larger than 25 km2. They were never found in areas where forest cover was less than approximately 10% (Mainguy 1991).
2.4 Characteristics contributing to vulnerability of species
West Virginia White has a number of limiting factors that make it vulnerable. NatureServe (2012) classifies it as "narrow" for environmental specificity. It is an oligophagus butterfly, meaning it eats only a few types of plant. It will only use members of the crucifer family (Brassicaceae) for egg-laying and subsequent larval development. It selects predominantly Two-leaved Toothwort over much of its range (Shapiro 1971, Cappuccino and Kareiva 1985, Bowden 1971) and in Ontario (Hess 1975, Brownell 1980). However the invasive herb Garlic Mustard (Alliaria petiolata) quickly out-competes toothwort once established, and is used by the female butterfly as the egg host. This is non-adaptive, as Garlic Mustard is poisonous, causing mortality to the developing larvae (Bowden 1971, Porter 1994).
West Virginia White lays only one egg at a time upon carefully selected egg laying sites (Doak et al. 2006). The lifespan of the adult female is brief, 5 to10 days, and there is only one brood per year. Cool, cloudy, spring weather during the flight period can result in a year of low reproductive success (Cappuccino and Kareiva 1985). Year-to-year unpredictability of Ontario springs can affect flight time, nectar sources, and the life span of toothwort (Mainguy 1991).
It is a rare species on a landscape level, occurring in small, often isolated colonies (NatureServe 2012), and can be absent from what appears to be otherwise suitable habitat (Hovanitz 1963, Porter 1994, Bess 2005, Doak et al. 2006). West Virginia White does not travel far, making it almost impossible for it to re-colonize patches of forest where it has been extirpated (Cappuccino and Kareiva 1985, NatureServe 2012).
For these reasons West Virginia White was listed as Endangered in Ontario in 1977 (Brownell 1980). At that time, it was observed in only two populations, one of which was threatened by aggregate development (Hess 1975). In 1990 the butterfly was down-listed to Special Concern after increased search efforts boosted the number of known sites in Ontario (Brownell 1980, Mainguy 1991) and the surrounding states of Michigan, Wisconsin and New York (Stanton 2001).
3.0 Threats
Natural ecosystems are continually evolving in response to a variety of forces and factors. But they are limited in their ability to adapt to rapid change, such as that introduced through human activities. Humans sometimes disrupt and degrade biodiversity through habitat loss, introduction of invasive species, population growth, pollution, unsustainable use and climate change. Our growing population combined with our rising levels of resource consumption can threaten biodiversity (OBC, 2011). Recently, an assessment of pressures on Ontario’s biodiversity showed that many threats are increasing (OBC, 2010b).
The West Virginia White currently faces the following threats.
Habitat loss
Fragmentation
Because the butterfly does not cross gaps in forest, fragmentation of its limited habitat in southern Ontario poses a significant threat (Stanton 2001, NatureServe 2012). Historical sites in Hamilton, London and Etobicoke (specimens in the Royal Ontario Museum and Canadian National Collection) (NHIC database 2012) have most likely been extirpated (Hess 1975) by the loss of forest cover as these cities grew. The historical population decline of West Virginia White is probably due to the disappearance of most of the forested landscape across its entire range (Cappuccino and Kareiva 1985, Morton and Tasker 1980, Chew 1981, Opler and Malikul 1992, Bess 2005, Porter 1994). Its refusal to cross open areas between forest patches (NatureServe 2012), low reproduction rates, brief adult lifespan and limited diet make it susceptible to a highly fragmented landscape and unable to recover quickly after local extirpation. Populations are, however, still scattered across Ontario, even within highly fragmented parts of the province (e.g. Halton Regional Forest). It remains unclear why the species remains absent from many forests that fulfil the minimum size requirements and have an abundance of the host plant (Cappuccino and Kareiva 1985).
The threat posed by fragmentation to the butterfly, its food plant, and its habitat is increased when the quality of old-growth hardwood forests is reduced. This can occur when:
- increased canopy light and reduced understory shade allow aggressive, light adapted, invasive plants such as Garlic Mustard to outcompete Two-leaved Toothwort;
- increased winds and higher temperatures dry the soil which could cause the host plant to die earlier, starving the larva before it can reach its dormant stage, or, the plant itself may be extirpated from a site; and
- forestry-related activities such as the tracks left by heavy machinery can destroy the host plant and expose soils that allow aggressive, invasive plants to become established. Invasive species such as Garlic Mustard enter woodlots most often along trails (or streams) via a human vector or on forestry equipment that has not been properly cleaned, further degrading sites.
Populations of West Virginia White may initially persist in sites that have experienced heavy tree removal, (Tasker 1975, D. Sutherland, pers. comm. 2012) due to an increased growth of toothworts. However, the increase in competition and light and decrease in humidity eventually limit the availability of the host plant over time and lead to site abandonment (Plath 1979).
Eastern White-tailed Deer (Odocoileus virginiainus)
Declines in native plant understories have been attributed to many factors, including overabundant deer populations (Alverson et al. 1988, Rooney 2001, Côté et al. 2004). Deer browse can pose a significant threat, particularly in woodlands with abundant early successional and "edge" habitat preferred by deer (Alverson et al. 1988). High deer densities can greatly modify fragmented landscapes. Deer wander widely and deer browsing can result in the almost complete elimination of woodland herbs, except for a few unpalatable species, including Garlic Mustard (Williams and Ward 2006). Deer can be a vector for invasive species, like Garlic Mustard (Myers et al. 2004, Williams and Ward 2006). They can also reduce the abundance and diversity of wildflowers, which can leave West Virginia whites without a steady supply of adult nectar food and larval food plants. Research on deer densities in comparison to the distribution and abundance of toothwort may provide information to help conserve West Virginia White colonies. More evidence is needed to determine how browsing by deer reduces the species richness and density of native plants such as toothwort, while increasing the density of browse-resistant plants such as Garlic Mustard (Frankland and Nelson 2003, Horsely et al. 2003).
Logging and understory herbs
In Ontario mesic hardwood forests, the understory is much more species-rich than the canopy, but our understanding of these herbaceous communities is limited. Declines in native plant species have been attributed to many factors, including logging (Whigham 2004), yet the relationship between harvest intensity and the response of the herbaceous layer is not well known (Gilliam and Turrill 1993). It is believed that the understory is influenced by a complex combination of factors, i.e. site structure, soil type, moisture level, topography, age, and disturbance history such as grazing, trails and previous logging regimes (Burke et al. 2008). Past management practices are important, and help shape current understory and canopy conditions in managed forests (Halpern and Spies 1995). The composition of understory plant communities is dynamic and variable between years, even in unmanaged sites (Burke et al. 2008). There is some suggestion that sun-loving plants, like toothworts and other spring ephemerals may benefit or be neutrally affected by the opening of the canopy (Collins et al. 1995). However, logging practices can compromise the integrity of rich, moist woodlands and must be carefully considered in any management plan for West Virginia White.
Invasive species
Garlic Mustard (Alliaria petiolata)
Other than outright destruction of habitat, Garlic Mustard is probably the most serious threat faced by the West Virginia White throughout its range (Nuzzo 1999, NatureServe 2012).
Garlic Mustard is a biennial invasive plant from Europe and Asia. It has been present in North America since the 1860's in the absence of competitors and plant predators (Renwick et al. 2001, Roberts and Anderson 2001, Carlson and Gorchov 2004). This invasive plant has become abundant in southern Ontario (Arnold 2007) and invades and dominates understory vegetation communities within deciduous and mixed hardwood forests (Nuzzo1999, Blossey et al. 2002 ).
The West Virginia White cannot tell the difference between the invasive Garlic Mustard and native plants from the mustard family (Huang et al. 1995, Courant et al. 1994) and mistakenly lay their eggs upon it (Chew 1982, Courant et al. 1994, Porter 1994). The larvae successfully hatch but then die by the second instar (Bowden 1971, Porter 1994) due to presence of chemical feeding deterrents and suspected growth inhibitors (Haribal et al. 2001, Renwick et al. 2001, Cipollini and Gruner 2009). There is currently no direct evidence that the larvae are able to adapt to use Garlic Mustard as a host plant (Porter 1994, NatureServe 2012). However, the larvae of a Mustard White population in New England exposed to Garlic Mustard for 35 to 55 years can now successfully feed on the plant during development (Courant et al. 1994, Keeler and Chew 2008). Therefore, it is possible that West Virginia White, could evolve a tolerance to Garlic Mustard, making it an alternate host plant (Bowden 1971, Porter 1994, Keeler and Chew 2008) in regions where Garlic Mustard dominates the understory and the "novelty" of the plant defences have worn off (Callaway et al. 2008, Barto et al. 2010b). Because the butterfly won't travel far and has only a single brood/year, the spread of such beneficial genes into nearby populations is unlikely (Porter 1994). The widespread tolerance of West Virginia White to Garlic Mustard remains doubtful at best.
Garlic Mustard produces on average 616 small persistent seeds per plant (Blossey et al. 2002) that remain dormant in the seedbank for up to four years (Solis 1998 as cited by Arnold 2007). Seeds are easily spread by humans on machinery, clothes, or shoes. Invasion into hardwood forests typically occurs along established trails created by forestry practices or ATV use, and along stream courses (Blossey et al. 2002, Arnold 2007). Garlic Mustard produces an allelopathic soil phytochemical (Barto et al. 2010b) that destroys native mycorrhizal root associations (arbuscular mycorrhizal fungi (AM)) that most native ephemeral forest plants require for nitrogen uptake (Vaughn and Berhow 1999, Roberts and Anderson 2001, Prati and Bassdorf 2004, Barto and Cipollini 2009, Barto et al. 2010a, Koch et al. 2011). This, combined with a lack of native herbivores able to feed on the plant, allows Garlic Mustard to outcompete native species in mature hardwood forests (Barto et al. 2010b, Lankau 2012). Foraging pressure from White-tailed Deer may increase negative impacts on native herbaceous cover (mainly nectar sources, (Bess 2005) because the deer actively avoid Garlic Mustard (Rogers et. al. 2008, NatureServe 2012). In addition, by disturbing forest soils with their tracks, deer may increase the suitability of soil conditions for Garlic Mustard invasions (Williams and Ward 2006, Rogers et al. 2008).
The potential future range of Garlic Mustard within Ontario (where requirements of Garlic Mustard to thrive occur (Welk et al. 2002)) overlaps the known range of West Virginia White almost completely. Large homogenous stands of Garlic Mustard are established in many old-growth hardwood forest fragments in southern Ontario (Arnold 2007), and the need to assess West Virginia White butterfly populations there has a high priority. Garlic Mustard is presently less common at the edge of the Canadian Shield but the current status of the butterfly and Garlic Mustard in those sites requires clarification. There is evidence of the Garlic Mustard present at the Bass Lake Rd. site in Peterborough (D. Sutherland, pers. comm. 2012) and several sites in Halton RM (B. Van Ryswyk, B. Yukich, pers. comm. 2012).
Effective control of Garlic Mustard requires multiple methods based on the spread of the plant in that site (see Arnold 2007). Typically a combination of manual removal with application of a glyphosate (Round-up TM) (Carlson and Gorchov 2004, as cited in Arnold 2007) during at least two stages of the annual cycle for up to five years is recommended. Control is most successful when surrounding sites are also managed and steps are taken to prevent re-colonization.
Research is ongoing into biological control using plant predators from Garlic Mustard’s native range. Several of these are weevils of the genus Ceutorhynchus that are specific to particular parts of the plant such as the leaves or roots (Blossey et al. 2002, Katovich et al. 2005, Arnold, 2007). Ceutorhynchus erysimi, which was accidentally introduced to North America in the mid 19th century has recently been observed in the wild predating Garlic Mustard in southern Ontario (Yates and Murphy 2008). Research about the possible negative impacts these weevils on commercial Brassicaceae crops and native crucifers is ongoing in the US and Switzerland (Blossey et al. 2002). Recent research also suggests that planting native forest plants capable of competing with Garlic Mustard (e.g. Bloodroot (Sanguinaria canadensis) (Arnold 2007)) along with the introduction of a specialist herbivore of Garlic Mustard, may be an especially effective method of control (Huang et al. 2012). Furthermore fitness of native competitors appears to increase with longer exposure time and lower allelopathic levels of Garlic Mustard (Lankau 2011, 2012).
Beech Bark Disease
The scale insect (Cryptococcus fagisuga) and an associated fungus (Nectria coccinea var. faginata) are recent invaders to Ontario that cause mortality in American Beech. This tree is an important component of the mature hardwood forests (McLaughlin and Griefenhagen 2012) where West Virginia White is found. The disease occurs when the scale insect colonizes a tree and causes wounds in the bark, allowing the later arriving fungus to penetrate the cambial layer and establish itself. The spread of the fungus around the trunk of the tree can cause partial or entire tree mortality (McLaughlin and Greifenhagen 2012). Nearby trees are eventually infected by the disease. This includes the re-sprouting from the remaining crown or roots of the originally damaged tree (Bess 2005, McLaughlin and Greifenhagen 2012). Damage from the disease in Ontario has been extensive and tree resistance is thought to be 1% of a given population. The scale and the fungus are wind dispersed and are quickly spreading across the range of American Beech westward all the way to Georgian Bay/Lake Huron and south of Algonquin Park (McLaughlin and Greifenhagen 2012). There is not much research on how to manage Beech Bark Disease due to its recent arrival to Ontario. Information from other jurisdictions outside of Ontario will be important as we try to determine long term effects of large Beech tree die-offs across the range of West Virginia White in Ontario. The effect of the removal of many canopy trees on its habitat is likely to cause negative effects on the butterfly and the host plant similar to those associated with forest fragmentation (Bess 2005).
Gypsy Moth (Lymantria dispar) and Gypsy Moth Control
Gypsy Moth was first recorded in the province in 1969 (Otvos pp.205-254. in Koul et al. 2004) and was widespread by 1992 (Canadian Forest Service 2001). However, outbreaks of the introduced moth in Ontario have increased in the past 30 years (T. Scarr, pers. comm. 2012). Although preferred food plants include oaks (Quercus spp.), birches (Betula spp.) and poplars (Populus spp.) it will eat more than 200 species in North America (Tobin et al. 2012). This includes Sugar Maple, a dominant species in mature hardwood forests of southern Ontario. In Ontario, severe defoliation is typically limited to forests dominated by oak (T. Scarr, pers. comm. 2012) which do not coincide with the preferred habitat of West Virginia White (C.D. Jones, pers. comm. 2012). However, several species of oak and poplar do occur in sites used by the butterfly. Gypsy Moth outbreaks can have potential impacts on West Virginia White butterfly populations when severe defoliation opens up the canopy, exposing the forest floor to prolonged periods of light. This can have negative effects on plant species in the understory that are sensitive to light, and result in shorter life-cycles of Two-leaved Toothwort (Bess 2005).
Ontario stopped its Gypsy Moth spraying control program in 1992 (T. Scarr, pers. comm. 2012) however several large municipalities such as Mississauga and Toronto still use operational spraying. Integrated Pest Management (IPM) guidelines for landowners, conservation authorities and public lands exist to aid in the control of Gypsy Moth in Ontario (OMNR 1991, Nealis and Erb 1993). This approach considers a suite of methods that can be used to control an outbreak of Gypsy Moth. The most effective and targeted treatment for a large outbreak is the use of Btk (Bacillus thuringiensis var. kurstaki). This virus attacks the gut lining of the larvae of various moths and butterflies (in the Lepidoptera order) through absorption into the digestive system, causing starvation and death. Btk is known to cause mortality in a range of lepidopteran larval hosts in lab conditions, including Pieris spp. (Peacock et al. 1998) and is shown to have long-lasting damaging effects on resident populations of non- target butterflies and moths (Boettner et al. 2000, Johnson et al. 1995, Wagner et al. 1996). Although its effects in the wild are unknown, it is probable that West Virginia White is at risk from Btk (NatureServe 2012, Bess 2005), due to the butterfly’s low densities and poor dispersal abilities.
Dispar virus or nuclear polyhedrosis virus (L-d NPV) or Gypchek is a Lymantridae-specific (moth-specific) insecticide that works directly with the naturally occurring virus found in soils. It builds up virus levels to a point which causes the collapse of a Gypsy Moth population. Gypchek is thought to be specific to Gypsy Moth and thus other non-target lepidopteran larvae are unaffected (Tobin et al. 2012), although this has not been tested directly upon West Virginia White. The timing of the spraying (two applications 7-10 days apart) of Btk or Gypchek is specifically aimed to overlap the time when half of the Gypsy Moth’s egg hatch, and mid-point in leaf out (T. Scarr, pers. comm. 2012). This narrow window of opportunity, in combination with expense, are considered drawbacks to some landowners (T. Scarr, pers. comm. 2012). As a cheaper alternative, single applications of the more general pesticide Carbaryl are sometimes used in smaller infestations. It is less discriminating, and will have a more far reaching effect within the ecosystem, including mortality of spiders, many other insects and reduced fertility in nesting birds (Cooper et al. 1990, Schweitzer 2004, Awkerman et al. 2011).
A fungus, Entomophaga maimaiga, occurs naturally in the soil and is most effective at control in years of wet, cool weather during the egg hatching and early instar development (April-May) of Gypsy Moth larvae (Hajek 2001). The fungus targets ground dwelling lepidopterans with granular skin or a coating of short dense hairs like Lymantriidae that are present in the spring (Hajek et al. 2000). In years of dry, warm spring weather, Gypsy Moth outbreaks may occur more widely than normal due to suppressed E. maimaiga activity (Hajek 2001, T. Scarr, pers. comm. 2012). Thus, the fungus is not dependant on moth densities, as it does not spread via infected individuals. The effects of E. maimaiga on West Virginia White larvae are not known (Bess 2005) but should be studied to see if this fungus interferes with the larval development of the butterfly.
Manual techniques to remove egg masses and burlap bags tied around tree trunks to collect larvae and adults are also suggested as control methods against Gypsy Moth (Schweitzer 2004, Otvos 2004). A further threat comes in the form of the Asian Gypsy Moth (L. dispar asiatica) which has been found in Ontario in the past (T. Scarr, pers. comm.), and is quite likely to reappear in the future. Unlike the European subspecies, the females are able to fly, which means faster and wider dispersal across Ontario.
Emerald Ash Borer (Agrilis planipennis)
First detected Windsor, Ontario in 2002, the Emerald Ash Borer has spread across southwestern Ontario, through the Greater Toronto area, as far east as Ottawa, Leeds- Grenville, Prescott-Russell and as far north as Sault Ste. Marie (Orr 2010). The developing larvae dwell in the cambial layer and feed on the xylem and phloem, which transport nutrients and water required for survival. This eventually damages and kills the tree (Lyons 2010). Successful Emerald Ash Borer development only occurs on trees in the Ash (Fraxinus) genus, with all five species of ash found in Ontario susceptible to the beetle. As such, it poses a threat to any forest containing these species.
Overall, the Emerald Ash Borer could cause significant changes to native forests due to an expansive host tree range with relatively few barriers limiting its spread. Some species-specific host preferences have been identified (i.e. higher densities of Emerald Ash Borer larvae are found on Green Ash (Fraxinus pennsylvanica) over White Ash (Fraxinus americana), and a similar host preference pattern is observed for White Ash over Blue Ash (Fraxinus quadrangulata) (Anulewicz et al. 2007, Tanis and McCullough 2012). Early detection of infestations may be determined by surveying the preferred host species (Anulewicz et al. 2007).
As Emerald Ash Borer continues to spread, new management strategies are being developed to minimize the economic and ecological impact of this forest pest. Control measures include: biological control through parasitoids, chemical control through systemic insecticides and firewood control which restricts a significant but passive form of beetle dispersion through the movement of infested firewood (Hausman 2010).
The full impacts of ash borer on forest stand structure, understory vegetation and sensitive forest species are still unknown. In general, ash mortality by Emerald Ash Borer results in isolated, but widely distributed canopy gaps, coupled with the extirpation of ash from the invaded forests (Gandhi and Herms 2010). It has been hypothesized that alien forest insects, including Emerald Ash Borer (Gandi and Herms 2010), can help invasive species become established and spread by creating canopy gaps that increase light availability (Simberloff and Von Holle 1999). Research in areas with ash removal protocols has shown that the resulting unnatural increase in gaps and use of heavy machinery to remove trees compacts soil and may promote the spread of other invasive species (Hausen 2010).
The negative effects of Emerald Ash Borer depend on the composition of the forest and the initial abundance of ash, particularly in the canopy. The loss of ash trees will be similar to logging impacts, and the gaps created through the dying ash trees can create dramatic changes on the forest understory. While there is variability in plant community response to silviculture treatments between sites, the non-native plant response, in general, is positively correlated with the level of disturbance (Sutherland and Nelson 2010). As Emerald Ash Borer spreads across Ontario, causing high rates of mortality to ash (Fraxinus) in hardwood forests (Orr 2010), changes to site structure and light regimes may harm the West Virginia White.
Other invasive species
The invasive species listed above are the most serious threats currently known to the West Virginia White and its habitat in Ontario. However, other invasive species in Ontario and possible related future threats may put the butterfly at risk.
The Cabbage White butterfly is an abundant introduced species that is well established in Ontario and feeds on both native and introduced crucifers, including Garlic Mustard. Research has shown it avoids forest interiors and thus does not compete with West Virginia White (Cromartie 1975). However, the spread of invasive plants into hardwood forests that have been altered structurally to allow more light and exposed soils will increase competition between West Virginia White and Cabbage White.
The Braconid wasps Cotesia glommerata and C. rubecula introduced for biological control of Cabbage White larvae (Benson et al. 2003) have been demonstrated to parasitize West Virginia White larvae under lab conditions. These wasps prefer open habitats and are not known to infiltrate the forest interiors (Benson et al. 2003), however, increasing fragmentation could expose West Virginia White to parasitism by the wasp (NatureServe 2012).
Viral diseases such as Granulosis virus, known to effect West Virginia White populations in the US (Bess 2004) are another unknown factor whose impact has not been assessed in Ontario.
Earthworms (Lumbricus spp.) could pose another risk to West Virginia White if they threaten survival of the butterfly’s host or nectaring plants. European earthworms are colonizing earthworm-free northern hardwood forests across North America (Hale et al. 2006). Without worms, fallen leaves decompose slowly, creating a spongy layer of organic "duff". This duff layer is the natural growing environment for native woodland wildflowers. Invasive earthworms eat the leaves and can eliminate the duff layer. At present, 17 non-native European and two North American earthworm species are found in the province (Eavers et al. 2012). Though it is unknown which plant species are most vulnerable, studies have found that earthworm invasions transform diverse herb-layer plant communities to simplified and sparser communities dominated by a few species (Hale et al. 2006, Holdsworth et al. 2007). They also cause the disappearance of species which include spring ephemerals such as Solomon’s seal (Polygonatum pubescens), Large-flowered Bellwort (Uvularia grandifolia), Large-flowered Trillium and Violets (Bohlen et al. 2004, Frelich et al. 2006). Additionally, earthworm invasions could alter competitive relationships among plant species, and may assist establishment of invasive plant species (Frelich et al. 2006, Eisenhauer et al. 2012). For example, earthworms may promote the establishment of European Buckthorn (Rhamnus cathartica) and Garlic Mustard, which are better adapted to earthworm activity than native species (Frelich et al. 2006).
Population growth
The ever increasing human population of Ontario has had a direct effect on the population of West Virginia White. Most of the sites where the butterfly was first recorded in the province have disappeared due to development (Hess 1975). As the population grows, there is increased pressure on the amount and quality of habitat of the West Virginia White. Not only are woodlots being fragmented by forestry practices, roadways, utility right-of-ways and land clearing, the habitat is declining due to growing residential development. This is most pronounced in the heavily fragmented landscape of southern Ontario, particularly the counties of Halton, Peel, Hamilton, Brant, Wellington and Waterloo, where population growth from 2006 to 2011 continued at a rate of 5.9 to 11.8% per year (Statistics Canada 2012). The key impact of this population growth is seen through rural housing development and associated activities (e.g. walking and vehicle trails, wood removal) that eliminate mature hardwood forest, the important habitat of West Virginia White.
In Connecticut, populations of West Virginia White became extirpated in woodlots where residential development reduced fragment size and caused a decline in habitat quality (Brownell 1980). Development is also threatening the butterfly’s habitat at the interface of the southern Canadian Shield and St. Lawrence Lowlands due to cottage and other residential developments. This area holds many known and potentially undiscovered populations of the butterfly. Increased housing and development of small roadways through the habitat in these areas may pose a risk to Ontario’s population of West Virginia White. The associated increase in recreational land use also contributes to deterioration of habitat through activities such as use of all-terrain vehicles, dirt bikes, and mountain bikes through suitable butterfly habitat (B.Van Ryswyck, pers. comm. 2012). These activities may destroy host plant populations and/or expose soils to invasive plant establishment along with direct mortality upon adults by collisions with motorized vehicles.
The growing human population places high demands on aggregate resources. Aggregate extraction in southern Ontario is an economically vital industry that meets the needs of modern infrastructure development. In 2009 the per capita demand was 14 tonnes per person per year (OMNR 2010). Recently, it has been determined that the demand for these materials far exceeds the available supply, based on existing quarries in the province (OMNR 2010). As such the government recommends that "formal acceptance of these high priority aggregate resource areas where license applications would be encouraged (or at least not unduly hindered) with the recognition that such high priority areas be as close to market areas as possible," (OMNR 2010). Extraction of aggregates occurs across the range of West Virginia White in Ontario, including areas with underlying limestone, dolostone, sandstone, granite, gravel, sand, clay earth and shale. Extraction activities at these quarries may have an impact on the butterfly’s habitat. Such was the case with a large quarry in Halton RM which was adjacent to a known population of West Virginia White. The quarry expansion resulted in the extirpation of the population in 1991. A proposed quarry in Harvey Township of Peterborough County may impact the species due to habitat removal and regional forest cover loss. Although there are currently no records of the butterfly from the property of the quarry, a total of nine records exist from surrounding areas within two km (Ontario Butterfly Atlas database 2012). Areas along Ledge, Quarry, Ties Mountain, and Bass Lake roads, which serve as access to the proposed quarry, are limestone/marble based forests that are suitable habitat for West Virginia White (C.D. Jones, D. Sutherland, pers. comm. 2012) and may be vital to the larger regional population. The habitat requirements and conservation needs of the West Virginia White should be considered during aggregate removal that may cause habitat loss.
Unsustainable use
Pressure upon populations of West Virginia White by insect collectors in Ontario is likely very small, as the butterfly is found in over 50 sites (Ontario Butterfly Atlas database 2012) and there is likely a number of undiscovered populations (C.D. Jones, pers. comm. 2012) in suitable habitat. The increase in popularity of photography of the butterfly has become a more concerning issue at sites in Halton (B. Van Ryswyk, pers. comm. 2012) due to trampling of understory vegetation. However if the species continues to decline due to habitat loss, invasive species and disease, threats posed by collecting could become an issue. West Virginia White is a specially protected invertebrate under the Fish and Wildlife Conservation Act and a permit is required to collect it.
Pressure on its host plant, the Two-leaved Toothwort, as a garden transplant is not high due to its drab appearance and widespread occurrence in Ontario. However, harvesting of other spring ephemerals (e.g. Wild Leek, Allium tricoccum) for food has been increasing in areas like the Halton Regional Forest (B. Van Ryswyk, B. Yukich, pers. comm. 2012). This type of activity during the butterfly’s flight period could negatively impact the habitat if left unmonitored and unmanaged. In addition to being potentially disruptive to the butterfly, excessive trampling and soil disturbance could promote introduction of Garlic Mustard.
Climate change
A warming climate may have negative implications for this single-brooded butterfly that is dependent on a short-lived spring ephemeral host plant (Bess 2005). Reproductive success requires a balance in timing of toothwort emergence with larval development, in order for larval development to be complete before the plant dies. Unfavorable events associated with climate change (e.g. unusually early spring emergence such as in 2012 when it was recorded 4 April almost four weeks ahead of the average first emergence recorded (Apr. 30 (n=33) Ontario Butterfly Atlas Database 2012)) may result in reproductive failure. The short life-span of the host plant due to exceptionally early warm weather may leave relatively young larva with no food source. Prolonged cloudy, cool conditions during flight times may disrupt the timing between the butterfly and the host plant, causing widespread reproductive failure (Cappuccino and Kareiva 1985). These conditions may encourage invasive species such as Garlic Mustard (Bradley et al. 2009), resulting in declining populations of critical host plants. Frequent, serious stresses to the habitat such as drought, severe Gypsy Moth defoliations and storm damage leading to canopy opening may be also be the result of a changing climate. Consideration of the variable responses of different life stages is essential to understanding ecological and evolutionary consequences of climate change on the butterfly (Kingsolver et al. 2011). The West Virginia White’s response to such climate change events in Ontario is currently unknown, but pose challenges to any risk management planning.
4.0 Management
4.1 Management goal and objectives
The goal of this management plan is to maintain or improve the health of Ontario’s West Virginia White population. To reach this goal a set of objectives have been determined with measurable, time-defined approaches that involve a number of interested organizations, landowners, institutions, and government. The key management objectives include further clarification of main threats to West Virginia White populations, the development of Best Management Practices aimed at reducing the impacts of these habitat-related threats and a better understanding of the current status and range of the butterfly in Ontario. Critical to our understanding of the threats are studies related to the butterfly’s relationship with Garlic Mustard and impacts associated with the growth in human recreational activities. Known populations should be monitored and a thorough provincial inventory must be done to determine the status of Ontario’s population.
Table 1. Management objectives
| No. | Management Objective |
|---|---|
| 1 | Determine distribution and abundance of known populations and survey for new populations |
| 2 | Initiate research and monitoring on known populations especially related to threats |
| 3 | Maintain the current distribution and abundance of West Virginia White through habitat protection/stewardship and other measures |
| 4 | Re-evaluate At Risk status both provincially and nationally |
4.2 Management actions completed or underway
West Virginia White was listed as Endangered under the Ontario Endangered Species Act in 1977 (Brownell 1980) due to knowledge of only two populations at the time (Hess 1975). The highest population density was located in Halton Regional Forest (Hess 1975), which was then protected from development and given a management directive of little or no disturbance to maintain the ecosystem features required by the butterfly and its host plant. Inventories of the plant community (Catling et al. 1974) and studies of the life history of the butterfly (Plath 1975) were made by the provincial government and the Toronto Entomologist’s Association (TEA) in order to understand the species' needs. Subsequent field work however, revealed a more widespread population across southern Ontario, which resulted in the down-listing of West Virginia White to Special Concern in Ontario in 1990. A two-year study in 1990 to 1991 was made to further our understanding of the Ontario distribution of West Virginia White. A total of 213 new sites were visited, in addition to 117 historical sites. The likelihood of finding butterflies at new sites was about 25%. However the chance of finding butterflies was highly dependent on the distance of the site from previously known sites. There was a 48% chance of finding butterflies in appropriate habitat within 10 km of a known site from the previous year but the probability declined (2.6 to 11%) at a distance greater than 10 km (Mainguy 1991).
In 1981, Brownell reported that West Virginia White occurred in several conservation areas (Foley Mountain, Gould Lake and Clendenan Dam), a regional forest (Halton RM), a provincial park (Frontenac), as well as private lands (in Manitoulin, Frontenac, and Leeds & Grenville). Since then, the butterfly has been found in four more conservation areas (Crawford Lake, Hilton Falls, Rattlesnake Point, Mountsberg) and an additional provincial park (Charleston Lake) (Ontario NHIC database 2012). Suggestion of an introduction of a population in Victoria County near Head Lake during the 1980's is unfounded and was determined to have been overlooked as a naturally occurring population (C.D. Jones, pers. comm. 2012) since the more recent discovery of populations found within 50 km to the east in Peterborough County.
The OMNR's Stand and Site Guide (OMNR 2010) specifically addresses standards and guidelines for forestry practices in the presence of West Virginia White. The document offers an operational prescription and the conditions for roads, landings and aggregate pits in the area of concern. It prioritizes protection of the forest understory, harvesting activity outside of the frost-free period and prevention of introduction and establishment of invasive plants via machinery.
4.3 Management plan approaches for action
Table 2. Management plan approaches for action for the West Virginia White in Ontario
1.0 Determine distribution and abundance of known populations and survey for new populations
| Management Theme | Management Approach | Relative Priority | Threats or Knowledge Gaps Addressed | Relative Timeframe |
|---|---|---|---|---|
| Inventory and Monitoring | 1.1 Undertake monitoring of known populations.
|
critical |
|
Short-term |
| Education and Outreach Stewardship |
1.2 Develop educational materials for distribution to landowners, forest managers, land stewards, naturalist organizations, conservation authorities, aggregates industries, cottager’s associations, Parks Canada about West Virginia White, importance of reporting it and threats to its survival. Emphasis on species identification, reporting directives, threats posed by Garlic Mustard, habitat fragmentation, sensitivity to human disturbance of both the butterfly and the host plant, Beech Bark Disease and spraying for Gypsy Moth. | necessary |
|
short-term |
| Research Inventory and Monitoring |
1.3 Address gaps in current known range to better assess the population size and distribution. Inventory sites for at least several consecutive years where the butterfly has not been recorded since 2010. Develop a protocol to identify potential areas occupied by the butterfly, such as through use of GIS and overlays of calcareous habitats with preferred ecological land classification (ELC) forest types. Inventory potential sites during adult flight period. Report to NHIC of any findings. Employ a tiered approach: target known sites, then those of high quality in proximity to known sites, then those of suitable habitat and quality in geologically targeted regions. Continue to mine data from institutions and individuals that have yet to contribute records of occurrence. |
critical |
|
short-term |
2.0 Initiate research and monitoring on known populations especially related to threats
| Management Theme | Management Approach | Relative Priority | Threats or Knowledge Gaps Addressed | Relative Timeframe |
|---|---|---|---|---|
| Research | 2.1 Examine feeding and development of late instars in response to Garlic Mustard across a representative range of sites in Ontario. Assess the plant’s impact on West Virginia White in Ontario. |
critical |
|
Short-term |
| Research | 2.2 Investigate effects of climate change on West Virginia White and its habitats across a representative range of sites in Ontario. Study effects of early emergence on breeding success and synchrony with its host plant(s) development. |
necessary |
|
Long-term |
| Research | 2.3 In addition to existing literature, continue to document the characteristics of suitable habitat (e.g. patch size, tree species composition, ELC type, etc.) so that potential habitat can be mapped. This will also help better determine the area of concern associated with known populations within a crown land forest management context. | critical |
|
Short-term |
| Research | 2.4 Evaluate the effects of forest management operations on host plant abundance and overall habitat suitability. This includes studying the impacts of overall site disturbance, extraction trails and of residual stand structure using Growth and Yield standards (e.g. residual basal area, diameter frequency distribution). Empirical information of this nature is needed to evaluate and refine current forest management prescriptions for West Virginia White. | critical |
|
Short-term |
| Communications | 2.5 Liaise with researchers working on control of relevant invasive species e.g. Ontario Invasive Plants Council. Establish connections with other jurisdictions (e.g. New York, Ohio, Indiana, Michigan) that also contend with Garlic Mustard, Beech Bark Disease and Gypsy Moth spraying as threats to their West Virginia White populations. |
necessary |
|
ongoing |
3.0 Maintain the current distribution and abundance of West Virginia White through habitat protection/stewardship and other measures
| Management Theme | Management Approach | Relative Priority | Threats or Knowledge Gaps Addressed | Relative Timeframe |
|---|---|---|---|---|
| Communications | 3.1 Encourage collaboration among relevant agencies such as Ontario Ministry of Natural Resources (OMNR), Environment Canada (EC), Parks Canada, NGOs, and scientific community to develop and implement habitat conservation for the butterfly. | critical |
|
short-term |
| Protection Stewardship Inventory and Monitoring |
3.2 Work with partners to conserve key habitats through securement, stewardship and other effective forms of protection across a representative range in Ontario; especially sites with large populations, large forest interiors, proximal sites of quality habitat, minimal threats and stable futures. Create a database of sites that are protected. Determine the value of the site to the overall population in Ontario. |
critical |
|
short-term |
| Management Inventory and Monitoring |
3.3 Manage prioritized sites to improve/maintain habitat. Remove invasive species that pose a risk to the population. Employ risk management to determine best approach (e.g. combined use of manual and chemical treatment for Garlic Mustard). Assess and monitor impacts of BMPs employed. |
critical |
|
Ongoing |
| Communications Management |
3.4 Ease the effects habitat decline due to human population growth and disturbances such as those posed by forest management, habitat removal and roadways. Encourage good forest management practices (i.e. development of a stand management plan and prescription, follow silviculture recommendations in existing OMNR documents such as the Stand and Site Guide and Silviculture Guide to Managing Southern Ontario Forests) to address the needs of West Virginia White on public and private lands. Provide input to road planning processes (i.e. Ministry of Transportation, forest access, municipal, etc.) Use site specific measures such as rerouting of trails, signage and fencing for prioritized sites on public lands. Protection of spring ephemeral wildflowers that have collecting pressures, such as Wild Leek should be addressed where these exist. Encourage municipalities to include West Virginia White occupied habitats as Significant Wildlife Habitat and/or Significant Woodland under the Provincial Policy Statement. |
necessary |
|
short-term |
| Education and Outreach Stewardship Communications |
3.5 Establish connections with other governments, forest managers, landowners, aggregates industry, conservation authorities, cottager’s associations and first nations and encourage Best Management Practices that are compatible with the needs of West Virginia White and other mature forest species on their lands. Collaborate with other conservation initiatives with similar habitat and management needs such as Cerulean Warbler, Louisiana Waterthrush and American Ginseng, which all require mesic, sub-climax to climax hardwood/mixed forest habitat. |
necessary |
|
Short-term |
4.0 Re-evaluate At Risk status both provincially and nationally
| Management Theme | Management Approach | Relative Priority | Threats or Knowledge Gaps Addressed | Relative Timeframe |
|---|---|---|---|---|
| Protection | 4.1 Re-assess the status of West Virginia White in Ontario after a determined period of time in which research, management, stewardship, protection, inventory, and monitoring has helped to clarify our gaps in knowledge. Encourage re-assessment of the species after 5 field seasons to determine whether to maintain as Special Concern, down-list to Not at risk or to change to Threatened or Endangered. Encourage COSEWIC to consider candidacy based on whether results of these efforts show continued declines. |
beneficial |
|
Long-term |
Glossary
Aestivation: the state of dormancy entered into by the pupa of the butterfly in response to the heat and dry conditions of the summer months.
Allelopathic: the production of biochemicals by an organism that affect the growth, survival or reproduction of another organism. These alleochemicals in Garlic Mustard usually cause these negative effects on native plants.
Arbuscular mycorrhizal fungi (AM): help plants to capture nutrients such as nitrogen, phosphorus, sulfur and micronutrients from the soil. This is a type of symbiosis between a fungi and a host plant commonly found in the roots of many spring ephemeral forest species.
Asynchronous: timing of events that are not at the same time. West Virginia White is evolved to be synchronous in its life cycle with members of the toothwort family so that it has a host plant to lay eggs and develop its larvae upon. Unpredictable cold spring weather or unusually hot weather may make the timing between the two organisms asynchronous.
Biennial: a plant which blooms in its second year and then dies. Garlic Mustard starts its first year as a basal rosette which then overwinters, and flowers the following spring.
Brassicaseae: This is a large family with many plants of major economic importance, including many familiar vegetables (Cabbage, Turnip), oil crops (Oil-seed Rape). They are found more or less all over the world, with most species occurring in the north temperate region. Native species found in mature hardwood forests in Ontario belong mainly to the genus Cardamine (formerly Dentarium) or Toothworts.
Cambial: the "living" part of the tree that is composed in part, of the vascular cambium, responsible for flow of nutrients and water to all parts of the plant.
Carbaryl: a commercially produced insecticide made from Carbamic Acid, popular for lawn care and some forestry applications. Carbaryl works by attacking the nervous system and has a broad scale effect on organisms.
Committee on the Status of Endangered Wildlife in Canada (COSEWIC): The committee responsible for assessing and classifying species at risk in Canada.
Committee on the Status of Species at Risk in Ontario (COSSARO): The committee established under section 3 of the Endangered Species Act, 2007 that is responsible for assessing and classifying species at risk in Ontario.
Conservation status rank: A rank assigned to a species or ecological community that primarily conveys the degree of rarity of the species or community at the global (G), national (N) or subnational (S) level. These ranks, termed G-rank, N-rank and S-rank, are not legal designations. The conservation status of a species or ecosystem is designated by a number from 1 to 5, preceded by the letter G, N or S reflecting the appropriate geographic scale of the assessment. The numbers mean the following:
1 = critically imperilled
2 = imperilled
3 = vulnerable
4 = apparently secure
5 = secure
Crucifer: any plant belonging to the family Brassicaceae, formerly called the Cruciferae.
Diapause: is the delay in development in response to regularly and recurring periods of adverse environmental conditions. West Virginia White enters into diapause, as a pupa, to endure the predictable seasons of summer, fall and winter where conditions are not favorable for growth or reproduction.
Dorsal: the top surface of a butterfly; in opposition to ventral, the bottom side from where the legs emerge.
Element Occurrence: an occurrence of an element of biodiversity on the landscape; an area of land on which an element (e.g. species or ecological community) is or was present. An EO has conservation value for the element: it is a location important to the conservation of the species or community. For a species, an EO is generally the habitat occupied by a local population.
Endangered Species Act, 2007 (ESA): The provincial legislation that provides protection to species at risk in Ontario.
Ephemeral: pertaining to a group of vascular plants that take advantage sunlight reaching the forest floor due to the leafless canopy trees in a forested habitat during the spring. They usually complete their reproductive cycle and then die back (senesce) when leaf out plunges the forest into shade.
Extirpated: occurs when the population of an organism is completely eliminated. Re-establishment requires immigration/introduction from another population.
Frontal prominence: an elongated structure at the anterior end of the pupa, distinctively long on West Virginia White compared to similar species such as Mustard White or Cabbage White.
Host plant: A species of plant upon which an insect, such as West Virginia White, lays its eggs upon, the larvae hatch, and then develop while using the plant as food. Thus the insect is closely tied to the host plant for survival. The host plant for the butterfly is Two-leaved Toothwort (Cardamine diphylla).
Forewing: The set of wings that emerge from the body of a butterfly anteriorly.
Hindwing: The set of wings that emerge from the body of a butterfly posteriorly.
Instar: The stages of growth and physiological development of an insect larvae, involving the shedding of skin and subsequent increase in size. West Virginia White has five instars in its larval development.
Invasive: An organism that adversely affects the habitats and landscapes they invade economically, environmentally, and/or ecologically. Often invasive species end up dominating a region due to various factors that make them successful.
k-strategist: an organism that expends high cost in reproduction for a low number of more difficult to produce offspring. Alternatively, an r-strategist is a species prone to numerous reproduction at low cost per individual offspring.
Lepidoptera: insects that belong to the butterfly and moth families.
Mesic: a descriptive term to indicate moist, well-watered conditions of a habitat.
Metapopulation: a set of individuals that make up a population which interact with each other within a specified area. A metapopulation of West Virginia White butterflies may comprise of several sites that are uninhibited by continuous habitat which allows them to exchange genetic traits.
Mustard Family: a member of the Brassicaceae Family.
Nectar: a verb used to describe the feeding behavior of butterflies on flowers; the process of gathering nectar as a food source.
Oligophagous: Feeding on a restricted range of food substances, especially a limited number of plants.
Pupa(e): In holometabolous insects, the part of the life cycle in which transformation occurs. In butterflies this is also called the chrysalis. The chrysalis is covered in a protective cocoon.
Phytochemical: Chemical compounds that plants naturally produce; some may be employed in plant defence, such as sinigrin produced by Garlic Mustard.
Prominence: a physical projection that is noticeable on the pupae (cocoon) of a butterfly. West Virginia White has a large prominence that differentiates it from similar species.
Risk management: management approaches taken to lower invasive species risk to an acceptable level. Furthermore, it involves evaluating different risk management options and determining which is the most appropriate for a given species and the species affected.
Seedbank: this is the naturally occurring store of seeds existing in the soil for a defined amount of time that are viable and may contribute to a plant population.
Senescence: the period of decline in the parts of an annual plant growing above ground (i.e. the leaves, stem and flowers).
Species at Risk Act (SARA): The federal legislation that provides protection to species at risk in Canada. This act establishes Schedule 1 as the legal list of wildlife species at risk to which the SARA provisions apply. Schedules 2 and 3 contain lists of species that at the time the Act came into force needed to be reassessed. After species on Schedule 2 and 3 are reassessed and found to be at risk, they undergo the SARA listing process to be included in Schedule 1.
Species at Risk in Ontario (SARO) List: The regulation made under section 7 of the Endangered Species Act, 2007 that provides the official status classification of species at risk in Ontario. This list was first published in 2004 as a policy and became a regulation in 2008.
Suffused: to be colored in muted tones, not brilliant or highly obvious tones.
Vein: Hollow structures formed from the coupling of the upper and lower walls of the wing. They provide both rigidity and flexibility to the wing. They run outwards from the body and in some species of butterfly are differentially colored (e.g./ Monarch or Mustard White).
References
Alverson, W.S., D. M. Waller and S. L. Solheim, S.L. 1988. Forests too deer: edge effects in northern Wisconsin. Conservation Biology 2: 348– 358.
Anulewicz, A.C., D.G. McCullough and D.L. Cappaert. 2007. Emerald ash borer (Agrilus planipennis) density and canopy dieback in three North American ash species. Arboriculture and Urban Forestry 33:338-349.
Arnold H., 2007. Control methods for the invasive plant Garlic Mustard (Alliaria petiolata) within Ontario Natural Areas. V 1.0. Southwestern Ontario Region, London, Ontario. 16 pp.
Awkerman, J.A., M.R. Marshall, A.B. Williams, G.A. Gale, R.J. Cooper and S. Raimondo. 2011. Assessment of indirect pesticide effects on Worm-eating Warbler populations in a managed forest ecosystem. Environmental Toxicology and Chemistry 30(8):1843-1851.
Barto, E.K. and D. Cipollini. 2009. Garlic Mustard (Alliaria petiolata) removal method affects native establishment. Invasive Plant Science and Management. 2:230-236.
Barto, E.K., P.M. Antunes, K. Stinson, A.M. Koch, J.N. Klironomos and D. Cipollini. 2010a. Differences in arbuscular mycorrhizal fungal communities associated with sugar maple seedlings in and outside of invaded Garlic Mustard forest patches. Biological Invasions 13:2755-2762.
Barto, E.K., J.R. Powell and D. Cipollini. 2010b. How novel are the chemical weapons of Garlic Mustard in North American forest understories? Biological Invasions 12:3465-3471.
Benson J., R.G. Van Dreische, A. Pasquale, J. Elkington. 2003. Introduced Braconic parasitoids and range reduction of a native butterfly in New England. Biological Control 28:197-213.
Bess, J. 2005. Conservation Assessment for the West Virginia White (Pieris virginiensis Edwards) USDA Forest Service, Eastern Region 39 pp.
Blossey,B., V.A. Nuzzo, H.L. Hinz and E. Gerber. 2002. Garlic Mustard in Biological Control of Invasive Plants in the Eastern United States. Authors R. Van Driesche, B. Blossey, M. Hoddle, S. Lyon and R. Reardon. USDA Forest Service Publication FHTET-2002-04.
Boettner, G., J. Elkington and C. Boettner. 2000. Effects of a biological control introduction on three nontarget native species of saturniid moths. Conservation Biology 14(6):1798-1806.
Bohlen, P.J., S. Scheu, C. M. Hale, M. A. McLean, S. Migge, P. M. Groffman and D. Parkinson. 2004. Non-native invasive earthworms as agents of change in northern temperate forests. Frontiers in Ecology and the Environment 2: 427-435.
Bowden,S.R. 1971. American white butterflies (Pieridae) and English host plants. Journal of the Lepidopterist’s Society 25:6-12.
Bradley, B.A., D.A. Blumenthal, D.S. Wilcove and L.H. Ziska. 2009. Predicting plant invasions in an era of global change. Trends in Ecology and Evolution 25(5):310-318.
Brownell, V.R. 1980. The West Virginia White (Artogeia virginiensis Edwards) in Canada: A Status Report. Nongame Program, Wildlife Branch, Ontario Ministry of Natural Resources. Toronto 55 pp.
Burke, D. M., K. A. Elliott, S. B. Holmes and D. Bradley. 2008. The effects of partial harvest on the understory vegetation of southern Ontario woodlands. Forest Ecology and Management 255: 2204–2212.
Callaway, R.M., D. Cipollini, K.E. Barto, G.C. Thelen, S.G. Hallett, D. Prati, K. Stinson and J. Klironomos. 2008. Novel weapons: invasive plants suppresses fungal mutualists in America but not in its native Europe. Ecology 89:1043-1055.
Canadian Forest Service. 2001. Front Line Express. Great Lakes Forestry Centre, Ontario Region, Sault Ste. Marie, Ontario.
Cappuccino, N. and P. Kareiva. 1985. Coping with a capricious environment: a population study of a rare pierid butterfly. Ecology 66:152-161.
Carlson, A.M. and D.L. Gorchov. 2004. Effects of Herbicide on the Invasive Biennial Alliaria petiolata (Garlic Mustard) and Initial Responses of Native Plants in a Southwestern Ohio Forest. Restoration Ecology 12(4):559-567.
Catling, P.M., K.L. McIntosh and S.M. McKay. 1974. Catling, P.M., McIntosh, K.L., McKay, S.M. A preliminary checklist of the Vascular plants of the Currie Tract, Halton County Forest, Ontario, In Hess Q.F. 1975. West Virginia White Edwards in Ontario. Toronto Entomologists' Association. Occasional Publication No. 5. Toronto, Ont.
Cech, R. and G. Tudor. 2005. Butterflies of the east coast: an Observer’s Guide. Princeton University Press, Princeton, New Jersey. 345 pp.
Chew, F. S. 1981. Coexistence and local extinction in two Pieris butterflies. American Naturalist 118: 655-672.
Cipollini, D., and B. Gruner. 2007. Cyanide in the chemical arsenal of Garlic Mustard (Alliaria petiolata). Journal of Chemical Ecology 33:85-94.
Collins, B.S., K. P. Dunne and S. T. A. Pickett. 1985. Responses of forest herbs to canopy gaps. pp. 218–34 In S. T. A. Pickett and P. S. White, eds., The ecology of Natural Disturbance and Patch Dynamics. London: Academic Press.
Cooper, G. 1980. A Study of the West Virginia White Butterfly Pieris virginiensis, in Frontenac County, Ontario – Ministry of Natural Resources, Wildlife Branch, Toronto, Ontario. Unpublished. 38 pp.
Cooper, R.J., K.M. Dodge, P.J. Martinat, S.B. Donahoe and R.C. Whitmore. 1990. Effect of Diflubenzuron Application on Eastern Deciduous Forest Birds. Journal of Wildlife Management 54 (3):486-493.
Côté, S.D., T. P. Rooney, T.P., J. P. Tremblay, C. Dussault and D. M. Waller. 2004. Ecological impacts of deer overabundance. Annual Review of Ecology Evolution and Systematics 35: 113–147.
Courant, A.V., A.E. Holbrook, E.D. Van der Reijden, and F.S. Chew. 1994. Native pierine butterfly (Pieridae) adapting to naturalized crucifer? Journal of the Lepidopterist’s Society 48(2):168-170.
Cromartie, W.J., Jr. 1975. Influence of Habitat on Colonization of Collard Plants by Pieris rapae. McGill University Ecological Agriculture Projects Website. Quebec.
Doak, P., P. Karieva and J. Kingsolver. 2006. Fitness Consequences of Choosy Oviposition for a Time-Limited Butterfly. Ecology 87(2):395-408.
Eavers, A. K., A. M. Gordon, P.A. Gray and W. I. Dunlop. 2012. Implications of a Potential Range Expansion of Invasive Earthworms in Ontario’s Forested Ecosystems: A Preliminary Vulnerability Analysis. Ministry of Natural Resources, Climate Change Research Report, CCRR-23. 36pp.
Eisenhauer, N., N. A. Fisichelli, L. E. Frelich and P. B. Reich. 2012. Interactive effects of global warming and 'global worming' on the initial establishment of native and exotic herbaceous plant species. Oikos 121: 1121-1133.
Frankland, F. and T. Nelson. 2003. Impacts of white-tailed deer on spring wildflowers in Illinois, USA. Natural Areas Journal 23: 341-348.
Frelich, L.E., C. Hale, S. Scheu, A. Holdsworth, L. Heneghan, P. Bohlen and P. Reich. 2006. Earthworm invasion into previously earthworm-free temperate and boreal forests. Biological Invasions 8: 1235–1245.
Gandhi, K. J. K. and D. A. Herms. 2010. Direct and indirect effects of alien insect herbivores on ecological processes and interactions in forests of eastern North America. Biological Invasions 12:389-405.
Gilliam, F. S., and N. L. Turrill. 1993. Herbaceous-layer cover and biomass in a young versus a mature stand of a Central Appalachian hardwood forest. Bulletin of the Torrey Botanical Club 120: 445-450.
Gilliam, F. S. and M. R. Roberts MR. 2003. The herbaceous layer in forests of Eastern North America. New York: Oxford University Press.
Glassberg, J. Butterflies through Binoculars. The East. 1999. Oxford University Press.
Gochfeld, M. and J. Burger. 1997. Butterflies of New Jersey. Rutgers University Press: Rutgers, New Jersey. 327 pp.
Hajek, A.E., L. Butler, J.K. Leibherr and M.M. Wheeler. 2000. Risk of Infection by the Fungal Pathogen Entomophaga maimaiga Among Lepidoptera on the Forest Floor. Environmental Entomology 29(3):645-650.
Hajek, A.E. 2001. Larval behavior in Lymantria dispar increases risk of fungal infection. Oecologia 126:285-291.
Hajek, A.E., L.Butler and M. Wheeler. 1995. Laboratory bioassays testing the host range of the Gypsy Moth fungal pathogen Entomophaga maimaiga. Biological Control 5:530-544.
Hale, C.M., L. E. Frelich and P. B. Reich. 2006. Changes in hardwood forest understory plant communities in response to European earthworm invasions. Ecology, 87: 1637– 1649.
Halpern, C. B. and T. A. Spies. 1995. Plant-species diversity in the natural and managed forests of the Pacific-Northwest. Ecological Applications 5: 913-934.
Haribal M., Z. Yang, A.B. Attygalle, R.A.A. Renwick and J. Meinwald. 2001. A cyanoallyl glucoside from Alliaria petiolata as a feeding deterrent for larvae of Pieris napi oleracea. Journal of Natural Productivity. 64:440-443.
Hausman, C. E. 2010. The Ecological impacts of the Emerald Ash Borer (Agrilus planipennis): Identification of conservation and forest management strategies. Ph. D. Thesis, Kent State University College of Arts and Sciences, 162 pages.
Hess, Q.F. 1975. West Virginia White Edwards in Ontario. Toronto Entomologists' Association Occasional Publication No. 5. Toronto, Ont.
Holdsworth, A.R., L. E. Frelich and P. E. Reich. 2007. Effects of earthworm invasion on plant species richness in northern hardwood forests. Conservation Biology 21: 997–1008.
Horseley, S. B., S. L. Stout and D. S. DeCalesta. 2003. White-tailed Deer impact on the vegetation dynamics of a northern hardwood forest. Ecological Applications 13: 98-118.
Hovanitz, W. 1963. the relation of Pieris viriginiensis Edw. to Pieris napi L.: Species formation in Pieris? Journal of Research on the Lepidoptera 1(2):124-134.
Huang, W., J. Carrillo, J. Ding and E. Siemann. 2012. Interactive effects of herbivory and competition intensity determine invasive plant performance. Oecologia 170:373- 382.
Huang, X.A., J.A.A. Renwick and F.S. Chew. 1995. Oviposition stimulants and deterrents control acceptance in Alliaria petiolata in Pieris rapae and P. napi oleracea. Chemoecology 5(6):79-87.
Johnson, K.S., J.M. Scriber, J.K.Nitao and D.R. Smitley. 1995. Toxicity of Bacillus thuringiensis var. kustaki to three nontarget Lepidoptera in field studies. Environmental Entomology 24:488-297.
Jones, C.D. 2012. Project Biologist, Natural Heritage Information Centre, OMNR. Personal communication.
Jones, C.D., Ross Layberry, and Alan Macnaughton. Ontario Butterfly Atlas Online. (5 November, 2012). Toronto Entomologists'.
Katovich, E., Becker, R.L., Ragsdale, D.W., and Skinner. L. 2005. Host testing of Garlic Mustard (Alliaria petiolata) biocontrol insects in Minnesota. Pp. 17-19 in L. Skinner (ed.). Proceedings: Symposium on the Biology, Ecology, and Management of Garlic Mustard (Alliaria petiolata) and European Buckthorn (Rhamnus cathartica), University of Minnesota, St. Paul Campus, St. Paul, MN. USDA Forest Service Publication FHTET-2005-09.
Keeler M.S. and F.S. Chew. 2008. Escaping an evolutionary trap: preference and performance of a native insect on an exotic invasive host. Oecologia 156:559-568.
Kingsolver, J.G., H.A. Woods, L.B. Buckley, K.A. Potter, H.J. McLean, J.K. Higgins. 2011. Complex Life Cycles and the Response of Insects to Climate Change. Integrative and Comparative Biology 51(5):719-732.
Klots, A.B. 1935. On the life history of West Virginia White Edwards (Lep., Pieridae). Journal of the Entomological Society 33:139-142.
Klots, A. B. 1954. A Field Guide to the Butterflies of North America East of the Great Plains. Peterson Field Guide Series. Houghton Mifflin Company, Boston, MA.
Koch, A.M., P.M. Antunes, E.K. Barto, D. Cipollini, D.L. Mummey and J.N. Klironomos. 2011. The effects of arbuscular mycorrhizal (AM) fungal and Garlic Mustard introductions on native AM fungal diversity. Biological Invasions 13:1627-1639.
Lankau, R.A. 2011. Interpopulation variation in allelopathic traits informs restoration of invaded landscapes. Evolutionary Applications. Blackwell Publishing Ltd. 5(2012) 270-282.
Lankau, R.A. 2012. Coevolution between invasive and native plants driven by chemical competition and soil biota. Proceedings of the National Academy of Natural Sciences of the United States of America. 109(28): 11240-11245.
Layberry R.A., P.W. Hall and J.D. Lafontaine. The Butterflies of Canada. 1998. University of Toronto Press Inc.
LeGrande, H. and T. Howard. 2010. Notes on the butterflies of North Carolina, 17th Approximation. Online. Available: http://149.168.1.196/nbnc/index.html [link no longer active]
Lyons, D.B. 2010. Emerald Ash Borer Biology pp. 4-8 in D.B. Lyons and T.A. Scarr. Workshop Proceedings: Guiding Principles for Managing the Emerald Ash Borer in Urban Environments. Canadian Forest Service. Sault Ste. Marie. 44 pp.
Mainguy, S. 1991. A Two Year Monitoring Study of the West Virginia White Butterfly: Final Report. Prepared for the Ontario Ministry of Natural Resources. The Landplan Collaborative Ltd., Guelph, Ontario. Unpublished report. 79 pp.
Martin, B.J., 1980. Report on the West Virginia White Butterfly (Pieris virginiensis Edwards), in Central and Southwestern Ontario. Ontario Ministry of Natural Resources, Wildlife Branch, Toronto, Ont. Unpublished. 59 pp.
McKay, S.M., 1976. Halton County Forest: The Flora, and The Habitat of the West Virginia White Butterfly (Pieris virginiensis). Ontario Ministry of Natural Resources. Toronto, Unpublished. 49 pp.
McLaughlin, J. and S. Greifenhagen. 2012. Beech Bark Disease in Ontario: A primer and Management Recommendations. Ontario Forest Research Institute, Ontario Ministry of Natural Resources, Sault Ste. Marie, Ontario.
Morton, J.K. and R.R. Tasker. 1980. The Status of the Virginia White Butterfly on Manitoulin Island. Canadian Field Naturalist. 94(3): 325-327.
Myers, J.A., M. Vellend, S. Gardescu and P.L. Marks. 2004. Seed dispersal by white- tailed deer: implications for long distance dispersal, invasion and migration of plants in eastern North America. Oecologia 139:35–44.
NatureServe, 2012. NatureServe Explorer: An online encyclopedia of life (web application). Version 7.1 D. F. Schweitzer. Pieris virginiensis – West Virginia White. NatureServe, Arlington, Virginia. (Accessed October 17, 2012).
Nealis V.G. and S. Erb. 1993. A sourcebook for the management of Gypsy Moth. Forestry Canada, Ontario Region, Great Lakes Forestry Centre, Sault Ste. Marie.
Nuzzo, V.A. 1999. Invasion pattern of the herb Garlic Mustard (Alliaria petiolata) in high quality forests. Biological Invasions 1:169-179.
Ontario Biodiversity Council (OBC). 2010b. State of Ontario’s Biodiversity 2010. A report of the Ontario Biodiversity Council, Peterborough, Ontario.
Ontario Biodiversity Council. 2011. Ontario’s Biodiversity Strategy, 2011: Renewing Our Commitment to Protecting What Sustains Us. Ontario Biodiversity Council, Peterborough, ON.
Ontario Butterfly Atlas Database online, 2012. Accessed 5 November, 2012. Toronto Entomologists'.
Ontario Ministry of Natural Resources. 1991. Gypsy Moth in Ontario: Facts about protecting property against Gypsy Moth infestation. Ontario Ministry of Natural Resources and the Gypsy Moth Management Committee.
OMNR. 2000. A Silvicultural Guide to Managing Southern Ontario Forests. Toronto: Queen’s Printer for Ontario. 661 pp.
Ontario Ministry of Natural Resources. 2010. State of the Aggregate Resource in Ontario Study (SAROS) Paper 5-Aggregate Reserves in Existing Operations. Report #09-1112-0064.
Ontario Ministry of Natural Resources, 2010. Forest Management Guide for Conserving Biodiversity at the Stand and Site Scales. Toronto: Queen’s Printer for Ontario. 211 pp.
Opler, P.A. 2004. Butterflies of North America: West Virginia White (Pieris virginiensis). USGS Northern Prairie Wildlife Research Center. Fargo, ND. http://www.npwrc.usgs.gov/resource/destr/lepid/bflyusa/usa/779.htm [link no longer active]
Opler, P.A. and V. Malikul. 1992. Eastern Butterflies (Peterson Field Guide). Houghton Mifflin Company, Boston, Massachusetts. 396 pp. + color plates.
Opler, P. A., K. Lotts, and T. Naberhaus, coordinators. 2012. Butterflies and Moths of North America. Data set accessed 21 November 2012.
Orr, M. 2010. Emerald Ash Borer Distribution Update and Regulatory Issues. Pp. 9-10 in D.B. Lyons and T.A. Scarr. Workshop Proceedings: Guiding Principles for Managing the Emerald Ash Borer in Urban Environments. Canadian Forest Service. Sault Ste. Marie. 44 pp.
Otvos, I.S. Integrated Pest Management in Forestry: Potential and Challenges. Pp. 205-254 as cited in Koul, O., G.S. Dhaliwal, G.W. Cuperus. 2004. Integraded Pest Management: Potential, Constraints and Challenges. Centre for Agricultural Bioscience International (CABI) publishing. Wallingford, Oxon, UK.
Peacock, J.W., D.F. Schweitzer, J.L. Carter and N.R. Dubois. 1998. Laboratory assessment of the effects of Bacillus thuringiensis on native Lepidoptera. Environmental Entomology 27(2):450-457.
Plath, W. Jr.. 1975. Life history of West Virginia White Edwards based on observations from 1969 to 1974 in the Halton County Forest Area. Toronto Entomologists' Association Occasional Publication No. 5, Toronto, Ont.
Porter, A. 1994. Implications of introduced Garlic Mustard (Alliaria petiolata) in the habitat of Pieris virginiensis (Pieridae). Journal of the Lepidopterist’s Society 48(2):171-172.
Prati, D. and O. Bossdorf. 2004. Allelopathic inhibition of germination by Alliaria petiolata (Brassicaceae). American Journal of Botany. 91:285-288.
Renwick, J.A.A., W.Q. Zhang, M. Haribal, A.B. Attygalle and K.D. Lopez. 2001. Dual chemical barriers protect a plant against different larval stages of an insect. Journal of Chemical Ecology. 27:1575-1583.
Roberts, K.J. and R.C. Anderson. 2001. Effects of Garlic Mustard [Alliaria petiolata (Bieb. Cavara & Grande)] extracts on plants and arbuscular mycorrhizal (AM) fungi. American Midland Naturalist. 146:146-152
Rooney, T.P. 2001. Deer impacts on forest ecosystems: a North American perspective. Forestry 74: 201–208.
Scarr, T.A., 2012. Provincial Forest Entomologist. Sault Ste. Marie. OMNR. Personal communication.
Scott, J. 1986. The Butterflies of North America: A Natural History and Field Guide. Stanford University Press, Stanford, California.
Schweitzer, D.F. 2004. Gypsy Moth (Lymantria dispar): Impacts and options for Biodiversity-Oriented Land Managers. 59 pp. NatureServe Explorer. Arlington, Virginia.
Shapiro, A.M. 1971. Occurrence of a latent polyphenism in West Virginia White Pieris virginiensis (Lepidoptera: Pieridae) Entomological News 82:13-16.
Shuey J.A. and J.W. Peacock. 1989. Host Plant Exploitation by an Oligophagous Population of Pieris virginiensis (Lepidoptera; Pieridae). American Midland Naturalist 122(2):255-261.
Simberloff, D. and B. Von Holle. 1999. Positive interactions of nonindigenous species: invasional meltdown? Biological Invasions 1:21–32.
Solis, K., 1998. Update: new results indicate flowering Garlic Mustard should be bagged and destroyed (Wisconsin). Restoration and Management Notes 16:223-224.
Stanton E.J. 2001. Status of Pieris virginiensis (Pieridae) in New York State. Journal of the Lepidopterists' Society 55(3): 122-123.
Statistics Canada, 2012. 2006 and 2011 Censuses of Canada. Produced by the Geography Division, Statistics Canada, 2012.
Stewart, W.G. 1988. The West Virginia White (Artogeia virginiensis W.H. Edwards) in Elgin and Middlesex Counties, Ontario. The Cardinal. 132:18-19. The McIlwraith Field Naturalists. London, Ontario, Canada.
Sutherland, D. 2012. Zoologist. Natural Heritage Information Centre, OMNR. Personal communication.
Sutherland, S. and C. R. Nelson. 2010. Nonnative plant response to silvicultural treatments: A model based on disturbance, propagule pressure, and competitive abilities. Western Journal of Applied Forestry 25:27-33.
Tanis, S.R. and D. G. McCullough. 2012. Differential persistence of blue ash and white ash following emerald ash borer invasion. Canadian Journal of Forest Research 42:1542-1550.
Tasker, R.R. 1975. West Virginia White Edwards in the Manitoulin Island area. In Hess, Q.F. 1975. West Virginia White Edwards in Ontario. Toronto Entomologists' Association Occasional Publication. No. 5. Toronto, Ont.
Tobin, P.C., B.B. Bai, D.E. Eggen and D.S. Leonard. 2012. The ecology, geopolitics, and economics of managing Lymantria dispar in the United States. International Journal of Pest Management 58(3): 195-210.
Van Ryswyk, B. 2012. Biologist. Conservation Halton. Personal communication.
Vaugh S.F. and M.A. Berhow 1999. Allelochemicals isolated from tissues of the invasive weed Garlic Mustard (Alliaria petiolata). Journal of Chemical Ecology 25:2495-2504.
Wagner, W.H. Jr. 1978. The Northern Great Lakes White, Pieris virginiensis, (Lepidoptera: Pieridae) in comparison with its southern Appalachian counterpart. Great Lakes Entomologist 11:53-57.
Wagner, D.L., J.W. Peacock, J.L. Carter and S.E. Talley. 1996. Field assessment of Bacillus thuringiensis on nontarget Lepidoptera. Environmental Entomology 25(6):1444- 1454.
Welk, E., K. Schubert and M.H. Hoffman. 2002. Present and potential distribution of invasive Garlic Mustard (Alliaria petiolata) in North America. Diversity and Distributions8:219-233.
Williams S. C. and J. S. Ward. 2006. Exotic seed dispersal by white-tailed deer in southern Connecticut. Natural Areas Journal 26: 383–390.
Whigham, D.F. 2004. Ecology of woodland herbs in temperate deciduous forests. Annual Review of Ecology Evolution Systematics 35: 583–621.
Yahner, R.H. 1998. Butterfly and skipper use of nectar sources in forested and agricultural landscapes of Pennsylvania. Journal of the Pennsylvania Academy of Science 71:104-108.
Yates, C.N. and S.D. Murphy. 2008. Observation of herbivore attack on Garlic Mustard (Alliaria petiolata) in southwestern Ontario, Canada. Biological Invasions. 10:757-760.
Yukich, B., 2012. Toronto Entomological Association. Toronto. Personal communication.